Teriparatide for Guided Bone Regeneration in Craniomaxillofacial Defects: A Systematic Review of Preclinical Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Focus Question
2.3. Search Strategy
2.4. Eligibility Criteria
- Population (P): Bone defects in the oral cavity and craniomaxillofacial bones treated with guided bone regeneration.
- Intervention (I): Use of teriparatide in guided bone regeneration.
- Comparison (C): Control groups receiving placebo, saline, or GBR without teriparatide.
- Outcome (O): Quantity and quality of regenerated bone, bone density, bone formation rates, and graft integration.
- Study design (S): Experimental and in vivo studies.
2.5. Study Selection
2.6. Data Extraction
2.7. Quality Assessment
3. Results
3.1. Study Selection
3.2. Characteristics of the Included Studies
3.3. Data Extraction
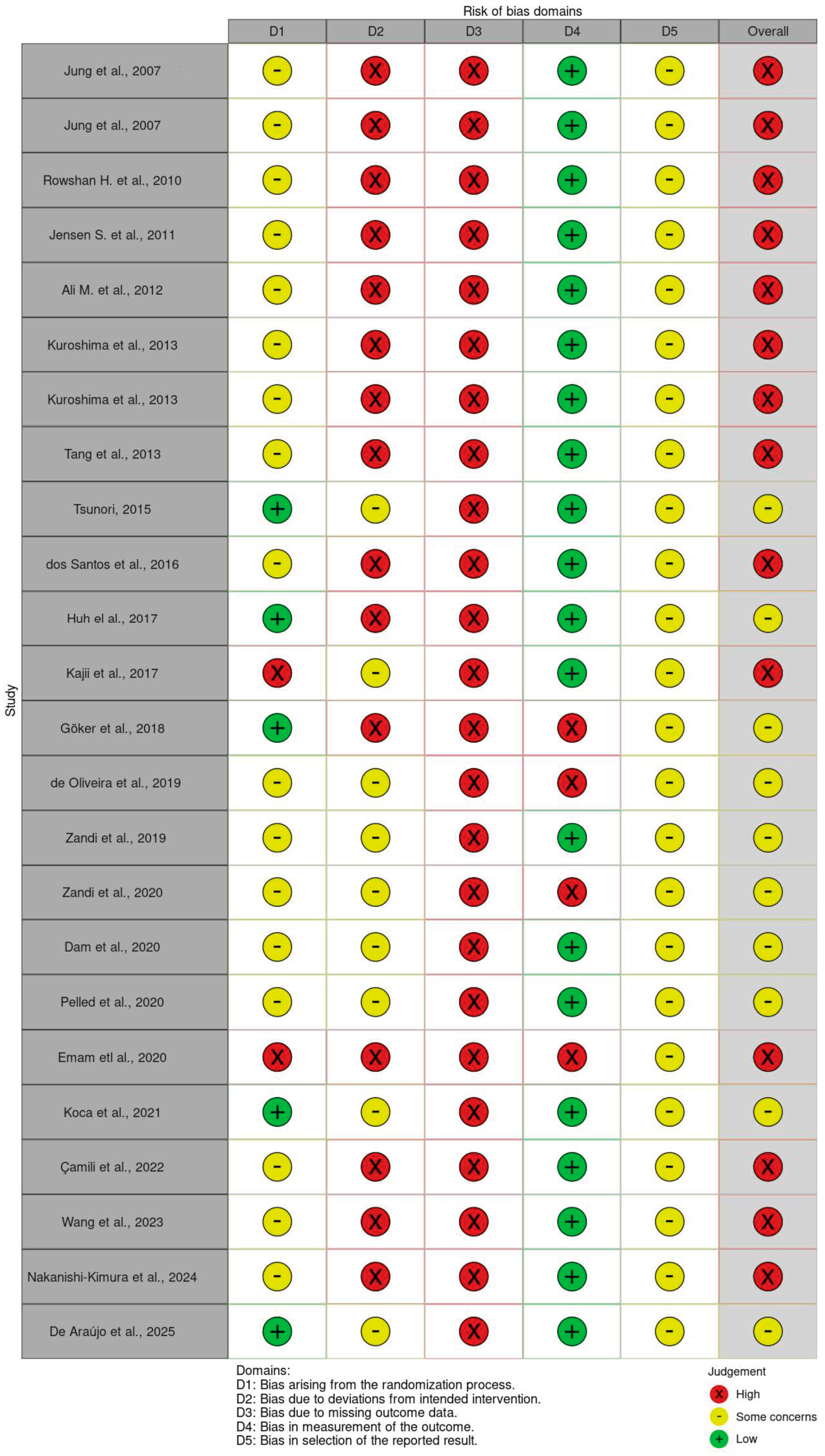
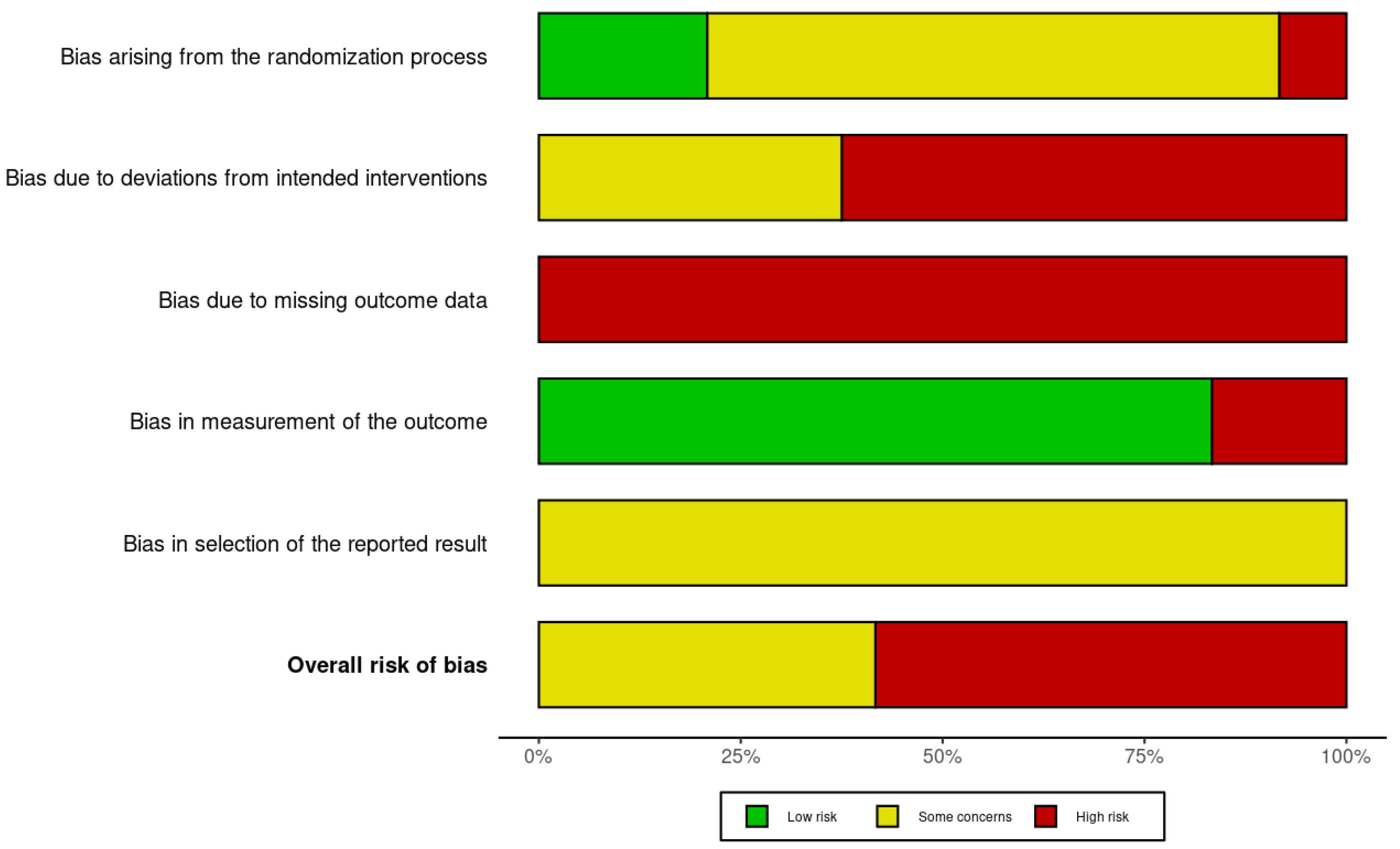
3.4. Risk of Bias Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| TP | teriparatide |
| GBR | guided bone regeneration |
| VEGF | vascular endothelial growth factor |
| BMP-2 | bone morphogenetic proteins |
| PDGF | platelet-derived growth factors |
| OCP/Col | octacalcium phosphate collagen |
| sr | stronim ranelate |
| px | poloxamer |
| mp | chitosan microparticles |
| Micro-CT | computerized microtomography |
| RANKL | receptor activator of nuclear factor ligand |
| OPG | osteoprotegerin |
| BMD | bone mineral density |
| peg | polyethylene glycol |
| HA/TCP | hydroxyapatite and tricalcium phosphate |
| RGD | arginine–glycine–aspartic acid |
| EMD | enamel matrix derivative |
| MA | mandibular advancement |
| ABL | abaloparatide |
| OVX | ovariectomized |
| BV/TV | bone volume/tissue volume |
References
- de Oliveira Puttini, I.; Gomes-Ferreira, P.H.d.S.; de Oliveira, D.; Hassumi, J.S.; Gonçalves, P.Z.; Okamoto, R. Teriparatide improves alveolar bone modelling after tooth extraction in orchiectomized rats. Arch. Oral Biol. 2019, 102, 147–154. [Google Scholar] [CrossRef]
- Zandi, M.; Dehghan, A.; Gheysari, F.; Rezaeian, L.; Mohammad Gholi Mezerji, N. Evaluation of teriparatide effect on healing of autografted mandibular defects in rats. J. Cranio-Maxillofac. Surg. 2019, 47, 120–126. [Google Scholar] [CrossRef]
- Seo, M.H.; Kim, S.M. Ridge augmentation in implant dentistry. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 208–210. [Google Scholar] [CrossRef]
- Chiapasco, M.; Zaniboni, M. Clinical outcomes of GBR procedures to correct peri-implant dehiscences and fenestrations: A systematic review. Clin. Oral Implants Res. 2009, 20, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Egusa, H. Current bone substitutes for implant dentistry. J. Prosthodont. Res. 2018, 62, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Thoma, D.S.; Hammerle, C.H.F. Assessment of the potential of growth factors for localized alveolar ridge augmentation: A systematic review. J. Clin. Periodontol. 2008, 35, 255–281. [Google Scholar] [CrossRef]
- Miron, R.J.; Zhang, Y. Autologous liquid platelet rich fibrin: A novel drug delivery system. Acta Biomater. 2018, 75, 35–51. [Google Scholar] [CrossRef]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.L.; et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]
- Miron, R.J.; Bosshardt, D.D.; Buser, D.; Zhang, Y.; Tugulu, S.; Gemperli, A.; Dard, M.; Caluseru, O.M.; Chandad, F.; Sculean, A. Comparison of the Capacity of Enamel Matrix Derivative Gel and Enamel Matrix Derivative in Liquid Formulation to Adsorb to Bone Grafting Materials. J. Periodontol. 2015, 86, 578–587. [Google Scholar] [CrossRef]
- Shin, W.C.; Moon, N.H.; Jang, J.H.; Seo, H.U.; Suh, K.T. A retrospective bicenter comparative study of surgical outcomes of atypical femoral fracture: Potential effect of teriparatide on fracture healing and callus formation. Bone 2019, 128, 115033. [Google Scholar] [CrossRef]
- Shin, C.J.; Kim, S.; Choi, C.S.; Shin, H.C.; Kwon, Y.J. Effectiveness of Osteoporosis Drug in Postmenopausal Women with Spinal Compression Fracture: Combined Consecutive Therapy of Teriparatide and Raloxifene versus Bisphosphonate Single. Korean J. Neurotrauma 2016, 12, 123–127. [Google Scholar] [CrossRef][Green Version]
- Agnihotri, R.; Gaur, S. Applications of teriparatide for alveolar bone regeneration: A systematic review. J. Int. Soc. Prev. Community Dent. 2021, 11, 639–643. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Akobeng, A.K. Principles of evidence based medicine. Arch. Dis. Child. 2005, 90, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Cochran, D.L.; Domken, O.; Seibl, R.; Jones, A.A.; Buser, D.; Hammerle, C.H.F. The effect of matrix bound parathyroid hormone on bone regeneration. Clin. Oral Implants Res. 2007, 18, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Hämmerle, C.H.; Kokovic, V.; Weber, F.E. Bone regeneration using a synthetic matrix containing a parathyroid hormone peptide combined with a grafting material. Int. J. Oral Maxillofac. Implants 2007, 22, 258–266. [Google Scholar]
- Rowshan, H.H.; Parham, M.A.; Baur, D.A.; McEntee, R.D.; Cauley, E.; Carriere, D.T.; Wood, J.C.; Demsar, W.J.; Pizarro, J.M. Effect of Intermittent Systemic Administration of Recombinant Parathyroid Hormone (1-34) on Mandibular Fracture Healing in Rats. J. Oral Maxillofac. Surg. 2010, 68, 260–267. [Google Scholar] [CrossRef]
- Jensen, S.S.; Chen, B.; Bornstein, M.M.; Bosshardt, D.D.; Buser, D. Effect of Enamel Matrix Derivative and Parathyroid Hormone on Bone Formation in Standardized Osseous Defects: An Experimental Study in Minipigs. J. Periodontol. 2011, 82, 1197–1205. [Google Scholar] [CrossRef]
- Ali, M.N.; Kobayashi, T.; Tanaka, M.; Ohshima, H.; Ejiri, S.; Saito, C. Effects of intermittent parathyroid hormone treatment on new bone formation during distraction osteogenesis in the rat mandible. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, e36–e42. [Google Scholar] [CrossRef]
- Kuroshima, S.; Al-Salihi, Z.; Yamashita, J. Parathyroid hormone related to bone regeneration in grafted and nongrafted tooth extraction sockets in rats. Implant Dent. 2013, 22, 71–76. [Google Scholar] [CrossRef]
- Kuroshima, S.; Kovacic, B.L.; Kozloff, K.M.; McCauley, L.K.; Yamashita, J. Intra-oral PTH administration promotes tooth extraction socket healing. J. Dent. Res. 2013, 92, 553–559. [Google Scholar] [CrossRef]
- Tang, Z.L.; Zhang, W.J.; Wang, D.X.; Chen, J.M.; Ma, H.; Wu, D.R. An experimental study addressing the promotion of mandibular defect repair through the intermittent subcutaneous injection of parathyroid hormone. J. Oral Maxillofac. Surg. 2014, 72, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Tsunori, K. Effects of parathyroid hormone dosage and schedule on bone regeneration. J. Oral Sci. 2015, 57, 131–136. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, R.A.; Ferreira, M.S.; Mafra, C.E.; Holzhausen, M.; de Lima, L.A.; Mendes Pannuti, C.; César Neto, J.B. Synthetic Parathyroid Hormone May Augment Bone Volume in Autogenous Grafts: A Study in Rats. J. Periodontol. 2016, 87, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.; Jung, U.W.; Park, K.M.; Kim, H.S.; Kim, K.D.; Park, W. Parathyroid Hormone (1-34) Might Not Improve Early Bone Healing after Sinus Augmentation in Healthy Rabbits. BioMed Res. Int. 2017, 2017, 6087676. [Google Scholar] [CrossRef]
- Kajii, F.; Iwai, A.; Tanaka, H.; Matsui, K.; Kawai, T.; Kamakura, S. Single-dose local administration of teriparatide with a octacalcium phosphate collagen composite enhances bone regeneration in a rodent critical-sized calvarial defect. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1851–1857. [Google Scholar] [CrossRef]
- Göker, F.; Ersanlı, S.; Arısan, V.; Cevher, E.; Güzel, E.E.; İşsever, H.; Ömer, B.; Durmuş Altun, G.; Morina, D.; Ekiz Yılmaz, T.; et al. Combined effect of parathyroid hormone and strontium ranelate on bone healing in ovariectomized rats. Oral Dis. 2018, 24, 1255–1269. [Google Scholar] [CrossRef]
- Zandi, M.; Dehghan, A.; Bigonah, N.; Doulati, S.; Mohammad Gholi Mezerji, N. Histological assessment of the effects of teriparatide therapy on mandibular fracture healing: A preclinical study. J. Craniomaxillofac. Surg. 2020, 48, 211–216. [Google Scholar] [CrossRef]
- Dam, C.; Jung, U.W.; Park, K.M.; Huh, J.; Park, W. Effect of teriparatide on early sinus graft healing in the ovariectomized rabbit. Clin. Oral Implants Res. 2020, 31, 264–273. [Google Scholar] [CrossRef]
- Pelled, G.; Lieber, R.; Avalos, P.; Cohn-Schwartz, D.; Tawackoli, W.; Roth, J.; Knapp, E.; Schwarz, E.M.; Awad, H.A.; Gazit, D.; et al. Teriparatide (recombinant parathyroid hormone 1–34) enhances bone allograft integration in a clinically relevant pig model of segmental mandibulectomy. J. Tissue Eng. Regen. Med. 2020, 14, 1037–1049. [Google Scholar] [CrossRef]
- Emam, H.; Leach, D.; Sun, Z.; Tee, B.C.; Karatas, B.; Kim, D.G.; Jatana, C. The effect of parathyroid hormone analogues when added to mineralized bone xenografts. J. Oral Implantol. 2020, 46, 372–379. [Google Scholar] [CrossRef]
- Koca, C.G.; Kösehasanoğulları, M. Evaluation of single-dose applied teriparatide effect on bone healing with histomorphometric and micro-ct analysis. J. Cranio-Maxillofac. Surg. 2021, 49, 98–103. [Google Scholar] [CrossRef]
- Çamili, Y.; Malkoç, S.; Taşlidere, A.; Ileri, Z.; Guler, O.C. Effects of teriparatide on bone formation in rats with experimentally induced premaxillary expansion. Dent. Press J. Orthod. 2022, 27, e2220370. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Qiao, H.; Qian, Y.; Zhen, G.; Zhao, Z.; Li, Y. Abaloparatide and teriparatide enhance mandibular growth in adolescent rats with site-specific and mechano-related effects. Eur. J. Orthod. 2023, 45, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi-Kimura, A.; Takakura, A.; Hoshi-Numahata, M.; Watanabe, H.; Nishiura, M.; Sato, Y.; Takao-Kawabata, R.; Iimura, T. Dynamic morphometric changes in the mandibular osteocytic lacunae of ovariectomized rats in response to teriparatide, as revealed by three-dimensional fluorescence analyses: Possible involvement of osteocytic perilacunar remodeling. J. Oral Biosci. 2024, 66, 49–60. [Google Scholar] [CrossRef]
- de Araújo, J.C.R.; Silva, L.A.S.; de Oliveira, E.F.S.; Vieira, M.N.; Lisboa, P.N.; Campos, T.M.B.; Okamoto, R.; de Vasconcellos, L.M.R. An Innovative Biomaterial: 45S5 Bioactive Glass Functionalized with PTH 1-34 In Vivo Bone Repair. BioNanoScience 2025, 15, 220. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2020, 12, 55–61. [Google Scholar] [CrossRef]

| Database | Search Strategy | Results |
|---|---|---|
| Pubmed | #1 teriparatide (Mesh terms) OR PTH 1–34 (Mesh terms) | 379 |
| #2 alveolar process (Mesh terms) | 1161 | |
| #3 bone regeneration (Mesh terms) | 1479 | |
| #4 craniomaxillofacial (all fields) | 3834 | |
| #1 and #2 | 5 | |
| #1, #2 and #3 | 1 | |
| #1, #2, #3 and 4 | 0 | |
| Web of Science | #1 teriparatide OR PTH 1-34 | 113 |
| #2 alveolar process | 2138 | |
| #3 bone regeneration | 8743 | |
| #4 craniomaxillofacial | 596 | |
| #1 and #2 | 36 | |
| #1, #2 and #3 | 35 | |
| #1, #2, #3 and 4 | 27 | |
| Scopus | #1 TITLE-ABS-KEY Teriparatide OR PTH AND 1-34 | 101 |
| #2 TITLE-ABS-KEY Alveolar AND Process | 6986 | |
| #3 TITLE-ABS-KEY Bone AND Regeneration | 8675 | |
| #4 craniomaxillofacial | 423 | |
| #1 and #2 | 4 | |
| #1, #2 and #3 | 0 | |
| #1, #2, #3 and 4 | 0 | |
| Scielo | #1 teriparatide | 17 |
| #2 alveolar process | 76 | |
| #3 bone regeneration | 152 | |
| #4 craniomaxillofacial | 30 | |
| #1 and #2 | 0 | |
| #1, #2 and #3 | 0 | |
| #1, #2, #3 and 4 | 0 | |
| Open Grey | 0 | |
| Research Gate | 5 | |
| Total | 35,01 6 |
| Charact Eristics of Included Studies | ||
|---|---|---|
| Experimental | a * | 24 |
| b ** | 1007 | |
| Publication Year | ||
| Before 2000 | a | 0 |
| b | 0 | |
| 2001–2005 | a | 0 |
| b | 0 | |
| 2006–2010 | a | 3 |
| b | 51 | |
| 2011–2015 | a | 6 |
| b | 230 | |
| 2016–2020 | a | 10 |
| b | 538 | |
| 2021–2025 | a | 5 |
| b | 188 |
| Author and Year | Study Design | Sample Size | Bone Defect Model | Outcome Measures | Teriparatide Dose Route of Administration | Control Group | Mean Bone Formation (TP Group) | Mean Bone Formation (Control Group) | Key Findings |
|---|---|---|---|---|---|---|---|---|---|
| Jung et al., 2007 [16] | New Zealand white rabbits | 16 | Critical-size bone defects in calvaria | Histomorphometric | 20 μg + peg + HA/TCP. 100 μg + peg + HA/TCP. Local. | Defect with no treatment; peg + HA/TCP. | 51.1 ± 22.6%. 53.5 ± 22.7% | 23.2 ± 10.1%. 34.3 ± 22.5% | TP + peg + HA/TCP increased the amount of bone regeneration compared with sites treated with peg + HA/TCP or empty control sites. |
| Jung et al., 2007 [17] | American foxhound dogs | 6 | Mandible | Histological, histomorphometry. | 20 μg + peg + 350 μg/mL RGD. Local | Peg alone. Autogenous bone (positive control). Empty defect (negative control) | 49.4 ± 7% | 39.3 ± 5.7%. 50.5 ± 3.4%. 38.7 ± 1.9% | Synthetic RGD-modified PEG-containing TP is an effective system to achieve bone regeneration in bone defects in mandible. |
| Rowshan H. et al., 2010 [18] | Male Sprague Dawley rats | 29 | Mandible | Histological, radiographic densitometry | 10 μg/kg/7 or 21 days. Subcutaneous. | 10 μg/mL saline solution for 7 or 21 days. Subcutaneous. | n/a | n/a | A low dosage of TP administration might enhance the healing process in the early phase of a mandibular fracture model in rats. |
| Jensen S. et al., 2011 [19] | Male Göttingen minipigs | 18 | Mandible | Histological, histomorphometry. | 20 μg + PEG + BCP. 20 μg/RGD + PEG + BCP. Local | Autogenous bone. BCP. PEG + BCP. EMD + PEG + BCP | 35.54%. 37.89% | 56.4%. 32.27%. 36.57%. 38.49%. | The study failed to demonstrate that EMD, TP, or TP + RGD, combined with experimental PEG hydrogel and BCP, stimulate bone formation. |
| Ali M. et al., 2012 [20] | Male Wistar rats | 18 | Mandible distraction | μCT at day 10, week 1, week 3 | 60 μg/kg, 3 times/week. Subcutaneous | Vehicle. (acetate buffer) | Day 10: 2.28 mm3 Week 1: 3.03 mm3 Week 3: 4.35 mm3 | Day 10: 2.28 mm3 Week 1: 3.03 mm3 Week 3: 4.35 mm3 | Intermittent TP boosted bone formation by 1.6–1.7 times, potentially reducing consolidation time. |
| Kuroshima et al., 2013 [21] | Sprague Dawley rats | 80 | Mandibular and maxillary extraction sockets | μCT, serum chemistry, histology | 80 μg/kg daily, subcutaneous or intra-oral injection | Saline (vehicle control) | n/a | n/a | TP accelerates hard and soft tissue healing, preserves the alveolar ridge, and is equally effective via intra-oral or subcutaneous administration. |
| Kuroshima et al., 2013 [22] | Sprague Dawley rats | 32 | Maxillary first molar extraction sockets, grafted and nongrafted | μCT | Xenograft + 80 μg/kg/day, subcutaneous injection, 14 days post-extraction (some groups also 7 days pre-extraction) | Xenograft + saline (vehicle control) | n/a | n/a | Intermittent TP post-extraction significantly enhanced bone formation in both grafted and nongrafted sockets; pre-extraction PTH alone had no significant effect. |
| Tang et al., 2013 [23] | Japanese white rabbits | 32 | 10 × 5 mm mandibular bone defect | X-ray, histology, histomorphometric, serum chemistry | 25 μg/day/4 weeks subcutaneous | Saline (vehicle control) | n/a | n/a | Intermittent TP promoted mandibular defect healing by increasing osteoblast activity, enhancing bone turnover, and upregulating OPG. |
| Tsunori, 2015 [24] | Male Fischer rats | 50 | Critical-size bone defects in rat calvaria | Serum calcium concentration, alkaline phosphatase activity, micro-CT analysis, histological and histomorphometric analyses | 15 μg/kg/day; 35 μg/kg/3 days/week; 105 μg/kg/day/week; 105 μg/kg/3 days/week Subcutaneous | Vehicle control; subcutaneous | 40.3 ± 15.2% 48.9 ± 12.4% 55.3 ± 4.2% 66.2 ± 9.3% | 10.3 ± 7.3% | TP groups showed greater and faster bone regeneration than controls in critical-size defects. |
| dos Santos et al., 2016 [25] | Wistar rats | 45 | Calvarial bone graft Histology fixed at mandibular angle | Histology, histomorphometric | 2 μg/kg or 40 μg/Kg 3×/week for 30 days. Subcutaneous. | Saline (vehicle control) | 76.42 ± 7.30% 80.75 ± 6.46% | 80.14 ± 4.80% | High-dose TP preserved graft volume, prevented resorption, and increased bone mass; low dose had no significant effect. |
| Huh el al., 2017 [26] | Female New Zealand white rabbits | 20 | Sinus augmentation in healthy rabbits | Radiographic and histomorphometric analysis | 10 μg/kg/5 days/2 weeks | 10 μg saline solution/kg/5 days/2 weeks | 10.85 ± 1.89% | 10.45 ± 4.4% | Intermittent TP might not stimulate new bone formation in healthy rabbits during the first 4 weeks of healing. |
| Kajii et al., 2017 [27] | Male Wistar rats | 18 | Critical-size bone defects in rat calvaria | Micro-CT, radiographic analysis, histological and histometric analyses | 0.1 μg/0.1 mL + ocp/col 1.0 μg/0.1 mL + ocp/col | ocp/col | 29.2 ± 6.0%. 38.1 ± 7.1% | 17.5% ± 3.6% | TP enhanced bone formation in a dose-dependent manner, confirming its osteoinductive effect with OCP/Col scaffolds |
| Göker et al., 2018 [28] | Female Wistar rats | 90 | Ovariectomy-induced calvaria defects | Measurement of bone turnover markers; dual-energy X-ray absorptiometry; microscopic observations | 2.5 μg + px + mp; 2.5 μg + px; 2.5 μg + 15 mg sr + px; 2.5 μg + 15 mg sr + px + mp | Defects with no treatment; 1.5 mg sr + px; | n/a | n/a | Bone formation increased over time, with TP + px and TP + px + mp groups outperforming controls. |
| Zandi et al., 2019 [2] | Male Wistar rats | 135 | Autografted mandibular defects | Histology, bone healing scores, micro-CT | Iliac graft + 2 μg PTH/kg/day. Subcutaneous. | Negative control (no graft), control (iliac graft) | 52.75 ± 2.83% | 18.00 ± 1.87%. 38.50 ± 3.27% | The results showed that systemic administration of TP significantly enhances bone regeneration in mandibular defects treated with autografts. |
| de Oliveira et al., 2019 [1] | Male Wistar rats | 78 | Orchiectomy-induced alveolar bone modeling | Micro-CT, immunolabeling for RANKL/OPG, bone volume | 0.5 μg/kg/day. Subcutaneous. | Orchiectomized without treatment; sham surgical procedures | 54.96 ± 4.16% | 41.23 ± 3.98%. 51.84 ± 3.29%. | Systemic TP enhanced alveolar bone modeling post-extraction in an androgen-deficient rat model. |
| Zandi et al., 2020 [29] | Albino Wistar rats | 120 | Unilateral mandibular fracture, surgically created | Histology | 2 μg/kg/day. Subcutaneous. | Saline (vehicle control) | n/a | n/a | TP significantly accelerated fracture healing, showing more trabecular and mature bone at all time points at days 10, 20, and 30. |
| Dam et al., 2020 [30] | Female New Zealand white rabbits | 20 | Sinus grafting in ovariectomized rabbits | Bone mineral density (BMD), radiographic and histometric analysis | 10 μg/kg/5 days/5 weeks. Subcutaneous. | Sterile saline solution in volume equivalent to the study group. | 29.45 ± 6.04% | 34.37 ± 5.33% | TP improved bone maturation, though not early volume, in estrogen-deficient sinus grafts. |
| Pelled et al., 2020 [31] | Yucatan minipigs | 6 | Segmental mandibulectomy in pigs | High-resolution X-ray imaging, biomechanical testing | 1.75 μg/kg/day/8 weeks. Subcutaneous. | Sterile phosphate-buffered saline in volume equivalent to the study group. | 46.3 ± 5.7% | 32.3 ± 4.8% | TP significantly enhanced bone allograft integration and mechanical properties. |
| Emam etl al, 2020 [32] | Domestic pigs | 6 | Mineralized bone xenografts in mandibular defects | BMD in CBCT and micro-CT, nanoindentation, histology | 20 μg + Bio-Oss. Local | Bio-Oss without PTH | n/a | n/a | Addition of TP to Bio-Oss improved mineralization, bone hardness, and regeneration. |
| Koca et al., 2021 [33] | Sprague Dawley rats | 48 | Critical-size defects in rat mandibles | Micro-CT, histomorphometry, osteoblast count | 40 μg + allograft. Local | Empty defects; autograft; allograft | 23.27 ± 0.15% | 8.3 ± 0.25% 12.38 ± 0.16% 18.43 ± 0.16% | A single local dose of TP significantly improved allograft integration and bone healing. |
| Çamili et al., 2022 [34] | Male Wistar rats | 20 | Experimentally induced premaxillary expansion using helical springs | Histology, immunohistochemistry | 60 μg/kg/7 days. Subcutaneous. | Saline injections, same volume | n/a | n/a | TP significantly enhanced bone formation at the midpalatal suture, increasing osteoblastic activity and upregulating osteonectin, osteocalcin, and VEGF |
| Wang et al., 2023 [35] | Male Sprague Dawley rats | 70 | Mandibular growth model | Morphometry, histology, immunohistochemistry PCR, immunofluorescence | 8 mg/kg/4 weeks. 800 μg/kg/4 weeks. 80 μg/kg/4 weeks. Abaloparatide: 8 mg/kg/4 weeks. 800 μg/kg/4 weeks. 80 μg/kg/4 weeks. Subcutaneous. | Saline injections, same volume. Subcutaneous. | n/a | n/a | TP promotes site-specific mandibular growth, enhances chondrogenesis, reduces hypertrophy, synergistic with MA. ABL generally more potent, especially at condyle and angle; limited effect at anterior alveolar bone unless combined with MA. |
| Nakanishi-Kimura et al., 2024 [36] | Ovariectomized (OVX) and sham female Sprague Dawley rats | 20 | Mandibular bone (alveolar, buccal, lingual sites) and parietal bone | Histomorphometry, 3D confocal fluorescence analysis | 30 μg/kg, 3×/4 weeks. Subcutaneous. | Saline injection (vehicle). Subcutaneous. OVX and sham-operated groups | n/a | n/a | TP induces site-specific perilacunar remodeling in mandibular alveolar bone, minimal effect in parietal bone; no net BV/TV change. |
| de Araújo et al., 2025 [37] | Female Wistar rats | 30 | Critical-size bone defects in rat calvaria | Histological, histomorphometry, immunohistochemical, and biomechanical | 45S5 bioactive glass | Empty defect, 45S5 bioactive glass + 10 μg teriparatide | 42.12 ± 5.34% | 18.14 ± 2.25% 29.01 ± 3.68% | TP associated with bioactive glass, was successfully incorporated and had a positive effect on bone repair. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canto, J.D.; Mourão, C.F.; Moraschini, V.; da Silva Bonato, R.; Sartoretto, S.C.; Calasans-Maia, M.D.; Granjeiro, J.M.; Louro, R.S. Teriparatide for Guided Bone Regeneration in Craniomaxillofacial Defects: A Systematic Review of Preclinical Studies. Curr. Issues Mol. Biol. 2025, 47, 582. https://doi.org/10.3390/cimb47080582
Canto JD, Mourão CF, Moraschini V, da Silva Bonato R, Sartoretto SC, Calasans-Maia MD, Granjeiro JM, Louro RS. Teriparatide for Guided Bone Regeneration in Craniomaxillofacial Defects: A Systematic Review of Preclinical Studies. Current Issues in Molecular Biology. 2025; 47(8):582. https://doi.org/10.3390/cimb47080582
Chicago/Turabian StyleCanto, Jessika Dethlefs, Carlos Fernando Mourão, Vittorio Moraschini, Rafael da Silva Bonato, Suelen Cristina Sartoretto, Monica Diuana Calasans-Maia, José Mauro Granjeiro, and Rafael Seabra Louro. 2025. "Teriparatide for Guided Bone Regeneration in Craniomaxillofacial Defects: A Systematic Review of Preclinical Studies" Current Issues in Molecular Biology 47, no. 8: 582. https://doi.org/10.3390/cimb47080582
APA StyleCanto, J. D., Mourão, C. F., Moraschini, V., da Silva Bonato, R., Sartoretto, S. C., Calasans-Maia, M. D., Granjeiro, J. M., & Louro, R. S. (2025). Teriparatide for Guided Bone Regeneration in Craniomaxillofacial Defects: A Systematic Review of Preclinical Studies. Current Issues in Molecular Biology, 47(8), 582. https://doi.org/10.3390/cimb47080582










