Bioelectrical Impedance Vector and Creatine Phosphokinase Changes Induced by a High-Intensity Training Session in Rink Hockey Players
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Procedures
2.3.1. Anthropometric Assessment
2.3.2. Bioimpedance Assessment
- -
- Whole-body assessment: The proximal electrode (sensor) of the arm was placed on the dorsal surface of the right wrist, between the ulna and radius. The proximal leg electrode was placed on the anterior surface of the right ankle, between the protruding portions of the bones. The distal electrodes (nozzles) were placed on the dorsal surface of the third proximal phalanx of the right hand and foot [16]. The proximal electrodes were spaced 5 cm from the distal to prevent interaction between electric fields, which could lead to an overestimation of the impedance values.
- -
- Rectus femoris muscle-localized assessment: The electrode placement procedures followed the recommendations of the Surface Electromyography for the Non-Invasive Assessment of Muscles (SENIAM) guidelines for the rectus femoris [39] and the ISAK standards and measurement protocol [35], as follows: 1. Locate the anterosuperior iliac spine (sartorius insertion), assuming that a more inferior (anteroinferior iliac spine) rectus femoris origin, and mark with a point; 2. Locate the superior pole of the patella with the knee in unforced extension, and mark with a point; 3. Connect both mark points with an anthropometric tape (running in a straight line along the immediately upper anterior thigh). Note the distance, in cm, between the two points and mark at 50% of this distance with a dot; 4. Placement of the electrodes: divide the distance between the origin and insertion by three. At one third of the origin (proximal) and one third of the insertion (distal), place the BIA sensor electrodes at a fixed distance of 5 cm (from the center of the electrodes) between the BIA injector electrodes (Figure 1).
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Bioelectrical Patterns in the Rink Hockey Players
4.2. Anthropometrical Changes Evoked by Training
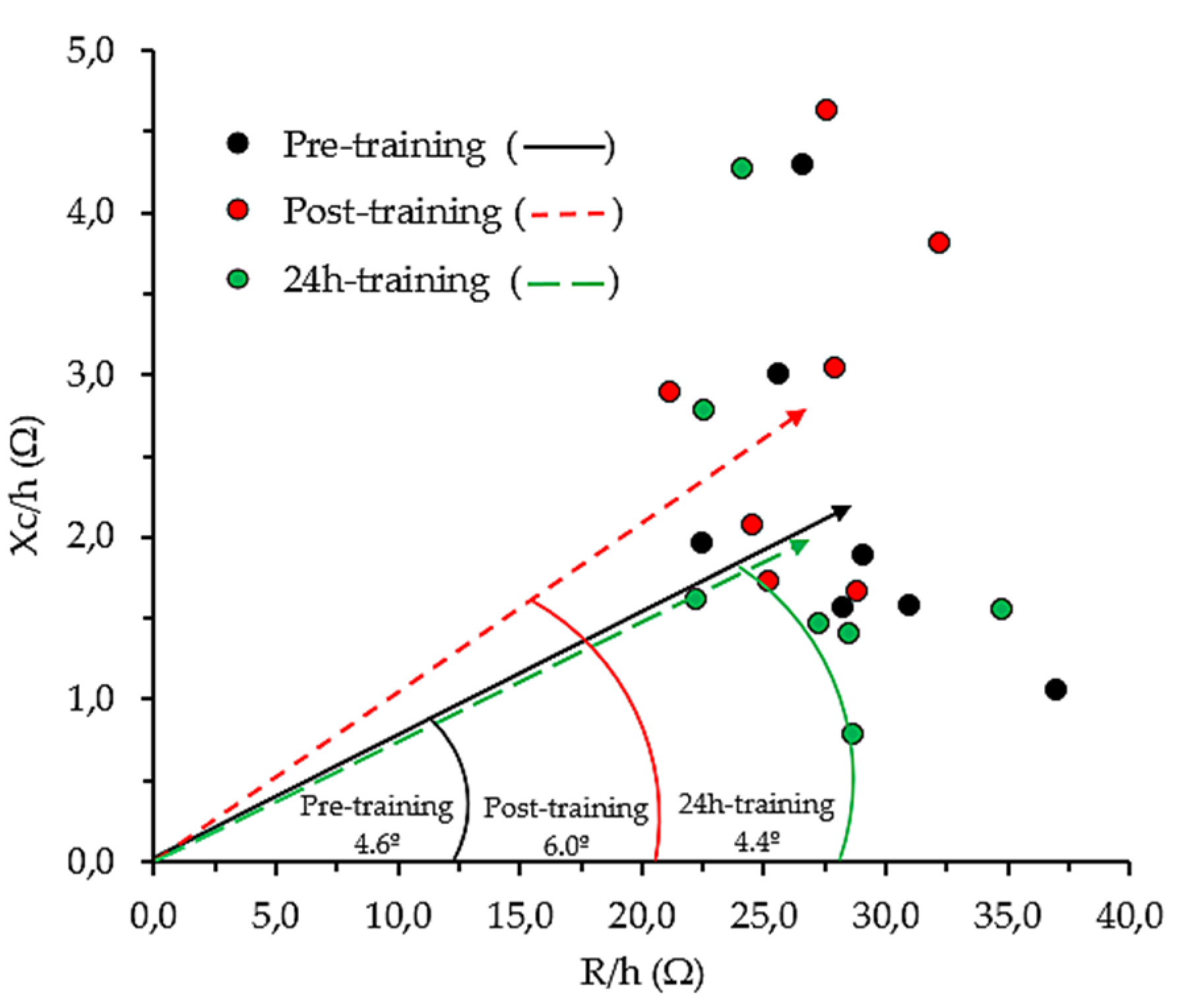
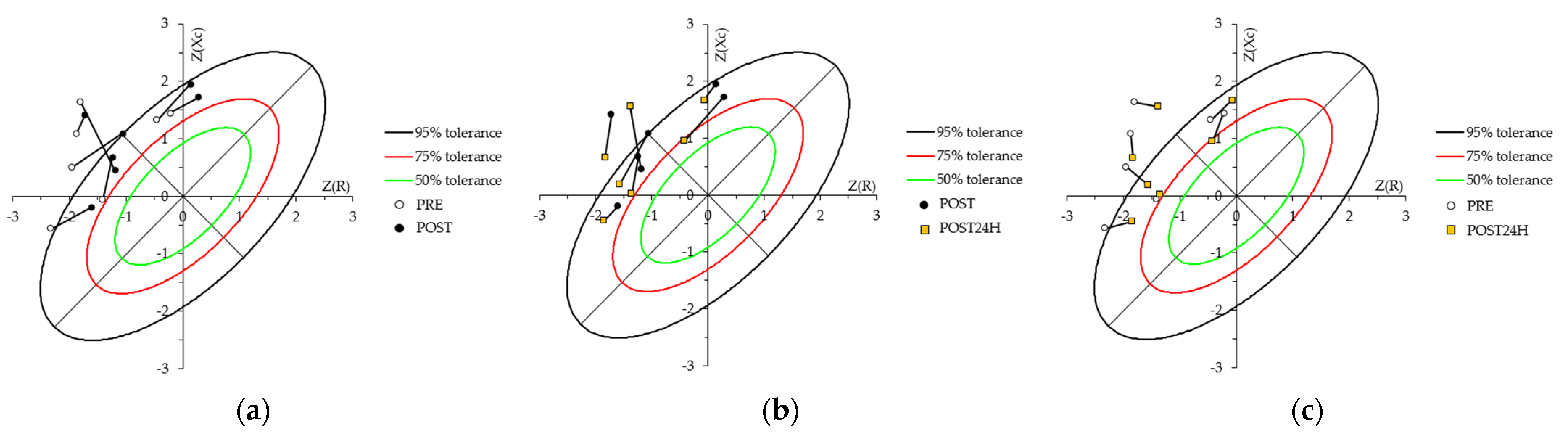
4.3. Whole-Body Bioelectrical Changes Evoked by Training
4.4. Localized Bioelectrical Changes Evoked by Training
4.5. Relationship between BIA Vector Changes and CK
5. Limitations of the Study
6. Practical Applications and Future Perspectives
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Skate Rink Hockey Technical Comission—Official Regulation. Available online: http://www.worldskate.org/rink-hockey/about/regulations.html (accessed on 26 November 2021).
- Ferraz, A.; Valente-Dos-santos, J.; Sarmento, H.; Duarte-Mendes, P.; Travassos, B. A Review of Players’ Characterization and Game Performance on Male Rink-Hockey. Int. J. Environ. Res. Public Health 2020, 17, 4259. [Google Scholar] [CrossRef] [PubMed]
- Yagüe Ares, P.L. Hockey Sobre Patines: Estudio de Las Demandas Fisiológicas en Competición, Análisis del Perfil Fisiológico Funcional, Desarrollo y Validación de un Modelo de Valoración Funcional Específica Orientado Al Jugador de Campo; University of Oviedo: Asturias, Spain, 2005. [Google Scholar]
- Hernández Mendo, A.; Anguera Argilaga, M.T. Behavioral Structure in Sociomotor Sports: Roller-Hockey. Qual. Quant. 2002, 36, 347–378. [Google Scholar] [CrossRef]
- Barranco, T.; Tvarijonaviciute, A.; Tecles, F.; Carrillo, J.M.; Sánchez-Resalt, C.; Jimenez-Reyes, P.; Rubio, M.; García-Balletbó, M.; Cerón, J.J.; Cugat, R. Changes in Creatine Kinase, Lactate Dehydrogenase and Aspartate Aminotransferase in Saliva Samples after an Intense Exercise: A Pilot Study. J. Sports Med. Phys. Fit. 2018, 58, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine Kinase Monitoring in Sport Medicine. Br. Med. Bull. 2007, 81–82, 209–230. [Google Scholar] [CrossRef]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-Kinase- and Exercise-Related Muscle Damage Implications for Muscle Performance and Recovery. J. Nutr. Metab. 2012, 2012, 960363. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.R.; Rumpf, M.C.; Hertzog, M.; Castagna, C.; Farooq, A.; Girard, O.; Hader, K. Acute and Residual Soccer Match-Related Fatigue: A Systematic Review and Meta-Analysis. Sport. Med. 2018, 48, 539–583. [Google Scholar] [CrossRef]
- Koutedakis, Y.; Raafat, A.; Sharp, N.C.; Rosmarin, M.N.; Beard, M.J.; Robbins, S.W. Serum Enzyme Activities in Individuals with Different Levels of Physical Fitness. J. Sports Med. Phys. Fit. 1993, 33, 252–257. [Google Scholar]
- Kim, J.; Lee, J. The Relationship of Creatine Kinase Variability with Body Composition and Muscle Damage Markers Following Eccentric Muscle Contractions. J. Exerc. Nutr. Biochem. 2015, 19, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical Impedance Analysis—Part I: Review of Principles and Methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical Impedance Analysis—Part II: Utilization in Clinical Practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef] [PubMed]
- Castizo-Olier, J.; Irurtia, A.; Jemni, M.; Carrasco-Marginet, M.; Fernández-García, R.; Rodríguez, F.A. Bioelectrical Impedance Vector Analysis (BIVA) in Sport and Exercise: Systematic Review and Future Perspectives. PLoS ONE 2018, 13, e0197957. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical Phase Angle and Impedance Vector Analysis—Clinical Relevance and Applicability of Impedance Parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef]
- Moon, J.R. Body Composition in Athletes and Sports Nutrition: An Examination of the Bioimpedance Analysis Technique. Eur. J. Clin. Nutr. 2013, 67, S54–S59. [Google Scholar] [CrossRef] [PubMed]
- Campa, F.; Toselli, S.; Mazzilli, M.; Gobbo, L.A.; Coratella, G. Assessment of Body Composition in Athletes: A Narrative Review of Available Methods with Special Reference to Quantitative and Qualitative Bioimpedance Analysis. Nutrients 2021, 13, 1620. [Google Scholar] [CrossRef]
- Micheli, M.L.; Pagani, L.; Marella, M.; Gulisano, M.; Piccoli, A.; Angelini, F.; Burtscher, M.; Gatterer, H. Bioimpedance and Impedance Vector Patterns as Predictors of League Level in Male Soccer Players. Int. J. Sports Physiol. Perform. 2014, 9, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, B.; Rutkove, S.B. Present Uses, Future Applications, and Technical Underpinnings of Electrical Impedance Myography. Curr. Neurol. Neurosci. Rep. 2017, 17, 86. [Google Scholar] [CrossRef]
- Cebrián-Ponce, Á.; Irurtia, A.; Carrasco-Marginet, M.; Saco-Ledo, G.; Girabent-Farrés, M.; Castizo-Olier, J. Electrical Impedance Myography in Health and Physical Exercise: A Systematic Review and Future Perspectives. Front. Physiol. 2021, 12, 740877. [Google Scholar] [CrossRef]
- Nescolarde, L.; Yanguas, J.; Lukaski, H.; Alomar, X.; Rosell-Ferrer, J.; Rodas, G. Localized Bioimpedance to Assess Muscle Injury. Physiol. Meas. 2013, 34, 237–245. [Google Scholar] [CrossRef]
- Nescolarde, L.; Yanguas, J.; Lukaski, H.; Alomar, X.; Rosell-Ferrer, J.; Rodas, G. Effects of Muscle Injury Severity on Localized Bioimpedance Measurements. Physiol. Meas. 2015, 36, 27–42. [Google Scholar] [CrossRef]
- Nescolarde, L.; Yanguas, J.; Terricabras, J.; Lukaski, H.; Alomar, X.; Rosell-Ferrer, J.; Rodas, G. Detection of Muscle Gap by L-BIA in Muscle Injuries: Clinical Prognosis. Physiol. Meas. 2017, 38, L1–L9. [Google Scholar] [CrossRef] [PubMed]
- Nescolarde, L.; Terricabras, J.; Mechó, S.; Rodas, G.; Yanguas, J. Differentiation Between Tendinous, Myotendinous and Myofascial Injuries by L-BIA in Professional Football Players. Front. Physiol. 2020, 11, 574124. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, V.C.; Bongiovanni, T.; Genovesi, F.; Minafra, P.; Francavilla, G. Localized Bioelectrical Impedance Analysis: How Useful Is It in the Folow-up of Muscle Injury? A Case Report. Med. Sport 2015, 68, 323–334. [Google Scholar]
- Silvestre, R.; West, C.; Maresh, C.M.; Kraemer, W.J. Body Composition and Physical Performance in Men’s Soccer: A Study of a National Collegiate Athletic Association Division I Team. J. Strength Cond. Res. 2006, 20, 177–183. [Google Scholar] [CrossRef]
- Cian, C.; Barraud, P.A.; Melin, B.; Raphel, C. Effects of Fluid Ingestion on Cognitive Function after Heat Stress or Exercise-Induced Dehydration. Int. J. Psychophysiol. 2001, 42, 243–251. [Google Scholar] [CrossRef]
- Maughan, R.J. Impact of Mild Dehydration on Wellness and on Exercise Performance. Eur. J. Clin. Nutr. 2003, 57, S19–S23. [Google Scholar] [CrossRef] [PubMed]
- Oppliger, R.A.; Bartok, C. Hydration Testing of Athletes. Sport. Med. 2002, 32, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Nescolarde, L.; Roca, E.; Bogónez-Franco, P.; Hernández-Hermoso, J.; Bayes-Genis, A.; Ara, J. Relationship Between Bioimpedance Vector Displacement and Renal Function After a Marathon in Non-Elite Runners. Front. Physiol. 2020, 11, 352. [Google Scholar] [CrossRef]
- Armstrong, L.E. Assessing Hydration Status: The Elusive Gold Standard. J. Am. Coll. Nutr. 2007, 26, 575S–584S. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, J.; Thompson, J.; Hanna, C.; Noakes, T.D.; Stewart, J.; Speedy, D. Sensitivity and Specificity of Clinical Signs for Assessment of Dehydration in Endurance Athletes. Br. J. Sports Med. 2010, 44, 716–719. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Piccoli, A. Bioelectrical Impedance Vector Analysis for Assessment of Hydration in Physiological States and Clinical Conditions. In Handbook of Anthropometry: Physical Measures of Human Form in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2012; pp. 287–305. ISBN 9781441917881. [Google Scholar]
- World Medical Association. WMA World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Borg, G. Perceived Exertion as an Indicator of Somatic Stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar] [PubMed]
- Marfell-Jones, M.; Esparza-Ros, F.; Vaquero-Cristóbal, R. ISAK Accreditation Handbook; International Society for the Advancement of Kinanthropometry, ISAK: Murcia, Spain, 2019; ISBN 9788416045259. [Google Scholar]
- Piccoli, A. Bioelectric Impedance Measurement for Fluid Status Assessment. Fluid Overload 2010, 164, 143–152. [Google Scholar] [CrossRef]
- Campa, F.; Gatterer, H.; Lukaski, H.; Toselli, S. Stabilizing Bioimpedance-Vector-Analysis Measures with a 10-Minute Cold Shower after Running Exercise to Enable Assessment of Body Hydration. Int. J. Sports Physiol. Perform. 2019, 14, 1006–1009. [Google Scholar] [CrossRef]
- Kushner, R.F.; Gudivaka, R.; Schoeller, D.A. Clinical Characteristics Influencing Bioelectrical Impedance Analysis Measurements. Am. J. Clin. Nutr. 1996, 64, 423S–427S. [Google Scholar] [CrossRef]
- Hermens, H.; Freriks, B.; Desselhorst-Klug, C.; Rau, G. Development of Recommendations for SEMG Sensors and Sensor Placement Procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Piccoli, A.; Nigrelli, S.; Caberlotto, A.; Bottazzo, S.; Rossi, B.; Pillon, L.; Maggiore, Q. Bivariate Normal Values of the Bioelectrical Impedance Vector in Adult and Elderly Populations. Am. J. Clin. Nutr. 1995, 61, 269–270. [Google Scholar] [CrossRef]
- Castizo-Olier, J.; Carrasco-Marginet, M.; Roy, A.; Chaverri, D.; Iglesias, X.; Pérez-Chirinos, C.; Rodríguez, F.; Irurtia, A. Bioelectrical Impedance Vector Analysis (BIVA) and Body Mass Changes in an Ultra-Endurance Triathlon Event. J. Sport. Sci. Med. 2018, 17, 571–579. [Google Scholar]
- Chertow, G.M.; Lowrie, E.G.; Wilmore, D.W.; Gonzalez, J.; Lew, N.L.; Ling, J.; Leboff, M.S.; Gottlieb, M.N.; Huang, W.; Zebrowski, B.; et al. Nutritional Assessment with Bioelectrical Impedance Analysis in Maintenance Hemodialysis Patients. J. Am. Soc. Nephrol. 1995, 6, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Marginet, M.; Castizo-Olier, J.; Rodríguez-Zamora, L.; Iglesias, X.; Rodríguez, F.A.; Chaverri, D.; Brotons, D.; Irurtia, A. Bioelectrical Impedance Vector Analysis (BIVA) for Measuring the Hydration Status in Young Elite Synchronized Swimmers. PLoS ONE 2017, 12, e0178819. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, A.; Vicini, M.; Pollastri, L.; Lombardi, E.; Magni, E.; Andreazzoli, A.; Orsini, M.; Bonifazi, M.; Lukaski, H.; Gatterer, H. Bioimpedance Patterns and Bioelectrical Impedance Vector Analysis (BIVA) of Road Cyclists. J. Sports Sci. 2018, 36, 2608–2613. [Google Scholar] [CrossRef] [PubMed]
- Campa, F.; Matias, C.; Gatterer, H.; Toselli, S.; Koury, J.C.; Andreoli, A.; Melchiorri, G.; Sardinha, L.B.; Silva, A.M. Classic Bioelectrical Impedance Vector Reference Values for Assessing Body Composition in Male and Female Athletes. Int. J. Environ. Res. Public Health 2019, 16, 5066. [Google Scholar] [CrossRef] [PubMed]
- Nuccio, R.P.; Barnes, K.A.; Carter, J.M.; Baker, L.B. Fluid Balance in Team Sport Athletes and the Effect of Hypohydration on Cognitive, Technical, and Physical Performance. Sport. Med. 2017, 47, 1951–1982. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.; Young, A.J.; Sawka, M.N. Bioelectrical Impedance to Estimate Changes in Hydration Status. Int. J. Sports Med. 2002, 23, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Heavens, K.R.; Charkoudian, N.; O’Brien, C.; Kenefick, R.W.; Cheuvront, S.N. Noninvasive Assessment of Extracellular and Intracellular Dehydration in Healthy Humans Using the Resistance-Reactance-Score Graph Method. Am. J. Clin. Nutr. 2016, 103, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Hirono, T.; Ikezoe, T.; Taniguchi, M.; Tanaka, H.; Saeki, J.; Yagi, M.; Umehara, J.; Ichihashi, N. Relationship Between Muscle Swelling and Hypertrophy Induced by Resistance Training. J. Strength Cond. Res. 2020. [Google Scholar] [CrossRef]
- Gatterer, H.; Schenk, K.; Laninschegg, L.; Schlemmer, P.; Lukaski, H.; Burtscher, M. Bioimpedance Identifies Body Fluid Loss after Exercise in the Heat: A Pilot Study with Body Cooling. PLoS ONE 2014, 9, e109729. [Google Scholar] [CrossRef] [PubMed]
- Marini, E.; Toselli, S. Bioelectrical Impedance Analysis of Body Composition-Applications in Sports Science; UNICApress: Cagliari, Italy, 2021; ISBN 9788833120324. [Google Scholar]
- Campa, F.; Piras, A.; Raffi, M.; Trofè, A.; Perazzolo, M.; Mascherini, G.; Toselli, S. The Effects of Dehydration on Metabolic and Neuromuscular Functionality during Cycling. Int. J. Environ. Res. Public Health 2020, 17, 1161. [Google Scholar] [CrossRef]
- Judelson, D.A.; Maresh, C.M.; Anderson, J.M.; Armstrong, L.E.; Casa, D.J.; Kraemer, W.J.; Volek, J.S. Hydration and Muscular Performance: Does Fluid Balance Affect Strength, Power and High-Intensity Endurance? Sport. Med. 2007, 37, 907–921. [Google Scholar] [CrossRef]
- Nabuco, H.C.G.; Silva, A.M.; Sardinha, L.B.; Rodrigues, F.B.; Tomeleri, C.M.; Ravagnani, F.C.P.; Cyrino, E.S.; Ravagnani, C.F.C. Phase Angle Is Moderately Associated with Short-Term Maximal Intensity Efforts in Soccer Players. Int. J. Sports Med. 2019, 40, 739–743. [Google Scholar] [CrossRef]
- Li, L.; Shin, H.; Li, X.; Li, S.; Zhou, P. Localized Electrical Impedance Myography of the Biceps Brachii Muscle during Different Levels of Isometric Contraction and Fatigue. Sensors 2016, 16, 581. [Google Scholar] [CrossRef]
- Fu, B.; Freeborn, T.J. Biceps Tissue Bioimpedance Changes from Isotonic Exercise-Induced Fatigue at Different Intensities. Biomed. Phys. Eng. Express 2018, 4, 025037. [Google Scholar] [CrossRef]
- Freeborn, T.J.; Fu, B. Time-Course Bicep Tissue Bio-Impedance Changes throughout a Fatiguing Exercise Protocol. Med. Eng. Phys. 2019, 69, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Freeborn, T.J.; Regard, G.; Fu, B. Localized Bicep Tissue Bioimpedance Alterations Following Eccentric Exercise in Healthy Young Adults. IEEE Access 2020, 8, 23100–23109. [Google Scholar] [CrossRef]
- Li, X.; Shin, H.; Li, L.; Magat, E.; Li, S.; Zhou, P. Assessing the Immediate Impact of Botulinum Toxin Injection on Impedance of Spastic Muscle. Med. Eng. Phys. 2017, 43, 97–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stagi, S.; Moroni, A.; Micheletti Cremasco, M.; Marini, E. Body Composition Symmetry in Long-Term Active Middle-Aged and Older Individuals. Int. J. Environ. Res. Public Health 2021, 18, 5956. [Google Scholar] [CrossRef]
- Mascherini, G.; Petri, C.; Galanti, G. Integrated Total Body Composition and Localized Fat-Free Mass Assessment. Sport Sci. Health 2015, 11, 217–225. [Google Scholar] [CrossRef]




| Subjects | HR (b/min) | HR Intensity Zones (min and %) | Total Session Time (Hours) | RPE—15-Point Borg Scale | Water Intake (L) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HRrest | HRmin | HRmean | HRmax | Very Light | Light | Moderate | High | Very High | Maximum | ||||||||||
| (Min–80 b/min) | (81–100 b/min) | (101–120 b/min) | (121–140 b/min) | (141–160 b/min) | (161–Max b/min) | ||||||||||||||
| Hours | % | Hours | % | Hours | % | Hours | % | Hours | % | Hours | % | ||||||||
| 1 | 61 | 72 | 132 | 189 | 0:30 | 19.9 | 0:32 | 21.2 | 0:24 | 15.9 | 0:27 | 17.9 | 0:29 | 19.2 | 0:09 | 6.0 | 2:31 | 15.0 | 1.40 |
| 2 | 56 | 71 | 134 | 190 | 0:19 | 12.6 | 0:49 | 32.5 | 0:35 | 23.2 | 0:20 | 13.2 | 0:16 | 10.6 | 0:12 | 7.9 | 2:31 | 16.0 | 1.20 |
| 3 | 52 | 63 | 129 | 183 | 0:16 | 10.7 | 0:33 | 22.0 | 0:35 | 23.3 | 0:23 | 15.3 | 0:30 | 20.0 | 0:13 | 8.7 | 2:30 | 15.0 | 1.50 |
| 4 | 56 | 68 | 128 | 190 | 0:22 | 15.3 | 0:40 | 27.8 | 0:29 | 20.1 | 0:19 | 13.2 | 0:23 | 16.0 | 0:11 | 7.6 | 2:24 | 17.0 | 2.25 |
| 5 | 53 | 61 | 117 | 180 | 0:12 | 7.9 | 0:27 | 17.8 | 0:27 | 17.8 | 0:33 | 21.7 | 0:35 | 23.0 | 0:18 | 11.8 | 2:32 | 12.0 | 2.25 |
| 6 | 55 | 65 | 122 | 177 | 0:10 | 6.8 | 0:15 | 10.2 | 0:24 | 16.3 | 0:36 | 24.5 | 0:37 | 25.2 | 0:25 | 17.0 | 2:27 | 13.0 | 1.60 |
| 7 | 58 | 66 | 129 | 184 | 0:20 | 13.2 | 0:30 | 19.9 | 0:33 | 21.9 | 0:24 | 15.9 | 0:28 | 18.5 | 0:16 | 10.6 | 2:31 | 15.0 | 1.60 |
| Average | 55.9 | 66.6 | 127.3 | 184.7 | 0:18 | 12.3 | 0:32 | 21.6 | 0:29 | 19.8 | 0:26 | 17.4 | 0:28 | 18.9 | 0:14 | 10.0 | 2:29 | 14.7 | 1.7 |
| SD | 3.0 | 4.0 | 5.9 | 5.2 | 0:06 | 4.5 | 0:10 | 7.1 | 0:04 | 3.2 | 0:06 | 4.3 | 0:07 | 4.7 | 0:05 | 3.7 | 0:02 | 1.7 | 0.4 |
| ANTHROPOMETRIC | ||||||||||||||||||
| PRE | POST | POST24H | Δ PRE-to-POST (%) | p | Δ POST-to-POST24H (%) | p | Δ PRE-to-POST24H (%) | p | ||||||||||
| BM (kg) | 79 ± 9.8 | 78.1 ± 9.7 | 79 ± 9.9 | −1.1 ± 0.6 * | 0.02 | 1.2 ± 0.8 * | 0.02 | 0.1 ± 0.6 | 0.73 | |||||||||
| P thigh (cm) | 59 ± 4.4 | 59.9 ± 4.4 | 59.1 ± 4.5 | 1.5 ± 0.4 * | 0.02 | −1.4 ± 0.6 * | 0.02 | 0.1 ± 0.3 | 0.39 | |||||||||
| BIOELECTRICAL | ||||||||||||||||||
| PRE | POST | POST24H | Δ PRE-to-POST (%) | p | Δ POST-to-POST24H (%) | p | Δ PRE-to-POST24H (%) | p | ||||||||||
| WB | LOC | WB | LOC | WB | LOC | WB | LOC | WB | LOC | WB | LOC | WB | LOC | WB | LOC | WB | LOC | |
| R (Ω) | 417.5 ± 48 | 50.7 ± 8.2 | 457.2 ± 49.2 | 47.4 ± 6.3 | 434.8 ± 43 | 47.6 ± 7.6 | 9.7 ± 5.8 * | −5.3 ± 13 | 0.02 | 0.27 | −4.8 ± 3.1 * | 0.5 ± 10.5 | 0.02 | 1 | 4.4 ± 4.9 | −4.5 ± 19.5 | 0.06 | 0.4 |
| R/h (Ω/m) | 236.8 ± 34.5 | 28.6 ± 4.6 | 259.1 ± 34.7 | 26.8 ± 3.5 | 246.3 ± 30 | 26.9 ± 4.4 | ||||||||||||
| Xc (Ω) | 64.1 ± 10.1 | 3.9 ± 2.1 | 67.4 ± 8.9 | 5 ± 2 | 62.8 ± 9.7 | 3.5 ± 2.2 | 6.1 ± 12.3 | 38.7 ± 44.7 * | 0.24 | 0.04 | −6.2 ± 13.8 | −30.8 ± 24.6 * | 0.24 | 0.02 | −1.8 ± 6 | −12.4 ± 12.3 * | 0.5 | 0.04 |
| Xc/h (Ω/m) | 36.3 ± 6 | 2.2 ± 1.1 | 38.1 ± 5.4 | 2.8 ± 1.1 | 35.5 ± 5.7 | 2 ± 1.2 | ||||||||||||
| |Z| (m) | 422.5 ± 48.1 | 50.9 ± 8.2 | 462.2 ± 49.5 | 47.7 ± 6.4 | 439.3 ± 43.4 | 47.8 ± 7.5 | 9.6 ± 5.7 * | −5.1 ± 13 | 0.02 | 0.31 | −4.8 ± 3.2 * | 0.3 ± 10.4 | 0.02 | 1 | 4.2 ± 4.8 | −4.5 ± 19.3 | 0.06 | 0.4 |
| |Z|/h (Ω/m) | 239.6 ± 34.6 | 28.7 ± 4.5 | 261.9 ± 34.8 | 27 ± 3.5 | 248.9 ± 30.3 | 27 ± 4.3 | ||||||||||||
| PhA (°) | 8.8 ± 1.3 | 4.6 ± 2.6 | 8.4 ± 1 | 6 ± 2.2 | 8.2 ± 1 | 4.4 ± 3 | −2.9 ± 12.8 | 48.1 ± 49.2 * | 0.55 | 0.03 | −1.6 ± 13.5 | −29.5 ± 29.1 | 0.24 | 0.06 | −5.8 ± 5 * | −4.4 ± 25.4 | 0.04 | 1 |
| r (R/h vs. Xc/h) | 0.61 | −0.79 | 0.57 | 0.23 | 0.68 | −0.7 | - | - | - | |||||||||
| HEMATOLOGICAL | ||||||||||||||||||
| PRE | POST | POST24H | Δ PRE-to-POST (%) | p | Δ POST-to-POST24H (%) | p | Δ PRE-to-POST24H (%) | p | ||||||||||
| CK (IU/L) | 193.3 ± 75.5 | 283.6 ± 84.3 | 381.3 ± 147.7 | 53.4 ± 29.8 * | 0.02 | 34.9 ± 29.5 * | 0.02 | 109.2 ± 76.4 * | 0.02 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cebrián-Ponce, Á.; Garnacho-Castaño, M.V.; Castellano-Fàbrega, M.; Castizo-Olier, J.; Carrasco-Marginet, M.; Serra-Paya, N.; Irurtia, A. Bioelectrical Impedance Vector and Creatine Phosphokinase Changes Induced by a High-Intensity Training Session in Rink Hockey Players. Appl. Sci. 2022, 12, 751. https://doi.org/10.3390/app12020751
Cebrián-Ponce Á, Garnacho-Castaño MV, Castellano-Fàbrega M, Castizo-Olier J, Carrasco-Marginet M, Serra-Paya N, Irurtia A. Bioelectrical Impedance Vector and Creatine Phosphokinase Changes Induced by a High-Intensity Training Session in Rink Hockey Players. Applied Sciences. 2022; 12(2):751. https://doi.org/10.3390/app12020751
Chicago/Turabian StyleCebrián-Ponce, Álex, Manuel V. Garnacho-Castaño, Mercè Castellano-Fàbrega, Jorge Castizo-Olier, Marta Carrasco-Marginet, Noemí Serra-Paya, and Alfredo Irurtia. 2022. "Bioelectrical Impedance Vector and Creatine Phosphokinase Changes Induced by a High-Intensity Training Session in Rink Hockey Players" Applied Sciences 12, no. 2: 751. https://doi.org/10.3390/app12020751
APA StyleCebrián-Ponce, Á., Garnacho-Castaño, M. V., Castellano-Fàbrega, M., Castizo-Olier, J., Carrasco-Marginet, M., Serra-Paya, N., & Irurtia, A. (2022). Bioelectrical Impedance Vector and Creatine Phosphokinase Changes Induced by a High-Intensity Training Session in Rink Hockey Players. Applied Sciences, 12(2), 751. https://doi.org/10.3390/app12020751







