Ethnomedicinal Uses, Phytochemistry, and Anticancer Potentials of African Medicinal Fruits: A Comprehensive Review
Abstract
1. Introduction
2. Fruits in Cancer Treatment
2.1. Biological Activities of Southern African Fruits
2.1.1. Marula
2.1.2. Kei Apple
2.1.3. Mobola Plum
2.1.4. Red Milkwood
2.1.5. Sour Fig
2.1.6. Wild Medlar
2.1.7. Wild Plum
2.1.8. Natal Plum (Num Num)
| Name | Indigenous Name (South Africa) | Distribution in Africa | Plants Parts Used for Treatment of Disease Conditions in Folk Medicine | References |
|---|---|---|---|---|
| Sclerocarya birrea | Marula, umganu (in Zulu) | South Africa, Botswana, Zimbabwe, Namibia, Zambia, Eswatini, Malawi, and Mozambique | (1) Bark: to treat diabetes, snake bite, pruritis, pharyngitis, splenomegaly, goiter, cholera, dysentery, diarrhea, proctitis, stomach ailments, ulcers, inflammation, arthritis, hypertension, skin diseases, fever, malaria, and proctitis; (2) roots: to treat snake bite, pruritis, pharyngitis, splenomegaly, goiter, fungal infections, and sore eye; (3) leaves: cold and flu, snake bite, pruritis, pharyngitis, splenomegaly, goiter, diarrhea, dysentery, proctitis, stomach ailments, ulcers, inflammation, arthritis, hypertension, skin diseases, fever, malaria, diabetes, fungal infections, and heartburn; (4) fruit: snake bite, pruritis, pharyngitis, splenomegaly, and goiter. | [40,43,44,56] |
| Dovyalis caffra | Kei Apple, umqokolo (in Zulu) | Eswatini, Malawi, South Africa, Zimbabwe, Mozambique, Tanzania, Lesotho, and Namibia | (1) Bark: to treat rheumatism; (2) root: to treat amenorrhea, chest pain, and rheumatism; (3) fruit: to treat coughing in different livestock; (4) thorns: amenorrhea and chest pain. | [55,56] |
| Parinari curatellifolia | Mobola Plum, mmola (in Sotho) | Zimbabwe, South Africa, Malawi, Nigeria, and Eswatini | (1) Bark: to treat pneumonia, cataracts, and earache; (2) leaves: to treat dislocated bones, broken bones, wound, and pneumonia. | [64,65,67] |
| Mimusops caffra | Red Milkwood, umThunzi (in Zulu) | Botswana, Mozambique, Tanzania, Eswatini, Angola, Zimbabwe, and South Africa | (1) Bark: to treat wounds and sores, and used as an emetic; (2) root: to treat sexually transmitted infections, such as gonorrhea, and other ailments, like candidiasis, tuberculosis, weight loss, and womb problems; (3) Leaf: antiplasmodial activity and used to manage malaria. | [74,75] |
| Carpobrotus edulis | Sour Fig, igcukuma (in Xhosa) | Coastal South Africa to coastal Mozambique | Leaves: to treat diarrhea, stomach upset, tuberculosis, skin infections (eczema, dermatitis, and sunburns), sinusitis, vaginal thrust burns, and toothache. | [37,81,82,83] |
| Vangueria infausta | Wild Medlar, umtulwa (in Zulu) | Malawi, Uganda, Tanzania, Mozambique, Kenya, Zimbabwe, Botswana, South Africa, Lesotho, Eswatini, and Namibia | (1) Roots: to treat abdominal pains, asthma, blood pressure, chest pain, cold and cough, diabetes, diarrhea and stomach problems, epilepsy, fever, headache, hernia, infertility, malaria, measles, male virility, menstrual problems, nervous system disorders, candidiasis, pneumonia, skin blisters, snake bites, stomach ulcers, and parasitic worms; (2) bark: skin blisters, syphilis, asthma, bloody stool, blood pressure, chest pain, cold and cough, diarrhea and stomach problems, infertility, and candidiasis; (3) leaf: to treat abscesses, asthma, blood pressure, chest pain, cold and cough, dermatitis, diabetes, fever, headache, hernia, malaria, candidiasis, parasitic worms, pleurisy, pneumonia, skin blisters, swellings, and toothache; (4) fruit: to treat menstrual problems and parasitic worms. | [56,88] |
| Harpephyllum caffrum | Wild Plum, umgwenya (in Zulu) | South Africa, Eswatini, Mozambique, and Zimbabwe | (1) Bark: to treat acne and eczema, sprains, and bone fractures; (2) root: to treat paralysis caused by sorcery. | [107,120] |
| Carissa macrocarpa | Num Num or Amatungula in IsiZulu | South Africa and Mozambique | Leaves: to treat venereal diseases and coughs. | [121,122] |
3. Anticancer Effects and Cellular Targets of Selected African Fruits
3.1. Tribulus terrestris L.
3.2. Xanthium strumarium L.
3.3. Withania somnifera (L.) Dunal
3.4. Xylopia aethiopica (Dunal) A. Rich
3.5. Abelmoschus esculentus (L.) Moench
3.6. Carissa macrocarpa (Eckl.) A.DC.
3.7. Carpobrotus edulis (L.) L. Bolus
3.8. Syzygium cumini (L.) Skeels
3.9. Kigelia Africana (Lam.) Benth.
3.10. Annona muricata L.
3.11. Persea americana Mill.
3.12. Punica granatum L.
| Human Cancer type | Cell Lines and In Vivo Models | Phytochemicals/Fruit or Seeds Extracts | Effective Concentration | Reference |
|---|---|---|---|---|
| Colorectal cancer | HCT116, DLD-1 | Extracts from X. strumarium and T. terrestris fruits | 100 mg/mL | [129] |
| Oral cancer | SAS, TW2.6 | Extracts from X. strumarium and T. terrestris fruits | 50 and 100 mg/mL | [130] |
| Liver cancer | HepG2 | Aqueous extract of T. terrestris fruits | 200 and 500 mg/L | [131] |
| Colorectal, breast, liver cancer, and T-cell lymphoma | Colo20, HCT116, DLD, MCF-7, Jurkat, HepG2 | Withania somnifera fruit extract | LC50 values of 287.8 (Colo20), 410.2 (HCT116), 226.3 (MCF7), 356.4 (Jurkat), and 164.7 (HepG2) µg/mL | [164] |
| Leukemia | Primary leukemia cells from patients | W. somnifera fruit extract | LD50 1.45 ± 0.05 IU | [159] |
| Liver, lung cancer, fibrosarcoma, and T-cell lymphoma | HepG2, A549, Jurkat, HepG2 | W. somnifera seed extract | Methanol extract LC50 values of 50.18 μg/mL (Jurkat), 112.9 μg/mL (HepG2), 163 μg/mL (L929) and ethyl acetate fraction LC50 48.73 μg/mL (Jurkat), 68.739 μg/mL (HepG2), and 111 μg/mL (L929) Both extracts were less effective against A549 | [146] |
| Liver, lung, and colon | HepG2, A549, HCT116, SW480 | Isolated pentacyclic triterpenoids from the fruits of Xanthium strumarium | IC50 range between 4.27 mM and >100 mM | [147] |
| Cervical cancer | HeLa | Hydro-alcoholic extract of X. strumarium fruits | Effective dose range of 12.5, 25, and 50 mg/mL | [149] |
| Cervical, colon, and gastric cancer | HeLa, HT-29, AGS | ent-kauranoid glycosides (fructusnoids A-C) from extract of X. strumarium fruits | IC50 range between 7.6 μM to >50 μM | [150] |
| Breast cancer | MDA-MB-231, MCF-7 | Ethanol extract of Xylopia aethiopica fruits | IC50 values of ~5 mg/mL | [180] |
| Cervical, oral, breast, and lung cancer | C-33A, KB, MCF-7, A549 | Methanol extract of X. aethiopica fruits | IC50 values of 60.2 mg/mL (MCF-7), 62.5 mg/mL (KB), 30.8 mg/mL (C-33A), and >100 mg/mL (A549) | [179] |
| Leukemia, colon cancer | HCT116, U937, KG1a | Ent-15-oxokaur-16-en-19-oic acid isolated from ethanol extract of X. aethiopica fruits | IC50 values of 12 μg/mL (HCT116), 7.5 μg/mL (U937), and >25 μg/mL (KG1a) | [181] |
| Pancreatic cancer, leukemia | MiaPaCa-2, CCRF-CEM, multidrug-resistant CEM/ADR5000 | Methanol extract of X. aethiopica fruits | IC50 values of 6.86 mg/mL (Mia PaCa2), 3.91 mg/mL (CCRF-CEM), and 7.4 mg/mL (CEM/ADR5000) | [182] |
| Leukemia, breast, liver and colon cancer, glioblastoma | Drug-sensitive CCRF-CEM and HL-60 cells, MDA-MB-231-pcDNA3, HCT116 (p53+/+), U87MG, HepG2 cells, multidrug-resistant HL-60/AR, MDA-MB-231-BCRP clone 23, HCT116 (p53−/−), U87MG.ΔEGFR | Methanol extracts of rhizomes of E. giganteus, roots of l. cylindrica, seeds of P. capense and X. aethiopica | IC50 values of 4.11 mg/mL (CCRF-CEM), 7.94 mg/mL (HL60), 30.60 mg/mL (HL60AR) 5.19 mg/mL (MDA-MB231) 10.04 mg/mL (MDA-MB231BCRP), 4.37 mg/mL (HCT116 p53+/+), and 18.28 mg/mL (HepG2) | [183] |
| Acute lymphoblastic leukemia, breast and colon cancer, glioblastoma | Drug-sensitive cell lines CCRF-CEM, MDA-MB-231-pcDNA3, HCT116 (p53+/+), U87MG and multidrug-resistant cell lines CEM/ADR5000, MDA-MB-231-BCRP clone 23, HCT116 (p53−/−), U87MG.ΔEGFR | Flavonoid 3,4′,5-trihydroxy-6”,6″-dimethylpyrano[2,3-γ] flavone, and alkaloid isotetrandrine derived from methanol crude extract of X. aethiopica fruits | IC50 range for flavonoid from 2.61 µM (CCRF-CEM) to 18.60 µM (U87MG.ΔEGFR) and alkaloid from 1.45 µM (HepG2) to 7.28 µM (MDA-MB-231-pcDNA) | [184] |
| Lung and gastric cancer | A549, AGS | Hydroethanol extracts of X. aethiopica fruits | Effective dose range of 500 mg/mL IC50 value for AGS of 151 mg/mL | [186] |
| Cervical and lung cancer | HeLa, MRC-5 SV2 | olean-12-en-3-O-β-d-glucopyranoside, isoquercitrin and 5,7,3′,4′-tetrahydroxy-flavonol-3-O-[β-d-glucopyranosyl-(1→6)]-β-d-glucopyranoside derived from methanolic fruit extract of Abelmoschus esculentus | Relatively nontoxic or mildly toxic at a concentration of 100 mg/mL | [194] |
| Nonsmall cell lung cancer, breast, cervical, and liver cancer | NCL-H460, MCF-7, HeLa, HepG2 | Extracts of A. esculentus pods | IC50 values of 49.62 mg/mL (NCL-H460), 56.40 mg/mL (MCF-7), 67.27 mg/mL (HeLa), and 167.95 mg/mL (HepG2) | [197] |
| Breast, liver, and cervical cancer | MCF-7, HepG2, HeLa | A. esculentus seed extract and ethanol, dichloromethane and petroleum fraction | Effective dose at 1000 μg/mL of seed extract | [198] |
| Breast cancer | MCF-7 | A. esculentus lectin from Abelmoschus esculentus | Effective dose at 0.1 mg/mL | [200] |
| Glioblastoma | U87MG | A. esculentus lectin from Abelmoschus esculentus | IC50 21 μg/mL | [201] |
| Breast, cervical, liver, and nonsmall cell lung cancer | MCF-7, HeLa, NCI-H460, HepG2 | Hydroethanolic extract of C. macrocarpa fruits | IC50 values of 66 μg/mL (HeLa), 57 μg/mL (NCI-H460), 109 μg/mL (MCF-7), and >400 μg/mL (HepG2) | [119] |
| Breast, cervical, liver, lung, and brain cancer | MCF-7, HeLa, HEPG2, H460, U251 | Alcoholic extract of Syzygium cumini fruits | IC50 values of 5.9 μg/mL (MCF-7) and >10 μg/mL (Hela, HEPG2, H460, and U251 | [220] |
| Nonsmall lung cancer cells | A549 | 75% aqueous ethanol fruit pulp and seed powder of S. cumini | IC50 values of 59 μg/mL (pulp) 38 μg/mL (seeds) | [219] |
| Breast cancer | MCF-7aro, MDA-MB-231 | Fruit pulp powder extract of S. cumini | IC50 values of 27 μg/mL (MCF-7aro) and 40 μg/mL (MDA-MB-231) | [221] |
| Cervical cancer | HeLa, SiHa | Crude and methanolic extract of S. cumini fruit skin | Crude extract inhibited 33.7% (HeLa) and 24.4% (SiHa) cell growth | [222] |
| Skin cancer | 7,12-dimethyl benz(a)anthracene induced skin papilloma in vivo | Hydroalcoholic extract of S. cumini seeds | 125 mg/kg/body wt./animal/day | [223] |
| Gastric cancer | Benzo[a]pyrene induced stomach carcinogenesis in vivo | Extract of S. cumini seeds | 25 mg/kg body wt./day | [224] |
| Melanoma | G361 | Dichloromethane extract and fractions from Kigelia Africana fruit | IC50 values of 2.1 mg/mL (extract), 1.2–15.4 mg/mL (fractions) | [240] |
| Melanoma | SK-MEL-28, MalMe-3M | Isolated phytocompounds from K. Africana fruit | IC50 value range of 0.3 to 180.5 mg/mL (SK-MEL-28), 0.5 to 75.5 mg/mL (MalMe-3M) | [241] |
| Colon cancer | Caco-2 | n-Hexane extract of seed oil from K. Africana fruit | Effective dose of 20–120 mg/mL | [242] |
| Liver, pancreatic, colorectal, gastric, and colon cancer | Huh-7, PANC-1, Colo-205, HT-29, SNU-16, SW620, HCT116 | Methanol and ethyl acetate extracts K. Africana fruit | IC50 range for methanolic extract from 6.79 µg/mL (SW620) to 91.32 µg/mL (HT-29) and ethyl acetate extract from 13.56 µg/mL (SNU-16) to 112.74 µg/mL (Huh-7) | [236] |
| Gastric cancer | Benzo[a]pyrene-induced stomach carcinogenesis in vivo | Ethanolic extract of K. Africana fruit | 2 mg/day for eight weeks | [243] |
| Leukemia | Benzene-induced Leukemia in vivo | Ethanol extract of K. Africana fruit | 0.5 mL of 100 mg/mL for three weeks | [244] |
| Prostate cancer | PC-3 | Muricins M and N, and muricenin isolated from Annona muricata fruit extract | Effective concentration of 20 μg/mL | [255] |
| Liver cancer | HepG2, Hep-G2/2.2. 15 | Muricin H, muricin I, and cis-annomontacin isolated from the seeds of A. muricata. | IC50 values for muricin H of 0.0951 μg/mL (HepG2), 0.01181 μg/mL (Hep-G2/2.2. 15), muricin I 0.0509 μg/mL (HepG2), 0.222 μg/mL (Hep-G2/2.2. 15), and cis-annomontacin 0.298 μg/mL (HepG2), and 0.0162 μg/mL (Hep-G2/2.2. 15) | [256] |
| Breast cancer | MDA-MB-468 | A. muricata fruit extract | IC50 value of 4.8 μg/mL | [257] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Aashima; Nanda, M.; Fronterre, C.; Sewagudde, P.; Ssentongo, A.E.; Yenney, K.; Arhin, N.D.; Oh, J.; Amponsah-Manu, F.; et al. Mapping Cancer in Africa: A Comprehensive and Comparable Characterization of 34 Cancer Types Using Estimates From GLOBOCAN 2020. Front. Public Health 2022, 10, 839835. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Parkin, D.M.; Gnangnon, F.; Tshisimogo, G.; Peko, J.-F.; Adoubi, I.; Assefa, M.; Bojang, L.; Awuah, B.; Koulibaly, M.; et al. Cancer in Sub-Saharan Africa in 2020: A Review of Current Estimates of the National Burden, Data Gaps, and Future Needs. Lancet Oncol. 2022, 23, 719–728. [Google Scholar] [CrossRef]
- Bahnassy, A.A.; Abdellateif, M.S.; Zekri, A.-R.N. Cancer in Africa: Is It a Genetic or Environmental Health Problem? Front. Oncol. 2020, 10, 2508. [Google Scholar] [CrossRef]
- Cairncross, L.; Parkes, J.; Craig, H.; Are, C. Cancer on the Global Stage: Incidence and Cancer-Related Mortality in South Africa—The ASCO Post. Available online: https://ascopost.com/issues/september-10-2021/cancer-on-the-global-stage-incidence-and-cancer-related-mortality-in-south-africa/ (accessed on 7 January 2023).
- Lukong, K.E.; Ogunbolude, Y.; Kamdem, J.P. Breast Cancer in Africa: Prevalence, Treatment Options, Herbal Medicines, and Socioeconomic Determinants. Breast Cancer Res. Treat. 2017, 166, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, Y.; Abdeljaoued-Tej, I.; Zatchi, A.A.; Abdelhak, S.; Boubaker, S.; Brown, J.S.; Benkahla, A. Cancer in Africa: The Untold Story. Front. Oncol. 2021, 11, 650117. [Google Scholar] [CrossRef] [PubMed]
- Jojo, L.W.; Nkutu, N.T. Experiences of Patients on Cancer Treatment Regarding Decentralization of Oncology Services at a Tertiary Hospital in the Eastern Cape. BMC Cancer 2023, 23, 453. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 20503121211034370. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Altun, İ.; Sonkaya, A. The Most Common Side Effects Experienced by Patients Were Receiving First Cycle of Chemotherapy. Iran. J. Public Health 2018, 47, 1218–1219. [Google Scholar]
- Remesh, A. Toxicities of Anticancer Drugs and Its Management. Int. J. Basic Clin. Pharmacol. 2012, 1, 2–12. [Google Scholar] [CrossRef]
- Brigden, M.; McKenzie, M. Treating Cancer Patients. Practical Monitoring and Management of Therapy-Related Complications. Can. Fam. Physician Med. Fam. Can. 2000, 46, 2258–2268. [Google Scholar]
- Chu, S.H.; Lee, Y.J.; Lee, E.S.; Geng, Y.; Wang, X.S.; Cleeland, C.S. Current Use of Drugs Affecting the Central Nervous System for Chemotherapy-Induced Peripheral Neuropathy in Cancer Patients: A Systematic Review. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2015, 23, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Ewertz, M.; Qvortrup, C.; Eckhoff, L. Chemotherapy-Induced Peripheral Neuropathy in Patients Treated with Taxanes and Platinum Derivatives. Acta Oncol. Stockh. Swed. 2015, 54, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Bidram, E.; Esmaeili, Y.; Ranji-Burachaloo, H.; Al-Zaubai, N.; Zarrabi, A.; Stewart, A.; Dunstan, D.E. A Concise Review on Cancer Treatment Methods and Delivery Systems. J. Drug Deliv. Sci. Technol. 2019, 54, 101350. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2019, 10, 1614. [Google Scholar] [CrossRef]
- Bouyahya, A.; Omari, N.E.; Bakrim, S.; Hachlafi, N.E.; Balahbib, A.; Wilairatana, P.; Mubarak, M.S. Advances in Dietary Phenolic Compounds to Improve Chemosensitivity of Anticancer Drugs. Cancers 2022, 14, 4573. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; AAl-Mana, F.; Mahmoud, E.A.; Zin El-Abedin, T.K.A.; Mattar, M.A.; Ekiert, H. Phenolic Compounds of Catalpa Speciosa, Taxus Cuspidata, and Magnolia Acuminata Have Antioxidant and Anticancer Activity. Molecules 2019, 24, 412. [Google Scholar] [CrossRef]
- Dobrzynska, M.; Napierala, M.; Florek, E. Flavonoid Nanoparticles: A Promising Approach for Cancer Therapy. Biomolecules 2020, 10, 1268. [Google Scholar] [CrossRef]
- ul Islam, B.; Suhail, M.; Khan, M.S.; Ahmad, A.; Zughaibi, T.A.; Husain, F.M.; Rehman, M.T.; Tabrez, S. Flavonoids and PI3K/Akt/MTOR Signaling Cascade: A Potential Crosstalk in Anticancer Treatment. Curr. Med. Chem. 2021, 28, 8083–8097. [Google Scholar] [CrossRef] [PubMed]
- Moyo, M.; Aremu, A.O.; Van Staden, J. Medicinal Plants: An Invaluable, Dwindling Resource in Sub-Saharan Africa. J. Ethnopharmacol. 2015, 174, 595–606. [Google Scholar] [CrossRef] [PubMed]
- The Use of African Medicinal Plants in Cancer Management—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9971233/ (accessed on 24 May 2023).
- Tsoka-Gwegwenia, J.M.; Cumber, S.N.; Nchanji, K.N. Breast Cancer among Women in Sub-Saharan Africa: Prevalence and a Situational Analysis. S. Afr. J. Gynaecol. Oncol. 2017, 9, 28–30. [Google Scholar] [CrossRef]
- Ma, J.; Jemal, A. Breast Cancer Statistics. In Breast Cancer Metastasis and Drug Resistance: Progress and Prospects; Ahmad, A., Ed.; Springer: New York, NY, USA, 2013; pp. 1–18. ISBN 978-1-4614-5647-6. [Google Scholar]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed]
- Daga, A.; Ansari, A.; Patel, S.; Mirza, S.; Rawal, R.; Umrania, V. Current Drugs and Drug Targets in Non-Small Cell Lung Cancer: Limitations and Opportunities. Asian Pac. J. Cancer Prev. APJCP 2015, 16, 4147–4156. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A. Phytochemicals as Potential Anticancer Drugs: Time to Ponder Nature’s Bounty. BioMed Res. Int. 2020, 2020, 8602879. [Google Scholar] [CrossRef] [PubMed]
- Egbuna, C.; Kumar, S.; Ifemeje, J.C.; Ezzat, S.M.; Kaliyaperumal, S. Phytochemicals as Lead Compounds for New Drug Discovery; Elsevier Science: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-817891-1. [Google Scholar]
- Berdigaliyev, N.; Aljofan, M. An Overview of Drug Discovery and Development. Future Med. Chem. 2020, 12, 939–947. [Google Scholar] [CrossRef]
- Shankar, M.G.; Swetha, M.; Keerthana, C.K.; Rayginia, T.P.; Anto, R.J. Cancer Chemoprevention: A Strategic Approach Using Phytochemicals. Front. Pharmacol. 2021, 12, 809308. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Twilley, D.; Rademan, S.; Lall, N. A Review on Traditionally Used South African Medicinal Plants, Their Secondary Metabolites and Their Potential Development into Anticancer Agents. J. Ethnopharmacol. 2020, 261, 113101. [Google Scholar] [CrossRef]
- Fouche, G.; Cragg, G.M.; Pillay, P.; Kolesnikova, N.; Maharaj, V.J.; Senabe, J. In Vitro Anticancer Screening of South African Plants. J. Ethnopharmacol. 2008, 119, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Mokganya, M.G.; Tshisikhawe, M.P. Medicinal Uses of Selected Wild Edible Vegetables Consumed by Vhavenda of the Vhembe District Municipality, South Africa. S. Afr. J. Bot. 2019, 122, 184–188. [Google Scholar] [CrossRef]
- Pfukwa, T.M.; Chikwanha, O.C.; Katiyatiya, C.L.F.; Fawole, O.A.; Manley, M.; Mapiye, C. Southern African Indigenous Fruits and Their Byproducts: Prospects as Food Antioxidants. J. Funct. Foods 2020, 75, 104220. [Google Scholar] [CrossRef]
- Pfukwa, T.M.; Fawole, O.A.; Manley, M.; Mapiye, C. Phenolic Profiling and Antioxidant Evaluation of Extracts from Southern African Indigenous Fruits Byproducts. Food Res. Int. 2022, 157, 111388. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Joshi, T.; Gupta, R.C.; Singh, I.P. Chapter 10—The Indian Blackberry (Jamun), Antioxidant Capacity, and Cancer Protection. In Cancer; Preedy, V., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 101–113. ISBN 978-0-12-405205-5. [Google Scholar]
- Kamanula, M.; Munthali, C.Y.; Kamanula, J.F. Nutritional and Phytochemical Variation of Marula (Sclerocarya birrea) (Subspecies Caffra and Birrea) Fruit among Nine International Provenances Tested in Malawi. Int. J. Food Sci. 2022, 2022, e4686368. [Google Scholar] [CrossRef] [PubMed]
- Nitcheu Ngemakwe, P.H.; Remize, F.; Thaoge, M.L.; Sivakumar, D. Phytochemical and Nutritional Properties of Underutilised Fruits in the Southern African Region. S. Afr. J. Bot. 2017, 113, 137–149. [Google Scholar] [CrossRef]
- Mashau, M.E.; Kgatla, T.E.; Makhado, M.V.; Mikasi, M.S.; Ramashia, S.E. Nutritional Composition, Polyphenolic Compounds and Biological Activities of Marula Fruit (Sclerocarya birrea) with Its Potential Food Applications: A Review. Int. J. Food Prop. 2022, 25, 1549–1575. [Google Scholar] [CrossRef]
- Ojewole, J.A.O.; Mawoza, T.; Chiwororo, W.D.H.; Owira, P.M.O. Sclerocarya birrea (a. Rich) Hochst. [‘Marula’] (Anacardiaceae): A Review of Its Phytochemistry, Pharmacology and Toxicology and Its Ethnomedicinal Uses. Phytother. Res. 2010, 24, 633–639. [Google Scholar] [CrossRef]
- Prinsloo, G.; Street, R.A. Marula [Sclerocarya birrea (A.Rich) Hochst]: A Review of Traditional Uses, Phytochemistry, and Pharmacology. In African Natural Plant Products Volume II: Discoveries and Challenges in Chemistry, Health, and Nutrition; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2013; Volume 1127, pp. 19–32. ISBN 978-0-8412-2804-7. [Google Scholar]
- Mariod, A.A.; Abdelwahab, S.I. Sclerocarya birrea (Marula), An African Tree of Nutritional and Medicinal Uses: A Review. Food Rev. Int. 2012, 28, 375–388. [Google Scholar] [CrossRef]
- Eloff, J.N. Antibacterial Activity of Marula (Sclerocarya birrea (A. Rich.) Hochst. Subsp. Caffra (Sond.) Kokwaro) (Anacardiaceae) Bark and Leaves. J. Ethnopharmacol. 2001, 76, 305–308. [Google Scholar] [CrossRef]
- Ojewole, J.A.O. Evaluation of the Anti-Inflammatory Properties of Sclerocarya birrea (A. Rich.) Hochst. (Family: Anacardiaceae) Stem-Bark Extracts in Rats. J. Ethnopharmacol. 2003, 85, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.; Hassan, L.G.; Dangoggo, S.M.; Hassan, S.W.; Umar, R.A.; Umar, K.J. Acute and Subchronic Toxicity Studies of Sclerocarya birrea Peels Extract in Rats. Int. J. Sci. Basic Appl. Res. IJSBAR 2014, 13, 111–118. [Google Scholar]
- McGaw, L.J.; Van der Merwe, D.; Eloff, J.N. In Vitro Anthelmintic, Antibacterial and Cytotoxic Effects of Extracts from Plants Used in South African Ethnoveterinary Medicine. Vet. J. 2007, 173, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Gathirwa, J.W.; Rukunga, G.M.; Njagi, E.N.M.; Omar, S.A.; Mwitari, P.G.; Guantai, A.N.; Tolo, F.M.; Kimani, C.W.; Muthaura, C.N.; Kirira, P.G.; et al. The in Vitro Anti-Plasmodial and in Vivo Anti-Malarial Efficacy of Combinations of Some Medicinal Plants Used Traditionally for Treatment of Malaria by the Meru Community in Kenya. J. Ethnopharmacol. 2008, 115, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Gondwe, M.; Kamadyaapa, D.R.; Tufts, M.; Chuturgoon, A.A.; Musabayane, C.T. Sclerocarya birrea [(A. Rich.) Hochst.] [Anacardiaceae] Stem-Bark Ethanolic Extract (SBE) Modulates Blood Glucose, Glomerular Filtration Rate (GFR) and Mean Arterial Blood Pressure (MAP) of STZ-Induced Diabetic Rats. Phytomedicine 2008, 15, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Borochov-Neori, H.; Judeinstein, S.; Greenberg, A.; Fuhrman, B.; Attias, J.; Volkova, N.; Hayek, T.; Aviram, M. Phenolic Antioxidants and Antiatherogenic Effects of Marula (Sclerocarrya birrea Subsp. Caffra) Fruit Juice in Healthy Humans. J. Agric. Food Chem. 2008, 56, 9884–9891. [Google Scholar] [CrossRef]
- Mpai, S.; du Preez, R.; Sultanbawa, Y.; Sivakumar, D. Phytochemicals and Nutritional Composition in Accessions of Kei-Apple (Dovyalis caffra): Southern African Indigenous Fruit. Food Chem. 2018, 253, 37–45. [Google Scholar] [CrossRef]
- du Preez, R.J. Fruits of Tropical Climates | Lesser-Known Fruits of Africa. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 2800–2810. ISBN 978-0-12-227055-0. [Google Scholar]
- Aremu, A.O.; Ncama, K.; Omotayo, A.O. Ethnobotanical Uses, Biological Activities and Chemical Properties of Kei-Apple [Dovyalis caffra (Hook.f. & Harv.) Sim]: An Indigenous Fruit Tree of Southern Africa. J. Ethnopharmacol. 2019, 241, 111963. [Google Scholar] [CrossRef]
- Omotayo, A.O.; Aremu, A.O. Underutilized African Indigenous Fruit Trees and Food–Nutrition Security: Opportunities, Challenges, and Prospects. Food Energy Secur. 2020, 9, e220. [Google Scholar] [CrossRef]
- Qanash, H.; Yahya, R.; Bakri, M.M.; Bazaid, A.S.; Qanash, S.; Shater, A.F.; T.M., A. Anticancer, Antioxidant, Antiviral and Antimicrobial Activities of Kei Apple (Dovyalis caffra) Fruit. Sci. Rep. 2022, 12, 5914. [Google Scholar] [CrossRef]
- El-Menshawi, B.S.; Fayad, W.; Mahmoud, K.; El-Hallouty, S.M.; El-Manawaty, M.; Olofsson, M.H.; Linder, S. Screening of Natural Products for Therapeutic Activity against Solid Tumors. Indian J. Exp. Biol. 2010, 48, 258–264. [Google Scholar] [PubMed]
- Moustafa, S.; Menshawi, B.M.; Wassel, G.; Mahmoud, K.; Mounier, M.M. Screening of Some Plants in Egypt for Their Cytotoxicity against Four Human Cancer Cell Lines. Int. J. PharmTech Res. 2014, 6, 1074–1084. [Google Scholar]
- Taher, M.A.; Tadros, L.K.; Dawood, D.H. Phytochemical Constituents, Antioxidant Activity and Safety Evaluation of Kei-Apple Fruit (Dovyalis caffra). Food Chem. 2018, 265, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Akweni, A.L.; Sibanda, S.; Zharare, G.E.; Zimudzi, C. Fruit-Based Allometry of Strychnos madagascariensis and S. Spinosa (Loganiaceae) in the Savannah Woodlands of the Umhlabuyalingana Municipality, KwaZulu-Natal, South Africa. Trees For. People 2020, 2, 100025. [Google Scholar] [CrossRef]
- Ogbonnia, S. Chapter 91—Extracts of Mobola Plum (Parinari curatellifolia Planch Ex Benth, Chrysobalanaceae) Seeds and Multiple Therapeutic Activities. In Nuts and Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 767–774. ISBN 978-0-12-375688-6. [Google Scholar]
- Parinari curatellifolia|PlantZAfrica. Available online: https://pza.sanbi.org/parinari-curatellifolia (accessed on 24 July 2023).
- Crown, O.O.; Olayeriju, O.S.; Kolawole, A.O.; Akinmoladun, A.C.; Olaleye, M.T.; Akindahunsi, A.A. Mobola Plum Seed Methanolic Extracts Exhibit Mixed Type Inhibition of Angiotensin Ⅰ-Converting Enzyme in Vitro. Asian Pac. J. Trop. Biomed. 2017, 7, 1079–1084. [Google Scholar] [CrossRef]
- Olaleye, M.T.; Amobonye, A.E.; Komolafe, K.; Akinmoladun, A.C. Protective Effects of Parinari curatellifolia Flavonoids against Acetaminophen-Induced Hepatic Necrosis in Rats. Saudi J. Biol. Sci. 2014, 21, 486–492. [Google Scholar] [CrossRef]
- Manuwa, T.R.; Akinmoladun, A.C.; Crown, O.O.; Komolafe, K.; Olaleye, M.T. Toxicological Assessment and Ameliorative Effects of Parinari curatellifolia Alkaloids on Triton-Induced Hyperlipidemia and Atherogenicity in Rats. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 611–623. [Google Scholar] [CrossRef]
- Nkosi, N.J.; Shoko, T.; Manhivi, V.E.; Slabbert, R.M.; Sultanbawa, Y.; Sivakumar, D. Metabolomic and Chemometric Profiles of Ten Southern African Indigenous Fruits. Food Chem. 2022, 381, 132244. [Google Scholar] [CrossRef]
- Studies of In Vitro Antioxidant and Cytotoxic Activities of Extracts and Isolated Compounds from Parinari curatellifolia (Chrysobalanaceae)|Semantic Scholar. Available online: https://www.semanticscholar.org/paper/Studies-of-In-vitro-Antioxidant-and-Cytotoxic-of-Halilu-October/d853e8e182761bb56d884215ed7eed96e6972433 (accessed on 25 July 2023).
- Ogbonnia, S.O.; Olayemi, S.O.; Anyika, E.N.; Enwuru, V.N.; Poluyi, O.O. Evaluation of Acute Toxicity in Mice and Subchronic Toxicity of Hydroethanolic Extract of Parinari curatellifolia Planch (Chrysobalanaceae) Seeds in Rats. Afr. J. Biotechnol. 2009, 8, 1800–1806. [Google Scholar]
- Halilu, E.M.; October, N.; Ugwah-Oguejiofor, C.J.; Jega, A.Y.; Nefai, M.S. Anti-Snake Venom and Analgesic Activities of Extracts and Betulinic and Oleanolic Acids Isolated from Parinari curatellifolia. J. Med. Plants Econ. Dev. 2020, 4, 8. [Google Scholar]
- Halilu, E. Toxicity Assessment of Methanol Extract of Parinari curatellifolia Planch Ex. Benth. Pak. J. Pharm. Sci. 2023, 36, 1233–1239. [Google Scholar]
- Mngadi, S.; Moodley, R.; Jonnalagadda, S.B. Elemental Composition and Nutritional Value of the Edible Fruits of Coastal Red-Milkwood (Mimusops caffra) and Impact of Soil Quality on Their Chemical Characteristics. J. Environ. Sci. Health Part B 2017, 52, 435–445. [Google Scholar] [CrossRef]
- Mashela, P.W.; Pofu, K.M.; Nzanza, B. Responses of Mmupudu (Mimusops zeyheri) Indigenous Fruit Tree to Three Soil Types. Afr. J. Agric. Res. 2013, 8, 1066–1069. [Google Scholar] [CrossRef]
- Chivandi, E.; Davidson, B.; Pretorius, B.; Erlwanger, K. Proximate, Mineral, Amino Acid, Fatty Acid, Vitamin E, Phytate Phosphate and Fibre Composition of Mimusops zeyheri (Red Milkwood) Seed. Int. J. Food Sci. Technol. 2011, 46, 555–560. [Google Scholar] [CrossRef]
- Chivandi, E.; Mukonowenzou, N.; Berliner, D. The Coastal Red-Milkwood (Mimusops caffra) Seed: Proximate, Mineral, Amino Acid and Fatty Acid Composition. S. Afr. J. Bot. 2016, 102, 137–141. [Google Scholar] [CrossRef]
- Omotayo, A.O.; Ijatuyi, E.J.; Ogunniyi, A.I.; Aremu, A.O. Exploring the Resource Value of Transvaal Red Milk Wood (Mimusops zeyheri) for Food Security and Sustainability: An Appraisal of Existing Evidence. Plants 2020, 9, 1486. [Google Scholar] [CrossRef] [PubMed]
- De Wet, H.; Nzama, V.N.; Van Vuuren, S.F. Medicinal Plants Used for the Treatment of Sexually Transmitted Infections by Lay People in Northern Maputaland, KwaZulu–Natal Province, South Africa. S. Afr. J. Bot. 2012, 78, 12–20. [Google Scholar] [CrossRef]
- Neuwinger, H.D. African Traditional Medicine: A Dictionary of Plant Use and Applications. With Supplement: Search System for Diseases; Medpharm: Guildford, UK, 2000. [Google Scholar]
- Madiehe, A.M.; Moabelo, K.L.; Modise, K.; Sibuyi, N.R.; Meyer, S.; Dube, A.; Onani, M.O.; Meyer, M. Catalytic Reduction of 4-Nitrophenol and Methylene Blue by Biogenic Gold Nanoparticles Synthesized Using Carpobrotus edulis Fruit (Sour Fig) Extract. Nanomater. Nanotechnol. 2022, 12, 18479804221108256. [Google Scholar] [CrossRef]
- Hafsa, J.; Hammi, K.M.; Khedher, M.R.B.; Smach, M.A.; Charfeddine, B.; Limem, K.; Majdoub, H. Inhibition of Protein Glycation, Antioxidant and Antiproliferative Activities of Carpobrotus edulis Extracts. Biomed. Pharmacother. 2016, 84, 1496–1503. [Google Scholar] [CrossRef]
- Malan, C.; Notten, A. Carpobrotus Edulis|PlantZAfrica. Available online: http://pza.sanbi.org/carpobrotus-edulis (accessed on 22 May 2023).
- Omoruyi, B.E.; Bradley, G.; Afolayan, A.J. Antioxidant and Phytochemical Properties of Carpobrotus edulis (L.) Bolus Leaf Used for the Management of Common Infections in HIV/AIDS Patients in Eastern Cape Province. BMC Complement. Altern. Med. 2012, 12, 215. [Google Scholar] [CrossRef]
- Otang-Mbeng, F.N.N.; Simeon, A. Materechera, Wilfred Carpobrotus edulis L. (Sour Fig): Phytochemistry, Pharmacology, and Toxicology. In The Therapeutic Properties of Medicinal Plants; Apple Academic Press: Palm Bay, FL, USA, 2019; ISBN 978-0-429-26520-4. [Google Scholar]
- Martins, A.; Vasas, A.; Viveiros, M.; Molnár, J.; Hohmann, J.; Amaral, L. Antibacterial Properties of Compounds Isolated from Carpobrotus edulis. Int. J. Antimicrob. Agents 2011, 37, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Omoruyi, B.E.; Afolayan, A.J.; Bradley, G. Chemical Composition Profiling and Antifungal Activity of the Essential Oil and Plant Extracts of Mesembryanthemum edule (L.) Bolus Leaves. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 19–30. [Google Scholar] [PubMed]
- Omoruyi, S.I.; Enogieru, A.B.; Ekpo, O.E. In Vitro Evaluation of the Antiproliferative Activity of Carpobrotus edulis on Human Neuroblastoma Cells. J. Herb. Med. 2021, 30, 100519. [Google Scholar] [CrossRef]
- Ordway, D.; Hohmann, J.; Viveiros, M.; Viveiros, A.; Molnar, J.; Leandro, C.; Arroz, M.J.; Gracio, M.A.; Amaral, L. Carpobrotus edulis Methanol Extract Inhibits the MDR Efflux Pumps, Enhances Killing of Phagocytosed S. Aureus and Promotes Immune Modulation. Phytother. Res. 2003, 17, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Maroyi, A. Nutraceutical and Ethnopharmacological Properties of Vangueria infausta Subsp. Infausta. Molecules 2018, 23, 1089. [Google Scholar] [CrossRef]
- Achilonu, M.C.; Ngubane, X.V.; Nkosi, S.M.; Jiyane, P.C. Phytochemicals, Bioactivity, and Ethnopharmacological Potential of Selected Indigenous Plants. S. Afr. J. Sci. 2023, 119, 5–11. [Google Scholar] [CrossRef]
- Vangueria Esculenta|PlantZAfrica. Available online: https://pza.sanbi.org/vangueria-esculenta (accessed on 24 July 2023).
- Raice, R.T.; Sjoholm, I.; Wang, H.; Bergenståhl, B. Characterization of Volatile Components Extracted from Vangueria infausta (African Medlar) by Using GC–MS. J. Essent. Oil Res. 2015, 27, 76–81. [Google Scholar] [CrossRef]
- Tshikalange, T.E.; Mophuting, B.C.; Mahore, J.; Winterboer, S.; Lall, N. An Ethnobotanical Study of Medicinal Plants Used in Villages under Jongilanga Tribal Council, Mpumalanga, South Africa. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 83–89. [Google Scholar] [CrossRef]
- Antibacterial Activity of Sixteen Plant Species from Phalaborwa, Limpopo Province, South Africa | Semantic Scholar. Available online: https://www.semanticscholar.org/paper/Antibacterial-activity-of-sixteen-plant-species-Shai-Chauke/ab9b7c34b448c3828f74136a9524aba7a214b3fc (accessed on 24 July 2023).
- Munodawafa, T.; Chagonda, L.S.; Moyo, S.R. Antimicrobial and Phytochemical Screening of Some Zimbabwean Medicinal Plants. J. Biol. Act. Prod. Nat. 2013, 3, 323–330. [Google Scholar] [CrossRef]
- Mbukwa, E.; Chacha, M.; Majinda, R.R.T. Phytochemical Constituents of Vangueria infausta: Their Radical Scavenging and Antimicrobial Activities. Arkivoc 2006, 2007, 104–112. [Google Scholar] [CrossRef]
- Mmushi, T.; Masoko, P.; Mdee, L.; Mokgotho, M.; Mampuru, L.; Howard, R. Antimycobacterial Evaluation of Fifteen Medicinal Plants in South Africa. Afr. J. Tradit. Complement. Altern. Med. 2009, 7, 34–39. [Google Scholar]
- Mahlo, S.M.; Chauke, H.R.; McGaw, L.; Eloff, J. Antioxidant and Antifungal Activity of Selected Medicinal Plant Extracts against Phytopathogenic Fungi. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 216–222. [Google Scholar] [CrossRef]
- Bapela, M.J.; Kaiser, M.; Meyer, J.J.M. Antileishmanial Activity of Selected South African Plant Species. S. Afr. J. Bot. 2017, 108, 342–345. [Google Scholar] [CrossRef]
- Abosl, A.O.; Mbukwa, E.; Majinda, R.R.; Raserok, B.H.; Yenesew, A.; Midiwo, J.O.; Akala, H.; Liyala, P.; Waters, N.C. Vangueria infausta Root Bark: In Vivo and in Vitro Antiplasmodial Activity. Br. J. Biomed. Sci. 2006, 63, 129–133. [Google Scholar] [PubMed]
- Bapela, M.J.; Heyman, H.; Senejoux, F.; Meyer, J.J.M. 1H NMR-Based Metabolomics of Antimalarial Plant Species Traditionally Used by Vha-Venda People in Limpopo Province, South Africa and Isolation of Antiplasmodial Compounds. J. Ethnopharmacol. 2019, 228, 148–155. [Google Scholar] [CrossRef]
- Morobe, I.C.; Mthethwa, N.S.; Bisi-Johnson, M.A.; Vasaikar, S.D.; Obi, C.L.; Oyedeji, A.O.; Kambizi, L.; Eloff, J.N.; Hattori, T. Cytotoxic Effects and Safety Profiles of Extracts of Active Medicinal Plants from South Africa. J. Microbiol. Res. 2012, 2, 176–182. [Google Scholar]
- Bapela, M.J.; Meyer, J.J.M.; Kaiser, M. In Vitro Antiplasmodial Screening of Ethnopharmacologically Selected South African Plant Species Used for the Treatment of Malaria. J. Ethnopharmacol. 2014, 156, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Aro, A.O.; Dzoyem, J.P.; Hlokwe, T.M.; Madoroba, E.; Eloff, J.N.; McGaw, L.J. Some South African Rubiaceae Tree Leaf Extracts Have Antimycobacterial Activity Against Pathogenic and Non-Pathogenic Mycobacterium Species. Phytother. Res. 2015, 29, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Moshi, M.J.; Innocent, E.; Magadula, J.J.; Otieno, D.F.; Weisheit, A.; Mbabazi, P.K.; Nondo, R.S.O. Brine Shrimp Toxicity of Some Plants Used as Traditional Medicines in Kagera Region, North Western Tanzania. Tanzan. J. Health Res. 2010, 12, 63–67. [Google Scholar] [CrossRef]
- Nawwar, M.; Hussein, S.; Ayoub, N.; Hashim, A.; El-Sharawy, R.; Lindequist, U.; Harms, M.; Wende, K. Constitutive Phenolics of Harpephyllum caffrum (Anacardiaceae) and Their Biological Effects on Human Keratinocytes. Fitoterapia 2011, 82, 1265–1271. [Google Scholar] [CrossRef]
- Olofinsan, K.A.; Salau, V.F.; Erukainure, O.L.; Islam, M.S. Harpephyllum caffrum Fruit (Wild Plum) Facilitates Glucose Uptake and Modulates Metabolic Activities Linked to Neurodegeneration in Isolated Rat Brain: An in Vitro and in Silico Approach. J. Food Biochem. 2022, 46, e14177. [Google Scholar] [CrossRef] [PubMed]
- Moodley, R.; Koorbanally, N.; Jonnalagadda, S.B. Elemental Composition and Nutritional Value of the Edible Fruits of Harpephyllum caffrum and Impact of Soil Quality on Their Chemical Characteristics. J. Environ. Sci. Health Part B 2013, 48, 539–547. [Google Scholar] [CrossRef]
- Harpephyllum Caffrum|PlantZAfrica. Available online: https://pza.sanbi.org/harpephyllum-caffrum (accessed on 24 July 2023).
- Shabana, M.M.; El Sayed, A.M.; Yousif, M.F.; El Sayed, A.M.; Sleem, A.A. Bioactive Constituents from Harpephyllum caffrum Bernh. and Rhus coriaria L. Pharmacogn. Mag. 2011, 7, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Mapunya, M.B.; Nikolova, R.V.; Lall, N. Melanogenesis and Antityrosinase Activity of Selected South African Plants. Evid. Based Complement. Alternat. Med. 2012, 2012, e374017. [Google Scholar] [CrossRef]
- Khalil, H.E.; Aljeshi, Y.M.; Saleh, F.A. Authentication of Carissa macrocarpa Cultivated in Saudi Arabia; Botanical, Phytochemical and Genetic Study. J. Pharm. Sci. Res. 2015, 7, 497–508. [Google Scholar]
- Mphaphuli, T.; Slabbert, R.M.; Sivakumar, D. Storage Temperature and Time Changes of Phenolic Compounds and Antioxidant Properties of Natal Plum (Carissa macrocarpa). Food Biosci. 2020, 38, 100772. [Google Scholar] [CrossRef]
- Souilem, F.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Harzallah-Skhiri, F.; Ferreira, I.C.F.R. Phenolic Profile and Bioactive Properties of Carissa macrocarpa (Eckl.) A.DC.: An In Vitro Comparative Study between Leaves, Stems, and Flowers. Molecules 2019, 24, 1696. [Google Scholar] [CrossRef]
- Seke, F.; Manhivi, V.E.; Slabbert, R.M.; Sultanbawa, Y.; Sivakumar, D. In Vitro Release of Anthocyanins from Microencapsulated Natal Plum (Carissa macrocarpa) Phenolic Extract in Alginate/Psyllium Mucilage Beads. Foods 2022, 11, 2550. [Google Scholar] [CrossRef]
- Venter, F.; Venter, J.A.; Joffe, P. Making the Most of Indigenous Trees, 3rd ed.; Briza Publications: Pretoria, South Africa, 2016; ISBN 978-1-920217-43-3. [Google Scholar]
- Food, Pharmaceutical and Industrial Potential of Carissa Genus: An Overview|SpringerLink. Available online: https://link.springer.com/article/10.1007/s11157-012-9306-7 (accessed on 25 July 2023).
- Kaunda, J.S.; Zhang, Y.-J. The Genus Carissa: An Ethnopharmacological, Phytochemical and Pharmacological Review. Nat. Prod. Bioprospecting 2017, 7, 181–199. [Google Scholar] [CrossRef]
- Moodley, R.; Chenia, H.; Jonnalagadda, S.B.; Koorbanally, N. Antibacterial and Anti-Adhesion Activity of the Pentacyclic Triterpenoids Isolated from the Leaves and Edible Fruits of Carissa macrocarpa. J. Med. Plants Res. 2011, 5, 4851–4858. [Google Scholar]
- Souilem, F.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Harzallah-Skhiri, F.; Ferreira, I.C.F.R. Amantagula Fruit (Carissa macrocarpa (Eckl.) A.DC.): Nutritional and Phytochemical Characterization. Plant Foods Hum. Nutr. 2019, 74, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Harpephyllum Caffrum—Useful Tropical Plants. Available online: https://tropical.theferns.info/viewtropical.php?id=Harpephyllum+caffrum (accessed on 24 July 2023).
- Sivakumar, D.; Remize, F.; Garcia, C. Bioactive Compounds in Southern African Fruits. In Bioactive Compounds in Underutilized Fruits and Nuts; Murthy, H.N., Bapat, V.A., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–17. ISBN 978-3-030-06120-3. [Google Scholar]
- Moodley, R. Phytochemical and Analytical Studies on Two Indigenous Medicinal Plants Found in KwaZulu-Natal, South Africa; Carissa macrocarpa and Harpephyllum caffrum. Ph.D. Thesis, University of Manchester, Manchester, UK.
- Tkachenko, K.; Frontasyeva, M.; Vasilev, A.; Avramov, L.; Shi, L. Major and Trace Element Content of Tribulus terrestris L. Wildlife Plants. Plants 2020, 9, 1764. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Du, Y.; Meng, H.; Dong, Y.; Li, L. A Review of Traditional Pharmacological Uses, Phytochemistry, and Pharmacological Activities of Tribulus terrestris. Chem. Cent. J. 2017, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Chhatre, S.; Nesari, T.; Somani, G.; Kanchan, D.; Sathaye, S. Phytopharmacological Overview of Tribulus terrestris. Pharmacogn. Rev. 2014, 8, 45–51. [Google Scholar] [CrossRef]
- Semerdjieva, I.B.; Zheljazkov, V.D. Chemical Constituents, Biological Properties, and Uses of Tribulus terrestris: A Review. Nat. Prod. Commun. 2019, 14, 1934578X19868394. [Google Scholar] [CrossRef]
- Yanala, S.R.; Sathyanarayana, D.; Kannan, K. A Recent Phytochemical Review—Fruits of Tribulus terrestris Linn. J Pharm Sci 2016, 8, 132. [Google Scholar]
- Yan, W.; Ohtani, K.; Kasai, R.; Yamasaki, K. Steroidal Saponins from Fruits of Tribulus terrestris. Phytochemistry 1996, 42, 1417–1422. [Google Scholar] [CrossRef]
- Chang, H.-W.; Liu, P.-F.; Tsai, W.-L.; Hu, W.-H.; Hu, Y.-C.; Yang, H.-C.; Lin, W.-Y.; Weng, J.-R.; Shu, C.-W. Xanthium strumarium Fruit Extract Inhibits ATG4B and Diminishes the Proliferation and Metastatic Characteristics of Colorectal Cancer Cells. Toxins 2019, 11, 313. [Google Scholar] [CrossRef]
- Shu, C.-W.; Weng, J.-R.; Chang, H.-W.; Liu, P.-F.; Chen, J.-J.; Peng, C.-C.; Huang, J.-W.; Lin, W.-Y.; Yen, C.-Y. Tribulus terrestris Fruit Extract Inhibits Autophagic Flux to Diminish Cell Proliferation and Metastatic Characteristics of Oral Cancer Cells. Environ. Toxicol. 2021, 36, 1173–1180. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.C.; Min, J.S.; Kim, M.; Kim, J.A.; Kor, M.H.; Yoo, H.S.; Ahn, J.K. Aqueous Extract of Tribulus terrestris Linn Induces Cell Growth Arrest and Apoptosis by Down-Regulating NF-ΚB Signaling in Liver Cancer Cells. J. Ethnopharmacol. 2011, 136, 197–203. [Google Scholar] [CrossRef]
- Kumar, M.; Soni, A.K.; Shukla, S.; Kumar, A. Chemopreventive Potential of Tribulus terrestris against 7,12- Dimethylbenz (a) Anthracene Induced Skin Papillomagenesis in Mice. Asian Pac. J. Cancer Prev. 2006, 7, 289. [Google Scholar] [PubMed]
- Hanif, M.A.; Yousaf, S.; Rehman, R.; Hanif, A.; Nadeem, R. Chapter 42—Puncture Vine. In Medicinal Plants of South Asia; Hanif, M.A., Nawaz, H., Khan, M.M., Byrne, H.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 571–585. ISBN 978-0-08-102659-5. [Google Scholar]
- Ștefănescu, R.; Tero-Vescan, A.; Negroiu, A.; Aurică, E.; Vari, C.-E. A Comprehensive Review of the Phytochemical, Pharmacological, and Toxicological Properties of Tribulus terrestris L. Biomolecules 2020, 10, 752. [Google Scholar] [CrossRef] [PubMed]
- El-Shaibany, A.; AL-Habori, M.; Al-Tahami, B.; Massarani, S.A. Anti-Hyperglycaemic Activity of Tribulus terrestris L Aerial Part Extract in Glucose-Loaded Normal Rabbits. Trop. J. Pharm. Res. 2015, 14, 2263–2268. [Google Scholar] [CrossRef]
- Hemalatha, S.; Hari, R. Acute and Subacute Toxicity Studies of the Saponin Rich Butanol Extracts of Tribulus terrestris Fruits in Wistar Rats. Int. J. Pharm. Sci. Rev. Res. 2014, 27, 307–313. [Google Scholar]
- Gandhi, S.; Srinivasan, B.P.; Akarte, A.S. Potential Nephrotoxic Effects Produced by Steroidal Saponins from Hydro Alcoholic Extract of Tribulus terrestris in STZ-Induced Diabetic Rats. Toxicol. Mech. Methods 2013, 23, 548–557. [Google Scholar]
- Aslani, M.R.; Movassaghi, A.R.; Mohri, M.; Pedram, M.; Abavisani, A. Experimental Tribulus terrestris Poisoning in Sheep: Clinical, Laboratory and Pathological Findings. Vet. Res. Commun. 2003, 27, 53–62. [Google Scholar] [CrossRef]
- Abudayyak, M.; Jannuzzi, A.T.; Özhan, G.; Alpertunga, B. Investigation on the Toxic Potential of Tribulus terrestris in Vitro. Pharm. Biol. 2015, 53, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Talasaz, A.H.; Abbasi, M.-R.; Abkhiz, S.; Dashti-Khavidaki, S. Tribulus terrestris-Induced Severe Nephrotoxicity in a Young Healthy Male. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2010, 25, 3792–3793. [Google Scholar] [CrossRef]
- Campanelli, M.; De Thomasis, R.; Tenaglia, R.L. Priapism Caused by “Tribulus terrestris”. Int. J. Impot. Res. 2016, 28, 39–40. [Google Scholar] [CrossRef]
- Ryan, M.; Lazar, I.; Nadasdy, G.M.; Nadasdy, T.; Satoskar, A.A. Acute Kidney Injury and Hyperbilirubinemia in a Young Male after Ingestion of Tribulus terrestris. Clin. Nephrol. 2015, 83, 177–183. [Google Scholar] [CrossRef]
- Factsheet—Xanthium strumarium (Large Cocklebur). Available online: https://keys.lucidcentral.org/keys/v3/eafrinet/weeds/key/weeds/Media/Html/Xanthium_strumarium_(Large_Cocklebur).htm (accessed on 24 May 2023).
- Fan, W.; Fan, L.; Peng, C.; Zhang, Q.; Wang, L.; Li, L.; Wang, J.; Zhang, D.; Peng, W.; Wu, C. Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics and Toxicology of Xanthium strumarium L.: A Review. Molecules 2019, 24, 359. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, A.; Saluja, A.K. Phytopharmacological Review of Xanthium strumarium L. (Cocklebur). Int. J. Green Pharm. IJGP 2010, 4, 133. [Google Scholar] [CrossRef]
- Al-Mekhlafi, F.A.; Abutaha, N.; Mashaly, A.M.A.; Nasr, F.A.; Ibrahim, K.E.; Wadaan, M.A. Biological Activity of Xanthium strumarium Seed Extracts on Different Cancer Cell Lines and Aedes Caspius, Culex Pipiens (Diptera: Culicidae). Saudi J. Biol. Sci. 2017, 24, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-W.; Xi, Y.-Y.; Chen, J.; Zhang, F.; Zheng, J.-J.; Zhang, P.-H. Phytochemical Investigation of the Fruits of Xanthium strumarium and Their Cytotoxic Activity. J. Nat. Med. 2022, 76, 468–475. [Google Scholar] [CrossRef]
- Didem Karagoz, I.; Cakir, A.; Ozaslan, M.; Halil Kilic, I.; Tepe, B.; Akdogan, E.; Kazaz, C. Anticancer Agents from Xanthium strumarium Fruits Against C6 Glioma Cells. Int. J. Pharmacol. 2022, 18, 437–454. [Google Scholar] [CrossRef]
- Vaishnav, K.; George, L.-B.; Highland, H.N. Induction of Cell Death through Alteration of Antioxidant Activity in HeLa Cervical Cancer Cells by Xanthium strumarium L. Extract. IOSR J. Pharm. Biol. Sci. 2015, 10, 33–42. [Google Scholar]
- Jiang, H.; Ma, G.-X.; Yang, L.; Xing, X.-D.; Yan, M.-L.; Zhang, Y.-Y.; Wang, Q.-H.; Kuang, H.-X.; Xu, X.-D. Rearranged Ent-Kauranoid Glycosides from the Fruits of Xanthium strumarium and Their Antiproliferative Activity. Phytochem. Lett. 2016, 18, 192–196. [Google Scholar] [CrossRef]
- Stuart, B.P.; Cole, R.J.; Gosser, H.S. Cocklebur (Xanthium strumarium, L. Var. Strumarium) Intoxication in Swine: Review and Redefinition of the Toxic Principle. Vet. Pathol. 1981, 18, 368–383. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, T.; Zhao, J.; Song, J.; Hua, H.; Li, L. [Comparative study on acute toxicity of four extracts from Xanthii Fructus in mice]. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Medica 2012, 37, 2228–2231. [Google Scholar]
- Jin, Y.; Liu, S.; Liu, Y.; Mou, H. Toxic Effects of Ethyl Acetate, n-Butanol, and Water Extracts from Alcohol Extractions of Cocklebur Fruit on Liver in Rats. Adverse Drug React. J. 2010, 12, 17-4. [Google Scholar]
- Wang, Y.; Han, T.; Xue, L.-M.; Han, P.; Zhang, Q.-Y.; Huang, B.-K.; Zhang, H.; Ming, Q.-L.; Peng, W.; Qin, L.-P. Hepatotoxicity of Kaurene Glycosides from Xanthium strumarium L. Fruits in Mice. Pharm. 2011, 66, 445–449. [Google Scholar]
- Xue, L.-M.; Zhang, Q.-Y.; Han, P.; Jiang, Y.-P.; Yan, R.-D.; Wang, Y.; Rahman, K.; Jia, M.; Han, T.; Qin, L.-P. Hepatotoxic Constituents and Toxicological Mechanism of Xanthium strumarium L. Fruits. J. Ethnopharmacol. 2014, 152, 272–282. [Google Scholar] [CrossRef]
- Mandal, S.C.; Dhara, A.K.; Ashok Kumar, C.K.; Maiti, B.C. Neuropharmacological Activity of Xanthium strumarium Linn. Extract. J. Herbs Spices Med. Plants 2001, 8, 69–77. [Google Scholar] [CrossRef]
- Xanthium strumarium, L. Extracts Produce DNA Damage Mediated by Cytotoxicity in in Vitro Assays but Does Not Induce Micronucleus in Mice—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/25025061/ (accessed on 26 July 2023).
- Saidi, H.; Mofidi, M. Toxic Effect of Xanthium strumarium as an Herbal Medicine Preparation. EXCLI J. 2009, 8, 115–117. [Google Scholar] [CrossRef]
- Oza, V.P.; Parmar, P.P.; Kumar, S.; Subramanian, R.B. Anticancer Properties of Highly Purified L-Asparaginase from Withania somnifera L. against Acute Lymphoblastic Leukemia. Appl. Biochem. Biotechnol. 2010, 160, 1833–1840. [Google Scholar] [CrossRef]
- Afewerky, H.K.; Ayodeji, A.E.; Tiamiyu, B.B.; Orege, J.I.; Okeke, E.S.; Oyejobi, A.O.; Bate, P.N.N.; Adeyemi, S.B. Critical Review of the Withania somnifera (L.) Dunal: Ethnobotany, Pharmacological Efficacy, and Commercialization Significance in Africa. Bull. Natl. Res. Cent. 2021, 45, 176. [Google Scholar] [CrossRef]
- Withania Somnifera|PlantZAfrica. Available online: http://pza.sanbi.org/withania-somnifera (accessed on 26 July 2023).
- New Withanolides and Other Constituents from the Fruit of Withania somnifera—Abou-Douh—2002—Archiv Der Pharmazie—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/1521-4184%28200208%29335%3A6%3C267%3A%3AAID-ARDP267%3E3.0.CO%3B2-E (accessed on 26 May 2023).
- Jayaprakasam, B.; Strasburg, G.A.; Nair, M.G. Potent Lipid Peroxidation Inhibitors from Withania somnifera Fruits. Tetrahedron 2004, 60, 3109–3121. [Google Scholar] [CrossRef]
- Abutaha, N. In Vitro Antiproliferative Activity of Partially Purified Withania somnifera Fruit Extract on Different Cancer Cell Lines. J. BUON Off. J. Balk. Union Oncol. 2015, 20, 625–630. [Google Scholar]
- Ichikawa, H.; Takada, Y.; Shishodia, S.; Jayaprakasam, B.; Nair, M.G.; Aggarwal, B.B. Withanolides Potentiate Apoptosis, Inhibit Invasion, and Abolish Osteoclastogenesis through Suppression of Nuclear Factor-ΚB (NF-ΚB) Activation and NF-ΚB–Regulated Gene Expression. Mol. Cancer Ther. 2006, 5, 1434–1445. [Google Scholar] [CrossRef]
- Patel, S.B.; Rao, N.J.; Hingorani, L.L. Safety Assessment of Withania somnifera Extract Standardized for Withaferin A: Acute and Sub-Acute Toxicity Study. J. Ayurveda Integr. Med. 2016, 7, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Prabu, P.C.; Panchapakesan, S.; Raj, C.D. Acute and Sub-Acute Oral Toxicity Assessment of the Hydroalcoholic Extract of Withania somnifera Roots in Wistar Rats. Phytother. Res. 2013, 27, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Sharada, A.C.; Solomon, F.E.; Devi, P.U. Toxicity of Withania somnifera Root Extract in Rats and Mice. Int. J. Pharmacogn. 1993, 31, 205–212. [Google Scholar] [CrossRef]
- Balkrishna, A.; Sinha, S.; Srivastava, J.; Varshney, A. Withania somnifera (L.) Dunal Whole-Plant Extract Demonstrates Acceptable Non-Clinical Safety in Rat 28-Day Subacute Toxicity Evaluation under GLP-Compliance. Sci. Rep. 2022, 12, 11047. [Google Scholar] [CrossRef]
- Raut, A.A.; Rege, N.N.; Tadvi, F.M.; Solanki, P.V.; Kene, K.R.; Shirolkar, S.G.; Pandey, S.N.; Vaidya, R.A.; Vaidya, A.B. Exploratory Study to Evaluate Tolerability, Safety, and Activity of Ashwagandha (Withania somnifera) in Healthy Volunteers. J. Ayurveda Integr. Med. 2012, 3, 111–114. [Google Scholar] [CrossRef]
- Tandon, N.; Yadav, S.S. Safety and Clinical Effectiveness of Withania somnifera (Linn.) Dunal Root in Human Ailments. J. Ethnopharmacol. 2020, 255, 112768. [Google Scholar] [CrossRef] [PubMed]
- Xylopia Aethiopica—Useful Tropical Plants. Available online: https://tropical.theferns.info/viewtropical.php?id=Xylopia+aethiopica (accessed on 26 July 2023).
- Fetse, J.P.; Kofie, W.; Adosraku, R.K. Ethnopharmacological Importance of Xylopia aethiopica (DUNAL) A. RICH (Annonaceae)—A Review. J. Pharm. Res. Int. 2016, 11, 1–21. [Google Scholar] [CrossRef]
- Earnest, E. Xylopia aethiopica: A Review of Its Ethnomedicinal, Chemical and Pharmacological Properties. Am. J. Pharmtech Res. 2014, 4, 22–37. [Google Scholar]
- Yin, X.; Chávez León, M.A.S.C.; Osae, R.; Linus, L.O.; Qi, L.-W.; Alolga, R.N. Xylopia aethiopica Seeds from Two Countries in West Africa Exhibit Differences in Their Proteomes, Mineral Content and Bioactive Phytochemical Composition. Molecules 2019, 24, 1979. [Google Scholar] [CrossRef]
- The Useful Plants of West Tropical Africa|SpringerLink. Available online: https://link.springer.com/article/10.1007/BF02859140 (accessed on 27 May 2023).
- Abubakar, I.B.; Ukwuani-Kwaja, A.N.; Olayiwola, F.S.; Malami, I.; Muhammad, A.; Ahmed, S.J.; Nurudeen, Q.O.; Falana, M.B. An Inventory of Medicinal Plants Used for Treatment of Cancer in Kwara and Lagos State, Nigeria. Eur. J. Integr. Med. 2020, 34, 101062. [Google Scholar] [CrossRef]
- Moreira, I.C.; Roque, N.F.; Vilegas, W.; Zalewski, C.A.; Lago, J.H.G.; Funasaki, M. Genus Xylopia (Annonaceae): Chemical and Biological Aspects. Chem. Biodivers. 2013, 10, 1921–1943. [Google Scholar] [CrossRef]
- Adaramoye, O.A.; Sarkar, J.; Singh, N.; Meena, S.; Changkija, B.; Yadav, P.P.; Kanojiya, S.; Sinha, S. Antiproliferative Action of Xylopia aethiopica Fruit Extract on Human Cervical Cancer Cells. Phytother. Res. 2011, 25, 1558–1563. [Google Scholar] [CrossRef]
- Choumessi, A.T.; Loureiro, R.; Silva, A.M.; Moreira, A.C.; Pieme, A.C.; Tazoacha, A.; Oliveira, P.J.; Penlap, V.B. Toxicity Evaluation of Some Traditional African Spices on Breast Cancer Cells and Isolated Rat Hepatic Mitochondria. Food Chem. Toxicol. 2012, 50, 4199–4208. [Google Scholar] [CrossRef] [PubMed]
- Choumessi, A.T.; Danel, M.; Chassaing, S.; Truchet, I.; Penlap, V.B.; Pieme, A.C.; Asonganyi, T.; Ducommun, B.; Valette, A. Characterization of the Antiproliferative Activity of Xylopia aethiopica. Cell Div. 2012, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Krusche, B.; Youns, M.; Voukeng, I.; Fankam, A.G.; Tankeo, S.; Lacmata, S.; Efferth, T. Cytotoxicity of Some Cameroonian Spices and Selected Medicinal Plant Extracts. J. Ethnopharmacol. 2011, 134, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Sandjo, L.P.; Wiench, B.; Efferth, T. Cytotoxicity and Modes of Action of Four Cameroonian Dietary Spices Ethno-Medically Used to Treat Cancers: Echinops Giganteus, Xylopia aethiopica, Imperata Cylindrica and Piper Capense. J. Ethnopharmacol. 2013, 149, 245–253. [Google Scholar] [CrossRef]
- Kuete, V.; Sandjo, L.P.; Mbaveng, A.T.; Zeino, M.; Efferth, T. Cytotoxicity of Compounds from Xylopia aethiopica towards Multi-Factorial Drug-Resistant Cancer Cells. Phytomedicine 2015, 22, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Asekun, O.T.; Adeniyi, B.A. Antimicrobial and Cytotoxic Activities of the Fruit Essential Oil of Xylopia aethiopica from Nigeria. Fitoterapia 2004, 75, 368–370. [Google Scholar] [CrossRef]
- Ribeiro, V.; Ferreres, F.; Macedo, T.; Gil-Izquierdo, Á.; Oliveira, A.P.; Gomes, N.G.M.; Araújo, L.; Pereira, D.M.; Andrade, P.B.; Valentão, P. Activation of Caspase-3 in Gastric Adenocarcinoma AGS Cells by Xylopia aethiopica (Dunal) A. Rich. Fruit and Characterization of Its Phenolic Fingerprint by HPLC-DAD-ESI(Ion Trap)-MSn and UPLC-ESI-QTOF-MS2. Food Res. Int. 2021, 141, 110121. [Google Scholar] [CrossRef]
- Akinloye, O.A.; Ayodele, P.F.; Ore, A. Median Lethality Dose of Xylopia aethiopica Fruit Ethanol Extract. J. Anal. Tech. Res. 2019, 1, 32–35. [Google Scholar] [CrossRef]
- Assih, M.; Badjabaïssi, E.; Bescond, J.; Mouzou, A.; Pakoussi, T.; Sanvee, S.C.J.; Yérima, M.; Diallo, A.; Dossou-Yovo, K.M.; Kaboua, K.; et al. Toxicological Studies of Hydroethanolic Leaf Extract of Xylopia aethiopica (Dunal) A. Rich. (Annonaceae) on Wistar Rats. J. Drug Deliv. Ther. 2022, 12, 8–13. [Google Scholar] [CrossRef]
- Keren, A.O.; Sani, G.H.; Vandi, Z.J. Acute Toxicity Study of Aqueous Fruit Extract of Xylopia aethiopica (Dunal) a. Rich. In Albino Rats. Kanem J. Med. Sci. 2022, 16, 92–100. [Google Scholar] [CrossRef]
- Physico-Chemical and Toxicological Studies On Xylopia Aethropica Leaves. Available online: https://www.researchgate.net/publication/275646964_PHYSICO-CHEMICAL_AND_TOXICOLOGICAL_STUDIES_ON_XYLOPIA_AETHROPICA_LEAVES (accessed on 26 July 2023).
- Ibitoye, D.O.; Kolawole, A.O. Farmers’ Appraisal on Okra [Abelmoschus esculentus (L.)] Production and Phenotypic Characterization: A Synergistic Approach for Improvement. Front. Plant Sci. 2022, 13, 787577. [Google Scholar] [CrossRef] [PubMed]
- CABI. Abelmoschus esculentus (Okra); CABI Compendium: Wallingford, CT, USA, 2022. [Google Scholar] [CrossRef]
- Elkhalifa, A.E.O.; Alshammari, E.; Adnan, M.; Alcantara, J.C.; Awadelkareem, A.M.; Eltoum, N.E.; Mehmood, K.; Panda, B.P.; Ashraf, S.A. Okra (Abelmoschus esculentus) as a Potential Dietary Medicine with Nutraceutical Importance for Sustainable Health Applications. Molecules 2021, 26, 696. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, B.J.; Popoola, T.D.; van Heerden, F.R.; Fatokun, A.A. Isolation and Characterisation of Two Quercetin Glucosides with Potent Anti-Reactive Oxygen Species (ROS) Activity and an Olean-12-En Triterpene Glucoside from the Fruit of Abelmoschus esculentus (L.) Moench. Chem. Biodivers. 2021, 18, e2000670. [Google Scholar] [CrossRef] [PubMed]
- Phytochemical Information and Pharmacological Activities of Okra (Abelmoschus esculentus): A Literature-based Review. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1002/ptr.6212 (accessed on 26 July 2023).
- Agregán, R.; Pateiro, M.; Bohrer, B.M.; Shariati, M.A.; Nawaz, A.; Gohari, G.; Lorenzo, J.M. Biological Activity and Development of Functional Foods Fortified with Okra (Abelmoschus esculentus). Crit. Rev. Food Sci. Nutr. 2022, 1–16. [Google Scholar] [CrossRef]
- Romdhane, M.H.; Chahdoura, H.; Barros, L.; Dias, M.I.; Corrêa, R.C.G.; Morales, P.; Ciudad-Mulero, M.; Flamini, G.; Majdoub, H.; Ferreira, I.C.F.R. Chemical Composition, Nutritional Value, and Biological Evaluation of Tunisian Okra Pods (Abelmoschus esculentus L. Moench). Molecules 2020, 25, 4739. [Google Scholar] [CrossRef]
- Chaemsawang, W.; Prasongchean, W.; Papadopoulos, K.I.; Ritthidej, G.; Sukrong, S.; Wattanaarsakit, P. The Effect of Okra (Abelmoschus esculentus (L.) Moench) Seed Extract on Human Cancer Cell Lines Delivered in Its Native Form and Loaded in Polymeric Micelles. Int. J. Biomater. 2019, 2019, e9404383. [Google Scholar] [CrossRef]
- Ping, M.-H. Hyperin Controls the Development and Therapy of Gastric Cancer via Regulating Wnt/β-Catenin Signaling. Cancer Manag. Res. 2020, 12, 11773–11782. [Google Scholar] [CrossRef]
- Monte, L.G.; Santi-Gadelha, T.; Reis, L.B.; Braganhol, E.; Prietsch, R.F.; Dellagostin, O.A.; e Lacerda, R.R.; Gadelha, C.A.A.; Conceição, F.R.; Pinto, L.S. Lectin of Abelmoschus esculentus (Okra) Promotes Selective Antitumor Effects in Human Breast Cancer Cells. Biotechnol. Lett. 2014, 36, 461–469. [Google Scholar] [CrossRef]
- Musthafa, S.A.; Muthu, K.; Vijayakumar, S.; George, S.J.; Murali, S.; Govindaraj, J.; Munuswamy-Ramanujam, G. Lectin Isolated from Abelmoschus esculentus Induces Caspase Mediated Apoptosis in Human U87 Glioblastoma Cell Lines and Modulates the Expression of Circadian Clock Genes. Toxicon 2021, 202, 98–109. [Google Scholar] [CrossRef]
- Ahmed, H.E.; Iqbal, Y.; Aziz, M.H.; Atif, M.; Batool, Z.; Hanif, A.; Yaqub, N.; Farooq, W.A.; Ahmad, S.; Fatehmulla, A.; et al. Green Synthesis of CeO2 Nanoparticles from the Abelmoschus esculentus Extract: Evaluation of Antioxidant, Anticancer, Antibacterial, and Wound-Healing Activities. Molecules 2021, 26, 4659. [Google Scholar] [CrossRef]
- Safety Evaluation of Abelmoschus Esculentus Polysaccharide|Request PDF. Available online: https://www.researchgate.net/publication/288097801_Safety_evaluation_of_Abelmoschus_esculentus_polysaccharide (accessed on 26 July 2023).
- Doreddula, S.K.; Bonam, S.R.; Gaddam, D.P.; Desu, B.S.R.; Ramarao, N.; Pandy, V. Phytochemical Analysis, Antioxidant, Antistress, and Nootropic Activities of Aqueous and Methanolic Seed Extracts of Ladies Finger (Abelmoschus esculentus L.) in Mice. Sci. World J. 2014, 2014, 519848. [Google Scholar] [CrossRef] [PubMed]
- Sabitha, V.; Ramachandran, S.; Naveen, K.R.; Panneerselvam, K. Investigation of in Vivo Antioxidant Property of Abelmoschus esculentus (L) Moench. Fruit Seed and Peel Powders in Streptozotocin-Induced Diabetic Rats. J. Ayurveda Integr. Med. 2012, 3, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilzadeh, D.; Razavi, B.M.; Hosseinzadeh, H. Effect of Abelmoschus esculentus (Okra) on Metabolic Syndrome: A Review. Phytother. Res. 2020, 34, 2192–2202. [Google Scholar] [CrossRef]
- Taniguchi-Fukatsu, A.; Yamanaka-Okumura, H.; Naniwa-Kuroki, Y.; Nishida, Y.; Yamamoto, H.; Taketani, Y.; Takeda, E. Natto and Viscous Vegetables in a Japanese-Style Breakfast Improved Insulin Sensitivity, Lipid Metabolism and Oxidative Stress in Overweight Subjects with Impaired Glucose Tolerance. Br. J. Nutr. 2012, 107, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.C.; Marchie, A.; Faulkner, D.A.; Wong, J.M.W.; de Souza, R.; Emam, A.; Parker, T.L.; Vidgen, E.; Trautwein, E.A.; et al. Direct Comparison of a Dietary Portfolio of Cholesterol-Lowering Foods with a Statin in Hypercholesterolemic Participants. Am. J. Clin. Nutr. 2005, 81, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Moradi, A.; Tarrahi, M.-J.; Ghasempour, S.; Shafiepour, M.; Clark, C.C.T.; Safavi, S.-M. The Effect of Okra (Abelmoschus esculentus) on Lipid Profiles and Glycemic Indices in Type 2 Diabetic Adults: Randomized Double Blinded Trials. Phytother. Res. 2020, 34, 3325–3332. [Google Scholar] [CrossRef]
- CABI. Carissa macrocarpa (Natal Plum); CABI Compendium: Wallingford, CT, USA, 2019. [Google Scholar] [CrossRef]
- Orabi, M.A.A.; Khalil, H.M.A.; Abouelela, M.E.; Zaafar, D.; Ahmed, Y.H.; Naggar, R.A.; Alyami, H.S.; Abdel-Sattar, E.-S.; Matsunami, K.; Hamdan, D.I. Carissa macrocarpa Leaves Polar Fraction Ameliorates Doxorubicin-Induced Neurotoxicity in Rats via Downregulating the Oxidative Stress and Inflammatory Markers. Pharmaceuticals 2021, 14, 1305. [Google Scholar] [CrossRef]
- Castañeda-Loaiza, V.; Placines, C.; Rodrigues, M.J.; Pereira, C.; Zengin, G.; Uysal, A.; Jeko, J.; Cziáky, Z.; Reis, C.P.; Gaspar, M.M.; et al. If You Cannot Beat Them, Join Them: Exploring the Fruits of the Invasive Species Carpobrotus edulis (L.) N.E. Br as a Source of Bioactive Products. Ind. Crops Prod. 2020, 144, 112005. [Google Scholar] [CrossRef]
- Mudimba, T.N.; Nguta, J.M. Traditional Uses, Phytochemistry and Pharmacological Activity of Carpobrotus edulis: A Global Perspective. J. Phytopharm. 2019, 8, 111–116. [Google Scholar] [CrossRef]
- Akhalwaya, S.; van Vuuren, S.; Patel, M. An in Vitro Investigation of Indigenous South African Medicinal Plants Used to Treat Oral Infections. J. Ethnopharmacol. 2018, 210, 359–371. [Google Scholar] [CrossRef]
- Cock, I.E.; van Vuuren, S.F. Anti-Proteus Activity of Some South African Medicinal Plants: Their Potential for the Prevention of Rheumatoid Arthritis. Inflammopharmacology 2014, 22, 23–36. [Google Scholar] [CrossRef]
- (PDF) Acute and Subacute Toxicity Evaluation of Aqueous Extracts of Carpobrotus edulis in Sprague Dawley Rats. Available online: https://www.researchgate.net/publication/343681218_Acute_and_subacute_toxicity_evaluation_of_aqueous_extracts_of_Carpobrotus_edulis_in_Sprague_Dawley_rats?_iepl%5BgeneralViewId%5D=BlZqYO6VIoqAmmtEkUdiIIV289LMJZ1wBNXD&_iepl%5Bcontexts%5D%5B0%5D=searchReact&_iepl%5BviewId%5D=ZQY1klgoMLqybb34NSG0QsBibNPeY1XMFwcU&_iepl%5BsearchType%5D=publication&_iepl%5Bdata%5D%5BcountLessEqual20%5D=1&_iepl%5Bdata%5D%5BinteractedWithPosition1%5D=1&_iepl%5Bdata%5D%5BwithoutEnrichment%5D=1&_iepl%5Bposition%5D=1&_iepl%5BrgKey%5D=PB%3A343681218&_iepl%5BtargetEntityId%5D=PB%3A343681218&_iepl%5BinteractionType%5D=publicationTitle (accessed on 27 July 2023).
- Ayyanar, M.; Subash-Babu, P. Syzygium cumini (L.) Skeels: A Review of Its Phytochemical Constituents and Traditional Uses. Asian Pac. J. Trop. Biomed. 2012, 2, 240–246. [Google Scholar] [CrossRef]
- Chagas, V.T.; França, L.M.; Malik, S.; Paes, A.M. Syzygium cumini (L.) Skeels: A Prominent Source of Bioactive Molecules against Cardiometabolic Diseases. Front. Pharmacol. 2015, 6, 259. [Google Scholar] [CrossRef]
- Aqil, F.; Gupta, A.; Munagala, R.; Jeyabalan, J.; Kausar, H.; Sharma, R.J.; Singh, I.P.; Gupta, R.C. Antioxidant and Antiproliferative Activities of Anthocyanin/Ellagitannin-Enriched Extracts From Syzygium cumini L. (Jamun, the Indian Blackberry). Nutr. Cancer 2012, 64, 428–438. [Google Scholar] [CrossRef] [PubMed]
- The Anthocyanin Components and Cytotoxic Activity of Syzygium cumini (L.) Fruits Growing in Egypt -Natural Product Sciences|Korea Science. Available online: https://koreascience.kr/article/JAKO200724737574177.page (accessed on 6 June 2023).
- Li, L.; Adams, L.S.; Chen, S.; Killian, C.; Ahmed, A.; Seeram, N.P. Eugenia Jambolana Lam. Berry Extract Inhibits Growth and Induces Apoptosis of Human Breast Cancer but Not Non-Tumorigenic Breast Cells. J. Agric. Food Chem. 2009, 57, 826–831. [Google Scholar] [CrossRef]
- Barh, D.; Viswanathan, G. Syzygium cumini Inhibits Growth and Induces Apoptosis in Cervical Cancer Cell Lines: A Primary Study. Available online: http://ecancer.org/en/journal/article/83-syzygium-cumini-inhibits-growth-and-induces-apoptosis-in-cervical-cancer-cell-lines-a-primary-study (accessed on 30 May 2023).
- Parmar, J.; Sharma, P.; Verma, P.; Goyal, P.K. Chemopreventive Action of Syzygium cumini on DMBA-Induced Skin Papillomagenesis in Mice. Asian Pac. J. Cancer Prev. 2010, 11, 261–265. [Google Scholar] [PubMed]
- Evaluation of Anti-Cancer and Anti-Oxidative Potential of Syzygium cumini Against Benzo[a]Pyrene (BaP) Induced Gastric Carcinogenesis in Mice. Asian Pac. J. Cancer Prev. 2010, 11, 753–758.
- Kumar, M.; Zhang, B.; Nishad, J.; Verma, A.; Sheri, V.; Dhumal, S.; Radha; Sharma, N.; Chandran, D.; Senapathy, M.; et al. Jamun (Syzygium cumini (L.) Skeels) Seed: A Review on Nutritional Profile, Functional Food Properties, Health-Promoting Applications, and Safety Aspects. Processes 2022, 10, 2169. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Kumar, M.M.; Bhawani, G.; Chaturvedi, H.; Kumar, M.; Goel, R.K. Effect of Ethanolic Extract of Eugenia Jambolana Seeds on Gastric Ulceration and Secretion in Rats. Indian J. Physiol. Pharmacol. 2007, 51, 131–140. [Google Scholar]
- Kumar, E.K.; Mastan, S.K.; Reddy, K.R.; Reddy, G.A.; Raghunandan, N.; Chaitanya, G. Antiarthrtic Property of Methanolic Extract of Syzygium cumini Seeds. Int. J. Integr. Biol. 2009, 4, 55–61. [Google Scholar]
- Silva, S.D.N.; Abreu, I.C.; Silva, G.F.C.; Ribeiro, R.M.; Lopes, A.D.S.; Cartágenes, M.D.S.D.S.; Freire, S.M.D.F.; Borges, A.C.R.; Borges, M.O.D.R. The Toxicity Evaluation of Syzygium cumini Leaves in Rodents. Rev. Bras. Farmacogn. 2012, 22, 102–108. [Google Scholar] [CrossRef][Green Version]
- Qamar, M.; Akhtar, S.; Ismail, T.; Wahid, M.; Ali, S.; Nazir, Y.; Murtaza, S.; Abbas, M.W.; Ziora, Z.M. Syzygium cumini (L.) Skeels Extracts; in Vivo Anti-Nociceptive, Anti-Inflammatory, Acute and Subacute Toxicity Assessment. J. Ethnopharmacol. 2022, 287, 114919. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.C.; Fuchs, F.D.; Weinert, L.S.; Esteves, J. The Efficacy of Folk Medicines in the Management of Type 2 Diabetes Mellitus: Results of a Randomized Controlled Trial of Syzygium cumini (L.) Skeels. J. Clin. Pharm. Ther. 2006, 31, 1–5. [Google Scholar] [CrossRef]
- Teixeira, C.C.; Rava, C.A.; Mallman da Silva, P.; Melchior, R.; Argenta, R.; Anselmi, F.; Almeida, C.R.C.; Fuchs, F.D. Absence of Antihyperglycemic Effect of Jambolan in Experimental and Clinical Models. J. Ethnopharmacol. 2000, 71, 343–347. [Google Scholar] [CrossRef]
- Shivaprakash, G.; Pai, M.R.S.M.; Mangalore, N.; Kumarchandra, R.; Acharya, S.; Kuppusamy, R.; Shirwaikar, A.; Adhikari, M.R.; Ganesh, J. Antioxidant Potential of Eugenia Jambolana Seed; A Randomized Clinical Trial in Type 2 Diabetes Mellitus. Int. J. Pharma Bio Sci. 2011, 2, 220–228. [Google Scholar]
- Acharya, S.; Shivaprakash, G.; Baliga, R.; Adhikari, P.; Jyothi, G.; Pai, M.R.S.M. Effect of Eugenia Jambolana on Plasma Glucose, Insulin Sensitivity and HDL-C Levels: Preliminary Results of a Randomized Clinical Trial. J. Pharm. Res. 2010, 3, 1268–1270. [Google Scholar]
- Kigelia Africana|PlantZAfrica. Available online: https://pza.sanbi.org/kigelia-africana (accessed on 28 July 2023).
- Bello, I.; Shehu, M.W.; Musa, M.; Asmawi, M.Z.; Mahmud, R. Kigelia africana (Lam.) Benth. (Sausage Tree): Phytochemistry and Pharmacological Review of a Quintessential African Traditional Medicinal Plant. J. Ethnopharmacol. 2016, 189, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, K.M.A.; El-Beltagi, H.S.; Mohamed, H.I.; Shalaby, T.A.; Galal, A.; Mansour, A.T.; Aboul Fotouh, M.M.; Bendary, E.S.A. Antioxidant, Anti-Cancer Activity and Phytochemicals Profiling of Kigelia pinnata Fruits. Separations 2022, 9, 379. [Google Scholar] [CrossRef]
- Osman, A.G.; Ali, Z.; Chittiboyina, A.G.; Khan, I.A. Kigelia africana Fruit: Constituents, Bioactivity, and Reflection on Composition Disparities. World J. Tradit. Chin. Med. 2017, 3, 1. [Google Scholar] [CrossRef]
- Gouda, Y.G.; Abdel-baky, A.M.; Darwish, F.M.; Mohamed, K.M.; Kasai, R.; Yamasaki, K. Iridoids from Kigelia pinnata DC. Fruits. Phytochemistry 2003, 63, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.; Jäger, A.K. The Sausage Tree (Kigelia pinnata): Ethnobotany and Recent Scientific Work. S. Afr. J. Bot. 2002, 68, 14–20. [Google Scholar] [CrossRef]
- Jackson, S.J.; Houghton, P.J.; Retsas, S.; Photiou, A. In Vitro Cytotoxicity of Norviburtinal and Isopinnatal from Kigelia pinnata Against Cancer Cell Lines. Planta Med. 2000, 66, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.A.; Bell, T.; Delbederi, Z.; Feutren-Burton, S.; McClean, B.; O’Dowd, C.; Watters, W.; Armstrong, P.; Waugh, D.; Berg, H. van den Growth Inhibitory Activity of Extracted Material and Isolated Compounds from the Fruits of Kigelia pinnata. Planta Med. 2010, 76, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Chivandi, E.; Cave, E.; Davidson, B.C.; Erlwanger, K.H.; Moyo, D.; Madziva, M.T. Suppression of Caco-2 and HEK-293 Cell Proliferation by Kigelia africana, Mimusops zeyheri and Ximenia Caffra Seed Oils. In Vivo 2012, 26, 99–105. [Google Scholar] [PubMed]
- Azuine, M.A.; Ibrahim, K.; Enwerem, N.M.; Wambebe, C.; Kolodziej, H. Protective Role of Kigelia africana Fruits against Benzo[a]Pyrene-Induced Forestomach Tumourigenesis in Mice and against Albumen-Induced Inflammation in Rats. Pharm. Pharmacol. Lett. 1997, 7, 67–70. [Google Scholar]
- Olufemi, A.; Omotayo, O.; David, A.; Monjeed, I.; Adebola, O.; Bilikis, S.; Temitope, A. Kigelia africana Stem Bark, Fruit and Leaf Extracts Alleviate Benzene-Induced Leukaemia in Rats. J. Pharm. Res. Int. 2017, 18, 1–10. [Google Scholar] [CrossRef]
- Plants Traditionally Used Individually and in Combination to Treat Sexually Transmitted Infections in Northern Maputaland, South Africa: Antimicrobial Activity and Cytotoxicity—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0378874113005072 (accessed on 28 July 2023).
- Khan, M.A.A.; Islam, M.T. Analgesic and Cytotoxic Activity of Acorus calamus L., Kigelia pinnata L., Mangifera indica L. and Tabernaemontana divaricata L. J. Pharm. Bioallied Sci. 2012, 4, 149–154. [Google Scholar] [CrossRef]
- Hassan, S.W.; Mshelia, P.Y.; Abubakar, M.G.; Adamu, Y.A.; Yakubu, A.S. Wound Healing, Antioxidants and Toxicological Properties of Root Extracts of Kigelia africana (Lam.) Benth. Int. J. Sci. Basic Appl. Res. IJSBAR 2015, 19, 251–268. [Google Scholar]
- Kh, H.K. Evaluation of anti-ulcer activity of methanolic extracts of Kigelia africana, Sophora interrupta and Holoptelea integrifolia leaves in experimental rats. Int. J. Curr. Pharm. Res. 2023, 4, 61–66. [Google Scholar]
- Fredrick, A.; Ebele, O.; Obi, P. Analgesic, Phytochemical and Toxicological Investigations Of Ethanol Extract Of The Leaves Of Kigelia africana (Lam.) Benth (Family Bignoniaceae)-Sausage Tree. J. Pharm. Biomed. Sci. 2014, 4, 588–595. [Google Scholar]
- Akah, P.A. Antidiarrheal Activity of Kigelia africana in Experimental Animals. J. Herbs Spices Med. Plants 1996, 4, 31–38. [Google Scholar] [CrossRef]
- Analgesic and Anti-Inflammatory Activities of Ethanolic Extract of the Stem Bark of Kigelia africana in Wistar Albino Mice and Rats. Available online: https://www.researchgate.net/publication/284182893_Analgesic_and_anti-inflammatory_activities_of_ethanolic_extract_of_the_stem_bark_of_Kigelia_africana_in_Wistar_albino_mice_and_rats (accessed on 28 July 2023).
- Abdul Wahab, S.M.; Jantan, I.; Haque, M.A.; Arshad, L. Exploring the Leaves of Annona muricata L. as a Source of Potential Anti-Inflammatory and Anticancer Agents. Front. Pharmacol. 2018, 9, 661. [Google Scholar] [CrossRef] [PubMed]
- Anticancer Properties of Graviola (Annona muricata): A Comprehensive Mechanistic Review—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6091294/ (accessed on 8 June 2023).
- Jaramillo, M.C.; Arango, G.J.; González, M.C.; Robledo, S.M.; Velez, I.D. Cytotoxicity and Antileishmanial Activity of Annona muricata Pericarp. Fitoterapia 2000, 71, 183–186. [Google Scholar] [CrossRef]
- Sun, S.; Liu, J.; Zhou, N.; Zhu, W.; Dou, Q.P.; Zhou, K. Isolation of Three New Annonaceous Acetogenins from Graviola Fruit (Annona muricata) and Their Anti-Proliferation on Human Prostate Cancer Cell PC-3. Bioorg. Med. Chem. Lett. 2016, 26, 4382–4385. [Google Scholar] [CrossRef] [PubMed]
- Liaw, C.-C.; Chang, F.-R.; Lin, C.-Y.; Chou, C.-J.; Chiu, H.-F.; Wu, M.-J.; Wu, Y.-C. New Cytotoxic Monotetrahydrofuran Annonaceous Acetogenins from Annona muricata. J. Nat. Prod. 2002, 65, 470–475. [Google Scholar] [CrossRef]
- Dai, Y.; Hogan, S.; Schmelz, E.M.; Ju, Y.H.; Canning, C.; Zhou, K. Selective Growth Inhibition of Human Breast Cancer Cells by Graviola Fruit Extract In Vitro and In Vivo Involving Downregulation of EGFR Expression. Nutr. Cancer 2011, 63, 795–801. [Google Scholar] [CrossRef]
- Mutakin, M.; Fauziati, R.; Fadhilah, F.N.; Zuhrotun, A.; Amalia, R.; Hadisaputri, Y.E. Pharmacological Activities of Soursop (Annona muricata Lin.). Molecules 2022, 27, 1201. [Google Scholar] [CrossRef]
- De Sousa, O.V.; Vieira, G.D.-V.; De Pinho, J.d.J.R.G.; Yamamoto, C.H.; Alves, M.S. Antinociceptive and Anti-Inflammatory Activities of the Ethanol Extract of Annona muricata L. Leaves in Animal Models. Int. J. Mol. Sci. 2010, 11, 2067–2078. [Google Scholar] [CrossRef]
- Arthur, F.K.N.; Woode, E.; Terlabi, E.O.; Larbie, C. Evaluation of Acute and Subchronic Toxicity of Annona muricata (Linn.) Aqueous Extract in Animals. Eur. J. Exp. Biol. 2011, 1, 115–124. [Google Scholar]
- Boyom, F.F.; Fokou, P.V.T.; Yamthe, L.R.T.; Mfopa, A.N.; Kemgne, E.M.; Mbacham, W.F.; Tsamo, E.; Zollo, P.H.A.; Gut, J.; Rosenthal, P.J. Potent Antiplasmodial Extracts from Cameroonian Annonaceae. J. Ethnopharmacol. 2011, 134, 717–724. [Google Scholar] [CrossRef]
- Quilez, A.M.; Montserrat-de la Paz, S.; De La Puerta, R.; Fernández-Arche, M.A.; Gargia-Gimenez, M.D. Validation of Ethnopharmacological Use as Anti-Inflammatory of a Decoction from Annona muricata Leaves. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 14–20. [Google Scholar] [CrossRef]
- Yasir, M.; Das, S.; Kharya, M.D. The Phytochemical and Pharmacological Profile of Persea americana Mill. Pharmacogn. Rev. 2010, 4, 77–84. [Google Scholar] [CrossRef] [PubMed]
- The Avocado (Persea americana, Lauraceae) Crop in Mesoamerica: 10,000 Years of History on JSTOR. Available online: https://www.jstor.org/stable/41761865 (accessed on 28 July 2023).
- Persea Americana—Useful Tropical Plants. Available online: https://tropical.theferns.info/viewtropical.php?id=Persea+americana (accessed on 28 July 2023).
- Ashande, C. A Mini-Review on the Phytochemistry and Pharmacology of the Medicinal Plant Species Persea americana Mill. (Lauraceae). J. Nat. Prod. Res. Ethnopharmacol. 2019, 6, 102–111. [Google Scholar]
- Bhuyan, D.J.; Alsherbiny, M.A.; Perera, S.; Low, M.; Basu, A.; Devi, O.A.; Barooah, M.S.; Li, C.G.; Papoutsis, K. The Odyssey of Bioactive Compounds in Avocado (Persea americana) and Their Health Benefits. Antioxidants 2019, 8, 426. [Google Scholar] [CrossRef]
- Jackson, M.D.; Walker, S.P.; Simpson-Smith, C.M.; Lindsay, C.M.; Smith, G.; McFarlane-Anderson, N.; Bennett, F.I.; Coard, K.C.M.; Aiken, W.D.; Tulloch, T.; et al. Associations of Whole-Blood Fatty Acids and Dietary Intakes with Prostate Cancer in Jamaica. Cancer Causes Control 2012, 23, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Gouegni, E.F.; Abubakar, H. Phytochemical, Toxicological, Biochemical and Haematological Studies on Avocado (Persea americana) in Experimental Animals. Niger. Food J. 2013, 31, 64–69. [Google Scholar] [CrossRef]
- Ozolua, R.; Anaka, O.; Okpo, S.; Idogun, S. Acute and Sub-Acute Toxicological Assessment of the Aqueous Seed Extract of Persea americana Mill (Lauraceae) in Rats. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Iheagwam, F.N.; Onyido, B.C.; Chinedu, S.N. Toxicological Profile of Dacryodes edulis (G. Don.) H. J. Lam. and Persea americana M. Seeds in Male Wistar Rats. Comp. Clin. Pathol. 2023, 32, 43–52. [Google Scholar] [CrossRef]
- Pahua-Ramos, M.E.; Ortiz-Moreno, A.; Chamorro-Cevallos, G.; Hernández-Navarro, M.D.; Garduño-Siciliano, L.; Necoechea-Mondragón, H.; Hernández-Ortega, M. Hypolipidemic Effect of Avocado (Persea americana Mill) Seed in a Hypercholesterolemic Mouse Model. Plant Foods Hum. Nutr. 2012, 67, 10–16. [Google Scholar] [CrossRef]
- Orabueze, I.C.; Babalola, R.; Azuonwu, O.; Okoko, I.-I.; Asare, G. Evaluation of Possible Effects of Persea americana Seeds on Female Reproductive Hormonal and Toxicity Profile. J. Ethnopharmacol. 2021, 273, 113870. [Google Scholar] [CrossRef] [PubMed]
- Rao, U.S.M.; Adinew, B. Remnant B-Cell-Stimulative and Anti-Oxidative Effects of Persea americana Fruit Extract Studied in Rats Introduced into Streptozotocin—Induced Hyperglycaemic State. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Nicolella, H.D.; Neto, F.R.; Corrêa, M.B.; Lopes, D.H.; Rondon, E.N.; dos Santos, L.F.R.; de Oliveira, P.F.; Damasceno, J.L.; Acésio, N.O.; Turatti, I.C.C.; et al. Toxicogenetic Study of Persea americana Fruit Pulp Oil and Its Effect on Genomic Instability. Food Chem. Toxicol. 2017, 101, 114–120. [Google Scholar] [CrossRef]
- Paul, R.; Kulkarni, P.; Ganesh, N. Avocado Fruit (Persea americana Mill) Exhibits Chemo-Protective Potentiality against Cyclophosphamide Induced Genotoxicity in Human Lymphocyte Culture. J. Exp. Ther. Oncol. 2011, 9, 221–230. [Google Scholar]
- Kulkarni, P.; Paul, R.; Ganesh, N. In Vitro Evaluation of Genotoxicity of Avocado (Persea americana) Fruit and Leaf Extracts in Human Peripheral Lymphocytes. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2010, 28, 172–187. [Google Scholar] [CrossRef]
- Oelrichs, P.B.; Ng, J.C.; Seawright, A.A.; Ward, A.; Schäffeler, L.; Macleod, J.K. Isolation and Identification of a Compound from Avocado (Persea americana) Leaves Which Causes Necrosis of the Acinar Epithelium of the Lactating Mammary Gland and the Myocardium. Nat. Toxins 1995, 3, 344–349. [Google Scholar] [CrossRef]
- Freitas, M.S.; Pereira, A.H.B.; Pereira, G.O.; Menezes, I.S.; Lucena, A.R.; Almeida, C.R.F.; Pereira, E.G.; Santos, L.A.; Tozin, L.R.S.; Alves, F.M.; et al. Acetogenin-Induced Fibrotic Heart Disease from Avocado (Persea americana, Lauraceae) Poisoning in Horses. Toxicon 2022, 219, 106921. [Google Scholar] [CrossRef]
- Craigmill, A.; Seawright, A.; Mattila, T.; Frost, A. Pathological Changes in the Mammary Gland and Biochemical Changes in Milk of the Goat Following Oral Dosing with Leaf of the Avocado (Persea americana). Aust. Vet. J. 1989, 66, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.; Basson, P.A.; Booker, H.H.; Hofherr, J.B.; Anthonissen, M. Cardiomyopathy Caused by Avocado (Persea americana Mill) Leaves. J. S. Afr. Vet. Assoc. 1991, 62, 21–22. [Google Scholar] [CrossRef]
- Buoro, I.B.; Nyamwange, S.B.; Chai, D.; Munyua, S.M. Putative Avocado Toxicity in Two Dogs. Onderstepoort J. Vet. Res. 1994, 61, 107–109. [Google Scholar]
- Aguirre, L.S.; Sandoval, G.V.; Medina, D.M.; Martinez, O.G.; Micheloud, J.F. Acute Heart Failure in Rabbits by Avocado Leaf Poisoning. Toxicon Off. J. Int. Soc. Toxinology 2019, 164, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Burger, W.P.; Naudé, T.W.; Van Rensburg, I.B.; Botha, C.J.; Pienaar, A.C. Cardiomyopathy in Ostriches (Struthio camelus) Due to Avocado (Persea americana Var. Guatemalensis) Intoxication. J. S. Afr. Vet. Assoc. 1994, 65, 113–118. [Google Scholar] [PubMed]
- Maphetu, N.; Unuofin, J.O.; Masuku, N.P.; Olisah, C.; Lebelo, S.L. Medicinal Uses, Pharmacological Activities, Phytochemistry, and the Molecular Mechanisms of Punica granatum L. (Pomegranate) Plant Extracts: A Review. Biomed. Pharmacother. 2022, 153, 113256. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, H.R.; Arastoo, M.; Ostad, S.N. A Comprehensive Review of Punica granatum (Pomegranate) Properties in Toxicological, Pharmacological, Cellular and Molecular Biology Researches. Iran. J. Pharm. Res. IJPR 2012, 11, 385–400. [Google Scholar]
- Medicinal Uses of Punica granatum and Its Health Benefits. Available online: https://www.phytojournal.com/vol1Issue5/6.html (accessed on 7 June 2023).
- Fakudze, N.T.; Aniogo, E.C.; George, B.P.; Abrahamse, H. The Therapeutic Efficacy of Punica granatum and Its Bioactive Constituents with Special Reference to Photodynamic Therapy. Plants 2022, 11, 2820. [Google Scholar] [CrossRef] [PubMed]
- Panth, N.; Manandhar, B.; Paudel, K.R. Anticancer Activity of Punica granatum (Pomegranate): A Review. Phytother. Res. 2017, 31, 568–578. [Google Scholar] [CrossRef]
- Zarfeshany, A.; Asgary, S.; Javanmard, S.H. Potent Health Effects of Pomegranate. Adv. Biomed. Res. 2014, 3, 100. [Google Scholar] [CrossRef]
- Freedland, S.J.; Carducci, M.; Kroeger, N.; Partin, A.; Rao, J.; Jin, Y.; Kerkoutian, S.; Wu, H.; Li, Y.; Creel, P.; et al. A Double-Blind, Randomized, Neoadjuvant Study of the Tissue Effects of POMx Pills in Men with Prostate Cancer Before Radical Prostatectomy. Cancer Prev. Res. 2013, 6, 1120–1127. [Google Scholar] [CrossRef]
- Pantuck, A.J.; Leppert, J.T.; Zomorodian, N.; Aronson, W.; Hong, J.; Barnard, R.J.; Seeram, N.; Liker, H.; Wang, H.; Elashoff, R.; et al. Phase II Study of Pomegranate Juice for Men with Rising Prostate-Specific Antigen Following Surgery or Radiation for Prostate Cancer. Clin. Cancer Res. 2006, 12, 4018–4026. [Google Scholar] [CrossRef]
- Paller, C.J.; Ye, X.; Wozniak, P.J.; Gillespie, B.K.; Sieber, P.R.; Greengold, R.H.; Stockton, B.R.; Hertzman, B.L.; Efros, M.D.; Roper, R.P.; et al. A Randomized Phase II Study of Pomegranate Extract for Men with Rising PSA Following Initial Therapy for Localized Prostate Cancer. Prostate Cancer Prostatic Dis. 2013, 16, 50–55. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Giménez-Bastida, J.A.; García-Conesa, M.T.; Gómez-Sánchez, M.B.; García-Talavera, N.V.; Gil-Izquierdo, A.; Sánchez-Álvarez, C.; Fontana-Compiano, L.O.; Morga-Egea, J.P.; Pastor-Quirante, F.A.; et al. Occurrence of Urolithins, Gut Microbiota Ellagic Acid Metabolites and Proliferation Markers Expression Response in the Human Prostate Gland upon Consumption of Walnuts and Pomegranate Juice. Mol. Nutr. Food Res. 2010, 54, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Setiadhi, R.; Sufiawati, I.; Zakiawati, D.; Nuraeny, N.; Hidayat, W.; Firman, D. Evaluation of Antibacterial Activity and Acute Toxicity of Pomegranate (Punica granatum l.) Seed Ethanolic Extracts in Swiss Webster Mice. J. Dentomaxillofacial Sci. 2017, 2, 119. [Google Scholar] [CrossRef]
- Vidal, A.; Fallarero, A.; Peña, B.R.; Medina, M.E.; Gra, B.; Rivera, F.; Gutierrez, Y.; Vuorela, P.M. Studies on the Toxicity of Punica granatum L. (Punicaceae) Whole Fruit Extracts. J. Ethnopharmacol. 2003, 89, 295–300. [Google Scholar] [CrossRef]
- Bassiri-Jahromi, S.; Pourshafie, M.; Mirabzadeh, E.; Tavasoli, A.; Katiraee, F.; Mostafavi, E.; Abbasian, S. Punica granatum Peel Extract Toxicity in Mice. Jundishapur J. Nat. Pharm. Prod. 2015, 10, 23770. [Google Scholar] [CrossRef]
- Patel, C.; Dadhaniya, P.; Hingorani, L.; Soni, M.G. Safety Assessment of Pomegranate Fruit Extract: Acute and Subchronic Toxicity Studies. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2008, 46, 2728–2735. [Google Scholar] [CrossRef] [PubMed]
- Vale, E.P.; do Rego, L.R.; Pureza, D.D.N.; de Barros Silva, P.G.; de Sousa, F.F.O.; Neto, M.D.A.B.M. Cytogenetic and Toxicological Effects of Punica granatum Linnaeus Fruit Peel Hydroethanolic Extract in Mice. S. Afr. J. Bot. 2020, 130, 465–470. [Google Scholar] [CrossRef]
- SciELO—Brazil—Assessment of Mutagenic and Antimutagenic Effects of Punica granatum in Mice Assessment of Mutagenic and Antimutagenic Effects of Punica granatum in Mice. Available online: https://www.scielo.br/j/bjps/a/Dbz4zXhPhCN4sK8FqQgy6dw/ (accessed on 29 July 2023).
- De Amorim, A.; Borba, H.R.; Armada, J.L. Test of Mutagenesis in Mice Treated with Aqueous Extracts from Punica granatum L.(Pomegranate). Rev. Bras. Farm. 1995, 76, 110–111. [Google Scholar]
- Read, E.; Deseo, M.; Hawes, M.; Rochfort, S. Identification of Potentially Cytotoxic Phenolics Present in Pomegranates (Punica granatum L.). Anim. Feed Sci. Technol. 2019, 251, 187–197. [Google Scholar] [CrossRef]
- Eghbali, S.; Askari, S.F.; Avan, R.; Sahebkar, A. Therapeutic Effects of Punica granatum (Pomegranate): An Updated Review of Clinical Trials. J. Nutr. Metab. 2021, 2021, 5297162. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Cottiglia, F.; Bueno, C.A.; Alché, L.E.; Leonti, M.; Vargiu, S.; Bifulco, E.; Menkissoglu-Spiroudi, U.; Caboni, P. Cytotoxic Tirucallane Triterpenoids from Melia Azedarach Fruits. Molecules 2010, 15, 5866–5877. [Google Scholar] [CrossRef]
- Adegoke, A.M.; Gota, V.; Gupta, S.; Gbadegesin, M.A.; Odunola1, O.A. Evaluation of Antioxidant and Anticancer Activities of Aqueous Extract of the Fruit Pulp of Adansonia Digitata Linn and Its Fractions. Afr. J. Med. Med. Sci. 2021, 50, 9–17. [Google Scholar]
- Ochwang’i, D.O.; Kimwele, C.N.; Oduma, J.A.; Gathumbi, P.K.; Mbaria, J.M.; Kiama, S.G. Medicinal Plants Used in Treatment and Management of Cancer in Kakamega County, Kenya. J. Ethnopharmacol. 2014, 151, 1040–1055. [Google Scholar] [CrossRef] [PubMed]
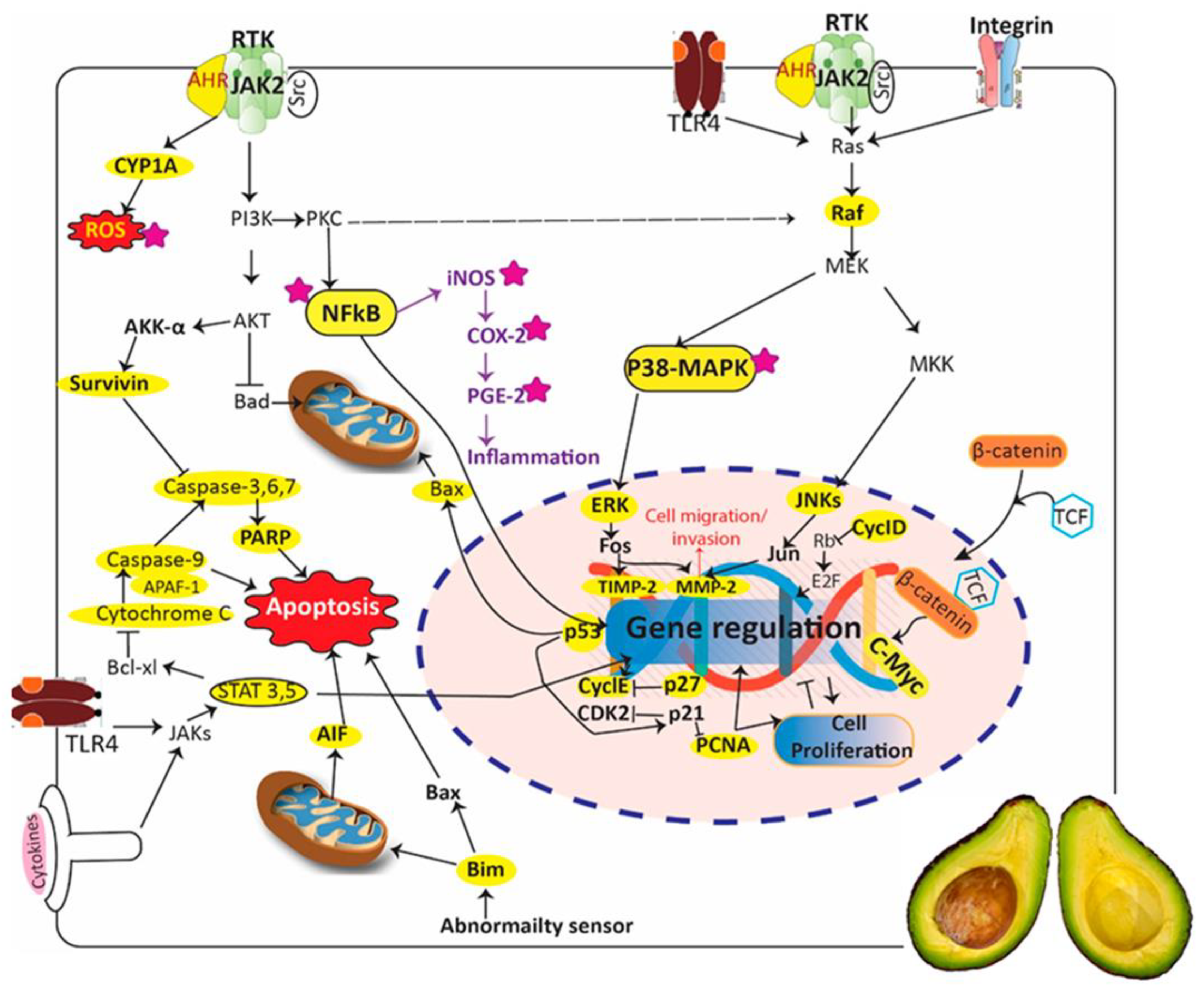
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakudze, N.T.; Sarbadhikary, P.; George, B.P.; Abrahamse, H. Ethnomedicinal Uses, Phytochemistry, and Anticancer Potentials of African Medicinal Fruits: A Comprehensive Review. Pharmaceuticals 2023, 16, 1117. https://doi.org/10.3390/ph16081117
Fakudze NT, Sarbadhikary P, George BP, Abrahamse H. Ethnomedicinal Uses, Phytochemistry, and Anticancer Potentials of African Medicinal Fruits: A Comprehensive Review. Pharmaceuticals. 2023; 16(8):1117. https://doi.org/10.3390/ph16081117
Chicago/Turabian StyleFakudze, Nosipho Thembekile, Paromita Sarbadhikary, Blassan P. George, and Heidi Abrahamse. 2023. "Ethnomedicinal Uses, Phytochemistry, and Anticancer Potentials of African Medicinal Fruits: A Comprehensive Review" Pharmaceuticals 16, no. 8: 1117. https://doi.org/10.3390/ph16081117
APA StyleFakudze, N. T., Sarbadhikary, P., George, B. P., & Abrahamse, H. (2023). Ethnomedicinal Uses, Phytochemistry, and Anticancer Potentials of African Medicinal Fruits: A Comprehensive Review. Pharmaceuticals, 16(8), 1117. https://doi.org/10.3390/ph16081117