Unveiling Therapeutic Powers of Indigenous Flora: Antimicrobial, Antioxidant, and Anticancer Properties of Horwoodia dicksoniae
Abstract
1. Introduction
2. Results
2.1. Antifungal and Antibacterial Activities of Horwoodia dicksoniae and Stipa capensis Extracts
2.2. Minimum Inhibitory Concentration (MIC)
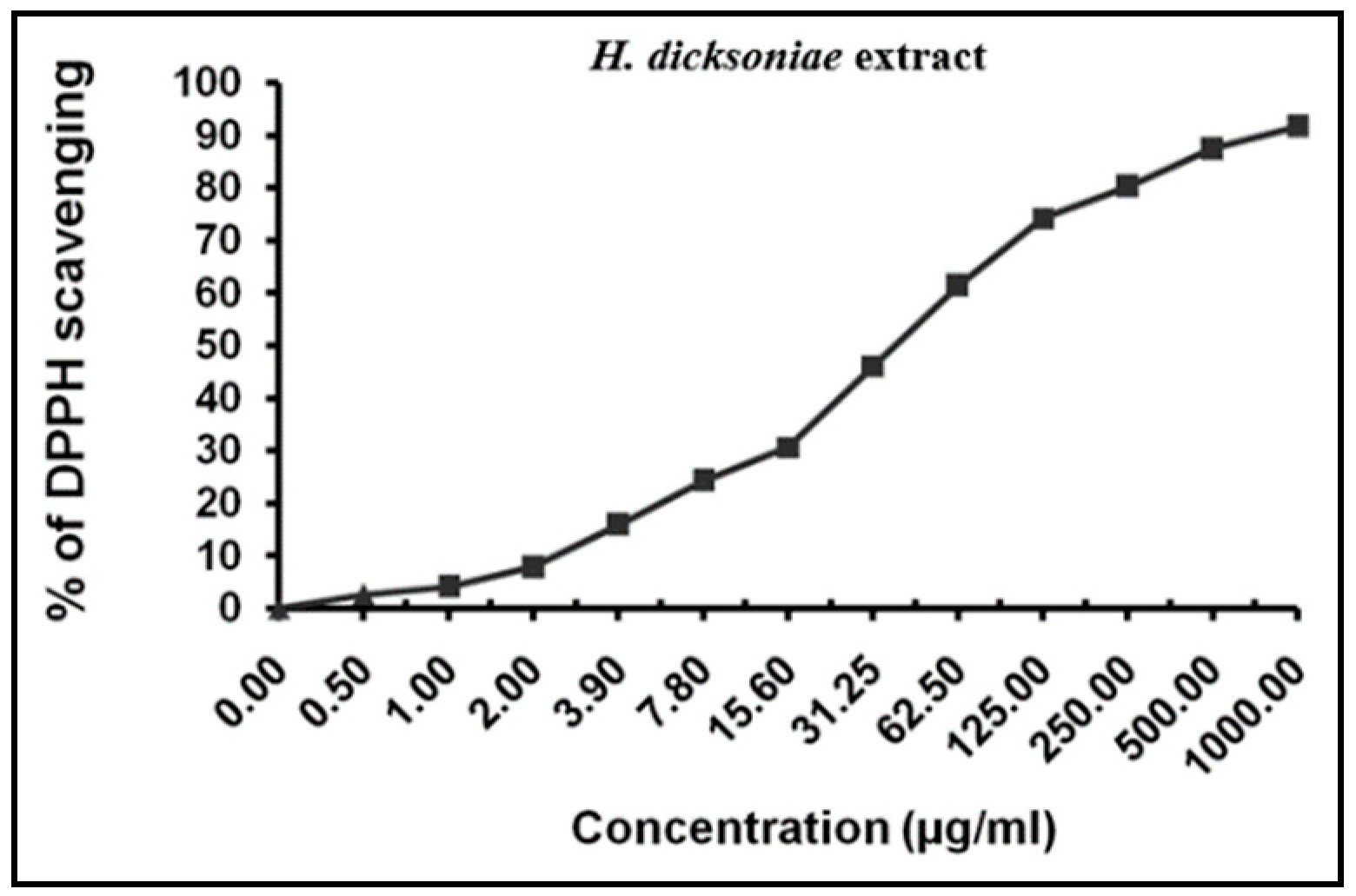
2.3. Total Antioxidant and Radical-Scavenging Activities of H. dicksoniae Extract
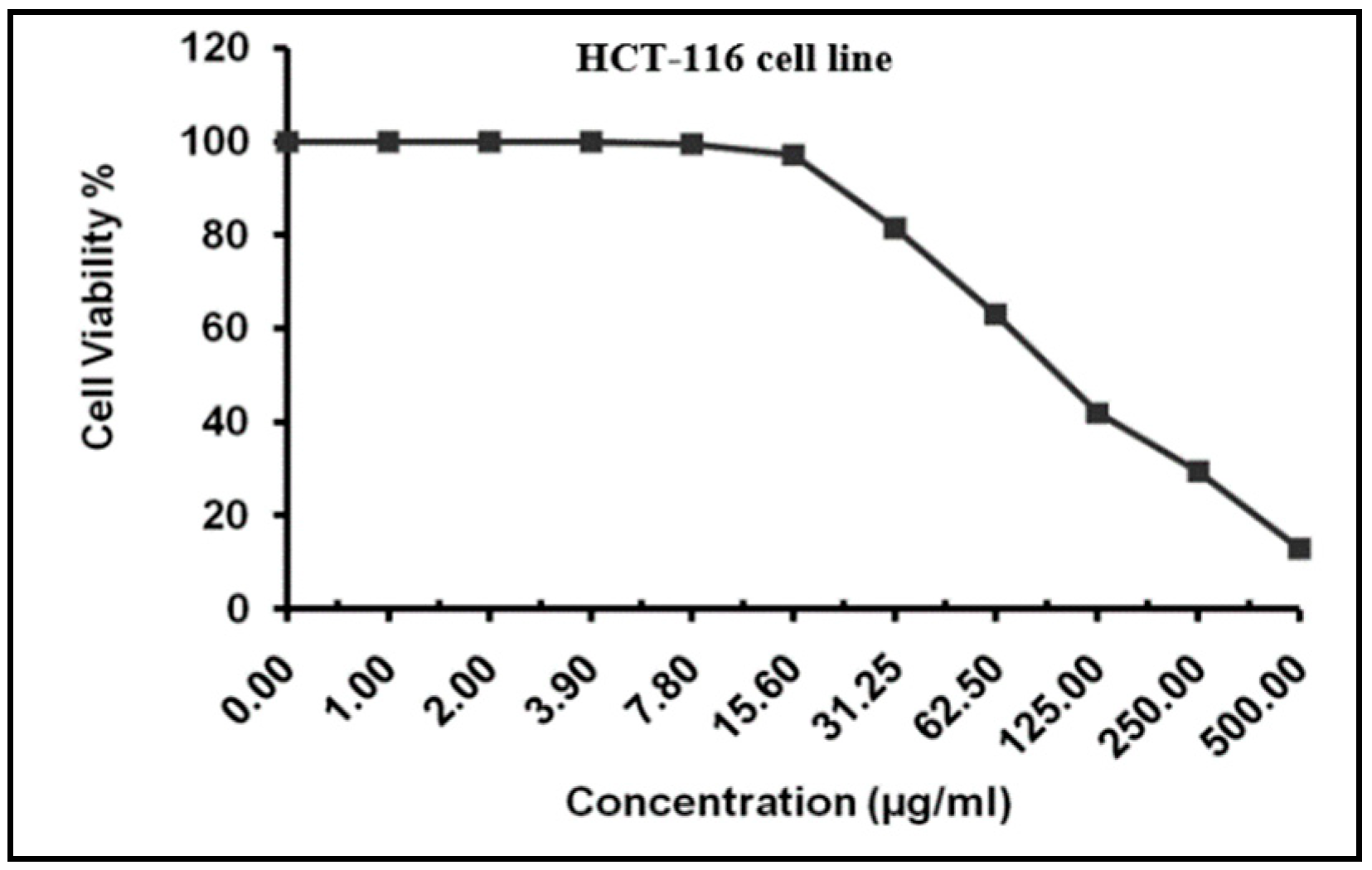
2.4. MTT Assay for Cytotoxicity Analysis
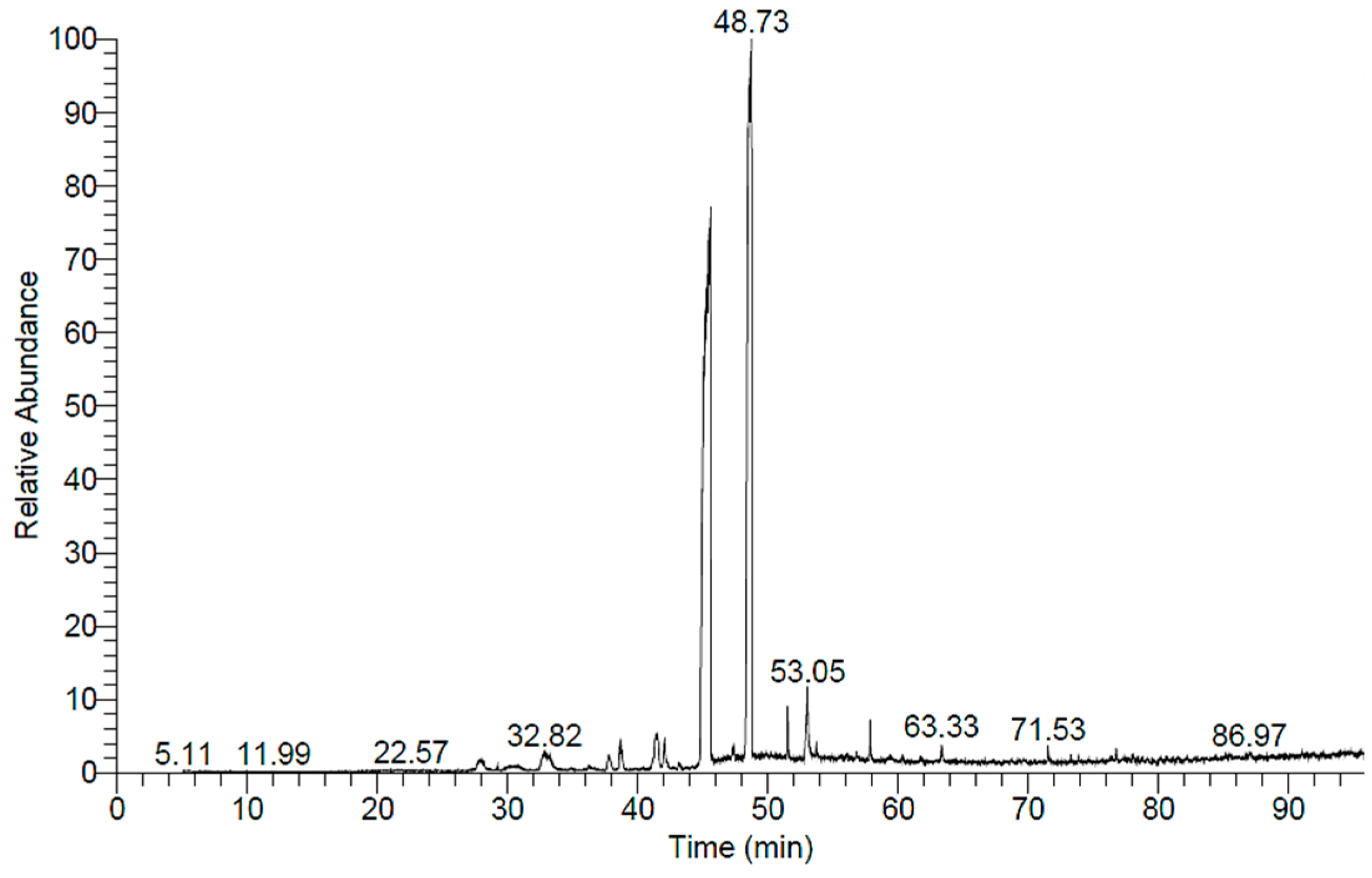
2.5. GC-MS Analysis of H. dicksoniae Ethanolic Extract
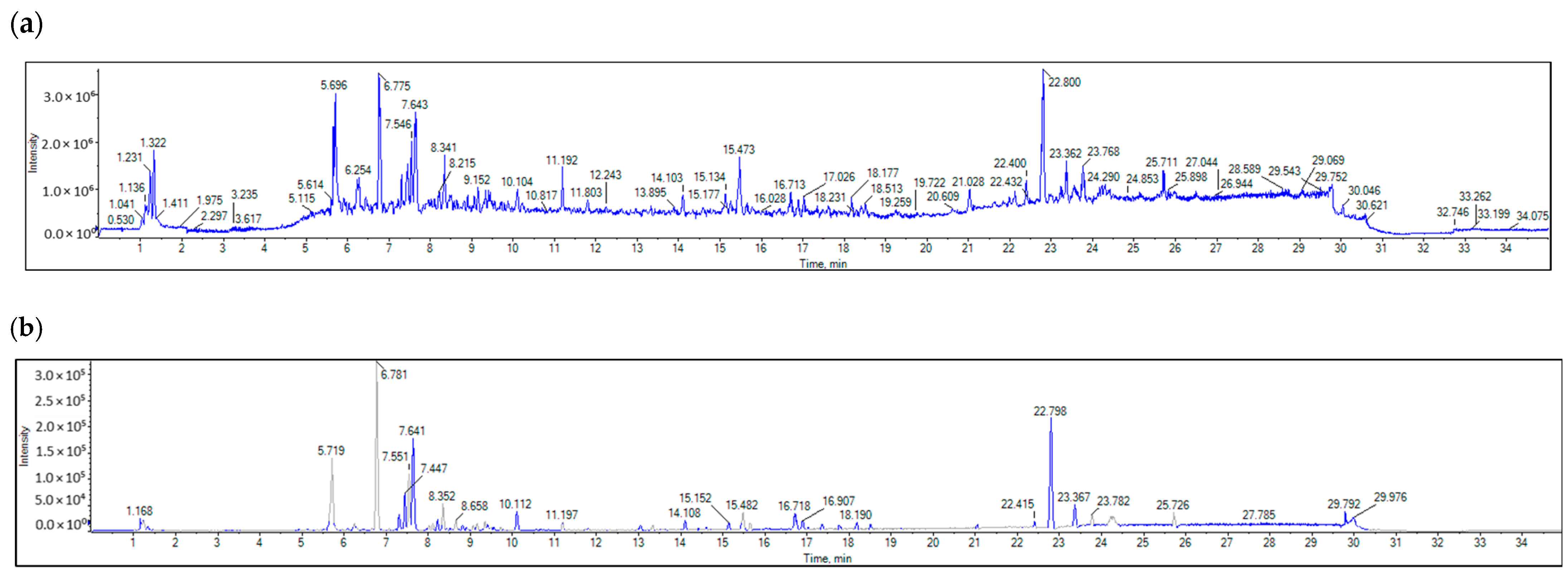
2.6. LC-MS/MS
2.7. Molecular Docking of Antimicrobial, Anticancer, and Antioxidant Target Proteins with Phytochemical Compounds (Ligands)
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Plant Extract Preparation
4.3. Antifungal and Antibacterial Activities
4.4. Antioxidant Activity Assay
4.5. MTT Assay-Based Anticancer Analysis
4.6. GC-MS Analysis of Crude H. dicksoniae Extract
4.7. LC-MS/MS Analysis of Crude H. dicksoniae Extract
4.7.1. Chemicals and Reagents
4.7.2. Sample Preparation
4.7.3. LC-MS/MS Analysis
4.7.4. Data Processing
4.8. Bioinformatics Experiments
4.8.1. Ligand Preparation
4.8.2. Protein Preparation
4.8.3. Molecular Docking
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fouda, H.; Abdulkader, O.; Sharaf, A.E.; Elhaw, M. Phytochemical constituents and gas chromatography with mass spectroscopy analysis of Euphorbia heterophylla’s aerial parts. Al-Azhar J. Pharm. Sci. 2022, 66, 42–55. [Google Scholar] [CrossRef]
- El-Sohaimy, S.; Hamad, G.; Mohamed, S.; Amar, M.; Al-Hindi, R. Biochemical and functional properties of Moringa oleifera leaves and their potential as a functional food. Glob. Adv. Res. J. Agric. Sci. 2015, 4, 188–199. [Google Scholar]
- Izuegbuna, O. Leukemia chemoprevention and therapeutic potentials: Selected medicinal plants with anti-leukemic activities. Nutr. Cancer 2022, 74, 437–449. [Google Scholar] [CrossRef]
- Muema, F.W.; Nanjala, C.; Oulo, M.A.; Wangchuk, P. Phytochemical content and antidiabetic properties of most commonly used antidiabetic medicinal plants of Kenya. Molecules 2023, 28, 7202. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.I.; Sheikh, W.M.; Rather, M.A.; Venkatesalu, V.; Muzamil Bashir, S.; Nabi, S.U. Medicinal plants: Treasure for antiviral drug discovery. Phytother. Res. 2021, 35, 3447–3483. [Google Scholar] [CrossRef]
- Qari, S.H.; Abdulmajeed, F.; Al Refaei, W.F.; Alaa, Q. Exploration of the Medicinal Flora of the Aljumum Region in Saudi Arabia. Appl. Sci. 2021, 11, 7620. [Google Scholar] [CrossRef]
- Abdelwahab, M.F.; Sangi, S.; Arafat, H.H.; Ragab, E.A. New phytochemical constituent and bioactivities of Horwoodia dicksoniae and Rumex cyprius. Pharmacogn. Mag. 2016, 12, 165. [Google Scholar] [CrossRef]
- Francis, A.; Lujan-Toro, B.E.; Warwick, S.I.; Macklin, J.A.; Martin, S.L. Update on the Brassicaceae species checklist. Biodivers. Data. J. 2021, 9, e58773. [Google Scholar] [CrossRef]
- Banan, S.A.; Al-Watban, A.A.; Doaigey, A.R.; Alsahli, A.A. Anatomical adaptations in species of Poaceae growing in Al-Ha’ir region of Riyadh, Saudi Arabia. Afr. J. Plant. Sci. 2019, 13, 201–208. [Google Scholar]
- Khalil, A.M.A.; Saleh, A.M.; Abo-El-Souad, S.M.S.; Mohamed, M.S.M. Plants from a semi-arid environment as a source of phytochemicals against Fusarium crown and foot rot in zucchini. AMB. Expr. 2023, 13, 6. [Google Scholar] [CrossRef]
- Fawzy, G.A.; Al-Taweel, A.M.; Abdel Baky, N.A.; Marzouk, M.S. Cytotoxic and renoprotective flavonoid glycosides from Horwoodia dicksoniae. AJPP 2012, 6, 1166–1175. [Google Scholar]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Yadav, S.; Pathak, P.; Verma, A.; Yadav, J.P. Harnessing Luteolin’s therapeutic potential in human disorders: Medicinal significance, biological, clinical properties and analytical aspects. Pharmacol. Res. Mod. Chin. Med. 2024, 10, 100401. [Google Scholar] [CrossRef]
- Yoo, H.S.; Won, S.B.; Kwon, Y.H. Luteolin induces apoptosis and autophagy in HCT116 colon cancer cells via p53-dependent pathway. Nutr. Cancer 2022, 74, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Ballash, G.A.; Parker, E.M.; Mollenkopf, D.F.; Wittum, T.E. The One Health dissemination of antimicrobial resistance occurs in both natural and clinical environments. J. Am. Vet. Med. Assoc. 2024, 262, 451–458. [Google Scholar] [CrossRef]
- Bertagnolio, S.; Dobreva, Z.; Centner, C.M.; Olaru, I.D.; Donà, D.; Burzo, S.; Huttner, B.D.; Chaillon, A.; Gebreselassie, N.; Wi, T.; et al. WHO global research priorities for antimicrobial resistance in human health. Lancet Microbe 2024, 5, 100902. [Google Scholar] [CrossRef]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef]
- Zouine, N.; El Ghachtouli, N.; El Abed, S.; Ibnsouda Koraichi, S. A comprehensive review on medicinal plant extracts as antibacterial agents: Factors, mechanism insights and future prospects. Sci. Afr. 2024, 26, e02395. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Saini, N.; Gahlawat, S.K.; Lather, V. Flavonoids: A nutraceutical and its role as anti-inflammatory and anticancer agent. In Plant Biotechnology: Recent Advancements and Developments; Gahlawat, S., Salar, R., Siwach, P., Duhan, J., Kumar, S., Kaur, P., Eds.; Springer: Singapore, 2017. [Google Scholar]
- Singh, S.; Gupta, P.; Meena, A.; Luqman, S. Acacetin, a flavone with diverse therapeutic potential in cancer, inflammation, infections and other metabolic disorders. Food Chem. Toxicol. 2020, 145, 111708. [Google Scholar] [CrossRef]
- Dbeibia, A.; Taheur, F.B.; Altammar, K.A.; Haddaji, N.; Mahdhi, A.; Amri, Z.; Mzoughi, R.; Jabeur, C. Control of Staphylococcus aureus methicillin resistant isolated from auricular infections using aqueous and methanolic extracts of Ephedra alata. Saudi J. Biol. Sci. 2022, 29, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Van Heijenoort, J. Formation of the glycan chains in the synthesis of bacterial peptidoglycan. Glycobiology 2001, 11, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Farkaš, V. Structure and biosynthesis of fungal cell walls Methodological approaches. Folia. Microbiol. 2003, 48, 469–478. [Google Scholar] [CrossRef]
- Abu-Shanab, B.; Adwan, G.; Abu Safiya, D.; Adwan, K.; Abu-Shanab, M. Antibacterial activity of Rhus coriaria extracts growing in Palestine. IUG Nat. Sci. Ser. 2005, 13, 147–153. [Google Scholar]
- Darah, I.; Lim, S.H.; Ninthianantham, K. Effects of methanolic extract of Wedelia chinensis Osbeck (Asteraceae) leaves against pathogenic bacteria with emphasize on Bacillus cereus. Ind. J. Pharm. Sci. 2013, 75, 533–539. [Google Scholar]
- Sahgal, G.; Ramanathan, S.; Sasidharan, S.; Mordi, M.N.; Ismail, S.; Mansor, S.M. In vitro and in vivo anticandidal activity of Swietenia mahogany methanolic seed extract. Trop. Biomed. 2011, 28, 132–137. [Google Scholar]
- Sohaib, M.; Al-Barakah, F.N.; Migdadi, H.M.; Husain, F.M. Comparative study among Avicennia marina, Phragmites australis, and Moringa oleifera based ethanolic-extracts for their antimicrobial, antioxidant, and cytotoxic activities. Saudi J. Biol. Sci. 2022, 29, 111–122. [Google Scholar] [CrossRef]
- Dzotam, J.K.; Touani, F.K.; Kuete, V. Antibacterial and antibiotic—Modifying activities of three food plants (Xanthosomamafaffa. Lam., Moringa oleifera (L.). Schott and Passiflora eduilis Sims) against multidrug resistant (MDR) Gram–negative bacteria. BMC Complement. Altern. Med. 2016, 16, 9. [Google Scholar]
- Bukar, A.M.; Kyari, M.Z.; Gwaski, P.A.; Gudusu, M.; Kuburi, F.S.; Abadam, Y.I. Evaluation of phytochemical and potential antibacterial activity of Ziziphus spina-christi against some medically important pathogenic bacteria. J. Pharmacogen. Phytochem. 2015, 3, 98–101. [Google Scholar]
- Shimada, T. Salivary proteins as a defense against dietary tannins. J. Chem. Ecol. 2006, 32, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- El Bouchti, M.; Bourhia, M.; Alotaibi, A.; Aghmih, K.; Majid, S.; Ullah, R.; Salamatullah, A.M.; El Achaby, M.; Oumam, M.; Hannache, H.; et al. Stipa tenacissima L.: A new promising source of bioactive compounds with antioxidant and anticancer potentials. Life 2021, 11, 757. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.S.; Hossain, M.A.; Said, S.A. Isolation and characterization of antimicrobial compound from the stem-bark of the traditionally used medicinal plant Adenium obesum. J. Tradit. Complement. Med. 2017, 7, 296–300. [Google Scholar] [CrossRef]
- Mehmood, F.; Hassan, F.; Sarfraz, R.; Khadim, Z.; Alamer, K.H.; Attia, H.; Saleh, M.A.; Al-Robai, S.A.; Zaman, Q.U.; Iftikhar, Z. Phytochemical screening, antibacterial, antioxidant, and cytotoxic activities of Geranium pusillum leaves. Microsc. Res. Tech. 2024, 87, 2171–2185. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tian, L. Diverse phytochemicals and Bioactivities in the ancient fruit and modern functional food pomegranate. Molecules 2017, 22, 1606. [Google Scholar] [CrossRef]
- Kumar, G.P.; Singh, S.B. Antibacterial and antioxidant activities of ethanol extracts from trans Himalayan medicinal plants. EJAS 2011, 3, 53–57. [Google Scholar] [CrossRef]
- Sun, M.Y.; Bhaskar, S.M.M. When two maladies meet: Disease burden and pathophysiology of stroke in cancer. Int. J. Mol. Sci. 2022, 23, 15769. [Google Scholar] [CrossRef]
- Yuan, M.; Zhang, G.; Bai, W.; Han, X.; Li, C.; Bian, S. The role of bioactive compounds in natural products extracted from plants in cancer treatment and their mechanisms related to anticancer effects. Oxidative Med. Cell. Longev. 2022, 2022, 1429869. [Google Scholar] [CrossRef]
- Khan, M.; Khan, M.; Adil, S.F.; Alkhathlan, H.Z. Screening of potential cytotoxic activities of some medicinal plants of Saudi Arabia. Saudi J. Biol. Sci. 2021, 29, 1801–1807. [Google Scholar] [CrossRef]
- Khan, S.U.; Ullah, F.; Mehmood, S.; Fahad, S.; Ahmad, R.A.; Althobaiti, F.; Dessoky, E.S.; Saud, S.; Danish, S.; Datta, R. Antimicrobial, antioxidant and cytotoxic properties of Chenopodium glaucum L. PLoS ONE 2022, 16, e0255502. [Google Scholar] [CrossRef]
- Lazzeri, L.; Malaguti, L.; Bagatta, M.; D’Avino, L. Characterization of the main glucosinolate content and fatty acid composition in no food Brassicaceae seeds. Acta Hortic. 2013, 1005, 331–338. [Google Scholar] [CrossRef]
- Das, U.N. Beneficial effect(s) of n-3 fatty acids in cardiovascular diseases: But, why and how? Prostaglandins Leukot. Essent. Fatty Acids 2000, 63, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. What is the desirable ratio of saturated, polyunsaturated, and monounsaturated fatty acids in the diet? Am. J. Clin. Nutr. 1997, 66, 988S–990S. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial fatty acids: An update of possible mechanisms of action and implications in the development of the next-generation of antibacterial agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef]
- Sehim, A.E.; Amin, B.H.; Yosri, M.; Salama, H.M.; Alkhalifah, D.H.; Alwaili, M.A.; Abd Elghaffar, R.Y. GC-MS analysis, antibacterial, and anticancer activities of Hibiscus sabdariffa L. methanolic extract: In vitro and in silico studies. Microorganisms 2023, 11, 1601. [Google Scholar] [CrossRef]
- Shaaban, M.T.; Ghaly, M.F.; Fahmi, S.M. Antibacterial activities of hexadecanoic acid methyl ester and green–synthesized silver nanoparticles against multidrug–resistant bacteria. J. Basic Microbiol. 2021, 61, 557–568. [Google Scholar] [CrossRef]
- Pakki, E.; Rewa, M.; Irma, N. The Effectiveness of Isopropyl Myristate as Enhancing Agent in the Antioxidant Cream of Kasumba Turate Seed (Carthamus tinctorius L.). J. Pharm. Med. Sci. 2020, 4, 44–50. [Google Scholar]
- Ringertz, S.; Ringertz, O. Antimicrobial effect of isopropil myristate when used as solvent in sterility testing. Pharm. Acta Helv. 1982, 57, 193–195. [Google Scholar]
- Huang, L.; Zhu, X.; Zhou, S.; Cheng, Z.; Shi, K.; Zhang, C.; Shao, H. Phthalic acid esters: Natural sources and biological activities. Toxins 2021, 13, 495. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef]
- Farooqi, S.S.; Naveed, S.; Qamar, F.; Sana, A.; Farooqi, S.H.; Sabir, N.; Mansoor, A.; Sadia, H. Phytochemical analysis, GC-MS characterization and antioxidant activity of Hordeum vulgare seed extracts. Heliyon 2024, 10, e27297. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Gerhart-Hines, Z.; Dominy, J.E.; Lee, Y.; Kim, S.; Tabata, M.; Xiang, Y.K.; Puigserver, P. Oleic acid stimulates complete oxidation of fatty acids through protein kinase A-dependent activation of SIRT1- PGC1α complex. J. Biol. Chem. 2013, 288, 7117–7126. [Google Scholar] [CrossRef] [PubMed]
- Wilsy, J.I.; Beschi, D.A.; Appavoo, M.R.; Wilsy, J.I. GC-MS analysis, collected from Kavalkinaru area, Tirunelveli District, Tamil Nadu, India. Eur. J. Mol. Clin. 2021, 7, 4287–4292. [Google Scholar]
- Zai-Chang, Y.; Bo-Chu, W.; Xiao-Sheng, Y.; Qiang, W. Chemical composition of the volatile oil from Cynanchum stauntonii and its activities of anti-influenza virus. Colloids Surf. B Biointerfaces 2005, 43, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Haq, N.; Muhammad, A.S.; Saima, M. Phytochemical composition and antioxidant potential of brassica. Brassica Germplasm. Charact. Breed. Util. 2018, 1, 7–26. [Google Scholar]
- Mattosinhos, P.D.S.; Sarandy, M.M.; Novaes, R.D.; Esposito, D.; Gonçalves, R.V. Anti-inflammatory, antioxidant, and skin regenerative potential of secondary metabolites from plants of the Brassicaceae family: A systematic review of in vitro and in vivo preclinical evidence (Biological activities brassicaceae skin diseases). Antioxidants 2022, 11, 1346. [Google Scholar] [CrossRef]
- Ecevit, K.; Barros, A.A.; Silva, J.M.; Reis, R.L. Preventing microbial infections with natural phenolic compounds. Future Pharmacol. 2022, 2, 460–498. [Google Scholar] [CrossRef]
- Patra, A.K. An overview of antimicrobial properties of different classes of phytochemicals. In Dietary Phytochemicals and Microbes; Springer: Dordrecht, The Netherlands, 2012; pp. 1–32. [Google Scholar]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Z.; Yu, Y.; Qian, C.; Lin, Y.; Jin, S.; Wu, L.; Li, S. Hesperetin promotes diabetic wound healing by inhibiting ferroptosis through the activation of SIRT3. Phytother Res. 2024, 38, 1478–1493. [Google Scholar] [CrossRef]
- De Lellis, L.; Cimini, A.; Veschi, S.; Benedetti, E.; Amoroso, R.; Cama, A.; Ammazzalorso, A. The anticancer potential of peroxisome proliferator-activated receptor antagonists. Chem. Med. Chem. 2018, 13, 209–219. [Google Scholar] [CrossRef]
- Chagas, M.D.S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and flavones as potential anti-inflammatory, antioxidant, and antibacterial compounds. Oxid. Med Cell. Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharifi-Rad, J.; Cappellini, F.; Reiner, Ž.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; El-Shazly, M.; Fahmy, N.M.; et al. The therapeutic potential of anthocyanins: Current approaches based on their molecular mechanism of action. Front. Pharmacol. 2020, 11, 1300. [Google Scholar] [CrossRef] [PubMed]
- Guaita-Esteruelas, S.; Gumà, J.; Masana, L.; Borràs, J. The peritumoural adipose tissue microenvironment and cancer. The roles of fatty acid binding protein 4 and fatty acid binding protein 5. Mol. Cell. Endocrinol. 2018, 462, 107–118. [Google Scholar] [CrossRef]
- Qattan, M.Y.; Khan, M.I.; Alharbi, S.H.; Verma, A.K.; Al-Saeed, F.A.; Abduallah, A.M.; Al Areefy, A.A. Therapeutic importance of kaempferol in the treatment of cancer through the modulation of cell signalling pathways. Molecules 2022, 27, 8864. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial properties, sources, clinical, and traditional applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, F.D.; Günal-Köroğlu, D.; Saricaoglu, B.; Ozkan, G.; Capanoglu, E.; Calina, D.; Sharifi-Rad, J. Anticancer potential of hydroxycinnamic acids: Mechanisms, bioavailability, and therapeutic applications. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 398, 469–495. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva-Rosario, A.C.R.; Da-Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Scott, A.C. Laboratory control of antimicrobial therapy. In Mackie & McCartney Practical Medical Microbiology; Churchill Livingstone: New York, NY, USA, 1989; Volume 13, pp. 161–181. [Google Scholar]
- Gardeli, C.; Papageorgiou, V.; Mallouchos, A.; Kibouris, T.; Komaitis, M. Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: Evaluation of antioxidant capacity of methanolic extracts. Food Chem. 2008, 107, 1120–1130. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M.; Mahmmoud, E.A.; Elaasser, M.M. Synthesis and anticancer activities of thiazoles, 1,3-thiazines, and thiazolidine using chitosan-Grafted-Poly (vinylpyridine) as basic catalyst. Heterocycles 2015, 91, 1227–1243. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Elaasser, M.M.; Abdel-Aziz, M.M.; El-Kassas, R.A. Antioxidant, antimicrobial, antiviral and antitumor activities of pyranone derivative obtained from Aspergillus candidus. J. Microbiol. Biotech. Res. 2011, 1, 5–17. [Google Scholar]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminf. 2012, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.; Blundell, T.L.; Ascher, D.B. Platinum: A database of computationally modelled mammalian transcription factor DNA-binding preferences. Nucleic Acids Res. 2015, 43, 84–92. [Google Scholar]
- Jiménez, J.; Doerr, S.; Martínez-Rosell, G.; Rose, A.S.; De-Fabritiis, G. DeepSite: Protein-binding site predictor using 3D-convolutional neural networks. Bioinformatics 2017, 33, 3036–3042. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Cabalu, J.; Malabanan, M.M.; Villarante, N.R.; Este, A.B.; Filomon, M.M.; Gutierrez, J.A. HDock: A Hadoop-based docking algorithm to tackle molecular docking at large scale. J. Mol. Model. 2020, 26, 1–14. [Google Scholar]
- Dassault Systèmes BIOVIA. Discovery Studio Visualizer; Dassault Systèmes BIOVIA: San Diego, CA, USA, 2020. [Google Scholar]

| Tested Microorganisms | H. dicksoniae | S. capensis | Standard Treatment | ||
|---|---|---|---|---|---|
| Aspergillus fumigatus | Fungi | NA | NA | 17.0 ± 0.1 | Amphotericin B |
| Candida albicans | 20.6 ± 1.5 | 16.3 ± 0.6 | 21.9 ± 0.1 | ||
| Staphylococcus aureus | Gram (+) Bacteria | 18.6 ± 0.6 | 17.8 ± 0.6 | 28.9 ± 0.1 | Ampicillin |
| Bacillus subtilis | 15.0 ± 0.6 | 13.0 ± 0.4 | 17.3 ± 0.1 | ||
| Escherichia coli | Gram (−) Bacteria | 22.4 ± 0.6 | 21.0 ± 0.3 | 25.3 ± 0.2 | Gentamicin |
| Proteus vulgaris | 8.0 ± 0.6 | NA | 17.3 ± 0.1 | ||
| Tested Microorganisms | Minimum Inhibitory Concentration (MIC) (μg/mL) | ||||
|---|---|---|---|---|---|
| H. dicksoniae | S. capensis | Standard Treatment | |||
| Candida albicans | Fungi | 1.95 | 31.25 | 0.98 | Amphotericin B |
| Staphylococcus aureus | Gram (+) Bacteria | 0.98 | 7.81 | 0.49 | Ampicillin |
| Bacillus subtilis | 7.81 | 12.81 | 0.49 | ||
| Escherichia coli | Gram (−) Bacteria | 1.95 | 5.60 | 0.49 | Gentamicin |
| Proteus vulgaris | 3.90 | NA | 0.49 | ||
| No. | Retention Time (min) | Compound | Area (%) | Formula | Molecular Weight |
|---|---|---|---|---|---|
| 1 | 37.75 | 2(3H)-furanone, 5-heptyldihydro | 0.70 | C11H20O2 | 184 |
| 2 | 38.64 | 1,2-benzenedicarboxylic acid, diethyl ester (diethyl phthalate) | 1.01 | C12H14O4 | 222 |
| 3 | 42.04 | 1-(4-isopropylphenyl)-2-methylpropyl acetate | 0.66 | C15H22O2 | 234 |
| 4 | 45.57 | Benzoic acid, phenylmethyl ester (benzyl benzoate) | 14.31 | C14H12O2 | 212 |
| 5 | 48.64 | Isopropyl myristate | 44.53 | C17H34O2 | 270 |
| 6 | 51.51 | Hexadecanoic acid, methyl ester | 1.00 | C17H34O2 | 270 |
| 7 | 53.04 | n-Hexadecanoic acid | 2.29 | C16H32O2 | 256 |
| 8 | 53.70 | Hexadecanoic acid, ethyl ester | 0.22 | C18H36O2 | 284 |
| 9 | 57.85 | Octadecanoic acid, methyl ester | 0.85 | C19H38O2 | 298 |
| 10 | 60.35 | Octadecanoic acid, ethyl ester | 0.14 | C20H40O2 | 312 |
| 11 | 63.33 | Glycidyl palmitate | 0.40 | C19H36O3 | 312 |
| 12 | 71.52 | Hexadecanoic acid, phenylmethyl ester | 0.32 | C23H38O2 | 346 |
| Target Proteins | Anticancer | Antioxidant | Antimicrobial | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ligands | CA2 | PPARA | PRKCE | FABP2 | FABP3 | FABP4 | C. albicans | B. subtilis | S. aureus | P. vulgaris | E. coli | ||||
| ERG2 | ERG3 | ERG5 | ERG11 | dacC | pbp | rpsA | rpsQ | ||||||||
| Benzoic acid, phenylmethyl ester | −6.0 | −6.8 | −7.1 | −8.2 | −7.0 | −6.8 | −8.3 | −7.0 | −7.9 | −7.2 | −6.1 | −6.6 | −5.0 | −4.9 | |
| 1-(4-isopropylphenyl)-2-methylpropyl acetate | −5.2 | −6.4 | −6.8 | −7.5 | −6.5 | −6.9 | −8.0 | −7.3 | −6.5 | −6.7 | −6.0 | −6.5 | −5.3 | −4.5 | |
| Hexadecanoic acid, phenylmethyl ester | −5.3 | −7.4 | −5.9 | −8.5 | −6.5 | −6.4 | −7.8 | −7.1 | −6.7 | −6.4 | −5.3 | −5.4 | −4.2 | −3.3 | |
| Dock Score (kcal/mol) of Ligand–Protein Interaction * | 2D Binding Interaction | 3D Binding Interaction |
|---|---|---|
| Hexadecanoic acid, phenylmethyl ester with PPARA (∆G = −7.4 kcal/mol) | 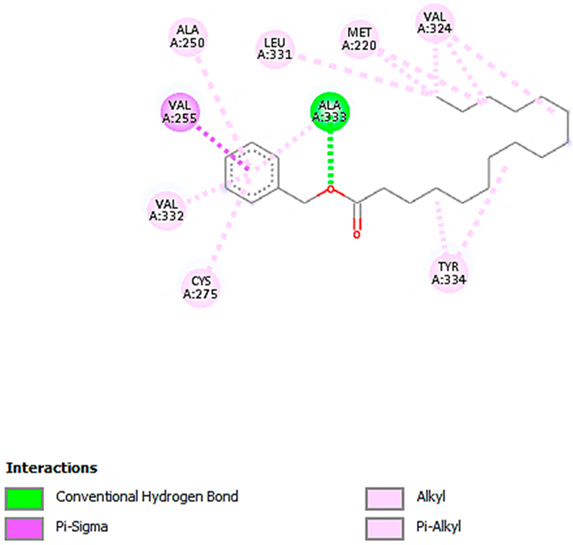 |  |
| Hexadecanoic acid, phenylmethyl ester with FABP2 (∆G = −8.5 kcal/mol) | 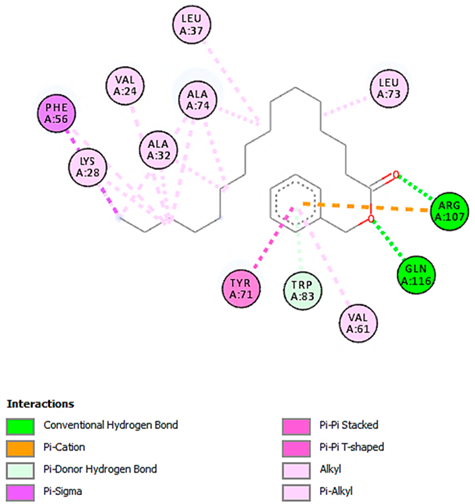 |  |
| Benzoic acid, phenylmethyl ester with ERG2-C. albicans (∆G = −8.3 kcal/mol) |  |  |
| Benzoic acid, phenylmethyl ester with pbp-S. aureus (∆G = −6.6 kcal/mol) | 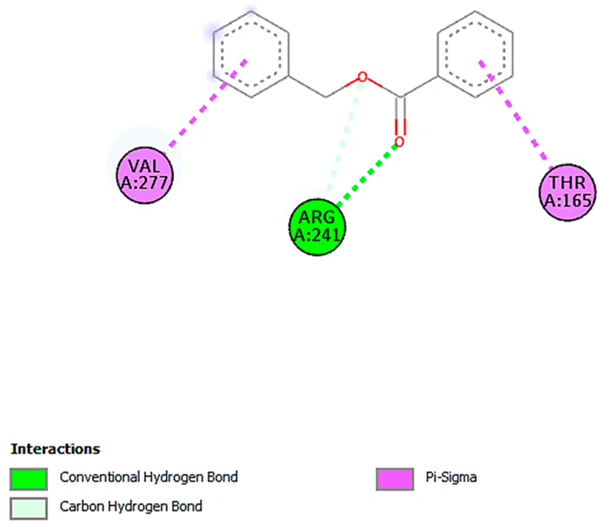 | 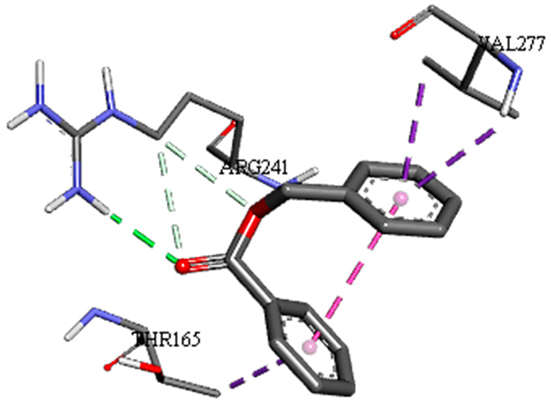 |
| 1-(4-isopropylphenyl)-2-methylpropyl acetate with rpsA-P. vulgaris (∆G = −5.3 kcal/mol) |  | 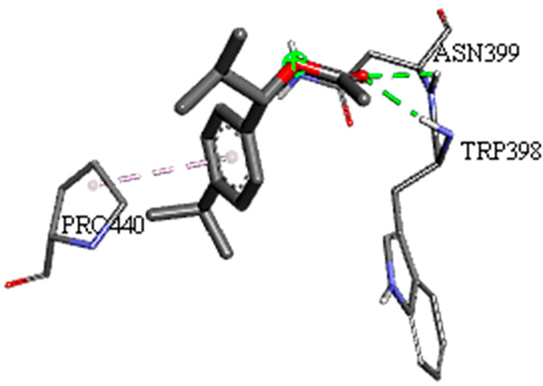 |
| Target Proteins | Anticancer | Antioxidant | Antimicrobial | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ligands | CA2 | PPARA | PRKCE | FABP2 | FABP3 | FABP4 | C. albicans | B. subtilis | S. aureus | P. vulgaris | E. coli | ||||
| ERG2 | ERG3 | ERG5 | ERG11 | dacC | pbp | rpsA | rpsQ | ||||||||
| Negative ion mode compounds (MS) | |||||||||||||||
| Acacetin | −6.3 | −7.0 | −8.0 | −8.2 | −7.8 | −8.1 | −9.2 | −8.7 | −8.1 | −7.9 | −7.9 | −8.1 | −6.5 | −5.9 | |
| Hesperetin | −6.5 | −7.5 | −8.6 | −8.5 | −8.3 | −8.3 | −8.9 | −8.7 | −7.7 | −7.8 | −7.8 | −8.3 | −6.7 | −6.0 | |
| Kaempferol-3-glucuronide | −6.8 | −8.3 | −8.4 | −9.3 | −9.2 | −9.5 | −7.0 | −6.5 | −8.8 | −8.7 | −8.7 | −7.8 | −6.6 | −5.6 | |
| Kaempferol-7-neohesperidoside | −8.0 | −7.8 | −9.8 | −9.1 | −6.5 | −9.4 | −8.0 | −6.6 | −9.4 | −9.1 | −9.1 | −9.0 | −7.3 | −6.4 | |
| Positive ion mode compounds (MS) | |||||||||||||||
| 3,5,7-trihydroxy-4′-methoxyflavone | −6.6 | −7.3 | −8.4 | −8.3 | −7.9 | −8.2 | −9.1 | −8.7 | −8.4 | −7.7 | −7.6 | −8.0 | −6.7 | −5.8 | |
| Apigenin 8-C-glucoside | −6.6 | −7.3 | −8.7 | −9.1 | −9.9 | −9.5 | −8.0 | −6.6 | −8.5 | −8.5 | −8.0 | −8.1 | −6.5 | −6.2 | |
| Cyanidin-3-O-rutinoside | −7.0 | −8.6 | −8.5 | −7.1 | −9.1 | −10.4 | −7.1 | −6.5 | −9.2 | −9.1 | −9.2 | −8.7 | −6.9 | −7.2 | |
| Luteolin-3′,7-di-O-glucoside | −7.2 | −9.2 | −8.9 | −7.0 | −6.9 | −9.2 | −7.3 | −6.9 | −9.7 | −9.6 | −8.7 | −9.6 | −7.9 | −6.6 | |
| Dock Score (kcal/mol) of Ligand–Protein Interaction * | 2D Binding Interaction | 3D Binding Interaction |
|---|---|---|
| Kaempferol-7-neohesperidoside with PRKCE (∆G = −9.8 kcal/mol) |  | 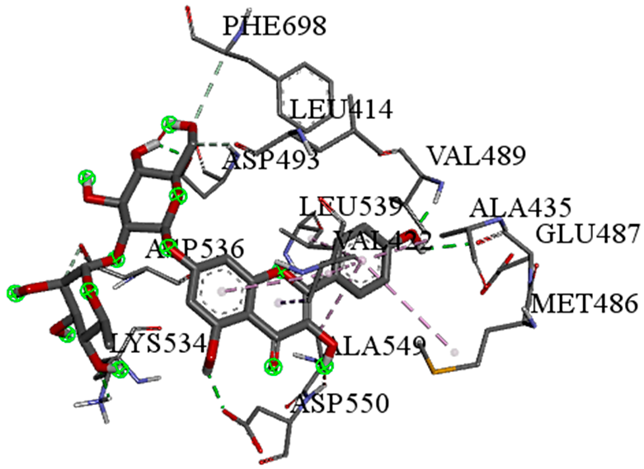 |
| Cyanidin-3-O-rutinoside with FABP4 (∆G = −10.4 kcal/mol) | 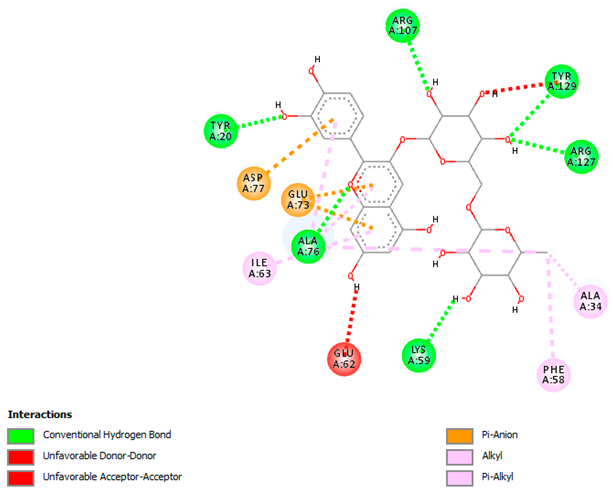 | 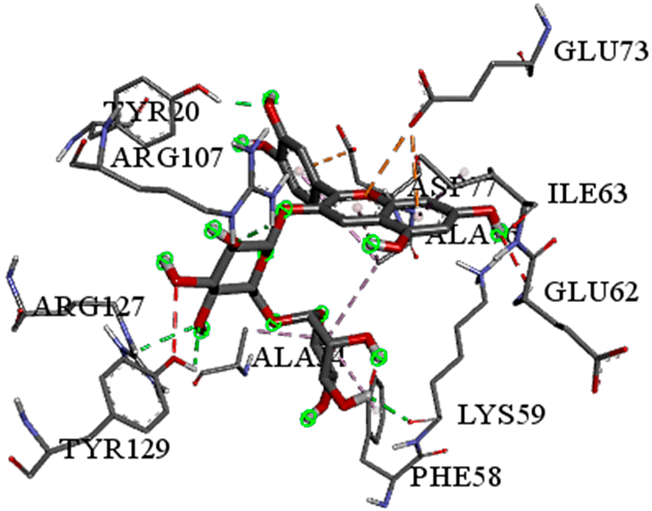 |
| Luteolin-3′,7-di-O-glucoside with ERG5-C. albicans (∆G = −9.7 kcal/mol) |  |  |
| Luteolin-3′,7-di-O-glucoside with pbp-S. aureus (∆G = −9.6 kcal/mol) | 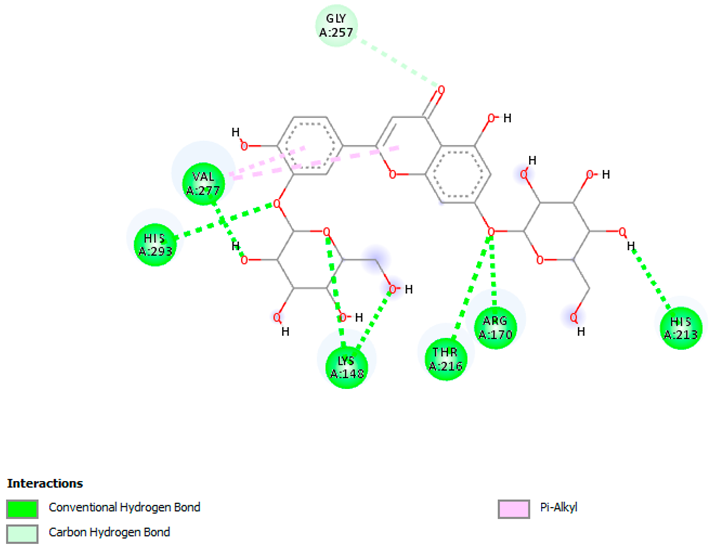 | 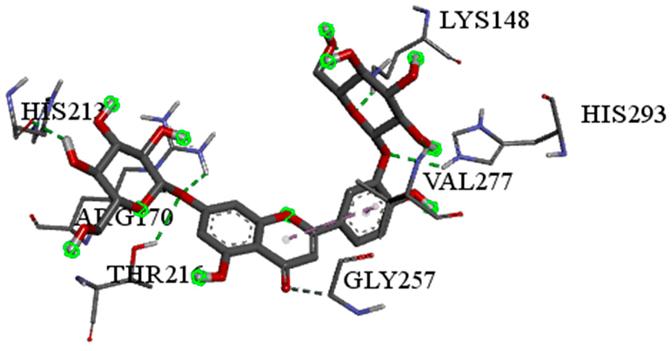 |
| Luteolin-3′,7-di-O-glucoside with rpsA-P. vulgaris (∆G = −7.9 kcal/mol) | 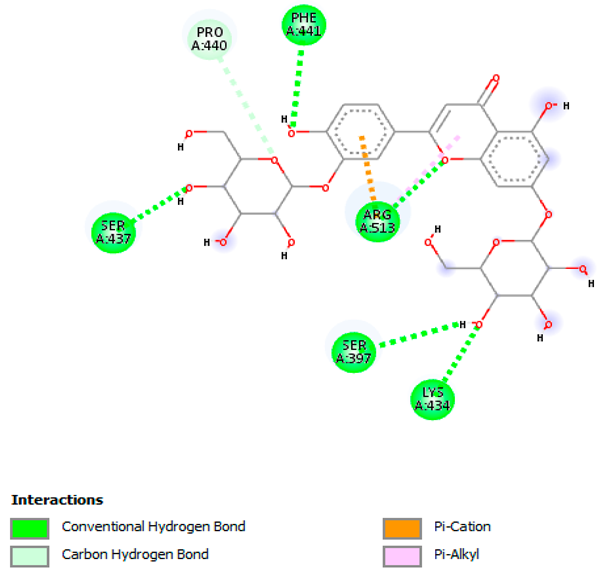 | 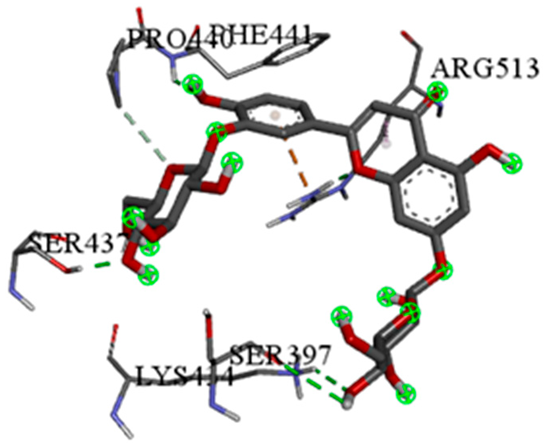 |
| Time (min) | Mobile Phase A (or B) | Mobile Phase C |
|---|---|---|
| 0 | 95 | 5 |
| 1 | 95 | 5 |
| 21 | 5 | 95 |
| 28 | 5 | 95 |
| 28.1 | 95 | 5 |
| 35 | 95 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altammar, K.A. Unveiling Therapeutic Powers of Indigenous Flora: Antimicrobial, Antioxidant, and Anticancer Properties of Horwoodia dicksoniae. Pharmaceuticals 2025, 18, 765. https://doi.org/10.3390/ph18050765
Altammar KA. Unveiling Therapeutic Powers of Indigenous Flora: Antimicrobial, Antioxidant, and Anticancer Properties of Horwoodia dicksoniae. Pharmaceuticals. 2025; 18(5):765. https://doi.org/10.3390/ph18050765
Chicago/Turabian StyleAltammar, Khadijah A. 2025. "Unveiling Therapeutic Powers of Indigenous Flora: Antimicrobial, Antioxidant, and Anticancer Properties of Horwoodia dicksoniae" Pharmaceuticals 18, no. 5: 765. https://doi.org/10.3390/ph18050765
APA StyleAltammar, K. A. (2025). Unveiling Therapeutic Powers of Indigenous Flora: Antimicrobial, Antioxidant, and Anticancer Properties of Horwoodia dicksoniae. Pharmaceuticals, 18(5), 765. https://doi.org/10.3390/ph18050765






