Abstract
A two-year (2018/19 and 2019/20) field experiment was carried out to evaluate the efficacy of recently developed fungicide combinations (with different modes of action) towards fungal diseases on seven bread and eight durum wheat varieties. The trial was performed at the FIELDLAB experimental station of the University of Perugia (Italy). The diseases were assessed under natural pressure except for Fusarium head blight (FHB) for which artificial inoculation with a Fusarium culmorum deoxynivalenol (DON)-producing strain was performed at the full flowering stage (BBCH 65). Fungicides were sprayed at the fully extended flag leaf (BBCH 39) and full flowering (BBCH 65) stages. The incidence of different fungal diseases was visually evaluated and other parameters [grains production (t/ha), protein content (%), test weight (kg/hL), and DON accumulation in grain (μg/kg)] were also determined. In the two years, characterized by different climatic conditions, the fungicide treatments showed efficacy in controlling the observed diseases (Septoria tritici blotch and FHB) as well as in reducing DON contamination. No significant differences were found between treatments. The results highlight that, in the present scenario of commercially available durum and bread wheat varieties, the timely application of the most common fungicides plays a crucial role for FHB and DON management in the presence of climatic conditions that are favorable to the disease. The impact of these results in an integrated disease management perspective is discussed.
1. Introduction
Bread and durum wheat [Triticum aestivum L. and T. turgidum subsp. durum (Desf.) Husn] are two of the most cultivated cereal crops worldwide. Italy is considered an important country for wheat cultivation [1], especially durum, and during 2020 a production of about 7 million tons, with 1.7 million hectares destined to wheat cultivation [2], was achieved. Across the Italian peninsula, these crops, with their derived products such as bread and pasta, are deeply connected to traditions, culture, and to the national economic system [3,4]. Considering the wheat production chain, there are two parameters that mainly affect the cereal market: quality and healthiness of the product [5]. In recent decades, increasing attention has been given to these parameters, especially by consumers, due to the intensification of plant diseases that are associated with the presence of fungal species producing mycotoxins in cereal crops [6].
The main threats for wheat crops in Europe and Italy are represented by fungal pathogens [7,8,9,10,11] that infect different plant tissues such as the leaves and heads. Powdery mildew (Blumeria graminis f.sp. tritici), yellow rust (Puccinia striiformis), leaf rust (Puccinia recondita; synonym Puccinia triticina), Septoria tritici blotch (STB) (Zymoseptoria tritici; synonym Septoria tritici), and Septoria nodorum blotch (Parastagonospora nodorum; synonym Septoria nodorum) are the most common foliar diseases of wheat in Europe [12]. Rusts (Puccinia spp.) were generally well controlled by varietal resistance until more aggressive physiological races, which overcame the resistances deployed in wheat varieties, emerged [13,14]. STB is considered one of the main diseases in most parts of the Europe [7,15,16] and it has been associated with yield losses, due to the reduction of the photosynthetic life of the canopy, especially of the flag leaf, during grain filling [17].
Regarding the head, the main threat is represented by Fusarium head blight (FHB), a complex disease caused by pathogenic fungi belonging to the genus Fusarium leading to yield losses and mycotoxin accumulation in the grain [6,18]. Many Fusarium species cause FHB [19] and, among them, Fusarium graminearum, Fusarium culmorum, Fusarium poae, and Fusarium avenaceum are considered the most widespread. However, other species such as Fusarium langsethiae, Fusarium sporotrichioides, and Fusarium tricinctum are also frequently found [20,21,22,23,24,25]. F. graminearum and F. culmorum are considered the main deoxynivalenol (DON) producers, a trichothecene that is considered the most common mycotoxin in wheat grain worldwide [26]. DON is toxic for humans and animals [27] and its accumulation makes wheat grains unsuitable for consumption [28]. For this reason, the European Commission (EC) has set maximum acceptable levels for DON (and other mycotoxins) in cereals and derived products for human consumption [29,30,31].
The management of wheat fungal diseases in Europe largely relies on the use of fungicides and less susceptible varieties [12]. The first systemic fungicides introduced in the market for the management of cereal diseases in the 1970s [32] were triazole fungicides, that belong to the demethylase inhibitors (DMI) group (FRAC Group 3) [33] and act as sterol biosynthesis inhibitors [34]. This mode of action [35] is still the most commonly used for the control of cereal diseases worldwide [36]. DMI fungicides also demonstrate a significant effect on preventing the decline of green leaf area in flag leaves [37] and favor grain yield [38]. Additionally, they showed to be the most active molecules against FHB and, therefore, they also prevent DON accumulation [39,40,41,42]. Strobilurins [quinone outside inhibitors (QoI), FRAC Group 11] [33], introduced in 1992, and succinate dehydrogenase inhibitors (SDHI, FRAC Group 7) [33], introduced in 2010, represent the other principal fungicides that are employed for cereal disease control and they are also usually mixed with the previously described triazole-based fungicides [36]. QoI and SDHI fungicide groups are mitochondrial inhibitors that block respiration in the fungal cell and inhibit the ability of spores to germinate. Both types of fungicides play an important role in the control of several wheat foliar diseases such as STB, powdery mildew, and rusts [43,44] and with their modes of action are protectant fungicides with different curative activity depending on the active ingredient [43,45]. These different fungicides (DMI, QoI, and SDHI) are often used in a mixture to maximize their effectiveness in disease control and to ensure a stronger anti-resistance strategy since QoIs and SDHIs have a higher risk for pathogen resistance development than DMI fungicides [36].
Integrated pest management (IPM) is defined as the use of different plant disease control strategies, considering profit, crop yield, and health profiles, as declared by the Food and Agriculture Organization of the United Nations (FAO) [46]. The European Union (EU), at the beginning of the twenty-first century, issued the Directive 2009/128/EC 2009 for minimizing the use of pesticides [11]. Agronomical practices (such as crop rotation, stubble management, sowing density, soil tillage), varietal choice, and the use of biological control agents, together with the use of fungicides, are currently available options for the integrated disease management of wheat [47,48]. In IPM, fungicides represent the last chance for controlling diseases, basing crop management mainly on the host genetic resistance/low susceptibility and agronomic practices [49]. However, the effective control of some wheat diseases in some geographic areas, are related only to the adoption of fungicide treatments [12]. In fact, fungicides play a crucial role in an IPM approach, obviously optimizing the rate and application timing.
Given this context, the object of this work was to evaluate, in a two-year (2018/2019 and 2019/2020) field plot experiment, the efficacy of commercially available fungicides, with three different modes of action (DMI, QoI, and SDHI), towards fungal diseases in several bread and durum wheat varieties. The diseases were evaluated under natural inoculum pressure, except for FHB for which artificial inoculation was performed. The visual incidence of different fungal diseases, grains production (t/ha), protein content (%), test weight (kg/hL), and DON accumulation (μg/kg) were determined. Climatic data in both experimental years were also collected. The obtained results provide information contributing to the effective implementation of the management of wheat fungal diseases as well as of DON contamination in the grains.
2. Materials and Methods
2.1. Field Trials and Experimental Design
The experiments were conducted during the 2018/2019 and 2019/2020 wheat growing seasons at the experimental station (FIELDLAB) of the Department of Agricultural, Food and Environmental Sciences of the University of Perugia, located at Papiano (Perugia, Central Italy) in the middle Tiber valley (42°57′ N, 12°22′ E, 165 asl). A total of seven bread (Altavista, Lancillotto, Metropolis, Nogal, Oregrain, Rebelde, Solehio) and eight durum wheat varieties (Antalis, Don Matteo, Egeo, Farah, Kanakis, Marakas, Monastir, Ramirez) were used in the study. All these varieties are commercially available and are widely used by farmers in Central Italy. The plots (1.5 m wide by 6 m long) were sown in mid-November each year and grown according to the standard agronomic practices of the experimental area as fully described in Supplementary Table S1. A total of three fungicide combinations plus non-inoculated and inoculated controls were compared in a randomized blocks design experiment with three replicates. Except for the non-inoculated controls, the plants were inoculated with a conidial suspension of an aggressive DON-producing strain of F. culmorum (see Section 2.3) after fungicide application. The experiment consisted of a total of 225 plots [15 wheat varieties (seven bread and eight durum) × five experimental treatments (3 fungicide combinations plus two controls) × three replicates].
2.2. Fungicide Application
To manage the main fungal wheat diseases, three different fungicide combinations were tested. Each strategy was composed of a combination of one fungicide that was applied at BBCH 39 (foliar diseases target) and one that was applied at BBCH 65 (FHB target). A total of six commercially available fungicides, with different modes of action (QoI, SDHI, and DMI) were employed. Information about the applied fungicides, doses, and application timings are described in Table 1 (further details are available in Supplementary Table S1). In brief: (1) Aviator Xpro (prothioconazole + bixafen) [BBCH 39] and Prosaro (prothioconazole + tebuconazole) [BBCH 65] were involved in the first fungicide combination (fungicide combination A); (2) Elatus Plus + Rivior (benzovindflupyr + tetraconazole) [BBCH 39] and Elatus Era (benzovindiflupyr + protioconazole) [BBCH 65] were applied for the second fungicide combination (fungicide combination B); and (3) Priaxor (fluxapyroxad + pyraclostrobin) [BBCH 39] and Caramba + Sportak (metconazole + prochloraz) [BBCH 65] were used for the third fungicide combination (fungicide combination C). The different fungicides were applied with a hand-pump sprayer equipped with 500 L/ha nozzles using the doses recommended on the commercial formulation labels.

Table 1.
Fungicide treatments applied in the two experimental years (2018/2019 and 2019/2020).
2.3. Inoculum Production and Application
The F. culmorum 74dw10 strain, belonging to the 3-acetyl-deoxynivalenol (3ADON) chemotype, was used in the 2018/2019 and 2019/2020 growing seasons for field inoculations. The 74dw10 strain was isolated from durum wheat kernels that were harvested in 2010 in Central Italy (Montecastrilli, Umbria, Central Italy), molecularly identified, characterized for its in vitro mycotoxigenic profile, and stored at −80 °C in the fungal collection of the Department of Agricultural, Food and Environmental Sciences of the University of Perugia [50]. The fungal strain was preliminarily cultured for six days in the dark at 21 °C on potato dextrose agar (PDA, Biolife Italiana, Milan, Italy) in 9 cm diameter Petri dishes (Aptaca SpA, Canelli, Asti, Italy). Then, the fungus was cultured in V8 liquid growth medium to obtain conidial inoculum. In detail, flasks (1 L) containing 200 mL of autoclaved 20% V8 juice (Campbell’s, Camden, NJ, USA) were inoculated with mycelium plugs (diameter 0.5 cm). The media were shaken on an orbital shaker (3591-I, Lab-line Instruments, IA, USA) at 100 rpm for 14 days at room temperature with 12/12 h light/dark. The samples were filtered (Miracloth, Millipore Corporation, Billerica, MA, USA) and centrifuged with a 5804 R centrifuge (Eppendorf, Hamburg, Germany) at 3000 rpm for 5 min at 4 °C. Finally, the concentration of conidia suspension was adjusted to 107 conidia/mL using a hemocytometer. The wheat varieties were artificially inoculated 24 h after the second fungicide application at full flowering stage (BBCH 65) (Supplementary Table S1) with the conidial suspension using a hand-pump sprayer equipped with 500 L/ha nozzles.
2.4. Disease Evaluation, Production Parameters and Weather Data Collection
The phytosanitary conditions of the tested varieties were monitored across their entire cycle and the presence of different fungal diseases was visually assessed. Foliar diseases were assessed starting from the beginning of tillering (BBCH 21) until the medium milk stage (BBCH 75), adopting a disease evaluation scale that considered:
- (a)
- incidence (%) from 0 (all plants healthy) to 100 (all plants symptomatic);
- (b)
- severity (%) from 0 (healthy leaves) to 100 (completely symptomatic leaves).
The “Foliar disease index”, assessed at the maximum leaf symptom expression (early milk, BBCH 73), for each plot was calculated using the following formula:
Foliar disease index = [disease incidence (%) × disease severity (%)]/100
FHB was monitored starting from the end of flowering (BBCH 69) until the medium milk stage (BBCH 75), adopting a disease evaluation that considered:
- (a)
- incidence (%), expressed as the percentage of symptomatic heads in the plot, from 0% (no symptomatic heads) to 100% (all spikes symptomatic);
- (b)
- severity (%), expressed as the average percentage of symptomatic spikelets per head.
The “FHB index”, assessed at the maximum head symptom expression (early milk stage, BBCH 75), was calculated for each plot using the following formula:
FHB index = [disease incidence (%) × disease severity (%)]/100
The full flowering (BBCH 65) date was recorded every year for each variety. The plots were harvested at full maturity (BBCH 99) using a plot combine (Wintersteiger Italia Srl, Bozen, Italy). After harvest, the grain yield (t/ha) of each plot was determined. Finally, grain subsamples of 500 g from each plot were analyzed with a specific grain analyzer (Infratec 1241, FOSS Headquarters, Hilleroed, Denmark) for protein content (%) and test weight (kg/hL).
Weather data (rainfall, relative humidity, and average temperatures) were recorded daily from September to June in both experimental years with a weather station at the above-mentioned experimental fields.
2.5. Deoxynivalenol Quantification in Grains
DON extraction was performed on a 500 g representative kernel subsample. In detail, the kernels were finely ground with a blender (IMETEC, Azzano San Paolo, Bergamo, Italy) and 5 g of each sample were added with 25 mL of methanol, vortexed for 3 min, filtered through filter paper (150 mm diameter, grade 1, Whatman, GE Healthcare, Amersham Place, UK) for separating the solid from the liquid phase. The DON present in the filtrate solution was quantified using an Enzyme-Linked Immunosorbent Assay (ELISA) (Bio-Shield DONTM kit) following the protocol provided by the manufacturer. Standard solutions of 0, 125, 500, 2000, and 5000 µg/kg were used for the construction of the calibration curve and, in each sample, DON concentration was automatically calculated by the software Prognosis-Data-Reader provided by the manufacturer. The limit of detection (LOD) of the kit was 50 µg/kg and the limit of quantification (LOQ) was 100 µg/kg.
2.6. Statistical Analysis
The data obtained were subject to the analysis of variance (ANOVA) using the “DSAASTAT ver. 1.0192” program [51]. Data were analyzed considering the “experimental treatment” (untreated-uninoculated, untreated-inoculated, treated with different fungicide combinations) as a factor and the different “parameters” analyzed as variables (foliar diseases index, FHB index, DON content in grain, and yield parameters). In addition, the controls were analyzed separately for foliar disease indexes, FHB index, DON content in grain, and yield for evidencing possible differences between the varieties. In detail: for the foliar disease indexes, the untreated-uninoculated (with F. culmorum) control was used. While, for FHB index, DON content and yield, the untreated-inoculated (with F. culmorum) control was considered. In case of significance, Tukey’s HSD multiple comparison tests to assess pairwise contrasts were performed (p ≤ 0.05) using the same program [51].
Finally, the correlations between FHB index (%) and DON (µg/kg) content in grains were evaluated using the Pearson correlation coefficient (r) for all the wheat varieties in the two different years.
3. Results
3.1. Climatic Conditions in the Two Experimental Years
The two experimental years were characterized by very different climatic conditions (Figure 1), particularly in terms of rainfall (mm). Considering the entire crop cycle (sowing-harvest), the rainfalls showed a similar daily mean value of 2.36 mm and 1.9 mm in 2018/2019 and in 2019/2020, respectively. However, in 2019/2020, the rainfalls mainly occurred in November and a prolonged drought period going from January to May was recorded (Figure 1A,B).
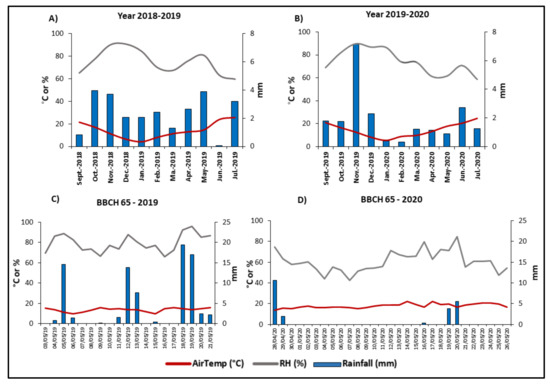
Figure 1.
Climatic conditions recorded in 2018/2019 and 2019/2020. The average air temperature (AirTemp °C), relative humidity (RH %), and rainfall (mm) during the two whole growing seasons (A,B) and during the flowering stage (BCCH 61-BBCH 69) of the tested varieties (C,D). The flowering stage was recorded from 03/05 to 21/05 in 2019 and from 28/04 to 26/05 in 2020. In these time frames, all tested varieties had scalarly reached the flowering stage.
Being full flowering (BBCH 65), the most susceptible wheat stage to FHB, a focus on the period during which this stage occurred in the different tested varieties was realized (Figure 1C,D). In general, rainfalls, and consequently relative humidity, were different in the two experimental years. In 2018/2019, the total (81.4 mm) and the mean (4.28 mm) values of rainfalls recorded during the flowering time of the tested varieties were higher compared to those recorded in 2019/2020 (22.4 mm and 0.4 mm, respectively). In addition, the mean humidity occurring during the flowering time was different in the two experimental years: 79.4% in 2018/2019 and 60.6% in 2019/2020.
3.2. Foliar Disease Symptoms Evaluation
STB was the only fungal foliar disease detected in both experimental years. The data relative to the presence of this disease and the effect of fungicides on STB symptoms during the two years are detailed in Supplementary Table S2. In general, considering the average STB index levels for all the varieties together, they were low in both years (3.18% and 1.04% recorded in the untreated control in 2018/2019 and 2019/2020, respectively). A significant reduction (p ≤ 0.05) of STB symptoms in the fungicide-treated plots in comparison to the untreated ones was observed in both experimental years. However, no significant differences between the different fungicides in reducing STB symptoms were detected. The same results were obtained also considering all durum wheat varieties and all bread wheat varieties as two separate groups.
Considering the varieties singularly, in 6 out of 15 varieties in 2018/2019 (Antalis, Don Matteo, Farah, Metropolis, Monastir and Ramirez) and in 9 out of 15 varieties in 2019/2020 (Antalis, Don Matteo, Egeo, Lancillotto, Marakas, Metropolis, Monastir, Ramirez and Rebelde), a significant reduction (p ≤ 0.05) of STB symptoms in the fungicide-treated plots in comparison to the untreated controls were detected. However, in all these varieties, no significant differences between the different tested fungicide treatments in reducing STB symptoms were found (Supplementary Table S2).
Within durum or bread wheat varieties, analyzing only the untreated controls, no significant differences (p ≥ 0.05) in terms of varietal susceptibility to STB were found in both experimental years (Supplementary Figure S1).
3.3. Fusarium Head Blight Symptoms Evaluation
Data relative to FHB presence and the effect of fungicides on FHB symptoms during the two experimental years, are detailed in Table 2.

Table 2.
Effect of fungicide treatments on Fusarium head blight symptoms, during the two experimental years.
In general, considering the average of all wheat varieties, the level of natural FHB disease observed was low, in particular in the second experimental year in which the FHB index detected in the untreated controls (0.07%) was significantly lower (p ≤ 0.05) than that recorded in the first year (2.74%) (Figure 2).
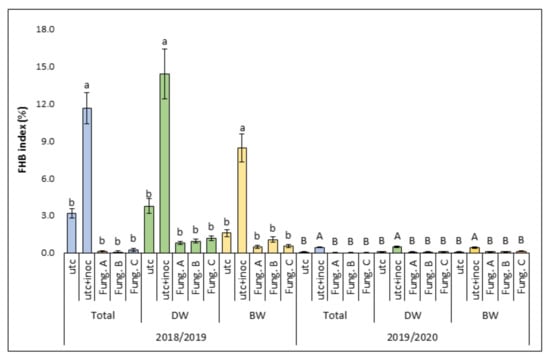
Figure 2.
Average level of Fusarium head blight (FHB) index (%) indicating FHB symptoms observed in the field during the two experimental years (2018/2019 and 2019/2020). Columns represent the average data (± standard error) of all wheat varieties (Total) or durum (DW) and bread (BW) wheat varieties as two separate groups. In each of these three categories (Total, DW, and BW), data are shown for each of the five experimental treatments: untreated control (utc), untreated-inoculated control (utc + inoc), fungicide combination A (Fung. A), fungicide combination B (Fung. B), and fungicide combination C (Fung. C). Within each category (Total, DW, and BW), the averages with different letters (lower case in 2018/2019 and upper case in 2019/2020) are significantly different at p ≤ 0.05 based on Tukey’s HSD adjustment for multiple comparison test.
The application of artificial inoculum significantly (p ≤ 0.05) increased the FHB index in the untreated-inoculated plots (11.61% and 0.45% in 2018/2019 and 2019/2020, respectively) in comparison to the untreated-uninoculated ones (2.74% and 0.07% in 2018/2019 and 2019/2020, respectively) (Figure 2). A significant reduction (p ≤ 0.05) of FHB symptoms in the fungicide-treated plots in comparison to the untreated-inoculated ones was observed in both experimental years. However, also in this case, no significant differences between the different fungicide treatments in reducing FHB symptoms were detected (Figure 2). The same results were obtained also considering all the durum wheat varieties and all the bread wheat varieties as two distinct groups (Figure 2).
Conversely, no significant reduction (p ≤ 0.05) of FHB symptoms in the fungicide-treated plots in comparison to the untreated-uninoculated ones was observed in both experimental years, highlighting that, in the presence of artificial inoculation, all fungicide treatments applied were able to keep FHB symptoms close to the natural pressure values. Fungicide treatments permitted to obtain a mean reduction of FHB index of 93% and 81% in 2018/2019 and in 2019/2020, respectively. In general, considering all the wheat varieties tested in the experiment in both years, the following FHB symptoms pattern was detected: untreated-inoculated > fungicide treated-inoculated = untreated-uninoculated.
In detail, considering the varieties singularly, in 14 out of 15 varieties in 2018/2019 and in 8 out of 15 varieties in 2019/2020, a significant reduction (p ≤ 0.05) of observed FHB symptoms in the fungicide-treated plots in comparison to the untreated-inoculated controls were detected. In all these varieties, no significant differences between the efficacies of the different fungicide treatments in reducing FHB symptoms were observed (Table 2).
Within durum wheat or bread wheat varieties, analyzing only the untreated-inoculated controls, significant differences (p ≥ 0.05) in terms of FHB symptoms were found (Supplementary Figure S2). In detail, in 2018/2019 the durum wheat variety Egeo was significantly (p ≤ 0.05) more affected by FHB than Don Matteo, Farah, Marakas, and Monastir. In the same season, the bread wheat variety Solehio was significantly (p ≤ 0.05) more affected by FHB than all the other tested bread wheat varieties except for Metropolis. In 2019/2020, probably due to a general low disease level, no significant differences (p ≥ 0.05) in terms of FHB index among durum wheat varieties were detected. Among bread wheat varieties, Nogal and Metropolis showed a higher (p ≤ 0.05) FHB index than Altavista.
3.4. Deoxynivalenol Quantification in Grains
The results about DON accumulation in wheat grains and the effect of fungicides on DON contamination during the two experimental years are detailed in Table 3.

Table 3.
Effect of fungicide treatments on deoxynivalenol accumulation in grains during the two experimental years.
Similarly to what observed for FHB symptoms, considering the average of all the wheat varieties tested, the level of DON contamination recorded in the untreated-uninoculated controls in the first experimental year (578.5 µg/Kg) was significantly higher (p ≤ 0.05) than that detected during the second one (97.2 µg/Kg) (Figure 3).
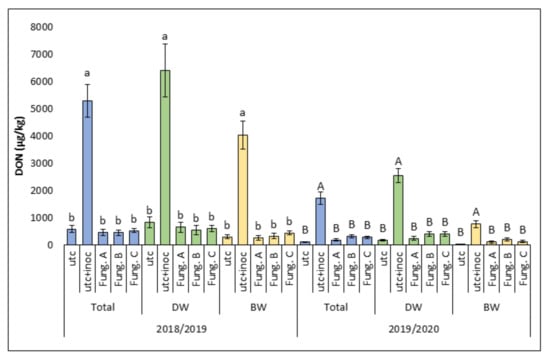
Figure 3.
Average level of deoxynivalenol (DON; µg/kg) detected in the grain during the two experimental years (2018/2019 and 2019/2020). Columns represent the average data (± standard error) of all wheat varieties (Total) or durum (DW) and bread (BW) wheat varieties as two separate groups. In each of these three categories (Total, DW, and BW), data are shown for each of the five experimental treatments: untreated control (utc), untreated-inoculated control (utc + inoc), fungicide combination A (Fung. A), fungicide combination B (Fung. B), and fungicide combination C (Fung. C). Within each category (Total, DW, and BW), the averages with different letters (lower case in 2018/2019 and upper case in 2019/2020) are significantly different at p ≤ 0.05 based on Tukey’s HSD adjustment for multiple comparison test.
Following the application of artificial inoculum, the DON levels in the untreated-inoculated plots (5297.3 µg/Kg and 1726.3 µg/Kg in 2018/2019 and 2019/2020, respectively) were significantly higher (p ≤ 0.05) than those detected in the untreated-uninoculated ones (Figure 3).
A significant reduction (p ≤ 0.05) of DON levels in the fungicide-treated plots in comparison to the untreated-inoculated ones was observed in both experimental years, with no significant differences among the different fungicide treatments applied (Figure 3). The same results were obtained also considering all durum wheat and all bread wheat varieties as two separate groups (Figure 3). As observed for FHB symptoms, in the presence of artificial inoculation, all the applied fungicide treatments were able to keep DON levels close to those present under FHB natural pressure in the absence of fungicides. Compared with the untreated-inoculated controls, fungicide treatments reduced DON by 91% in 2018/2019 and 85% in 2019/2020. In conclusion, also for DON contamination, the same FHB pattern was detected: untreated-inoculated > fungicide treated-inoculated = untreated-uninoculated.
Considering the different varieties, in 12 out of 15 varieties in 2018/2019 and 2019/2020, a significant reduction (p ≤ 0.05) of DON levels in all the fungicide-treated plots in comparison to the untreated-inoculated controls was detected. In all these varieties, no significant differences between the efficacies of the different tested fungicide treatments in reducing FHB symptoms were observed (Table 2).
Within durum or bread wheat varieties, analyzing only the untreated-inoculated controls, significant differences (p ≥ 0.05) in terms of DON levels were found (Supplementary Figure S3). In detail, in 2018/2019, the durum wheat grain of Egeo and Kanakis varieties was significantly (p ≤ 0.05) more contaminated with DON than that of all other durum wheat varieties. In the same season, no significant differences (p ≥ 0.05) of DON accumulation were detected in the grain of the different tested bread wheat varieties. In 2019/2020, the durum wheat grain of the Don Matteo variety was significantly (p ≤ 0.05) more contaminated with DON than that of Farah and Antalis varieties. In the same season, the bread wheat grain of the Metropolis variety was significantly (p ≤ 0.05) more contaminated with DON than that of Nogal, Oregrain, and Solehio varieties.
3.5. Relationship between Deoxynivalenol Accumulation in Grain and Fusarium Head Blight Symptoms
Correlations between DON content (µg/kg) and FHB index (%) observed in the field were performed taking the average value of both parameters of all the varieties in the two different experimental years (Figure 4).
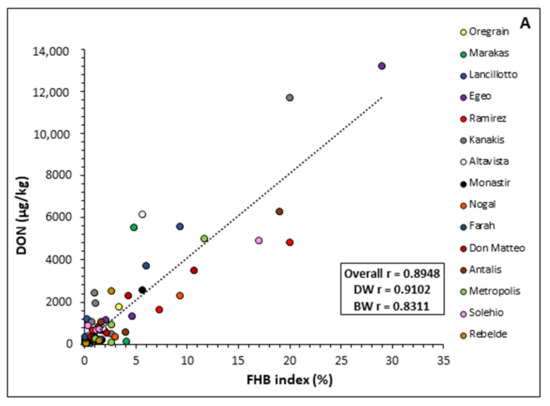
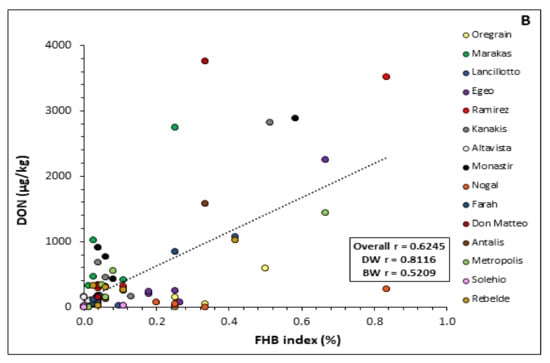
Figure 4.
Correlation between Fusarium head blight (FHB) index (%) and deoxynivalenol (DON, µg/kg) grain contamination recorded for each durum and bread wheat variety tested in 2018/2019 (A) and 2019/2020 (B). Pearson correlation coefficient (r) is shown in the box for the average of all varieties (Total) and for durum (DW) or bread wheat (BW) as two separate groups.
The two parameters were positively related in both years. However, the relationship between DON accumulation in grain and FHB symptoms was stronger in the first year (r = 0.89) than in the second one (r = 0.62). A strong relationship was also observed for the durum wheat varieties (0.91 and 0.81 in 2018/2019 and 2019/2020, respectively) in comparison to the bread wheat varieties (0.83 and 0.52 in 2018/2019 and 2019/2020, respectively) in both years (Figure 4).
3.6. Evaluation of Grain Yield (t/ha), Test Weight (kg/hL) and Protein Content (%)
Data about the effect of fungicides on grain yield (t/ha) during the two experimental years are detailed in Supplementary Table S3.
Considering the average yield levels of all the varieties tested in the experiment, the grain yield recorded in the untreated controls in the first year (7.73 t/ha) was significantly higher (p ≤ 0.05) than that detected during the second one (6.01 t/ha) (Figure 5). Artificial inoculation significantly reduced (p ≤ 0.05) the grain yield in the untreated-inoculated plots (6.44 t/ha) in comparison to the untreated-uninoculated ones (7.73 t/ha) only in the 2018/2019 season (Figure 5), during which, following fungicides application, grain yield of the treated-inoculated plots was significantly higher (p ≤ 0.05) than that of untreated-inoculated ones, with no significant differences between the different fungicides tested (Figure 5). In 2018/2019, the same results were obtained also considering all durum wheat and all bread wheat varieties as two distinct groups (Figure 5). Compared to the untreated-inoculated controls, fungicide treatments increased grain yield by 22.8%. Regarding grain yield averaged over wheat species and varieties the following pattern was detected in 2018/2019: untreated-inoculated < untreated-uninoculated ≤ fungicide treated-inoculated. On the contrary, in 2019/2020 differences between untreated-inoculated and untreated-uninoculated plots were not significant. Fungicide treatments confirmed to significantly increase total grain yield as compared to the untreated-inoculated control, even though such increase was very low (+3.3%) (Figure 5).
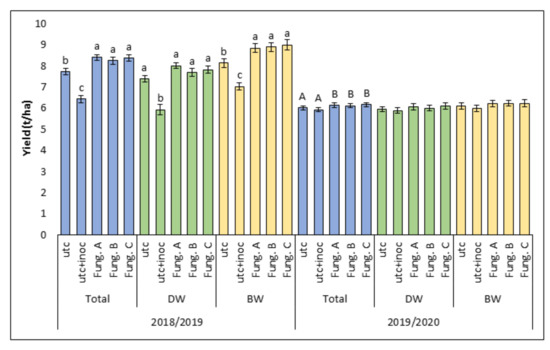
Figure 5.
Average grain yield (t/ha) recorded during the two experimental years (2018/2019 and 2019/2020). Columns represent the average data (± standard error) of all wheat varieties (Total) or durum (DW) and bread (BW) wheat varieties as two separate groups. In each of these three categories (Total, DW, and BW), the data are shown for each of the five experimental treatments: untreated control (utc), untreated-inoculated control (utc + inoc), fungicides combination A (Fung. A), fungicide combination B (Fung. B), and fungicide combination C (Fung. C). Within each category (Total, DW, and BW), averages with different letters (lower case in 2018/2019 and upper case in 2019/2020) are significantly different at p ≤ 0.05 based on Tukey’s HSD adjustment for multiple comparison test. The absence of letters means the absence of significant differences.
Considering the single varieties, in 7 out of 15 varieties in 2018/2019, a significant increase (p ≤ 0.05) of grain yield in all fungicide-treated plots in comparison to the untreated-inoculated control was detected. However, in all these varieties, no significant differences between the efficacies of the different tested fungicide treatments in increasing grain yield were observed (Supplementary Table S3). In 2019/2020, for each variety, no significant increase of grain yield in the fungicide-treated plots in comparison to untreated-inoculated control was recorded.
Within durum or bread wheat varieties, analyzing only the untreated-inoculated controls, significant differences (p ≥ 0.05) in terms of grain yield were found (Supplementary Figure S4). In detail, in 2018/2019, the yield of the durum wheat variety Monastir was significantly (p ≤ 0.05) higher than those of all the other varieties except for Farah and Marakas. In the same season, no significant differences (p ≥ 0.05) in terms of grain yield were detected in the bread wheat varieties tested. In 2019/2020, the yield of the durum wheat variety Egeo was significantly (p ≤ 0.05) lower than that of all the other varieties except Kanakis, Monastir, and Marakas. In the same season, the bread wheat variety Oregrain showed a significantly (p ≤ 0.05) higher yield with respect to Altavista, Nogal, and Rebelde varieties.
The results relative to the effect of fungicides on test weight (kg/hL) during the two experimental seasons are detailed in Supplementary Table S4.
Considering the average of all the varieties tested in the experiment, in 2018/2019 the artificial inoculation significantly (p ≤ 0.05) decreased test weight. In this season, following the application of fungicides, the test weight in the treated-inoculated plots was significantly higher (p ≤ 0.05) than that of the untreated-inoculated ones, but no significant differences between the different fungicide treatments were recorded. Similar results were obtained also considering all durum and bread wheat varieties as two separate groups. In 2018/2019, the test weight followed the pattern: untreated-inoculated < untreated-uninoculated = fungicide treated-inoculated. In 2019/2020, no significant differences in the test weight were recorded.
The results about the effect of fungicides on the grain protein content (%) during the two experimental seasons are detailed in Supplementary Table S5.
Considering all the varieties, in 2018/2019 artificial inoculation significantly (p ≤ 0.05) increased the grain protein content, but the application of fungicides significantly reduced it. Also in this case, no significant (p ≥ 0.05) differences between the different fungicide treatments were recorded. In this season, similar results only in the durum wheat varieties group were obtained. In general, in 2018/2019, the protein content followed the pattern: untreated-inoculated > untreated-uninoculated = fungicide treated-inoculated. In 2019/2020, no significant (p ≥ 0.05) differences in the protein content were recorded.
4. Discussion
The objective of the present study was to evaluate, in a two-year (2018/2019 and 2019/2020) field experiment, the efficacy of several commercially available fungicides, with three different modes of action (DMI, QoI, and SDHI), towards fungal diseases on seven bread wheat and eight durum wheat varieties that are widely cultivated in the experimental area (Central Italy).
Wheat diseases were assessed under natural pressure, except for FHB, for which artificial inoculation with a F. culmorum DON-producing strain was performed. Fungicides were sprayed at two different application timings: fully extended flag leaf (BBCH 39) and full flowering (BBCH 65).
So far, several field experiments on the management strategies for the integrated control of wheat fungal diseases have been carried in North-Western Italy [39,52,53,54,55]. To our knowledge, this is the only recent study carried out in the Central Italian environment in which different commercially available fungicide combinations (with different modes of action) and different durum and bread wheat varieties were deployed in the field to prevent fungal diseases and DON accumulation in grains, as well as to assess yield parameters (yield, test weight, and protein content).
The two investigated years (2018/2019 and 2019/2020) were different in terms of climatic conditions. The first season (2018/2019) was more favorable to disease development, in particular for FHB infection, than the second one (2019/2020). This was specifically related to the higher occurrence of rainfall and relative humidity during the flowering time of the tested varieties (Figure 1). With flowering being the most favorable wheat phenological stage for Fusarium spp. infection, high humidity during this time greatly favors FHB disease [56,57,58]. In our study, this was confirmed by the higher FHB symptoms that were observed in the field, as well as by DON contamination detected in grain, during the 2018/2019 season with respect to the following one (Figure 2; Figure 3).
A certain effect of the more favorable climatic conditions during the first experimental year was also observed for STB presence. However, none of the two experimental years was advantageous for the development of fungal foliar diseases of wheat. In fact, except for the low STB presence in 2018/2019, no other fungal foliar diseases were observed. This could be related to the lack of rainfall during the phenological stages of tillering (BBCH 20-29) and stem elongation (BBCH 30-39) (Figure 1) that usually promotes the presence of the most important foliar diseases of this crop. Research conducted so far showed that also tillage method, fertilization rate, and cultivar choice affect the incidence of wheat fungal diseases. However, the occurrence of fungal pathogens mainly depends on weather conditions during plant growth [59].
Considering STB, the only recorded foliar disease in this study, all the fungicide treatments applied at BBCH 39 showed the same good ability to manage it (Supplementary Table S2) proving the efficacy of the three active ingredient combinations deployed (DMI + SDHI, SDHI + DMI, and SDHI + QoI) at this growth stage. This highlights that the correct fungicide application timing [36] and combination of active ingredients with different modes of action (DMI, SDHI, and QoI), allow to achieve a satisfactory control of the main leaf fungal diseases of wheat also in the central Italian environment as already demonstrated for other sites and climatic conditions of this country [53,60,61,62].
The fungicide treatments applied in this study at BBCH 65 were all able to well-manage FHB infection and, consequently, DON contamination in grain (Table 2; Table 3; Figure 2; Figure 3). In agreement with previous studies [38,42,63,64,65,66] this research shows the efficacy of DMI fungicides, applied at BBCH 65, towards FHB and associated DON accumulation in wheat grain. In fact, also in the presence of a very strong FHB pressure, all the applied fungicides were able to greatly reduce symptom development and mycotoxin accumulation. This was particularly appreciable during the first experimental year (2018/2019), during which favorable climatic conditions also showed a positive effect on promoting infections following artificial inoculation. These findings confirm that climatic factors play a fundamental role for FHB development [59] and that its management strategies are strictly related to them during the growing season.
The different climatic conditions in the two experimental years have also produced interesting dissimilarities in the relationship between FHB symptoms in the field and DON grain contamination of the 15 wheat varieties tested. The strongest positive association (Figure 4) between these two parameters, were obtained in the presence of the highest level of symptom expression (e.g., during 2018/2019 and considering durum wheat varieties only). FHB symptoms evaluation is considered, in general, a good tool to predict DON contamination in kernels [67,68,69]. However, in some instances, the accumulation of DON in grains may not be correlated to disease severity in the field [70]. The results that were obtained in this study suggest that the lack of a strong correlation between DON accumulation and FHB in the field could be particularly observed in the presence of low levels of symptoms.
Considering grain yield, despite a significant effect on symptoms reduction in both the investigated seasons, fungicide applications led to significant benefits in terms of grain yield only in the season in which the highest FHB pressure occurred (Figure 5). This aspect was observed also by other authors for other wheat diseases, such as STB [53]. In an integrated disease management strategy, this indicates that fungicides represent a suitable tool for operators, especially when climate conditions are favorable to disease development [71]. However, some diseases have an impact not just on yield but also on grain safety and quality. This is the case of FHB, which is associated with mycotoxin accumulation in grains, seriously threatening final consumers’ health [72]. In cases like these, the rational use of fungicides shall be considered also as capable to reduce the accumulation of harmful compounds in the grain. For example, other authors suggested that when the main target of a protection program is FHB control and the environment as well as other factors (e.g., susceptible variety, adoption of minimum tillage) may lead to a higher FHB risk, the application of DMI fungicides at flowering is essential to keep DON contamination below the EU regulatory limits [53].
In the presence of Fusarium artificial inoculation, a significant protein content increase was recorded (Supplementary Table S5). This aspect was confirmed also by previous research [73,74,75]. Some authors [76,77] explained such increase as the consequence of the decreased carbohydrates that are used by developing pathogens. However, other studies observed a decrease [78,79] or no effect on the protein level by Fusarium infection [76,80].
The different combinations of fungicides as well as the durum or bread wheat varieties tested in this experiment represent the most recent options offered in the market for farmers in Central Italy. The results obtained highlight that in the present scenario of commercially available durum and bread wheat varieties, the timely application of the most common and commercially available fungicides is able to play a crucial role in FHB and DON management. However, this study remarks that fungicide application needs to be subordinated to different considerations on climate conditions and disease monitoring activities. In fact, the results highlight the strong relationship between environmental conditions and fungal disease management, suggesting that the use of the fungicides could be reduced or even avoided in the presence of low disease pressure.
Regarding the susceptibility of the different tested varieties to FHB, some differences emerged in the two experimental years (Supplementary Figures S2–S3), however, the response of different genotypes to FHB needs a longer validation time. The use of less susceptible varieties should be the main approach to reducing FHB risk and mycotoxin accumulation. This is also in accordance with the European strategies and policies and with the request of consumers and supply chains to decrease and minimize the use of chemicals [81]. In fact, the future of FHB integrated management, as well as of other wheat diseases, should consist in the selection/adoption of less susceptible/resistant genotypes, the use of early-warning/detection systems involving regular pathogen monitoring and disease prediction, the adoption of appropriate cultural practices, as well as the correct use of fungicide treatments, in terms of application timing and mode of action [82].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12040840/s1, Supplementary Figure S1: Average level of Septoria tritici blotch (STB) index (%) indicating STB symptoms observed in the field only for durum and bread wheat untreated controls in 2018/2019 (A, B) and 2019/2020 (B, C). Columns represent the average data (± standard error) for each variety. No significant differences among the varieties were recorded.; Supplementary Figure S2: The average level of Fusarium head blight (FHB) index (%) indicating FHB symptoms observed in the field only for durum wheat and bread wheat untreated-inoculated controls in 2018/2019 (A, B) and 2019/2020 (B, C). Columns represent the average data (± standard error) for each variety. Averages with different letters are significantly different at p ≤ 0.05 based on the Tukey’s HSD adjustment for multiple comparison tests. The absence of letters means the absence of significant differences; Supplementary Figure S3: Average level of deoxynivalenol (DON, µg/kg) grain contamination of durum wheat and bread wheat untreated-inoculated controls in 2018/2019 (A, B) and 2019/2020 (B, C). Columns represent the average data (± standard error) for each variety. Averages with different letters are significantly different at p ≤ 0.05 based on the Tukey’s HSD adjustment for multiple comparison tests. The absence of letters means the absence of significant differences; Supplementary Figure S4: Average yield (t/ha) of durum and bread wheat untreated-inoculated controls in 2018/2019 (A, B) and 2019/2020 (B, C). Columns represent the average data (± standard error) for each variety. Averages with different letters are significantly different at p ≤ 0.05 based on the Tukey’s HSD adjustment for multiple comparison tests. The absence of letters means the absence of significant differences. Supplementary Table S1: Detailed information on agronomic practices and fungicide treatments carried out in the field trials in 2018/2019 and 2019/2020; Supplementary Table S2: Effect of fungicide treatments on Septoria tritici blotch symptoms, during the two experimental years.; Supplementary Table S3: Effect of fungicide treatments on the yield (t/ha) of bread and durum wheat varieties in the two experimental years; Supplementary Table S4: Effect of fungicide treatments on the test weight (kg/hL) of bread and durum wheat varieties in the two experimental years; Supplementary Table S5: Effect of fungicide treatments on the protein content (%) of bread and durum wheat varieties in the two experimental years.
Author Contributions
Conceptualization, G.B., F.T. and L.C.; methodology, G.B., F.T. and L.C.; validation, G.B. and L.C.; formal analysis, E.B.; investigation, G.B., F.T., E.B., G.R., M.C.-B. and M.O.; resources, L.C.; data curation, E.B. and G.B.; writing—original draft preparation, E.B.; writing—review and editing, G.B., F.T., M.C.-B., M.G. and L.C.; visualization, E.B. and G.B. All authors have read and agreed to the published version of the manuscript.
Funding
The two-year experiment was funded by “Fondazione Cassa di Risparmio di Perugia” (Project 2018.0505.026) and by the following partners: Bayer Crop Science Italia Srl, BASF Italia SpA, Syngenta Italia SpA, Bavicchi SpA, Manganelli SpA, CGS Sementi SpA, Grigi Cereali Srl, Molini Popolari Riuniti SocCoopArl, Colussi Group SpA, Molino Bigazzi Srl, and Fondazione per l’Istruzione Agraria (Perugia, Italy).
Acknowledgments
The authors wish to thank Daniele Luchetti, Maria Vittoria Consalvi, and Luca Ceccarelli for their technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAOSTAT. Statistic Division. Food and Agriculture Organization of the UN. Database 2019. Available online: http://faostat.fao.org (accessed on 9 November 2021).
- Istituto Nazionale di Statistica (ISTAT). Available online: http://dati.istat.it (accessed on 10 December 2020).
- Martínez-Moreno, F.; Solís, I.; Noguero, D.; Blanco, A.; Özberk, İ.; Nsarellah, N.; Elias, E.; Mylonas, I.; Soriano, J.M. Durum wheat in the Mediterranean Rim: Historical evolution and genetic resources. Genet. Resour. Crop Evol. 2020, 67, 1415–1436. [Google Scholar] [CrossRef]
- D’Alessandro, A.; De Pergola, G. Mediterranean diet pyramid: A proposal for italian people. Nutrients 2014, 6, 4302–4316. [Google Scholar] [CrossRef] [PubMed]
- De Vita, P.; Li Destri Nicosia, O.; Nigro, F.; Platani, C.; Riefolo, C.; Di Fonzo, N.; Cattivelli, L. Breeding progress in morpho-physiological, agronomical and qualitative, traits of durum wheat cultivars released in Italy during the 20th century. Eur. J. Agron. 2007, 26, 39–53. [Google Scholar] [CrossRef]
- Goswani, R.S.; Kistler, H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.D.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evo. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases-a field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef]
- Savary, S.; Djurle, A.; Yuen, J.; Ficke, A.; Rossi, V.; Esker, P.D.; Ferandes, J.M.C.; Del Ponte, E.M.; Kumar, J.; Madden, L.V.; et al. A white paper on global wheat health based on scenario development and analysis. Phytopathology 2017, 107, 1109–1122. [Google Scholar] [CrossRef]
- Singh, R.P.; Singh, P.K.; Rutkoski, J.; Hodson, D.P.; He, X.; Jørgensen, L.N.; Hovmøller, M.S.; Huerta-Espino, J. Disease impact on wheat yield potential and prospects of genetic control. Annu. Rev. Phytopathol. 2016, 54, 303–322. [Google Scholar] [CrossRef]
- Jørgensen, L.N.; Hovmøller, M.S.; Hansen, J.G.; Lassen, P.; Clark, B.; Bayles, R.; Rodemann, B.; Flath, K.; Jahn, M.; Tomas Goral, T.; et al. IPM Strategies and Their Dilemmas Including an Introduction to www.eurowheat.org. J. Integr. Agric. 2014, 13, 265–281. [Google Scholar] [CrossRef]
- Willocquet, L.; Meza, W.R.; Dumont, B.; Klocke, B.; Feike, T.; Kersebaum, K.C.; Meriggi, P.; Rossi, P.; Ficke, A.; Djurle, A.; et al. An outlook on wheat health in Europe from a network of field experiments. Crop Prot. 2021, 139, 105335. [Google Scholar] [CrossRef]
- Hovmøller, M.S.; Walter, S.; Bayles, R.A.; Hubbard, A.; Flath, K.; Sommerfeldt, N.; Leconte, M.; Czembor, P.; Rodriguez-Algaba, J.; Thach, T.; et al. Replacement of the European wheat yellow rust population by new races from the centre of diversity in the near-Himalayan region. Plant Pathol. 2016, 65, 402–411. [Google Scholar] [CrossRef]
- Hovmøller, M.S.; Yahyaoui, A.H.; Milus, E.A.; Justesen, A.F. Rapid global spread of two aggressive strains of a wheat rust fungus. Mol. Ecol. 2008, 17, 3818–3826. [Google Scholar] [CrossRef] [PubMed]
- Fones, H.; Gurr, S. The impact of Septoria tritici Blotch disease on wheat: An EU perspective. Fungal Genet. Biol. 2015, 79, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Torriani, S.F.; Melichar, J.P.; Mills, C.; Pain, N.; Sierotzki, H.; Courbot, M. Zymoseptoria tritici: A major threat to wheat production, integrated approaches to control. Fungal Genet. Biol. 2015, 79, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Puppala, V.; Herrman, T.J.; Bockus, W.W.; Loughin, T.M. Quality responses of twelve hard red winter wheat cultivars to foliar disease across four locations in central Kansas. Cereal Chem. 1998, 75, 94–99. [Google Scholar] [CrossRef]
- Da Rocha, M.E.B.; Freire, F.C.O.; Maia, F.E.F.; Guedes, M.I.F.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- O’Donnell, K.; Rooney, A.P.; Proctor, R.H.; Brown, D.W.; McCormick, S.P.; Ward, T.J.; Frandsen, R.J.N.; Lysøe, E.; Rehner, S.A.; Aoki, T.; et al. Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet. Biol. 2013, 52, 20–31. [Google Scholar] [CrossRef]
- Beccari, G.; Prodi, A.; Senatore, M.T.; Balmas, V.; Tini, F.; Onofri, A.; Pedini, L.; Sulyok, M.; Brocca, L.; Covarelli, L. Cultivation Area Affects the Presence of Fungal Communities and Secondary Metabolites in Italian Durum Wheat Grains. Toxins 2020, 12, 9. [Google Scholar] [CrossRef]
- Birr, T.; Hasler, M.; Verreet, J.; Klink, H. Composition and predominance of Fusarium species causing Fusarium Head Blight in winter wheat grain depending on cultivar susceptibility and meteorological factors. Microorganisms 2020, 8, 617. [Google Scholar] [CrossRef]
- Beccari, G.; Prodi, A.; Tini, F.; Bonciarelli, U.; Onofri, A.; Oueslati, S.; Limayma, M.; Covarelli, L. Changes in the Fusarium Head Blight Complex of Malting Barley in a Three-Year Field Experiment in Italy. Toxins 2017, 9, 120. [Google Scholar] [CrossRef]
- Czaban, J.; Wróblewska, B.; Sułek, A.; Mikos, M.; Boguszewska, E.; Podolska, G.; Nieróbca, A. Colonisation of winter wheat grain by Fusarium spp. and mycotoxin content as dependent on a wheat variety, crop rotation, a crop management system and weather conditions. Food Addit. Contam. Part A 2015, 32, 874–910. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.K.; Jensen, J.D.; Nielsen, G.C.; Jensen, J.E.; Spliid, N.H.; Thomsen, I.K.; Justesen, A.F.; Collinge, D.B.; Jørgensen, L.N. Fusarium head blight of cereals in Denmark: Species complex and related mycotoxins. Phytopathology 2011, 101, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-M.; Parry, D.W.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Edwards, S.G.; Cooke, B.M.; Doohan, F.M.; Brennan, J.M.; Moretti, A.; et al. Predominance and association of pathogenic fungi causing Fusarium ear blight in wheat in four European countries. Eur. J. Plant Pathol. 2005, 112, 143–154. [Google Scholar] [CrossRef]
- Kimura, M.; Tokai, T.; Takahashi-Ando, N.; Ohsato, S.; Fujimura, M. Molecular and genetics studies of Fusarium trichothecene biosynthesis: Pathways, genes, and evolution. Biosci. Biotechnol. Biochem. 2007, 71, 2105–2123. [Google Scholar] [CrossRef] [PubMed]
- Buerstmayr, H.; Adam, G.; Lemmens, M. Resistance to head blight caused by Fusarium spp. in wheat. In Disease Resistance in Wheat; Sharma, I., Ed.; CABI: Wallingford, UK, 2012; pp. 236–276. [Google Scholar]
- Foroud, N.A.; Eudes, F. Trichothecenes in cereal grains. Int. J. Mol. Sci. 2009, 10, 147–173. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No. 1126/2007 of 28 September 2007 amending Regulation (EC) No. 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. Off. J. Eur. Union 2007, L255, 14–17. [Google Scholar]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- European Commission. Commission Recommendation of 17 august 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, L229, 7–29. [Google Scholar]
- Morton, V.; Staub, T. A short history of fungicides. In APSnet Features; American Phytopathological Society: Saint Paul, MN, USA, 2008. [Google Scholar] [CrossRef]
- Shah, L.; Ali, A.; Yahya, M.; Zhu, Y.; Wang, S.; Si, H.; Bahma, H.; Ma, C. Integrated control of Fusarium head blight and deoxynivalenol mycotoxin in wheat. Plant. Pathol. 2018, 67, 532–548. [Google Scholar] [CrossRef]
- Audenaert, K.; Landschoot, S.; Vanheule, A.; Waegeman, W.; De Baets, B.; Haesaert, G. Impact of fungicide timing on the composition of the Fusarium head blight disease complex and the presence of deoxynivalenol (DON) in Wheat. In Fungicides—Beneficial and Harmful Aspects; Thajuddin, N., Ed.; InTech: Rijeka, Croatia, 2011; pp. 79–98. [Google Scholar]
- FRAC. Fungicide Resistance Action Committee (FRAC) Code List 2021: Fungal Control Agents Sorted by Cross Resistance Pattern and Mode of Action (Including Coding for FRAC Groups on Product Labels). 2021. Available online: https://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2021--final.pdf?sfvrsn=f7ec499a_2 (accessed on 9 November 2021).
- Poole, N.F.; Arnaudin, M.E. The role of fungicides for effective disease management in cereal crops. Can. J. Plant Pathol. 2014, 36, 1–11. [Google Scholar] [CrossRef]
- Kettlewell, P.S.; Davies, W.P.; Hocking, T.J. Disease development and senescence of the flag leaf of winter wheat in response to propiconazole. J. Agric. Sci. 1984, 99, 661–663. [Google Scholar] [CrossRef]
- Matthies, A.; Buchenauer, H. Effect of tebuconazole (Folicur) and prochloraz (Sportak) treatments on Fusarium head scab development, yield, and deoxynivalenol (DON) content in grains of wheat following artificial inoculation with Fusarium culmorum. J. Plant Dis. Prot. 2000, 107, 33–52. [Google Scholar]
- Scarpino, V.; Reyneri, A.; Sulyok, M.; Krska, R.; Blandino, M. Effect of fungicide application to control Fusarium head blight and 20 Fusarium and Alternaria mycotoxins in winter wheat (Triticum aestivum L.). World Mycotoxin J. 2015, 8, 499–510. [Google Scholar] [CrossRef]
- Lehoczki-Krsjak, K.; Szabo-Hever, A.; Toth, B.; Kotai, C.; Bartok, T.; Varga, M.; Faràdy, L.; Masterházy, A. Prevention of Fusarium mycotoxin contamination by breeding and fungicide application to wheat. Food. Addit. Contam. 2010, 27, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.A.; Lipps, P.E.; Hershman, D.E.; McMullen, M.P.; Draper, M.A.; Madden, L.V. Efficacy of triazole-based fungicides for Fusarium head blight and deoxynivalenol control in wheat: A multivariate meta-analysis. Phytopathology 2008, 98, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Maria Menniti, A.; Pancaldi, D.; Maccaferri, M.; Casalini, L. Effect of Fungicides on Fusarium Head Blight and Deoxynivalenol Content in Durum Wheat Grain. Eur. J. Plant Pathol. 2003, 109, 109–115. [Google Scholar] [CrossRef]
- McKay, A.H.; Hagerty, G.C.; Follas, G.B.; Moore, M.S.; Christie, M.S.; Beresford, R.M. Succinate dehydrogenase inhibitor (SDHI) fungicide resistance prevention strategy. N. Z. Plant Protec. 2011, 64, 119–124. [Google Scholar] [CrossRef][Green Version]
- Oerke, E.C.; Beck, C.; Dehne, H.W. Physiological effects of strobilurins on wheat yield. Phytopathology 2001, 91, 67–71. [Google Scholar]
- Bartlett, D.W.; Clough, J.M.; Godfrey, C.R.A.; Godwin, J.R.; Hall, A.A.; Heaney, S.P.; Maund, S.J. Understanding the strobilurin fungicides. Pestic. Outlook 2001, 12, 143–148. [Google Scholar] [CrossRef]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P.A. Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef]
- Jalli, M.; Kaseva, J.; Andersson, B.; Ficke, A.; Jørgensen, L.N.; Ronis, A.; Kaukoranta, T.; Ørum, J.; Djurle, A. Yield increases due to fungicide control of leaf blotch diseases in wheat and barley as a basis for IPM decision-making in the Nordic-Baltic region. Eur. J. Plant. Pathol. 2020, 158, 315–333. [Google Scholar] [CrossRef]
- Newitt, J.T.; Prudence, S.M.M.; Hutchings, M.I.; Worsley, S.F. Biocontrol of cereal crop diseases using Streptomycetes. Pathogens 2019, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Brent, K.J.; Hollomon, D.W. Fungicide Resistance in Crop Pathogens: How Can it be Managed? Frac Monograph, N., Ed.; Cropelife International: Brussels, Belgium, 2007; pp. 3–50. [Google Scholar]
- Covarelli, L.; Beccari, G.; Prodi, A.; Generotti, S.; Etruschi, F.; Juan, C.; Ferrer, E.; Mañes, J. Fusarium species, chemotype characterisation and trichothecene contamination of durum and soft wheat in an area of central Italy. J. Sci. Food Agric. 2015, 95, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Onofri, A.; Pannacci, E. Spreadsheet Tools for Biometry Classes in Crop Science Programmes. Commun. Biometry Crop. Sci. 2014, 9, 43–53. [Google Scholar]
- Scarpino, V.; Blandino, M. Effects of durum wheat cultivars with different degrees of FHB susceptibility grown under different meteorological conditions on the contamination of regulated, modified and emerging mycotoxins. Microorganisms 2021, 9, 408. [Google Scholar] [CrossRef] [PubMed]
- Capo, L.; Blandino, M. Minimizing yield losses and sanitary risks through an appropriate combination of fungicide seed and foliar treatments on wheat in different production situations. Agronomy 2021, 4, 725. [Google Scholar] [CrossRef]
- Blandino, M.; Pascale, M.; Haidukowski, M.; Reyneri, A. Influence of agronomic conditions on the efficacy of different fungicides applied to wheat at heading: Effect on flag leaf senescence, Fusarium head blight attack, grain yield and deoxynivalenol contamination. Ital. J. Agron. 2011, 6, e32. [Google Scholar] [CrossRef]
- Blandino, M.; Pilati, A.; Reyneri, A. Effect of foliar treatments to durum wheat on flag leaf senescence, grain yield, quality and deoxynivalenol contamination in North Italy. Field Crops Res. 2009, 2, 214–222. [Google Scholar] [CrossRef]
- Beccari, G.; Arellano, C.; Covarelli, L.; Tini, F.; Sulyok, M.; Cowger, C. Effect of wheat infection timing on Fusarium head blight causal agents and secondary metabolites in grain. Int. J. Food Microbiol. 2019, 290, 214–225. [Google Scholar] [CrossRef]
- Kelly, A.C.; Clear, R.M.; O’Donnell, K.; McCormick, S.; Turkington, T.K.; Tekauz, A.; Gilbert, J.; Kistler, H.C.; Busman, M.; Ward, T.J. Diversity of Fusarium head blight populations and trichothecene toxin types reveals regional differences in pathogen composition and temporal dynamics. Fungal Genet. Biol. 2015, 82, 22–31. [Google Scholar] [CrossRef]
- Oerke, E.C.; Meier, A.; Dehne, H.W.; Sulyok, M.; Krska, R.; Steiner, U. Spatial variability of Fusarium head blight pathogens and associated mycotoxins in wheat crops. Plant Pathol. 2010, 59, 671–682. [Google Scholar] [CrossRef]
- Różewicz, M.; Wyzińska, M.; Grabiński, J. The Most Important Fungal Diseases of Cereals—Problems and Possible Solutions. Agronomy 2021, 11, 714. [Google Scholar] [CrossRef]
- Alvisi, G.; Ponti, D.; Cristiani, C.; Pradolesi, G.; Donati, G.; Pelliconi, F.; Allegri, A.; Consolani, E.; Tarlazzi, S. Verifica dell’efficacia de Fluxapyroxad+Pyraclostrobin nel controllo del complesso della septoriosi del frumento in Emilia-Romagna. Atti Giornate Fitopatol. 2018, 2, 131–138. [Google Scholar]
- Serra, L.; Ricci, V.; Gualco, A. Benzovindiflupyr: Caratteristiche della nuova sostanza attiva e attività nel controllo delle malattie fungine di frumento e orzo. Atti Giornate Fitopatol. 2016, 2, 123–130. [Google Scholar]
- Lazzari, A.; Arangeli, G.; Boebel, A.; Gualco, A.; Lazzati, S.; Risi, C.; Cantoni, A. Bixafen: Una nuova sostanza attiva fungicida per il controllo delle malattie fogliari del frumento e orzo. Atti Giornate Fitopatol. 2012, 2, 213–218. [Google Scholar]
- Tini, F.; Beccari, G.; Onofri, A.; Ciavatta, E.; Gardiner, D.M.; Covarelli, L. Fungicides may have differential efficacies towards the main causal agents of Fusarium head blight of wheat. Pest Manag. Sci. 2020, 76, 3738–3748. [Google Scholar] [CrossRef]
- Haidukowski, M.; Visconti, A.; Perrone, G.; Vanadia, S.; Pancaldi, D.; Covarelli, L.; Balestrazzi, R.; Pascale, M. Effect of prothioconazole-based fungicides on Fusarium head blight, grain yield and deoxynivalenol accumulation in wheat under field conditions. Phytopathol. Mediterr. 2012, 51, 236–246. [Google Scholar]
- Haidukowski, M.; Pascale, M.; Perrone, G.; Pancaldi, D.; Campagna, C.; Visconti, A. Effect of fungicides on the development of Fusarium head blight, yield and deoxynivalenol accumulation in wheat inoculated under field conditions with Fusarium graminearum and Fusarium culmorum. J. Sci. Food Agric. 2005, 85, 191–198. [Google Scholar] [CrossRef]
- Edwards, S.G.; Pirgozliev, S.R.; Hare, M.C.; Jenikson, P. Quantification of trichothecene-producing Fusarium species in harvest grain by competitive PCR to determine the efficacy of fungicides against Fusarium head blight of winter wheat. Appl. Environ. Microbiol. 2001, 67, 1575–1580. [Google Scholar] [CrossRef]
- Pascale, M.; Visconti, A.; Chelkowski, J. Ear rot susceptibility and mycotoxin contamination of maize hybrids inoculated with Fusarium species under field conditions. Eur. J. Plant. Pathol. 2002, 108, 645–651. [Google Scholar] [CrossRef]
- Bai, G.H.; Plattner, R.; Desjardins, A.; Kolb, F. Resistance to Fusarium head blight and deoxynivalenol accumulation in wheat. Plant Breed 2001, 120, 1–6. [Google Scholar] [CrossRef]
- Nowicki, T. Vomitoxin and Fusarium damaged kernels-is there a relationship in Canadian wheat? In Proceedings of the 2nd Canadian Workshop on Fusarium Head Blight, Ottawa, ON, Canada, 3 November 2001. [Google Scholar]
- Mesterházy, Á.; Bartók, T.; Mirocha, C.G.; Komoróczy, R. Nature of wheat resistance to Fusarium head blight and the role of deoxynivalenol for breeding. Plant Breed. 1999, 118, 97–110. [Google Scholar] [CrossRef]
- Ransom, J.K.; McMullen, M.V. Yield and Disease Control on Hard Winter Wheat Cultivars with Foliar Fungicides. Agron. J. 2008, 100, 1130–1137. [Google Scholar] [CrossRef]
- Janik, E.; Niemcewicz, M.; Ceremuga, M.; Stela, M.; Saluk-Bijak, J.; Siadkowski, A.; Bijak, M. Molecular aspects of mycotoxins—A serious problem for human health. Int. J. Mol. Sci. 2020, 21, 8187. [Google Scholar] [CrossRef] [PubMed]
- Siuda, R.; Grabowski, A.; Lenc, L.; Ralcewicz, M.; Spychaj-Fabisiak, E. Influence of the degree of fusariosis on technological traits of wheat grain. Int. J. Food Sci. Technol. 2010, 45, 2596–2604. [Google Scholar] [CrossRef]
- Brinkmeyer, U.; Dänicke, S.; Lehmann, M.; Valenta, H.; Lebzien, P.; Schollenberger, M.; Südekem, K.H.; Weinert, J.; Flachowsky, G. Influence of a Fusarium culmorum inoculation of wheat on the progression of mycotoxin accumulation, ingredient concentrations and ruminal in sacco dry matter degradation of wheat residues. Arch. Anim. Nutr. 2006, 60, 141–157. [Google Scholar] [CrossRef]
- Matthäus, K.; Dänicke, S.; Vahjen, W.; Simon, O.; Wang, J.; Valenta, H.; Meyer, K.; Strumpf, A.; Ziesenib, H.; Flachowsky, G. Progression of mycotoxin and nutrient concentrations in wheat after inoculation with Fusarium culmorum. Arch. Anim. Nutr. 2004, 58, 19–35. [Google Scholar] [CrossRef]
- Wang, J.; Wieser, H.; Pawelzik, E.; Weinert, J.; Keuteng, A.J.; Wolf, G.A. Impact of the fungal protease produced by Fusarium culmorum on the protein quality and breadmaking properties of winter wheat. Eur. Food Res. Technol. 2005, 220, 552–559. [Google Scholar] [CrossRef]
- Wasowicz, E. Changes of chemical grain components, especially lipids, during their deterioration by fungi. In Cereal Grains, Mycotoxins, Fungi and Quality in Drying and Storage; Chelkowski, J., Ed.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 259–280. [Google Scholar]
- Gärtner, B.H.; Munich, M.; Kleijer, G.; Mascher, F. Characterisation of kernel resistance against Fusarium infection in spring wheat by baking quality and mycotoxin assessments. Eur. J. Plant Pathol. 2008, 120, 61–68. [Google Scholar] [CrossRef]
- Prange, A.; Birzele, B.; Krämer, J.; Meier, A.; Modrow, H.; Köhler, P. Fusarium-inoculated wheat: Deoxynivalenol contents and baking quality in relation to infection time. Food Control. 2005, 8, 39–745. [Google Scholar] [CrossRef]
- Terzi, V.; Morcia, C.; Faccioli, P.; Faccini, N.; Rossi, V.; Cigolini, M.; Corbellini, M.; Scudellari, D.; Delogu, G. Fusarium DNA traceability along the bread production chain. Int. J. Food Sci. Technol. 2007, 42, 1390–1396. [Google Scholar] [CrossRef]
- European Commission. Farm to Fork Strategy—For a Fair, Healthy and Environmentally-Friendly Food System. Available online: https://ec.europa.eu/food/horizontal-topics/farm-fork-strategy_en (accessed on 19 January 2022).
- Carmona, M.; Sautua, F.; Pérez-Hérnandez, O.; Reis, E.M. Role of Fungic. applications on the integrated management of wheat stripe rust. Front. Plant Sci. 2020, 11, 733. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).