Overview of Caffeine Effects on Human Health and Emerging Delivery Strategies
Abstract
1. Introduction
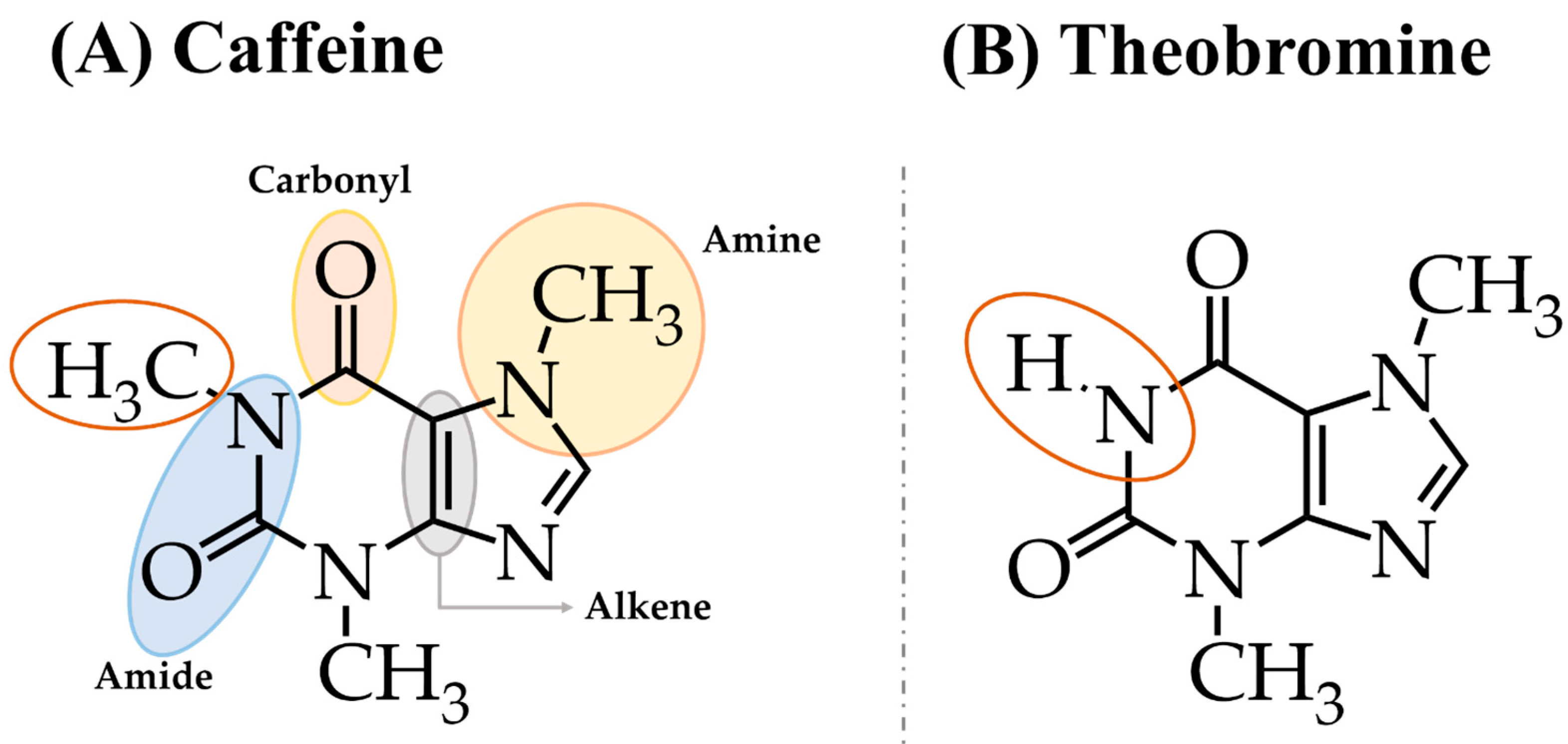
2. Chemical Structure and Main Natural Sources of Caffeine
3. Benefits of Caffeine on Health
3.1. Cancer
3.2. Anti-Inflammatory and Immunomodulation
3.2.1. Autoimmune Diseases and Immunomodulation
3.2.2. Ocular Diseases
3.2.3. Respiratory Diseases
3.3. Neurodegenerative Diseases
3.4. Cardiovascular Diseases
4. Caffeine Impact on Sports Performance
4.1. Optimal Dosage
4.2. Timing of Intake
4.3. Abstinence
4.4. Training Time vs. Caffeine Consumption
4.5. Physiological Factors
4.6. Gender
4.7. Caffeine Consumers or Not
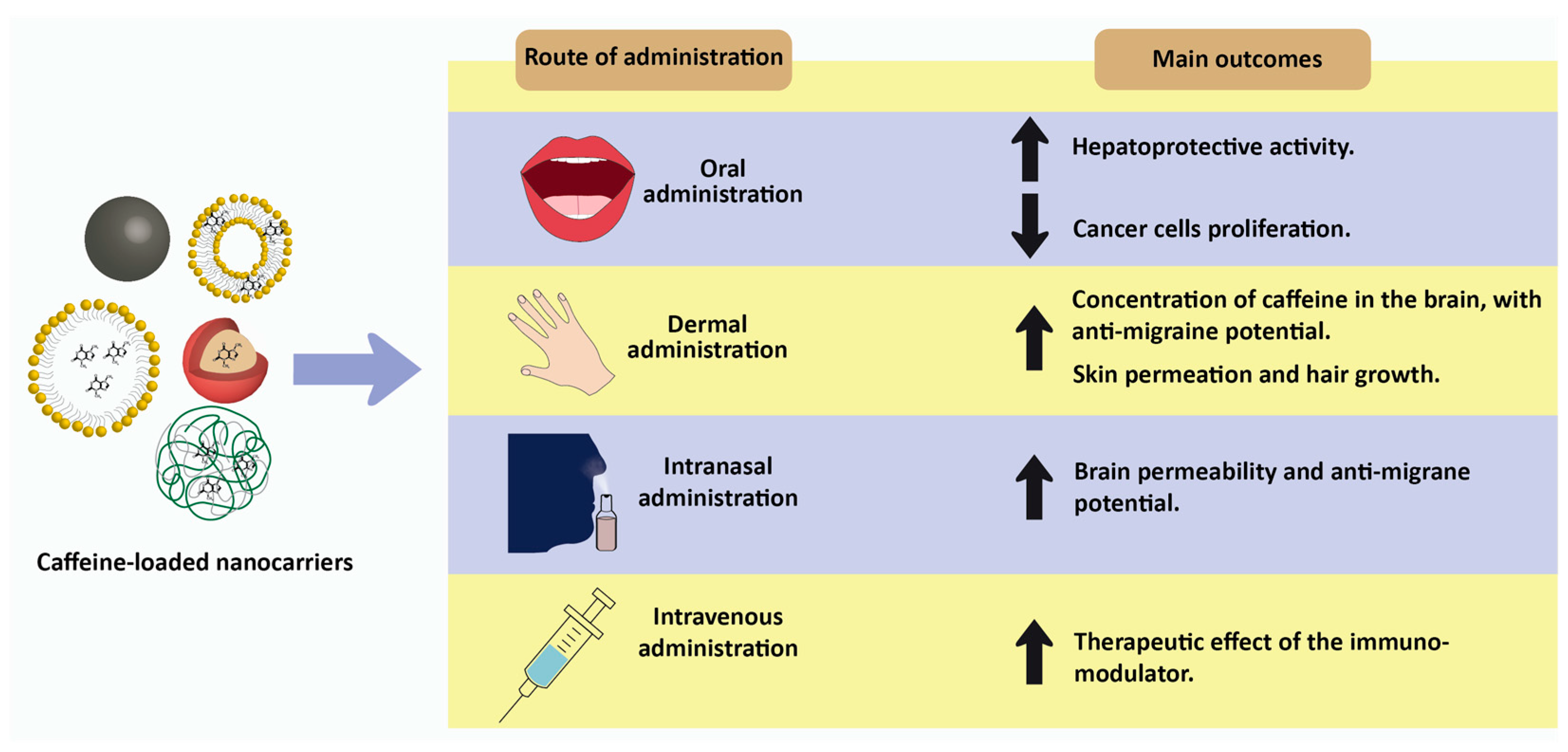
5. Future Directions: Nanotechnology-Based Delivery Strategies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Falcão, A.; Alves, G.; Lopes, J.A.; Silva, L.R. Employ of Anthocyanins in Nanocarriers for Nano Delivery: In Vitro and In Vivo Experimental Approaches for Chronic Diseases. Pharmaceutics 2022, 14, 2272. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Q.; Lei, H.M.; Hu, Q.Y.; Li, G.H.; Zhao, P.J. Recent Advances in the Synthetic Biology of Natural Drugs. Front. Bioeng. Biotechnol. 2021, 9, 691152. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Ozleyen, A.; Boyunegmez Tumer, T.; Oluwaseun Adetunji, C.; El Omari, N.; Balahbib, A.; Taheri, Y.; Bouyahya, A.; Martorell, M.; Martins, N.; et al. Natural Products and Synthetic Analogs as a Source of Antitumor Drugs. Biomolecules 2019, 9, 679. [Google Scholar] [CrossRef]
- Cristina-Souza, G.; Santos, P.S.; Santos-Mariano, A.C.; Coelho, D.B.; Rodacki, A.; De-Oliveira, F.R.; Bishop, D.J.; Bertuzzi, R.; Lima-Silva, A.E. Caffeine Increases Endurance Performance via Changes in Neural and Muscular Determinants of Performance Fatigability. Med. Sci. Sports Exerc. 2022, 54, 1591–1603. [Google Scholar] [CrossRef]
- Jodra, P.; Lago-Rodríguez, A.; Sánchez-Oliver, A.J.; López-Samanes, A.; Pérez-López, A.; Veiga-Herreros, P.; San Juan, A.F.; Domínguez, R. Effects of caffeine supplementation on physical performance and mood dimensions in elite and trained-recreational athletes. J. Int. Soc. Sport. Nutr. 2020, 17, 2. [Google Scholar] [CrossRef]
- Sampaio-Jorge, F.; Morales, A.P.; Pereira, R.; Barth, T.; Ribeiro, B.G. Caffeine increases performance and leads to a cardioprotective effect during intense exercise in cyclists. Sci. Rep. 2021, 11, 24327. [Google Scholar] [CrossRef] [PubMed]
- San Juan, A.F.; López-Samanes, Á.; Jodra, P.; Valenzuela, P.L.; Rueda, J.; Veiga-Herreros, P.; Pérez-López, A.; Domínguez, R. Caffeine Supplementation Improves Anaerobic Performance and Neuromuscular Efficiency and Fatigue in Olympic-Level Boxers. Nutrients 2019, 11, 2120. [Google Scholar] [CrossRef] [PubMed]
- White, J.R., Jr.; Padowski, J.M.; Zhong, Y.; Chen, G.; Luo, S.; Lazarus, P.; Layton, M.E.; McPherson, S. Pharmacokinetic analysis and comparison of caffeine administered rapidly or slowly in coffee chilled or hot versus chilled energy drink in healthy young adults. Clin. Toxicol. 2016, 54, 308–312. [Google Scholar] [CrossRef]
- Błaszczyk-Bębenek, E.; Jagielski, P.; Schlegel-Zawadzka, M. Caffeine Consumption in a Group of Adolescents from South East Poland—A Cross Sectional Study. Nutrients 2021, 13, 2084. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.S.; Speck, A.E.; Canas, P.M.; Cunha, R.A. Neuronal adenosine A2A receptors signal ergogenic effects of caffeine. Sci. Rep. 2020, 10, 13414. [Google Scholar] [CrossRef] [PubMed]
- James, J.E. Maternal caffeine consumption and pregnancy outcomes: A narrative review with implications for advice to mothers and mothers-to-be. BMJ Evid.-Based Med. 2021, 26, 114–115. [Google Scholar] [CrossRef]
- Karcz-Kubicha, M.; Antoniou, K.; Terasmaa, A.; Quarta, D.; Solinas, M.; Justinova, Z.; Pezzola, A.; Reggio, R.; Müller, C.E.; Fuxe, K.; et al. Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology 2003, 28, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.G.; Arceneaux, K.P., III; Chu, J.T.; Jacob, G., Jr.; Schreiber, A.L.; Tipton, R.C.; Yu, Y.; Johnson, W.D.; Greenway, F.L.; Primeaux, S.D. The effect of caffeine and albuterol on body composition and metabolic rate. Obesity 2015, 23, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.P.; Pliássova, A.; Cunha, R.A. The physiological effects of caffeine on synaptic transmission and plasticity in the mouse hippocampus selectively depend on adenosine A1 and A2A receptors. Biochem. Pharmacol. 2019, 166, 313–321. [Google Scholar] [CrossRef] [PubMed]
- McCreedy, A.; Bird, S.; Brown, L.J.; Shaw-Stewart, J.; Chen, Y.F. Effects of maternal caffeine consumption on the breastfed child: A systematic review. Swiss Med. Wkly. 2018, 148, w14665. [Google Scholar] [CrossRef]
- Burke, T.M.; Markwald, R.R.; McHill, A.W.; Chinoy, E.D.; Snider, J.A.; Bessman, S.C.; Jung, C.M.; O’Neill, J.S.; Wright, K.P., Jr. Effects of caffeine on the human circadian clock in vivo and in vitro. Sci. Transl. Med. 2015, 7, 305ra146. [Google Scholar] [CrossRef]
- Staack, A.; Distelberg, B.; Moldovan, C.; Belay, R.E.; Sabaté, J. The Impact of Caffeine Intake on Mental Health Symptoms in Postmenopausal Females with Overactive Bladder Symptoms: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Women’s Health 2022, 31, 819–825. [Google Scholar] [CrossRef]
- Agostoni, C.; Canani, R.B.; Fairweather-Tait, S.; Heinonen, M.; Korhonen, H.; La Vieille, S.; Marchelli, R. Scientific Opinion on the safety of caffeine. EFSA J. 2015, 13, 4102. [Google Scholar] [CrossRef]
- Addicott, M.A.; Yang, L.L.; Peiffer, A.M.; Burnett, L.R.; Burdette, J.H.; Chen, M.Y.; Hayasaka, S.; Kraft, R.A.; Maldjian, J.A.; Laurienti, P.J. The effect of daily caffeine use on cerebral blood flow: How much caffeine can we tolerate? Hum. Brain Mapp. 2009, 30, 3102–3114. [Google Scholar] [CrossRef]
- Jahrami, H.; Al-Mutarid, M.; Penson, P.E.; Al-Islam Faris, M.; Saif, Z.; Hammad, L. Intake of Caffeine and Its Association with Physical and Mental Health Status among University Students in Bahrain. Foods 2020, 9, 473. [Google Scholar] [CrossRef] [PubMed]
- Rios-Leyvraz, M.; Bochud, M.; Tabin, R.; Genin, B.; Russo, M.; Rossier, M.F.; Eap, C.B.; Bovet, P.; Chiolero, A. Monitoring caffeine intake in children with a questionnaire and urine collection: A cross-sectional study in a convenience sample in Switzerland. Eur. J. Nutr. 2020, 59, 3537–3543. [Google Scholar] [CrossRef]
- Weibel, J.; Lin, Y.S.; Landolt, H.P.; Berthomier, C.; Brandewinder, M.; Kistler, J.; Rehm, S.; Rentsch, K.M.; Meyer, M.; Borgwardt, S.; et al. Regular Caffeine Intake Delays REM Sleep Promotion and Attenuates Sleep Quality in Healthy Men. J. Biol. Rhythm. 2021, 36, 384–394. [Google Scholar] [CrossRef]
- Food and Drug Administration. Pure and Highly Concentrated Caffeine; Food and Drug Administration: Silver Spring, MD, USA, 2023.
- Center for Food Safety and Applied Nutrition, Food and Drug Administration, U.S. Departments of Agriculture and Health and Human Services. Highly Concentrated Caffeine in Dietary Supplements: Guidance for Industry; Food and Drug Administration: Silver Spring, MD, USA, 2018.
- Abrahão, F.R.; Rocha, L.C.R.; Santos, T.A.; Carmo, E.L.d.; Pereira, L.A.S.; Borges, S.V.; Pereira, R.G.F.A.; Botrel, D.A. Microencapsulation of bioactive compounds from espresso spent coffee by spray drying. LWT 2019, 103, 116–124. [Google Scholar] [CrossRef]
- Khazaeli, P.; Pardakhty, A.; Shoorabi, H. Caffeine-Loaded Niosomes: Characterization and in Vitro Release Studies. Drug Deliv. 2007, 14, 447–452. [Google Scholar] [CrossRef]
- Milkova, V.; Goycoolea, F.M. Encapsulation of caffeine in polysaccharide oil-core nanocapsules. Colloid Polym. Sci. 2020, 298, 1035–1041. [Google Scholar] [CrossRef]
- Mohammadi, N.; Ehsani, M.R.; Bakhoda, H. Development of caffeine-encapsulated alginate-based matrix combined with different natural biopolymers, and evaluation of release in simulated mouth conditions. Flavour Fragr. J. 2018, 33, 357–366. [Google Scholar] [CrossRef]
- Shao, M.; Li, S.; Tan, C.P.; Kraithong, S.; Gao, Q.; Fu, X.; Zhang, B.; Huang, Q. Encapsulation of caffeine into starch matrices: Bitterness evaluation and suppression mechanism. Int. J. Biol. Macromol. 2021, 173, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Mena, P.; Calani, L.; Cid, C.; Del Rio, D.; Lean, M.E.; Crozier, A. Variations in caffeine and chlorogenic acid contents of coffees: What are we drinking? Food Funct. 2014, 5, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Ashihara, H.; Suzuki, T. Distribution and biosynthesis of caffeine in plants. Front. Biosci. 2004, 9, 1864–1876. [Google Scholar] [CrossRef]
- Ogita, S.; Uefuji, H.; Morimoto, M.; Sano, H. Application of RNAi to confirm theobromine as the major intermediate for caffeine biosynthesis in coffee plants with potential for construction of decaffeinated varieties. Plant Mol. Biol. 2004, 54, 931–941. [Google Scholar] [CrossRef]
- Tavagnacco, L.; Schnupf, U.; Mason, P.E.; Saboungi, M.-L.; Cesàro, A.; Brady, J.W. Molecular Dynamics Simulation Studies of Caffeine Aggregation in Aqueous Solution. J. Phys. Chem. B 2011, 115, 10957–10966. [Google Scholar] [CrossRef] [PubMed]
- Usabiaga, I.; Camiruaga, A.; Calabrese, C.; Maris, A.; Fernández, J.A. Exploring Caffeine–Phenol Interactions by the Inseparable Duet of Experimental and Theoretical Data. Chem.—A Eur. J. 2019, 25, 14230–14236. [Google Scholar] [CrossRef]
- Vella-Zarb, L.; Baisch, U. Crystal water as the molecular glue for obtaining different co-crystal ratios: The case of gallic acid tris-caffeine hexa hydrate. Acta Crystallogr. Sect. E Crystallogr. Commun. 2018, 74, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Coleman, W.F. Chocolate: Theobromine and Caffeine. J. Chem. Educ. 2004, 81, 1232. [Google Scholar] [CrossRef]
- Judelson, D.A.; Preston, A.G.; Miller, D.L.; Muñoz, C.X.; Kellogg, M.D.; Lieberman, H.R. Effects of theobromine and caffeine on mood and vigilance. J. Clin. Psychopharm. 2013, 33, 499–506. [Google Scholar] [CrossRef]
- Knapik, J.J.; Steelman, R.A.; Trone, D.W.; Farina, E.K.; Lieberman, H.R. Prevalence of caffeine consumers, daily caffeine consumption, and factors associated with caffeine use among active duty United States military personnel. Nutr. J. 2022, 21, 22. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.C.; Knight, C.A.; Hockenberry, J.; Teplansky, R.; Hartman, T.J. Beverage caffeine intakes in the U.S. Food Chem. Toxicol. 2014, 63, 136–142. [Google Scholar] [CrossRef]
- Informer, C. Caffeine Content of Drinks. Available online: https://www.caffeineinformer.com/the-caffeine-database (accessed on 26 November 2022).
- Jeon, J.-S.; Kim, H.-T.; Jeong, I.-H.; Hong, S.-R.; Oh, M.-S.; Yoon, M.-H.; Shim, J.-H.; Jeong, J.H.; Abd El-Aty, A.M. Contents of chlorogenic acids and caffeine in various coffee-related products. J. Adv. Res. 2019, 17, 85–94. [Google Scholar] [CrossRef]
- McCusker, R.R.; Fuehrlein, B.; Goldberger, B.A.; Gold, M.S.; Cone, E.J. Caffeine content of decaffeinated coffee. J. Anal. Toxicol. 2006, 30, 611–613. [Google Scholar] [CrossRef]
- Mills, C.E.; Oruna-Concha, M.J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P. The effect of processing on chlorogenic acid content of commercially available coffee. Food Chem. 2013, 141, 3335–3340. [Google Scholar] [CrossRef] [PubMed]
- Ridley, C.P.-M. Water Joe Caffeine Content. Available online: https://caffeinepark.com/water-joe-caffeine-content-1599/ (accessed on 26 November 2022).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Tran, K.B.; Lang, J.J.; Compton, K.; Xu, R.; Acheson, A.R.; Henrikson, H.J.; Kocarnik, J.M.; Penberthy, L.; Aali, A.; Abbas, Q.; et al. The global burden of cancer attributable to risk factors, 2010-19: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 563–591. [Google Scholar] [CrossRef]
- Iragorri, N.; de Oliveira, C.; Fitzgerald, N.; Essue, B. The Out-of-Pocket Cost Burden of Cancer Care—A Systematic Literature Review. Curr. Oncol. 2021, 28, 1216–1248. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Cadoná, F.C.; Dantas, R.F.; de Mello, G.H.; Silva, F.P., Jr. Natural products targeting into cancer hallmarks: An update on caffeine, theobromine, and (+)−catechin. Crit. Rev. Food Sci. Nutr. 2022, 62, 7222–7241. [Google Scholar] [CrossRef] [PubMed]
- Gaascht, F.; Dicato, M.; Diederich, M. Coffee provides a natural multitarget pharmacopeia against the hallmarks of cancer. Genes Nutr. 2015, 10, 51. [Google Scholar] [CrossRef]
- Cui, W.Q.; Wang, S.T.; Pan, D.; Chang, B.; Sang, L.X. Caffeine and its main targets of colorectal cancer. World J. Gastrointest. Oncol. 2020, 12, 149–172. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Y.; Tang, L. Caffeine induces sustained apoptosis of human gastric cancer cells by activating the caspase-9/caspase-3 signalling pathway. Mol. Med. Rep. 2017, 16, 2445–2454. [Google Scholar] [CrossRef]
- El-Far, A.H.; Darwish, N.H.E.; Mousa, S.A. Senescent Colon and Breast Cancer Cells Induced by Doxorubicin Exhibit Enhanced Sensitivity to Curcumin, Caffeine, and Thymoquinone. Integr. Cancer Ther. 2020, 19, 1534735419901160. [Google Scholar] [CrossRef] [PubMed]
- Machado, K.L.; Marinello, P.C.; Silva, T.N.X.; Silva, C.F.N.; Luiz, R.C.; Cecchini, R.; Cecchini, A.L. Oxidative Stress in Caffeine Action on the Proliferation and Death of Human Breast Cancer Cells MCF-7 and MDA-MB-231. Nutr. Cancer 2021, 73, 1378–1388. [Google Scholar] [CrossRef]
- Fagundes, T.R.; Madeira, T.B.; Melo, G.P.; Bordini, H.P.; Marinello, P.C.; Nixdorf, S.L.; Cecchini, A.L.; Luiz, R.C. Caffeine improves the cytotoxic effect of dacarbazine on B16F10 murine melanoma cells. Bioorg. Chem. 2022, 120, 105576. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Rasà, D.M.; Saccone, S.; Federico, C.; Magro, G.; Cavallaro, S.; D’Agata, V. Caffeine Effect on HIFs/VEGF Pathway in Human Glioblastoma Cells Exposed to Hypoxia. Anti-Cancer Agents Med. Chem. 2018, 18, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Wrześniok, D.; Rzepka, Z.; Respondek, M.; Beberok, A.; Rok, J.; Szczepanik, K.; Buszman, E. Caffeine modulates growth and vitality of human melanotic COLO829 and amelanotic C32 melanoma cells: Preliminary findings. Food Chem. Toxicol. 2018, 120, 566–570. [Google Scholar] [CrossRef]
- Venkata Charan Tej, G.N.; Neogi, K.; Verma, S.S.; Chandra Gupta, S.; Nayak, P.K. Caffeine-enhanced anti-tumor immune response through decreased expression of PD1 on infiltrated cytotoxic T lymphocytes. Eur. J. Pharmacol. 2019, 859, 172538. [Google Scholar] [CrossRef]
- Eini, H.; Frishman, V.; Yulzari, R.; Kachko, L.; Lewis, E.C.; Chaimovitz, C.; Douvdevani, A. Caffeine promotes anti-tumor immune response during tumor initiation: Involvement of the adenosine A2A receptor. Biochem. Pharmacol. 2015, 98, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Hammami, A.; Allard, D.; Allard, B.; Stagg, J. Targeting the adenosine pathway for cancer immunotherapy. Semin. Immunol. 2019, 42, 101304. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hu, L.; Liu, T.; Chen, F.; Li, J.; Xu, J.; Jiang, L.; Xiang, Z.; Wang, X.; Sheng, J. Caffeine Targets G6PDH to Disrupt Redox Homeostasis and Inhibit Renal Cell Carcinoma Proliferation. Front. Cell Dev. Biol. 2020, 8, 556162. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Nahar, U.; Dahiya, D.; Mukherjee, S.; Dey, P.; Gupta, R.; Radotra, B.; Sachdeva, N.; Sood, A.; Bhadada, S.K.; et al. Role of cytotoxic T cells and PD-1 immune checkpoint pathway in papillary thyroid carcinoma. Front. Endocrinol. 2022, 13, 931647. [Google Scholar] [CrossRef]
- Song, J.; Sun, H.; Zhang, S.; Shan, C. The Multiple Roles of Glucose-6-Phosphate Dehydrogenase in Tumorigenesis and Cancer Chemoresistance. Life 2022, 12, 271. [Google Scholar] [CrossRef]
- Kaur, B.; Sohrabi, Y.; Achreja, A.; Lisanti, M.P.; Martinez-Outschoorn, U.E. Editorial: Hallmark of cancer: Reprogramming of cellular metabolism. Front. Oncol. 2023, 12, 1126913. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-C.; Hwang, J.-H. Caffeine Inhibits Growth of Temozolomide-Treated Glioma via Increasing Autophagy and Apoptosis but Not via Modulating Hypoxia, Angiogenesis, or Endoplasmic Reticulum Stress in Rats. Nutr. Cancer 2022, 74, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, P.; Kiang, K.M.Y.; Cheng, Y.S.; Leung, G.K.K. Caffeine Sensitizes U87-MG Human Glioblastoma Cells to Temozolomide through Mitotic Catastrophe by Impeding G2 Arrest. BioMed Res. Int. 2018, 2018, 5364973. [Google Scholar] [CrossRef]
- Lin, C.-K.; Liu, S.-T.; Wu, Z.-S.; Wang, Y.-C.; Huang, S.-M. Mechanisms of Cisplatin in Combination with Repurposed Drugs against Human Endometrial Carcinoma Cells. Life 2021, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Stern, L.; Giese, N.; Hackert, T.; Strobel, O.; Schirmacher, P.; Felix, K.; Gaida, M.M. Overcoming chemoresistance in pancreatic cancer cells: Role of the bitter taste receptor T2R10. J. Cancer 2018, 9, 711–725. [Google Scholar] [CrossRef]
- Higuchi, T.; Kawaguchi, K.; Miyake, K.; Han, Q.; Tan, Y.; Oshiro, H.; Sugisawa, N.; Zhang, Z.; Razmjooei, S.; Yamamoto, N.; et al. Oral Recombinant Methioninase Combined with Caffeine and Doxorubicin Induced Regression of a Doxorubicin-resistant Synovial Sarcoma in a PDOX Mouse Model. Anticancer Res. 2018, 38, 5639–5644. [Google Scholar] [CrossRef]
- Pascua, S.M.; McGahey, G.E.; Ma, N.; Wang, J.J.; Digman, M.A. Caffeine and Cisplatin Effectively Targets the Metabolism of a Triple-Negative Breast Cancer Cell Line Assessed via Phasor-FLIM. Int. J. Mol. Sci. 2020, 21, 2443. [Google Scholar] [CrossRef] [PubMed]
- Tonkaboni, A.; Lotfibakhshaiesh, N.; Danesh, P.; Tajerian, R.; Ziaei, H. Evaluation of Inhibitory Effects of Caffeine on Human Carcinoma Cells. Nutr. Cancer 2021, 73, 1998–2002. [Google Scholar] [CrossRef] [PubMed]
- Meisaprow, P.; Aksorn, N.; Vinayanuwattikun, C.; Chanvorachote, P.; Sukprasansap, M. Caffeine Induces G0/G1 Cell Cycle Arrest and Inhibits Migration through Integrin αv, β3, and FAK/Akt/c-Myc Signaling Pathway. Molecules 2021, 26, 7659. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, L.; Wan, Z.; He, Y.; Huang, H.; Xiang, H.; Wu, X.; Zhang, K.; Liu, Y.; Goodin, S.; et al. Atorvastatin and Caffeine in Combination Regulates Apoptosis, Migration, Invasion and Tumorspheres of Prostate Cancer Cells. Pathol. Oncol. Res. 2020, 26, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gu, C.; Wang, X.; Lang, Y.; Wu, Y.; Wu, X.; Zhu, X.; Wang, K.; Yang, H. Caffeine enhances the anti-tumor effect of 5-fluorouracil via increasing the production of reactive oxygen species in hepatocellular carcinoma. Med. Oncol. 2019, 36, 97. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Tsuchiya, H. Caffeine citrate enhanced cisplatin antitumor effects in osteosarcoma and fibrosarcoma in vitro and in vivo. BMC Cancer 2019, 19, 689. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kawaguchi, K.; Zhao, M.; Kiyuna, T.; Miyake, K.; Miyake, M.; Nelson, S.D.; Dry, S.M.; Li, Y.; Yamamoto, N.; et al. Exquisite Tumor Targeting by Salmonella A1-R in Combination with Caffeine and Valproic Acid Regresses an Adult Pleomorphic Rhabdomyosarcoma Patient-Derived Orthotopic Xenograft Mouse Model. Transl. Oncol. 2020, 13, 393–400. [Google Scholar] [CrossRef]
- Bartolomeu, A.R.; Romualdo, G.R.; Lisón, C.G.; Besharat, Z.M.; Corrales, J.A.M.; Chaves, M.Á.G.; Barbisan, L.F. Caffeine and Chlorogenic Acid Combination Attenuate Early-Stage Chemically Induced Colon Carcinogenesis in Mice: Involvement of oncomiR miR-21a-5p. Int. J. Mol. Sci. 2022, 23, 6292. [Google Scholar] [CrossRef]
- Popović, D.; Lalošević, D.; Miljković, D.; Popović, K.; Čapo, I.; Popović, J. Caffeine induces metformin anticancer effect on fibrosarcoma in hamsters. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2461–2467. [Google Scholar] [CrossRef]
- Tej, G.; Neogi, K.; Nayak, P.K. Caffeine-enhanced anti-tumor activity of anti-PD1 monoclonal antibody. Int. Immunopharm. 2019, 77, 106002. [Google Scholar] [CrossRef]
- Higuchi, T.; Oshiro, H.; Miyake, K.; Sugisawa, N.; Han, Q.; Tan, Y.; Park, J.; Zhang, Z.; Razmjooei, S.; Yamamoto, N.; et al. Oral Recombinant Methioninase, Combined with Oral Caffeine and Injected Cisplatinum, Overcome Cisplatinum-Resistance and Regresses Patient-derived Orthotopic Xenograft Model of Osteosarcoma. Anticancer Res. 2019, 39, 4653–4657. [Google Scholar] [CrossRef]
- Xiao, T.S. Innate immunity and inflammation. Cell. Mol. Immunol. 2017, 14, 1–3. [Google Scholar] [CrossRef]
- Turvey, S.E.; Broide, D.H. Innate immunity. J. Allergy Clin. Immunol. 2010, 125, S24–S32. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Chow, M.T.; Möller, A.; Smyth, M.J. Inflammation and immune surveillance in cancer. Semin. Cancer Biol. 2012, 22, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Al Reef, T.; Ghanem, E. Caffeine: Well-known as psychotropic substance, but little as immunomodulator. Immunobiology 2018, 223, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Sharif, K.; Watad, A.; Bragazzi, N.L.; Adawi, M.; Amital, H.; Shoenfeld, Y. Coffee and autoimmunity: More than a mere hot beverage! Autoimmun. Rev. 2017, 16, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.E.; Falk, J.L. Dose-dependent surmountability of locomotor activity in caffeine tolerance. Pharmacol. Biochem. Behav. 1995, 52, 139–143. [Google Scholar] [CrossRef]
- Laux, D.C.; Klesius, P.H.; Jeter, W.S. Suppressive effects of caffeine on the immune response of the mouse to sheep erythrocytes. Proc. Soc. Exp. Biol. Med. 1973, 144, 633–638. [Google Scholar] [CrossRef]
- Rosenthal, L.A.; Taub, D.D.; Moors, M.A.; Blank, K.J. Methylxanthine-induced inhibition of the antigen- and superantigen-specific activation of T and B lymphocytes. Immunopharmacology 1992, 24, 203–217. [Google Scholar] [CrossRef]
- Açıkalın, B.; Sanlier, N. Coffee and its effects on the immune system. Trends Food Sci. Technol. 2021, 114, 625–632. [Google Scholar] [CrossRef]
- Horrigan, L.A.; Kelly, J.P.; Connor, T.J. Immunomodulatory effects of caffeine: Friend or foe? Pharmacol. Ther. 2006, 111, 877–892. [Google Scholar] [CrossRef]
- Wang, H.Q.; Song, K.Y.; Feng, J.Z.; Huang, S.Y.; Guo, X.M.; Zhang, L.; Zhang, G.; Huo, Y.C.; Zhang, R.R.; Ma, Y.; et al. Caffeine Inhibits Activation of the NLRP3 Inflammasome via Autophagy to Attenuate Microglia-Mediated Neuroinflammation in Experimental Autoimmune Encephalomyelitis. J. Mol. Neurosci. 2022, 72, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Moases Ghaffary, E.; Abtahi Froushani, S.M. Immunomodulatory benefits of mesenchymal stem cells treated with Caffeine in adjuvant-induced arthritis. Life Sci. 2020, 246, 117420. [Google Scholar] [CrossRef]
- Sorenson, C.M.; Song, Y.-S.; Zaitoun, I.S.; Wang, S.; Hanna, B.A.; Darjatmoko, S.R.; Gurel, Z.; Fisk, D.L.; McDowell, C.M.; McAdams, R.M.; et al. Caffeine Inhibits Choroidal Neovascularization Through Mitigation of Inflammatory and Angiogenesis Activities. Front. Cell Dev. Biol. 2021, 9, 737426. [Google Scholar] [CrossRef] [PubMed]
- Dabouz, R.; Cheng, C.W.H.; Abram, P.; Omri, S.; Cagnone, G.; Sawmy, K.V.; Joyal, J.-S.; Desjarlais, M.; Olson, D.; Weil, A.G.; et al. An allosteric interleukin-1 receptor modulator mitigates inflammation and photoreceptor toxicity in a model of retinal degeneration. J. Neuroinflamm. 2020, 17, 359. [Google Scholar] [CrossRef] [PubMed]
- Krogh Nielsen, M.; Subhi, Y.; Molbech, C.R.; Falk, M.K.; Nissen, M.H.; Sørensen, T.L. Systemic Levels of Interleukin-6 Correlate With Progression Rate of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2019, 60, 202–208. [Google Scholar] [CrossRef]
- Conti, F.; Lazzara, F.; Romano, G.L.; Platania, C.B.M.; Drago, F.; Bucolo, C. Caffeine Protects Against Retinal Inflammation. Front. Pharmacol. 2022, 12, 824885. [Google Scholar] [CrossRef]
- Rutkowska, M.; Hożejowski, R.; Helwich, E.; Borszewska-Kornacka, M.K.; Gadzinowski, J. Severe bronchopulmonary dysplasia—Incidence and predictive factors in a prospective, multicenter study in very preterm infants with respiratory distress syndrome. J. Matern.-Fetal Neonatal Med. 2019, 32, 1958–1964. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, Y.; Lei, X.; Dong, W. Caffeine and bronchopulmonary dysplasia: Clinical benefits and the mechanisms involved. Pediatr. Pulmonol. 2022, 57, 1392–1400. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 inflammasome activation and cell death. Cell. Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef]
- Liao, J.; Kapadia, V.S.; Brown, L.S.; Cheong, N.; Longoria, C.; Mija, D.; Ramgopal, M.; Mirpuri, J.; McCurnin, D.C.; Savani, R.C. The NLRP3 inflammasome is critically involved in the development of bronchopulmonary dysplasia. Nat. Commun. 2015, 6, 8977. [Google Scholar] [CrossRef]
- Mesek, I.; Nellis, G.; Lass, J.; Metsvaht, T.; Varendi, H.; Visk, H.; Turner, M.A.; Nunn, A.J.; Duncan, J.; Lutsar, I. Medicines prescription patterns in European neonatal units. Int. J. Clin. Pharm. 2019, 41, 1578–1591. [Google Scholar] [CrossRef] [PubMed]
- Shenk, E.E.; Bondi, D.S.; Pellerite, M.M.; Sriram, S. Evaluation of Timing and Dosing of Caffeine Citrate in Preterm Neonates for the Prevention of Bronchopulmonary Dysplasia. J. Pediatr. Pharmacol. Ther. 2018, 23, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, Q.; Zhong, D.; Li, C.; Du, L. Caffeine prevents hyperoxia-induced lung injury in neonatal mice through NLRP3 inflammasome and NF-κB pathway. Respir. Res. 2020, 21, 140. [Google Scholar] [CrossRef] [PubMed]
- Endesfelder, S.; Strauß, E.; Bendix, I.; Schmitz, T.; Bührer, C. Prevention of Oxygen-Induced Inflammatory Lung Injury by Caffeine in Neonatal Rats. Oxid. Med. Cell. Longev. 2020, 2020, 3840124. [Google Scholar] [CrossRef] [PubMed]
- Iris, M.; Tsou, P.-S.; Sawalha, A.H. Caffeine inhibits STAT1 signaling and downregulates inflammatory pathways involved in autoimmunity. Clin. Immunol. 2018, 192, 68–77. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, L.; Cai, C.; Gong, X. Caffeine Inhibits NLRP3 Inflammasome Activation by Suppressing MAPK/NF-κB and A2aR Signaling in LPS-Induced THP-1 Macrophages. Int. J. Biol. Sci. 2019, 15, 1571–1581. [Google Scholar] [CrossRef]
- Kovács, E.G.; Alatshan, A.; Budai, M.M.; Czimmerer, Z.; Bíró, E.; Benkő, S. Caffeine Has Different Immunomodulatory Effect on the Cytokine Expression and NLRP3 Inflammasome Function in Various Human Macrophage Subpopulations. Nutrients 2021, 13, 2409. [Google Scholar] [CrossRef]
- Abbasi, A.; Froushani, S.M.A.; Delirezh, N.; Mostafaei, A. Caffeine alters the effects of bone marrow-derived mesenchymal stem cells on neutrophils. Adv. Clin. Exp. Med. 2018, 27, 463–468. [Google Scholar] [CrossRef]
- Abbasi, A.; Kukia, N.R.; Froushani, S.M.A.; Hashemi, S.M. Nicotine and caffeine alter the effects of the LPS-primed mesenchymal stem cells on the co-cultured neutrophils. Life Sci. 2018, 199, 41–47. [Google Scholar] [CrossRef]
- Tabolacci, C.; Cordella, M.; Rossi, S.; Bonaccio, M.; Eramo, A.; Mischiati, C.; Beninati, S.; Iacoviello, L.; Facchiano, A.; Facchiano, F. Targeting Melanoma-Initiating Cells by Caffeine: In Silico and In Vitro Approaches. Molecules 2021, 26, 3619. [Google Scholar] [CrossRef]
- Markova, E.V.; Knyazheva, M.A.; Tikhonova, M.A.; Amstislavskaya, T.G. Structural and functional characteristics of the hippocampus in depressive-like recipients after transplantation of in vitro caffeine-modulated immune cells. Neurosci. Lett. 2022, 786, 136790. [Google Scholar] [CrossRef] [PubMed]
- De Alcântara Almeida, I.; Mancebo Dorvigny, B.; Souza Tavares, L.; Nunes Santana, L.; Vitor Lima-Filho, J. Anti-inflammatory activity of caffeine (1,3,7-trimethylxanthine) after experimental challenge with virulent Listeria monocytogenes in Swiss mice. Int. Immunopharm. 2021, 100, 108090. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-W.; Tsai, H.-C.; Huang, C.-C.; Tsai, C.-Y.; Su, Y.-B.; Lin, M.-W.; Lee, K.-C.; Hsieh, Y.-C.; Li, T.-H.; Huang, S.-F.; et al. Effects and mechanisms of caffeine to improve immunological and metabolic abnormalities in diet-induced obese rats. Am. J. Phys.-Endocrinol. Metab. 2018, 314, E433–E447. [Google Scholar] [CrossRef] [PubMed]
- Farokhi-Sisakht, F.; Farhoudi, M.; Mahmoudi, J.; Farajdokht, F.; Kahfi-Ghaneh, R.; Sadigh-Eteghad, S. Effect of intranasal administration of caffeine on mPFC ischemia-induced cognitive impairment in BALB/c mice. Acta Neurobiol. Exp. 2022, 82, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Eraky, S.M.; El-Mesery, M.; El-Karef, A.; Eissa, L.A.; El-Gayar, A.M. Silymarin and caffeine combination ameliorates experimentally-induced hepatic fibrosis through down-regulation of LPAR1 expression. Biomed. Pharmacother. 2018, 101, 49–57. [Google Scholar] [CrossRef]
- Olopade, F.; Femi-Akinlosotu, O.; Ibitoye, C.; Shokunbi, T. Probing Caffeine Administration as a Medical Management for Hydrocephalus: An Experimental Study. Pediatr. Neurol. 2022, 135, 12–21. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Descovi, S.N.; Petrolli, T.G.; da Silva, A.S.; Baldisserotto, B. Caffeine modulates brain purinergic signaling in Nile tilapia (Oreochromis niloticus) under hypoxia conditions: Improvement of immune and inflammatory responses. Fish Phys. Biochem. 2019, 45, 551–560. [Google Scholar] [CrossRef]
- Rossetto, I.M.U.; Cagnon, V.H.A.; Kido, L.A.; Lizarte Neto, F.S.; Tirapelli, L.F.; Tirapelli, D.P.d.C.; de Almeida Chuffa, L.G.; Martinez, F.E.; Martinez, M. Caffeine consumption attenuates ethanol-induced inflammation through the regulation of adenosinergic receptors in the UChB rats cerebellum. Toxicol. Res. 2021, 10, 835–849. [Google Scholar] [CrossRef]
- Wadhwa, M.; Chauhan, G.; Roy, K.; Sahu, S.; Deep, S.; Jain, V.; Kishore, K.; Ray, K.; Thakur, L.; Panjwani, U. Caffeine and Modafinil Ameliorate the Neuroinflammation and Anxious Behavior in Rats during Sleep Deprivation by Inhibiting the Microglia Activation. Front. Cell. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef]
- Raoofi, A.; Delbari, A.; Nasiry, D.; Eslampour, H.; Golmohammadi, R.; Javadinia, S.S.; Sadrzadeh, R.; Mojadadi, M.-S.; Rustamzadeh, A.; Akhlaghi, M.; et al. Caffeine modulates apoptosis, oxidative stress, and inflammation damage induced by tramadol in cerebellum of male rats. J. Chem. Neuroanat. 2022, 123, 102116. [Google Scholar] [CrossRef]
- Hosny, E.N.; Sawie, H.G.; Elhadidy, M.E.; Khadrawy, Y.A. Evaluation of antioxidant and anti-inflammatory efficacy of caffeine in rat model of neurotoxicity. Nutr. Neurosci. 2019, 22, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Förderreuther, S.; Lampert, A.; Hitier, S.; Lange, R.; Weiser, T. The Impact of Baseline Pain Intensity on the Analgesic Efficacy of Ibuprofen/Caffeine in Patients with Acute Postoperative Dental Pain: Post Hoc Subgroup Analysis of a Randomised Controlled Trial. Adv. Ther. 2020, 37, 2976–2987. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.C.; Chen, S. Translational Neurodegeneration in the era of fast growing international brain research. Transl. Neurodegener. 2022, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Bekhit, A.E.-D.A.; Wu, Q.; Chen, M.; Liao, X.; Wang, J.; Ding, Y. Bioactive peptides and gut microbiota: Candidates for a novel strategy for reduction and control of neurodegenerative diseases. Trends Food Sci. Technol. 2021, 108, 164–176. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Wei, D.; Zhang, X.; Wang, J.; Wu, X.; Chang, J. Mitochondria-targeted nanoparticles in treatment of neurodegenerative diseases. Exploration 2021, 1, 20210115. [Google Scholar] [CrossRef]
- Kolahdouzan, M.; Hamadeh, M.J. The neuroprotective effects of caffeine in neurodegenerative diseases. CNS Neurosci. Ther. 2017, 23, 272–290. [Google Scholar] [CrossRef]
- Silvestro, S.; Sindona, C.; Bramanti, P.; Mazzon, E. A State of the Art of Antioxidant Properties of Curcuminoids in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 3168. [Google Scholar] [CrossRef]
- Herden, L.; Weissert, R. The Effect of Coffee and Caffeine Consumption on Patients with Multiple Sclerosis-Related Fatigue. Nutrients 2020, 12, 2262. [Google Scholar] [CrossRef]
- Houghton, V.; Du Preez, A.; Lefèvre-Arbogast, S.; de Lucia, C.; Low, D.Y.; Urpi-Sarda, M.; Ruigrok, S.R.; Altendorfer, B.; González-Domínguez, R.; Andres-Lacueva, C.; et al. Caffeine Compromises Proliferation of Human Hippocampal Progenitor Cells. Front. Cell Dev. Biol. 2020, 8, 806. [Google Scholar] [CrossRef]
- Gupta, R.C.; Srivastava, A.; Lall, R. Toxicity Potential of Nutraceuticals. Methods Mol. Biol. 2018, 1800, 367–394. [Google Scholar] [CrossRef]
- Pereira-Figueiredo, D.; Brito, R.; Araújo, D.S.M.; Nascimento, A.A.; Lyra, E.S.B.; Cheibub, A.M.S.S.; Pereira Netto, A.D.; Ventura, A.L.M.; Paes-de-Carvalho, R.; Calaza, K.C. Caffeine exposure ameliorates acute ischemic cell death in avian developing retina. Purinergic Signal. 2020, 16, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Figueiredo, D.; Nascimento, A.A.; Cunha-Rodrigues, M.C.; Brito, R.; Calaza, K.C. Caffeine and Its Neuroprotective Role in Ischemic Events: A Mechanism Dependent on Adenosine Receptors. Cell. Mol. Neurobiol. 2022, 42, 1693–1725. [Google Scholar] [CrossRef]
- Ruggiero, M.; Calvello, R.; Porro, C.; Messina, G.; Cianciulli, A.; Panaro, M.A. Neurodegenerative Diseases: Can Caffeine Be a Powerful Ally to Weaken Neuroinflammation? Int. J. Mol. Sci. 2022, 23, 12958. [Google Scholar] [CrossRef]
- Manalo, R.V.M.; Medina, P.M.B. Caffeine Protects Dopaminergic Neurons from Dopamine-Induced Neurodegeneration via Synergistic Adenosine-Dopamine D2-Like Receptor Interactions in Transgenic Caenorhabditis elegans. Front. Neurosci. 2018, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Bagchi, A. Study of the Effects of Nicotine and Caffeine for the Treatment of Parkinson’s Disease. Appl. Biochem. Biotechnol. 2022, 195, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.-L.; Dawson, V.L.; Dawson, T.M. Parkin-mediated lysine 63-linked polyubiquitination: A link to protein inclusions formation in Parkinson’s and other conformational diseases? Neurobiol. Aging 2006, 27, 524–529. [Google Scholar] [CrossRef]
- Wilkaniec, A.; Lenkiewicz, A.M.; Babiec, L.; Murawska, E.; Jęśko, H.M.; Cieślik, M.; Culmsee, C.; Adamczyk, A. Exogenous Alpha-Synuclein Evoked Parkin Downregulation Promotes c. Implications for Parkinson’s Disease Pathology. Front. Aging Neurosci. 2021, 13, 591475. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Ren, X.; Zheng, W.; Zeng, Z.; Guo, Y.; Hou, Z.; Guo, W.; Chen, X.; Li, F.; Chen, J.-F. Chronic Caffeine Treatment Protects Against α-Synucleinopathy by Reestablishing Autophagy Activity in the Mouse Striatum. Front. Neurosci. 2018, 12, 301. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, J.; Park, H.-J.; Park, E.S.; Oh, S.; Zheng, H.; Junn, E.; Voronkov, M.; Stock, J.B.; Mouradian, M.M. Synergistic neuroprotection by coffee components eicosanoyl-5-hydroxytryptamide and caffeine in models of Parkinson’s disease and DLB. Proc. Natl. Acad. Sci. USA 2018, 115, E12053–E12062. [Google Scholar] [CrossRef]
- Gupta, S.; Dasmahapatra, A.K. Caffeine destabilizes preformed Aβ protofilaments: Insights from all atom molecular dynamics simulations. Phys. Chem. Chem. Phys. 2019, 21, 22067–22080. [Google Scholar] [CrossRef] [PubMed]
- Farrokhi, M.R.; Emamghoreishi, M.; Amiri, A.; Keshavarz, M. Neuroprotective effects of caffeine against beta-amyliod neurotoxicity: The involvement of glycogen synthase kinase-3β protein. Phys. Pharm. 2019, 23, 150–153. [Google Scholar]
- Janitschke, D.; Nelke, C.; Lauer, A.A.; Regner, L.; Winkler, J.; Thiel, A.; Grimm, H.S.; Hartmann, T.; Grimm, M.O.W. Effect of Caffeine and Other Methylxanthines on Aβ-Homeostasis in SH-SY5Y Cells. Biomolecules 2019, 9, 689. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, C.; Murray, A.P.; Corradi, J.; Antollini, S.S. A novel pharmacological activity of caffeine in the cholinergic system. Neuropharmacology 2018, 135, 464–473. [Google Scholar] [CrossRef]
- Khan, A.; Ikram, M.; Muhammad, T.; Park, J.; Kim, M.O. Caffeine Modulates Cadmium-Induced Oxidative Stress, Neuroinflammation, and Cognitive Impairments by Regulating Nrf-2/HO-1 In Vivo and In Vitro. J. Clin. Med. 2019, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Molska, G.R.; Paula-Freire, L.I.G.; Sakalem, M.E.; Köhn, D.O.; Negri, G.; Carlini, E.A.; Mendes, F.R. Green coffee extract attenuates Parkinson’s-related behaviors in animal models. An. Acad. Bras. Cienc. 2021, 93, e20210481. [Google Scholar] [CrossRef]
- Sun, H.; Gonzalez, F.; McQuillen, P.S. Caffeine Restores Background EEG Activity Independent of Infarct Reduction after Neonatal Hypoxic Ischemic Brain Injury. Dev. Neurosci. 2020, 42, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.F.; Tassi, C.C.; Amaral, G.P.; Stefanello, S.T.; Dalla Corte, C.L.; Soares, F.A.; Posser, T.; Franco, J.L.; Carvalho, N.R. Effects of caffeine on brain antioxidant status and mitochondrial respiration in acetaminophen-intoxicated mice. Toxicol. Res. 2020, 9, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, S.S.; Uthaythas, S.; Govindarajulu, M.; Ramesh, S.; Parameshwaran, K.; Dhanasekaran, M. Caffeine, a natural methylxanthine nutraceutical, exerts dopaminergic neuroprotection. Neurochem. Int. 2021, 148, 105066. [Google Scholar] [CrossRef]
- Di Martino, E.; Bocchetta, E.; Tsuji, S.; Mukai, T.; Harris, R.A.; Blomgren, K.; Ådén, U. Defining a Time Window for Neuroprotection and Glia Modulation by Caffeine after Neonatal Hypoxia-Ischaemia. Mol. Neurobiol. 2020, 57, 2194–2205. [Google Scholar] [CrossRef]
- Soontarapornchai, K.; Cai, C.L.; Ahmad, T.; Aranda, J.V.; Hand, I.; Beharry, K.D. Pharmacodynamic Effects of Standard versus High Caffeine Doses in the Developing Brain of Neonatal Rats Exposed to Intermittent Hypoxia. Int. J. Mol. Sci. 2021, 22, 3473. [Google Scholar] [CrossRef]
- Garcez, M.L.; Damiani, A.P.; Pacheco, R.; Rodrigues, L.; de Abreu, L.L.; Alves, M.C.; de Andrade, V.M.; Boeck, C.R. Caffeine Neuroprotection Decreases A2A Adenosine Receptor Content in Aged Mice. Neurochem. Res. 2019, 44, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Badshah, H.; Ikram, M.; Ali, W.; Ahmad, S.; Hahm, J.R.; Kim, M.O. Caffeine May Abrogate LPS-Induced Oxidative Stress and Neuroinflammation by Regulating Nrf2/TLR4 in Adult Mouse Brains. Biomolecules 2019, 9, 719. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.M.N.; Skoug, C.; Silva, H.B.; Carvalho, R.A.; Gruetter, R.; Cunha, R.A. Impact of Caffeine Consumption on Type 2 Diabetes-Induced Spatial Memory Impairment and Neurochemical Alterations in the Hippocampus. Front. Neurosci. 2019, 12, 1015. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.L.; Arantes, L.P.; da Silveira, T.L.; Zamberlan, D.C.; Cordeiro, L.M.; Obetine, F.B.B.; da Silva, A.F.; da Cruz, I.B.M.; Soares, F.A.A.; Oliveira, R.d.P. Ilex paraguariensis extract provides increased resistance against oxidative stress and protection against Amyloid beta-induced toxicity compared to caffeine in Caenorhabditis elegans. Nutr. Neurosci. 2021, 24, 697–709. [Google Scholar] [CrossRef]
- Sharma, K.; Fallon, S.J.; Davis, T.; Ankrett, S.; Munro, G.; Christopher, G.; Coulthard, E. Caffeine and attentional control: Improved and impaired performance in healthy older adults and Parkinson’s disease according to task demands. Psychopharmacology 2022, 239, 605–619. [Google Scholar] [CrossRef]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Turnbull, D.; Rodricks, J.V.; Mariano, G.F.; Chowdhury, F. Caffeine and cardiovascular health. Regul. Toxicol. Pharmacol. 2017, 89, 165–185. [Google Scholar] [CrossRef]
- Zhou, A.; Hyppönen, E. Long-term coffee consumption, caffeine metabolism genetics, and risk of cardiovascular disease: A prospective analysis of up to 347,077 individuals and 8368 cases. Am. J. Clin. Nutr. 2019, 109, 509–516. [Google Scholar] [CrossRef]
- Zhou, A.; Hyppönen, E. Habitual coffee intake and plasma lipid profile: Evidence from UK Biobank. Clin. Nutr. 2021, 40, 4404–4413. [Google Scholar] [CrossRef]
- Ruggiero, E.; Di Castelnuovo, A.; Costanzo, S.; Persichillo, M.; De Curtis, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; Bonaccio, M.; et al. Daily Coffee Drinking Is Associated with Lower Risks of Cardiovascular and Total Mortality in a General Italian Population: Results from the Moli-sani Study. J. Nutr. 2020, 151, 395–404. [Google Scholar] [CrossRef]
- Said, M.A.; Vegte, Y.J.v.d.; Verweij, N.; Harst, P.v.d. Associations of Observational and Genetically Determined Caffeine Intake with Coronary Artery Disease and Diabetes Mellitus. J. Am. Heart Assoc. 2020, 9, e016808. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, J.; Jose, M.; Seo, Y.; Feng, L.; Ge, S. Association between Caffeine Intake and All-Cause and Cause-Specific Mortality: An Analysis of the National Health and Nutrition Examination Survey (NHANES) 1999–2014 Database. Nurs. Rep. 2021, 11, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Del Giorno, R.; Scanzio, S.; De Napoli, E.; Stefanelli, K.; Gabutti, S.; Troiani, C.; Gabutti, L. Habitual coffee and caffeinated beverages consumption is inversely associated with arterial stiffness and central and peripheral blood pressure. Int. J. Food Sci. Nutr. 2022, 73, 106–115. [Google Scholar] [CrossRef]
- D’Elia, L.; La Fata, E.; Galletti, F.; Scalfi, L.; Strazzullo, P. Coffee consumption and risk of hypertension: A dose–response meta-analysis of prospective studies. Eur. J. Nutr. 2019, 58, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Ngueta, G. Caffeine and caffeine metabolites in relation to hypertension in U.S. adults. Eur. J. Clin. Nutr. 2020, 74, 77–86. [Google Scholar] [CrossRef]
- Crooks, E.; Hansen, D.A.; Satterfield, B.C.; Layton, M.E.; Van Dongen, H.P.A. Cardiac autonomic activity during sleep deprivation with and without caffeine administration. Phys. Behav. 2019, 210, 112643. [Google Scholar] [CrossRef]
- Tripathi, M.; Singh, B.K.; Liehn, E.A.; Lim, S.Y.; Tikno, K.; Castano-Mayan, D.; Rattanasopa, C.; Nilcham, P.; Abdul Ghani, S.A.B.; Wu, Z.; et al. Caffeine prevents restenosis and inhibits vascular smooth muscle cell proliferation through the induction of autophagy. Autophagy 2022, 18, 2150–2160. [Google Scholar] [CrossRef]
- Subendran, S.; Wang, Y.-C.; Lu, Y.-H.; Chen, C.-Y. The evaluation of zebrafish cardiovascular and behavioral functions through microfluidics. Sci. Rep. 2021, 11, 13801. [Google Scholar] [CrossRef]
- Ferreira, R.E.S.; Pacheco, R.L.; de Oliveira Cruz Latorraca, C.; Riera, R.; Eid, R.G.; Martimbianco, A.L.C. Effects of Caffeine Supplementation on Physical Performance of Soccer Players: Systematic Review and Meta-Analysis. Sports Health 2021, 13, 347–358. [Google Scholar] [CrossRef]
- Grgic, J. Exploring the minimum ergogenic dose of caffeine on resistance exercise performance: A meta-analytic approach. Nutrition 2022, 97, 111604. [Google Scholar] [CrossRef]
- Martins, G.L.; Guilherme, J.; Ferreira, L.H.B.; de Souza-Junior, T.P.; Lancha, A.H., Jr. Caffeine and Exercise Performance: Possible Directions for Definitive Findings. Front. Sports Act. Living 2020, 2, 574854. [Google Scholar] [CrossRef]
- Trexler, E.T.; Smith-Ryan, A.E.; Roelofs, E.J.; Hirsch, K.R.; Mock, M.G. Effects of coffee and caffeine anhydrous on strength and sprint performance. Eur. J. Sport Sci. 2016, 16, 702–710. [Google Scholar] [CrossRef]
- Chieng, D.; Kistler, P.M. Coffee and tea on cardiovascular disease (CVD) prevention. Trends Cardiovasc. Med. 2022, 32, 399–405. [Google Scholar] [CrossRef]
- Grgic, J.; Grgic, I.; Pickering, C.; Schoenfeld, B.J.; Bishop, D.J.; Pedisic, Z. Wake up and smell the coffee: Caffeine supplementation and exercise performance-an umbrella review of 21 published meta-analyses. Br. J. Sports Med. 2020, 54, 681–688. [Google Scholar] [CrossRef]
- Burke, L.M. Caffeine and sports performance. Appl. Phys. Nutr. Metab. 2008, 33, 1319–1334. [Google Scholar] [CrossRef] [PubMed]
- Marcou, J.; Savva, R.-M. Does Caffeine Enhance Athletic Performance? Arab J. Nutr. Exerc. 2017, 1, 56–62. [Google Scholar] [CrossRef]
- Pickering, C.; Kiely, J. What Should We Do about Habitual Caffeine Use in Athletes? Sports Med. 2019, 49, 833–842. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A. Caffeine and creatine use in sport. Ann. Nutr. Metab. 2010, 57 (Suppl. S2), 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kamimori, G.H.; Karyekar, C.S.; Otterstetter, R.; Cox, D.S.; Balkin, T.J.; Belenky, G.L.; Eddington, N.D. The rate of absorption and relative bioavailability of caffeine administered in chewing gum versus capsules to normal healthy volunteers. Int. J. Pharm. 2002, 234, 159–167. [Google Scholar] [CrossRef]
- Grgic, J. Effects of Caffeine on Resistance Exercise: A Review of Recent Research. Sports Med. 2021, 51, 2281–2298. [Google Scholar] [CrossRef] [PubMed]
- Guest, N.S.; Van Dusseldorp, T.A.; Nelson, M.T.; Grgic, J.; Schoenfeld, B.J.; Jenkins, N.D.M.; Arent, S.M.; Antonio, J.; Stout, J.R.; Trexler, E.T.; et al. International society of sports nutrition position stand: Caffeine and exercise performance. J. Int. Soc. Sports Nutr. 2021, 18, 1. [Google Scholar] [CrossRef]
- Lara, B.; Gutiérrez-Hellín, J.; García-Bataller, A.; Rodríguez-Fernández, P.; Romero-Moraleda, B.; Del Coso, J. Ergogenic effects of caffeine on peak aerobic cycling power during the menstrual cycle. Eur. J. Nutr. 2020, 59, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.S.; Painelli, V.S.; Yamaguchi, G.; Oliveira, L.F.; Saunders, B.; da Silva, R.P.; Maciel, E.; Artioli, G.G.; Roschel, H.; Gualano, B. Dispelling the myth that habitual caffeine consumption influences the performance response to acute caffeine supplementation. J. Appl. Phys. 2017, 123, 213–220. [Google Scholar] [CrossRef]
- Aboumanei, M.H.; Mahmoud, A.F. Design and development of a proniosomal transdermal drug delivery system of caffeine for management of migraine: In vitro characterization, 131I-radiolabeling and in vivo biodistribution studies. Process Biochem. 2020, 97, 201–212. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Komes, D.; Karlović, S.; Djaković, S.; Špoljarić, I.; Mršić, G.; Ježek, D. Improving the controlled delivery formulations of caffeine in alginate hydrogel beads combined with pectin, carrageenan, chitosan and psyllium. Food Chem. 2015, 167, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bhaskar, L.V.K.S.; Nair, M.P.; Verma, H.K. Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. J. Drug Deliv. Sci. Technol. 2021, 63, 102487. [Google Scholar] [CrossRef]
- Shaddel, R.; Akbari-Alavijeh, S.; Cacciotti, I.; Yousefi, S.; Tomas, M.; Capanoglu, E.; Tarhan, O.; Rashidinejad, A.; Rezaei, A.; Bhia, M.; et al. Caffeine-loaded nano/micro-carriers: Techniques, bioavailability, and applications. Crit. Rev. Food Sci. Nutr. 2022, 1–26. [Google Scholar] [CrossRef]
- Dali, P.; Shende, P. Self-Assembled Lipid Polymer Hybrid Nanoparticles Using Combinational Drugs for Migraine via Intranasal Route. AAPS PharmSciTech 2022, 24, 20. [Google Scholar] [CrossRef]
- Khater, D.; Nsairat, H.; Odeh, F.; Saleh, M.; Jaber, A.; Alshaer, W.; Al Bawab, A.; Mubarak, M.S. Design, Preparation, and Characterization of Effective Dermal and Transdermal Lipid Nanoparticles: A Review. Cosmetics 2021, 8, 39. [Google Scholar] [CrossRef]
- Sakdiset, P.; Okada, A.; Todo, H.; Sugibayashi, K. Selection of phospholipids to design liposome preparations with high skin penetration-enhancing effects. J. Drug Deliv. Sci. Technol. 2018, 44, 58–64. [Google Scholar] [CrossRef]
- Kalvodová, A.; Zbytovská, J. Lipid nanocapsules enhance the transdermal delivery of drugs regardless of their physico-chemical properties. Int. J. Pharm. 2022, 628, 122264. [Google Scholar] [CrossRef]
- Abd, E.; Gomes, J.; Sales, C.C.; Yousef, S.; Forouz, F.; Telaprolu, K.C.; Roberts, M.S.; Grice, J.E.; Lopes, P.S.; Leite-Silva, V.R.; et al. Deformable liposomes as enhancer of caffeine penetration through human skin in a Franz diffusion cell test. Int. J. Cosmet. Sci. 2021, 43, 1–10. [Google Scholar] [CrossRef]
- Amasya, G.; Ozturk, C.; Aksu, B.; Tarimci, N. QbD based formulation optimization of semi-solid lipid nanoparticles as nano-cosmeceuticals. J. Drug Deliv. Sci. Technol. 2021, 66, 102737. [Google Scholar] [CrossRef]
- Ramezani, V.; Honarvar, M.; Seyedabadi, M.; Karimollah, A.; Ranjbar, A.M.; Hashemi, M. Formulation and optimization of transfersome containing minoxidil and caffeine. J. Drug Deliv. Sci. Technol. 2018, 44, 129–135. [Google Scholar] [CrossRef]
- Völker, J.M.; Koch, N.; Becker, M.; Klenk, A. Caffeine and Its Pharmacological Benefits in the Management of Androgenetic Alopecia: A Review. Skin Pharm. Phys. 2020, 33, 153–169. [Google Scholar] [CrossRef]
- Abd, E.; Benson, H.A.E.; Roberts, M.S.; Grice, J.E. Follicular Penetration of Caffeine from Topically Applied Nanoemulsion Formulations Containing Penetration Enhancers: In vitro Human Skin Studies. Skin Pharm. Phys. 2018, 31, 252–260. [Google Scholar] [CrossRef]
- Liu, T.-I.; Tsai, Y.-C.; Wang, T.-M.; Chang, S.-H.; Yang, Y.-C.; Chen, H.-H.; Chiu, H.-C. Development of a nano-immunomodulator encapsulating R837 and caffeine for combined radio-/immunotherapy against orthotopic breast cancer. Prog. Nat. Sci. Mater. Int. 2020, 30, 697–706. [Google Scholar] [CrossRef]
- Chen, P.-R.; Chuang, Y.-J. Study of Caffeine-Loaded Gelatin Nanoparticles for Treatment of Melanoma and Fibroblast Cells. Lett. Appl. NanoBioScience 2020, 11, 4243–4254. [Google Scholar] [CrossRef]
- Gajare, S.P.; Bansode, P.A.; Patil, P.V.; Patil, A.D.; Pore, D.M.; Sonawane, K.D.; Dhanavade, M.J.; Khot, V.M.; Rashinkar, G.S. Anticancer, Antibacterial and Hyperthermia Studies of a Caffeine-Based N-Heterocyclic Carbene Silver Complex Anchored on Magnetic Nanoparticles. ChemistrySelect 2021, 6, 1958–1968. [Google Scholar] [CrossRef]
- Khan, F.; Park, S.-K.; Bamunuarachchi, N.I.; Oh, D.; Kim, Y.-M. Caffeine-loaded gold nanoparticles: Antibiofilm and anti-persister activities against pathogenic bacteria. Appl. Microbiol. Biotechnol. 2021, 105, 3717–3731. [Google Scholar] [CrossRef]
- Hansen, S.E.; Marxen, E.; Janfelt, C.; Jacobsen, J. Buccal delivery of small molecules—Impact of levulinic acid, oleic acid, sodium dodecyl sulfate and hypotonicity on ex vivo permeability and spatial distribution in mucosa. Eur. J. Pharm. Biopharm. 2018, 133, 250–257. [Google Scholar] [CrossRef]
- Elmotasem, H.; Farag, H.K.; Salama, A.A.A. In vitro and in vivo evaluation of an oral sustained release hepatoprotective caffeine loaded w/o Pickering emulsion formula—Containing wheat germ oil and stabilized by magnesium oxide nanoparticles. Int. J. Pharm. 2018, 547, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Calheiros, T.F.; Furtado, L.M.; Carmona-Ribeiro, A.M.; Ando, R.A.; Petri, D.F.S. Physicochemical and antifungal properties of waterborne polymer nanoparticles synthesized with caffeine. Coll. Polym. Sci. 2020, 298, 341–353. [Google Scholar] [CrossRef]
- Barbasz, A.; Czyżowska, A.; Piergies, N.; Oćwieja, M. Design cytotoxicity: The effect of silver nanoparticles stabilized by selected antioxidants on melanoma cells. J. Appl. Toxicol. 2022, 42, 570–587. [Google Scholar] [CrossRef] [PubMed]
- Breuckmann, P.; Meinke, M.C.; Jaenicke, T.; Krutmann, J.; Rasulev, U.; Keck, C.M.; Müller, R.H.; Klein, A.L.; Lademann, J.; Patzelt, A. Influence of nanocrystal size on the in vivo absorption kinetics of caffeine after topical application. Eur. J. Pharm. Biopharm. 2021, 167, 57–64. [Google Scholar] [CrossRef]
| Source | Volume (mL) | Caffeine Range (mg) | References |
|---|---|---|---|
| Coffee | |||
| Americano coffee | 100.0 | 91.7–213.3 | [42] |
| Decaffeinated coffee | 500.0 | 0.0–13.9 | [43] |
| Instant coffee | 125.0 | 8.7–120.0 | [31,41,42,44] |
| Plain coffee | 200.0 | 68.4–136.9 | [42] |
| Scotland espresso | 13.0–90.0 | 66.0–276.0 | [31] |
| Italy espresso | 13.0–31.0 | 54.0–150.0 | [31] |
| Spain espresso | 34.0–104.0 | 82.0–139.0 | [31] |
| Tea | |||
| Black tea | 236.0 | 42.0 | [41] |
| Green tea | 236.0 | 18.0 | [41] |
| Yerba Mate | 236.0 | 40.0 | [41] |
| Soft drinks | |||
| Coca-Cola classic | 354.0 | 34.0 | [41] |
| Coca-Cola Energy | 354.0 | 38.0 | [41] |
| Diet Coke | 354.0 | 46.0 | [41] |
| Pepsi | 354.0 | 38.0 | [41] |
| Mountain Dew | 354.0 | 54.0 | [41] |
| Mountain Dew Rise | 473.0 | 180.0 | [41] |
| Ski | 354.0 | 69.0 | [41] |
| Sunkist | 354.0 | 19.0 | [41] |
| Energy drinks | |||
| Mountain Dew Amp | 473.0 | 142.0 | [41] |
| Full Throttle | 473.0 | 160.0 | [41] |
| Monster Dragon Tea | 680.0 | 60.0 | [41] |
| Java Monster 300 | 443.0 | 300.0 | [41] |
| Red Bull | 250.0 | 80.0 | [41] |
| Rockstar Boom | 473.0 | 160.0 | [41] |
| Rockstar XDurance | 473.0 | 300.0 | [41] |
| Juice | |||
| Cran Energy | 295.0 | 70.0 | [41] |
| Energy shots | |||
| Bang Shot | 88.0 | 300.0 | [41] |
| 5-Hour Energy | 57.0 | 200.0 | [41] |
| TruBrain Extra | 29.0 | 100.0 | [41] |
| Spike Energy Double Shot | 125.0 | 350.0 | [41] |
| Other beverages | |||
| Water Joe | 591.0 | 70.0 | [41,45] |
| Chocolate | |||
| Dark chocolate | 10.0 * | 8.0 | [41] |
| Guarana | 1.0 * | 47.0 | [41] |
| Target Cancer | Study Type | Model | Caffeine Exposure | Result | Reference |
|---|---|---|---|---|---|
| Breast | In vitro | MCF-7 and MDA-MB-231cells | 1–10 mM | Caffeine reduced the cell viability in concentrations greater than 2.5 mM for MCF7 and for 5 and 10 mM for MDA-MB-231 cell lines. At the latter concentrations, caffeine induces apoptosis and necrosis in both cell lines. | [57] |
| Breast | In vitro | MDA-MB-231, MCF7 and MCF10A cells | 0.000125 mM | After MDA-MB-231 and MCF7 cells’ treatment with caffeine, there was a change in metabolism towards respiratory-chain phosphorylation with low ratio of free to bound NADH. In combination with cisplatin, there was a decrease in viability and preference of cancer cells over normal breast cells. | [73] |
| Breast and colon | In vitro | HCT116 and MCF7 cells | 0–60 mM | Apoptosis increased in both proliferative and senescent cells after treatment with caffeine at a concentration of 15 mM. | [56] |
| Carcinoma squamous cells | In vitro | HN5 and KYSE30 cells | 0.5–70 mM | Caffeine at concentrations of 20, 50, and 70 mM presented an inhibitory effect and decreased the proliferation rate of both cell lines. | [74] |
| Endometrial | In vitro | RL95-2, HEC-1-A and KLE cells | 0–40 mM | Therapeutic concentration of cisplatin decreased from 4.1 to 1.1 µM and from 163 to 6.6 µM, with caffeine concentrations of 1.1 and 5.3 mM, respectively. | [70] |
| Glioblastoma multiforme | In vitro | Human GBM and U87-MG cells | 1 mM | Pre-treatment of cells with caffeine followed by combined treatment of temozolomide and caffeine significantly decreased cell viability compared to the other groups. | [69] |
| Glioblastoma multiforme | In vitro | Human GBM, U87MG and T98G 101 cells | 0.5–10 mM | In both cell lines, caffeine at 2.5 mM was able to reduce cellular viability, which was more pronounced under hypoxia. | [59] |
| Lung | In vitro | NCI-H23 and MLC15 cells | 0–0.5 mM | After of NCl-H23 cells’ treatment with 0.25 and 0.50 mM caffeine, the size of colonies decreased by 78.1% and 63.9%, respectively. In addition, caffeine induced cell arrest in the G0/G1 phase, reduced the S phase of the cell cycle, and suppressed cell invasion. | [75] |
| Melanoma | In vitro | Normal human melanocytes COLO829 and C32 cells | 100–1000 mM | The results showed the ability of caffeine to reduce the viability of COLO829 and C32 cells by 5–35% and 1–16%, respectively. In addition, it also led to a decrease in thiol degradation and pro-apoptotic effects and did not affect normal melanocytes cells. | [60] |
| Melanoma | In vitro | B16F10 cells | 0.001–0.04 mM | Cells’ pre-treatment with caffeine enhanced the cytotoxic effects induced by dacarbazine. In addition, caffeine increased oxidative stress in a dose-dependent manner. | [58] |
| Pancreatic ductal adenocarcinoma | In vitro | AsPC-1, BxPC-3, Capan-1, COLO-357, MiaPaCa-2, SU.86.86, PANC-1, and T3M4 pancreatic cancer cells | 0.1, 0.2 mM | Caffeine enhanced cell death induced by 5-fluorouracil and gemcitabine, and also decreased the IC50 of both chemotherapeutic agents. | [71] |
| Prostate | In vitro | PC-3 cells | 0.5 mM | Caffeine affected cell viability in a dose-dependent manner. Cell migration and invasion ability was more affected by the combination of atorvastatin and caffeine than by caffeine alone. The same was observed for the formation of tumor spheres. | [76] |
| Glioma | In vitro and in vivo | RT2 cells-induced glioma in male Fischer 344 inbred rat | 100 mg/kg/day orally (2 weeks) plus temozolomide given once daily (5 days) | The combination of caffeine with temozolomide inhibited tumor growth compared to the control group. | [68] |
| Hepatocellular carcinoma | In vitro and in vivo | SMMC-7721 and Hep3 cell lines and Male BALB/c nude mice | 0–32 mM (in vitro) 20 mg/kg/day injected IP every other day for (2 weeks) | Caffeine decreased the viability of both cell lines and had a synergistic effect with 5-fluorouracil. In addition, tumor growth was suppressed, and tumor weight was reduced in mice treated with caffeine alone or in combination with 5-fluorouracil. | [77] |
| Osteosarcoma, fibrosarcoma | In vitro and in vivo | HOS, HT1080 and LM8 cells and athymic nude mice | 0.5 mM (in vitro) 100 mg/kg injected IP on days 2 to 4 to the treatment (1 week). The treatment was performed two times. | The combination of cisplatin and caffeine decreased cell viability compared with cisplatin alone. In vivo, after implantation of LM8 and HT1080 cells, the combination of cisplatin + caffeine decreased tumor volume and weight. | [78] |
| Pleomorphic rhabdomyosarcoma | In vitro and in vivo | RMS cells, Athymic nu/nu nude mice | 0.5 and 1 mM (in vitro) 100 mg/kg/day injected IP daily (3 weeks) | Caffeine showed the ability to enhance the antiproliferative effects of valproic acid. In vivo, the group treated with caffeine and valproic acid showed a reduction in tumor volume compared to the control group. This was also confirmed in the group treated with Salmonella typhimurium A1 receptor in combination with caffeine and valproic acid. | [79] |
| Renal cell carcinoma | In silico, in vitro, and in vivo | ACHN and 786-O cells, and BALB/c nude mice | 0–0.016 mM intragastrically administered for 34 consecutive days | The molecular docking studies demonstrated that caffeine was able to bind to G6PDH at the NADP+ binding site, which is a biomarker and potential therapeutic target for renal cell carcinoma. In addition, caffeine was able to decrease the viability and proliferation of both cell lines and in the in vivo studies. | [64] |
| Colorectal | In vivo and in silico | Swiss Webster mice | 50 mg/kg/day, intragastrically 5 times a week (10 weeks) | Mice treated with caffeine alone or in combination with chlorogenic acid decreased the expression of IL-6, IL-17, and TNF-α. | [80] |
| Fibrosarcoma | In vivo | Adult albino mice | 1.030, 2.060 and 4.120 mM in drinking water administered daily (8 weeks) | In caffeine-treated mice, tumor incidence, size, and growth rate decreased with the increase in caffeine concentration. In addition, caffeine-treated mice had a higher percentage of cytotoxic T cells and higher TNF-α and IFN-γ levels. | [61] |
| Fibrosarcoma | In vivo | Adult Syrian golden hamsters | 100 mg/kg/day, intragastrical administration; treatment started 3 days before inoculation with sarcoma cells and continued for 14 days | Administration of metformin and caffeine resulted in inhibition of fibrosarcoma growth. | [81] |
| Melanoma | In vivo | Albino mice and C57BL/6J mice | 4.120 mM daily in drinking water (3 or 6 weeks) | In the carcinogen-induced tumor model, the groups treated with caffeine alone decreased the tumor growth rate from 5.3 mm2/day to 2.6 mm2/day. The combination with anti-PD1 led to a more pronounced decrease (0.9 mm2/day). | [82] |
| Osteosarcoma | In vivo | Athymic nu/nu nude mice | 100 kg/kg/day, orally administered for 14 consecutive days | The osteosarcoma mice model (patient-derived orthotopic xenograft) treated with cisplatinum + oral recombinant methioninase + caffeine, showed the most marked decrease in comparison to the other groups. | [83] |
| Synovial sarcoma | In vivo | Athymic nu/nu nude mice | 100 mg/kg/day, orally administered for 14 consecutive days | The combination of oral recombinant methioninase and caffeine reduced tumor volume. | [72] |
| Target/Disease | Study Type | Model | Caffeine Exposure | Result | Reference |
|---|---|---|---|---|---|
| Anti-inflammatory effect and immunomodulation | In vitro | Human peripheral blood mononuclear cells | 1.16 mM | Caffeine reduced the levels of several cytokines (IL-8, MIP-1β, IL-6, IFN-γ, GM-CSF, TNF-α, IL-2, IL-4, MCP-1, and IL-10. It also inhibited STAT1 signaling. | [109] |
| Bronchopulmonary dysplasia | In vitro | THP-1-derived macrophages | 100–800 μM | There was a decrease in NLRP3 inflammasome activation, ASC speck formation, and caspase 1 cleavage. In addition, IL-1β and IL-18 secretion decreased, as well as the phosphorylation of MAPK and NF-kB pathway members. | [110] |
| Immunomodulation | In vitro | Monocytes and macrophage | 300–1000 µM | Caffeine suppressed TNF-α and Akt signaling in both LPS-activated macrophage subtypes, inhibited STAT/IL-10 signaling in macrophage colony-stimulating factor, and significantly increased the expression of A2a and downregulated mTOR phosphorylation in M-macrophages. | [111] |
| Immunomodulation | In vitro | Mesenchymal stem cells and neutrophiles | 0.1–1 mM | Caffeine-treated mesenchymal stem cells produced fewer reactive oxygen species and increased phagocytosis of neutrophils co-cultured with mesenchymal stem cells. | [112] |
| Immunomodulation | In vitro | Mesenchymal stem cells and neutrophiles | 0.1–1 mM | Caffeine treatment increased the viability of co-cultured neutrophils. | [113] |
| Melanoma | In vitro and in silico | Mel1 and Mel3 cells | 1 and 2 mM | After caffeine treatment, there was a decrease in the levels of IL-1β, IP-10, macrophage inflammatory protein 1-α, and CCL4. On the other hand, the expression of regulated and normal T cells decreased in the Mel3 cell line. | [114] |
| Autoimmune encephalomyelitis | In vitro and in vivo | Primary microglia and BV2 cells C57BL/6 mice were immunized to induce autoimmune encephalomyelitis | 2 mM (in vitro) 10, 20 and 30 mg/kg/day in drinking water (30 days) after immunization with MOG35–55 | Caffeine decreased clinical score, inflammatory cell infiltration degree of the demyelination, and microglia stimulation in mice. In addition, it increased LC3-II/LC3-I levels and decreased NLRP3 and P62 levels. | [95] |
| Choroidal neovascularization | In vitro and in vivo | Laser photocoagulation C57BL/6j mice model | 200, 400 µM (in vitro); before laser photocoagulation (day 9): 20 mg/kg at day 0 and 10 mg/kg at day 1–4 and day 7 to 8; after laser photocoagulation: 10 mg/kg for 2 weeks (excluding weekends) | Significantly reduced the migration of retinal and choroidal endothelial cells (in vitro). Decreased choroidal neovascularization and inflammatory (mononuclear phagocytes) cells recruitment to the lesion area. | [97] |
| Depression | In vitro and in vivo | CBA × C57BL/6 F1 mice and syngeneic splenocytes | Transplantation (IV injection) with 15 × 106 splenocytes previously treated with 100 µg of caffeine for 25 min | Immune cells treated with caffeine and transplanted into depressive-like mice resulted in an increase in neuronal density and anti-inflammatory cytokines (IL-10 and IL-4) and a decrease in proinflammatory cytokines (IL-1β, INF-γ, and TNF-α). | [115] |
| Infection | In vitro and in vivo | Peritoneal macrophages and Swiss mice infected with L. Monocytogenes | 0.0257–25.7 μM (in vitro) 0.05, 0.5, 5 mg/Kg of caffeine IV injected 30 min after mice infection | In mice, the leucocyte infiltration in the peritoneal cavity decreased after caffeine treatment. In addition, mRNA expression of IL-1β, IL-6, and the enzyme inducible nitric oxide synthase were decreased, whereas IL-10 was increased. | [116] |
| Immunological and metabolic anomalies in obesity | In vitro and in vivo | Male Sprague-Dawley rat, RAW 264.7 macrophage and HepG2 cells | 50, 100, 150 mΜ (in vitro) High-fat-diet (6 weeks) induced hepatic steatosis mice were treated with 20 mg/kg/day by oral gavage (6 weeks) | In caffeine-treated mice, the profiles of TNF−α, MCP-1, IL-6, intercellular adhesion molecule, and nitrite were suppressed. In addition, live white adipose tissue and muscle macrophages and their cytokine levels also decreased. | [117] |
| Retinal inflammation | In vitro and in vivo | Ischemia reperfusion (I/R) injury mice model | 1–100 µM (in vitro); 10 µL at 97.8 mM instilled 60 min before and after I/R reperfusion, twice a day for 72 h | Caffeine reduced the secretion of IL-1β, IL-6, and TNF-α and restored the integrity of retinal cell monolayer (in vitro). Instilled caffeine reduced IL-6 mRNA levels and maintained BDNF physiological levels in the retina. | [100] |
| Rheumatoid arthritis | In vitro and in vivo | Mesenchymal stem cells and Wistar rats | 0–1 mM (in vitro); 14 days after rheumatoid arthritis induction, mice were injected IP with 2 × 106 cells previously treated with 0.5 mM caffeine for 48 h | Caffeine at a concentration of 0.5 mM promoted lower levels of cytokines, such as IFN-γ, IL-6, and IL-1β, and higher levels of IDO and TGF-β. In addition, cells treated with caffeine diminished the severity of rheumatoid arthritis in vivo and caused a decrease in serum levels of C-reactive protein, nitric oxide, myeloperoxidase, and TNF-α. | [96] |
| Cognitive impairment | In vivo | BALB/c mice | 0.025, 0.05, 0.1 mg of caffeine intranasally administered (10 µL) 1 day before ischemia-induced cognitive impairment in mice, and the next 7 consecutive days | Caffeine improved the behavior outcomes of ischemic mice and reduced the expression of proinflammatory biomarkers (TNF-α, IL-6) and increased the levels of anti-inflammatory cytokines (IL-10). | [118] |
| Hepatic fibrosis—antioxidant and anti-inflammatory | In vivo | Hepatic fibrosis Sprague Dawley rats | 50 mg/kg/day orally administered (8 weeks) | Decreased fibrosis and necro-inflammation; decreased LPAR1, TGF-β1, CTGF, α-SMA, and LPAR1 expression; improved liver function. | [119] |
| Hydrocephalus | In vivo | Kaolin-induced hydrocephalus mice neonates | 50 mg/kg/day of caffeine were administered to dams by gavage or water (21 days) and lactated the neonates | Administration of caffeine to dams reduced cell death and increased the neurons dendritic arborization in the sensorimotor cortex and striatum of the mice neonates and improved hydrocephalic deficits and behavioral development. | [120] |
| Immunomodulation and anti-inflammatory effect | In vivo | Nile tilapia | Diet containing 5 and 8% w/w (21 days) | Caffeine supplemented diet prevented alterations caused by hypoxia, such as ATP hydrolysis and consequent accumulation in the extracellular environment. | [121] |
| Inflammation and adenosinergic system in cerebellum | In vivo | Ethanol-induced inflammation in Wistar and UChB rats | 15.4 mM/day in 10% ethanol solution (55 days) | Caffeine reduced gene expression of A1 and A2a receptors and increased and reduced A1 and A2a protein levels, respectively, in the cerebellum. Caffeine also attenuated the inflammation, demonstrating a neuroprotective role. | [122] |
| Neuroinflammation | In vivo | Sprague Dawley rats | 60 mg/kg/day administered orally by gavage (2 days) | Caffeine/modafinil increased the levels of anti-inflammatory (IL-4 and IL-10) and decreased proinflammatory (TNF-α, IL-1β) cytokines in the hippocampus. Treatment decreased microglial immunoreactivity and improved inflammatory response and anxious behavior. | [123] |
| Neurotoxicity | In vivo | Tramadol-induced damage in cerebellum rat model | 37.5 mg/kg/day administered orally by gavage (21 days) | Caffeine upregulated autophagy-related genes and reduced the expression of inflammatory and apoptosis markers, demonstrating neuroprotective effects in the cerebellum. | [124] |
| Neurotoxicity—antioxidant and anti-inflammatory | In vivo | Albino rats | 20 mg/kg/day IP injected (30 days) | Caffeine reduced oxidative stress and restored TNF-α levels in cerebral tissues. | [125] |
| Oxygen-induced inflammatory lung injury | In vivo | Neonatal rats | 10 mg/kg IP injected every 48h (15 days) | Under hyperoxia, caffeine decreased pro-inflammatory mediators (TNF-α, IL-1α, IL-1β, IFN-γ) and NF-kB, and decreased infiltrating cells in the lung. Opposite effects were observed in normoxiaconditions. | [108] |
| Dental pain | Clinical Trial | Patients with acute postoperative dental pain | 100 mg (single dose) | Caffeine improved the effect of ibuprofen in the treatment of moderate postoperative dental pain. | [126] |
| Disease | Study Type | Model | Caffeine Exposure | Result | Reference |
|---|---|---|---|---|---|
| Parkinson’s | In silico | Molecular docking simulations | N/A | Caffeine was able to bind at position 28 in both wild-type and mutant parkin proteins. | [139] |
| Alzheimer’s | In silico | Molecular docking simulations | N/A | In the presence of caffeine, the distances between the inter-residual increased, leading to the breakdown of hydrophobic contacts, ultimately destabilizing the Aβ protofibrils. | [144] |
| Parkinson’s | In vitro | Transgenic Caenorhabditis elegans | 10 mM | Caffeine was able to prevent neuronal cell loss in 96% of dopaminergic neurons. | [138] |
| Alzheimer’s | In vitro | SHSY5Y cells | 0.6 and 1 mM | Both concentrations were able to reduce beta-amyloid neurotoxicity. | [145] |
| Alzheimer’s | In vitro | SH-SY5Y wild-type and N2a cells | 100 µM | In the presence of caffeine, the level of ADAM10 protein increased to 138.5 ± 9.2%, and the levels of APP protein level and ROS decreased to 85.4 ± 3.6% and 48.8 ± 3.2%, respectively. | [146] |
| Alzheimer’s | In vitro | HEK293 cells | 0.1–10 mM | Caffeine induces conformational changes in muscle nicotinic acetylcholine receptors, which are molecular targets of Alzheimer’s disease. | [147] |
| Synaptic transmission and plasticity | In vitro | Dorsal hippocampus slices of C57bl\6j mice and A2aR knockout mice | 50 μM | Caffeine increased synaptic transmission by 40%, decreased facilitation of paired pulse, and decreased the amplitude of long-term potentiation by 35%. | [15] |
| Cd-induced neurodegeneration | In vitro and in vivo | HT-22 and BV-2 cells and wild-type C57BL/6N male mice | 30 mg/kg/day IP injected (2 weeks) | Caffeine reduced ROS, lipid peroxidation and 8-dihydro-8-oxoguanine levels. It also attenuated neuronal loss, synaptic dysfunction, and learning and cognitive deficits. | [148] |
| Parkinson’s | In vivo | Swiss mice and Wistar rats | 31.2 mg/kg given orally by gavage | Caffeine administration reduced the catalepsy index and increased the number of ipsilateral rotations. | [149] |
| Hypoxic ischemia | In vivo | Sprague Dawley mice | 1.5 mM in drinking water until 16 postnatal days | Pre-treatment with caffeine reduced brain infarct after hypoxia ischemia and also restored brain activity. | [150] |
| Acetaminophen-induced neurotoxicity | In vivo | Swiss albino mice | 20 mg/kg IP injected 30 min after treatment with acetaminophen | Treatment with caffeine and acetaminophen reduced the formation of ROS compared with the acetaminophen group. In addition, the survival time of caffeine-treated mice increased by 33%. | [151] |
| Parkinson’s | In vivo | C57BL/6 mice with motor behavioral deficit induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine | 20 mg/kg/day, 7 days before MPTP-induced neurodegeneration and 7 days after | Caffeine improved behavioral and neurotransmitter recovery against the induced toxicity. It was also able to restore antioxidant levels and suppress neuroinflammation. | [152] |
| Hypoxic ischemia | In vivo | Wild-type C57/bl6 specific pathogen-free mice | 5 mg/kg IP injected (120 days) | Caffeine administration after hypoxic ischemic brain injury reduced lesions in the gray and white matter and the number of amoeboid microglia and apoptotic cells. The expression of pro-inflammatory cytokines also decreased. | [153] |
| Apnea of prematurity | In vivo | Infection-free pregnant Sprague Dawley rats | 20 mg/kg 1 day followed by 5 mg/kg/day over 14 days or 80 mg/kg 1 day followed by 20 mg/kg/day over 14 days, IP injected | Caffeine administration in normoxia reduced oxidative stress and hypermyelination, and increased Golgi bodies. Caffeine at standard and high doses could provide neuroprotective effects. | [154] |
| Parkinson’s | In vivo | C57BL/6 male mice | 5.1 mM in drinking water | Caffeine protected against synucleinopathy by modulating α-syn-induced apoptosis, microglial, and astrocytic activation in the striatum. | [142] |
| Neuroprotection | In vivo | Male Swiss mice | 1.5 mM in drinking water (4 weeks) | The number of A2a receptors was decreased in the hippocampus of mice that consumed caffeine. The aged mice treated with caffeine presented more pyknotic neurons in the hippocampus and reduced damage. | [155] |
| LPS-induced oxidative stress and neuroinflammation | In vivo | C57BL/6N male mice | 3 mg/kg/day IP injected (6 weeks) | The LPS-injected group had enhanced expression of Bax and caspase-3. On the other hand, these markers were reduced in the group treated with caffeine, and this treatment also caused a restoration of the synaptic markers. | [156] |
| Diabetes | In vivo | Male GK and Wistar–Hannover–Galas rats | 5.1 mM in drinking water (4 months) | Caffeine prevented the GFAP, vimentin, and SNAP25 alterations caused by diabetes, and also improved memory deficits. | [157] |
| Alzheimer’s | In vivo | Wild-type N2 and CL2006 worms | Worms were cultured in 200 and 400 μM caffeine-treated plates | The treatment prevented amyloid beta-peptide paralysis, decreased acetylcholinesterase activity, and decreased amyloid beta-peptide mRNA levels. | [158] |
| Parkinson’s | In vivo | C57BL/6J mice | 50 mg/kg/day in drinking water | The co-administration of caffeine and eicosanoyl-5-hydroxytryptamide resulted in decreased accumulation of phosphorylated α-synuclein, maintenance of neuronal integrity and function, reduction in neuroinflammation, and improvement in behavioral performance. | [143] |
| Parkinson’s | Clinical trial | Parkinson’s disease patients | 100 mg (single dose) | Caffeine treatment reduced the number of errors in patients and controls on the Stroop and Choice reaction time and enhanced dual item accuracy on the rapid visual serial presentation task. | [159] |
| Study Type | Model | Result | Reference |
|---|---|---|---|
| Systematic review | Review of prospective studies | Regular and moderate coffee consumption (1–2 cups/day) is not associated with hypertension risk. Higher coffee consumption has a protective effect. | [168] |
| Prospective | 347,077 volunteers (37–73 years old, UK Biobank) | Coffee consumption may lead to a slight increase in CVD risk. | [162] |
| Prospective | 2278 volunteers (18–80 years old) | Caffeine metabolites are responsible for lowering the risk of hypertension. | [169] |
| Prospective | 20,487 (35–94 years old) | Moderate coffee consumption (3–4 cups/day) has been associated with lower CVD mortality. | [164] |
| Prospective | >500,000 individuals (40–69 years old) | The consumption of 2–3 cups of coffee per day (121–182 mg caffeine/day) was associated with a low risk of coronary artery disease. | [165] |
| Prospective | 23,878 individuals (>20 years old) | Higher caffeine intake (>100 mg/day) was associated with lower CVD mortality. | [166] |
| Prospective | 362,571 individuals (37–73 years old, UK Biobank) | High coffee consumption (>6 cups/day) increases levels of low-density lipoprotein cholesterol, total cholesterol, and apolipoprotein B, thereby increasing the risk for CVD. | [163] |
| Prospective | 1095 individuals (mean age 53 ± 14 years old) | Moderate coffee consumption (>3 cups/day) reduces CVD risk factors such as arterial stiffness and high blood pressure | [167] |
| Randomized Controlled Trial | 12 volunteers (19–39 years old) | Administration of caffeine (200 mg, 12 h intervals) during sleep deprivation reduced HR and increased HF-HRV. The concentration effect was nonlinear. No significant interaction between sleep deprivation and caffeine intake | [170] |
| In vitro in vivo | Primary human and mouse aortic VSMCs, immortalized mouse aortic VSMCs; restenosis mice model (apoe−/−C57BL/6 J) | In vitro, caffeine (2 mM) induced autophagy by inhibiting mTOR signaling and decreased proliferation of VMCs by inhibiting WNT signaling. In vivo, caffeine at 2.57 mM (in drinking water, 2 weeks before and after injury) decreased vascular restenosis. | [171] |
| In vivo | Zebrafish | Caffeine (128 and 334 µM in zebrafish culture water) caused a similar decrease in HR. | [172] |
| Nanosystem | Method | Composition | Application | Model | Result | Reference |
|---|---|---|---|---|---|---|
| Lipid-based nanosystems | ||||||
| Liposomes | Thin-film hydration | Lecithin, polysorbate 80, polysorbate 20 | Alopecia | Wistar rats | Improves skin delivery, weight, and hair length. | [198] |
| Liposomes | Thin film hydration | Phospholipid, cholesterol | Skin drug delivery | Abdominal skin of WBN/ILA-Ht hairless rats | DPPG liposomes enhanced skin penetration by disrupting the lipidic barrier of stratum corneum. | [194] |
| Liposomes | High-pressure homogenization | Phosphatidylcholine, propylene glycol | Skin drug delivery | Full-thickness abdominal human skin | Propylene glycol increased liposome deformability and improved skin permeation of caffeine. | [196] |
| Lipidic nanosystems | High-pressure homogenization | Trilaurin, oleic acid, pluronic F68, imiquimod | Cancer | Orthotopic breast cancer mice model | Caffeine slightly improved antitumor activity. | [201] |
| Lipid nanocapsules | Phase inversion temperature | Miglyol 812 N, Kolliphor HS 15, Phospholipon 90G | Skin drug delivery | Porcine skin | Caffeine was not successfully encapsulated. Nanocapsules improved the transdermal permeation of caffeine. | [195] |
| Semi-solid nanostructured lipid carriers | Two-stage homogenization method, high shear homogenization, ultrasonication | Compritol® 888 ATO and Precirol® ATO 5, argan oil, Poloxamer 407 | Cosmetics, skin drug delivery | Wistar rat full-thickness dorsal skin | NLCs exhibited a high capacity for deposition and permeation through the skin. | [197] |
| Proniosomes | Coacervation phase separation | Cholesterol, span 60, lecithin | Brain delivery—migraine | Swiss albino mouse abdominal skin and albino rabbit ear | Increased caffeine permeation through the skin and caffeine levels in blood and brain compared to orally administered caffeine. No evidence of skin irritation. | [188] |
| Nanoemulsions | Low energy emulsification | Dicaprylyl ether, ethylhexyl isononanoate, potassium lauroyl wheat amino acids, palm glycerides and capryloyl glycine | Cosmetics, skin drug delivery | Abdominal human epidermis | Did not improve skin permeation of caffeine compared to emulsion. | [205] |
| Nanoemulsions | Low energy emulsification | Volpo-N10, oleic acid or eucalyptol | Skin drug delivery | Human full-thickness skin | Increased permeation and retention of caffeine in hair follicles and skin. | [200] |
| Pickering emulsions stabilized by magnesium oxide NPs | High shear homogenization | Wheat germ oil, magnesium oxide NPs | Oral drug delivery—hepatoprotective | Wistar rats intoxicated with CCl4 | Decreased proliferation of cancer cells, moderate reduction in oxidative stress and inflammatory markers, similar to caffeine solution. Increased catalase levels compared to caffeine. | [206] |
| Polymer-based nanosystems | ||||||
| Polymeric nanoparticles | Emulsion polymerization | Methyl methacrylate, CTAB or sodium dodecyl sulfate | Antifungal | C. albicans | CTAB–caffeine nanoparticles inhibited the growth of C. albicans. | [207] |
| Polymeric nanoparticles | Desolvation | Gelatin | Cancer | B16F10, L929 cell lines | Inhibited the proliferation of murine melanoma cells (B16F10) and induced apoptosis without causing cytotoxic effects on normal fibroblast cells (L929). | [202] |
| Metal-based nanosystems | ||||||
| Silver complexes anchored to magnetic NPs | Covalent conjugation and complexation | Chloro-functionalized Fe3O4 magnetic NPs, caffeine N-heterocyclic carbene-silver complex | Cancer | HepG2, WRL-68 cell lines; E. coli, P. aeruginosa, S. aureus, L. monocytogenes | Enhanced cytotoxic effects against HepG2 cells and antibacterial activity against E. coli, S. aureus and B. cereus. Hyperthermia studies showed that the nanosystems reached a temperature of 47 °C, which is suitable for anticancer applications | [203] |
| Silver nanoparticles | Chemical reduction | Silver nitrate, gallic acid, (-)-epicatechin-3-gallate or caffeine | Cancer | B16-F0, COLO 679 cell lines | EGCG- and caffeine-stabilized AgNPs were the most and less effective against the tested cancer cell lines. | [208] |
| Gold nanoparticles | Chemical reduction | Gold (III) chloride trihydrate | Antibacterial | E. coli, P. aeruginosa, S. aureus, L. monocytogenes | Inhibition of biofilm formation and removal of mature biofilms. Antibacterial activity against resistant pathogenic bacteria. | [204] |
| Crystal-based nanosystems | ||||||
| Nanocrystals | Pearl-milling | Carbopol® 981, propylene glycol | Skin drug delivery | Human volunteers, arm skin | Nanocrystals with a size of 694 nm showed a delayed, but higher and longer delivery of caffeine, being detected in serum for at least 5 days. | [209] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saraiva, S.M.; Jacinto, T.A.; Gonçalves, A.C.; Gaspar, D.; Silva, L.R. Overview of Caffeine Effects on Human Health and Emerging Delivery Strategies. Pharmaceuticals 2023, 16, 1067. https://doi.org/10.3390/ph16081067
Saraiva SM, Jacinto TA, Gonçalves AC, Gaspar D, Silva LR. Overview of Caffeine Effects on Human Health and Emerging Delivery Strategies. Pharmaceuticals. 2023; 16(8):1067. https://doi.org/10.3390/ph16081067
Chicago/Turabian StyleSaraiva, Sofia M., Telma A. Jacinto, Ana C. Gonçalves, Dário Gaspar, and Luís R. Silva. 2023. "Overview of Caffeine Effects on Human Health and Emerging Delivery Strategies" Pharmaceuticals 16, no. 8: 1067. https://doi.org/10.3390/ph16081067
APA StyleSaraiva, S. M., Jacinto, T. A., Gonçalves, A. C., Gaspar, D., & Silva, L. R. (2023). Overview of Caffeine Effects on Human Health and Emerging Delivery Strategies. Pharmaceuticals, 16(8), 1067. https://doi.org/10.3390/ph16081067









