Clinical Results of a Massive Blood Transfusion Protocol for Postpartum Hemorrhage in a University Hospital in Japan: A Retrospective Study
Abstract
:1. Introduction
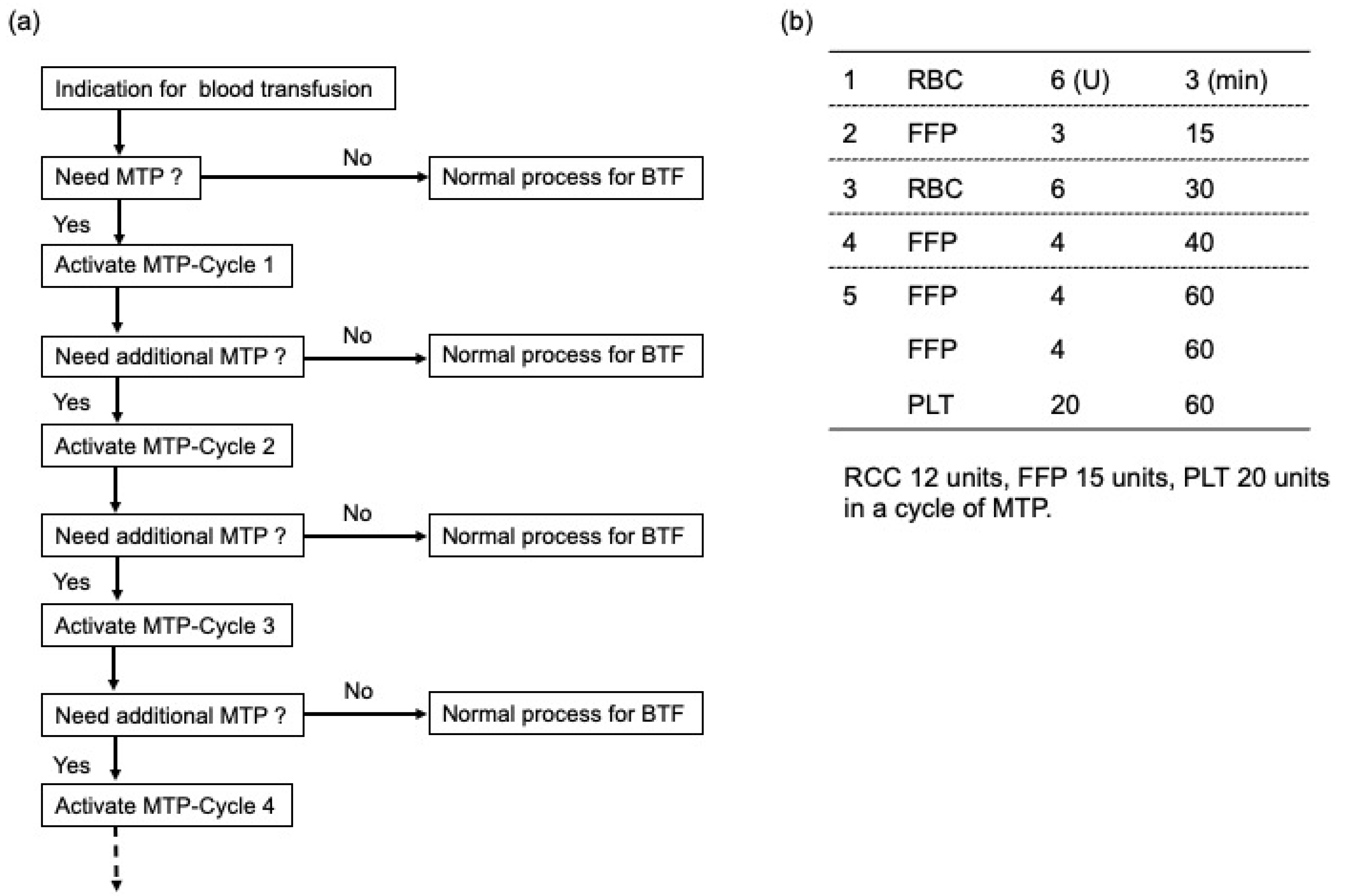
2. Materials and Methods
3. Results
3.1. Patient Characteristics, Obstetric Outcomes, and Intrapartum Management
3.1.1. Patient Characteristic and Obstetric Outcomes
3.1.2. Intrapartum Management
3.2. Analysis of the Data in Patients Receiving Blood Products for MTP
3.2.1. Patients Receiving Blood Products for MTP
3.2.2. The Timing of MTP Activation after Delivery
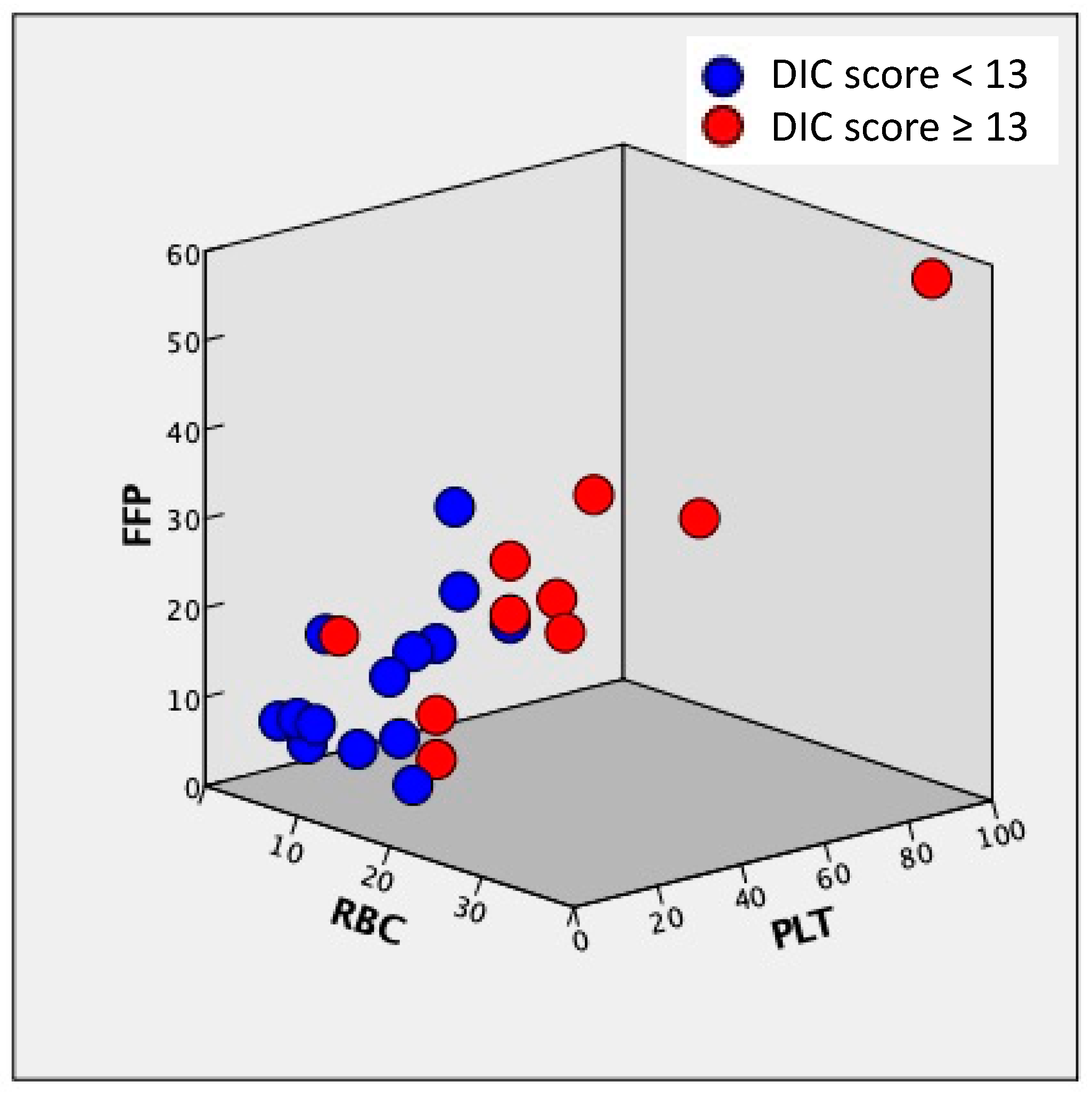
3.2.3. Correlation of the Amount of RBC, FFP, and PLT Transfusion, Obstetrical DIC Score, and Blood Loss
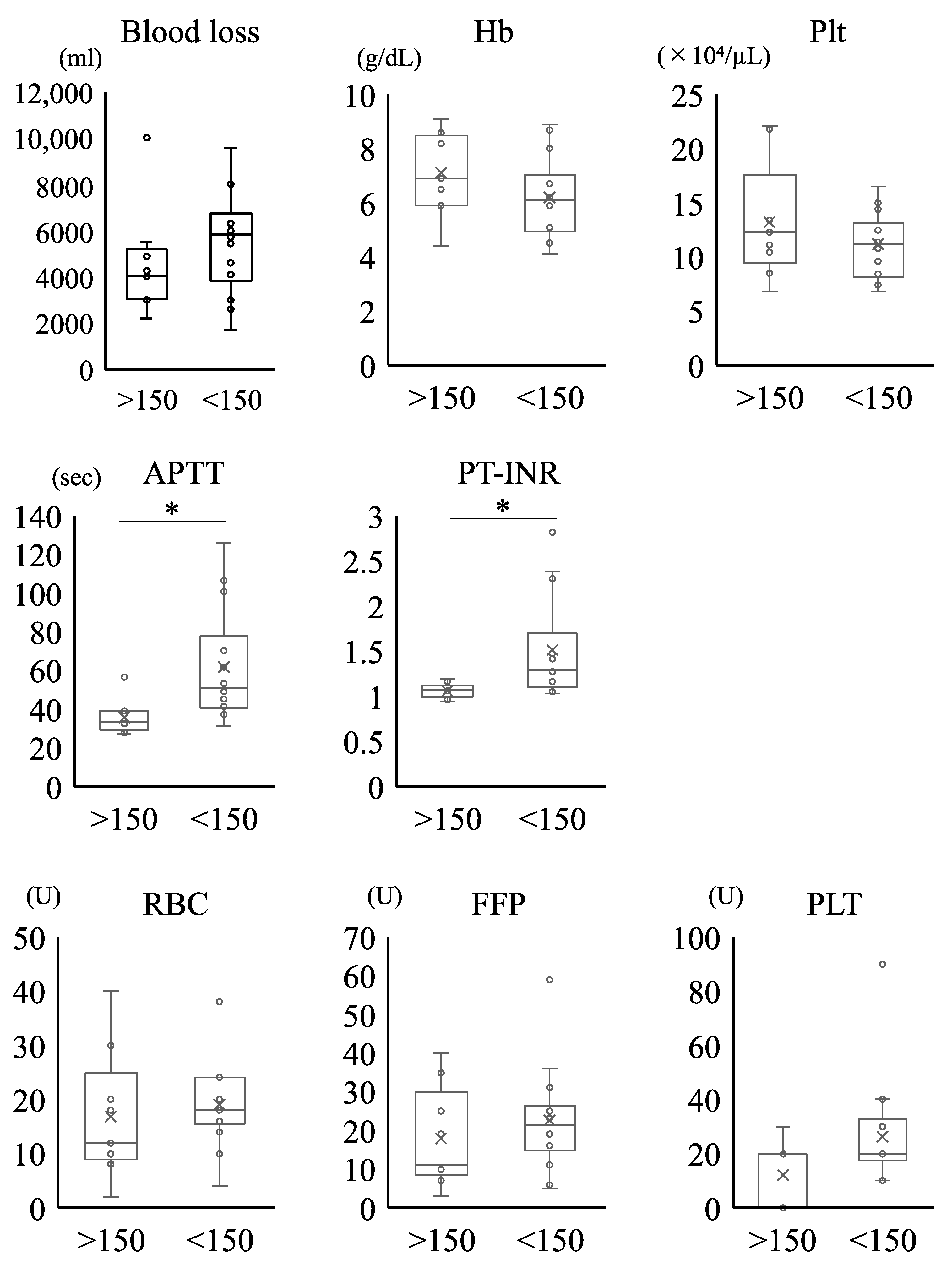
3.2.4. Clinical and Laboratory Parameters at the Onset of PPH in Patients Who Received MTP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thurn, L.; Wikman, A.; Westgren, M.; Lindqvist, P.G. Massive blood transfusion in relation to delivery: Incidence, trends and risk factors: A population-based cohort study. BJOG 2019, 126, 1577–1586. [Google Scholar] [CrossRef]
- Khan, K.S.; Wojdyla, D.; Say, L.; Gulmezoglu, A.M.; Van Look, P.F. WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef]
- Kramer, M.S.; Berg, C.; Abenhaim, H.; Dahhou, M.; Rouleau, J.; Mehrabadi, A.; Joseph, K.S. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am. J. Obstet. Gynecol. 2013, 209, 449.e1–449.e7. [Google Scholar] [CrossRef] [PubMed]
- Mitra, B.; Cameron, P.A.; Gruen, R.L.; Mori, A.; Fitzgerald, M.; Street, A. The definition of massive transfusion in trauma: A critical variable in examining evidence for resuscitation. Eur. J. Emerg. Med. 2011, 18, 137–142. [Google Scholar] [CrossRef] [PubMed]
- British Committee for Standards in Haematology; Stainsby, D.; MacLennan, S.; Thomas, D.; Isaac, J.; Hamilton, P.J. Guidelines on the management of massive blood loss. Br. J. Haematol. 2006, 135, 634–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malone, D.L.; Hess, J.R.; Fingerhut, A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J. Trauma Acute Care Surg. 2006, 60, S91–S96. [Google Scholar] [CrossRef] [Green Version]
- Mhyre, J.M.; Shilkrut, A.; Kuklina, E.V.; Callaghan, W.M.; Creanga, A.A.; Kaminsky, S.; Bateman, B.T. Massive blood transfusion during hospitalization for delivery in New York State, 1998–2007. Obstet. Gynecol. 2013, 122, 1288–1294. [Google Scholar] [CrossRef] [Green Version]
- Nunez, T.C.; Young, P.P.; Holcomb, J.B.; Cotton, B.A. Creation, implementation, and maturation of a massive transfusion protocol for the exsanguinating trauma patient. J. Trauma 2010, 68, 1498–1505. [Google Scholar] [CrossRef] [Green Version]
- Holcomb, J.B.; Tilley, B.C.; Baraniuk, S.; Fox, E.E.; Wade, C.E.; Podbielski, J.M.; Del Junco, D.J.; Brasel, K.J.; Bulger, E.M.; Callcut, R.A.; et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: The PROPPR randomized clinical trial. JAMA 2015, 313, 471–482. [Google Scholar] [CrossRef]
- Minakami, H.; Maeda, T.; Fujii, T.; Hamada, H.; Iitsuka, Y.; Itakura, A.; Itoh, H.; Iwashita, M.; Kanagawa, T.; Kanai, M.; et al. Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2014 edition. J. Obstet. Gynaecol. Res. 2014, 40, 1469–1499. [Google Scholar] [CrossRef]
- Kanayama, N.; Tamura, N. Amniotic fluid embolism: Pathophysiology and new strategies for management. J. Obstet. Gynaecol. Res. 2014, 40, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T. Obstetrical disseminated intravascular coagulation score. J. Obstet. Gynaecol. Res. 2014, 40, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Green, L.; Knight, M.; Seeney, F.M.; Hopkinson, C.; Collins, P.W.; Collis, R.E.; Simpson, N.; Weeks, A.; Stanworth, S.S. The epidemiology and outcomes of women with postpartum haemorrhage requiring massive transfusion with eight or more units of red cells: A national cross-sectional study. BJOG 2016, 123, 2164–2170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez, M.C.; Goodnough, L.T.; Druzin, M.; Butwick, A.J. Postpartum hemorrhage treated with a massive transfusion protocol at a tertiary obstetric center: A retrospective study. Int. J. Obstet. Anesth. 2012, 21, 230–235. [Google Scholar] [CrossRef]
- Butwick, A.J.; Goodnough, L.T. Transfusion and coagulation management in major obstetric hemorrhage. Curr. Opin. Anaesthesiol. 2015, 28, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Charbit, B.; Mandelbrot, L.; Samain, E.; Baron, G.; Haddaoui, B.; Keita, H.; Sibony, O.; Mahieu-Caputo, D.; Hurtaud-Roux, M.F.; Huisse, M.G.; et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J. Thromb. Haemost. 2007, 5, 266–273. [Google Scholar] [CrossRef]
- Burtelow, M.; Riley, E.; Druzin, M.; Fontaine, M.; Viele, M.; Goodnough, L.T. How we treat: Management of life-threatening primary postpartum hemorrhage with a standardized massive transfusion protocol. Transfusion 2007, 47, 1564–1572. [Google Scholar] [CrossRef]
- Rahe-Meyer, N.; Hanke, A.; Schmidt, D.S.; Hagl, C.; Pichlmaier, M. Fibrinogen concentrate reduces intraoperative bleeding when used as first-line hemostatic therapy during major aortic replacement surgery: Results from a randomized, placebo-controlled trial. J. Thorac. Cardiovasc. Surg. 2013, 145, S178–S185. [Google Scholar] [CrossRef] [Green Version]
- Makino, S.; Takeda, S.; Kobayashi, T.; Murakami, M.; Kubo, T.; Hata, T.; Masuzaki, H. National survey of fibrinogen concentrate usage for post-partum hemorrhage in Japan: Investigated by the Perinatology Committee, Japan Society of Obstetrics and Gynecology. J. Obstet. Gynaecol. Res. 2015, 41, 1155–1160. [Google Scholar] [CrossRef] [Green Version]
- Halmin, M.; Chiesa, F.; Vasan, S.K.; Wikman, A.; Norda, R.; Rostgaard, K.; Vesterager Pedersen, O.B.; Erikstrup, C.; Nielsen, K.R.; Titlestad, K.; et al. Epidemiology of Massive Transfusion: A Binational Study from Sweden and Denmark. Crit. Care Med. 2016, 44, 468–477. [Google Scholar] [CrossRef]
- Young, P.P.; Cotton, B.A.; Goodnough, L.T. Massive transfusion protocols for patients with substantial hemorrhage. Transfus. Med. Rev. 2011, 25, 293–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Como, J.J.; Dutton, R.P.; Scalea, T.M.; Edelman, B.B.; Hess, J.R. Blood transfusion rates in the care of acute trauma. Transfusion 2004, 44, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Matsunaga, S.; Yamashita, T.; Okutomi, T.; Sakurai, A.; Sekizawa, A.; Hasegawa, J.; Terui, K.; Miyake, Y.; Murotsuki, J.; et al. A systematic review of massive transfusion protocol in obstetrics. Taiwan J. Obstet. Gynecol. 2017, 56, 715–718. [Google Scholar] [CrossRef] [PubMed]

| Maternal age (years) | 36.8 (31–44) |
| Body mass index (kg/m2) | 21.0 (15.4–32.4) |
| Primiparous | 20 (83.3) |
| Spontaneous pregnancy | 12 (50.0) |
| Singleton pregnancy | 21 (87.5) |
| Obstetric complication | |
| Hypertension disorder of pregnancy | 4 (16.7) |
| Gestational diabetes | 6 (25.0) |
| Placenta previa | 5 (20.8) |
| Gestational age at delivery (weeks) | 37.4 (33.3–40.7) |
| Birth weight (g) | 2771 (1709–3572) |
| Mode of delivery | |
| Cesarean section | 20 (83.3) |
| Spontaneous vaginal delivery | 2 (8.3) |
| Instrumental delivery | 2 (8.3) |
| Estimated blood loss (mL) | 5017 (2200–10,000) |
| Obstetric DIC score | 11.7 (5–24) |
| ≥8 | 17 (70.8) |
| ≥13 | 10 (41.7) |
| SI ≥ 1 | 14 (58.3) |
| Blood transfusion | |
| RBC (U) | 17.9 (2–40) |
| FFP (U) | 20.2 (3–59) |
| PLT (U) | 20.4 (0–90) |
| FFP/RBC ratio (mean) | 1.13 |
| ≥1.0 | 16 (66.7) |
| 0.8–1.0 | 3 (12.5) |
| <0.8 | 5 (20.8) |
| Additional procedure to control bleeding | |
| Transarterial embolization | 18 (75.0) |
| Balloon tamponade | 2 (8.4) |
| Hysterectomy | 1 (4.2) |
| ICU admission | 15 (62.5) |
| No. | Etiology of Hemorrhage | Blood Loss (mL) | Obstetrics DIC Score | SI ≥ 1 | RBC (U) | FFP (U) | PLT (U) | Autologous Blood (U) | FFP/RBC Ratio |
|---|---|---|---|---|---|---|---|---|---|
| 1 | AFE (DIC-type), Uterine atony | 8000 | 17 | + | 24 | 36 | 40 | 0 | 1.5 |
| 2 | AFE (DIC-type), Uterine atony | 2600 | 17 | + | 24 | 25 | 20 | 0 | 1.04 |
| 3 | Uterine atony | 10,000 | 24 | + | 40 | 40 | 30 | 0 | 1.00 |
| 4 | Placenta previa, PAS | 5500 | 15 | + | 30 | 25 | 20 | 2 | 0.83 |
| 5 | PAS | 4900 | 7 | + | 8 | 10 | 0 | 0 | 1.25 |
| 6 | Placenta previa, Uterine atony | 8000 | 9 | + | 16 | 19 | 20 | 4 | 1.19 |
| 7 | AFE (DIC-type), Uterine atony | 5700 | 15 | + | 16 | 6 | 20 | 0 | 0.38 |
| 8 | PAS | 3000 | 10 | − | 4 | 16 | 20 | 6 | 4.00 |
| 9 | AFE (DIC-type), Uterine atony | 4100 | 13 | + | 20 | 23 | 40 | 0 | 1.20 |
| 10 | Vaginal hematoma | 6000 | 14 | − | 16 | 11 | 20 | 0 | 0.69 |
| 11 | Uterine atony | 2200 | 7 | − | 2 | 3 | 20 | 0 | 1.50 |
| 12 | Uterine atony | 4000 | 7 | − | 10 | 11 | 0 | 0 | 1.10 |
| 13 | AFE (DIC-type), Uterine atony | 4600 | 12 | − | 18 | 20 | 10 | 0 | 1.11 |
| 14 | Uterine rupture | 2300 | 6 | − | 12 | 7 | 10 | 0 | 0.58 |
| 15 | Placenta previa, HELLP syndrome | 9600 | 23 | + | 38 | 59 | 90 | 3 | 1.55 |
| 16 | AFE (DIC-type), Uterine atony | 6000 | 14 | + | 24 | 31 | 20 | 0 | 1.29 |
| 17 | Vaginal hematoma | 4000 | 5 | − | 20 | 19 | 0 | 0 | 0.95 |
| 18 | AFE (DIC-type), Uterine atony, Placenta previa, PAS | 6150 | 9 | + | 18 | 5 | 10 | 6 | 0.28 |
| 19 | AFE (DIC-type), Placental abruption | 6300 | 11 | + | 24 | 24 | 20 | 0 | 1.00 |
| 20 | Uterine atony, Placenta previa | 5433 | 10 | − | 14 | 23 | 30 | 4 | 1.64 |
| 21 | Uterine atony | 3000 | 6 | − | 12 | 7 | 20 | 0 | 0.58 |
| 22 | AFE (DIC-type) | 1700 | 16 | + | 10 | 19 | 10 | 0 | 1.90 |
| 23 | PAS | 4240 | 5 | − | 18 | 35 | 20 | 0 | 1.94 |
| 24 | Uterine atony | 3090 | 8 | + | 12 | 11 | 0 | 0 | 0.92 |
| mean or number (range or %) | 5017 | 11.7 | 14 | 17.9 | 20.2 | 20.4 | 1.04 | 1.13 | |
| (2200–10,000) | (5–24) | (58.3) | (2–40) | (3–59) | (0–90) | (0–6) | (0.28–4.00) |
| <2 h | 15 (62.5%) |
| 2–24 h | 9 (37.5%) |
| >24 h | 0 (0%) |
| RBC | FFP | PLT | Obstetrical DIC Score | Blood Loss | |
|---|---|---|---|---|---|
| RBC | 1.000 | - | - | - | - |
| FFP | 0.892 | 1.000 | - | - | - |
| PLT | 0.765 | 0.864 | 1.000 | - | - |
| Obstetrical DIC score | 0.861 | 0.817 | 0.806 | 1.000 | - |
| Blood loss | 0.861 | 0.792 | 0.757 | 0.774 | 1.000 |
| No. | Etiology of Hemorrhage | Blood Loss (mL) | Hb (g/dL) | Plt (×104/μL) | FNG (mg/dL) | APTT (s) | PT-INR |
|---|---|---|---|---|---|---|---|
| 1 | AFE (DIC-type), Uterine atony | 8000 | 6.2 | 7.4 | <50 | 125.7 | 2.82 |
| 2 | AFE (DIC-type), Uterine atony | 2600 | 6.7 | 12.7 | <50 | 55.7 | 2.38 |
| 3 | Uterine atony | 10,000 | 4.4 | 8.5 | 173 | 38.9 | 1.08 |
| 4 | Placenta previa, PAS | 5500 | 6.9 | 6.8 | 203 | 27.1 | 0.96 |
| 5 | PAS | 4900 | 8.2 | 10.4 | 172 | 30.3 | 1.08 |
| 6 | Placenta previa, Uterine atony | 8000 | 4.5 | 10.8 | 94 | 53.1 | 1.3 |
| 7 | AFE (DIC-type), Uterine atony | 5700 | 6 | 15 | 146 | 37.3 | 1.05 |
| 8 | PAS | 3000 | 8.9 | 12.5 | 136 | 45.3 | 1.16 |
| 9 | AFE (DIC-type), Uterine atony | 4100 | 5.9 | 16.5 | 147 | 41.6 | 1.1 |
| 10 | Vaginal hematoma | 6000 | 4.5 | 8.4 | 144 | 101.1 | 1.41 |
| 11 | Uterine atony | 2200 | 8.6 | 12.6 | 267 | 35.2 | 1.01 |
| 12 | Uterine atony | 4000 | 5.9 | 12.3 | 177 | 56.6 | 1.07 |
| 13 | AFE (DIC-type), Uterine atony | 4600 | 5.1 | 6.8 | 91 | 70.1 | 1.27 |
| 14 | Uterine rupture | 2300 | ND | ND | ND | ND | ND |
| 15 | Placenta previa, HELLP syndrome | 9600 | 4.1 | 14.4 | 118 | 48.9 | 1.29 |
| 16 | AFE (DIC-type), Uterine atony | 6000 | 6.4 | 12.5 | 131 | 38 | 1.09 |
| 17 | Vaginal hematoma | 4000 | 9.1 | 22.1 | 332 | 27.7 | 0.94 |
| 18 | AFE (DIC-type), Uterine atony, Placenta previa, PAS | 6150 | 8.7 | 7.4 | 64 | 61.8 | 1.48 |
| 19 | AFE (DIC-type), Placental abruption | 6300 | 6.4 | 9.6 | 72 | 47.3 | 1.49 |
| 20 | Uterine atony, Placenta previa | 5433 | 5.2 | 11.4 | 137 | 31.1 | 1.03 |
| 21 | Uterine atony | 3000 | 8.4 | 21.8 | 328 | 33.7 | 1.16 |
| 22 | AFE (DIC-type) | 1700 | 8 | 11 | <50 | 106.4 | 2.3 |
| 23 | PAS | 4240 | 6.5 | 11.1 | 153 | 32.7 | 1.19 |
| 24 | Uterine atony | 3090 | 5.9 | 13.4 | 218 | 39.1 | 1.06 |
| mean (range) | 5017 (2200–10,000) | 6.5 (4.1–9.1) | 11.9 (6.8–22.1) | 150.1 (<50–332) | 51.5 (27.1–125.7) | 1.34 (0.94–2.82) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochiai, D.; Abe, Y.; Yamazaki, R.; Uemura, T.; Toriumi, A.; Matsuhashi, H.; Tanaka, Y.; Ikenoue, S.; Kasuga, Y.; Tanosaki, R.; et al. Clinical Results of a Massive Blood Transfusion Protocol for Postpartum Hemorrhage in a University Hospital in Japan: A Retrospective Study. Medicina 2021, 57, 983. https://doi.org/10.3390/medicina57090983
Ochiai D, Abe Y, Yamazaki R, Uemura T, Toriumi A, Matsuhashi H, Tanaka Y, Ikenoue S, Kasuga Y, Tanosaki R, et al. Clinical Results of a Massive Blood Transfusion Protocol for Postpartum Hemorrhage in a University Hospital in Japan: A Retrospective Study. Medicina. 2021; 57(9):983. https://doi.org/10.3390/medicina57090983
Chicago/Turabian StyleOchiai, Daigo, Yushi Abe, Rie Yamazaki, Tomoe Uemura, Ayako Toriumi, Hiroko Matsuhashi, Yuya Tanaka, Satoru Ikenoue, Yoshifumi Kasuga, Ryuji Tanosaki, and et al. 2021. "Clinical Results of a Massive Blood Transfusion Protocol for Postpartum Hemorrhage in a University Hospital in Japan: A Retrospective Study" Medicina 57, no. 9: 983. https://doi.org/10.3390/medicina57090983
APA StyleOchiai, D., Abe, Y., Yamazaki, R., Uemura, T., Toriumi, A., Matsuhashi, H., Tanaka, Y., Ikenoue, S., Kasuga, Y., Tanosaki, R., & Tanaka, M. (2021). Clinical Results of a Massive Blood Transfusion Protocol for Postpartum Hemorrhage in a University Hospital in Japan: A Retrospective Study. Medicina, 57(9), 983. https://doi.org/10.3390/medicina57090983






