Protective Effect of Obeticholic Acid on Sepsis-Induced Liver Dysfunction via Regulating Bile Acid Homeostasis
Abstract
1. Introduction
2. Results
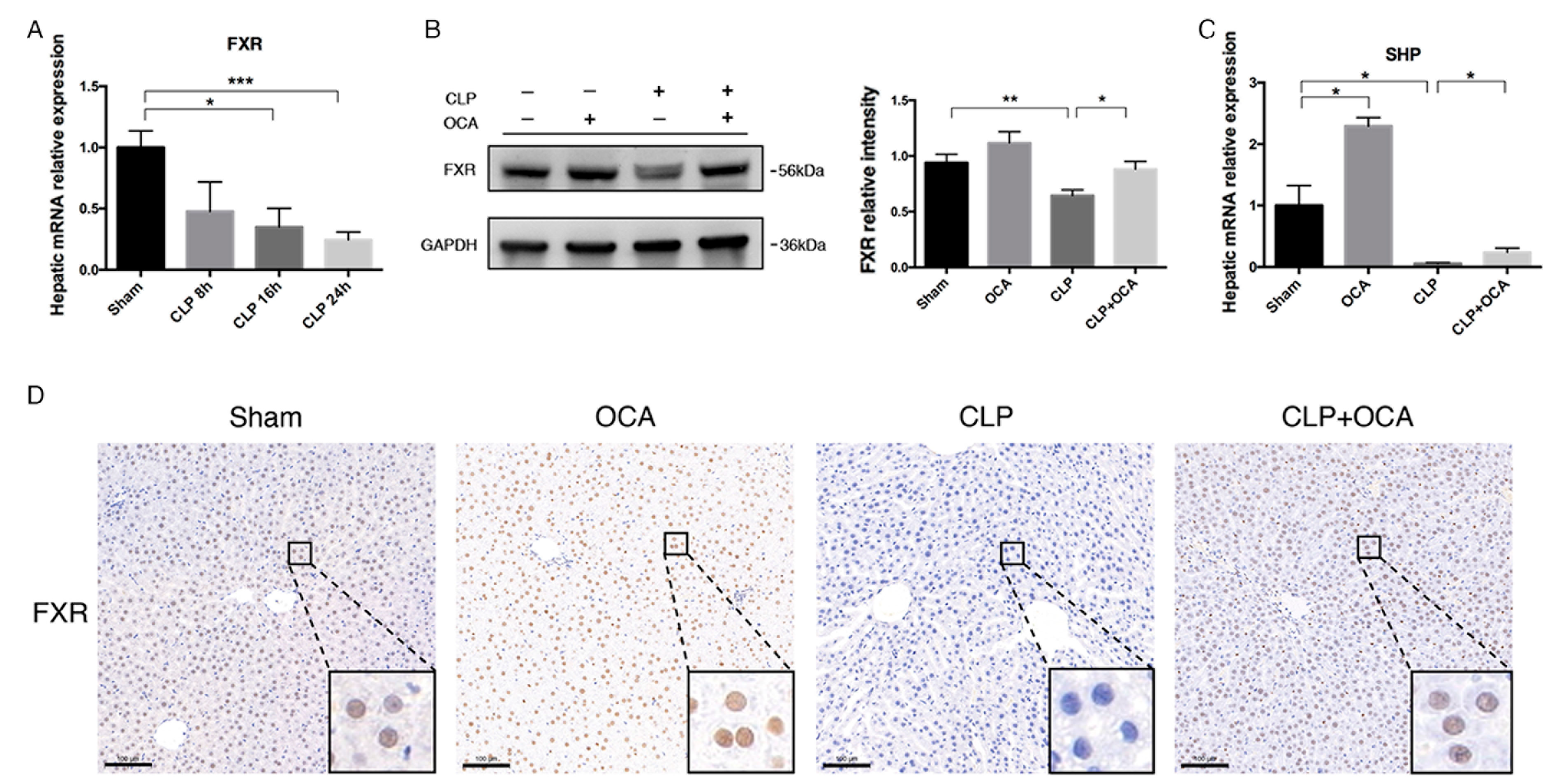
2.1. FXR Agonist OCA Upregulates Hepatic FXR in CLP Rats
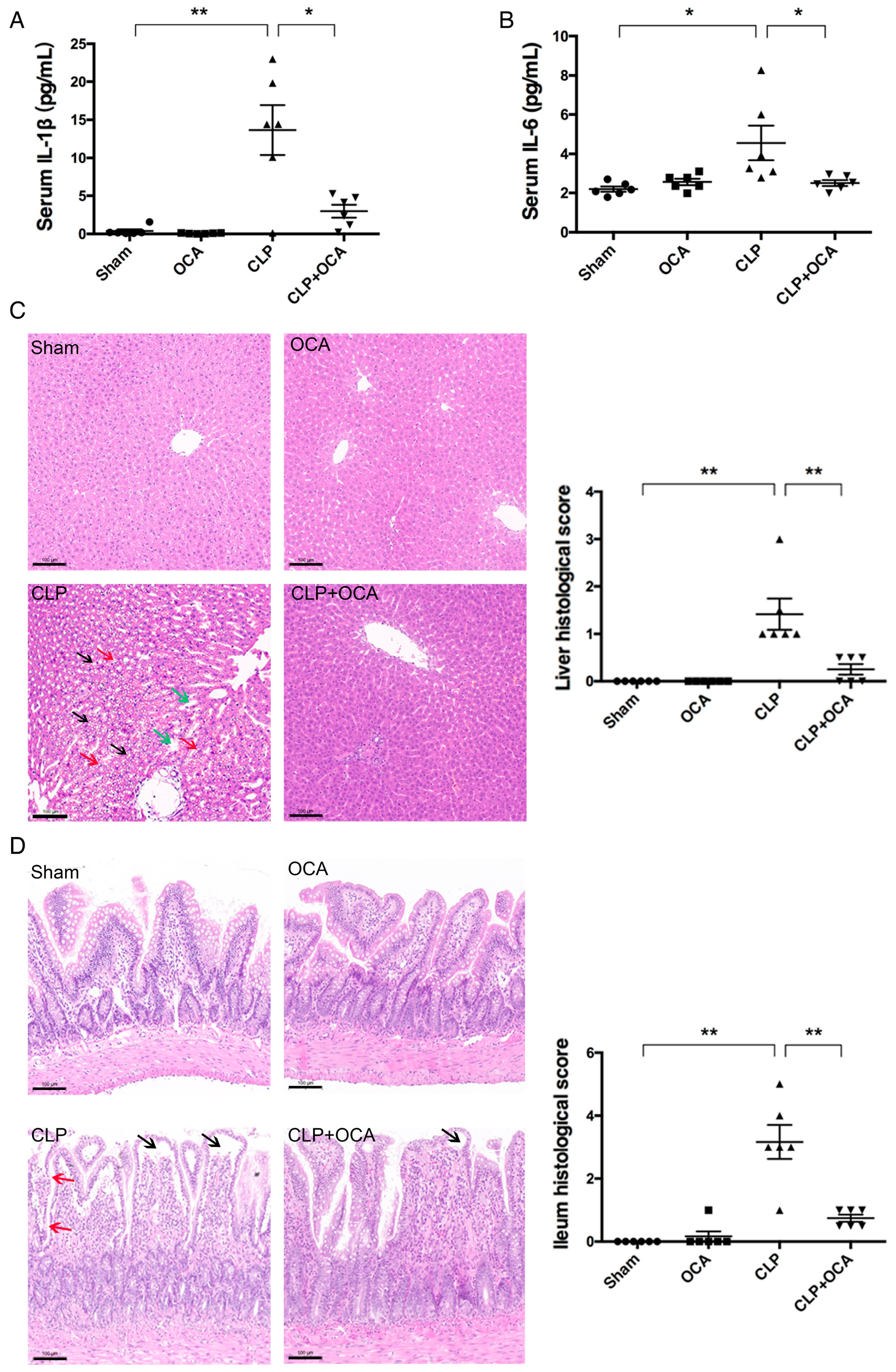
2.2. Hepatic FXR Activation Ameliorates Inflammation and Tissue Injury in CLP Rats
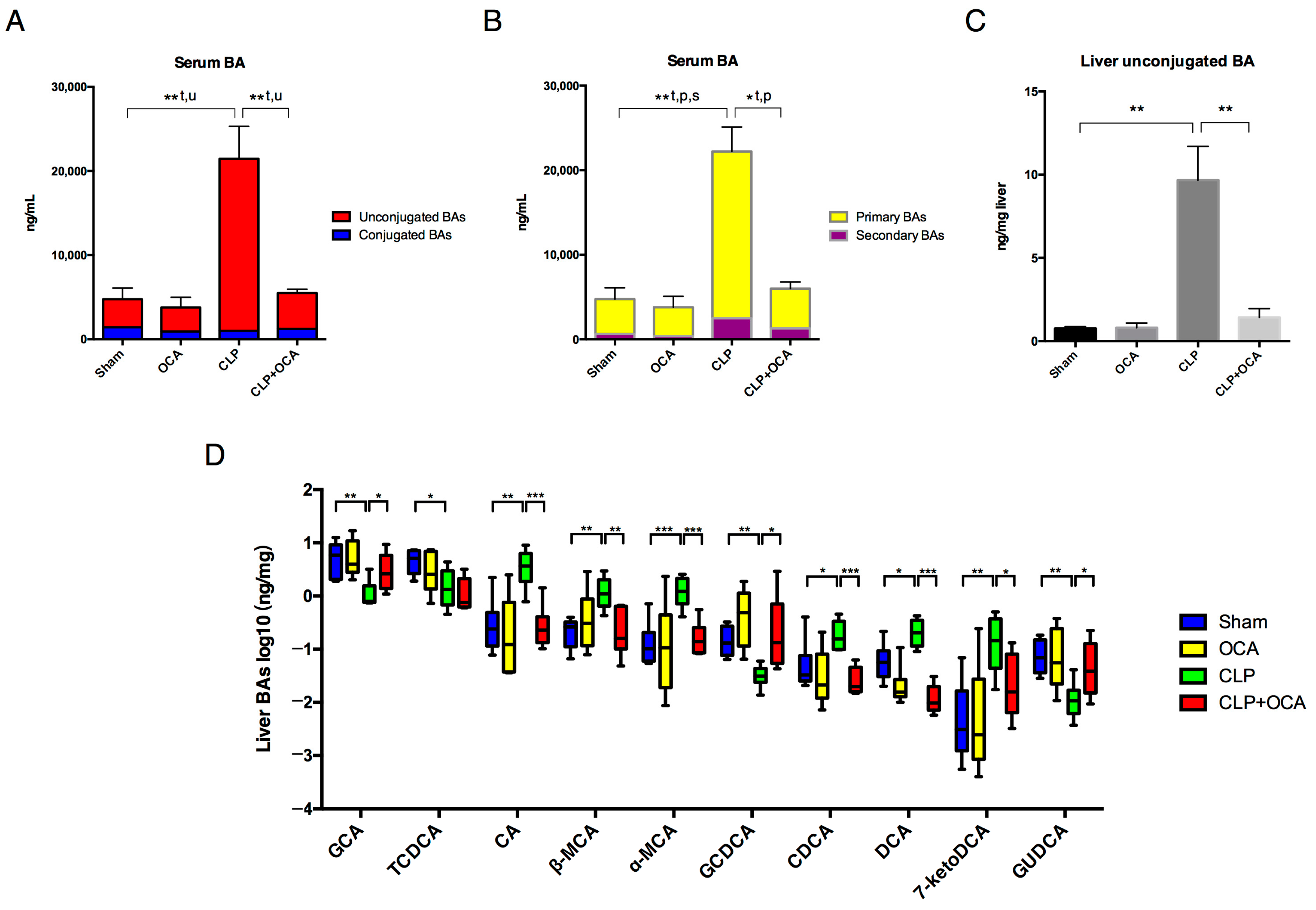
2.3. Hepatic FXR Activation Modulate Liver Function and Bile Acid Metabolism in CLP Rats
2.4. Hepatic FXR Activation Maintains BA Homeostasis in CLP Rats
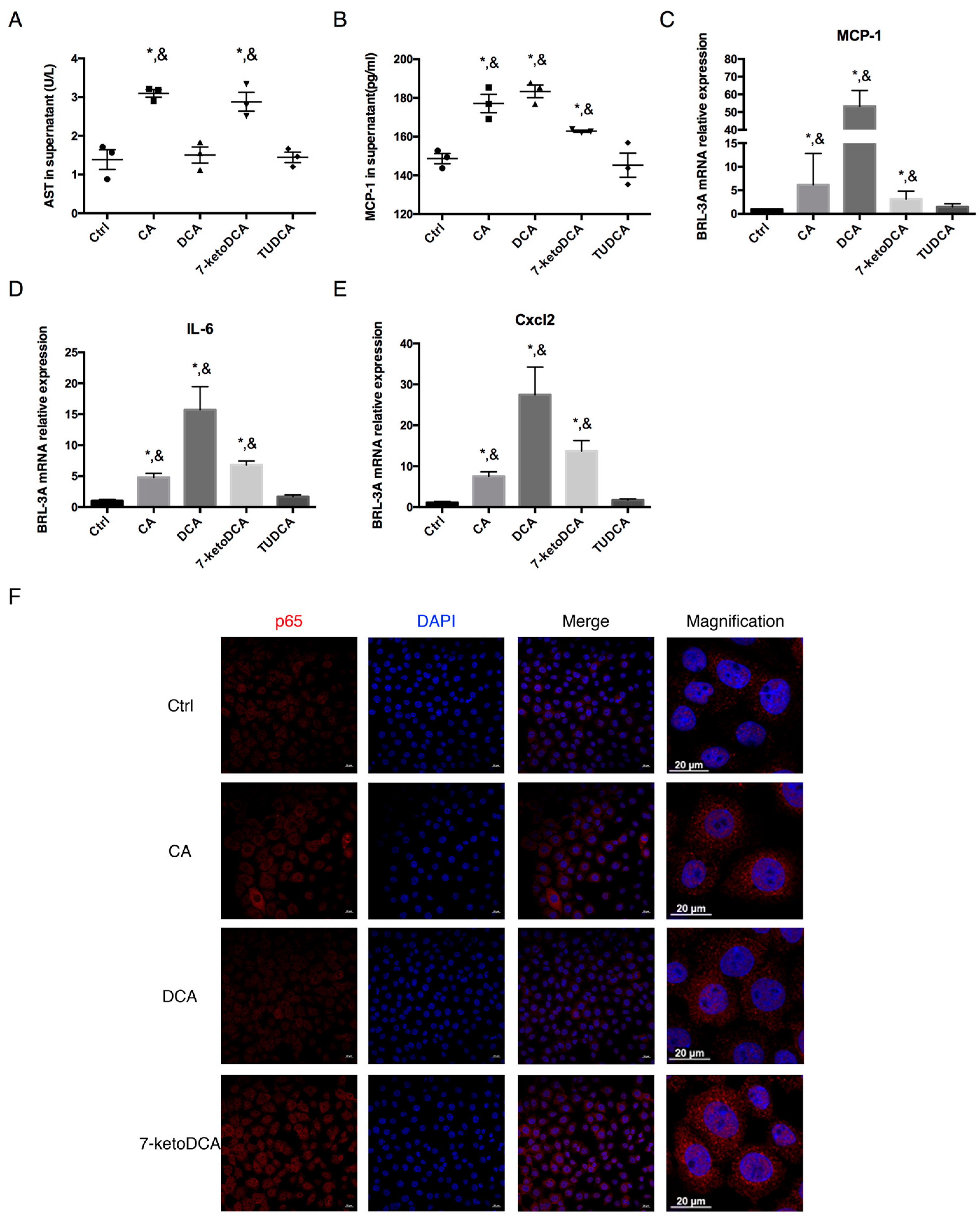
2.5. CA, DCA, and 7-ketoDCA Promote Inflammatory Phenotype in Rat Hepatocytes
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals and Treatments
4.3. Establishment of Sepsis Model by CLP Operation
4.4. Cell Culture
4.5. Histopathology, Immunohistochemical, and Immunofluorescence Staining
4.6. Western Blotting
4.7. Analysis of Biochemical Indicators
4.8. RNA Extraction and RT-qPCR
4.9. Enzyme-Linked Immunosorbent Assay (ELISA)
4.10. Measurement of Bile Acids
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Jenniskens, M.; Langouche, L.; Vanwijngaerden, Y.M.; Mesotten, D.; Van den Berghe, G. Cholestatic liver (dys)function during sepsis and other critical illnesses. Intensive Care Med. 2016, 42, 16–27. [Google Scholar] [CrossRef]
- Nesseler, N.; Launey, Y.; Aninat, C.; Morel, F.; Malledant, Y.; Seguin, P. Clinical review: The liver in sepsis. Crit. Care 2012, 16, 235. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, J.; Wang, X.; Ji, F.; Ronco, C.; Tian, J.; Yin, Y. Gut-liver crosstalk in sepsis-induced liver injury. Crit. Care 2020, 24, 614. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Bile Acids as Metabolic Regulators and Nutrient Sensors. Annu. Rev. Nutr. 2019, 39, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Maillette de Buy Wenniger, L.; Beuers, U. Bile salts and cholestasis. Dig. Liver Dis. 2010, 42, 409–418. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Garruti, G.; Lunardi Baccetto, R.; Molina-Molina, E.; Bonfrate, L.; Wang, D.Q.; Portincasa, P. Bile Acid Physiology. Ann. Hepatol. 2017, 16 (Suppl. 1), S4–S14. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Li, Z.J.; Gou, H.Z.; Song, X.J.; Zhang, L. The gut microbiota-bile acid axis: A potential therapeutic target for liver fibrosis. Front. Cell Infect. Microbiol. 2022, 12, 945368. [Google Scholar] [CrossRef]
- Fiorucci, S.; Distrutti, E. Chenodeoxycholic Acid: An Update on Its Therapeutic Applications. Handb. Exp. Pharmacol. 2019, 256, 265–282. [Google Scholar]
- Fuchs, C.D.; Trauner, M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 432–450. [Google Scholar] [CrossRef]
- Jansen, P.L.; Ghallab, A.; Vartak, N.; Reif, R.; Schaap, F.G.; Hampe, J.; Hengstler, J.G. The ascending pathophysiology of cholestatic liver disease. Hepatology 2017, 65, 722–738. [Google Scholar] [CrossRef]
- Hegade, V.S.; Speight, R.A.; Etherington, R.E.; Jones, D.E. Novel bile acid therapeutics for the treatment of chronic liver diseases. Therap Adv. Gastroenterol. 2016, 9, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Fan, J.; Zhou, H. Bile acid-mediated signaling in cholestatic liver diseases. Cell Biosci. 2023, 13, 77. [Google Scholar] [CrossRef]
- Hohenester, S.; Kanitz, V.; Kremer, A.E.; Paulusma, C.C.; Wimmer, R.; Kuehn, H.; Denk, G.; Horst, D.; Elferink, R.O.; Beuers, U. Glycochenodeoxycholate Promotes Liver Fibrosis in Mice with Hepatocellular Cholestasis. Cells 2020, 9, 281. [Google Scholar] [CrossRef]
- Allen, K.; Jaeschke, H.; Copple, B.L. Bile acids induce inflammatory genes in hepatocytes: A novel mechanism of inflammation during obstructive cholestasis. Am. J. Pathol. 2011, 178, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Recknagel, P.; Gonnert, F.A.; Westermann, M.; Lambeck, S.; Lupp, A.; Rudiger, A.; Dyson, A.; Carre, J.E.; Kortgen, A.; Krafft, C.; et al. Liver dysfunction and phosphatidylinositol-3-kinase signalling in early sepsis: Experimental studies in rodent models of peritonitis. PLoS Med. 2012, 9, e1001338. [Google Scholar] [CrossRef] [PubMed]
- Horvatits, T.; Drolz, A.; Trauner, M.; Fuhrmann, V. Liver Injury and Failure in Critical Illness. Hepatology 2019, 70, 2204–2215. [Google Scholar] [CrossRef]
- Jenniskens, M.; Langouche, L.; Van den Berghe, G. Cholestatic Alterations in the Critically Ill: Some New Light on an Old Problem. Chest 2018, 153, 733–743. [Google Scholar] [CrossRef]
- Horvatits, T.; Drolz, A.; Rutter, K.; Roedl, K.; Langouche, L.; Van den Berghe, G.; Fauler, G.; Meyer, B.; Hulsmann, M.; Heinz, G.; et al. Circulating bile acids predict outcome in critically ill patients. Ann. Intensive Care 2017, 7, 48. [Google Scholar] [CrossRef]
- Kosyakovsky, L.B.; Somerset, E.; Rogers, A.J.; Sklar, M.; Mayers, J.R.; Toma, A.; Szekely, Y.; Soussi, S.; Wang, B.; Fan, C.S.; et al. Machine learning approaches to the human metabolome in sepsis identify metabolic links with survival. Intensive Care Med. Exp. 2022, 10, 24. [Google Scholar] [CrossRef]
- Long, X.; Mu, S.; Zhang, J.; Xiang, H.; Wei, W.; Sun, J.; Kuang, Z.; Yang, Y.; Chen, Y.; Zhao, H.; et al. Global Signatures of the Microbiome and Metabolome during Hospitalization of Septic Patients. Shock 2023, 59, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S.; Distrutti, E. The Pharmacology of Bile Acids and Their Receptors. Handb. Exp. Pharmacol. 2019, 256, 3–18. [Google Scholar] [PubMed]
- Jiang, L.; Zhang, H.; Xiao, D.; Wei, H.; Chen, Y. Farnesoid X receptor (FXR): Structures and ligands. Comput. Struct. Biotechnol. J. 2021, 19, 2148–2159. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Yang, J.; Liu, L.; Yu, H.; Gong, X.; Liu, D. The regulation of tissue-specific farnesoid X receptor on genes and diseases involved in bile acid homeostasis. Biomed. Pharmacother. 2023, 168, 115606. [Google Scholar] [CrossRef]
- Chiang, J.Y. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [CrossRef]
- Han, C.Y. Update on FXR Biology: Promising Therapeutic Target? Int. J. Mol. Sci. 2018, 19, 2069. [Google Scholar] [CrossRef]
- Lin, S.; Wang, S.; Wang, P.; Tang, C.; Wang, Z.; Chen, L.; Luo, G.; Chen, H.; Liu, Y.; Feng, B.; et al. Bile acids and their receptors in regulation of gut health and diseases. Prog. Lipid Res. 2023, 89, 101210. [Google Scholar] [CrossRef]
- Liu, Y.J.; Mao, E.Q.; Ouyang, B.; Chen, J.; Tang, Y.Q.; Huang, S.W.; Guan, X.D. Effect of biliary tract external drainage on cytokine expression and histomorphology of intestine, liver, and lung in rats with hemorrhagic shock. Crit. Care Med. 2009, 37, 2800–2806. [Google Scholar] [CrossRef]
- Pellicciari, R.; Fiorucci, S.; Camaioni, E.; Clerici, C.; Costantino, G.; Maloney, P.R.; Morelli, A.; Parks, D.J.; Willson, T.M. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J. Med. Chem. 2002, 45, 3569–3572. [Google Scholar] [CrossRef]
- Fiorucci, S.; Di Giorgio, C.; Distrutti, E. Obeticholic Acid: An Update of Its Pharmacological Activities in Liver Disorders. Handb. Exp. Pharmacol. 2019, 256, 283–295. [Google Scholar]
- Kramer, L.; Jordan, B.; Druml, W.; Bauer, P.; Metnitz, P.G.; Austrian Epidemiologic Study on Intensive Care, A.S.G. Incidence and prognosis of early hepatic dysfunction in critically ill patients—A prospective multicenter study. Crit. Care Med. 2007, 35, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, T.A.; Zalzala, M.H. Impact of Nuclear Receptors on the Control of Bile Acid Metabolism and Synthesis: A Comprehensive Review. Curr. Pharmacol. Rep. 2025, 11, 25. [Google Scholar] [CrossRef]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef]
- Shin, D.J.; Wang, L. Bile Acid-Activated Receptors: A Review on FXR and Other Nuclear Receptors. Handb. Exp. Pharmacol. 2019, 256, 51–72. [Google Scholar]
- Anderson, K.M.; Gayer, C.P. The Pathophysiology of Farnesoid X Receptor (FXR) in the GI Tract: Inflammation, Barrier Function and Innate Immunity. Cells 2021, 10, 3206. [Google Scholar] [CrossRef] [PubMed]
- Gadaleta, R.M.; Oldenburg, B.; Willemsen, E.C.; Spit, M.; Murzilli, S.; Salvatore, L.; Klomp, L.W.; Siersema, P.D.; van Erpecum, K.J.; van Mil, S.W. Activation of bile salt nuclear receptor FXR is repressed by pro-inflammatory cytokines activating NF-kappaB signaling in the intestine. Biochim. Biophys. Acta 2011, 1812, 851–858. [Google Scholar] [CrossRef]
- Vanwijngaerden, Y.M.; Wauters, J.; Langouche, L.; Vander Perre, S.; Liddle, C.; Coulter, S.; Vanderborght, S.; Roskams, T.; Wilmer, A.; Van den Berghe, G.; et al. Critical illness evokes elevated circulating bile acids related to altered hepatic transporter and nuclear receptor expression. Hepatology 2011, 54, 1741–1752. [Google Scholar] [CrossRef]
- Hao, H.; Cao, L.; Jiang, C.; Che, Y.; Zhang, S.; Takahashi, S.; Wang, G.; Gonzalez, F.J. Farnesoid X Receptor Regulation of the NLRP3 Inflammasome Underlies Cholestasis-Associated Sepsis. Cell Metab. 2017, 25, 856–867.e5. [Google Scholar] [CrossRef]
- Yan, N.; Yan, T.; Xia, Y.; Hao, H.; Wang, G.; Gonzalez, F.J. The pathophysiological function of non-gastrointestinal farnesoid X receptor. Pharmacol. Ther. 2021, 226, 107867. [Google Scholar] [CrossRef]
- Xiong, X.; Ren, Y.; Cui, Y.; Li, R.; Wang, C.; Zhang, Y. Obeticholic acid protects mice against lipopolysaccharide-induced liver injury and inflammation. Biomed. Pharmacother. 2017, 96, 1292–1298. [Google Scholar] [CrossRef]
- Verbeke, L.; Mannaerts, I.; Schierwagen, R.; Govaere, O.; Klein, S.; Vander Elst, I.; Windmolders, P.; Farre, R.; Wenes, M.; Mazzone, M.; et al. FXR agonist obeticholic acid reduces hepatic inflammation and fibrosis in a rat model of toxic cirrhosis. Sci. Rep. 2016, 6, 33453. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.N.; Yu, H.T.; Li, Y.Q.; Zeng, Y.; Yang, S.; Yang, G.P.; Pei, Q.; Huang, J. Bioequivalence and Pharmacokinetic Profiles of Generic and Branded Obeticholic Acid in Healthy Chinese Subjects Under Fasting and Fed Conditions. Drug Des. Devel Ther. 2021, 15, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; LaCerte, C.; Edwards, J.; Poordad, F.; Lawitz, E.; Lee, L.; Karan, S.; Sawhney, S.; Erickson, M.; MacConell, L.; et al. Safety, pharmacokinetics and pharmacodynamics of obeticholic acid in subjects with fibrosis or cirrhosis from NASH. Liver Int. 2024, 44, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, D.; Arab, J.P.; Arrese, M. UDCA, NorUDCA, and TUDCA in Liver Diseases: A Review of Their Mechanisms of Action and Clinical Applications. Handb. Exp. Pharmacol. 2019, 256, 237–264. [Google Scholar]
- Denson, L.A.; Sturm, E.; Echevarria, W.; Zimmerman, T.L.; Makishima, M.; Mangelsdorf, D.J.; Karpen, S.J. The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology 2001, 121, 140–147. [Google Scholar] [CrossRef]
- Ghenu, M.I.; Dragos, D.; Manea, M.M.; Ionescu, D.; Negreanu, L. Pathophysiology of sepsis-induced cholestasis: A review. JGH Open 2022, 6, 378–387. [Google Scholar] [CrossRef]
- Leonhardt, J.; Dorresteijn, M.J.; Neugebauer, S.; Mihaylov, D.; Kunze, J.; Rubio, I.; Hohberger, F.S.; Leonhardt, S.; Kiehntopf, M.; Stahl, K.; et al. Immunosuppressive effects of circulating bile acids in human endotoxemia and septic shock: Patients with liver failure are at risk. Crit. Care 2023, 27, 372. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Bile Acid Metabolism in Liver Pathobiology. Gene Expr. 2018, 18, 71–87. [Google Scholar] [CrossRef]
- Cai, S.Y.; Ouyang, X.; Chen, Y.; Soroka, C.J.; Wang, J.; Mennone, A.; Wang, Y.; Mehal, W.Z.; Jain, D.; Boyer, J.L. Bile acids initiate cholestatic liver injury by triggering a hepatocyte-specific inflammatory response. JCI Insight 2017, 2, e90780. [Google Scholar] [CrossRef]
- Li, C.; Lan, M.; Lv, J.; Zhang, Y.; Gao, X.; Gao, X.; Dong, L.; Luo, G.; Zhang, H.; Sun, J. Screening of the Hepatotoxic Components in Fructus Gardeniae and Their Effects on Rat Liver BRL-3A Cells. Molecules 2019, 24, 3920. [Google Scholar] [CrossRef]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef]
- Biagioli, M.; Carino, A. Signaling from Intestine to the Host: How Bile Acids Regulate Intestinal and Liver Immunity. Handb. Exp. Pharmacol. 2019, 256, 95–108. [Google Scholar]
- Bauer, M. The liver-gut-axis: Initiator and responder to sepsis. Curr. Opin. Crit. Care 2022, 28, 216–220. [Google Scholar] [CrossRef]
- Shulpekova, Y.; Shirokova, E.; Zharkova, M.; Tkachenko, P.; Tikhonov, I.; Stepanov, A.; Sinitsyna, A.; Izotov, A.; Butkova, T.; Shulpekova, N.; et al. A Recent Ten-Year Perspective: Bile Acid Metabolism and Signaling. Molecules 2022, 27, 1983. [Google Scholar] [CrossRef] [PubMed]
- Morotomi, M.; Kawai, Y.; Mutai, M. Intestinal microflora and bile acids. In vitro cholic acid transformation by mixed fecal culture of rats. Microbiol. Immunol. 1979, 23, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, S.; Li, Y.; Zhao, M.; Kuang, J.; Liang, D.; Wang, J.; Wei, M.; Rajani, C.; Ma, X.; et al. Gut microbiota-bile acid crosstalk contributes to the rebound weight gain after calorie restriction in mice. Nat. Commun. 2022, 13, 2060. [Google Scholar] [CrossRef] [PubMed]
- Blesl, A.; Stadlbauer, V. The Gut-Liver Axis in Cholestatic Liver Diseases. Nutrients 2021, 13, 1018. [Google Scholar] [CrossRef]
- Urbani, G.; Rondini, E.; Distrutti, E.; Marchiano, S.; Biagioli, M.; Fiorucci, S. Phenotyping the Chemical Communications of the Intestinal Microbiota and the Host: Secondary Bile Acids as Postbiotics. Cells 2025, 14, 595. [Google Scholar] [CrossRef]
- Camilleri, M. Bile acid detergency: Permeability, inflammation, and effects of sulfation. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G480–G488. [Google Scholar] [CrossRef]
- Milivojac, T.; Grabez, M.; Krivokuca, A.; Malicevic, U.; Gajic Bojic, M.; Dukanovic, D.; Uletilovic, S.; Mandic-Kovacevic, N.; Cvjetkovic, T.; Barudzija, M.; et al. Ursodeoxycholic and chenodeoxycholic bile acids attenuate systemic and liver inflammation induced by lipopolysaccharide in rats. Mol. Cell Biochem. 2025, 480, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Palladini, G.; Cagna, M.; Di Pasqua, L.G.; Adorini, L.; Croce, A.C.; Perlini, S.; Ferrigno, A.; Berardo, C.; Vairetti, M. Obeticholic Acid Reduces Kidney Matrix Metalloproteinase Activation Following Partial Hepatic Ischemia/Reperfusion Injury in Rats. Pharmaceuticals 2022, 15, 524. [Google Scholar] [CrossRef] [PubMed]
- Rittirsch, D.; Huber-Lang, M.S.; Flierl, M.A.; Ward, P.A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 2009, 4, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Dejager, L.; Pinheiro, I.; Dejonckheere, E.; Libert, C. Cecal ligation and puncture: The gold standard model for polymicrobial sepsis? Trends Microbiol. 2011, 19, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.J.; McArdle, A.H.; Brown, R.; Scott, H.J.; Gurd, F.N. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch. Surg. 1970, 101, 478–483. [Google Scholar] [CrossRef]
- Lee, C.C.; Lee, R.P.; Subeq, Y.M.; Lee, C.J.; Chen, T.M.; Hsu, B.G. Fluvastatin attenuates severe hemorrhagic shock-induced organ damage in rats. Resuscitation 2009, 80, 372–378. [Google Scholar] [CrossRef]

| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| β-actin | GCTGTGCTATGTTGCCCTAGACTTC | GGAACCGCTCATTGCCGATAGTG |
| FXR | AGGATAGAGAGGCAGTGGAGAAGC | AGCGTGGTGATGGTTGAATGTCC |
| SHP | GGCACTATCCTCTTCAACCCAGATG | GGGCTCCAGGACTTCACACAATG |
| Bsep | TTTGTTGGAAGCAGTGGGTGTGG | TGGAACGGAGGAACTGAATGTTGAC |
| Ntcp | CTTACTGGCTACCTCCTCCCTGATG | TGCAGCTTGGATTGAGTTGGAAGAG |
| IL-6 | ACTTCCAGCCAGTTGCCTTCTTG | TGGTCTGTTGTGGGTGGTATCCTC |
| MCP-1 | CTCACCTGCTGCTACTCATTCACTG | CTTCTTTGGGACACCTGCTGCTG |
| CXCL2 | ATGCTGTACTGGTCCTGCTCCTC | GTCACCGTCAAGCTCTGGATGTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Ma, L.; An, Y.; Ge, Y.; Xu, D.; Mao, E. Protective Effect of Obeticholic Acid on Sepsis-Induced Liver Dysfunction via Regulating Bile Acid Homeostasis. Pharmaceuticals 2025, 18, 763. https://doi.org/10.3390/ph18050763
Wang J, Ma L, An Y, Ge Y, Xu D, Mao E. Protective Effect of Obeticholic Acid on Sepsis-Induced Liver Dysfunction via Regulating Bile Acid Homeostasis. Pharmaceuticals. 2025; 18(5):763. https://doi.org/10.3390/ph18050763
Chicago/Turabian StyleWang, Jiahui, Li Ma, Yuan An, Yan Ge, Dan Xu, and Enqiang Mao. 2025. "Protective Effect of Obeticholic Acid on Sepsis-Induced Liver Dysfunction via Regulating Bile Acid Homeostasis" Pharmaceuticals 18, no. 5: 763. https://doi.org/10.3390/ph18050763
APA StyleWang, J., Ma, L., An, Y., Ge, Y., Xu, D., & Mao, E. (2025). Protective Effect of Obeticholic Acid on Sepsis-Induced Liver Dysfunction via Regulating Bile Acid Homeostasis. Pharmaceuticals, 18(5), 763. https://doi.org/10.3390/ph18050763







