Solitary Fibrous Tumor of the Prostate: A Case Report and Literature Review
Abstract
:1. Introduction
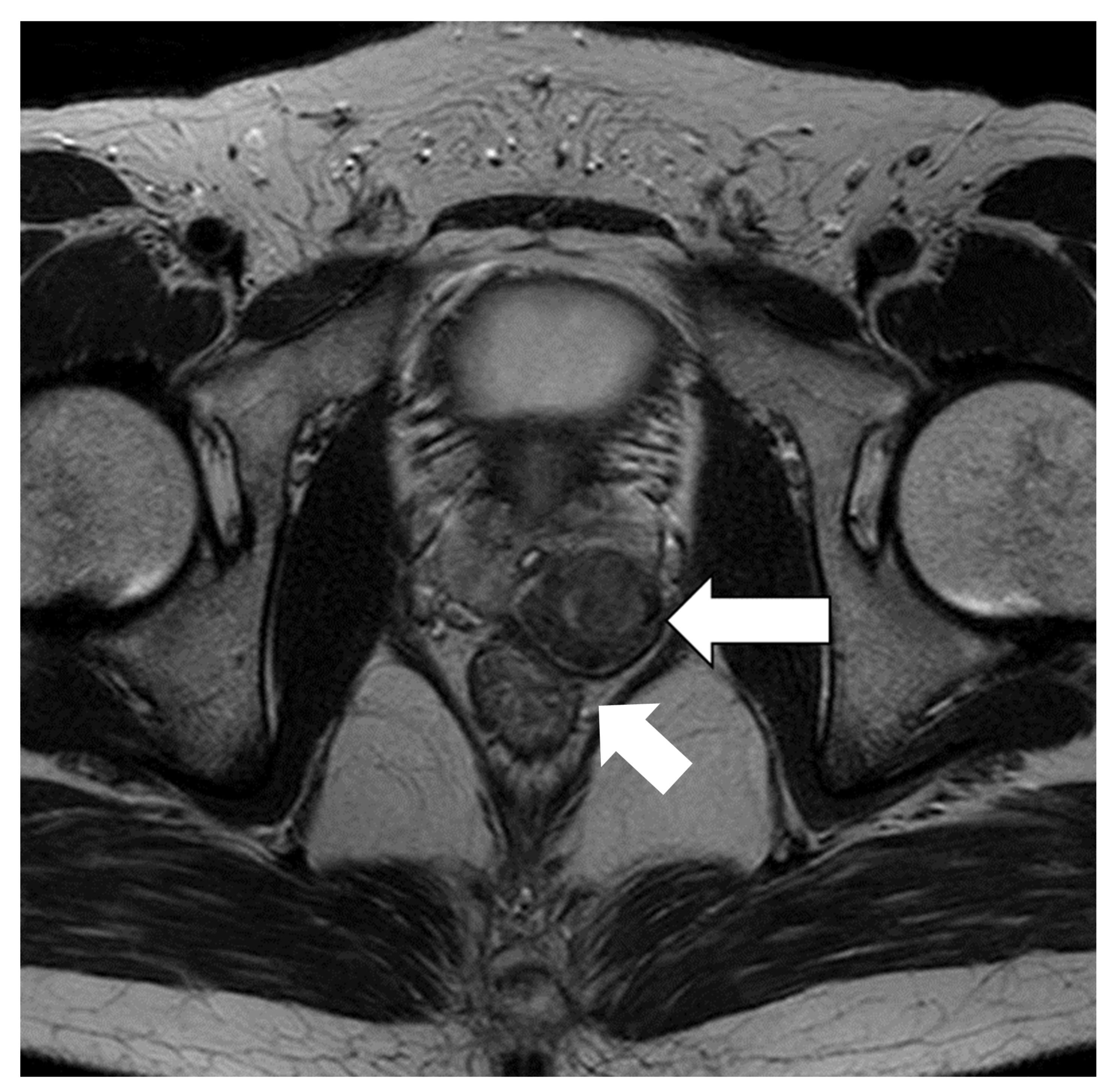
2. Case Report
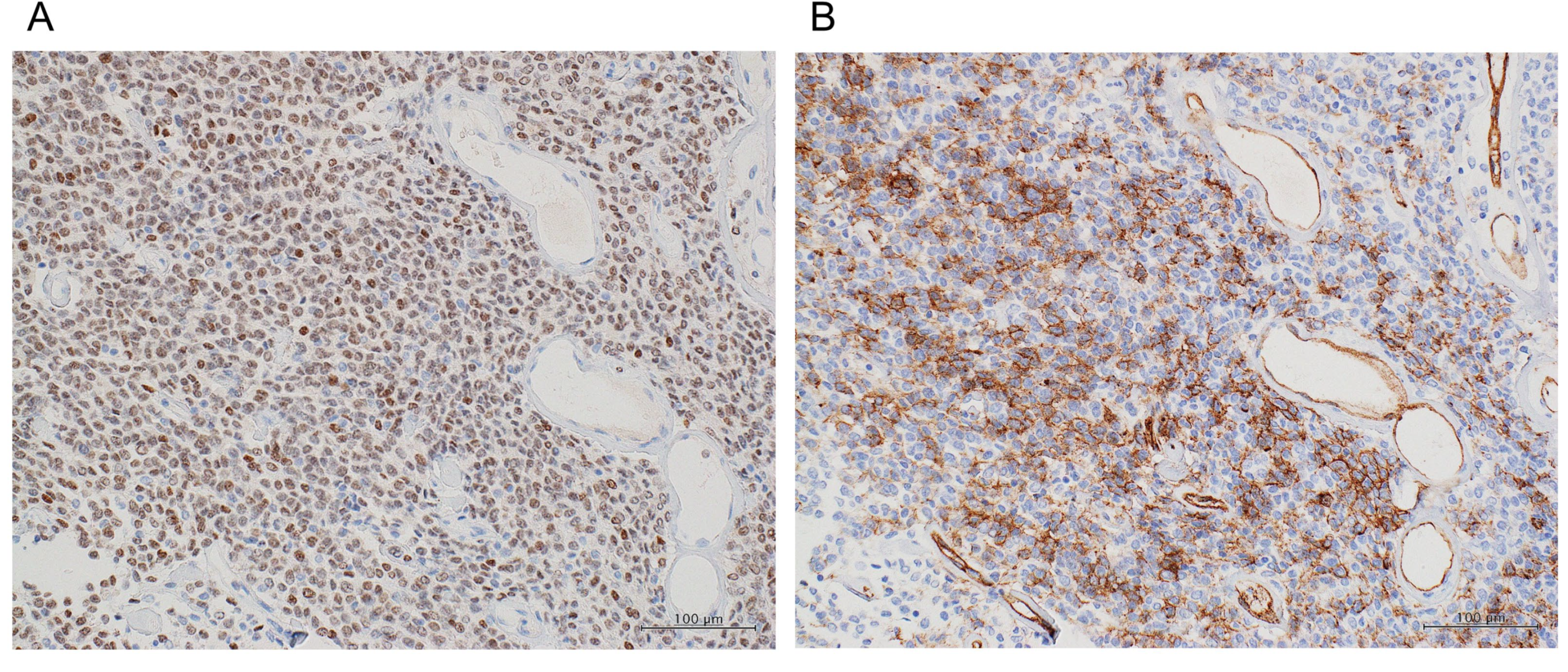
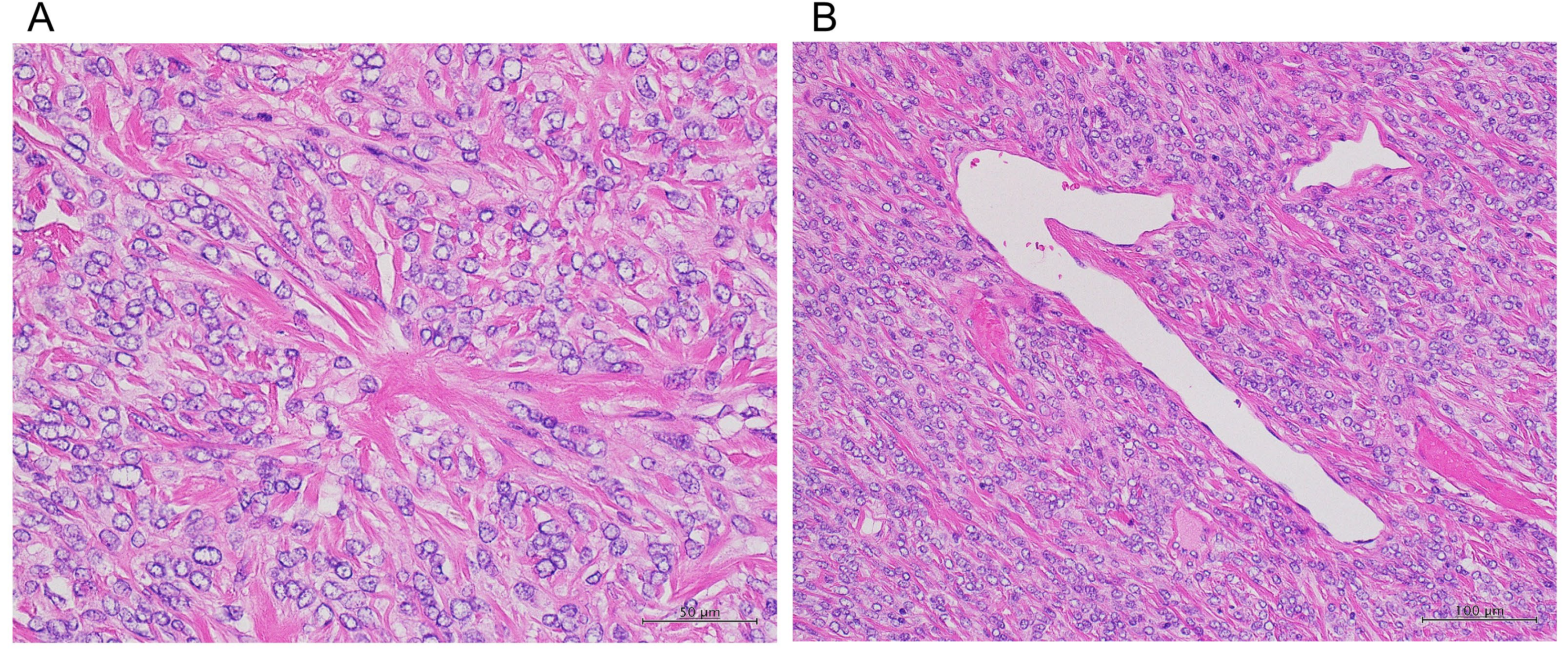
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fletcher, C.D.M.; Bridge, J.A.; Lee, J.C. Extrapleural solitary fibrous tumor. In WHO Classification of Tumours of Soft Tissue and Bone; Fletcher, C.D.M., Ed.; The International Agency for Research on Cancer Press: Lyon, France, 2013; pp. 80–82. [Google Scholar]
- Klemperer, P.; Rabin, C.B. Primary neoplasms of the pleura. A report of the five cases. Arch. Pathol. 1931, 11, 385–412. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.S.; Antonescu, C.R.; Hajdu, C.; Ferrone, C.R.; Hissain, M.; Lewis, J.J.; Brennan, M.F.; Coit, D.G. Clinicopathologic correlates of solitary fibrous tumors. Cancer 2002, 94, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Chmielecki, J.; Crago, A.M.; Rosenberg, M.; O’Connor, R.; Walker, S.R.; Ambrogio, L.; Auclair, D.; McKenna, A.; Heinrich, M.C.; Frank, D.A.; et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat. Genet. 2013, 45, 131–132. [Google Scholar] [CrossRef] [Green Version]
- Robinson, D.R.; Wu, Y.M.; Kalyana-Sundaram, S.; Cao, X.; Lonigro, R.J.; Sung, Y.S.; Chen, C.L.; Zhang, L.; Wang, R.; Su, F.; et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat. Genet. 2013, 45, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Corkum, M.T.; Pautler, S.E.; Wehrli, B.; Winquist, E. Images—Solitary fibrous tumor of the prostate. Can. Urol. Assoc. J. 2020, 14, E613–E614. [Google Scholar] [CrossRef] [PubMed]
- Nishith, N.; Gupta, M.; Kaushik, N.; Sen, R. Solitary Fibrous Tumor of the Prostate: A Diagnostic Challenge: A Case Report. Iran. J. Pathol. 2020, 15, 41–44. [Google Scholar]
- Osamu, S.; Murasawa, H.; Imai, A.; Hatakeyama, S.; Yoneyama, T.; Hashimoto, Y.; Koie, T.; Ohyama, C. Solitary Fibrous Tumor of the Prostate Which Was Initially Misdiagnosed as Prostate Cancer. Case Rep. Urol. 2017, 2017, 3594914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronchi, A.; La Mantia, E.; Gigantino, V.; Perdonà, S.; De Sio, M.; Facchini, G.; Franco, R.; De Chiara, A. A rare case of malignant solitary fibrous tumor in prostate with review of the literature. Diagn. Pathol. 2017, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Bakhshwin, A.; Berry, R.S.; Cox, R.M.; LI, R.; Reynolds, J.P.; Rubin, B.P.; McKenney, J.K. Malignant solitary fibrous tumour of the prostate: Four cases emphasising significant histological and immunophenotypical overlap with sarcomatoid carcinoma. Pathology 2020, 52, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Quarto, G.; Grimaldi, G.; Izzo, A.; Muscariello, R.; Castaldo, L.; Di Caprio, B.; Bimonte, S.; Del Prete, P.; Cuomo, A.; et al. Neuropathic painful complications due to endopelvic nerve lesions after robot-assisted laparoscopic prostatectomy: Three case reports. Medicine (Baltimore) 2019, 98, e18011. [Google Scholar] [CrossRef] [PubMed]
- McKenney, J.K. Mesenchymal tumors of the prostate. Mod. Pathol. 2018, 31, S133–S142. [Google Scholar] [CrossRef] [PubMed]
- Galosi, A.B.; Mazzucchelli, R.; Scarpelli, M.; Lopez-Beltran, A.; Cheng, L.; Muzzonigro, G.; Montironi, R. Solitary fibrous tumour of the prostate identified on needle biopsy. Eur. Urol. 2009, 56, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Guner, G.; Bishop, J.A.; Bezerra, S.M.; Taheri, D.; Zahavi, D.; Mendoza Rodriguez, M.A.; Sharma, R.; Epstein, J.I.; Netto, G.J. The utility of STAT6 and ALDH1 expression in the differential diagnosis of solitary fibrous tumor versus prostate-specific stromal neoplasms. Hum. Pathol. 2016, 54, 184–188. [Google Scholar] [CrossRef]
- Cheah, A.L.; Billings, S.D.; Goldblum, J.R.; Carver, P.; Tanas, M.Z.; Rubin, B.P. STAT6 rabbit monoclonal antibody is a robust diagnostic tool for the distinction of solitary fibrous tumour from its mimics. Pathology 2014, 46, 389–395. [Google Scholar] [CrossRef]
- Yoshida, A.; Tsuta, K.; Ohno, M.; Yoshida, M.; Narita, Y.; Kawai, A.; Asamura, H.; Kushima, R. STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumor. Am. J. Surg. Pathol. 2014, 38, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Kohashi, K.; Kinoshita, I.; Yamamoto, H.; Iwasaki, T.; Yoshimoto, M.; Ishihara, S.; Toda, Y.; Itou, Y.; Koga, Y.; et al. Clinicopathological review of solitary fibrous tumors: Dedifferentiation is a major cause of patient death. Virchows Arch. 2019, 475, 467–477. [Google Scholar] [CrossRef]
- Olson, N.J.; Linos, K. Dedifferentiated Solitary Fibrous Tumor: A Concise Review. Arch. Pathol. Lab. Med. 2018, 142, 761–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demicco, E.G.; Park, M.S.; Araujo, D.M.; Fox, P.S.; Bassett, R.L.; Pollock, R.E.; Lazar, A.J.; Wang, W.L. Solitary fibrous tumor: A clinicopathological study of 110 cases and proposed risk assessment model. Mod. Pathol. 2012, 25, 1298–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demicco, E.G.; Wagner, M.J.; Maki, R.G.; Gupta, V.; Iofin, I.; Lazar, A.; Wang, W. Risk assessment in solitary fibrous tumors: Validation and refinement of a risk stratification model. Mod. Pathol. 2017, 30, 1433–1442. [Google Scholar] [CrossRef]
- Bahrami, A.; Lee, S.; Schaefer, I.M.; Boland, J.M.; Patton, K.T.; Pounds, S.; Fletcher, C.D. TERT promoter mutations and prognosis in solitary fibrous tumor. Mod. Pathol. 2016, 29, 1511–1522. [Google Scholar] [CrossRef] [Green Version]
- Moureau-Zabotto, L.; Chetaille, B.; Bladou, F.; Bladou, F.; Dauvergne, P.Y.; Marcy, M.; Perrot, D.; Guiramand, J.; Sarran, A.; Bertucci, F. Solitary fibrous t umor of the prostate: Case report and review of the literature. Case Rep. Oncol. 2012, 5, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.A. Solitary fibrous tumors of the pleura. Curr. Opin. Pulm. Med. 2012, 18, 339–346. [Google Scholar] [CrossRef] [PubMed]


| Tumors | Morphology |
|---|---|
| SFT | (1) Fibroblast-like cells arranged in a disorganized non-repetitive pattern, (2) scattered angulated hemangiopericytic vessels, (3) variable stromal collagenization, (4) occasional cellular and myxoid forms |
| STUMP | (1) Scattered atypical stromal cells associated with benign glands, (2) resemblance to glandular-stromal hyperplasia yet with hypercellular stroma, (3) extensive myxoid stroma, (4) phyllodes pattern |
| Stromal sarcoma | (1) Solid growth of overtly malignant cells with storiform, epithelioid, fibrosarcomatous, or patternless pattern, (2) malignant phyllodes-like |
| Sarcomatoid carcinoma | Mixture of frank epithelial and sarcomatous components such as ‘undifferentiated’ with atypical giant cells or pleomorphic spindle cells |
| Leiomyoma | Well-organized smooth muscle fascicles lacking mitotic activity |
| Leiomyosarcoma | Intersecting fascicles showing mitoses, cytologic atypia, and necrosis |
| Rhabdomyosarcoma | (1) Often spindled, but fusiform or more rounded cells and well-developed rhabdomyoblasts may be present; (2) embryonal subtype consisting of cartilaginous differentiation |
| IMT | (1) Typical myoblastic features with elongated or ‘stretched-out’ spindle cells, often with a loose myxoid background; (2) nuclei with significant variation in size; (3) nucleoli that may be quite prominent or multiple without nuclear hyperchromasia |
| GIST | Rectal-based spindle cells that compress and displace the prostate gland |
| SFT | STUMP | SS | Leiomyo-Sarcoma | Rhabdomyo-Sarcoma | IMT | GIST | |
|---|---|---|---|---|---|---|---|
| CD34 | + | + | + | - | - | - | + |
| SMA | - | ± | - | + | + | + | ± |
| Desmin | - | ± | - | + | + | + | ± |
| Myogenin | - | - | - | - | + | - | - |
| c-kit | - | - | - | - | - | ± | + |
| ALK-1 | - | - | - | - | - | + | - |
| PRs | ± | + | + | ± | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeuchi, Y.; Kato, D.; Nakane, K.; Kawase, K.; Takai, M.; Iinuma, K.; Saigo, C.; Miyazaki, T.; Koie, T. Solitary Fibrous Tumor of the Prostate: A Case Report and Literature Review. Medicina 2021, 57, 1152. https://doi.org/10.3390/medicina57111152
Takeuchi Y, Kato D, Nakane K, Kawase K, Takai M, Iinuma K, Saigo C, Miyazaki T, Koie T. Solitary Fibrous Tumor of the Prostate: A Case Report and Literature Review. Medicina. 2021; 57(11):1152. https://doi.org/10.3390/medicina57111152
Chicago/Turabian StyleTakeuchi, Yasumichi, Daiki Kato, Keita Nakane, Kota Kawase, Manabu Takai, Koji Iinuma, Chiemi Saigo, Tatsuhiko Miyazaki, and Takuya Koie. 2021. "Solitary Fibrous Tumor of the Prostate: A Case Report and Literature Review" Medicina 57, no. 11: 1152. https://doi.org/10.3390/medicina57111152
APA StyleTakeuchi, Y., Kato, D., Nakane, K., Kawase, K., Takai, M., Iinuma, K., Saigo, C., Miyazaki, T., & Koie, T. (2021). Solitary Fibrous Tumor of the Prostate: A Case Report and Literature Review. Medicina, 57(11), 1152. https://doi.org/10.3390/medicina57111152







