Abstract
Glaucoma is a leading cause of irreversible blindness worldwide. To date, intraocular pressure (IOP) is the only modifiable risk factor in glaucoma treatment, but even in treated patients, the disease can progress. Cannabinoids, which have been known to lower IOP since the 1970s, have been shown to have beneficial effects in glaucoma patients beyond their IOP-lowering properties. In addition to the classical cannabinoid receptors CB1 and CB2, knowledge of non-classical cannabinoid receptors and the endocannabinoid system has increased in recent years. In particular, the CB2 receptor has been shown to mediate anti-inflammatory, anti-apoptotic, and neuroprotective properties, which may represent a promising therapeutic target for neuroprotection in glaucoma patients. Due to their vasodilatory effects, cannabinoids improve blood flow to the optic nerve head, which may suggest a vasoprotective potential and counteract the altered blood flow observed in glaucoma patients. The aim of this review was to assess the available evidence on the effects and therapeutic potential of cannabinoids in glaucoma patients. The pharmacological mechanisms underlying the effects of cannabinoids on IOP, neuroprotection, and ocular hemodynamics have been discussed.
1. Introduction
Cannabis or marijuana is one of the most widely used drugs in the world. In many countries, cannabis remains an illegal drug, while other countries allow medical use or consumption. Currently, there is a growing interest in cannabis and both medical and recreational use is gradually being legalized [1]. The cannabis plant, Cannabis sativa, has been used as a medicinal and psychotropic drug for many centuries [1,2,3]. It is one of the earliest cultivated plants, domesticated around 12,000 years ago [1,2]. It was first cultivated in Central or Southeast Asia [2]. Cannabis provided fibers for ropes and nets, food, and seeds for oil [1,3]. Early civilizations, including China, Egypt, Greek, India, and the Roman Empire also used Cannabis for its medicinal and psychoactive properties [1]. In the twentieth century, the psychoactive effects of tetrahydrocannabinol (THC) led to the prohibition of cannabis use in the United Kingdom with the “Misuse of Drugs Act 1971” and in the United States with “The Marihuana Tax Act” in 1937, as the drug was considered to be significantly harmful and had no medicinal use. [2,3]. To date, cannabinoids exhibit therapeutic potential for chronic pain, spastic disorders, such as those associated with multiple sclerosis, unwanted weight loss, and nausea and vomiting resulting from chemotherapy [4,5]. The potential use of cannabinoids in glaucoma due to their hypotensive effect on intraocular pressure (IOP) has been explored since the 1970s [6,7]. Several studies have investigated the effects of cannabinoids in glaucoma in terms of their IOP-lowering and potential neuroprotective effects [8,9,10,11,12]. As public and scientific interest of cannabinoids has increased in recent years, there is a growing need for a better understanding of the ocular mechanisms underlying cannabinoids. Therefore, we have provided an overview of the current evidence on the effects and therapeutic potential of cannabinoids in glaucoma.
2. Glaucoma
Glaucoma is the leading cause of irreversible blindness worldwide, affecting approximately 70 million people, with its prevalence predicted to further increase in the future [13]. Glaucoma is an umbrella term for multifactorial optic nerval neuropathies associated with the progressive loss of retinal ganglion cells (RGCs) resulting in characteristic visual field defects [14]. Despite the burden on affected patients, glaucoma also imposes high economic costs, especially in the late stages [15]. Therefore, early diagnosis and adequate treatment are vital to prevent future disability. An elevated IOP is considered to be the most important risk factor underlying the development and progression of the disease and lowering the IOP remains the only proven treatment option [16]. Drug therapies, such as prostaglandin derivatives, β-receptor antagonists, α2-receptor agonists, carbonic anhydrase inhibitors, and cholinergic agonists, are used to lower IOP as a single or combined application [17,18,19]. In 2017, the US Food and Drug Administration (FDA), and more recently the European Medical Agency (EMA), approved the Rho-kinase inhibitor netarsudil to lower elevated IOP in patients with open-angle glaucoma or ocular hypertension [18,19]. Rho-kinase inhibitors are a new class of ocular hypotensive drugs that exhibit cytoskeletal modulatory effects, inducing their effect mainly through increased aqueous humor outflow via the trabecular meshwork and adjacent tissues [19].
Although several anti-glaucoma medications and surgical procedures are currently available to lower IOP, a considerable number of patients progress despite adequate treatment [20]. Therefore, therapy aimed at more than just lowering IOP would be required [17]. However, despite the identification of IOP as the main risk factor for progression, the molecular mechanisms for optic nerve damage in glaucoma are not fully understood. Since a significant number of primary open-angle glaucoma patients do not have a statistically elevated IOP, this suggests the contribution of other mechanisms than IOP to optical neuropathy [21]. Among others, potential contributing mechanisms involve tissue ischemia caused by vascular dysregulation, inflammatory mediators, including oxidative stress, and abnormal effects of endogenous substances, such as glucocorticoids, glutamate, nitric oxide, endothelin, neurotrophic factors, and caspases [22,23,24]. These pathomechanisms have not yet been understood in detail, but therapies targeting components other than IOP are actively being researched. Currently, nutrients and nutraceuticals with anti-apoptotic, anti-inflammatory, and anti-oxidant properties, like vitamins, palmitoylethanolamide (PEA), melatonin, citicoline, coenzyme Q10, or ginkgo biloba extract, are being investigated for their neuroprotective effect in glaucoma therapy [25,26]. Available data from previous studies have shown that nutritional supplementation may play a role as a coadjutant in the therapeutic management of glaucoma, but the clinical significance of these data remains to be demonstrated [25]. As cannabinoids are thought to have effects beyond lowering IOP, this review evaluates their potential role in glaucoma treatment.
3. Marijuana and Endocannabinoids
Cannabinoids can be classified into phytocannabinoids, synthetic cannabinoids, and endocannabinoids (eCBs, Figure 1). The term “cannabinoids” is used for any chemical substance that binds to cannabinoid receptors and has effects similar to those produced by the cannabis plant [27].
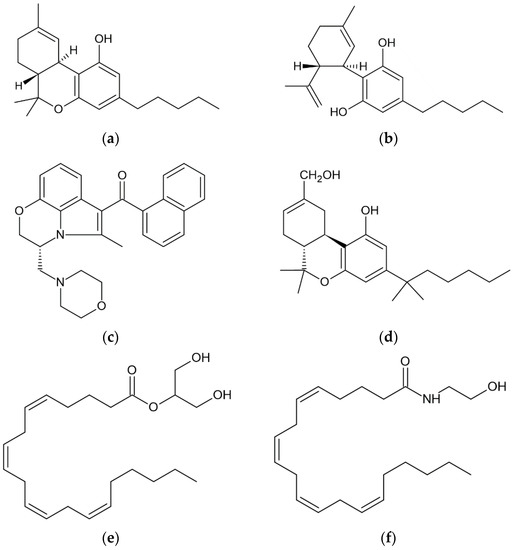
Figure 1.
Chemical structures of six cannabinoids: the phytocannabinoids (a) tetrahydrocannabinol (THC) and (b) cannabidiol (CBD); the synthetic cannabinoids (c) WIN55212-2 and (d) HU-210; and the endocannabinoids (e) 2-arachidonylgylcerol (2-AG) and (f) arachidonoyl ethanolamide (anandamide, AEA).
3.1. Phytocannabinoids
Cannabis sativa L. (C. sativa), contains about 120 phytocannabinoids, of which 11 different chemical classes have been identified [28]. Phytocannabinoids are mainly found in the resin secreted by the trichomes of female plants [3]. The most studied ones are Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) [29]. They bind to cannabinoid receptors resulting in their characteristic clinical or psychoactive effects [29]. Their targets include the classical cannabinoid receptors type 1 and 2 (CB1 and CB2), along with numerous other targets [29].
From a pharmacological perspective, Δ9-THC acts as partial agonist at the CB1 and CB2 receptors [28,29]. As characteristic of a partial agonist, Δ9-THC exhibits agonist–antagonist effects. Whether Δ9-THC exerts more agonistic or antagonistic effects depends on the proportion of cannabinoid receptors in the tissues, the presence of other cannabinoid receptor agonists, and the species [28]. Δ9-THC exhibits neuroprotective, anti-spasmodic, and anti-inflammatory effects [30]. The psychotropic effects of cannabinoids and the cannabinoid-induced “tetrad” that includes hypolocomotion, hypothermia, catalepsy, and analgesia are attributed to the partial agonist activity of Δ9-THC at CB1 receptors [28,29,30,31].
Since the psychotropic effects of Δ9-THC are of concern, there is growing interest in cannabinoids without psychotropic effects, like CBD or cannabidivarin (CBDV). Anti-inflammatory, anti-oxidative, anti-convulsive, analgesic, anti-emetic, and neuroprotective effects have been described for CBD [30]. Other pre-clinical studies showed anti-tumor, anti-invasive, and anti-angiogenic effects in cancer research [30]. CBD is known to have a low affinity for CB1 and CB2 receptors and to act as an inverse agonist of the classical cannabinoid receptors [28,30]. There is evidence that CBD acts as a negative allosteric modulator of CB1 at concentrations lower than those required to act as an inverse agonist. In this way, CBD does not activate the receptor itself but reduces the potency and efficacy of orthosteric ligands, such as Δ9-THC [32]. CBD has also been shown to act as a negative allosteric modulator of CB2 receptors [33]. These results implicate that CBD could be used to counteract several adverse effects of Δ9-THC and increase the therapeutic index of Δ9-THC [29].
3.2. Synthetic Cannabinoids
Synthetic cannabinoids are a structurally diverse group of psychoactive substances produced by chemical means [27,34]. In recent years, more than 450 synthetic compounds have been synthesized and often these substances bear the initials of the person/institution that performed their synthesis [27]. They can be chemically classified into classical, non-classical, aminoalkylindoles, eicosanoids, hybrids, and others [34]. Synthetic cannabinoids from the eicosanoid class are structurally similar to eCBs, whereas classical synthetic cannabinoids are more similar to phytocannabinoids [34]. This group of substances can bind to both cannabinoid and non-cannabinoid receptors, but has a higher affinity for cannabinoid receptors than Δ9-THC as they are mostly full agonists [34]. This results in greater potency, but also stronger psychoactive effects [27,34]. The FDA has approved three synthetic cannabis-related drug products which contain dronabinol and nabilone, as well as one cannabis-derived drug product containing CBD [35]. Cannabis-related compounds are created in a laboratory setting and some synthetic compounds may also occur naturally in the plant, such as dronabinol, while others are exclusively synthetically produced,, such as nabilone [35]. In contrast to cannabis-related compounds, cannabis-derived compounds, such as CBD and THC, are extracted directly from the cannabis plant [35]. The product containing CBD has been approved for the treatment of seizures associated with Lennox–Gastaut and Dravet syndrome [35]. Dronabinol and nabilone are used to treat nausea associated with cancer chemotherapy and/or anorexia associated with weight loss in AIDS patients [35]. While not a synthetic cannabinoid, a cannabis extract oral spray, containing equal quantities of THC and CBD, has been approved in Canada, New Zealand, and several European countries for the treatment of spasticity associated with multiple sclerosis when the patients have an inadequate response to other treatments or cannot tolerate their side effects [36].
3.3. Endocannabinoids
The endocannabinoid system (ECS) in the eye refers to a complex network of signaling molecules, receptors, and enzymes that play a crucial role in maintaining ocular homeostasis and in regulating various physiological processes [37]. Within the eye, the ECS is found in different ocular structures, including the cornea, conjunctiva, retina, and various cells, such as the RGCs, ciliary body, and trabecular meshwork [37,38,39,40]. Activation of the ECS in these structures can modulate various functions, including inflammation, IOP, neurotransmission, photoreception, and vascular regulation. Considering the pharmacological aspect, the ECS comprises endogenous cannabinoids (endocannabinoids, eCBs), cannabinoid receptors, and the proteins that transport, synthesize, and degrade eCBs [41,42]. In recent years, it has become clear that the ECS is more complex than originally thought as the number of identified components has increased. The most studied eCBs are arachidonoyl ethanolamide (anandamide, AEA), 2-arachidonoylglycerol (2-AG), and PEA, an anandamide congener [8,37,40]. As stated above, eCBs are present in many parts of the mammalian eye, containing the retina, choroid, iris, ciliary body, and trabecular meshwork [40]. In glaucoma patients, there is evidence that concentrations of 2-AG and PEA are decreased in the ciliary body, suggesting that eCBs may involve IOP regulation [43]. While 2-AG reacts as a full agonist and AEA as a partial agonist for the CB1 and CB2 receptors [44], PEA only binds them with weak activity [45]. eCBs and eCB-like molecules target the receptors GPR18, GPR55, GPR119, TRPV1, and PPARα-γ with different binding activity [45]. In order to maintain the balance of eCBs in the body, they are synthesized or degraded as needed [40]. eCBs are synthesized from membrane phospholipids by diacylglycerol lipase (DGL) α/β and N-arachidonoyl phosphatidylethanolamine phospholipase D (NAPE-PLD) [30]. Degradative enzymes mainly include fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), but also cyclooxygenase-2 (COX-2) and lipoxygenases (LOXs) [30]. Synthesizing and degrading enzymes have been described in the retina and trabecular meshwork [40]. This indicates IOP modulating and neuroprotective capacities via the activity of metabolizing enzymes, such as COX-2, FAAH, and MAGL [37,46]. The targets of eCBs and phytocannabinoids overlap to some extent [42]. An overview to the interactions of phytocannabinoids with the ECS system has been outlined in the excellent review of Di Marzo and Piscitelli [42].
4. Molecular Targets and Mechanisms for Cannabinoid-Induced Ocular Effects
So far, two main types of cannabinoid receptors, CB1 and CB2, have been identified as promising therapeutic targets. In addition to binding to the classical CB1 and CB2 receptors, cannabinoids and their metabolites also interact with non-cannabinoid receptors. The following overview presents the most important cannabinoid and non-cannabinoid receptors with a specific focus on glaucomatous disease.
4.1. CB1 and CB2 Receptors
CB1 and CB2 receptors are the natural receptors for eCBs and can interact with phytocannabinoids and synthetic cannabinoids. The CB1 receptor was first discovered back in 1990 [47]; a second CB2 receptor was successfully cloned in 1993 [48]. Pharmacologically, the CB1 and CB2 receptors are members of the G protein-coupled receptor family. They are defined by seven transmembrane-spanning helices with intervening intracellular loops and a C-terminal domain that can couple with G proteins. Signal transduction is mostly negatively stimulated via adenylyl cyclase and positively stimulated to mitogen-activated protein kinase (MAPK) [49,50]. CB1 further regulates ion channels, such as potassium and calcium channels [49,50]. CB1 receptors are mainly found in the human central nervous system (CNS) and are involved in the release of neurotransmitters [29,51]. The CB1 receptor is also known to be the main receptor underlying psychotropic effects [52]. CB2 receptors are predominantly present in peripheral tissues, such as the immune system, and regulate the release of cytokines [29,53].
In the human eye, CB1 receptors have been detected in the cornea, iris, ciliary body including the epithelium, ciliary muscle and blood vessels of the ciliary body, trabecular meshwork, Schlemm’s canal, and retina [54]. The location and critical anatomical distribution of cannabinoid receptors indicate that cannabinoids may influence IOP through both increasing aqueous humor efflux and decreasing aqueous humor production [55]. A possible influence of cannabinoids on conventional aqueous humor outflow has been suggested by the existence of CB1 receptors in the trabecular meshwork and in the canal of Schlemm [56]. Aqueous humor production and/or uveoscleral outflow imply an effect via the CB1 receptors in the ciliary pigment epithelium and ciliary muscle [56]. The distribution and precise roles of CB2 receptors in the human eye appear to be less well known, but CB2 receptors have been found in the cornea, trabecular meshwork, and retina, particularly in animal models [57,58,59,60].
In the retina, CB1 receptors have been detected in the ganglion layer, the inner and outer plexiform layers, the inner nuclear layer, and the outer segments of photoreceptors [54,60]. CB2 receptors have also been described in retinal cell types and layers, like amacrine, bipolar, Müller, microglial, and RGCs, or in the retinal pigment epithelium [59,60]. CB1 and CB2 receptors are responsible for the functional modulation of neuroretinal cells in animal tissues and may therefore be a potential target for neuroprotection [60].
4.2. Non-Cannabinoid Receptors
The most commonly described G protein-coupled receptors, in addition to the CB1 and CB2 receptors, to which cannabinoids bind are GPR18, GPR55, and GPR119. GPR55, also known as the putative “type 3” cannabinoid receptor, interacts with eCBs, phytocannabiniods, and synthetic cannabinoids [61,62]. CBD binds GPR55 and GPR18 as an antagonist, while Δ9-THC acts as an agonist [5]. GPR55 has been described to be present in the trabecular meshwork and rods of the retina [58,63,64]. Its signaling pathway differs from the classical cannabinoid receptors by activating Ras homolog gene family member A (Rho A) and Rho-associated protein kinase (ROCK) [62]. GPR55 is widely distributed in many tissues, including in the CNS and immune system. Showing anti-inflammatory properties, GPR55 and CB2 receptors can cross-talk to regulate neutrophil responses [65]. GPR18, a G protein-coupled receptor, can be activated by N-arachidonoyl glycine, a carboxylic metabolite of the endocannabinoid AEA, or phytocannabinoids, such as Δ9-THC or CBD [66,67]. GPR18 has been found to be expressed in the ciliary and corneal epithelium, the trabecular meshwork, and retina [68,69]. In addition, GPR18 mRNA and protein were found in the endothelium of retinal vessels, suggesting its contribution to cannabinoid-mediated retinal vasoactivity [69]. GPR119, an orphan receptor for endogenous lipids with eCB-like structures, has not been described in detail for the eye, as its interest is more in the regulation of insulin secretion. Protein expression of GPR119 is most pronounced in structures near the chamber angle associated with sites of outflow, including the trabecular meshwork, and GPR119 has also been shown to modulate IOP in female mice [70].
Cannabinoids interact with transient receptor potential (TRP) ion channels. The TRP protein superfamily are trans-membrane proteins which are mostly expressed in the nervous system and are involved in cellular processes related to mechano-, chemo-, thermo-, and photosensation, and ion homeostasis [71,72]. TRP channels mainly control the flux of calcium across the plasma membrane and influence other ion channels sensitive to calcium [72]. Currently six TRP channels can mediate cannabinoid activity, including TRPV1–4 (TRP vanilloid), TRPA1 (TRP ankyrin), and TRPM8 (TRP melastatin) [72]. Δ9-THC acts with TRPV2–4 channels as an agonist, while CBD also binds to TRPV1 and TRPV2 as an agonist, indicating that the affinity for TRP receptors depends on the selected cannabinoid [5,73]. TRPV1, a capsaicin-sensitive vanilloid TRP subunit 1, can be targeted by the endocannabinoid AEA and is expressed in the retina, including the RGCs and retinal microglia [74]. TRPV4 has been reported as a promising molecular target of glaucoma as it is expressed in the trabecular meshwork showing a crucial role in IOP regulation [75,76], and is also found in RGCs and retinal plexiform layers [77,78]. TRPV1 and TRPV4 have been found in the optic nerve head, indicating that calcium handling may influence optic nerve function [79]; their activation leads to the apoptosis of RGCs [74,77,78,80].
It has been suggested that not only the classical cannabinoid receptors regulate anti-inflammatory effects. Nuclear peroxisome proliferator-activated receptors (PPARs) are members of the nuclear receptor superfamily. PPARs are ligand-regulated transcription factors that control gene expression related to inflammation, glucose and lipid metabolism, metabolic homeostasis, adipogenesis, and cell proliferation [81,82]. Both Δ9-THC and CBD exhibit agonistic effects on PPARγ [5]. In addition to the aforementioned receptors, CBD can decrease pain scores and neutrophil infiltration in corneal hyperalgesia through the involvement of serotonin (5-HT) receptors and reduce the release of TNF-α in retinal microglia via adenosine receptors [83,84,85]. The interaction of cannabinoids and their metabolites with numerous receptors makes understanding their mechanisms of action complex and the development of new therapies difficult.
5. Therapeutic Approaches of Cannabinoids on IOP
As IOP is the only modifiable risk factor that can slow down or prevent the progression of the disease, pharmacological IOP-lowering is still the mainstay of glaucoma therapy. Pharmacological targets of IOP-lowering drugs include aqueous humor production and aqueous humor outflow facility via the trabecular meshwork as well as the uveoscleral pathway [17].
There is evidence that cannabinoids decrease aqueous humor production and increase trabecular and uveoscleral outflow, thereby affecting IOP [86]. As previously described, IOP-lowering effects appear to be mainly mediated via the CB1 receptor [8,56]. In contrast to the CB1 receptor, the CB2 receptor does not appear to mediate IOP-lowering properties [87]. Other mechanisms that contribute to lowering the IOP are the contraction of the ciliary muscle and reduced secretion from the ciliary process through decreasing the release of noradrenaline [55]. Beyond this, the metabolites of the ECS are reported to lower the IOP due to the induction of COX-2-dependent prostaglandins and matrix metalloproteinases in the non-pigmentary ciliary epithelium [46]. It has been demonstrated that AEA and Δ9-THC can act as COX-2-inducing cannabinoids [46]. Via the cyclooxygenase (COX) pathway, the hydrolysis of eCBs to arachidonic acid, the precursor of prostanoid synthesis, occurs [56]. COX-2 oxidates AEA and 2-AG into a series of prostaglandin ethanolamides (prostamides) and prostaglandin glyceryl esters [88]. These are similar to the prostaglandins formed from arachidonic acid [88]. Prostamides exert potent effects in glaucoma, such as the prostamide analogue bimatoprost [88]. Prostamides and prostaglandins exert effects through the uveoscleral signaling pathway [37,89]. COX-1 and COX-2 are normally found in the non-pigmented secretory epithelium of the ciliary body [90]. However, a specific loss of COX-2 occurs in patients with primary open-angle glaucoma [90]. Cannabinoids stimulate the expression of COX-2 and matrix metalloproteinases [46]. These mechanisms induce improved outflow using increased dimensions of Schlemm’s canal or through remodeling the extracellular matrix [46,86,91,92].
5.1. Clinical Studies on the IOP-Lowering Effect of Cannabinoids
There have been several human studies on healthy subjects and patients with ocular hypertension and glaucoma which executed the effect of cannabinoids, especially Δ9-THC, on IOP [8,10]. These studies indicated that not all cannabinoids show the same effect on IOP [8,10]. The reason for this differing effect has not yet been fully elucidated but may be at least partially related to the complex interaction between IOP regulation and cannabinoid receptors [67]. Different routes of administration have been used, including oral, inhalation, intravenous intake, and topical application [8,10]. One of the first studies assessing the IOP-lowering potential of cannabinoids was conducted in 1971 by Hepler and Frank. The authors of this study reported an approximately 25% decrease in IOP in 11 healthy subjects one hour after smoking 18 mg of Δ9-THC [6]. This IOP-lowering capacity of systemically administered THC has later been confirmed in both glaucoma patients and in healthy subjects [8]. In the following, we provide a brief overview on the literature concerning the effects of cannabinoids on IOP. A more detailed overview of the IOP-lowering potential of cannabinoids has been provided in the previously published comprehensive reviews of Passani et al. [8] and Wang and Danesh-Meyer [10].
Most clinical trials have examined orally administered THC. The advantage of orally administered cannabinoids is that exact dosing of the drug is possible. Bioavailability shows 10–20% as there is a high first-pass metabolism [10,88]. Δ9-THC has been administered in doses ranging from 5 mg to 80 mg in previous studies [8]. As such, a variety of clinical studies have investigated the effects of oral Δ9-THC and synthetic cannabinoids, such as nabilone, BW146Y, or dronabinol, and demonstrated transient ocular hypotensive effects [93,94,95,96,97]. This IOP-lowering effect was at its strongest 2–4 h after administration, reaching approximately 10–30% [8]. This absolute change in IOP seemed to be a dose–response relationship showing greater IOP reductions from baseline with increased dosage, but no dose–duration relationship [98,99]. However, these studies only reported transient effects of cannabinoids, raising the issue that frequent daily doses are required for a therapeutic effect. Based on the IOP-lowering effects of 3–4 h [95], medical marijuana would need to be consumed at least 6–8 times a day and glaucoma patients would be placed at risk for substance dependence [99].
In contrast to oral Δ9-THC, orally administered CBD failed to lower IOP. In a randomized, double-masked, placebo-controlled four-way crossover study by Tomida et al., patients with ocular hypertension or early primary open-angle glaucoma received a single sublingual dose of 5 mg Δ9-THC, 20 mg CBD, 40 mg CBD, or placebo [95]. In this study, CBD did not exert an IOP-lowering effect, and the higher dose of 40 mg CBD even resulted in a transient increase in IOP 4 h after administration [95]. However, the latter study was limited to only six subjects [95]. Further evidence by a study from Miller et al. indicated that Δ9-THC lowered IOP in mice, whereas CBD had opposite effects and interfered with the Δ9-THC effects [67]. This can be attributed to the effect of CBD acting as a negative allosteric modulator at CB1 receptors and should be considered as a potential side effect in concern of long-term use [67]. A more recent interest has also developed towards orally administered PEA; it showed IOP-lowering effects in patients with ocular hypertension, glaucoma, and after prophylactic iridotomy [100,101,102].
In addition to orally administered cannabinoids, intravenous and inhaled applications of Δ9-THC showed ocular hypotensive effects [8]. Dosages of inhaled Δ9-THC ranged from 12 mg to 80 mg and IOP reduction ranged from 13% to 34%, respectively [8]. The maximum IOP-lowering effect was reached approximately 90 min after inhaled administration [8]. Inhalation of cannabinoids is limited by their variable bioavailability reaching from 2% to 56% depending on the frequency of puffs, depth of inhalation, and breath hold [10,103]. This makes inhalation difficult for clinical use. Intravenous administration has only been tested on 12 healthy subjects and not yet on glaucoma patients. At doses of 0.022 mg/kg and 0.044 mg/kg or 3.0 mg and 6.7 mg THC, IOP was reduced between 29% and 62%, respectively. The peak IOP reduction was described between 30 min and 90 min after administration [8]. In both documented studies, significant side effects, like euphoria, dizziness, confusion, and pre-syncopal symptoms, were reported [8,104,105].
5.2. Side Effects
The side effects of cannabinoids are related to the route of administration. Many of the reported studies in which cannabinoids were administered to lower IOP reported adverse effects in neurological, cardiovascular, ophthalmological, pulmonary, gastrointestinal, hepatic, renal, dermatological, or muscular systems after administration [34,106,107]. Although cannabinoids exert multiple effects on a variety of different organ systems, the acute psychological effects generally resolve within 2–6 h after application [10,106]. Neurologic side effects include dizziness, drowsiness, anxiety, euphoria, or hallucinations. Frequent digestive or cardiovascular reactions have been reported to include abdominal pain, nausea, vomiting, tachycardia, hyper- or hypotension, or syncope. As for ophthalmological side effects, conjunctival hyperemia, photosensitivity, and blurry vision may occur, thereby limiting long-term clinical use [34].
5.3. Novel Topical Formulations
Systemic side effects may be prevented or at least reduced by topical administration. However, to date, the topical application of cannabinoids continues to face limitations. Cannabinoids are highly lipophilic molecules with low aqueous solubility. After instillation, less than 5% of the applied dose reaches the intraocular tissues, as precorneal factors, such as drainage, non-corneal absorption, or induced lacrimation, limit absorption [56]. Studies which investigated the IOP-lowering effects of topical cannabinoids mostly failed to show significant effects [108,109,110,111]. Different galenical approaches to improve local availability include the use of sesame oil or mineral oil as vehicles for higher solubility, and newer drug delivery systems based on cyclodextrin, prodrugs, and nanoparticles for increased tissue penetration [56,112]. However, these tested formulations suffered from limited tolerability, such as burning sensation or lid swelling, which limited their long time use and enforced the development of better drug delivery vehicles [113,114].
Three studies that showed promising IOP-lowering or tissue-penetrating effects of cyclodextrins, prodrugs, and/or nanoparticles are presented below. In eight glaucoma patients, topical administration of WIN55212-2, a synthetic and selective CB1 receptor agonist, in combination with 2-hydroxylpropyl-β-cyclodextrin resulted in an IOP-lowering effect [91]. The authors described no major side effects and their solution demonstrated good stability and tolerability [8,91]. IOP was significantly reduced by 15 ± 0.5% (25 μg group) and 23 ± 0.9% (50 μg group) 30 min after administration [91]. The IOP-lowering effect was maximal 1 h after administration and tended to dissolve 2 h after administration [91]. The prodrug concept increases water solubility of Δ9-THC without undergoing great chemical modification. An investigated prodrug of Δ9-THC is Δ9-THC-valine-hemisuccinate (THC-VHS). Taskar et al. improved the effect of THC-VHS administered to normotensive rabbits using solid lipid nanoparticles (SLNs), which reportedly resulted in a reduction of IOP which was longer (480 min) than for 2.5% w/v pilocarpine hydrochloride (120 min) and 0.2% w/v timolol maleate (180 min) [115]. The tissue concentration of THC-VHS in the iris-ciliary bodies and retina–choroid was significantly higher with SLNs which may not only hold therapeutic potential for effects on IOP, but also neuroprotection [115]. Another nanoparticle formulation was used by Kabiri et al. [113]. The authors described a nanoparticle-laden hydrogel, where the hydrogel is composed of hyaluronic acid and methylcellulose, along with the amphiphilic nanoparticles of poly(ethylene oxide) and poly(lactic acid) [113]. They loaded the nanoparticles with cannabigerolic acid (CBGA), a close cannabinoid mimic molecule [113]. Corneal drug penetration was 300% higher in comparison to the control group [113]. The authors reported that only 0.015% of the applied CBGA load permeated through the cornea, which was justified through the absence of lachrymal drainage [113]. This method improved both bioavailability and corneal permeation, and reportedly reduced ocular irritation [113]. The amphiphilicity of these nanoparticles is well suited to transport lipophilic molecules, such as cannabinoids, through the cornea [113]. Recent developments of formulations improving the penetration of topically administered lipophilic molecules may be promising to evaluate the role of topically administered cannabinoids on IOP.
6. Neuroprotective Actions of Cannabinoids
Axonal injury can be mediated by ischemic effects, like peripapillary circulation abnormalities, or compression, e.g., through high IOP [116]. Neuroprotection is a strategy to prevent neuronal death, as neurons are postmitotic and cannot be replaced when lost [116]. Since glaucoma leads to a loss of RGCs and degeneration of the optic nerve head via apoptosis, much emphasis has been put into the development of neurodegenerative agents. To date, despite many efforts, no neuroprotective agent has been able to display clinically significant neuroprotective properties in a large, controlled trial or received drug approval from the regulatory agencies. This may be partly due to the fact that we do not yet fully understand why ganglion cells die in glaucoma and what would be an appropriate drug target for a potential neuroprotective approach. Among others, excitotoxicity, mitochondrial dysfunction, protein misfolding, oxidative stress, inflammation, and neurotrophin deprivation have been described as potential targets for neuroprotection in glaucoma [117,118].
6.1. Targeting Mechanisms in Neuroprotection
In the context of cannabinoids as potential neuroprotective agents, glutamate-induced excitotoxicity may be of particular interest. It has been well described that excessive stimulation of neurons by neurotransmitters, such as glutamate, can lead to neuronal death and is usually referred to as glutamate excitotoxicity [117,118]. Glutamate excess and N-methyl-D-aspartate (NMDA) receptor activation induces calcium influx and releases reactive oxygen species (ROS), leading to apoptosis [119]. Several studies have shown that cannabinoids prevent glutamate- or NMDA-induced neurotoxicity in isolated neurons or brains by activating CB1 receptors [120,121,122,123]. Thus, it has been hypothesized that activation of CB1 receptors may mediate neuroprotective effects in neurodegenerative diseases [124]. In contrast, CB1 receptors, which are not expressed in glutamatergic neurons, are thought to trigger cell death pathways and promote a pro-inflammatory response [125]. In glial cells, they may trigger reactive oxygen species/reactive nitrogen species (ROS/RNS) production and inflammatory cell activation [125]. Due to the diversity of these results, further studies are needed to evaluate the role of CB1 receptor signaling and ROS formation, which currently depends on the cell type and context [125].
In addition, the strong anti-inflammatory potential of the CB2 receptor signaling pathway makes cannabinoids another promising approach in neuroprotection [124]. Neuroinflammation is associated with microglial and/or astroglial cell activation triggered by pro-inflammatory factors, like cyto-/chemokines or elevated levels of ROS/RNS, ultimately leading to neuronal death [125]. The neuroprotective effect of CB2 receptor agonists in neurodegenerative diseases attenuates microglial activation by reducing ROS/RNS production, preventing leucocyte recruitment, attenuating vascular inflammation, and improving blood–brain barrier dysfunction [125]. Perivascular microglia exhibit high CB2 expression levels, suggesting that CB2 receptor activation improves the cerebral blood flow in neuroinflammatory diseases [126].
TRPV receptors are novel targets for developing treatment strategies in glaucoma. Since the TRPV1 receptor is located in neuronal tissues, such as the retina, this receptor has been mostly studied for its neuroprotective effects [60]. The death of RGCs is triggered at high doses of TRPV1 agonists, which can be explained by the following two mechanisms. Leonelli et al. suggested that this pro-apoptotic effect of TRPV1 under stress is dependent on nitric oxide synthase and NMDA-mediated glutamatergic excitotoxicity [127,128]. TRPV1 activation can be either be achieved through direct activation or indirectly through other endogenous ligands in the retina, such as endothelin-1 (ET-1) [129]. ET-1, which is upregulated in the glaucomatous eye, can potentiate the activity of TRPV1 [130]. In glaucoma patients, local expression of TRPV1 receptors in the retina is increased due to the high IOP [60,131]. TRPV1 receptors lead to an extracellular calcium influx, which in return leads to a net hyperpolarization on ganglion cell firing rates [60,131]. Overall, this protects the RGCs by reducing excessive neuronal cell activity and alleviating stress and metabolic demands on the cells [74,131]. Weitlauf et al. proved this hypothesis using mice that lacked TRPV1 receptors and showed that when increasing the IOP, TRPV1 -/- RGCs did not show any compensatory increase of TRPV receptors along with no according increase in firing rates [131]. This supports the idea that TRPV1 receptors promote neuronal survival in response to disease-relevant stressors through transient enhancement of local excitation at postsynaptic sites [131].
6.2. Evidence for Neuroprotection
As stated above, clinical evidence for the neuroprotective effects of cannabinoids in human studies is sparse. There are conceptual and methodological problems that make it difficult to transfer preclinical results to clinical glaucoma practice [132]. Reasons include the paucity of adequate animal models that can fully mimic human diseases along with the difficulty to predict the ocular bioavailability of drugs in humans [132,133]. Moreover, in human trials, the intervention is performed after diagnosis, whereas in most experimental studies, the neuroprotective agent is administered at the time of or prior to the injury [132]. Furthermore, outcome measures in the clinic are, for ethical reasons, functional outcomes that take weeks or months to show changes, whereas animal studies mostly employ histopathologic endpoints [132,133].
Preclinical studies have demonstrated neuroprotective effects of cannabinoids, but the specific mechanisms and therapeutic potential of the targeted receptors are not yet fully understood. Agonism and antagonism of the same receptors showed neuroprotective effects, which may depend on the used model, time of treatment, dosage, or individual differences [60]. Cannabinoid administration reduced RGC loss in rat animal models with episcleral vessel cauterization-induced glaucoma or saline-induced acute IOP elevation via CB1 receptor agonism [134,135,136,137]. Fourteen rats with unilateral glaucoma induced through cauterization of episcleral vessels received 5 mg/kg Δ9-THC or ethanol solution via intraperitoneal administration weekly for 20 weeks [134]. In this study, Δ9-THC induced a 10–20% reduction of RGC loss, while RGC loss was 40–50% in the control group [134]. Further animal models on rats examining optic nerve head neuroprotection were performed on optic nerve crush injury [138,139]. In an experimental rat model, reduction of injury-induced metabolic and electrophysiological deficits were achieved during a course of six hours after optic nerve axotomy [139]. A synthetic non-psychotropic cannabinoid and NMDA agonist, HU-211, was administered once intraperitoneally and yielded the positive result of regenerative growth and axonal sprouting 30 days after the initial injury, which in return was absent in control animals [138]. The neuroprotective effects of cannabinoids have been investigated in different animal models of ocular diseases and neurodegeneration [9,60]. In a streptozotocin-induced diabetic retinopathy rat model, CBD was found to reduce neurotoxicity, oxidative stress, damage to the blood–retinal barrier, inflammation, and retinal cell death through the inhibition of p38 MAP kinase [140]. In retinitis pigmentosa animal models, the synthetic cannabinoid HU-210 and a CB1 receptor antagonist SR141716A attenuated photoreceptor and retinal degeneration [141,142]. CB1 and CB2 receptor agonism via eCBs, 2-AG, or AEA hampers microglial activation and RGC loss in rat models, including the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway [143,144]. In contrary, Maccarone et al. suggested the CB1, and more pronounced CB2 receptor antagonism as a possible neuroprotective mechanism since they reduced photoreceptor neurodegeneration [145]. CB1/CB2 receptor antagonism is similarly described and therefore it suggests a common pattern of response in retinal damage [145]. Since TRPV1 increases in RGCs due to elevated pressure in the eye, antagonism of the channel was able to reduce RGC apoptosis [146]. In contrast to antagonizing TRVP1, others have shown that TRPV1 receptor agonists can inhibit apoptosis in RGCs and protect against RGC inactivation [137,147]. Contrary to the studies mentioned, some models also reported neurodysfunctional effects due to cannabinoids and their receptors. Two months of systemic treatment with Δ9-THC resulted in toxic effects on photoreceptor cells in mice through functional loss in electroretinography and apoptosis [148]. For a detailed overview of other studies on neuroprotection in ocular diseases, the reader is referred to more extensive reviews [9,20,60]. An excellent overview on in vivo animal glaucoma models is provided by Gallo Afflitto et al. [60].
To date, the evidence from clinical trials for a potential neuroprotective effect of cannabinoids is sparse. PEA, an endocannabinoid-like molecule, showed promising effects in a single center, randomized, prospective, single-blinded, two treatment, two period crossover study by Rossi et al. [149]. The authors demonstrated that orally administered PEA doses of 600 mg/day for four months increased the electric activity of RGCs and retina that were measured using pattern evoked electroretinograms (PERGs) in patients with stable primary open-angle glaucoma and normal tension glaucoma. In addition, this study showed that PEA lowered the IOP significantly [149]. These authors discussed that both IOP-lowering and direct neuroprotective effects are involved in RGC survival, and in this way, influence each other [149]. It has previously been shown that PEA reduces IOP in glaucoma patients and that this indirectly contributes to neuroprotection [149]. Alternatively, PEA showed vasoactive properties through vasorelaxation of the ophthalmic artery via PPARα transcription factors in other studies, which suggests that the neuroprotective effect of cannabinoids may be related to their vasodilatation capabilities [8,150].
Challenging the hypothesis of a neuroprotective effect of cannabinoids, there is also evidence indicating that cannabinoids might be harmful and may lead to neuroretinal dysfunction. Studies by Schwitzer et al. showed delayed responses in ganglion cells and bipolar cells in electroretinography and delayed transmission of visual information in regular cannabis users [151,152,153]. Increased retinal background noise, as resting-state neural activity, was increased in regular cannabis users [154]. In most studies, the dosage of the cannabinoid was difficult to compare as it was self-reported by the subjects [155]. However, these studies might be limited by the concomitant use of other drugs, such as alcohol and tobacco, which may thereby limit the general applicability of the results [155]. As mentioned for the preclinical studies, the conflicting results of these clinical studies also suggest that the molecular mechanisms and effects of cannabinoids on ocular structures and visual function are poorly understood, and further trials are required.
7. Vascular Targets of Cannabinoids and the Effect on Ocular Hemodynamics
In addition to IOP, it has been hypothesized that vascular risk factors play a role in the pathogenesis in glaucoma [156]. The hypothesis that vascular impairment may contribute to the pathogenesis of glaucomatous optic neuropathy implies that ganglion cell damage is a consequence of vascular dysregulation and/or insufficient blood supply in the optic nerve head [14,157,158,159,160]. Using a variety of different methods, it has been shown that ocular perfusion and oxygen extraction are reduced in patients with glaucoma, indicating that local hypoxia may play a role in the pathogenesis of this disease [161,162,163,164,165]. However, it has also been suggested that glaucoma is associated with dysregulated perfusion rather than reduced blood flow [159,166]. As such, there is evidence that vascular autoregulation, an intrinsic mechanism of the retinal vasculature to keep the perfusion pressure constant, may be compromised in patients with glaucoma [167]. Along this line of thought, previous evidence indicated an abnormal correlation between optic nerve head perfusion and ocular perfusion pressure in patients with glaucoma [168], which was normalized when IOP was lowered [169]. Further, neuro-vascular coupling is reduced in patients with glaucoma, an effect that seems to be largely independent from IOP [170]. For an in-depth discussion regarding the role of ocular perfusion in glaucoma, the reader is referred to previously published articles (as outlined in Scheme 1) [14,159,171].
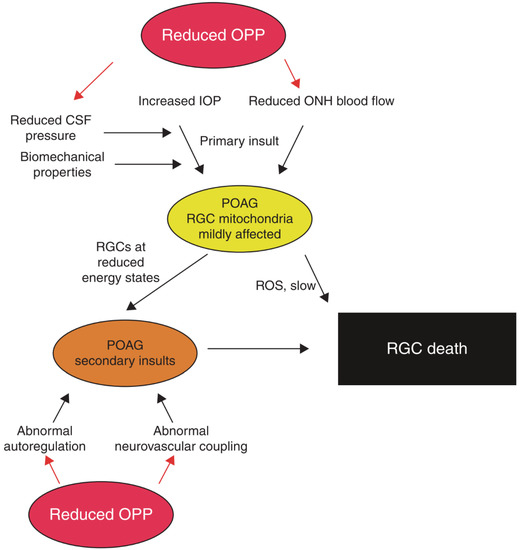
Scheme 1.
This overview shows the primary and secondary insults in glaucoma pathogenesis influenced by reduced ocular perfusion pressure (OPP). Elevated intraocular pressure (IOP) and optic nerve head (ONH) ischemia lead to the primary insult at the optic nerve head. Biomechanical properties of ocular tissues and reduced cerebrospinal fluid (CSF) can alter susceptibility. In patients with primary open-angle glaucoma (POAG), retinal ganglion cells (RGCs) then exhibit reduced energy states as the mitochondria are affected. The combination of oxidative stress caused by reactive oxygen species (ROS) and secondary insults, like abnormal autoregulation and abnormal neurovascular coupling, may lead to RGC death, which is the end of the pathway. “Reprinted with permission form Ref. [14]. 2013, Elsevier”.
7.1. Vascular Targets
The effects of cannabinoids on the ocular vasculature are insufficiently described. A full overview of the vascular targets of cannabinoids is beyond the scope of this review and the reader is referred to comprehensive recent review articles [172,173,174]. In the following, a short overview of the most important vascular drug targets and mechanisms has been provided.
Vascular responses seem to be mainly mediated through the involvement of the cannabinoid receptors CB1 and CB2, as well as non-cannabinoid receptors, such as the endothelial cannabinoid receptor (CBe) and vanilloid receptors [172,174,175]. In addition, cannabinoids influence the actions of vasoactive compounds, like angiotensin II, acetylcholine, methoxamine, and thromboxane receptor agonists [172]. Cannabinoids induce the lowering of blood pressure and vasorelaxation [175]. The proposed mechanisms have been mainly studied with regard to CB1 receptors in resistance vessels [173]. Most studies have shown that cannabinoids mediate vasorelaxant effects at least partially via CB1 receptors [172]. The definitive pathway is not completely understood but depending on receptor location in the perivascular nerves or in the blood vessels, two possible mechanisms are suggested. When the CB1 receptors are located in the perivascular nerves, they inhibit the release of norepinephrine from sympathetic nerve terminals [175]. Depending on the location of the CB1 receptors in the smooth muscle cell layer or in the endothelial cells, different intracellular pathways will be activated. Pathways that activate the CB1 receptor in the smooth muscle cells include the inhibition of L-type calcium channels, adenylyl cyclase, and initiation of MAPK-mediated cascades. Activation of the CB1 receptor in endothelial cells induce the synthesis or release of nitric oxide, endothelial-derived hyperpolarizing factors, or prostacyclin [173].
CB2 receptors also promote vasorelaxation, but not much is currently known about the molecular pathways. Here, it has been hypothesized that activation of phospholipase C and formation of inositol 1,4,5-triphosphate mediates vasorelaxation [176].
As previously mentioned, CB1 and CB2 receptors inhibit adenylyl cyclase. In contrast, noradrenaline, as part of the sympathetic nervous system, activates adenylyl cyclase, which results in the release of calcium and vasoconstriction. These two counterregulatory systems indicate that cannabinoids through the activation of one system lead to the inhibition of the second counterregulatory system [177]. With simultaneous upregulation of the ECS, the sympathetic nervous system and vasoconstrictors of the renin–angiotensin–aldosterone system (RAAS) appear to be oppositely regulated [177].
Furthermore, eCBs regulate vasoactivity not only in the peripheral vasculature, but also in the CNS microvasculature [69]. Previous studies described that the effects of the eCB AEA on vessels are not only attributed to the CB1 receptor [178]. As the vascular tissue tone depends on ET-1 and NO, an in vitro study by Ronco et al. examined the effects of the endogenous cannabinoid receptor agonist AEA in human endothelial cells. The authors speculated that AEA leads to vasodilatation via non-CB1 receptor-dependent inhibition of ET-1 production and CB1-mediated increase of NO, in this way, increasing the vasodilatation/vasoconstriction ratio [179]. In another in vitro study by MacIntyre et al., ET-1-mediated vasoconstriction in retinal arterioles was shown to be inhibited by abnormal CBD (Abn-CBD), a CBD analog and agonist at the putative O-1918-sensitive endothelial cannabinoid receptor (CBe). Virtually similar effects have been demonstrated for N-arachidonoyl glycine, an endogenous eCB and putative agonist for GPR18, which the authors detected in the endothelium of retinal vessels [69]. This indicates that cannabinoids mediate changes in vascular resistance through endothelium-dependent signaling mechanisms on a local basis independent of nitric oxide and involving novel endothelial non-CB1/CB2 receptor targets [180]. These findings may be of particular clinical interest since ET-1 is a highly potent vasoconstrictor and its levels are elevated in glaucoma patients and animal models of glaucoma [22]. The endothelin receptors 1A and 1B are both present in the region of the optic nerve head [181].
7.2. Evidence for Hemodynamic Effects
When cannabinoids and eCBs are applied to isolated arteries or perfused vascular beds, they exhibit changes in vascular resistance [172,174]. In vitro studies with eCBs and synthetic cannabinoid agonists in isolated arteries showed predominantly vasorelaxant effects, but there is also evidence for vasoconstrictor responses [172,174]. In the bovine ophthalmic artery, the endogenous cannabinoid ligand AEA and the synthetic cannabinoid agonist WIN55212-2 induced relaxation through involving the CB1 receptor-sensitive pathway [182]. Furthermore, it has been described that the effect on the vessels depends on the basal vascular tone, since in isolated retinal arterioles vasodilatory actions were only shown in precontracted vessels [180].
In anesthetized animals, cannabinoids induced prolonged hypotension, whereas pressor responses were noted in conscious animals [174]. The reason for these differing results may be related to the fact that the effects of cannabinoids appear to be dependent on the species, type of cannabinoids, and experimental preparation [172,183]. In a study with rabbits, Δ9-THC increased blood flow in the iris, ciliary processes, and choroid, but not the retina [184]. This was interpreted by the authors as dilatation of vessels leading away from the anterior uvea. Administration of Δ9-THC leads to vasodilatation of conjunctival vessels and redness of the eye, demonstrating that cannabinoids exert vasoactive effects on the anterior eye segment [185].
Much evidence of blood flow alteration using cannabinoids has been studied in the cerebral circulation. As the optic nerve is part of the CNS, these findings may be of interest for blood flow in the retina or optic nerve head. A large systematic review by Ogunbiyi et al. summarized the effects of inhaled and intravenous THC on cerebral blood flow in human and animal studies [186]. Notably, acute THC administration caused an increase in the cerebral blood flow, whereas chronic cannabis use resulted in a decrease in the cerebral blood flow [186]. The authors attributed a vasodilatory effect for the increase in blood flow and a downregulation of CB1 receptors as tolerance after prolonged exposed activation, resulting in an opposite effect. This effect of decreased cerebral blood flow in chronic cannabis users can be reversed after prolonged abstinence from the drug [186].
With respect to retinal hemodynamics, it has been reported that orally administered 7.5 mg dronabinol resulted in a significant decreased retinal arteriovenous passage time from 1.77 ± 0.35 s to 1.57 ± 0.31 s (p = 0.028) in eight healthy subjects without adverse respiratory or cardiovascular side effects [96], indicating an increase in retinal blood flow. In another study, the arteriolar and venular diameters of 55 frequent cannabis users and 51 control subjects were compared using retinal imaging [187]. Cannabis users had, on average, larger arteriolar diameters, which was interpreted as a retinal vasodilatory effect of cannabinoids [187]. A study performed in our laboratory revealed that the oral intake of 5 mg dronabinol significantly increased optic nerve head blood flow in 24 healthy subjects without affecting the retinal autoregulatory response to increased perfusion pressure (Figure 2) [188]. Whether cannabinoids may be a future option to therapeutically increase ocular perfusion in patents with glaucoma or other ocular vascular disorders has yet to be clarified [188].
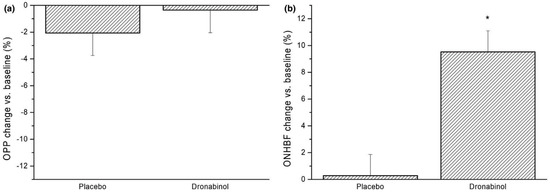
Figure 2.
Dronabinol and optic nerve head blood flow in healthy subjects. On the left side (a), the relative changes in OPP after administration of placebo and dronabinol (−2.1 ± 8.4% vs. −0.4 ± 8.2%, respectively, p = 0.48 between study days) are shown. On the right side (b), the relative change in optic nerve head blood flow (ONHBF) at rest was increased by 9.5 ± 8.1% after dronabinol intake (p < 0.001). Data are presented as mean ± SD (n = 24). * Significant changes vs. baseline. “Reprinted from Ref. [188]. This work is licensed under the Creative Commons Attribution 4.0 International (CC BY 4.0) license. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/”.
8. Conclusions and Future Perspectives
The potential of cannabinoids to lower IOP is limited due to their short-lasting effects and systemic toxicity, as high doses are often required. Overall, there is weak evidence that cannabinoids other than THC, which is mainly responsible for psychotropic side effects, can lower intraocular pressure. For this reason, cannabinoids are not currently recommended for clinical use in glaucoma. To counteract the psychotropic effects, further research is required on non-psychotropic cannabinoids in neurodegenerative diseases. Moreover, new promising molecules for topical application of cannabinoids are in their late stages of testing. In addition, neuroprotective effects of cannabinoids appear promising in animal models, but conflicting results indicate that the mechanisms on neuronal survival are poorly understood. Nevertheless, the anti-oxidative, anti-inflammatory, and vasodilatory properties may represent a new opportunity for neurodegenerative diseases, including glaucoma. Further studies under clinical conditions with larger populations and longer follow-up times would be needed to find out more about the molecular mechanisms and long-term effects of cannabinoids in glaucoma patients.
Author Contributions
Conceptualization, T.L., D.S. and G.G.; Literature Research and Writing—Original Draft Preparation, T.L., L.P. and G.G.; Writing—Review and Editing, T.L., D.S., L.P., V.P., A.P.-C., J.C., L.S. and G.G.; Visualization, T.L. and L.P.; Supervision, D.S. and G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Austrian Science Fund FWF (project KLI 854).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Open Access Funding by the Austrian Science Fund (FWF).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schilling, S.; Melzer, R.; McCabe, P.F. Cannabis sativa. Curr. Biol. 2020, 30, R8–R9. [Google Scholar] [CrossRef]
- Crocq, M.A. History of cannabis and the endocannabinoid system. Dialogues Clin. Neurosci. 2020, 22, 223–228. [Google Scholar] [CrossRef]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef]
- MacDonald, E.; Adams, A. CADTH Rapid Response Reports. In The Use of Medical Cannabis with Other Medications: A Review of Safety and Guidelines—An Update; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2019. [Google Scholar]
- Stasiłowicz, A.; Tomala, A.; Podolak, I.; Cielecka-Piontek, J. Cannabis sativa L. as a Natural Drug Meeting the Criteria of a Multitarget Approach to Treatment. Int. J. Mol. Sci. 2021, 22, 778. [Google Scholar] [CrossRef] [PubMed]
- Hepler, R.S.; Frank, I.R. Marihuana smoking and intraocular pressure. JAMA 1971, 217, 1392. [Google Scholar] [CrossRef]
- Tomida, I.; Pertwee, R.G.; Azuara-Blanco, A. Cannabinoids and glaucoma. Br. J. Ophthalmol. 2004, 88, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Passani, A.; Posarelli, C.; Sframeli, A.T.; Perciballi, L.; Pellegrini, M.; Guidi, G.; Figus, M. Cannabinoids in Glaucoma Patients: The Never-Ending Story. J. Clin. Med. 2020, 9, 3978. [Google Scholar] [CrossRef] [PubMed]
- Rapino, C.; Tortolani, D.; Scipioni, L.; Maccarrone, M. Neuroprotection by (endo)Cannabinoids in Glaucoma and Retinal Neurodegenerative Diseases. Curr. Neuropharmacol. 2018, 16, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.T.M.; Danesh-Meyer, H.V. Cannabinoids and the eye. Surv. Ophthalmol. 2021, 66, 327–345. [Google Scholar] [CrossRef]
- Nucci, C.; Bari, M.; Spanò, A.; Corasaniti, M.; Bagetta, G.; Maccarrone, M.; Morrone, L.A. Potential roles of (endo)cannabinoids in the treatment of glaucoma: From intraocular pressure control to neuroprotection. Prog. Brain Res. 2008, 173, 451–464. [Google Scholar] [CrossRef]
- Cairns, E.A.; Baldridge, W.H.; Kelly, M.E. The Endocannabinoid System as a Therapeutic Target in Glaucoma. Neural Plast. 2016, 2016, 9364091. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Cherecheanu, A.P.; Garhofer, G.; Schmidl, D.; Werkmeister, R.; Schmetterer, L. Ocular perfusion pressure and ocular blood flow in glaucoma. Curr. Opin. Pharmacol. 2013, 13, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Pezzullo, L.; Streatfeild, J.; Simkiss, P.; Shickle, D. The economic impact of sight loss and blindness in the UK adult population. BMC Health Serv. Res. 2018, 18, 63. [Google Scholar] [CrossRef]
- Coleman, A.L.; Miglior, S. Risk factors for glaucoma onset and progression. Surv. Ophthalmol. 2008, 53 (Suppl. S1), S3–S10. [Google Scholar] [CrossRef]
- Schmidl, D.; Schmetterer, L.; Garhöfer, G.; Popa-Cherecheanu, A. Pharmacotherapy of glaucoma. J. Ocul. Pharmacol. Ther. 2015, 31, 63–77. [Google Scholar] [CrossRef]
- Lu, L.J.; Tsai, J.C.; Liu, J. Novel Pharmacologic Candidates for Treatment of Primary Open-Angle Glaucoma. Yale J. Biol. Med. 2017, 90, 111–118. [Google Scholar]
- Shalaby, W.S.; Shankar, V.; Razeghinejad, R.; Katz, L.J. Current and new pharmacotherapeutic approaches for glaucoma. Expert Opin. Pharmacother. 2020, 21, 2027–2040. [Google Scholar] [CrossRef]
- Wang, S.Y.; Singh, K. Management of the glaucoma patient progressing at low normal intraocular pressure. Curr. Opin. Ophthalmol. 2020, 31, 107–113. [Google Scholar] [CrossRef]
- Bengtsson, B. The prevalence of glaucoma. Br. J. Ophthalmol. 1981, 65, 46–49. [Google Scholar] [CrossRef]
- Yorio, T.; Krishnamoorthy, R.; Prasanna, G. Endothelin: Is it a contributor to glaucoma pathophysiology? J. Glaucoma 2002, 11, 259–270. [Google Scholar] [CrossRef]
- Guo, Y.; Shrestha, M.; Marshak-Rothstein, A.; Gregory-Ksander, M.S. Caspase-8-mediated inflammation but not apoptosis drives the development of glaucoma. Investig. Ophthalmol. Vis. Sci. 2021, 62, 2350. [Google Scholar]
- Evangelho, K.; Mogilevskaya, M.; Losada-Barragan, M.; Vargas-Sanchez, J.K. Pathophysiology of primary open-angle glaucoma from a neuroinflammatory and neurotoxicity perspective: A review of the literature. Int. Ophthalmol. 2019, 39, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Adornetto, A.; Rombolà, L.; Morrone, L.A.; Nucci, C.; Corasaniti, M.T.; Bagetta, G.; Russo, R. Natural Products: Evidence for Neuroprotection to Be Exploited in Glaucoma. Nutrients 2020, 12, 3158. [Google Scholar] [CrossRef] [PubMed]
- Sim, R.H.; Sirasanagandla, S.R.; Das, S.; Teoh, S.L. Treatment of Glaucoma with Natural Products and Their Mechanism of Action: An Update. Nutrients 2022, 14, 534. [Google Scholar] [CrossRef]
- Alves, V.L.; Gonçalves, J.L.; Aguiar, J.; Teixeira, H.M.; Câmara, J.S. The synthetic cannabinoids phenomenon: From structure to toxicological properties. A review. Crit. Rev. Toxicol. 2020, 50, 359–382. [Google Scholar] [CrossRef]
- Turner, S.E.; Williams, C.M.; Iversen, L.; Whalley, B.J. Molecular Pharmacology of Phytocannabinoids. Prog. Chem. Org. Nat. Prod. 2017, 103, 61–101. [Google Scholar] [CrossRef]
- An, D.; Peigneur, S.; Hendrickx, L.A.; Tytgat, J. Targeting Cannabinoid Receptors: Current Status and Prospects of Natural Products. Int. J. Mol. Sci. 2020, 21, 5064. [Google Scholar] [CrossRef]
- Alves, P.; Amaral, C.; Teixeira, N.; Correia-da-Silva, G. Cannabis sativa: Much more beyond Δ9-tetrahydrocannabinol. Pharmacol. Res. 2020, 157, 104822. [Google Scholar] [CrossRef]
- Metna-Laurent, M.; Mondésir, M.; Grel, A.; Vallée, M.; Piazza, P.V. Cannabinoid-Induced Tetrad in Mice. Curr. Protoc. Neurosci. 2017, 80, 9–59. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef]
- Martínez-Pinilla, E.; Varani, K.; Reyes-Resina, I.; Angelats, E.; Vincenzi, F.; Ferreiro-Vera, C.; Oyarzabal, J.; Canela, E.I.; Lanciego, J.L.; Nadal, X.; et al. Binding and Signaling Studies Disclose a Potential Allosteric Site for Cannabidiol in Cannabinoid CB2 Receptors. Front. Pharmacol. 2017, 8, 744. [Google Scholar] [CrossRef]
- Roque-Bravo, R.; Silva, R.S.; Malheiro, R.F.; Carmo, H.; Carvalho, F.; da Silva, D.D.; Silva, J.P. Synthetic Cannabinoids: A Pharmacological and Toxicological Overview. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 187–209. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. FDA and Cannabis: Research and Drug Approval Process. Available online: https://www.fda.gov/news-events/public-health-focus/fda-and-cannabis-research-and-drug-approval-process (accessed on 29 June 2023).
- Accelerated Cure Project for Multiple Sclerosis. Sativex—The Road to FDA Approval. Available online: https://www.acceleratedcure.org/sites/default/files/images/Sativex-TheRoadtoFDAApproval.pdf (accessed on 29 June 2023).
- Yazulla, S. Endocannabinoids in the retina: From marijuana to neuroprotection. Prog. Retin. Eye. Res. 2008, 27, 501–526. [Google Scholar] [CrossRef] [PubMed]
- Aiello, F.; Gallo Afflitto, G.; Li, J.O.; Martucci, A.; Cesareo, M.; Nucci, C. CannabinEYEds: The Endocannabinoid System as a Regulator of the Ocular Surface Nociception, Inflammatory Response, Neovascularization and Wound Healing. J. Clin. Med. 2020, 9, 4036. [Google Scholar] [CrossRef] [PubMed]
- Schwitzer, T.; Schwan, R.; Angioi-Duprez, K.; Giersch, A.; Laprevote, V. The Endocannabinoid System in the Retina: From Physiology to Practical and Therapeutic Applications. Neural Plast. 2016, 2016, 2916732. [Google Scholar] [CrossRef]
- Cairns, E.A.; Toguri, J.T.; Porter, R.F.; Szczesniak, A.M.; Kelly, M.E. Seeing over the horizon-targeting the endocannabinoid system for the treatment of ocular disease. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615. [Google Scholar] [CrossRef]
- Di Marzo, V.; Piscitelli, F. The Endocannabinoid System and its Modulation by Phytocannabinoids. Neurotherapeutics 2015, 12, 692–698. [Google Scholar] [CrossRef]
- Chen, J.; Matias, I.; Dinh, T.; Lu, T.; Venezia, S.; Nieves, A.; Woodward, D.F.; Di Marzo, V. Finding of endocannabinoids in human eye tissues: Implications for glaucoma. Biochem. Biophys. Res. Commun. 2005, 330, 1062–1067. [Google Scholar] [CrossRef]
- Haspula, D.; Clark, M.A. Cannabinoid Receptors: An Update on Cell Signaling, Pathophysiological Roles and Therapeutic Opportunities in Neurological, Cardiovascular, and Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 7693. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.; Kendall, D.A. The complications of promiscuity: Endocannabinoid action and metabolism. Br. J. Pharmacol. 2007, 152, 602–623. [Google Scholar] [CrossRef] [PubMed]
- Rösch, S.; Ramer, R.; Brune, K.; Hinz, B. R(+)-methanandamide and other cannabinoids induce the expression of cyclooxygenase-2 and matrix metalloproteinases in human nonpigmented ciliary epithelial cells. J. Pharmacol. Exp. Ther. 2006, 316, 1219–1228. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Pertwee, R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997, 74, 129–180. [Google Scholar] [CrossRef]
- Howlett, A.C. Cannabinoid receptor signaling. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2005; pp. 53–79. [Google Scholar] [CrossRef]
- Szabo, B.; Schlicker, E. Effects of cannabinoids on neurotransmission. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2005; pp. 327–365. [Google Scholar] [CrossRef]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef]
- Straiker, A.J.; Maguire, G.; Mackie, K.; Lindsey, J. Localization of cannabinoid CB1 receptors in the human anterior eye and retina. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2442–2448. [Google Scholar]
- Somvanshi, R.K.; Zou, S.; Kadhim, S.; Padania, S.; Hsu, E.; Kumar, U. Cannabinol modulates neuroprotection and intraocular pressure: A potential multi-target therapeutic intervention for glaucoma. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166325. [Google Scholar] [CrossRef]
- Järvinen, T.; Pate, D.W.; Laine, K. Cannabinoids in the treatment of glaucoma. Pharmacol. Ther. 2002, 95, 203–220. [Google Scholar] [CrossRef]
- Murataeva, N.; Miller, S.; Dhopeshwarkar, A.; Leishman, E.; Daily, L.; Taylor, X.; Morton, B.; Lashmet, M.; Bradshaw, H.; Hillard, C.J.; et al. Cannabinoid CB2R receptors are upregulated with corneal injury and regulate the course of corneal wound healing. Exp. Eye Res. 2019, 182, 74–84. [Google Scholar] [CrossRef]
- Zhong, L.; Geng, L.; Njie, Y.; Feng, W.; Song, Z.H. CB2 cannabinoid receptors in trabecular meshwork cells mediate JWH015-induced enhancement of aqueous humor outflow facility. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1988–1992. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, X.; Wang, L. Presence and regulation of cannabinoid receptors in human retinal pigment epithelial cells. Mol. Vis. 2009, 15, 1243–1251. [Google Scholar]
- Gallo Afflitto, G.; Aiello, F.; Scuteri, D.; Bagetta, G.; Nucci, C. CB1R, CB2R and TRPV1 expression and modulation in in vivo, animal glaucoma models: A systematic review. Biomed. Pharmacother. 2022, 150, 112981. [Google Scholar] [CrossRef]
- Morales, P.; Jagerovic, N. Advances Towards The Discovery of GPR55 Ligands. Curr. Med. Chem. 2016, 23, 2087–2100. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, J.; Lehmann, C. GPR55—A putative “type 3” cannabinoid receptor in inflammation. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 297–302. [Google Scholar] [CrossRef]
- Kumar, A.; Qiao, Z.; Kumar, P.; Song, Z.H. Effects of palmitoylethanolamide on aqueous humor outflow. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4416–4425. [Google Scholar] [CrossRef]
- Bouskila, J.; Javadi, P.; Casanova, C.; Ptito, M.; Bouchard, J.F. Rod photoreceptors express GPR55 in the adult vervet monkey retina. PLoS ONE 2013, 8, e81080. [Google Scholar] [CrossRef]
- Balenga, N.A.; Aflaki, E.; Kargl, J.; Platzer, W.; Schröder, R.; Blättermann, S.; Kostenis, E.; Brown, A.J.; Heinemann, A.; Waldhoer, M. GPR55 regulates cannabinoid 2 receptor-mediated responses in human neutrophils. Cell Res. 2011, 21, 1452–1469. [Google Scholar] [CrossRef]
- Kohno, M.; Hasegawa, H.; Inoue, A.; Muraoka, M.; Miyazaki, T.; Oka, K.; Yasukawa, M. Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem. Biophys. Res. Commun. 2006, 347, 827–832. [Google Scholar] [CrossRef]
- Miller, S.; Daily, L.; Leishman, E.; Bradshaw, H.; Straiker, A. Δ9-Tetrahydrocannabinol and Cannabidiol Differentially Regulate Intraocular Pressure. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5904–5911. [Google Scholar] [CrossRef]
- Caldwell, M.D.; Hu, S.S.; Viswanathan, S.; Bradshaw, H.; Kelly, M.E.; Straiker, A. A GPR18-based signalling system regulates IOP in murine eye. Br. J. Pharmacol. 2013, 169, 834–843. [Google Scholar] [CrossRef]
- MacIntyre, J.; Dong, A.; Straiker, A.; Zhu, J.; Howlett, S.E.; Bagher, A.; Denovan-Wright, E.; Yu, D.Y.; Kelly, M.E. Cannabinoid and lipid-mediated vasorelaxation in retinal microvasculature. Eur. J. Pharmacol. 2014, 735, 105–114. [Google Scholar] [CrossRef]
- Miller, S.; Hu, S.S.; Leishman, E.; Morgan, D.; Wager-Miller, J.; Mackie, K.; Bradshaw, H.B.; Straiker, A. A GPR119 Signaling System in the Murine Eye Regulates Intraocular Pressure in a Sex-Dependent Manner. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2930–2938. [Google Scholar] [CrossRef]
- Montell, C. The TRP superfamily of cation channels. Sci. STKE 2005, 2005, re3. [Google Scholar] [CrossRef]
- Yang, T.J.; Yu, Y.; Yang, J.Y.; Li, J.J.; Zhu, J.Y.; Vieira, J.A.C.; Jiang, Q. Involvement of transient receptor potential channels in ocular diseases: A narrative review. Ann. Transl. Med. 2022, 10, 839. [Google Scholar] [CrossRef]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2018, 11, 487. [Google Scholar] [CrossRef]
- Sappington, R.M.; Sidorova, T.; Long, D.J.; Calkins, D.J. TRPV1: Contribution to retinal ganglion cell apoptosis and increased intracellular Ca2+ with exposure to hydrostatic pressure. Investig. Ophthalmol. Vis. Sci. 2009, 50, 717–728. [Google Scholar] [CrossRef]
- Luo, N.; Conwell, M.D.; Chen, X.; Kettenhofen, C.I.; Westlake, C.J.; Cantor, L.B.; Wells, C.D.; Weinreb, R.N.; Corson, T.W.; Spandau, D.F.; et al. Primary cilia signaling mediates intraocular pressure sensation. Proc. Natl. Acad. Sci. USA 2014, 111, 12871–12876. [Google Scholar] [CrossRef]
- Patel, P.D.; Chen, Y.L.; Kasetti, R.B.; Maddineni, P.; Mayhew, W.; Millar, J.C.; Ellis, D.Z.; Sonkusare, S.K.; Zode, G.S. Impaired TRPV4-eNOS signaling in trabecular meshwork elevates intraocular pressure in glaucoma. Proc. Natl. Acad. Sci. USA 2021, 118, e2022461118. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, Y.; Zhang, S.; Sun, X.; Wu, J. TRPV4-induced Müller cell gliosis and TNF-α elevation-mediated retinal ganglion cell apoptosis in glaucomatous rats via JAK2/STAT3/NF-κB pathway. J. Neuroinflamm. 2021, 18, 271. [Google Scholar] [CrossRef]
- Taylor, L.; Arnér, K.; Ghosh, F. Specific inhibition of TRPV4 enhances retinal ganglion cell survival in adult porcine retinal explants. Exp. Eye Res. 2017, 154, 10–21. [Google Scholar] [CrossRef]
- Choi, H.J.; Sun, D.; Jakobs, T.C. Astrocytes in the optic nerve head express putative mechanosensitive channels. Mol. Vis. 2015, 21, 749–766. [Google Scholar]
- Ryskamp, D.A.; Witkovsky, P.; Barabas, P.; Huang, W.; Koehler, C.; Akimov, N.P.; Lee, S.H.; Chauhan, S.; Xing, W.; Rentería, R.C.; et al. The polymodal ion channel transient receptor potential vanilloid 4 modulates calcium flux, spiking rate, and apoptosis of mouse retinal ganglion cells. J. Neurosci. 2011, 31, 7089–7101. [Google Scholar] [CrossRef]
- Burstein, S. PPAR-gamma: A nuclear receptor with affinity for cannabinoids. Life Sci. 2005, 77, 1674–1684. [Google Scholar] [CrossRef]
- Berger, J.; Moller, D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef]
- Aebersold, A.S.; Song, Z.H. The Effects of Cannabidiol on Aqueous Humor Outflow and Trabecular Meshwork Cell Signaling. Cells 2022, 11, 3006. [Google Scholar] [CrossRef]
- Thapa, D.; Cairns, E.A.; Szczesniak, A.M.; Toguri, J.T.; Caldwell, M.D.; Kelly, M.E.M. The Cannabinoids Δ8THC, CBD, and HU-308 Act via Distinct Receptors to Reduce Corneal Pain and Inflammation. Cannabis Cannabinoid Res. 2018, 3, 11–20. [Google Scholar] [CrossRef]
- Liou, G.I.; Auchampach, J.A.; Hillard, C.J.; Zhu, G.; Yousufzai, B.; Mian, S.; Khan, S.; Khalifa, Y. Mediation of cannabidiol anti-inflammation in the retina by equilibrative nucleoside transporter and A2A adenosine receptor. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5526–5531. [Google Scholar] [CrossRef]
- Rocha-Sousa, A.; Rodrigues-Araújo, J.; Gouveia, P.; Barbosa-Breda, J.; Azevedo-Pinto, S.; Pereira-Silva, P.; Leite-Moreira, A. New therapeutic targets for intraocular pressure lowering. ISRN Ophthalmol. 2013, 2013, 261386. [Google Scholar] [CrossRef]
- Laine, K.; Järvinen, K.; Järvinen, T. Topically administered CB2-receptor agonist, JWH-133, does not decrease intraocular pressure (IOP) in normotensive rabbits. Life Sci. 2003, 72, 837–842. [Google Scholar] [CrossRef]
- Woodward, D.F.; Carling, R.W.; Cornell, C.L.; Fliri, H.G.; Martos, J.L.; Pettit, S.N.; Liang, Y.; Wang, J.W. The pharmacology and therapeutic relevance of endocannabinoid derived cyclo-oxygenase (COX)-2 products. Pharmacol. Ther. 2008, 120, 71–80. [Google Scholar] [CrossRef]
- Smid, S.D. Role of prostaglandins and specific place in therapy of bimatoprost in the treatment of elevated intraocular pressure and ocular hypertension: A closer look at the agonist properties of bimatoprost and the prostamides. Clin. Ophthalmol. 2009, 3, 663–670. [Google Scholar] [CrossRef][Green Version]
- Maihöfner, C.; Schlötzer-Schrehardt, U.; Gühring, H.; Zeilhofer, H.U.; Naumann, G.O.; Pahl, A.; Mardin, C.; Tamm, E.R.; Brune, K. Expression of cyclooxygenase-1 and -2 in normal and glaucomatous human eyes. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2616–2624. [Google Scholar]
- Porcella, A.; Maxia, C.; Gessa, G.L.; Pani, L. The synthetic cannabinoid WIN55212-2 decreases the intraocular pressure in human glaucoma resistant to conventional therapies. Eur. J. Neurosci. 2001, 13, 409–412. [Google Scholar] [CrossRef]
- Njie, Y.F.; Kumar, A.; Qiao, Z.; Zhong, L.; Song, Z.H. Noladin ether acts on trabecular meshwork cannabinoid (CB1) receptors to enhance aqueous humor outflow facility. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1999–2005. [Google Scholar] [CrossRef]
- Tiedeman, J.S.; Shields, M.B.; Weber, P.A.; Crow, J.W.; Cocchetto, D.M.; Harris, W.A.; Howes, J.F. Effect of synthetic cannabinoids on elevated intraocular pressure. Ophthalmology 1981, 88, 270–277. [Google Scholar] [CrossRef]
- Flach, A.J. Delta-9-tetrahydrocannabinol (THC) in the treatment of end-stage open-angle glaucoma. Trans. Am. Ophthalmol. Soc. 2002, 100, 215–222; discussion 222–214. [Google Scholar]
- Tomida, I.; Azuara-Blanco, A.; House, H.; Flint, M.; Pertwee, R.G.; Robson, P.J. Effect of sublingual application of cannabinoids on intraocular pressure: A pilot study. J. Glaucoma 2006, 15, 349–353. [Google Scholar] [CrossRef]
- Plange, N.; Arend, K.O.; Kaup, M.; Doehmen, B.; Adams, H.; Hendricks, S.; Cordes, A.; Huth, J.; Sponsel, W.E.; Remky, A. Dronabinol and retinal hemodynamics in humans. Am. J. Ophthalmol. 2007, 143, 173–174. [Google Scholar] [CrossRef]
- Newell, F.W.; Stark, P.; Jay, W.M.; Schanzlin, D.J. Nabilone: A pressure-reducing synthetic benzopyran in open-angle glaucoma. Ophthalmology 1979, 86, 156–160. [Google Scholar] [CrossRef]
- Green, K. Marijuana smoking vs cannabinoids for glaucoma therapy. Arch. Ophthalmol. 1998, 116, 1433–1437. [Google Scholar] [CrossRef]
- Sun, X.; Xu, C.S.; Chadha, N.; Chen, A.; Liu, J. Marijuana for Glaucoma: A Recipe for Disaster or Treatment? Yale J. Biol. Med. 2015, 88, 265–269. [Google Scholar]
- Strobbe, E.; Cellini, M.; Campos, E.C. Effectiveness of palmitoylethanolamide on endothelial dysfunction in ocular hypertensive patients: A randomized, placebo-controlled cross-over study. Investig. Ophthalmol. Vis. Sci. 2013, 54, 968–973. [Google Scholar] [CrossRef]
- Pescosolido, N.; Librando, A.; Puzzono, M.; Nebbioso, M. Palmitoylethanolamide effects on intraocular pressure after Nd:YAG laser iridotomy: An experimental clinical study. J. Ocul. Pharmacol. Ther. 2011, 27, 629–635. [Google Scholar] [CrossRef]
- Gagliano, C.; Ortisi, E.; Pulvirenti, L.; Reibaldi, M.; Scollo, D.; Amato, R.; Avitabile, T.; Longo, A. Ocular hypotensive effect of oral palmitoyl-ethanolamide: A clinical trial. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6096–6100. [Google Scholar] [CrossRef]
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef]
- Cooler, P.; Gregg, J.M. Effect of delta-9-tetrahydrocannabinol on intraocular pressure in humans. South. Med. J. 1977, 70, 951–954. [Google Scholar] [CrossRef]
- Purnell, W.D.; Gregg, J.M. Delta(9)-tetrahydrocannabinol,, euphoria and intraocular pressure in man. Ann. Ophthalmol. 1975, 7, 921–923. [Google Scholar]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.; Weizman, A.; Weinstein, A. Positive and Negative Effects of Cannabis and Cannabinoids on Health. Clin. Pharmacol. Ther. 2019, 105, 1139–1147. [Google Scholar] [CrossRef]
- Merritt, J.C.; Perry, D.D.; Russell, D.N.; Jones, B.F. Topical delta 9-tetrahydrocannabinol and aqueous dynamics in glaucoma. J. Clin. Pharmacol. 1981, 21, 467s–471s. [Google Scholar] [CrossRef]
- Merritt, J.C.; Olsen, J.L.; Armstrong, J.R.; McKinnon, S.M. Topical delta 9-tetrahydrocannabinol in hypertensive glaucomas. J. Pharm. Pharmacol. 1981, 33, 40–41. [Google Scholar] [CrossRef] [PubMed]
- Green, K.; Roth, M. Ocular effects of topical administration of delta 9-tetrahydrocannabinol in man. Arch. Ophthalmol. 1982, 100, 265–267. [Google Scholar] [CrossRef]
- Jay, W.M.; Green, K. Multiple-drop study of topically applied 1% delta 9-tetrahydrocannabinol in human eyes. Arch. Ophthalmol. 1983, 101, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Ashkar, S.; Jain, S.; Marchand, M.; Tran, S.D. Breaking Barriers in Eye Treatment: Polymeric Nano-Based Drug-Delivery System for Anterior Segment Diseases and Glaucoma. Polymers 2023, 15, 1373. [Google Scholar] [CrossRef]
- Kabiri, M.; Kamal, S.H.; Pawar, S.V.; Roy, P.R.; Derakhshandeh, M.; Kumar, U.; Hatzikiriakos, S.G.; Hossain, S.; Yadav, V.G. A stimulus-responsive, in situ-forming, nanoparticle-laden hydrogel for ocular drug delivery. Drug Deliv. Transl. Res. 2018, 8, 484–495. [Google Scholar] [CrossRef]
- Saraiva, S.M.; Martín-Banderas, L.; Durán-Lobato, M. Cannabinoid-Based Ocular Therapies and Formulations. Pharmaceutics 2023, 15, 1077. [Google Scholar] [CrossRef]
- Taskar, P.S.; Patil, A.; Lakhani, P.; Ashour, E.; Gul, W.; ElSohly, M.A.; Murphy, B.; Majumdar, S. Δ9-Tetrahydrocannabinol Derivative-Loaded Nanoformulation Lowers Intraocular Pressure in Normotensive Rabbits. Transl. Vis. Sci. Technol. 2019, 8, 15. [Google Scholar] [CrossRef]
- Levin, L.A. Direct and indirect approaches to neuroprotective therapy of glaucomatous optic neuropathy. Surv. Ophthalmol. 1999, 43 (Suppl. S1), S98–S101. [Google Scholar] [CrossRef] [PubMed]
- Bagli, E.; Kitsos, G. Neuroprotective agents in glaucoma. In The Mistery of Glaucoma; InTech: London, UK, 2011; pp. 115–144. [Google Scholar]
- Baltmr, A.; Duggan, J.; Nizari, S.; Salt, T.E.; Cordeiro, M.F. Neuroprotection in glaucoma—Is there a future role? Exp. Eye Res. 2010, 91, 554–566. [Google Scholar] [CrossRef]
- Coyle, J.T.; Puttfarcken, P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993, 262, 689–695. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Khalil, I.E.; Matragoon, S.; Abou-Mohamed, G.; Tsai, N.J.; Roon, P.; Caldwell, R.B.; Caldwell, R.W.; Green, K.; Liou, G.I. Neuroprotective effect of (-)Delta9-tetrahydrocannabinol and cannabidiol in N-methyl-D-aspartate-induced retinal neurotoxicity: Involvement of peroxynitrite. Am. J. Pathol. 2003, 163, 1997–2008. [Google Scholar] [CrossRef] [PubMed]
- van der Stelt, M.; Veldhuis, W.B.; Bär, P.R.; Veldink, G.A.; Vliegenthart, J.F.; Nicolay, K. Neuroprotection by Delta9-tetrahydrocannabinol, the main active compound in marijuana, against ouabain-induced in vivo excitotoxicity. J. Neurosci. 2001, 21, 6475–6479. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Thayer, S.A. Cannabinoid receptor agonists protect cultured rat hippocampal neurons from excitotoxicity. Mol. Pharmacol. 1998, 54, 459–462. [Google Scholar] [CrossRef]
- Nagayama, T.; Sinor, A.D.; Simon, R.P.; Chen, J.; Graham, S.H.; Jin, K.; Greenberg, D.A. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J. Neurosci. 1999, 19, 2987–2995. [Google Scholar] [CrossRef]
- Tadijan, A.; Vlašić, I.; Vlainić, J.; Đikić, D.; Oršolić, N.; Jazvinšćak Jembrek, M. Intracellular Molecular Targets and Signaling Pathways Involved in Antioxidative and Neuroprotective Effects of Cannabinoids in Neurodegenerative Conditions. Antioxidants 2022, 11, 2049. [Google Scholar] [CrossRef]
- Paloczi, J.; Varga, Z.V.; Hasko, G.; Pacher, P. Neuroprotection in Oxidative Stress-Related Neurodegenerative Diseases: Role of Endocannabinoid System Modulation. Antioxid. Redox Signal. 2018, 29, 75–108. [Google Scholar] [CrossRef]
- Benyó, Z.; Ruisanchez, É.; Leszl-Ishiguro, M.; Sándor, P.; Pacher, P. Endocannabinoids in cerebrovascular regulation. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H785–H801. [Google Scholar] [CrossRef] [PubMed]
- Leonelli, M.; Martins, D.O.; Britto, L.R. Retinal cell death induced by TRPV1 activation involves NMDA signaling and upregulation of nitric oxide synthases. Cell Mol. Neurobiol. 2013, 33, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Leonelli, M.; Martins, D.O.; Britto, L.R. TRPV1 receptors are involved in protein nitration and Müller cell reaction in the acutely axotomized rat retina. Exp. Eye Res. 2010, 91, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Kawamata, T.; Ninomiya, T.; Omote, K.; Namiki, A. Endothelin-1 enhances capsaicin-evoked intracellular Ca2+ response via activation of endothelin a receptor in a protein kinase Cepsilon-dependent manner in dorsal root ganglion neurons. Neuroscience 2006, 137, 949–960. [Google Scholar] [CrossRef]
- Lau, J.; Dang, M.; Hockmann, K.; Ball, A.K. Effects of acute delivery of endothelin-1 on retinal ganglion cell loss in the rat. Exp. Eye Res. 2006, 82, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Weitlauf, C.; Ward, N.J.; Lambert, W.S.; Sidorova, T.N.; Ho, K.W.; Sappington, R.M.; Calkins, D.J. Short-term increases in transient receptor potential vanilloid-1 mediate stress-induced enhancement of neuronal excitation. J. Neurosci. 2014, 34, 15369–15381. [Google Scholar] [CrossRef]
- Nucci, C.; Martucci, A.; Giannini, C.; Morrone, L.A.; Bagetta, G.; Mancino, R. Neuroprotective agents in the management of glaucoma. Eye 2018, 32, 938–945. [Google Scholar] [CrossRef]
- Vasudevan, S.K.; Gupta, V.; Crowston, J.G. Neuroprotection in glaucoma. Indian J. Ophthalmol. 2011, 59 (Suppl. S1), S102–S113. [Google Scholar] [CrossRef]
- Crandall, J.; Matragoon, S.; Khalifa, Y.M.; Borlongan, C.; Tsai, N.T.; Caldwell, R.B.; Liou, G.I. Neuroprotective and intraocular pressure-lowering effects of (-)Delta9-tetrahydrocannabinol in a rat model of glaucoma. Ophthalmic Res. 2007, 39, 69–75. [Google Scholar] [CrossRef]
- Pinar-Sueiro, S.; Zorrilla Hurtado, J.; Veiga-Crespo, P.; Sharma, S.C.; Vecino, E. Neuroprotective effects of topical CB1 agonist WIN 55212-2 on retinal ganglion cells after acute rise in intraocular pressure induced ischemia in rat. Exp. Eye Res. 2013, 110, 55–58. [Google Scholar] [CrossRef]
- Hui-Feng, L.; Yuan, H.; Jun, J.; Ming-Li, J.; Jin-Wei, X. Clinical study on intravitreal injection of cannabinoid HU-211 for optic nerve damage in glaucoma rats. Int. Eye Sci. 2014, 14, 1584–1586. [Google Scholar]
- Nucci, C.; Gasperi, V.; Tartaglione, R.; Cerulli, A.; Terrinoni, A.; Bari, M.; De Simone, C.; Agrò, A.F.; Morrone, L.A.; Corasaniti, M.T.; et al. Involvement of the endocannabinoid system in retinal damage after high intraocular pressure-induced ischemia in rats. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2997–3004. [Google Scholar] [CrossRef]
- Zalish, M.; Lavie, V. Dexanabinol (HU-211) has a beneficial effect on axonal sprouting and survival after rat optic nerve crush injury. Vision Res. 2003, 43, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Yoles, E.; Belkin, M.; Schwartz, M. HU-211, a nonpsychotropic cannabinoid, produces short- and long-term neuroprotection after optic nerve axotomy. J. Neurotrauma 1996, 13, 49–57. [Google Scholar] [CrossRef] [PubMed]
- El-Remessy, A.B.; Al-Shabrawey, M.; Khalifa, Y.; Tsai, N.T.; Caldwell, R.B.; Liou, G.I. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am. J. Pathol. 2006, 168, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Lax, P.; Esquiva, G.; Altavilla, C.; Cuenca, N. Neuroprotective effects of the cannabinoid agonist HU210 on retinal degeneration. Exp. Eye Res. 2014, 120, 175–185. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, X.; Liu, S.; Shen, Y. Neuroprotective effect of cannabinoid receptor 1 antagonist in the MNU-induced retinal degeneration model. Exp. Eye Res. 2018, 167, 145–151. [Google Scholar] [CrossRef]
- Kokona, D.; Spyridakos, D.; Tzatzarakis, M.; Papadogkonaki, S.; Filidou, E.; Arvanitidis, K.I.; Kolios, G.; Lamani, M.; Makriyannis, A.; Malamas, M.S.; et al. The endocannabinoid 2-arachidonoylglycerol and dual ABHD6/MAGL enzyme inhibitors display neuroprotective and anti-inflammatory actions in the in vivo retinal model of AMPA excitotoxicity. Neuropharmacology 2021, 185, 108450. [Google Scholar] [CrossRef]
- Slusar, J.E.; Cairns, E.A.; Szczesniak, A.M.; Bradshaw, H.B.; Di Polo, A.; Kelly, M.E. The fatty acid amide hydrolase inhibitor, URB597, promotes retinal ganglion cell neuroprotection in a rat model of optic nerve axotomy. Neuropharmacology 2013, 72, 116–125. [Google Scholar] [CrossRef]
- Maccarone, R.; Rapino, C.; Zerti, D.; di Tommaso, M.; Battista, N.; Di Marco, S.; Bisti, S.; Maccarrone, M. Modulation of Type-1 and Type-2 Cannabinoid Receptors by Saffron in a Rat Model of Retinal Neurodegeneration. PLoS ONE 2016, 11, e0166827. [Google Scholar] [CrossRef]
- Sappington, R.M.; Sidorova, T.; Ward, N.J.; Chakravarthy, R.; Ho, K.W.; Calkins, D.J. Activation of transient receptor potential vanilloid-1 (TRPV1) influences how retinal ganglion cell neurons respond to pressure-related stress. Channels 2015, 9, 102–113. [Google Scholar] [CrossRef]
- Sakamoto, K.; Kuroki, T.; Okuno, Y.; Sekiya, H.; Watanabe, A.; Sagawa, T.; Ito, H.; Mizuta, A.; Mori, A.; Nakahara, T.; et al. Activation of the TRPV1 channel attenuates N-methyl-D-aspartic acid-induced neuronal injury in the rat retina. Eur. J. Pharmacol. 2014, 733, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, R.; Lu, H.; Zhang, X. Systemic administration with tetrahydrocannabinol causes retinal damage in BALB/c mice. Hum. Exp. Toxicol. 2020, 39, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.C.M.; Scudeller, L.; Lumini, C.; Bettio, F.; Picasso, E.; Ruberto, G.; Briola, A.; Mirabile, A.; Paviglianiti, A.; Pasinetti, G.M.; et al. Effect of palmitoylethanolamide on inner retinal function in glaucoma: A randomized, single blind, crossover, clinical trial by pattern-electroretinogram. Sci. Rep. 2020, 10, 10468. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.R.; Lograno, M.D. Involvement of the peroxisome proliferator-activated receptor (PPAR) alpha in vascular response of endocannabinoids in the bovine ophthalmic artery. Eur. J. Pharmacol. 2012, 683, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Schwitzer, T.; Schwan, R.; Albuisson, E.; Giersch, A.; Lalanne, L.; Angioi-Duprez, K.; Laprevote, V. Association Between Regular Cannabis Use and Ganglion Cell Dysfunction. JAMA Ophthalmol. 2017, 135, 54–60. [Google Scholar] [CrossRef]
- Schwitzer, T.; Schwan, R.; Angioi-Duprez, K.; Giersch, A.; Lalanne, L.; Albuisson, E.; Laprevote, V. Delayed bipolar and ganglion cells neuroretinal processing in regular cannabis users: The retina as a relevant site to investigate brain synaptic transmission dysfunctions. J. Psychiatr. Res. 2018, 103, 75–82. [Google Scholar] [CrossRef]
- Schwitzer, T.; Henrion, M.L.; Sarre, D.; Albuisson, E.; Angioi-Duprez, K.; Giersch, A.; Lalanne, L.; Schwan, R.; Laprevote, V. Spatial localization of retinal anomalies in regular cannabis users: The relevance of the multifocal electroretinogram. Schizophr. Res. 2020, 219, 56–61. [Google Scholar] [CrossRef]
- Lucas, A.; Thirion, A.; Schwan, R.; Krieg, J.; Angioi-Duprez, K.; Laprevote, V.; Schwitzer, T. Association between increased retinal background noise and co-occurrent regular cannabis and alcohol use. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 89, 335–340. [Google Scholar] [CrossRef]
- Zantut, P.R.A.; Veras, M.M.; Yariwake, V.Y.; Takahashi, W.Y.; Saldiva, P.H.; Young, L.H.; Damico, F.M.; Fajersztajn, L. Effects of cannabis and its components on the retina: A systematic review. Cutan. Ocul. Toxicol. 2020, 39, 1–9. [Google Scholar] [CrossRef]
- Arend, O.; Plange, N.; Sponsel, W.E.; Remky, A. Pathogenetic aspects of the glaucomatous optic neuropathy: Fluorescein angiographic findings in patients with primary open angle glaucoma. Brain Res. Bull. 2004, 62, 517–524. [Google Scholar] [CrossRef]
- Flammer, J. The vascular concept of glaucoma. Surv. Ophthalmol. 1994, 38, S3–S6. [Google Scholar] [CrossRef]
- Resch, H.; Garhofer, G.; Fuchsjäger-Mayrl, G.; Hommer, A.; Schmetterer, L. Endothelial dysfunction in glaucoma. Acta Ophthalmol. 2009, 87, 4–12. [Google Scholar] [CrossRef]
- Schmidl, D.; Garhofer, G.; Schmetterer, L. The complex interaction between ocular perfusion pressure and ocular blood flow-relevance for glaucoma. Exp. Eye Res. 2011, 93, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Schmetterer, L.; Kiel, J. Ocular Blood Flow; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Yaoeda, K.; Shirakashi, M.; Fukushima, A.; Funaki, S.; Funaki, H.; Abe, H.; Tanabe, N. Relationship between optic nerve head microcirculation and visual field loss in glaucoma. Acta Ophthalmol. Scand. 2003, 81, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Shiga, Y.; Kunikata, H.; Aizawa, N.; Kiyota, N.; Maiya, Y.; Yokoyama, Y.; Omodaka, K.; Takahashi, H.; Yasui, T.; Kato, K.; et al. Optic Nerve Head Blood Flow, as Measured by Laser Speckle Flowgraphy, Is Significantly Reduced in Preperimetric Glaucoma. Curr. Eye Res. 2016, 41, 1447–1453. [Google Scholar] [CrossRef]
- Harris, A.; Zarfati, D.; Zalish, M.; Biller, J.; Sheets, C.W.; Rechtman, E.; Migliardi, R.; Garzozi, H.J. Reduced cerebrovascular blood flow velocities and vasoreactivity in open-angle glaucoma. Am. J. Ophthalmol. 2003, 135, 144–147. [Google Scholar] [CrossRef]
- Calzetti, G.; Mursch-Edlmayr, A.S.; Bata, A.M.; Ungaro, N.; Mora, P.; Chua, J.; Schmidl, D.; Bolz, M.; Garhöfer, G.; Gandolfi, S.; et al. Measuring optic nerve head perfusion to monitor glaucoma: A study on structure-function relationships using laser speckle flowgraphy. Acta Ophthalmol. 2022, 100, e181–e191. [Google Scholar] [CrossRef] [PubMed]
- Garhöfer, G.; Bata, A.M.; Popa-Cherecheanu, A.; Hommer, A.; Vass, C.; Resch, H.; Schmidl, D.; Werkmeister, R.M.; Schmetterer, L. Retinal Oxygen Extraction in Patients with Primary Open-Angle Glaucoma. Int. J. Mol. Sci. 2022, 23, 10152. [Google Scholar] [CrossRef]
- Mozaffarieh, M.; Flammer, J. Is there more to glaucoma treatment than lowering IOP? Surv. Ophthalmol. 2007, 52 (Suppl. S2), S174–S179. [Google Scholar] [CrossRef] [PubMed]
- Bata, A.M.; Fondi, K.; Witkowska, K.J.; Werkmeister, R.M.; Hommer, A.; Vass, C.; Resch, H.; Schmidl, D.; Popa-Cherecheanu, A.; Chua, J.; et al. Optic nerve head blood flow regulation during changes in arterial blood pressure in patients with primary open-angle glaucoma. Acta Ophthalmol. 2019, 97, e36–e41. [Google Scholar] [CrossRef]
- Fuchsjäger-Mayrl, G.; Wally, B.; Georgopoulos, M.; Rainer, G.; Kircher, K.; Buehl, W.; Amoako-Mensah, T.; Eichler, H.G.; Vass, C.; Schmetterer, L. Ocular blood flow and systemic blood pressure in patients with primary open-angle glaucoma and ocular hypertension. Investig. Ophthalmol. Vis. Sci. 2004, 45, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Fuchsjäger-Mayrl, G.; Georgopoulos, M.; Hommer, A.; Weigert, G.; Pemp, B.; Vass, C.; Garhöfer, G.; Schmetterer, L. Effect of dorzolamide and timolol on ocular pressure: Blood flow relationship in patients with primary open-angle glaucoma and ocular hypertension. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1289–1296. [Google Scholar] [CrossRef]
- Garhöfer, G.; Resch, H.; Weigert, G.; Lung, S.; Simader, C.; Schmetterer, L. Short-term increase of intraocular pressure does not alter the response of retinal and optic nerve head blood flow to flicker stimulation. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1721–1725. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Oh, S.; Baek, S.U.; Ahn, S.J.; Park, K.H.; Jeoung, J.W. Ocular Perfusion Pressure and the Risk of Open-Angle Glaucoma: Systematic Review and Meta-analysis. Sci. Rep. 2020, 10, 10056. [Google Scholar] [CrossRef]
- Stanley, C.; O’Sullivan, S.E. Vascular targets for cannabinoids: Animal and human studies. Br. J. Pharmacol. 2014, 171, 1361–1378. [Google Scholar] [CrossRef] [PubMed]
- Lípez-Miranda, V.; Herradón, E.; Martín, M.I. Vasorelaxation caused by cannabinoids: Mechanisms in different vascular beds. Curr. Vasc. Pharmacol. 2008, 6, 335–346. [Google Scholar] [CrossRef]
- Randall, M.D.; Kendall, D.A.; O’Sullivan, S. The complexities of the cardiovascular actions of cannabinoids. Br. J. Pharmacol. 2004, 142, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J. Endocannabinoids and vascular function. J. Pharmacol. Exp. Ther. 2000, 294, 27–32. [Google Scholar] [PubMed]
- Zoratti, C.; Kipmen-Korgun, D.; Osibow, K.; Malli, R.; Graier, W.F. Anandamide initiates Ca2+ signaling via CB2 receptor linked to phospholipase C in calf pulmonary endothelial cells. Br. J. Pharmacol. 2003, 140, 1351–1362. [Google Scholar] [CrossRef]
- Alswailmi, F.K. A Cross Talk between the Endocannabinoid System and Different Systems Involved in the Pathogenesis of Hypertensive Retinopathy. Pharmaceuticals 2023, 16, 345. [Google Scholar] [CrossRef]
- Chataigneau, T.; Félétou, M.; Thollon, C.; Villeneuve, N.; Vilaine, J.P.; Duhault, J.; Vanhoutte, P.M. Cannabinoid CB1 receptor and endothelium-dependent hyperpolarization in guinea-pig carotid, rat mesenteric and porcine coronary arteries. Br. J. Pharmacol. 1998, 123, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Ronco, A.M.; Llanos, M.; Tamayo, D.; Hirsch, S. Anandamide inhibits endothelin-1 production by human cultured endothelial cells: A new vascular action of this endocannabinoid. Pharmacology 2007, 79, 12–16. [Google Scholar] [CrossRef]
- Su, E.N.; Kelly, M.E.; Cringle, S.J.; Yu, D.Y. Role of Endothelium in Abnormal Cannabidiol-Induced Vasoactivity in Retinal Arterioles. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4029–4037. [Google Scholar] [CrossRef] [PubMed]
- Ripodas, A.; de Juan, J.A.; Roldán-Pallarés, M.; Bernal, R.; Moya, J.; Chao, M.; López, A.; Fernández-Cruz, A.; Fernández-Durango, R. Localisation of endothelin-1 mRNA expression and immunoreactivity in the retina and optic nerve from human and porcine eye. Evidence for endothelin-1 expression in astrocytes. Brain Res. 2001, 912, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.R.; Lograno, M.D. Cannabinoid agonists induce relaxation in the bovine ophthalmic artery: Evidences for CB1 receptors, nitric oxide and potassium channels. Br. J. Pharmacol. 2006, 147, 917–925. [Google Scholar] [CrossRef]
- Sultan, S.R.; Millar, S.A.; O’Sullivan, S.E.; England, T.J. A Systematic Review and Meta-Analysis of the In Vivo Haemodynamic Effects of Δ9-Tetrahydrocannabinol. Pharmaceuticals 2018, 11, 13. [Google Scholar] [CrossRef]
- Green, K.; Wynn, H.; Padgett, D. Effects of delta9-tetrahydrocannabinol on ocular blood flow and aqueous humor formation. Exp. Eye Res. 1978, 26, 65–69. [Google Scholar] [CrossRef]
- Ohlsson, A.; Lindgren, J.E.; Wahlen, A.; Agurell, S.; Hollister, L.E.; Gillespie, H.K. Plasma delta-9 tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin. Pharmacol. Ther. 1980, 28, 409–416. [Google Scholar] [CrossRef]
- Ogunbiyi, M.O.; Hindocha, C.; Freeman, T.P.; Bloomfield, M.A.P. Acute and chronic effects of Δ9-tetrahydrocannabinol (THC) on cerebral blood flow: A systematic review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 101, 109900. [Google Scholar] [CrossRef]
- Hill, M.; Wong, T.Y.; Davis, M.; Meier, M.H. Associations between cannabis use and retinal vessel diameter in young adults. Schizophr. Res. 2020, 219, 62–68. [Google Scholar] [CrossRef]
- Hommer, N.; Kallab, M.; Szegedi, S.; Puchner, S.; Stjepanek, K.; Bauer, M.; Werkmeister, R.M.; Schmetterer, L.; Abensperg-Traun, M.; Garhöfer, G.; et al. The Effect of Orally Administered Dronabinol on Optic Nerve Head Blood Flow in Healthy Subjects-A Randomized Clinical Trial. Clin. Pharmacol. Ther. 2020, 108, 155–161. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).