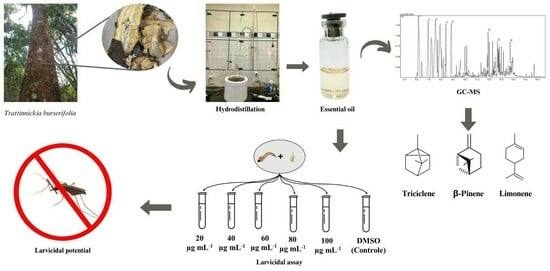
Larvicidal Potential of Trattinnickia Burserifolia Mart. Essential Oil in Controlling the Malaria Vector in the Amazon
Abstract
1. Introduction
2. Results
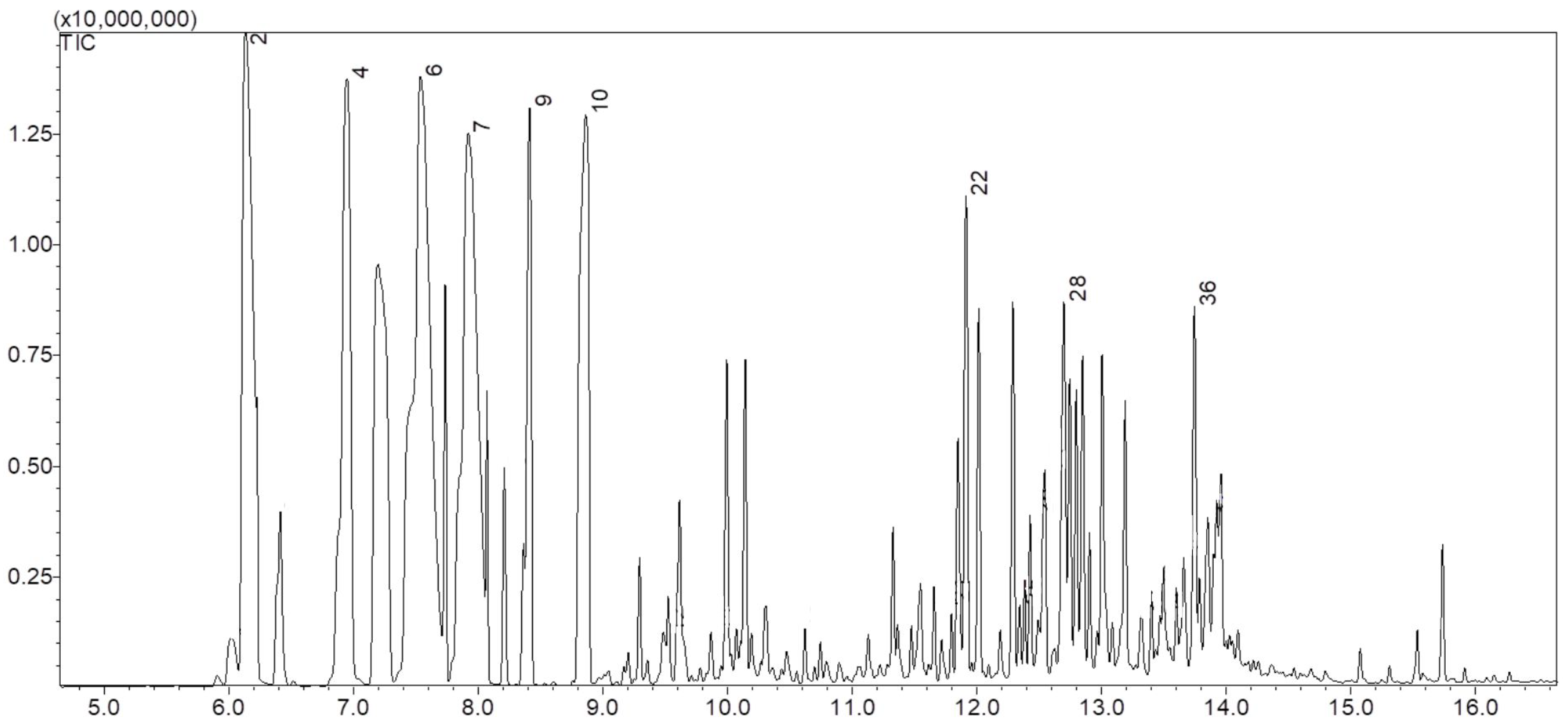
2.1. Characterization by GC-MS
2.2. Larvicidal Bioassay
3. Discussion
4. Materials and Methods
4.1. Recording and Collection of the Species
4.2. Essential Oil Extraction
4.3. Identification of Chemical Constituents by Gas Chromatography Coupled withMass Spectrometry
4.4. Collection of An. Darlingi Larvae
4.5. Larvicidal Assay
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Health Organization. Malária. Available online: https://www.who.int/news-room/questions-and-answers/item/malaria (accessed on 3 September 2024).
- Brazil Ministry of Health. Epidemiological Bulletin—Epidemiological Overview of Malaria in 2021: Seeking the Path to Malaria Elimination in Brazil. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/m/malaria (accessed on 9 August 2024).
- Veronesi, R.; Focaccia, R. Treatise on Infectious Diseases, 6th ed.; Atheneu Editora: Rio de Janeiro, Brazil, 2021; pp. 2025–2034. [Google Scholar]
- Ministry of Health. Health Surveillance Secretariat. Department of Immunization and Communicable Diseases. Malaria Treatment Guide in Brazil; Ministry of Health: Brasília, Brazil, 2021.
- dos Santos, F.; Xu, M.; Bravo de Guenni, L.; Lourenço-de-Oliveira, R.; Rubio-Palis, Y. Characterization of larval habitats of Anopheles (Nyssorhynchus) darlingi and associated species in malaria areas in western Brazilian Amazon. Mem. Inst. Oswaldo Cruz 2024, 119, e240116. [Google Scholar] [CrossRef] [PubMed]
- Barreto, T.M.A.C.; Ferko, G.P.S.; Rodrigues, F.S. Hospital Costs of Diseases Attributable to Environmental Factors among Residents of Boa Vista and the Increase in Care for Venezuelan Migrants. Cad. Saúde Coletiva 2022, 30, 235–243. [Google Scholar] [CrossRef]
- Adhikari, K.; Khanikor, B.; Sarma, R. Persistent Susceptibility of Aedes aegypti to Eugenol. Sci. Rep. 2022, 12, 2277. [Google Scholar] [CrossRef]
- Medeiros, J.F.D.; Acayaba, R.D.A.; Montagner, C.C. Chemistry in the Assessment of Human Health Impact from Pesticide Exposure. Química Nova 2021, 44, 584–598. [Google Scholar] [CrossRef]
- Mossa, A.T.; Mohafrash, S.; Chandrasekaran, N. Safety of Natural Insecticides: Toxic Effects on Experimental Animals. BioMed Res. Int. 2018, 18, 4308054. [Google Scholar] [CrossRef]
- Vale, E.S.M.; Rodrigues, I.B.; Tadei, W.P. Evaluation of the Use of Plant Extracts in Aedes spp. Breeding Sites on the Campus of the National Institute for Amazonian Research (INPA), Manaus, Amazonas. In Fundamentals and Research in Environmental and Agricultural Sciences, 1st ed.; Andrade, J.K.B., Ed.; Editora Licuri: Paraíba, Brazil, 2024; Volume 1, pp. 142–149. [Google Scholar] [CrossRef]
- Souza, A.L.D.C.; Rebouças, C.K.D.O.; Albuquerque, C.C.D.; Moura, C.D.C.F.L.; Torres, T.M.; Batista, J.I.L.; Bezerra, A.C.D.S. In Vitro Anthelmintic Activity of Lippia gracilis Schauer Essential Oil Against Egg-Hatching of Goat Gastrointestinal Nematodes. Arq. Inst. Biológico 2020, 87, e0522019. [Google Scholar] [CrossRef]
- Torres-Santos, P.T.; Farias, I.F.; Almeida, M.D.; Passos, G.S.; Ribeiro, L.A.; Rolim, L.A.; Horta, M. Acaricidal Efficacy and Chemical Study of Hexane Extracts of the Leaves of Neoglaziovia variegata (Bromeliaceae) Against the Tick Rhipicephalus microplus. Exp. Appl. Acarol. 2021, 84, 263–270. [Google Scholar] [CrossRef]
- Sampaio, M.G.V.; Bezerra Dos Santos, C.R.; Cortez Sombra Vandesmet, L.; Souza Dos Santos, B.; Bianca Da Silva Santos, I.; Correia, M.T.D.S.; Da Silva, M.V. Chemical Composition, Antioxidant, and Antiprotozoal Activity of Eugenia gracillima Kiaersk. Leaves Essential Oil. Nat. Prod. Res. 2021, 35, 1914–1918. [Google Scholar] [CrossRef]
- Alcantara, I.S.; Martins, A.O.B.P.B.; de Oliveira, M.R.C.; Coronel, C.; Gomez, M.C.V.; Rolon, M.; de Menezes, I.R.A. Cytotoxic Potential and Antiparasitic Activity of the Croton rhamnifolioides Pax Leaves. & K. Hoffm. Essential Oil and Its Inclusion Complex (EOCr/β-CD). Polym. Bull. 2021, 79, 1175–1185. [Google Scholar] [CrossRef]
- Pereira, J.R.; da Silva, S.M.P.; Marques, M.O.M. Efficacy of Essential Oil of Lippia sidoides (Verbenaceae) for Controlling the Cattle Tick Rhipicephalus (Boophilus) microplus on Naturally Parasitized Animals under Field Conditions. Vet. Parasitol. 2022, 311, 109788. [Google Scholar] [CrossRef]
- Gomes, G.A.; Rondon, F.C.M.R.M.; Carneiro-Torres, D.S.C.T.; Fampa, P.F.; Bevilaqua, C.M.L.B.L.; Bandeira, P.N.B.; de Carvalho Carvalho, M.G. Croton pulegiodorus Baill and Croton piauhiensis Mull. Arg. (Euphorbiaceae) Essential Oils: Chemical Composition and Anti-Leishmania Activity. Rev. Virtual Química 2022, 14, 938–946. [Google Scholar] [CrossRef]
- Bieski, I.G.C.; Santos, J.L.U.D.; Ferreira, M.D.L.; Garcia, P.C.; Dourado, S.H.A.; Januário, A.B.; Apolinário, J.M.D.S.D.S. Economic and Therapeutic Potential of the Most Used Essential Oils in Brazil. Rev. Fitos 2020, 15, 125–137. [Google Scholar] [CrossRef]
- Daly, D.C.; Perdiz, R.O.; Fine, P.V.A.; Damasco, G.; Martínez-Habibe, M.C.; Calvillo-Canadell, L. A Review of Neotropical Burseraceae. Braz. J. Bot. 2022, 45, 103–137. [Google Scholar] [CrossRef]
- Thulin, M.; Beier, B.A.; Razafimandimbison, S.G.; Banks, H.I. Ambilobea, a new genus from Madagascar, the position of Aucoumea, and comments on the tribal classification of the frankincense and myrrh family (Burseraceae). Nord. J. Bot. 2008, 26, 218–229. [Google Scholar] [CrossRef]
- Passero, L.F.; Laurenti, M.D.; Santos-Gomes, G.; Soares Campos, B.L.; Sartorelli, P.; Lago, J.H. Plants used in traditional medicine: Extracts and secondary metabolites that exhibit antileishmanial activity. Curr. Clin. Farmacol. 2014, 9, 187–204. [Google Scholar] [CrossRef]
- de Souza, A.A.; Ortíz, B.L.S.; de Carvalho Rocha Koga, R.; Sales, P.F.; da Cunha, D.B.; Guerra, A.L.M.; Carvalho, J.C.T. Secondary Metabolites Found among the Species Trattinnickia rhoifolia Willd. Molecules 2021, 26, 7661. [Google Scholar] [CrossRef]
- Albino, R.C.; Braz, M.M.; Bizzo, H.R.; Santana da Silva, R.V.; Leitão, S.G.; Ribeiro de Oliveira, D. Amazonian Medicinal Smokes: Chemical Analysis of Burseraceae Pitch (Breu) Oleoresin Smokes and Insights into Their Use on Headache. J. Ethnopharmacol. 2021, 276, 114165. [Google Scholar] [CrossRef]
- Larocca, D.G.; Júnior, N.G.R.; Vicente, R.E.; da Silva, I.V. Ethnobotanical Treatment of Tropical Diseases, Malaria and Dengue, Prescribed by Bioenergético Practitioners and Profile of the Involved Population in Meridional Amazon. Etnobiología 2021, 19, 114–128. [Google Scholar]
- Cierto, L.E.O.; Zevallos, A.W.; Valencia-Reyes, Z.L.; Vilchez-Ochoa, G.L.; Salas-Zeballos, V.R.; Curo, G.G.; Dumont, J.R.D. Diversity of Plasmodium Vectors and Functional Traits of Trees in the Disturbed Forest in Tingo María. Bol. Malariol. Environ. Health 2022, 62, 190–201. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef]
- Batista, L.; Stangerlin, D.M.; Melo, R.R.D.; Souza, A.P.D.; Silva, E.D.S.; Pariz, E. Mechanical resistance and chemical characterization of Amazonian deteriorated woods in field tests. Madera Bosques 2021, 27, 218–229. [Google Scholar] [CrossRef]
- Silva, E.D.S.; Júnior, E.A.B.; Silva, G.A.D.O.; Curvo, K.R.; Stangerlin, D.M.; Melo, R.R.D.; Souza, A.P.D. Surface deterioration of five amazonian wood exposed to natural weathering. Madera Bosques 2022, 28, e2822405. [Google Scholar] [CrossRef]
- de Souza, A.A.; Ortíz, B.L.S.; Borges, S.F.; Pinto, A.V.P.; Ramos, R.D.S.; Pena, I.C.; Tavares Carvalho, J.C. Acute Toxicity and Anti-Inflammatory Activity of Trattinnickia rhoifolia Willd (Sucuruba) Using the Zebrafish Model. Molecules 2022, 27, 7741. [Google Scholar] [CrossRef] [PubMed]
- Bimestre, T.A.; Silva, F.S.; Tuna, C.E.; dos Santos, J.C.; de Carvalho, J.A., Jr.; Canettieri, E.V. Physicochemical Characterization and Thermal Behavior of Different Wood Species from the Amazon Biome. Energias 2023, 16, 2257. [Google Scholar] [CrossRef]
- Magalhães, L.M. Identification of Flavonoids by LC-MS/MS in Leaf Extract of Trattinnickia rhoifolia (Willd) and Evaluation of Antioxidant Activity. Master’s Dissertation, Federal University of Mato Grosso, Sinop, Brazil, 2017. Available online: http://ri.ufmt.br/handle/1/4169 (accessed on 20 March 2025).
- Canetti, A.; Braz, E.M.; de Mattos, P.P.; Basso, R.O.; Filho, A.F. A novel approach to maximize wood production in sustainable management of the Amazon rainforest. Ann. For. Sci. 2021, 78, 67. [Google Scholar] [CrossRef]
- Delascio-Chitty, F.; Brewer-Carías, C. Aspectos generales de la vegetación, flora y plantas útiles del valle del río Kanarakuni, Alto río Caura, estado Bolívar, Venezuela. Mem. Fund. La Salle Cienc. Nat. 2023, 81, 109–147. [Google Scholar]
- Muñoz-Romo, M.; Ramoni-Perazzi, P. Unraveling the leaf-dropping behavior behind bat folivory: Do bats use biological control against roost parasites? Mammalia 2020, 84, 195–200. [Google Scholar] [CrossRef]
- Silveira, Z.D.S.; Macêdo, N.S.; Bezerra, S.R.; Siyadatpanah, A.; Coutinho, H.D.M.; Seifi, Z.; Balbino, V.D.Q. Phytochemistry and Biological Activities of Amburana cearensis (Allemão) ACSm. Molecules 2022, 27, 505. [Google Scholar] [CrossRef]
- Asbahani, E.A. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Schindler, B.; Silva, D.T.D.; Heinzmann, B.M. Effect of seasonality on the essential oil yield of Piper gaudichaudianu m kunth. Cienc. Florest. 2018, 28, 263–273. [Google Scholar] [CrossRef]
- Ribeiro, M.S.; Bonilla, H.O.; Lucena, P.M.E. Influence of Seasonality and the Circadian Cycle on the Yield and Chemical Composition of Essential Oils from Croton spp. of the Caatinga. Iheringia Série Botânica 2018, 73, 31–38. [Google Scholar] [CrossRef]
- de Oliveira, G.G.; Da Silva, H.B.; Vital, E.S. Chemical constituents and antimicrobial activity of essential oil of Trattinnickia burserifolia, Tepequém, Roraima, Brazil. Planta Med. 2014, 80, LP86. [Google Scholar] [CrossRef]
- Brazil INPE—National Institute for Space Research. TerraBrasilis Platform: Comparative Annual Table of Brazilian States in 2023. Available online: https://terrabrasilis.dpi.inpe.br/queimadas/situacao-atual/situacao_atual/ (accessed on 7 February 2025).
- Hassiotis, C.N. Chemical compounds and essential oil release through decomposition process from Lavandula stoechas in Mediterranean region. Biochem. Syst. Ecol. 2010, 38, 493–501. [Google Scholar] [CrossRef]
- Alsalhi, M.S.; Elumalai, K.; Devanesan, S.; Govindarajan, M.; Krishnappa, K.; Maggi, F. The aromatic ginger Kaempferia galanga L. (Zingiberaceae) essential oil and its main compounds are effective larvicidal agents against Aedes vittatus and Anopheles maculatus without toxicity on the non-target aquatic fauna. Ind. Crops Prod. 2020, 158, 113012. [Google Scholar] [CrossRef]
- dos Anjos, R.C.L.; Cortes, B.A.G.; Alves, E.M.; de Farias, L.H.M.; de Melo Cazal, C.; Pereira, P.S.; Christofoli, M. Chemical Composition and Antifungal Activity of the Essential Oil from the Flowers of Bauhinia forficata (Link) and Its Properties on the Germination of Cucurbita maxima (Duchesne) Seeds. Res. Soc. Dev. 2022, 11, e14211225591. [Google Scholar] [CrossRef]
- National Library of Medicine (EUA); National Center for Biotechnology Information. PubChem Resumo do Composto Para CID 79035, Tricyclene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tricyclene (accessed on 20 March 2025).
- de Andrade, F.F.; da Silva, A.V.C.; de Araújo, L.M.; da Silva, L.G.M.; da Silva, J.K.; da Silva, R.N.A. Essential oils from the Amazon: Potential and future perspectives for Andiroba, Breu-Branco, Buriti, and Copaíba. Stud. Eng. Exact Sci. 2025, 6, e14133. [Google Scholar] [CrossRef]
- National Library of Medicine (EUA); National Center for Biotechnology Information. PubChem Resumo do Composto Para CID 14896, beta-Pinene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/beta-Pinene (accessed on 20 March 2025).
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; [S.l.: S.n.]; Allured Business Media: Carol Stream, IL, USA, 2007. [Google Scholar]
- Santana, R.C.; dos Santos Rosa, A.; da Silva Mateus, M.H.; Soares, D.C.; Atella, G.; Guimaraes, A.C.; Pinto-da-Silva, L.H. In vitro leishmanicidal activity of monoterpenes present in two species of Protium (Burseraceae) on Leishmania amazonensis. J. Ethnopharmacol. 2020, 259, 112981. [Google Scholar] [CrossRef]
- National Library of Medicine (EUA); National Center for Biotechnology Information. PubChem Resumo do Composto Para CID 22311, Limonene, (+/-). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Limonene (accessed on 20 March 2025).
- de Santana, B.P.; de Deus, R.G.; de Oliveira, O.G.L.; Vieira, T.M.; Roner, M.N.B. Óleos essenciais com atividade carrapaticida contra Rhipicephalus (Boophilus) microplus e Rhipicephalus sanguineus: Uma revisão. Res. Soc. Dev. 2022, 11, e178111537030. [Google Scholar] [CrossRef]
- National Library of Medicine (EUA); National Center for Biotechnology Information. PubChem Resumo do Composto Para CID 6654, alfa-PINENO. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/alpha-PINENE (accessed on 20 March 2025).
- da Silva, R.C.S.; de Souza Arruda, I.R.; Malafaia, C.B.; de Moraes, M.M.; Beck, T.S.; da Camara, C.A.G.; Machado, G. Synthesis, characterization and antibiofilm/antimicrobial activity of nanoemulsions containing Tetragastris catuaba (Burseraceae) essential oil against disease-causing pathogens. J. Drug Deliv. Sci. Technol. 2022, 67, 102795. [Google Scholar] [CrossRef]
- National Library of Medicine (EUA); National Center for Biotechnology Information. PubChem Resumo do Composto Para CID 7462, alfa-Terpineno. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/alpha-Terpinene (accessed on 20 March 2025).
- Umeh, N.E.; Onuorah, R.T.; Ekweogu, C.N.; Ijioma, S.N.; Egeduzu, O.G.; Nwaru, E.C.; Ugbogu, E.A. Chemical profiling, toxicity assessment, anti-diarrhoeal, anti-inflammatory and antinociceptive activities of Canarium schweinfurthii Engl. (Burseraceae) bark in rats. J. Ethnopharmacol. 2024, 333, 118460. [Google Scholar] [CrossRef]
- Acikgul, F.C.; Duran, N.; Kutlu, T.; Ay, E.; Tek, E.; Bayraktar, S. The therapeutic potential and molecular mechanism of Alpha-pinene, Gamma-terpinene, and P-cymene against melanoma cells. Heliyon 2024, 10, e36223. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (EUA); National Center for Biotechnology Information. PubChem Resumo do Composto Para CID 7461, gama-Terpineno. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/gamma-Terpinene (accessed on 20 March 2025).
- Hernández-Ruiz, R.; Solas, M.; Suárez-Pantiga, S.; Pedrosa, M.R.; Sanz, R. γ-Terpinene: Biorenewable Reductant for the Molybdenum-Catalyzed Reduction of Sulfoxides, N-Oxides and Nitroarenes. Adv. Synth. Catal. 2025, 367, e202401387. [Google Scholar] [CrossRef]
- de Castro Borba, E.R.; Mubárack, T.C.; Luz, T.R.S.A.; Silveira, D.P.B.; Silva, A.Z.; Figueiredo, P.D.M.S.; Coutinho, D.F. Chemical characteristics and antimicrobial activity of the essential oil of Plectranthus amboinicus (lour.) Spreng. cultivated with different fertilizers. Res. Soc. Dev. 2021, 10, e28210111717. [Google Scholar] [CrossRef]
- National Library of Medicine (EUA); National Center for Biotechnology Information. PubChem Resumo do Composto Para CID 6431138, 6-epi-alpha-Cubebene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6-epi-alpha-Cubebene (accessed on 20 March 2025).
- Jaramillo-Colorado, B.E.; Arroyo-Salgado, B.; Palacio-Herrera, F.M. Antifungal activity of four Piper genus essential oils against postharvest fungal Fusarium spp. isolated from Mangifera indica L. and Persea americana Mill. fruits. Ind. Crops Prod. 2025, 223, 120170. [Google Scholar] [CrossRef]
- National Library of Medicine (EUA); National Center for Biotechnology Information. PubChem Resumo do Composto Para CID 86608, alfa-Bergamoteno. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/alpha-Bergamotene (accessed on 20 March 2025).
- Wen, Y.H.; Chen, T.J.; Jiang, L.Y.; Li, L.; Guo, M.; Peng, Y.; Zhu, P. Unusual (2R,6R)-bicyclo [3.1.1] heptane ring construction in fungal α-trans-bergamotene biosynthesis. Iscience 2022, 25, 104030. [Google Scholar] [CrossRef]
- Lima, S.C.; Oliveira, A.C.; Costa, M.L.L.; Abensur, D.D.; dos Santos Andrade, A.T.; Souza, H.V.; Roque, R.A. Larvicidal Effect and Mechanism of Action of the Essential Oil and Major Compound from Piper brachypetiolatum (Piperaceae) Against Aedes aegypti (Linnaeus, 1762) Larvae, with Protection of Non-Target Aquatic Animals. 2024. [S.l.: S.n.]. Available online: https://www.researchsquare.com/article/rs-5233897/v1 (accessed on 20 March 2025).
- Mendonça, J.F.; Sousa, A.H.D.; Faroni, L.R.; Fernandes, C.C.; Santos, A.C.D.; Lopes, L.M.; Prates, L.H. Yield, composition and toxicity of piperaceae volatiles to pest insects. Rev. Caatinga 2024, 37, e12298. [Google Scholar] [CrossRef]
- National Library of Medicine (EUA); National Center for Biotechnology Information. PubChem Resumo do Composto Para CID 3084311, delta-Cadinol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/delta-Cadinol (accessed on 20 March 2025).
- Veloso, C.A.G.; de Souza, P.H.S.; Nóbrega, F.P.; de Medeiros, A.C.D.; Fechine, I.M.; de Melo, J.I.M.; Tavares, J.F. Chemical composition of Varronia dardani (Taroda) J.S. Mill essential oil and its antibiofilm activity. Braz. J. Dev. 2020, 6, 12887–12898. [Google Scholar] [CrossRef]
- Carvalho, R.A.; da Conceição Lima, A.M.; Pereira, Á.I.S.; Sobrinho, O.P.L.; Ribeiro, F.A.A.; da Silva Costa, S.T.; Lopes, T.Y.A. Pharmacological Potentials of Aloe Vera: A Study Conducted Using Scientific and Technological Prospecting Techniques. Cad. Prospecção 2020, 13, 184. [Google Scholar] [CrossRef]
- Pereira, S.A.; de Mendonça, M.S.; Barbalho, C.R.S.; de Alencar, M.S.M.; de Souza, C.D.M. Scientific and Technological Prospecting of the Genus jatropha (Euphorbiaceae). Cad. Prospecção Salvador 2015, 8, 355–364. [Google Scholar] [CrossRef]
- Barroso, J.A. Evaluation of the Insecticidal Activity Potential of Essential Oils Extracted from Azadirachta indica and Ricinus communis L. on Anopheles spp. and Aedes spp. Larvae. Rev. Ibero-Am. Humanidades Ciências Educ. 2022, 8, 1250–1275. [Google Scholar] [CrossRef]
- Bezerra, A.L.F.M.; Pinheiro, E.B.F. Essential Oils as an Alternative for the Control of Anopheles Genus Larvae: A Review. Res. Soc. Dev. 2021, 10, e37101119384. [Google Scholar] [CrossRef]
- Gurunathan, A.; Senguttuvan, J.; Paulsamy, S. Evaluation of mosquito repellent activity of isolated oleic acid, eicosyl ester from Thalictrum javanicum. Indian J. Pharm. Sci. 2016, 78, 103. [Google Scholar] [CrossRef]
- Borges, A.D.C.; de Carvalho, C.E.G.; de Souza, J.R.L.; Morato, E.F.; Cadaxo-Sobrinho, E.S.; Marques, D.D. Evaluation of the Chemical Composition and Larvicidal Activity of Cymbopogon nardus Essential Oil in the Control of Aedes aegypti in Southwestern Amazon. Holos 2021, 5, 1–13. [Google Scholar] [CrossRef]
- Benelli, G.; Rajeswary, M.; Vijayan, P.; Senthilmurugan, S.; Alharbi, N.S.; Kadaikunnan, S.; Govindarajan, M. Essential Oil of Boswellia ovalifoliolata (Burseraceae) as an Ecological Larvicide? Toxicity Against Six Mosquito Vectors of Public Health Importance, Non-Target Mosquito Fishes, Back Swimmers, and Water Bugs. Environ. Sci. Pollut. Res. 2018, 25, 10264–10271. [Google Scholar] [CrossRef]
- Ayinde, A.A.; Morakinyo, O.M.; Sridhar, M.K.C. Repellency and larvicidal activities of Azadirachta indica seed oil on Anopheles gambiae in Nigeria. Heliyon 2020, 6, e03920. [Google Scholar] [CrossRef]
- de Oliveira, A.C.; Simões, R.C.; Tavares, C.P.; Lima, C.A.; Sá, I.S.C.; da Silva, F.M.; Roque, R.A. Toxicity of the essential oil from Tetradenia riparia (Hochstetter.) Codd (Lamiaceae) and its principal constituent against malaria and dengue vectors and non-target animals. Pestic. Biochem. Physiol. 2022, 188, 105265. [Google Scholar] [CrossRef]
- Simas, N.K.; Lima, E.D.C.; Conceição, S.D.R.; Kuster, R.M.; Oliveira Filho, A.M.D.; Lage, C.L.S. Produtos naturais para o controle da transmissão da dengue: Atividade larvicida de Myroxylon balsamum (óleo vermelho) e de terpenóides e fenilpropanóides. Química Nova 2004, 27, 46–49. [Google Scholar] [CrossRef]
- Cruz, I.L.S.; Pimentel, M.A.G.; Nascimento, T.A.; Alves, S.P.; Maleck, M.; Queiroz, M.M.C. Larvicidal activity and chemical composition of four essential oils against Aedes aegypti (Diptera: Culicidae). Braz. J. Biol. 2024, 84, e283724. [Google Scholar] [CrossRef]
- de Menezes Torres, M.d.C.; da Luz, M.A.; de Oliveira, F.B.; Barbosa, A.J.C.; de Araújo, L.G. Chemical composition of essential oil from Croton Heliotropiifolius kunth (Euphorbiaceae) Leaves. Braz. J. Dev. 2021, 7, 15862–15872. [Google Scholar] [CrossRef]
- Suppajariyawat, P.; Andrade, A.F.B.D.; Elie, M.; Baron, M.; Gonzalez-Rodriguez, J. The use of chemical composition and additives to classify petrol and diesel using gas chromatography–mass spectrometry and chemometric analysis: A UK study. Open Chem. 2019, 17, 183–197. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for laboratory and field testing of mosquito larvicides. In World Health Organization Communicable Disease Control, Prevention and Eradication Who Pesticide Evaluation Scheme; World Health Organization (WHO): Geneva, Switzerland, 2005; pp. 1–41. [Google Scholar]
- Giatropoulos, A.; Kimbaris, A.; Michaelakis, A.; Papachristos, D.P.; Polissiou, M.G.; Emmanouel, N. Chemical composition and assessment of larvicidal and repellent capacity of 14 Lamiaceae essential oils against Aedes albopictus. Parasitol. Res. 2018, 117, 1953–1964. [Google Scholar] [CrossRef] [PubMed]


| Article Title | Author, Journal and Year |
|---|---|
| Ethnobotanical treatment of tropical diseases prescribed by practitioners and the bioenergetic profile of the population involved in southern Amazonas. | [23] |
| Diversity of Plasmodium vectors and functional traits of trees in a disturbed forest in Tingo María, 2022. | [24] |
| Corresponding Peak | Constituents | Molecular Formula | Retention Time (min) | Area (%) |
|---|---|---|---|---|
| 2 | α-Pinene | C10H16 | 6.133 | 9.38 |
| 4 | β-Pinene | C10H16 | 6.948 | 14.21 |
| 6 | Tricyclene | C10H16 | 7.537 | 15.86 |
| 7 | Limonene | C10H16 | 7.925 | 12.23 |
| 9 | γ-Terpinene | C10H16 | 8.415 | 3.83 |
| 10 | α-Terpinene | C10H16 | 8.867 | 6.84 |
| 22 | α-Cubebene | C15H24 | 11.917 | 3.58 |
| 28 | α-Bergamotene | C15H24 | 12.702 | 3.50 |
| 29 | α-Muurolene | C15H24 | 12.852 | 2.82 |
| 36 | δ-Cadinol | C15H26O | 13.749 | 2.00 |
| - | Others Total | - | - | 25.75 100.00 |
| Time (h) | LC50 (µg mL−1) (LCL-LCU) | LC90 (µg mL−1) (LCL-LCU) | χ2 (Df) | Linear Equation | RP |
|---|---|---|---|---|---|
| 24 | 96.35 (68.714–289.888) | 366.78 (170.175–471.410) | 0.079 (3) * | Y =−4.380 + 1.353x | 0.003 |
| 48 | 27.74 (2.691–43.715) | 189.35 (94.024–371.725) | 5.011 (3) * | Y =−2.236 + 1.066x | 0.011 |
| 72 | 14.51 (2.784–35.984) | 77.87 (49.174–831.514) | 3.810 (3) * | Y =−2.041 + 1.176x | 0.022 |
| α-Cypermethrin | 0.33 (0.313–0.357) | 0.60 (0.565–0.668) | 0.03 (4) * | 4.950 ± 0.351 | 1 |
| Chemical Constituent | Chemical Grade | Share |
|---|---|---|
| Tricyclene | Monoterpene with three additional methyl substituents [43] | Potential pharmacological activity against protozoa of the genus Plasmodium [44]. |
| β-Pinene | Monoterpene, isomer of pinene with an exocyclic double bond [45,46] | It has larvicidal activity. It has a role as a plant metabolite [47]. |
| Limonene | Substituted monoterpene [46,48] | One of the most commonly found compounds in plant species. It is a monoterpene that has larvicidal potential. It plays a role as a human metabolite [49]. |
| α-Pinene | Methyl-substituted monoterpenes [50] | Anxiolytic, antioxidant, anti-inflammatory, analgesic, antimicrobial, and anticoagulant effects. It has a role as a plant metabolite [51]. |
| α-Terpinene | Isomeric monoterpene [52] | It has biological activities, including high therapeutic potential. It has a role as a volatile oil component and a plant metabolite [53,54]. |
| γ-Terpinene | Isomeric monoterpenes [55] | Catalysis process. Antimicrobial activity. Has a role as an antioxidant and a plant metabolite. A volatile component of the oil is a human xenobiotic metabolite [56,57]. |
| α-Cubebene | Tricyclic sesquiterpene [58] | It has fungicidal action. It has a role as a plant metabolite. A human metabolite is a volatile oil component [59]. |
| α-Bergamotene | Sesquiterpene [60] | Used in biosynthesis of products. It has a role as a plant metabolite and a volatile oil component [61]. |
| α-Muurolene | Sesquiterpene [62] | Insecticidal action [63]. |
| δ-Cadinol | Cadinane sesquiterpenoid [64] | Antimicrobial activity. It has a role as an algal metabolite and a plant metabolite [65]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, G.G.; da Silva, S.M.D.; de Souza, A.P.; da Silva, L.V.A.; Silva, A.L.e.; de Melo, A.C.G.R.; Roque, R.A.; de Oliveira, A.C.; de Melo Filho, A.A.; Soares, A.M. Larvicidal Potential of Trattinnickia Burserifolia Mart. Essential Oil in Controlling the Malaria Vector in the Amazon. Pharmaceuticals 2025, 18, 604. https://doi.org/10.3390/ph18050604
de Oliveira GG, da Silva SMD, de Souza AP, da Silva LVA, Silva ALe, de Melo ACGR, Roque RA, de Oliveira AC, de Melo Filho AA, Soares AM. Larvicidal Potential of Trattinnickia Burserifolia Mart. Essential Oil in Controlling the Malaria Vector in the Amazon. Pharmaceuticals. 2025; 18(5):604. https://doi.org/10.3390/ph18050604
Chicago/Turabian Stylede Oliveira, Gisele Guimarães, Stherfany Mac Donald da Silva, Alessandro Pereira de Souza, Leticia Vieira Anchieta da Silva, Anauara Lima e Silva, Ana Cristina Gonçalves Reis de Melo, Rosemary Aparecida Roque, André Correa de Oliveira, Antonio Alves de Melo Filho, and Andreimar Martins Soares. 2025. "Larvicidal Potential of Trattinnickia Burserifolia Mart. Essential Oil in Controlling the Malaria Vector in the Amazon" Pharmaceuticals 18, no. 5: 604. https://doi.org/10.3390/ph18050604
APA Stylede Oliveira, G. G., da Silva, S. M. D., de Souza, A. P., da Silva, L. V. A., Silva, A. L. e., de Melo, A. C. G. R., Roque, R. A., de Oliveira, A. C., de Melo Filho, A. A., & Soares, A. M. (2025). Larvicidal Potential of Trattinnickia Burserifolia Mart. Essential Oil in Controlling the Malaria Vector in the Amazon. Pharmaceuticals, 18(5), 604. https://doi.org/10.3390/ph18050604