Potential Use of Antioxidant Compounds for the Treatment of Inflammatory Bowel Disease
Abstract
1. Introduction
2. The Role of Oxidative Stress in IBD
2.1. Mechanisms of the Formation of Reactive Oxygen Species in the Cell
2.2. Development of Oxidative Stress in IBD
3. Natural Antioxidant Cellular Protection
3.1. Superoxide Dismutase (SOD)
3.2. Catalase (CAT)
3.3. Glutathione Peroxidase (GSH-Px)
3.4. Glutathione Reductase (GR)
3.5. Thioredoxin (Trx)
3.6. Cellular Antioxidant Regulation
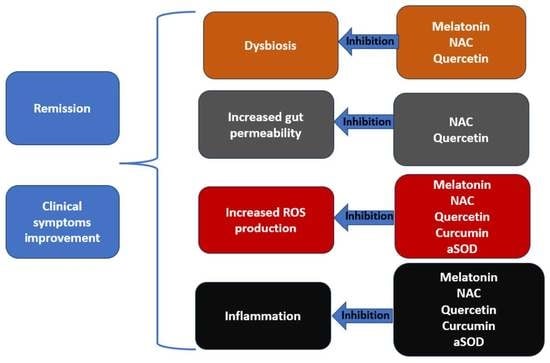
4. Natural Antioxidants in the Treatment of IBD
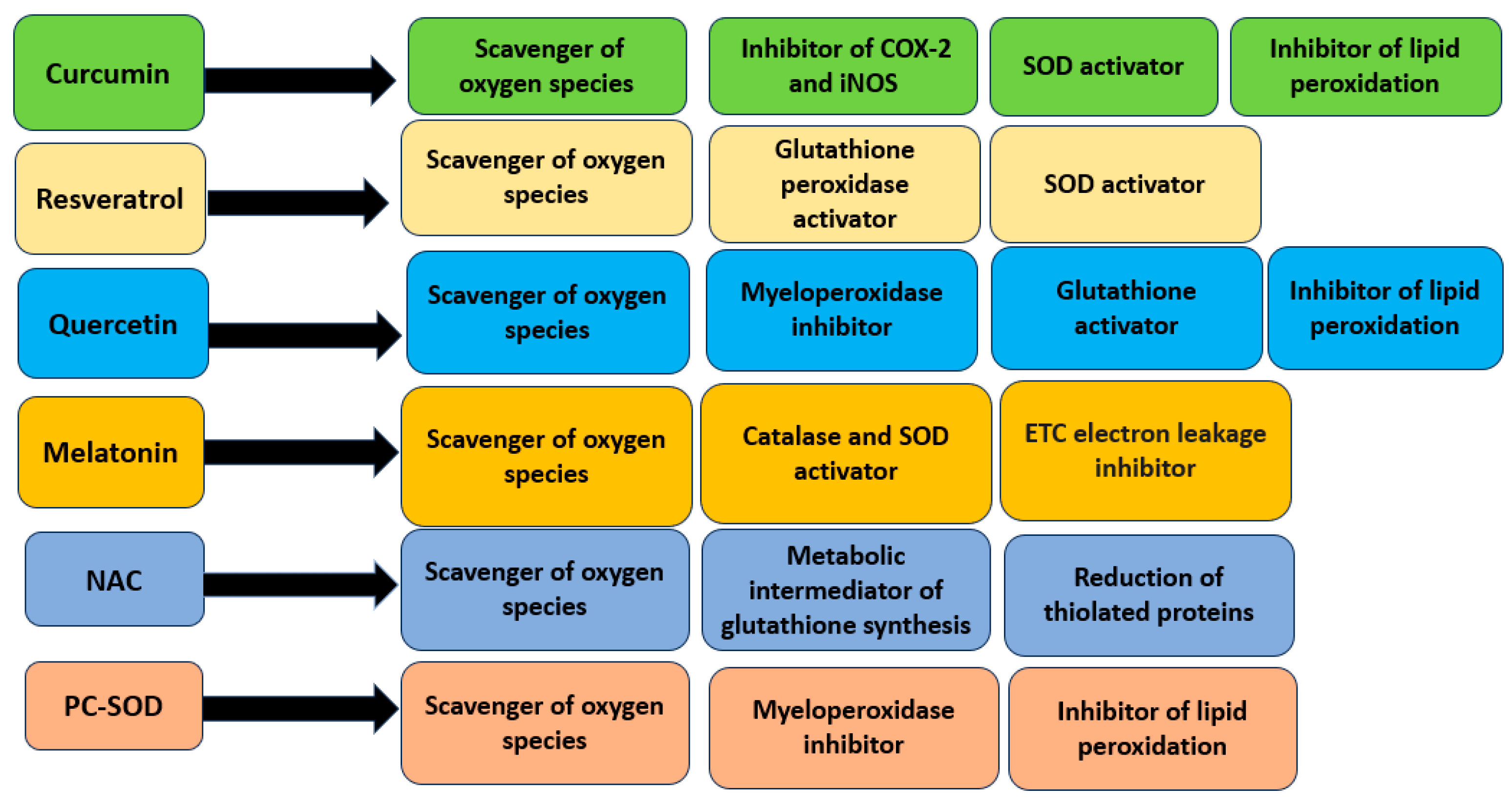
4.1. Curcumin
4.2. Resveratrol
4.3. Quercetin
4.4. Melatonin
5. Artificial Antioxidants in the Treatment of IBD
5.1. N-acetylcysteine
5.2. Artificial Superoxide Dismutase
6. Discussion
7. Conclusions
8. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V. ECCO-ESGAR Guideline for Diagnostic Assessment in Inflammatory Bowel Disease. J. Crohn’s Colitis 2019, 13, 144–164. [Google Scholar] [CrossRef]
- Dmochowska, N.; Wardill, H. Hughes Advances in Imaging Specific Mediators of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2018, 19, 2471. [Google Scholar] [CrossRef]
- Chung, A.E.; Vu, M.B.; Myers, K.; Burris, J.; Kappelman, M.D. Crohn’s and Colitis Foundation of America Partners Patient-Powered Research Network. Med. Care 2018, 56, S33. [Google Scholar] [CrossRef]
- Colombel, J.-F.; Shin, A.; Gibson, R. Functional gastrointestinal symptoms in patients with inflammatory bowel disease: A clinical challenge. Clin. Gastroenterol. Hepatol. 2019, 17, 380–390. [Google Scholar] [CrossRef]
- McDowell, C.; Farooq, U.; Haseeb, M. Inflammatory Bowel Disease. [Updated 2023 April 16]. StatPearls. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470312/ (accessed on 25 July 2023).
- Su, H.-J.; Chiu, Y.-T.; Chiu, C.-T.; Lin, Y.-C.; Wang, C.-Y.; Hsieh, J.-Y.; Wie, S.-C. Inflammatory bowel disease and its treatment in 2018: Global and Taiwanese status updates. J. Formos. Med. Assoc. 2019, 118, 1083–1092. [Google Scholar] [CrossRef]
- Hazel, K.; O’Connor, A. Emerging treatments for inflammatory bowel disease. Ther. Adv. Chronic Dis. 2019, 11, 2040622319899297. [Google Scholar] [CrossRef]
- McKenzie, S.J.; Baker, M.S.; Buffinton, G.D.; Doe, W.F. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J. Clin. Investig. 1996, 98, 136–141. [Google Scholar] [CrossRef]
- Lih-Brody, L.; Powell, S.R.; Collier, K.; Reddy, G.M.; Cerchia, R.; Kahn, E.; Weissman, G.S.; Katz, S.; Floyd, R.A.; McKinley, M.J.; et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig. Dis. Sci. 1996, 41, 2078–2086. [Google Scholar] [CrossRef]
- Salim, A.S. Role of oxygen-derived free radical scavengers in the management of recurrent attacks of ulcerative colitis: A new approach. J. Lab. Clin. Med. 1992, 119, 710–717. [Google Scholar]
- Järnerot, G.; Ström, M.; Danielsson, A.; Kilander, A.; Lööf, L.; Hultcrantz, R.; Löfberg, R.; Florén, C.; Nilsson, A.; Broström, O. Allopurinol in addition to 5-aminosalicylic acid based drugs for the maintenance treatment of ulcerative colitis. Aliment. Pharmacol. Ther. 2000, 14, 1159–1162. [Google Scholar]
- Ferreira, C.A.; Ni, D.; Rosenkrans, Z.T.; Cai, W. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res. 2018, 11, 4955–4984. [Google Scholar] [CrossRef]
- Nogueira, V.; Hay, N. Molecular pathways: Reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 4309–4314. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Tauffenberger, A.; Magistretti, J. Reactive Oxygen Species: Beyond Their Reactive Behavior. Neurochem. Res. 2021, 46, 77–87. [Google Scholar] [CrossRef]
- Onukwufor, J.O.; Berry, B.J.; Wojtovich, A. Physiologic Implications of Reactive Oxygen Species Production by Mitochondrial Complex I Reverse Electron Transport. Antioxidants 2019, 8, 285. [Google Scholar] [CrossRef]
- Zhao, R.; Jiang, S.; Zhang, L.; Yu, Z. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Nickel, A.; Kohlhaas, M.; Maack, C. Mitochondrial reactive oxygen species production and elimination. J. Mol. Cell Cardiol. 2014, 73, 26–33. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Chausse, B.; da Cunha, F.M.; Luévano-Martínez, L.A.; Marazzi, T.B.M.; Pessoa, S.; Kowaltowski, A.J. Mitochondrial compartmentalization of redox processes. Free Radic. Biol. Med. 2012, 52, 2201–2208. [Google Scholar] [CrossRef]
- Zeeshan, H.; Lee, G.; Kim, H.-R.; Chae, H.-J. Endoplasmic Reticulum Stress and Associated ROS. Int. J. Mol. Sci. 2016, 17, 327. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Cytochrome P450 and Oxidative Stress in the Liver. Liver 2017, 401–419. [Google Scholar]
- Del Rio, L.A.; López-Huertas, E. ROS Generation in Peroxisomes and its Role in Cell Signaling. Plant Cell Physiol. 2016, 57, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. Am. Physiol. Soc. 2014, 94, 329. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.; Caldwell, J.; Phan, V.; Prescott, D.; Nazli, A.; Wang, A.; Soderhölm, J.D.; Perdue, M.H.; Sherman, P.M.; McKay, D.M. Decreased epithelial barrier function evoked by exposure to metabolic stress and nonpathogenic E. coli is enhanced by TNF-alpha. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, 3. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.; Farrugia, G. Oxidative stress: Key player in gastrointestinal complications of diabetes. Neurogastroenterol. Motil. 2011, 23, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Than, G.; Aytac, E.; Aytekin, H.; Gunduz, F.; Dogusoy, G.; Aydin, S.; Tahan, V.; Uzun, H. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid–induced ulcerative colitis in rats. Can. J. Surg. Can. Med. Assoc. 2011, 54, 333. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Peng, P.; Ding, N.; Jia, W.; Huang, C.; Tang, Y. Oxidative Stress, Inflammation, Gut Dysbiosis: What Can Polyphenols Do in Inflammatory Bowel Disease? Antioxidants 2023, 12, 967. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzian, A.; Banan, A.; Farhadi, A.; Komanduri, S.; Mutlu, E.; Zhang, Y.; Fields, J.Z. Increases in free radicals and cytoskeletal protein oxidation and nitration in the colon of patients with inflammatory bowel disease. Gut. BMJ Publ. Group 2003, 52, 720. [Google Scholar] [CrossRef] [PubMed]
- Horvath, B.; Liu, G.; Wu, X.; Lai, K.K.; Shen, B.; Liu, X. Overexpression of p53 predicts colorectal neoplasia risk in patients with inflammatory bowel disease and mucosa changes indefinite for dysplasia. Gastroenterol. Rep. 2015, 3, 344. [Google Scholar] [CrossRef]
- Mangerich, A.; Dedon, P.C.; Fox, J.G.; Tannenbaum, S.R.; Wogan, G.N. Chemistry meets biology in colitis-associated carcinogenesis. Free. Radic. Res. 2013, 47, 958–986. [Google Scholar] [CrossRef]
- Banning, A.; Freihaut, B.; Henry, R.; Pierce, S.; Bayer, W.L. GPx2 counteracts PGE2 production by dampening COX-2 and mPGES-1 expression in human colon cancer cells. Antioxid. Redox Signal. 2008, 10, 1491–1500. [Google Scholar] [CrossRef]
- Moura, F.A.; de Andrade, K.Q.; Dos Santos, J.C.F.; Araújo, O.R.P.; Goulart, M.O.F. Antioxidant therapy for treatment of inflammatory bowel disease: Does it work? Redox Biol. 2015, 6, 617. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.-F. Superoxide dismutases: Ancient enzymes and new insights. FEBS Lett. 2011, 586, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Unsal, V.; Dalkıran, T.; Çiçek, M.; Kölükçü, E. The Role of Natural Antioxidants Against Reactive Oxygen Species Produced by Cadmium Toxicity: A Review. Adv. Pharm. Bull. 2020, 10, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Unsal, V.; Belge-Kurutaş, E. Experimental Hepatic Carcinogenesis: Oxidative Stress and Natural Antioxidants. Open Access Maced. J. Med. Sci. 2017, 5, 686. [Google Scholar] [CrossRef] [PubMed]
- Unsal, V. Natural Phytotherapeutic Antioxidants in the Treatment of Mercury Intoxication-A Review. Adv. Pharm. Bull. 2018, 8, 365–376. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free. Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Elmallah, M.; Elkhadragy, M.; Al-Olayan, E.; Abdel Moneim, A. Protective Effect of Fragaria ananassa Crude Extract on Cadmium-Induced Lipid Peroxidation, Antioxidant Enzymes Suppression, and Apoptosis in Rat Testes. Int. J. Mol. Sci. 2017, 18, 957. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Collins, A.E.; Saleh, T.M.; Kalisch, B.E. Naturally Occurring Antioxidant Therapy in Alzheimer’s Disease. Antioxidants 2022, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. Thioredoxin System in Cell Death Progression. Antioxid. Redox Signal. 2012, 17, 1738–1747. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.K.; Khatri, R.; Jaiswal, A.K. Regulation of Nrf2—An update. Free Radic Biol Med. 2014, 66, 36–44. [Google Scholar] [CrossRef]
- Shi, L.; Wu, L.; Chen, Z.; Yang, J.; Chen, X.; Yu, F.; Lin, X. MiR-141 Activates Nrf2-Dependent Antioxidant Pathway via Down-Regulating the Expression of Keap1 Conferring the Resistance of Hepatocellular Carcinoma Cells to 5-Fluorouracil. Cell Physiol. Biochem. 2015, 35, 2333–2348. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Rinker, L.; Peng, J.; Chilian, W.M. Reactive Oxygen Species: The Good and the Bad. React. Oxyg. Species (ROS) Living Cells 2018, 7. [Google Scholar] [CrossRef]
- Brumatti, L.V.; Marcuzzi, A.; Tricarico, P.M.; Zanin, V.; Girardelli, M.; Bianco, A.M. Curcumin and Inflammatory Bowel Disease: Potential and Limits of Innovative Treatments. Molecules 2014, 19, 21127. [Google Scholar] [CrossRef] [PubMed]
- Singla, V.; Pratap Mouli, V.; Garg, S.K.; Rai, T.; Choudhury, B.N.; Verma, P.; Deb, R.; Tiwari, V.; Rohatgi, S.; Dhingra, R. Induction with NCB-02 (curcumin) enema for mild-to-moderate distal ulcerative colitis—A randomized, placebo-controlled, pilot study. J. Crohns. Colitis. 2014, 8, 208–214. [Google Scholar] [CrossRef]
- Lang, A.; Salomon, N.; Wu, J.C.; Kopylov, U.; Lahat, A.; Har-Noy, O.; Ching, J.Y.; Cheong, P.K.; Avidan, B.; Gamus, D. Curcumin in Combination with Mesalamine Induces Remission in Patients with Mild-to-Moderate Ulcerative Colitis in a Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1444–1449.e1. [Google Scholar] [CrossRef]
- Banerjee, R.; Pal, P.; Penmetsa, A.; Kathi, P.; Girish, G.; Goren, I.; Reddy, D.N. Novel Bioenhanced Curcumin with Mesalamine for Induction of Clinical and Endoscopic Remission in Mild-to-Moderate Ulcerative Colitis: A Randomized Double-Blind Placebo-controlled Pilot Study. J. Clin. Gastroenterol. 2021, 55, 702–708. [Google Scholar] [CrossRef]
- Rahal, K.; Chmiedlin-Ren, P.; Adler, J.; Dhanani, M.; Sultani, V.; Rittershaus, A.C.; Reingold, L.; Zhu, J.; McKenna, B.J.; Christman, G.M.; et al. Resveratrol has anti-inflammatory and antifibrotic effects in the peptidoglycan-polysaccharide rat model of Crohn’s disease. Inflamm. Bowel Dis. 2012, 18, 613. [Google Scholar] [CrossRef]
- Yildiz, G.; Yildiz, Y.; Ulutas, P.A.; Yaylali, A.; Ural, M. Resveratrol Pretreatment Ameliorates TNBS Colitis in Rats. Recent Pat. Endocr. Metab. Immune Drug Discov. 2015, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Samsamikor, M.; Daryani, N.E.; Asl, P.R.; Hekmatdoost, A. Resveratrol Supplementation and Oxidative/Anti-Oxidative Status in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch. Med. Res. 2016, 47, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Samsami-Kor, M.; Daryani, N.E.; Asl, R.; Hekmatdoost, A. Anti-Inflammatory Effects of Resveratrol in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch. Med. Res. 2015, 46, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chaudhury, A.; Ng, K.Y. Preparation and evaluation of zinc-pectin-chitosan composite particles for drug delivery to the colon: Role of chitosan in modifying in vitro and in vivo drug release. Int. J. Pharm. 2011, 406, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Posadino, A.M.; Cossu, A.; Giordo, R.; Zinellu, A.; Sotgia, S.; Vardeu, A.; Hoa, P.T.; Van Nguyen, L.V.; Carru, C.; Pintus, G. Resveratrol alters human endothelial cells redox state and causes mitochondrial-dependent cell death. Food Chem. Toxicol. 2015, 78, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Ge, Y.; Li, P.; Tian, X.; Wang, H.; Zheng, X.; Ju, S. Dietary quercetin ameliorates experimental colitis in mouse by remodeling the function of colonic macrophages via a heme oxygenase-1-dependent pathway. Cell Cycle. 2018, 17, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Dodda, D.; Chhajed, R.; Mishra, J.; Padhy, M. Targeting oxidative stress attenuates trinitrobenzene sulphonic acid induced inflammatory bowel disease like symptoms in rats: Role of quercetin. Indian J. Pharmacol. 2014, 46, 286. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hara, H. Role of flavonoids in intestinal tight junction regulation. J. Nutr. Biochem. 2011, 22, 401–408. [Google Scholar] [CrossRef]
- Kamishikiryo, J.; Matsumura, R.; Takamori, T.; Sugihara, N. Effect of quercetin on the transport of N-acetyl 5-aminosalicylic acid. J. Pharm. Pharmacol. 2013, 65, 1037–1043. [Google Scholar] [CrossRef]
- Sánchez, A.; Calpena, A.C.; Clares, B. Evaluating the Oxidative Stress in Inflammation: Role of Melatonin. Int. J. Mol. Sci. 2015, 16, 16981. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, C. Evaluation of Melatonin Effectiveness in the Adjuvant Treatment of Ulcerative Colitis. Cochrane Library. Available online: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-00836249/full (accessed on 1 September 2022).
- Zhu, D.; Ma, Y.; Ding, S.; Jiang, H.; Fang, J. Effects of Melatonin on Intestinal Microbiota and Oxidative Stress in Colitis Mice. Biomed Res. Int. 2018, 2018, 2607679. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Hu, C.A.A. Therapeutic Potential of Amino Acids in Inflammatory Bowel Disease. Nutrients 2017, 9, 920. [Google Scholar] [CrossRef] [PubMed]
- Elberry, A.A.; Sharkawi, S.M.Z.; Wahba, M.R. Antinociceptive and anti-inflammatory effects of N-acetylcysteine and verapamil in Wistar rats. Korean J. Pain. 2019, 32, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Seril, D.N.; Liao, J.; Ho, K.L.; Yang, C.S.; Yang, G.Y. Inhibition of chronic ulcerative colitis-associated colorectal adenocarcinoma development in a murine model by N-acetylcysteine. Carcinogenesis 2002, 23, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- 68. Zheng, J.; Yuan, X.; Zhang, C.; Jia, P.; Jiao, S.; Zhao, X.; Yin, H.; Du, Y.; Liu, H. N-Acetylcysteine alleviates gut dysbiosis and glucose metabolic disorder in high-fat diet-fed mice. J. Diabetes 2019, 11, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Ancha, H.R.; Kurella, R.R.; McKimmey, C.C.; Lightfoot, S.; Harty, R.F. Effects of N-acetylcysteine plus mesalamine on prostaglandin synthesis and nitric oxide generation in TNBS-induced colitis in rats. Dig. Dis. Sci. 2009, 54, 758–766. [Google Scholar] [CrossRef]
- Masnadi Shirazi, K.; Sotoudeh, S.; Masnadi Shirazi, A.; Moaddab, S.Y.; Nourpanah, Z.; Nikniaz, Z. Effect of N-acetylcysteine on remission maintenance in patients with ulcerative colitis: A randomized, double-blind controlled clinical trial. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101532. [Google Scholar] [CrossRef]
- Guijarro, L.G.; Mate, J.; Gisbert, J.; Perez-Calle, J.L.; Marin-Jimenez, I.; Arriaza, E.; Olleros, T.; Delgado, M.; Castillejo, M.S.; Prieto-Merino, D.; et al. N-acetyl-L-cysteine combined with mesalamine in the treatment of ulcerative colitis: Randomized, placebo-controlled pilot study. World J. Gastroenterol. 2008, 14, 2851–2857. [Google Scholar] [CrossRef]
- Hou, C.L.; Zhang, J.; Liu, X.T.; Liu, H.; Zeng, X.F.; Qiao, S.Y. Superoxide dismutase recombinant Lactobacillus fermentum ameliorates intestinal oxidative stress through inhibiting NF-κB activation in a trinitrobenzene sulphonic acid-induced colitis mouse model. J. Appl. Microbiol. 2014, 116, 1621–1631. [Google Scholar] [CrossRef]
- Suzuki, Y.; Matsumoto, T.; Okamoto, S.; Hibi, T.A. A lecithinized superoxide dismutase (PC-SOD) improves ulcerative colitis. Color. Dis. 2008, 10, 931. [Google Scholar] [CrossRef] [PubMed]
- Al-Howail, H.A.; Hakami, H.A.; Al-Otaibi, B.; Al-Mazrou, A.; Daghestani, M.H.; Al-Jammaz, I.; Al-Khalaf, H.H.; Aboussekhra, A. PAC down-regulates estrogen receptor alpha and suppresses epithelial-to-mesenchymal transition in breast cancer cells. BMC Cancer. 2016, 16, 540. [Google Scholar]
- Semlali, A.; Contant, C.; Al-Otaibi, B.; Al-Jammaz, I.; Chandad, F. The curcumin analog (PAC) suppressed cell survival and induced apoptosis and autophagy in oral cancer cells. Sci. Rep. 2021, 11, 11701. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, J.; Vemu, B.; Tocmo, R.; Nauman, M.C.; Johnson, J.J. Pharmacokinetic Analysis of Carnosic Acid and Carnosol in Standardized Rosemary Extract and the Effect on the Disease Activity Index of DSS-Induced Colitis. Nutrients 2021, 13, 773. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, S.; Jayachandran, I.; Subramanian, S.C.; Anjana, R.M.; Balasubramanyam, M.; Mohan, V.; Venkatesan, B.; Manickam, N. Decreased Sestrin levels in patients with type 2 diabetes and dyslipidemia and their association with the severity of atherogenic index. J. Endocrinol. Investig. 2021, 44, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Dehnoo, M.; Safari, A.A.; Rahimi-Madiseh, M.; Lorigooini, Z.; Moradi, M.T.; Amini-Khoei, H. Anethole Ameliorates Acetic Acid-Induced Colitis in Mice: Anti-Inflammatory and Antioxidant Effects. Evid Based Complement Altern. Med. 2022, 2022, 9057451. [Google Scholar] [CrossRef]
- Fu, T.T.; Shen, L. Ergothioneine as a Natural Antioxidant Against Oxidative Stress-Related Diseases. Front. Pharmacol. 2022, 13, 850813. [Google Scholar] [CrossRef]

| Compound | Chemical Structure | Mechanism of Action | Clinical Manifestation |
|---|---|---|---|
| Curcumin | 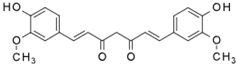 | Inhibition of NF-κB and STAT3 pathways [47]. Inhibition of COX-2 and iNOS expression [47]. | Reducing the clinical symptoms of IBD and increasing the achievement of remission [48,49]. |
| Resveratrol | 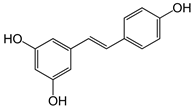 | Increase in glutathione peroxidase and SOD activity [51,52]. Inhibition of NF-κB pathway [52]. | Lowering the DAI value [53]. |
| Quercetin | 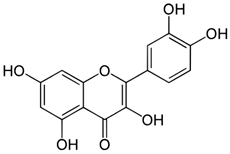 | Decrease in the activity of myeloperoxidase [57,58]. Increase in the concentration of glutathione [58]. Inhibition of TNF-α expression [57]. Antibacterial activation of macrophages [57]. | Reducing the clinical symptoms [60]. Improving the composition of intestinal microflora [57]. Reducing intestinal permeability [59]. Activating tight junction repair [59]. |
| Melatonin | 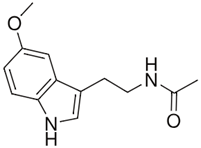 | Increasing the activity of SOD and catalase [61]. Limiting the production of pro-inflammatory cytokines [62]. Enhancing the production of anti-inflammatory cytokines [62]. | Reducing the clinical symptoms [63]. Modulation of intestinal dysbiosis [64]. |
| N-acetylcysteine | 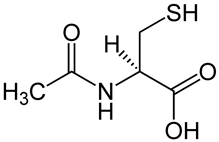 | Inhibition of NF-κB pathway [66]. Inhibition of apoptosis by acting on caspases [67]. Promotion of intestinal cell proliferation [67]. Modulation claudin and occluding activity [67]. | Reducing the clinical symptoms [69,70]. Improving the composition of the intestinal microflora [68]. Reducing intestinal permeability [68]. |
| Artificial superoxide dismutase | 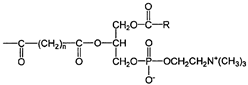 | Direct inhibition of lipid peroxidation [72]. Decrease in the level of MPO [72]. Inhibition of NF-κB pathway [72]. | Reducing the clinical symptoms of IBD [73]. Lowering the DAI value [73]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blagov, A.V.; Orekhova, V.A.; Sukhorukov, V.N.; Melnichenko, A.A.; Orekhov, A.N. Potential Use of Antioxidant Compounds for the Treatment of Inflammatory Bowel Disease. Pharmaceuticals 2023, 16, 1150. https://doi.org/10.3390/ph16081150
Blagov AV, Orekhova VA, Sukhorukov VN, Melnichenko AA, Orekhov AN. Potential Use of Antioxidant Compounds for the Treatment of Inflammatory Bowel Disease. Pharmaceuticals. 2023; 16(8):1150. https://doi.org/10.3390/ph16081150
Chicago/Turabian StyleBlagov, Alexander V., Varvara A. Orekhova, Vasily N. Sukhorukov, Alexandra A. Melnichenko, and Alexander N. Orekhov. 2023. "Potential Use of Antioxidant Compounds for the Treatment of Inflammatory Bowel Disease" Pharmaceuticals 16, no. 8: 1150. https://doi.org/10.3390/ph16081150
APA StyleBlagov, A. V., Orekhova, V. A., Sukhorukov, V. N., Melnichenko, A. A., & Orekhov, A. N. (2023). Potential Use of Antioxidant Compounds for the Treatment of Inflammatory Bowel Disease. Pharmaceuticals, 16(8), 1150. https://doi.org/10.3390/ph16081150