Phenotyping of Southern United States Soybean Cultivars for Potential Seed Weight and Seed Quality Compositions
Abstract
:1. Introduction
2. Materials and Methods
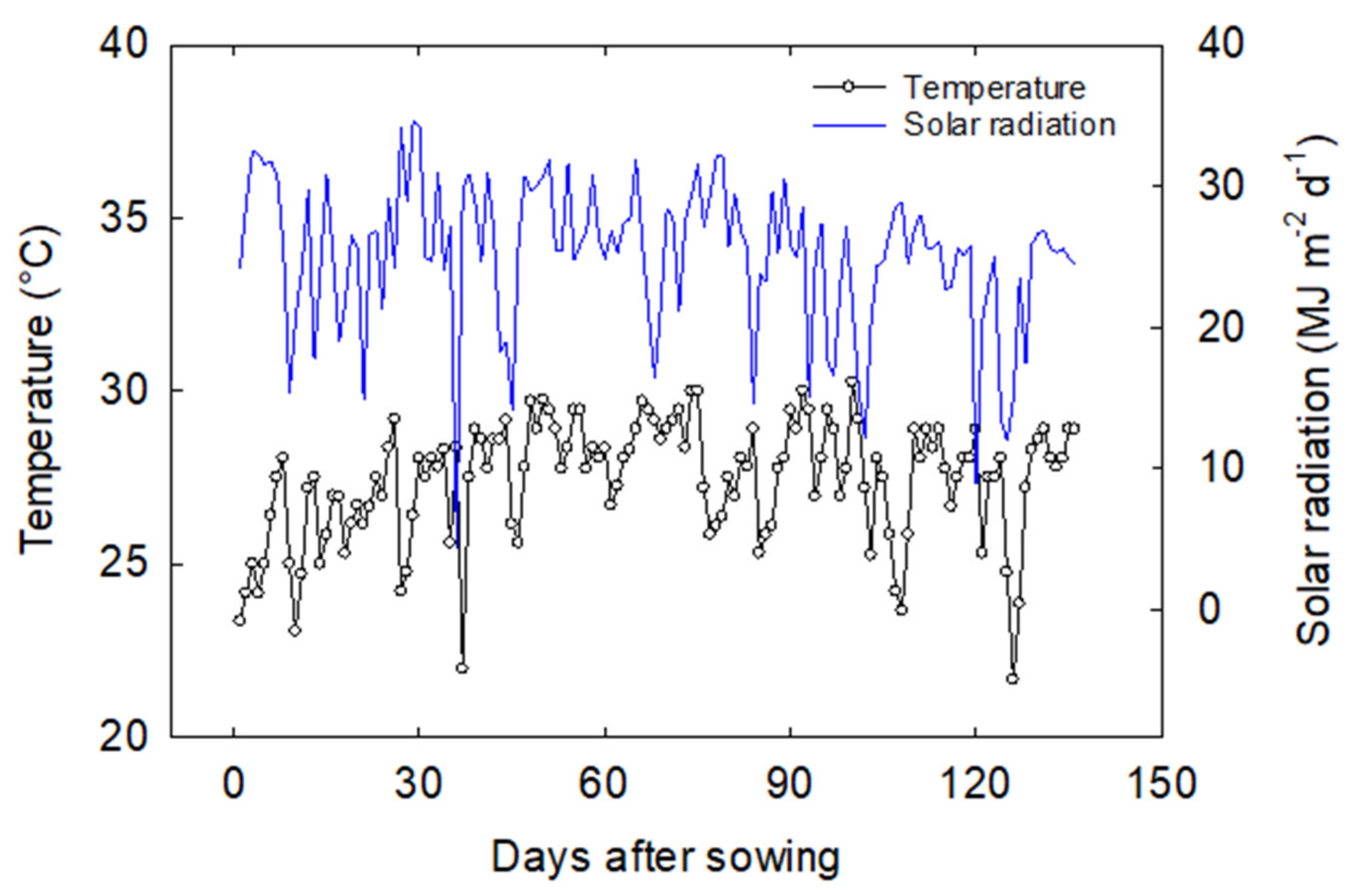
2.1. Plant Material and Growing Condition
2.2. Flowering and Yield Components
2.3. Seed Quality Using NIR Scanning
2.4. Statistical Analysis
3. Results and Discussion
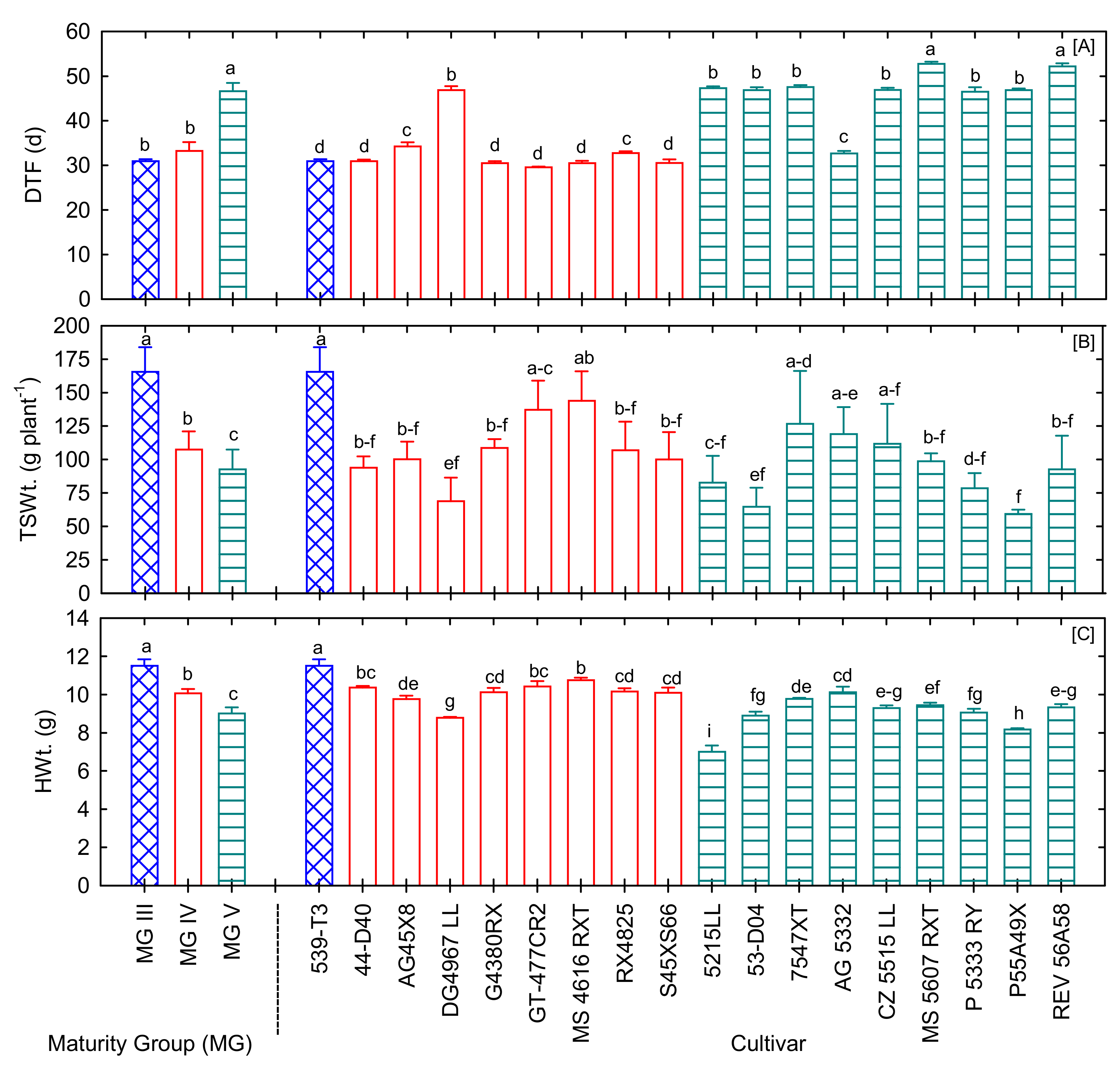
3.1. Variations in Flowering, Total Seed Weight, and 100-Seed Weight
3.2. Phenotypic Variations of Soybean Seed Quality Compositions
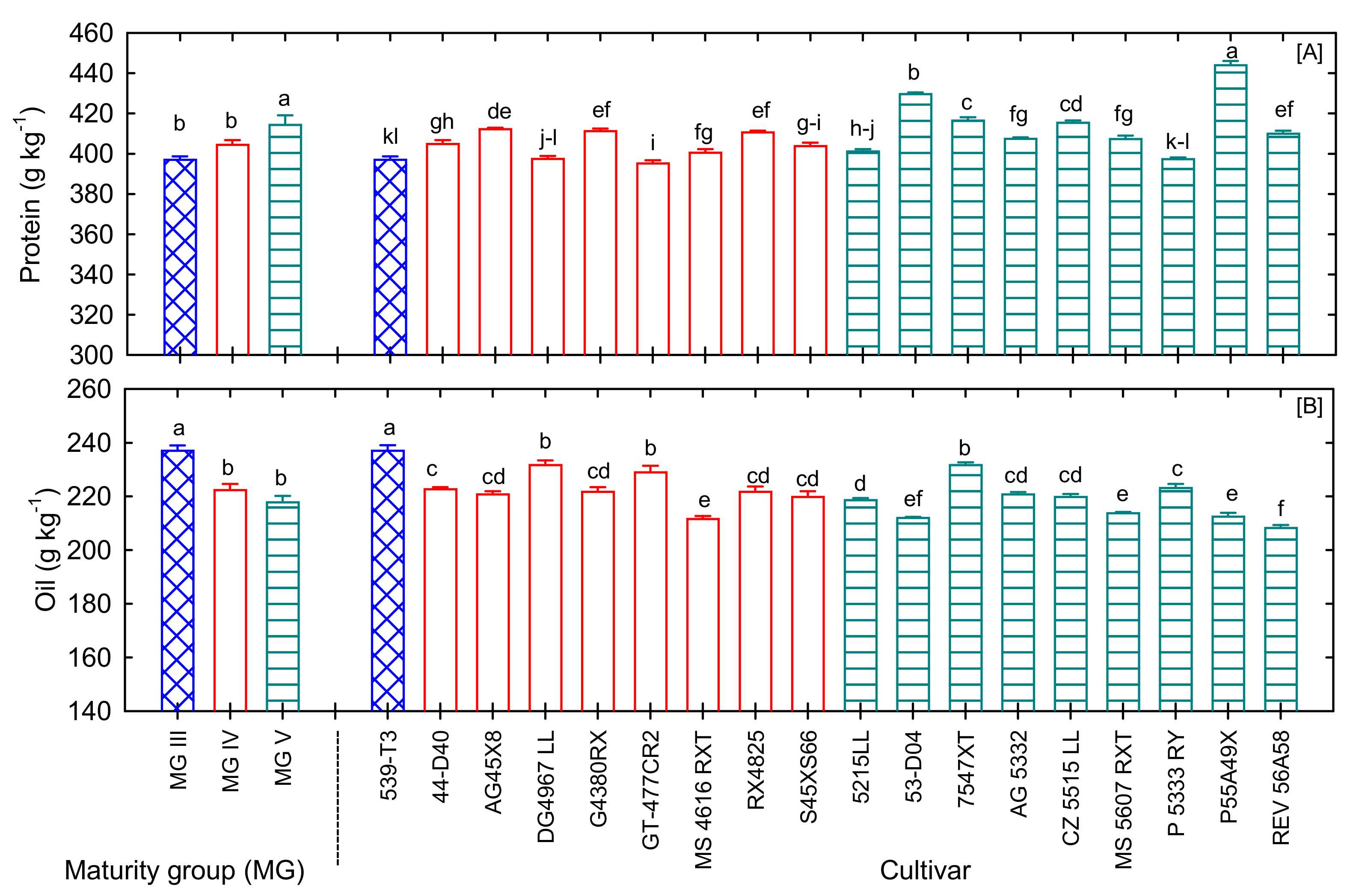
3.2.1. Soybean Seed Protein and Oil
3.2.2. Soybean Seed Fatty Acids
3.2.3. Soybean Seed Sugars
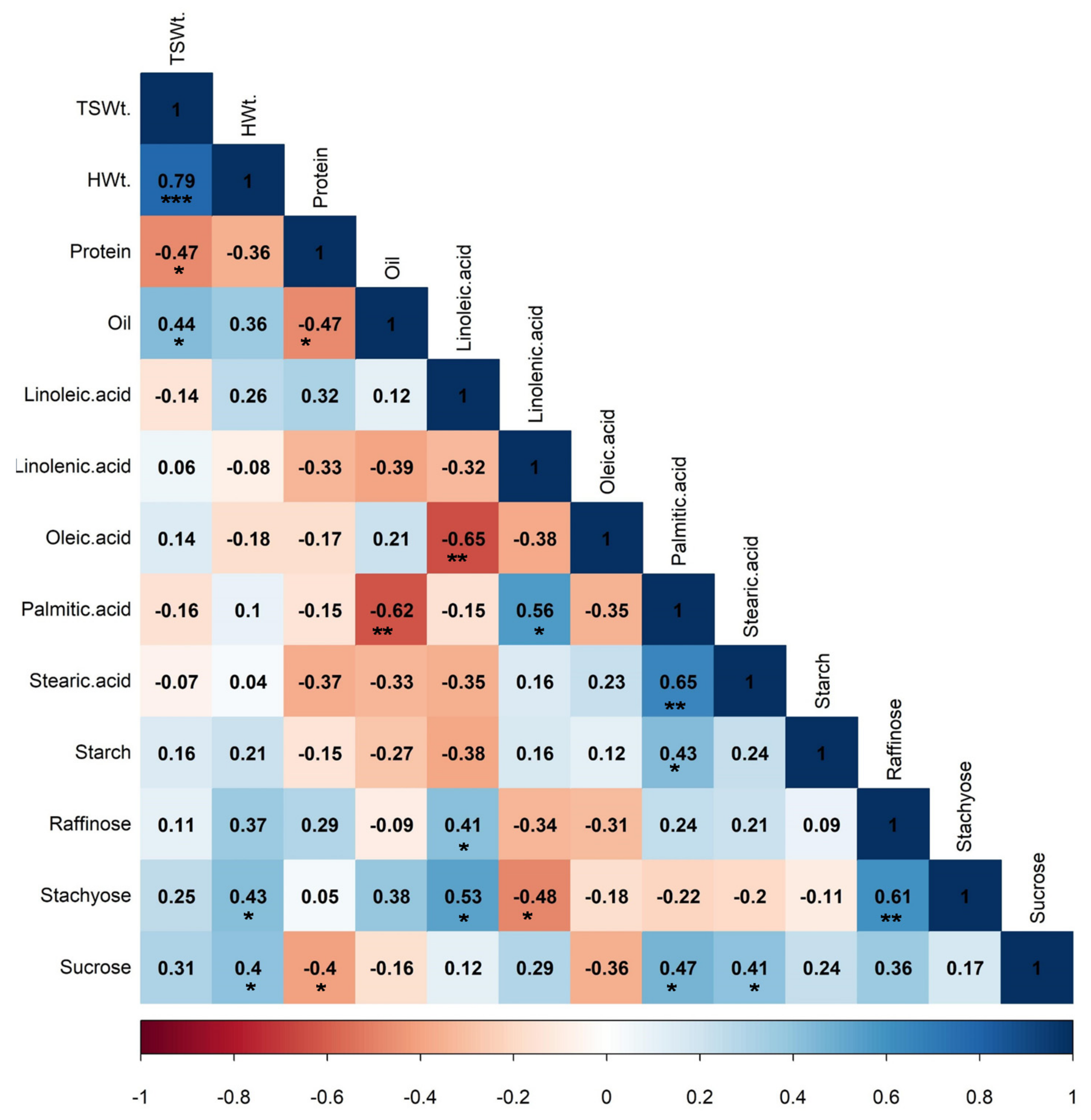
3.3. Correlations between Yield and Quality Composition Traits
3.4. Variability Assessment and Selection of Promising High Yielding Soybean Cultivars with High Protein and Oil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 1 March 2022).
- Voora, V.; Larrea, C.; Bermúdez, S. Global Market Report: Soybeans Sustainable Commodities Marketplace Series 2019. Available online: https://www.iisd.org/system/files/2020-10/ssi-global-market-report-soybean.pdf (accessed on 29 November 2021).
- Annual Soy Stats Results. Available online: https://soygrowers.com/education-resources/publications/soy-stats/ (accessed on 29 November 2021).
- Robert, J.W. The Soybean Solution: Meeting World Food Needs; NIT-College of Agriculture, University of Illinois at Urbana: Champaign, IL, USA, 1986; pp. 4–27. [Google Scholar]
- Painkra, P.; Shrivatava, R.; Nag, S.K.; Kute, I. Correlation analysis for seed yield and its attributing traits in soybean (Glycine Max L. Merrill). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2034–2040. [Google Scholar] [CrossRef]
- Lee, T.; Tran, A.; Hansen, J.; Ash, M. Major Factors Affecting Global Soybean and Products Trade Projections. Available online: https://ageconsearch.umn.edu/record/244273?ln=en (accessed on 28 November 2021).
- Vogel, J.T.; Liu, W.; Olhoft, P.; Crafts-Brandner, S.J.; Pennycooke, J.C.; Christiansen, N. Soybean yield formation physiology—A foundation for precision breeding based improvement. Front. Plant Sci. 2021, 12, 719706. [Google Scholar] [CrossRef] [PubMed]
- Todeschini, M.H.; Milioli, A.S.; Rosa, A.C.; Dallacorte, L.V.; Panho, M.C.; Marchese, J.A.; Benin, G. Soybean genetic progress in South Brazil: Physiological, phenological and agronomic traits. Euphytica 2019, 215, 124. [Google Scholar] [CrossRef]
- Chiorato, A.F.; Carbonell, S.A.M.; Vencovsky, R.; Fonseca Júnior, N.D.S.; Pinheiro, J.B. Genetic gain in the breeding program of common beans at IAC from 1989 to 2007. Crop Breed. Appl. Biotechnol. 2010, 10, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Assefa, Y.; Purcell, L.C.; Salmeron, M.; Naeve, S.; Casteel, S.N.; Kovács, P.; Archontoulis, S.; Licht, M.; Below, F.; Kandel, H.; et al. Assessing variation in us soybean seed composition (Protein and Oil). Front. Plant Sci. 2019, 10, 298. [Google Scholar] [CrossRef]
- Reddy, K.R.; Patro, H.; Lokhande, S.; Bellaloui, N.; Gao, W. Ultraviolet-B radiation alters soybean growth and seed quality. Food Nutr. Sci. 2016, 7, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Alsajri, F.A.; Wijewardana, C.; Irby, J.T.; Bellaloui, N.; Krutz, L.J.; Golden, B.; Gao, W.; Reddy, K.R. Developing functional relationships between temperature and soybean yield and seed quality. Agron. J. 2020, 112, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Lord, N.; Shang, C.; Rosso, L.; Zhang, B. Development of near-infrared reflectance spectroscopy calibration for sugar content in ground soybean seed using Perten DA7250 analyzer. Crop Sci. 2021, 61, 966–975. [Google Scholar] [CrossRef]
- Ficht, A.; Bruce, R.; Torkamaneh, D.; Grainger, C.M.; Eskandari, M.; Rajcan, I. Genetic analysis of sucrose concentration in soybean seeds using a historical soybean genomic panel. Theor. Appl. Genet. 2022. [Google Scholar] [CrossRef]
- Kakar, N.; Bheemanahalli, R.; Jumaa, S.; Redoña, E.; Warburton, M.L.; Reddy, K.R. Assessment of agro-morphological, physiological and yield traits diversity among tropical rice. PeerJ 2021, 9, e11752. [Google Scholar] [CrossRef]
- Bheemanahalli, R.; Sunoj, V.S.J.; Saripalli, G.; Prasad, P.V.V.; Balyan, H.S.; Gupta, P.K.; Grant, N.; Gill, K.S.; Jagadish, S.V.K. Quantifying the impact of heat stress on pollen germination, seed set, and grain filling in spring wheat. Crop Sci. 2019, 59, 684–696. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Boote, K.J.; Kimball, B.A.; Ziska, L.H.; Izaurralde, R.C.; Ort, D.; Thomson, A.M.; Wolfe, D. Climate impacts on agriculture: Implications for crop production. Agron. J. 2011, 103, 351–370. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Wu, T.; Zhang, X.; Song, W.; Xu, C.; Sun, S.; Hou, W.; Jiang, B.; Han, T.; Wu, C. Critical photoperiod measurement of soybean genotypes in different maturity groups. Crop Sci. 2019, 59, 2055–2061. [Google Scholar] [CrossRef]
- Song, W.; Sun, S.; Ibrahim, S.E.; Xu, Z.; Wu, H.; Hu, X.; Jia, H.; Cheng, Y.; Yang, Z.; Jiang, S.; et al. Standard cultivar selection and digital quantification for precise classification of maturity groups in soybean. Crop Sci. 2019, 59, 1997–2006. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.H. Physiological and ecological characteristics of late maturing soybean [Glycine max] in northern Kyushu of Japan. Coast. Bioenviron. Saga Univ. Jpn. 2005, 4, 29–36. [Google Scholar]
- Zhang, Y.; He, J.; Wang, Y.; Xing, G.; Zhao, J.; Li, Y.; Yang, S.; Palmer, R.G.; Zhao, T.; Gai, J. Establishment of a 100-seed weight quantitative trait locus–allele matrix of the germplasm population for optimal recombination design in soybean breeding programmes. J. Exp. Bot. 2015, 66, 6311–6325. [Google Scholar] [CrossRef] [Green Version]
- Vann, R. North Carolina Soybean Yield Contest. Available online: https://soybeans.ces.ncsu.edu/north-carolina-soybean-contest/ (accessed on 1 March 2022).
- Rincker, K.; Nelson, R.; Specht, J.; Sleper, D.; Cary, T.; Cianzio, S.R.; Casteel, S.; Conley, S.; Chen, P.; Davis, V.; et al. Genetic improvement of U.S. soybean in maturity groups II, III, and IV. Crop Sci. 2014, 54, 1419–1432. [Google Scholar] [CrossRef]
- Boehm, J.D.; Abdel-Haleem, H.; Schapaugh, W.T.; Rainey, K.; Pantalone, V.R.; Shannon, G.; Klein, J.; Carter, T.E.; Cardinal, A.J.; Shipe, E.R.; et al. Genetic improvement of US soybean in maturity groups V, VI, and VII. Crop Sci. 2019, 59, 1838–1852. [Google Scholar] [CrossRef]
- Poysa, V.; Woodrow, L. Stability of soybean seed composition and its effect on soymilk and tofu yield and quality. Food Res. Int. 2002, 35, 337–345. [Google Scholar] [CrossRef]
- Robinson, E.H.; Li, M.H. Plant-based catfish feeds. Miss. Agric. For. Exp. Stn. Res. Rep. 2014, 24, 4. [Google Scholar]
- Morris, T.C. Optimizing Soybean Yield and Quality Through Planting Date and Maturity Group Selection in North Carolina; North Carolina State University: Raleigh, NC, USA, 2021. [Google Scholar]
- USDA/NASS 2021 State Agriculture Overview for Mississippi. Available online: https://www.nass.usda.gov/Quick_Stats/Ag_Overview/stateOverview.php?state=MISSISSIPPI (accessed on 1 March 2022).
- Wijewardana, C.; Reddy, K.R.; Bellaloui, N. Soybean seed physiology, quality, and chemical composition under soil moisture stress. Food Chem. 2019, 278, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Yaklich, R.W.; Vinyard, B.; Camp, M.; Douglass, S. Analysis of seed protein and oil from soybean northern and southern region uniform tests. Crop Sci. 2002, 42, 1504–1515. [Google Scholar] [CrossRef]
- Fehr, W.R. Breeding for modified fatty acid composition in soybean. Crop Sci. 2007, 47, S-72–S-87. [Google Scholar] [CrossRef]
- La, T.C.; Pathan, S.M.; Vuong, T.; Lee, J.-D.; Scaboo, A.M.; Smith, J.R.; Gillen, A.M.; Gillman, J.; Ellersieck, M.R.; Nguyen, H.T.; et al. Effect of high-oleic acid soybean on seed oil, protein concentration, and yield. Crop Sci. 2014, 54, 2054–2062. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated fatty acids and risk of coronary heart disease: Modulation by replacement nutrients. Curr. Atheroscler. Rep. 2010, 12, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Bellaloui, N.; Bruns, H.A.; Abbas, H.K.; Mengistu, A.; Fisher, D.K.; Reddy, K.N. Agricultural practices altered soybean seed protein, oil, fatty acids, sugars, and minerals in the midsouth USA. Front. Plant Sci. 2015, 6, 31. [Google Scholar] [CrossRef]
- Cherry, J.H.; Bishop, L.; Hasegawa, P.M.; Lefflert, H.R. Differences in the fatty acid composition of soybean seed produced in northern and southern areas of the U.S.A. Phytochemistry 1985, 24, 237–241. [Google Scholar] [CrossRef]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Rimm, E.; Colditz, G.A.; Rosner, B.A.; Hennekens, C.H.; Willett, W.C. Dietary fat intake and the risk of coronary heart disease in women. N. Engl. J. Med. 1997, 337, 1491–1499. [Google Scholar] [CrossRef]
- Hou, A.; Chen, P.; Shi, A.; Zhang, B.; Wang, Y.-J. Sugar variation in soybean seed assessed with a rapid extraction and quantification method. Int. J. Agron. 2009, 2009, 484571. [Google Scholar] [CrossRef] [Green Version]
- Liu, K. Chemistry and nutritional value of soybean components. In Soybeans: Chemistry, Technology, and Utilization; Liu, K., Ed.; Springer US: Boston, MA, USA, 1997; pp. 25–113. ISBN 978-1-4615-1763-4. [Google Scholar]
- Burton, J.W. Quantitative genetics: Results relevant to soybean breeding. Agron. USA 1987, 16, 211–247. [Google Scholar]
- Geater, C.W.; Fehr, W.R. Association of total sugar content with other seed traits of diverse soybean cultivars. Crop Sci. 2000, 40, 1552–1555. [Google Scholar] [CrossRef]
- Hymowitz, T.; Collins, F.I.; Panczner, J.; Walker, W.M. Relationship between the content of oil, protein, and sugar in soybean seed. Agron. J. 1972, 64, 613–616. [Google Scholar] [CrossRef]
- Halloran, G.M. Grain yield and protein relationships in a wheat cross. Crop Sci. 1981, 21, 699–701. [Google Scholar] [CrossRef]
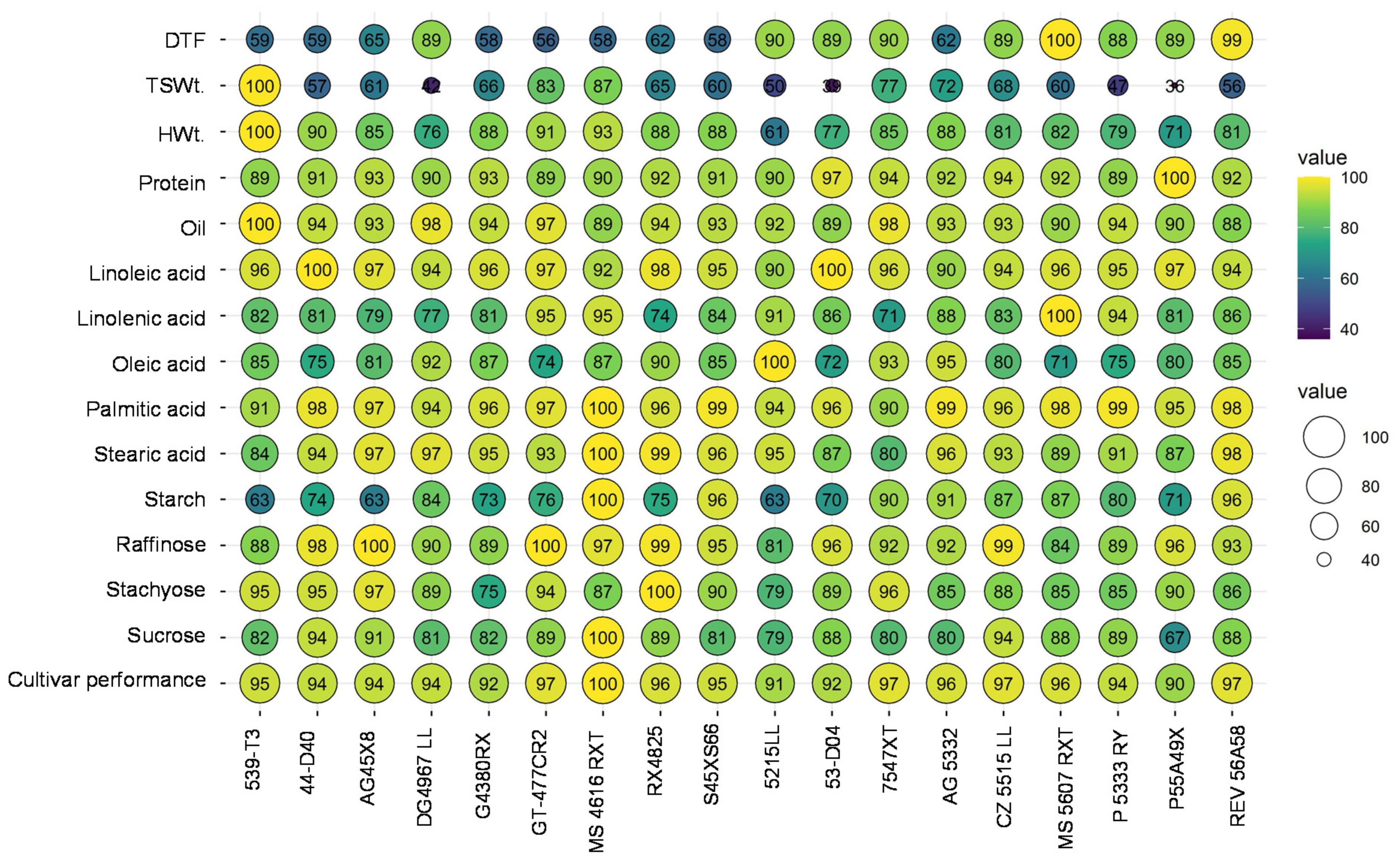
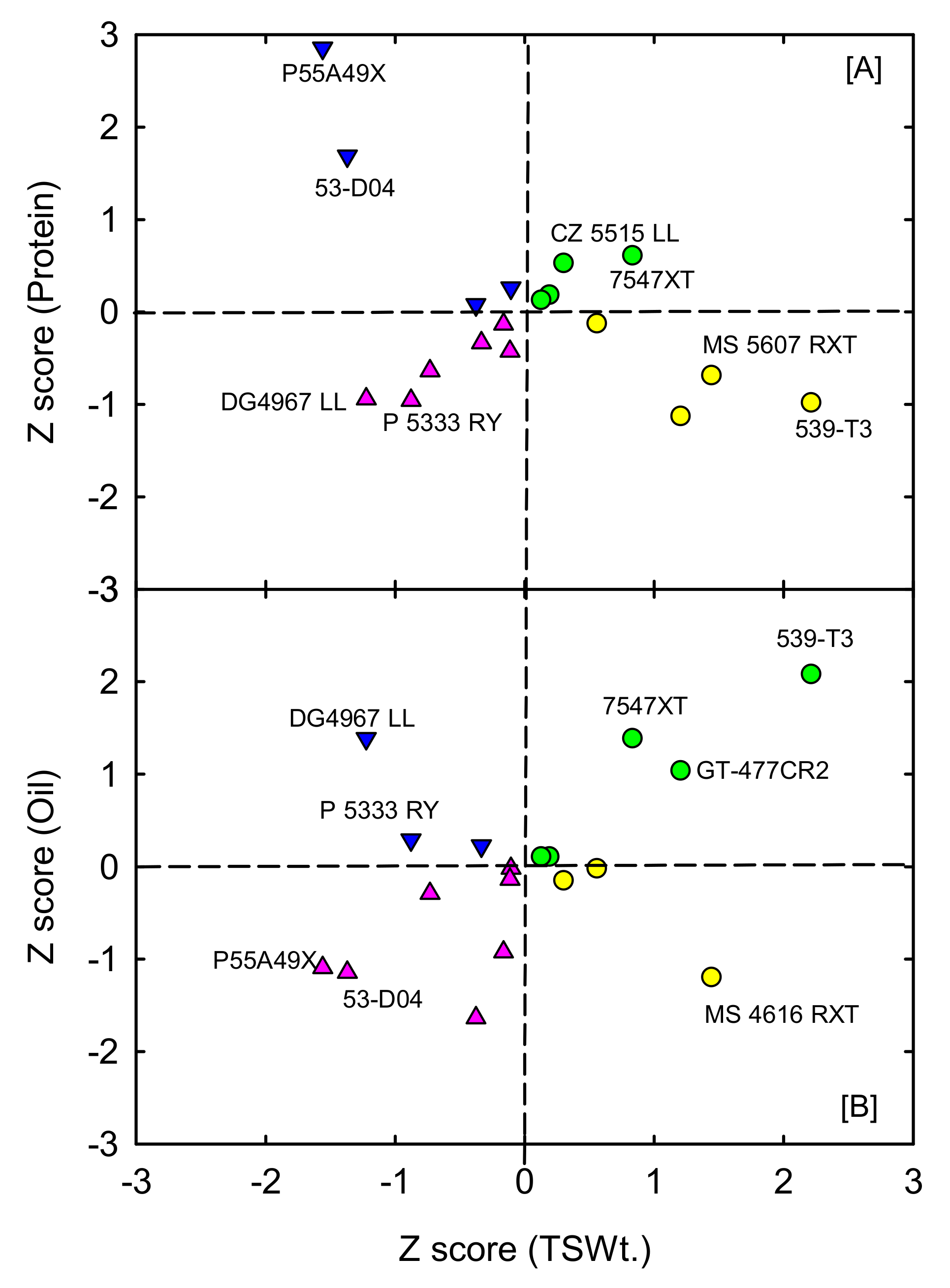
| Trait | Cultivar | MG | Minimum | Maximum | Mean |
|---|---|---|---|---|---|
| Yield components | |||||
| Days to fifty flowering (%) | *** | *** | 29.6 | 52.6 | 39.8 |
| Total seed weight (g plant−1) | * | ** | 59.5 | 165.6 | 103.4 |
| 100-seed weight (g) | *** | *** | 7.01 | 11.5 | 9.6 |
| Seed quality compositions | |||||
| Protein (g kg−1) | *** | *** | 395.2 | 444.0 | 409.0 |
| Oil (g kg−1) | *** | *** | 208.3 | 237.1 | 221.0 |
| Linoleic acid (g kg−1) | *** | ns | 501.7 | 557.7 | 532.5 |
| Linolenic acid (g kg−1) | *** | ns | 64.7 | 91.7 | 77.8 |
| Oleic acid (g kg−1) | *** | ns | 183.2 | 256.7 | 214.8 |
| Palmitic acid (g kg−1) | *** | *** | 119.1 | 131.9 | 127.0 |
| Stearic acid (g kg−1) | *** | *** | 38.1 | 47.7 | 44.4 |
| Starch (g kg−1) | *** | * | 26.5 | 42.3 | 33.9 |
| Raffinose (g kg−1) | *** | ** | 8.7 | 10.7 | 9.9 |
| Stachyose (g kg−1) | *** | * | 32.9 | 43.9 | 39.0 |
| Sucrose (g kg−1) | *** | * | 38.9 | 57.9 | 49.7 |
| Cultivar | Brand | MG | Linoleic Acid | Linolenic Acid | Oleic Acid | Palmitic Acid | Stearic Acid | Starch | Raffinose | Sucrose | Stachyose |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 539-T3 | NK | III | 537.9 (c–e) | 75.6 (e–h) | 217.2 (ef) | 119.7 (i) | 40.2 (i) | 26.8 (g) | 9.4 (fg) | 47.3 (d) | 41.5 (b) |
| 44-D40 | Armor | IV | 556.0 (ab) | 74.1 (f–h) | 192.5 (g–i) | 128.8 (b–e) | 45.1 (d–f) | 31.4 (d–g) | 10.4 (a–c) | 54.7 (b) | 41.7 (ab) |
| AG45X8 | Asgrow | IV | 539.4 (b–e) | 72.4 (g–i) | 207.5 (fg) | 128.4 (c–f) | 46.5 (a–d) | 26.8 (g) | 10.7 (a) | 52.4 (bc) | 42.3 (ab) |
| DG4967 LL | Delta Grow | IV | 527.0 (d–f) | 70.3 (h–j) | 236.8 (b–d) | 123.5 (h) | 46.2 (a–e) | 35.7 (b–e) | 9.6 (d–g) | 47.0 (d) | 39.1 (cd) |
| G4380RX | AgriGold | IV | 537.6 (c–e) | 74.4 (f–h) | 222.2 (c–f) | 126.1 (f–h) | 45.6 (b–e) | 30.9 (e–g) | 9.5 (e–g) | 47.6 (d) | 32.9 (e) |
| GT-477CR2 | Great Heart Seed | IV | 541.8 (a–d) | 86.8 (ab) | 189.1 (g–i) | 127.9 (d–f) | 44.4 (ef) | 32.4 (d–f) | 10.6 (a) | 51.6 (c) | 41.1 (bc) |
| MS 4616 RXT | MorSoy | IV | 515.5 (fg) | 86.7 (ab) | 222.1 (c–f) | 131.9 (a) | 47.7 (a) | 42.3 (a) | 10.3 (a–c) | 57.9 (a) | 38.2 (d) |
| RX4825 | Croplan | IV | 547.6 (a–c) | 67.9 (ij) | 230.1 (b–e) | 126.9 (e–g) | 47.3 (ab) | 31.7 (d–g) | 10.6 (ab) | 51.7 (c) | 43.9 (a) |
| S45XS66 | Dyna-Gro | IV | 529.1 (d–f) | 76.8 (e–g) | 217.9 (d–f) | 131.2 (ab) | 46.0 (a–e) | 40.7 (ab) | 10.1 (a–e) | 47.0 (d) | 39.3 (cd) |
| 5215LL | Go Soy | V | 503.3 (g) | 83.9 (b–d) | 256.7 (a) | 124.1 (h) | 45.4 (c–e) | 26.5 (g) | 8.7 (h) | 46.1 (d) | 34.4 (e) |
| 53-D04 | Armor | V | 557.7 (a) | 79.2 (d–f) | 186.0 (hi) | 126.9 (e–g) | 41.6 (hi) | 29.7 (fg) | 10.2 (a–d) | 51.2 (c) | 38.8 (d) |
| 7547XT | USG | V | 537.2 (c–e) | 64.7 (j) | 239.1 (a–c) | 119.1 (i) | 38.1 (j) | 38.2 (a–c) | 9.8 (c–f) | 46.6 (d) | 42.3 (ab) |
| AG 5332 | Asgrow | V | 501.7 (g) | 80.7 (c–e) | 244.3 (ab) | 130.3 (a–d) | 46.0 (a–e) | 38.6 (a–c) | 9.8 (c–f) | 46.4 (d) | 37.1 (d) |
| CZ 5515 LL | Credenz | V | 521.8 (ef) | 76.2 (e–g) | 205.9 (fg) | 126.1 (f–h) | 44.4 (ef) | 36.8 (b–d) | 10.6 (a) | 54.7 (b) | 38.6 (d) |
| MS 5607 RXT | Morsoy | V | 536.4 (c–e) | 91.7 (a) | 183.2 (i) | 129.3 (b–e) | 42.5 (gh) | 36.7 (b–d) | 8.9 (gh) | 51.2 (c) | 37.4 (d) |
| P 5333 RY | Progeny | V | 530.5 (c–f) | 86.2 (a–c) | 191.6 (g–i) | 130.9 (a–c) | 43.6 (fg) | 33.7 (c–f) | 9.5 (e–g) | 51.4 (c) | 37.4 (d) |
| P55A49X | Pioneer | V | 540.1 (a–d) | 73.8 (f–h) | 204.5 (f–h) | 125.1 (gh) | 41.3 (hi) | 30.1 (fg) | 10.3 (a–c) | 39.0 (e) | 39.3 (cd) |
| REV 56A58 | Terral | V | 524.4 (d–f) | 79.1 (d–f) | 219.4 (d–f) | 129.8 (a–d) | 46.8 (a–c) | 40.8 (ab) | 9.9 (b–f) | 51.0 (c) | 37.5 (d) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bheemanahalli, R.; Poudel, S.; Alsajri, F.A.; Reddy, K.R. Phenotyping of Southern United States Soybean Cultivars for Potential Seed Weight and Seed Quality Compositions. Agronomy 2022, 12, 839. https://doi.org/10.3390/agronomy12040839
Bheemanahalli R, Poudel S, Alsajri FA, Reddy KR. Phenotyping of Southern United States Soybean Cultivars for Potential Seed Weight and Seed Quality Compositions. Agronomy. 2022; 12(4):839. https://doi.org/10.3390/agronomy12040839
Chicago/Turabian StyleBheemanahalli, Raju, Sadikshya Poudel, Firas A. Alsajri, and Kambham Raja Reddy. 2022. "Phenotyping of Southern United States Soybean Cultivars for Potential Seed Weight and Seed Quality Compositions" Agronomy 12, no. 4: 839. https://doi.org/10.3390/agronomy12040839







