1. Introduction
Multiple sclerosis (MS) is a chronic, inflammatory, and immune-mediated [
1] central nervous system (CNS) disease that results in the destruction of the myelin sheath of neuronal axons and can even lead to neurodegeneration [
2]. MS is the most prevalent chronic inflammatory disease of the CNS [
3], with an incidence three times higher in women than in men [
4]. The clinical manifestations of MS can vary, with complex symptoms in the sphere of sensitivity, proprioception, balance, vision, mobility, muscle performance, sphincter control, and cognition [
5]. Exacerbations occur frequently over time, followed by partial or total functional recovery (relapsing–remitting MS, RRMS), although MS can also present with a progressive accumulation of physical and cognitive deficits after a period of exacerbations (secondary progressive, SPMS) or without a prior phase of exacerbations (primary progressive, PPMS). In any of the evolutionary forms of MS, the accumulation of these deficits often leads to a progressive reduction in quality of life and deterioration of functional capacity [
6].
From a physiological point of view, the manifestation of muscular force is the result of tension produced from contraction by the sarcomeres of the muscle cells. The magnitude of the tension generated by the contraction of a muscle or group of muscles is related, in part, to the type of motor units recruited, the amount of existing muscle mass, and the length and arrangement of muscle fascicles [
7]. Muscle strength and size are significantly correlated (correlation adjusted for age and sex with a value of r = 0.26 [
8]), but the magnitude of the correlation between muscle size and strength varies depending on the muscle group analyzed [
9] and the type of strength evaluated (muscular power, maximum dynamic strength, and maximum isometric strength) [
10,
11,
12]. The loss of muscle mass is associated with a loss of strength and functionality (e.g., in healthy elderly [
13] and hemodialysis patients) [
14]. However, the degree of strength loss is higher than the degree of muscle mass loss [
15]. On the other hand, several studies found that the degree of disability is more strongly associated with a loss of strength than of muscle mass [
16], but also noted an association between the degree of functionality in physical activities related to daily life (e.g., walking speed or getting up and sitting in a chair) and the degree of muscle quality (MQ), expressed as the manifested strength divided by the activated muscle mass [
17].
Muscular performance (isometric strength, dynamic strength, and muscular power) is frequently affected in MS patients, especially in the lower extremities [
18,
19]. Factors such as gender, age, type of MS, and degree of neurological disability assessed according to the Expanded Disability Status Scale (EDSS) [
20] have also been related to the ability to produce force, and some reference values are currently available for different manifestations of force among MS patients (men and women) with different types and degrees of neurological disability [
21]. Compared to healthy individuals, patients with MS present a loss of muscle mass, a lower pennation angle, thinner muscle fibers on the basis of diameter, and a higher proportion of type II fibers, as well as the reduced ability to fully activate motor units and a reduced rate of force development [
18,
22,
23,
24]. The lower muscular performance of MS patients is accentuated in dynamic contraction exercises performed at high speed [
25,
26]. Muscle power (load × displacement/time) is a strong predictor of the degree of functional disability [
27], which is why interest has grown in analyzing the effects of physical exercise programs based on muscular power work (exercises developed at high execution speeds) of the lower limbs (MMII) [
28]. For these reasons, physical exercise is widely recommended for patients with MS [
29,
30]; in recent years, the importance of strength training has been especially emphasized among these patients [
31].
The degree of functionality is not associated with muscle mass to the same extent as with muscle strength; indeed, throughout a patient’s functional decline, mass loss does not follow the same trajectory as strength loss [
32]. These observations have contributed to increased interest in the study of MQ in recent years, especially in the context of aging, sarcopenia, or disability [
33,
34,
35,
36,
37].
The concept of muscle quality (MQ) is interesting, as MQ provides an index for the level of functionality of muscle tissue relative to its mass. For example, this index was studied in relation to the ability to use glucose, oxidative damage, metabolism proteins, and muscle density [
38]. However, publications that use the force production index in relation to muscle mass are more common [
39]. This relationship is a key determinant of muscle function in adulthood [
27]. MQ is considered a better marker of functional capacity than absolute strength or muscle mass [
40]. One of the difficulties presented by the study of MQ is the lack of a widely accepted methodology for its evaluation. From the perspective of physical capacity, studies related to MQ have determined muscle mass (actually lean mass or fat-free mass) and cross-sectional muscle (CSA) using ultrasonography, bioimpedance analysis (BIA), dual-energy X-ray absorptiometry (DXA), computed tomography (CT), and magnetic resonance imaging (MRI) [
41]. These studies have measured the strength manifested as isokinetic strength [
42], isometric strength [
43], or maximum strength (one rep maximum, 1 RM) [
44]. Published scientific articles related to MQ have frequently used the elderly as study subjects, but not patients with MS. The interest shown in scientific publications on MQ research and, on the other hand, the lack of studies carried out on this topic in MS patients, highlight the need and relevance of such studies.
The purpose of this study was to compare the MQ of the knee extensors in the main manifestations of strength (isometric, maximum dynamic strength, and muscular power) between patients with differing degrees of neurological disability and evolutionary forms of the illness. We also provide reference values for muscle quality in patients with MS.
3. Results
Given the higher prevalence of MS in women, and since gender was not a selection criterion for participation in the study, the proportion of women in the sample was higher (64%), as expected, but there were no differences between the sexes in terms of age, BMI, years of disease evolution since diagnosis, or EDSS value (
Table 1).
Most of the patients in the sample presented RRMS (70%) or, less frequently, PPMS (13%). The degree of disability presented by the patients was mostly mild or moderate (77% overall), and only 3% had a very severe degree of illness (3%) (
Table 2). An association was found between the type of MS and the degree of EDSS (χ
2 (6, N = 250) = 23.721,
p < 0.001), with RRMS presenting a lower degree of neurological disability and SPMS a higher degree of severity.
There was no association observed between sex and the evolutionary type of MS (χ
2 (2, N = 250) = 4.520,
p < 0.104) or between gender and EDSS grade (χ
2 (3, N = 250) = 2.817,
p < 0.421). For this reason, data in
Table 2,
Table 3,
Table 4,
Table 5,
Table 6,
Table 7 and
Table 8 are not broken down by gender. In the
supplementary material, the data of tables are disaggregated by gender.
The lean mass of the selected ROIs is shown in
Table 3 by MS type and in
Table 4 by EDSS level. Only a few (seven) patients in the sample presented very severe EDSS scores, so they were not considered for the comparison of the variables between groups of neurological disability. Lean mass was similar between patients with different evolutionary types of the disease, and it was also similar between patients with different degrees of EDSS.
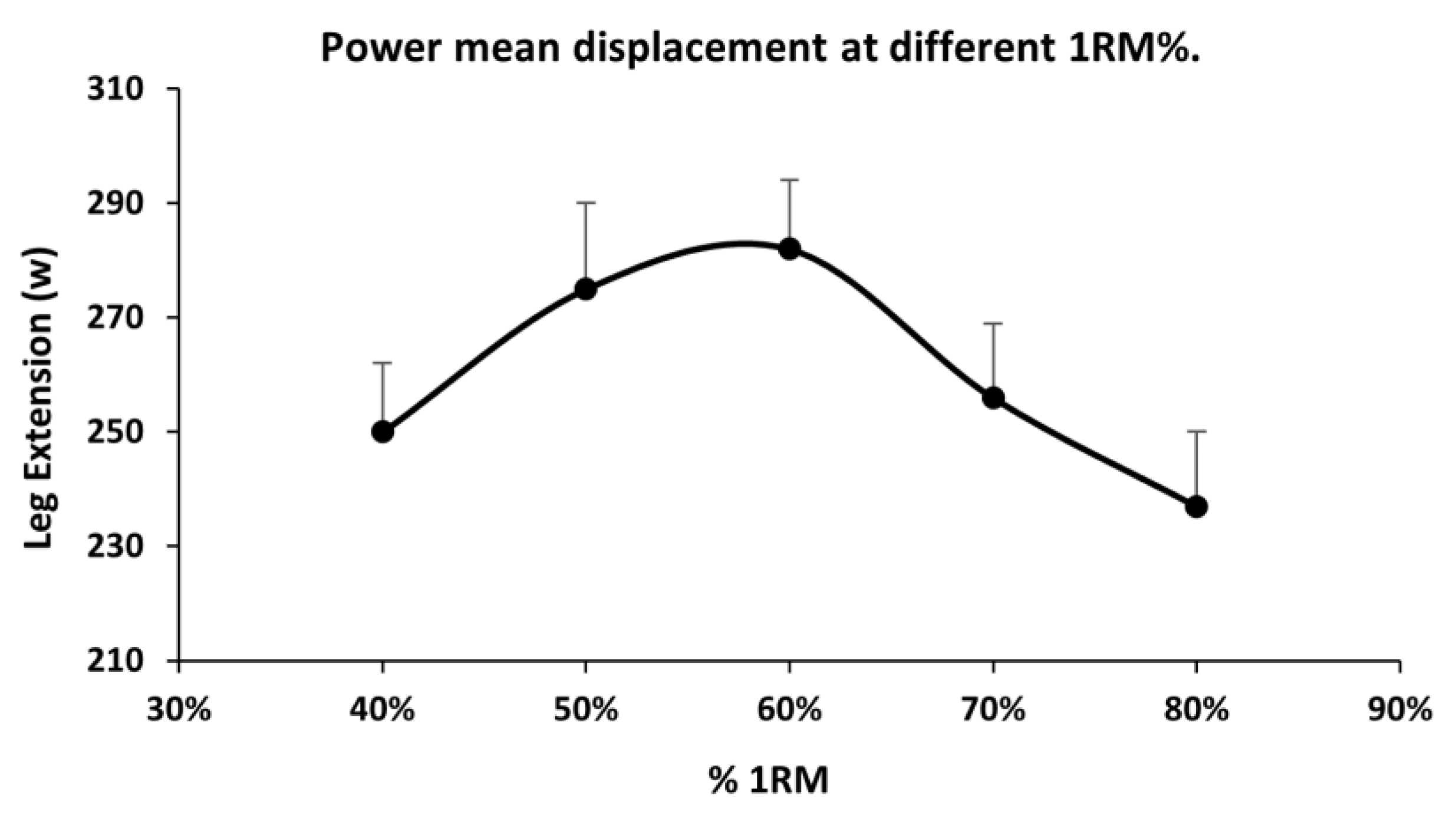
Table 5 shows the descriptive values of the study variables grouped by MS type. The patients in the RRMS group were younger, and those in the PPMS group were the oldest. The strength values manifested by knee extension in the three types of strength studied were similar between all disease groups. In general, the muscle quality of the different manifestations of strength was observed to be similar between RRMS and PPMS patients and lower in the group of patients showing SPMS compared to RRMS patients. However, the effect sizes were found to be very small; the proportion of variance explained by the type of MS varied between only 5% and 7%.
Table 6 shows the descriptive values of the study variables grouped by EDSS grade. Patients with mild EDSS scores presented greater strength compared to patients with a moderate or severe degree of neurological disability. The degree of disability can explain between 13% and 17% of the variance in manifestations of strength. Moreover, patients with mild EDSS scores presented better muscle quality indices compared to patients with more severe neurological functional impairment and, in general, patients with a severe degree presented the worst values. Between 23% and 27% of variance in muscle quality is explained by the degree of the EDSS.
Table 7 and
Table 8 summarize the threshold values used to establish the reference quartiles for the manifestations of muscle strength and quality depending on the type of MS and the level of neurological disability. The
supplementary materials provide the reference values grouped by sex, type of MS, and EDSS.
4. Discussion
After analyzing muscle quality in a wide sample of 250 patients with confirmed MS, differences in muscle quality were found to be greater when patients were compared according to the degree of neurological disability than when compared according to the disease evolutionary type. In total, only 36% of the sample was male because the incidence of this disease is three times higher in women than in men [
54]. Overall, 70% of the sample had an RRMS course, which is explained by the fact that more than 80% of MS patients begin with an RR course, and only a minority have PPMS when diagnosed [
55].
The average age in the sample was around 47 years—that is, a young adult population. However, since MS is a chronic disease with a tendency to progress, the time elapsed since the initial diagnosis of the disease was more important [
56]. In the studied sample, the average time since diagnosis was around 11 years. As the sample was young, it is clear that MS is often diagnosed in a very young population [
57].
The degree of neurological disability is the most widely used clinical parameter to monitor disease progression [
56], and the most commonly used instrument for evaluating disability in clinical trials is the EDSS [
58,
59], which provides a score that ranges from 0 (no apparent neurological disability) to 10 (death from MS). However, there are no universally accepted cut-off points to categorize the degree of neurological disability based on the score obtained, which is why some authors use their own categories [
30]. Based on the categories used in our center, 77% of the sample had a mild or moderate neurological disability. In total, 3% of our sample had a very severe degree of disability. This group of patients was not used in the present study for comparisons between patients based on disability because very few patients had a severe disability, especially considering the sizes of the other disability groups. Thus, comparisons with this group would have very poor statistical power. In our sample, the degree of the EDSS was associated with the evolutionary disease type, with RRMS having a higher proportion of patients presenting mild disability and patients with SPMS having a higher proportion of severe disability. Although our study showed a small correlation between age and EDSS score (Pearson’s
r = 0.267), and the RRMS group was younger, these factors do not seem to provide an explanation for this difference in the proportion of disability since the correlation between the years of evolution of the disease and the EDSS score in our study was slightly higher (Pearson’s
r = 0.323), and the time of evolution between the groups was similar. Our results agree with previous longitudinal studies which found that patients with RRMS tended to present with a slower progression of neurological disability [
60]. One of the criticisms made of the universally used EDSS is that some important manifestations of MS patients, such as fatigue, upper extremity functions, and cognitive impairment, have little weight in the finally obtained score; thus, this instrument is not very sensitive to change [
61]. The differences that we found in the EDSS between the groups were likely due to different affectations of nonmotor functions explored with the EDSS (vision, brainstem, sphincter control, or skin sensitivity), since we did not find differences between the groups in maximum isometric force, neither in dynamic force nor in the mean power manifested at 60% of 1 RM.
Some authors have studied lean mass in people with MS because lean mass is considered a key concept in force production [
62]. However, these studies often focus on comparisons between patients with MS and a healthy population [
63,
64]. Very few studies have compared the lean mass determined by DXA between patients with different EDSS scores [
30]. In our study, there were no differences between the type of MS and the lean mass of the pelvic–thigh area. There were also no differences observed between the different groups of degree of disability, unlike the results of another study [
30], which observed that patients with a greater degree of disability presented a lower lean mass. However, this previous study determined the total lean body mass and only grouped patients into two disability groups (26 patients with EDSS scores less than 4 versus 21 patients with scores of more than 4.5). Moreover, the group with the greatest disability had about 17 years of disease evolution compared to 9 years of evolution in the group with the lowest disability.
The muscle quality of patients with MS, understood as the manifested force relativized to the activated muscle mass, is an aspect little explored in the scientific literature. We collected data on the ROIs defined by DXA in studies related to muscle quality of the knee extensors in different populations without MS. Some studies determined the lean mass of the entire limb [
65,
66,
67], while others focused on the thigh region [
68,
69,
70]. The ROIs used in our study were limited to obtain the majority of the lean mass of the main muscle groups that intervene as agonists, synergists, antagonists, or fixators in the extension movement of the knee, with an angulation of 110° at the hip [
71].
The results of the analysis of muscle quality in the three evolutionary types of disease show that the MQ_Isometric was significantly higher in the group with RRMS (15.8% lower in the PPMS and 13.4% in the SPMS), although the effect size was relatively small, explaining 5% of the variance by evolutionary type of the disease. For the MQ_MDF, the RRMS group also presented significantly better quality than the SPMS group, which provided 17.7% worse quality and was not significant compared to the PPMS group, explaining 6% of the variability by evolutionary group of the disease. The difference in muscle quality was greatest in MQ_Power, which was 29.5% better in the RRMS group than in the SPMS group. Although this result was not significant, it was 16.1% better than the result for PPMS, explaining 7% of variance due to the evolutionary type of the disease. This greater difference is likely because a power exercise is neuromuscularly more complex, requiring more rapid interaction of agonists, synergists, and antagonists [
72], and neural involvement has a greater impact on more complex exercises. Our study was cross-sectional, but considering that patients with SPMS of an earlier stage presented RRMS [
6], our results suggest that the evolution of RRMS to SPMS tends to worsen neurological disability and muscle quality, despite not being accompanied by a loss of strength or muscle mass.
When muscle quality was compared between MS patients with different degrees of neurological disability, the differences were clearer than those for the evolutionary type of the disease. Patients with a mild degree of EDSS scores presented significantly higher MQ_Isometric values than patients with moderate or severe involvement (11% and 24.8% better, respectively) and, in turn, severe EDSS scores were worse than those of moderate EDSS scores. The degree of disability ultimately explained 27.1% of the variance in MQ_Isometric. The group with a mild EDSS grade also presented significantly better muscle quality for the manifestation of maximum dynamic force—14.1% better than the moderate EDSS group and 26.9% better than the severe EDSS group. These differences were even greater in relation to MQ_Power, for which the quality in the mild EDSS group was 26.7% better than that in the moderate impairment group, and 40.3% better than that in the group with a severe degree of neurological disability. Overall, there were no differences in lean mass between the groups with different neurological disabilities, but there were differences in manifested strength, which confirmed that decreased muscle quality was caused by a lower capacity to generate force per unit of muscular mass. Further studies are needed to identify whether the observed loss in force generation is caused by changes in the muscle fibers or by changes in the neural components of muscle contraction.
Muscle quality in patients with MS has rarely been studied. To fill this gap, the present study provides values that can be used as a reference. These values were obtained using reproducible and sensitive methods to determine the segmental muscle mass of the knee extensors by applying DXA and measuring the main manifestations of force involved in the actions of daily life, including isometric force, dynamic force, and muscular power. In addition, the values were obtained from a large sample of patients and categorized according to both the evolutionary type of the disease and the degree of neurological disability. These muscle quality values are also provided in the
supplementary materials, where they are further disaggregated by sex.
However, the present study has some limitations. The main limitation is that this study utilized a cross-sectional approach. Although it can help establish hypotheses, this approach cannot elucidate the causes of observed differences. These causes should be investigated in future observational or longitudinal studies. Moreover, the determination of segmental lean mass through DXA, despite its excellent repeatability and reproducibility, does not allow for separate analysis of each muscle group or muscle.