HIV-1 Integrase Inhibition Activity by Spiroketals Derived from Plagius flosculosus, an Endemic Plant of Sardinia (Italy) and Corsica (France)
Abstract
1. Introduction
2. Results and Discussion
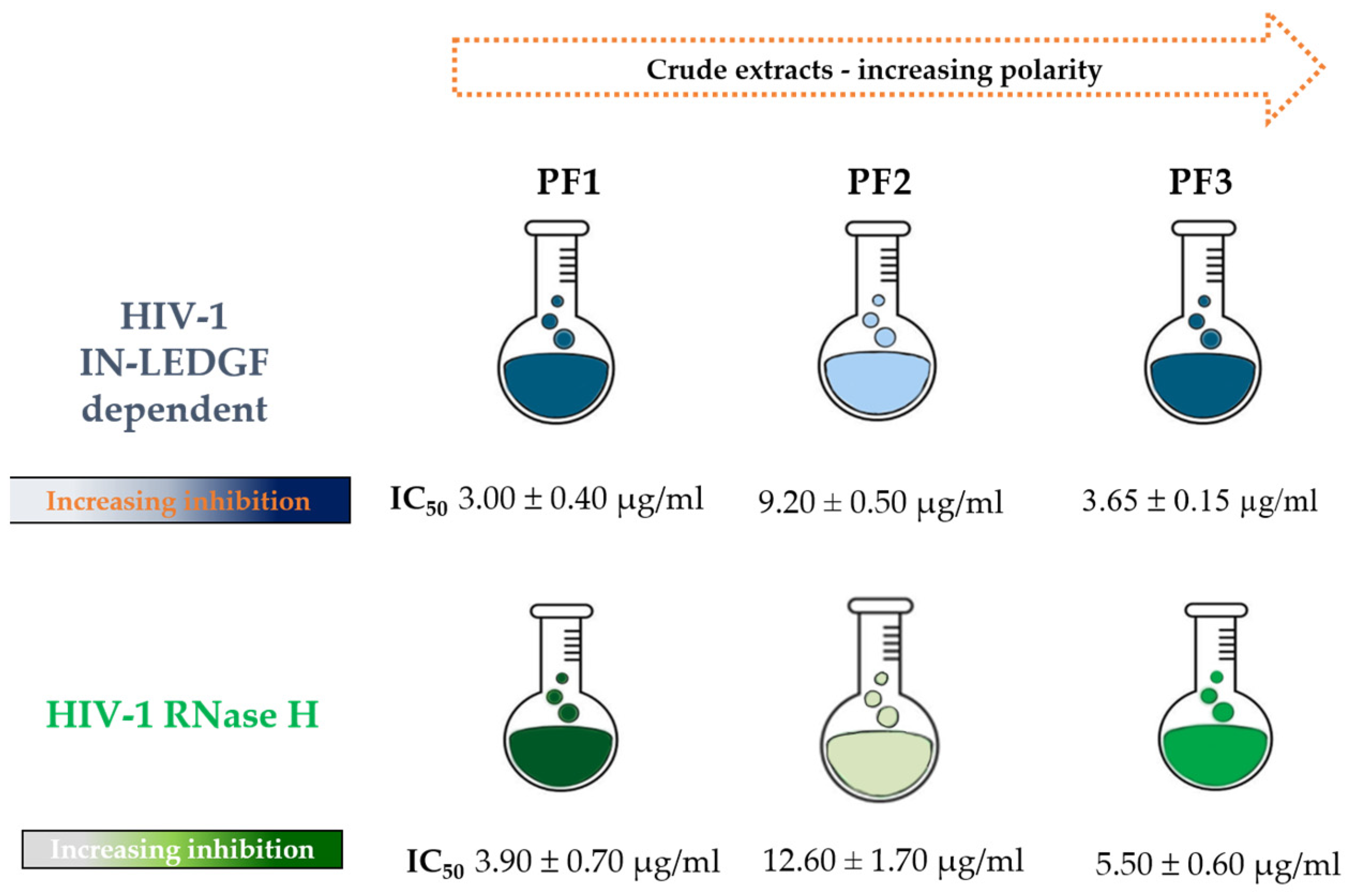
2.1. Effect of P. flosculosus Extracts on HIV-1 IN Activity in the Presence of LEDGF/p75 Cellular Cofactor
2.2. Effect of P. flosculosus Extracts on HIV-1 RT-Associated RNase H Activity
2.3. Chemical Characterization of Bioactive Crude Extract
2.4. Effects of Pure Compounds on HIV-1 IN Activity in the Presence of LEDGF/p75 Cellular Cofactor and on HIV-1 RNase H Activity
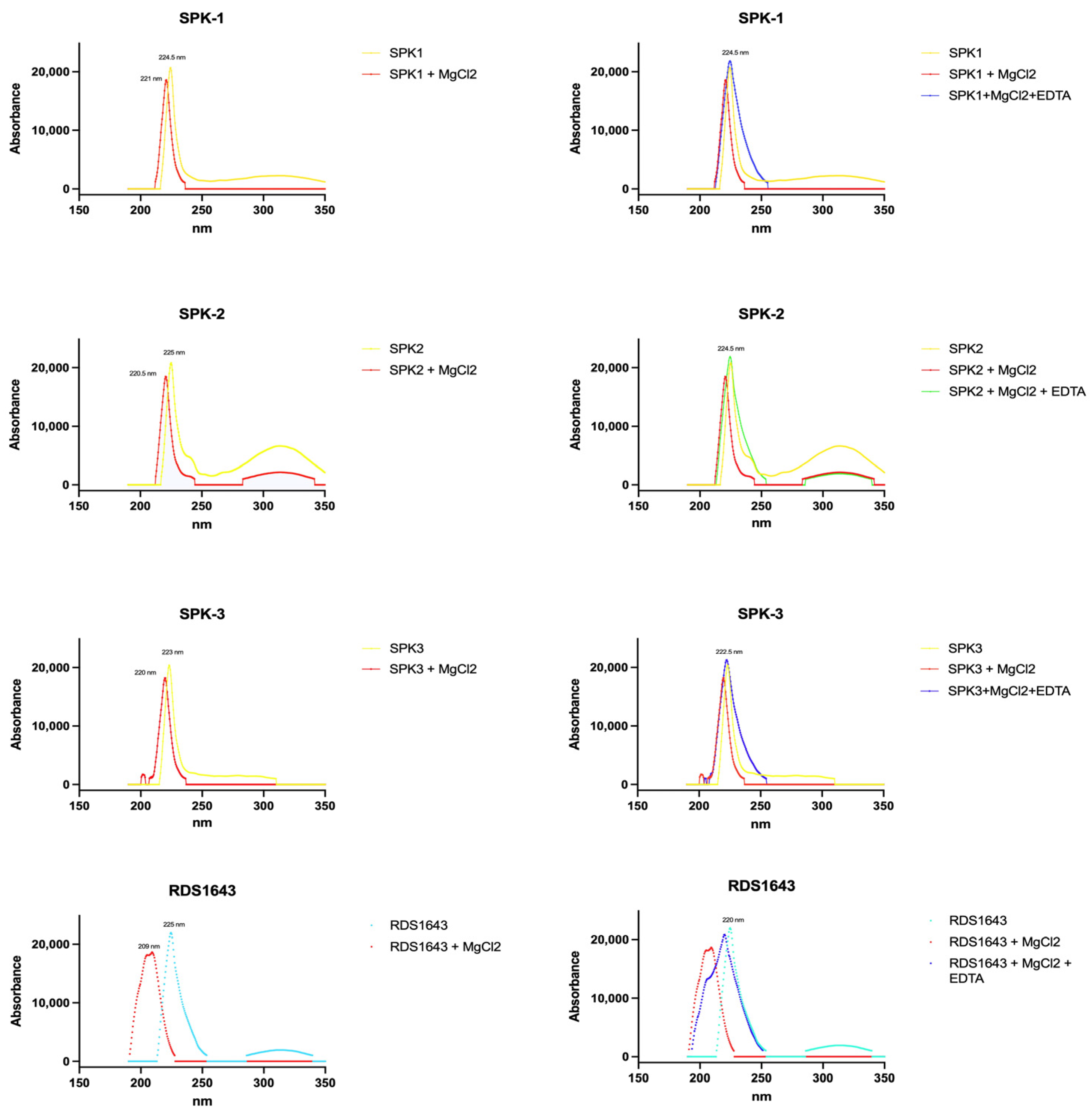
2.5. Pure Compounds’ Spectra in the Presence of Magnesium
2.6. Evaluation of Inhibition of Pure Compounds against HIV-1 IN Activities
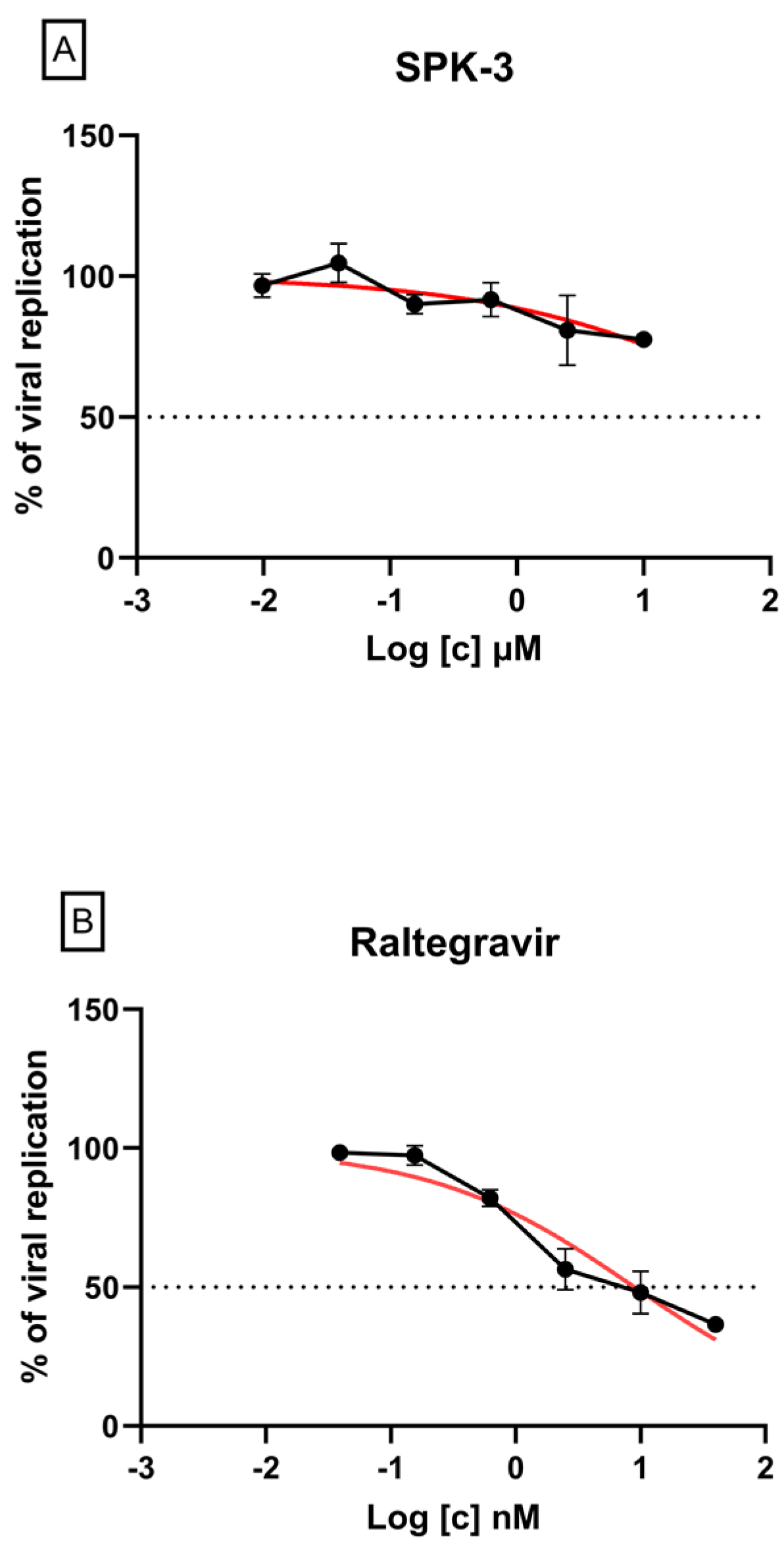
2.7. Inhibition of HIV-1 Replication in a Cell-Based Assay by SPK3
3. Materials and Methods
3.1. Plant Material and Sample Preparation
3.2. Preparation of Crude Extracts for Bioassay
3.3. Bioguided-Fractionation of the Active Extract and Compounds Purification
3.3.1. General Chromatographic Procedures
3.3.2. NMR Experiments
3.3.3. Compound Purification
3.4. Anti-HIV Biochemical Assays
3.4.1. Recombinant Proteins Preparation
3.4.2. HTRF LEDGF-Dependent and -Independent Assays
3.4.3. IN–LEDGF Binding Assay
3.4.4. IN–IN Binding Assay
3.4.5. RT-Associated RNase H Assay
3.4.6. Compound Spectra in the Presence of MgCl2
3.5. Cytotoxicity Assay
3.6. Determination of Anti-HIV-1 Activity in TZM-bl Cell Line
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fact Sheet—Latest Global and Regional Statistics on the Status of the AIDS Epidemic. UNAIDS. Available online: https://www.unaids.org/en/resources/documents/2022/UNAIDS_FactSheet (accessed on 3 April 2023).
- Hu, W.; Hughes, S.H. HIV-1 Reverse Transcription. Cold Spring Harb. Perspect. Med. 2012, 2, a006882. [Google Scholar] [CrossRef]
- Yang, W.E.I.; Steitz, T.A. Recombining the Structures of HIV Integrase, RuvC and RNase H The Recently Reported Crystal Structures of Two Recombination Enzymes, Endonuclease, Are Surprisingly Similar to That of Ribonuclease H Suggesting the Possibility That They Have a Common Enz. Structure 1995, 3, 131–134. [Google Scholar] [CrossRef]
- Delelis, O.; Carayon, K.; Saïb, A.; Deprez, E.; Mouscadet, J.F. Integrase and Integration: Biochemical Activities of HIV-1 Integrase. Retrovirology 2008, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Engelman, A.; Kessl, J.J.; Kvaratskhelia, M. Allosteric Inhibition of HIV-1 Integrase Activity. Curr. Opin. Chem. Biol. 2013, 17, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Rozman, M.; Zidovec-Lepej, S.; Jambrosic, K.; Babić, M.; Drmić Hofman, I. Role of TLRs in HIV-1 Infection and Potential of TLR Agonists in HIV-1 Vaccine Development and Treatment Strategies. Pathogens 2023, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Finzi, D.; Blankson, J.; Siliciano, J.D.; Margolick, J.B.; Chadwick, K.; Pierson, T.; Smith, K.; Lisziewicz, J.; Lori, F.; Flexner, C.; et al. Latent Infection of CD4+ T Cells Provides a Mechanism for Lifelong Persistence of HIV-1, Even in Patients on Effective Combination Therapy. Nat. Med. 1999, 5, 512–517. [Google Scholar] [CrossRef]
- Ndashimye, E.; Reyes, P.S.; Arts, E.J. New Antiretroviral Inhibitors and HIV-1 Drug Resistance: More Focus on 90% HIV-1 Isolates? FEMS Microbiol. Rev. 2023, 47, fuac040. [Google Scholar] [CrossRef]
- Esposito, F.; Ambrosio, F.A.; Maleddu, R.; Costa, G.; Rocca, R.; Maccioni, E.; Catalano, R.; Romeo, I.; Eleftheriou, P.; Karia, D.C.; et al. Chromenone Derivatives as a Versatile Scaffold with Dual Mode of Inhibition of HIV-1 Reverse Transcriptase-Associated Ribonuclease H Function and Integrase Activity. Eur. J. Med. Chem. 2019, 182, 111617. [Google Scholar] [CrossRef]
- Mahboubi-Rabbani, M.; Abbasi, M.; Hajimahdi, Z.; Zarghi, A. HIV-1 Reverse Transcriptase/Integrase Dual Inhibitors: A Review of Recent Advances and Structure-Activity Relationship Studies. Iran. J. Pharm. Res. 2021, 20, 333–369. [Google Scholar] [CrossRef]
- Métifiot, M.; Leon, O.; Tarrago-Litvak, L.; Litvak, S.; Andréola, M.L. Targeting HIV-1 Integrase with Aptamers Selected against the Purified RNase H Domain of HIV-1 RT. Biochimie 2005, 87, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Kessl, J.J.; Jena, N.; Koh, Y.; Taskent-Sezgin, H.; Slaughter, A.; Feng, L.; De Silva, S.; Wu, L.; Le Grice, S.F.J.; Engelman, A.; et al. Multimode, Cooperative Mechanism of Action of Allosteric HIV-1 Integrase Inhibitors. J. Biol. Chem. 2012, 287, 16801–16811. [Google Scholar] [CrossRef]
- Le Rouzic, E.; Bonnard, D.; Chasset, S.; Bruneau, J.M.; Chevreuil, F.; Le Strat, F.; Nguyen, J.; Beauvoir, R.; Amadori, C.; Brias, J.; et al. Dual Inhibition of HIV-1 Replication by Integrase-LEDGF Allosteric Inhibitors Is Predominant at the Post-Integration Stage. Retrovirology 2013, 10, 144. [Google Scholar] [CrossRef]
- Dinh, L.P.; Sun, J.; Glenn, C.D.; Patel, K.; Pigza, J.A.; Donahue, M.G.; Yet, L.; Kessl, J.J. Multi-Substituted Quinolines as HIV-1 Integrase Allosteric Inhibitors. Viruses 2022, 14, 1466. [Google Scholar] [CrossRef] [PubMed]
- Christ, F.; Voet, A.; Marchand, A.; Nicolet, S.; Desimmie, B.A.; Marchand, D.; Bardiot, D.; Van Der Veken, N.J.; Van Remoortel, B.; Strelkov, S.V.; et al. Rational Design of Small-Molecule Inhibitors of the LEDGF/P75-Integrase Interaction and HIV Replication. Nat. Chem. Biol. 2010, 6, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Slaughter, A.; Jena, N.; Feng, L.; Kessl, J.J.; Fadel, H.J.; Malani, N.; Male, F.; Wu, L.; Poeschla, E.; et al. A New Class of Multimerization Selective Inhibitors of HIV-1 Integrase. PLoS Pathog. 2014, 10, e1004171. [Google Scholar] [CrossRef]
- Ohata, Y.; Tomonaga, M.; Watanabe, Y.; Tomura, K.; Kimura, K.; Akaki, T.; Adachi, K.; Kodama, E.N.; Matsuzaki, Y.; Hayashi, H. Antiviral Activity and Resistance Profile of the Novel HIV-1 Non-Catalytic Site Integrase Inhibitor JTP-0157602. J. Virol. 2022, 96, e01843-21. [Google Scholar] [CrossRef] [PubMed]
- Parcella, K.; Wang, T.; Eastman, K.; Zhang, Z.; Yin, Z.; Patel, M.; Tu, Y.; Zheng, B.Z.; Walker, M.A.; Saulnier, M.G.; et al. Discovery and Preclinical Profiling of GSK3839919, a Potent HIV-1 Allosteric Integrase Inhibitor. ACS Med. Chem. Lett. 2022, 13, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Engelman, A.N.; Kvaratskhelia, M. Multimodal Functionalities of HIV-1 Integrase. Viruses 2022, 14, 926. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Maes, L.; Vlietinck, A.; Pieters, L. Plant-Derived Leading Compounds for Chemotherapy of Human Immunodeficiency Virus (HIV) Infection—An Update (1998–2007). Planta Med. 2008, 74, 1323–1337. [Google Scholar] [CrossRef]
- Sanna, C.; Maxia, A.; Fenu, G.; Loi, M.C. So Uncommon and so Singular, but Underexplored: An Updated Overview on Ethnobotanical Uses, Biological Properties and Phytoconstituents of Sardinian Endemic Plants. Plants 2020, 9, 958. [Google Scholar] [CrossRef]
- Guzzo, F.; Russo, R.; Sanna, C.; Celaj, O.; Caredda, A.; Corona, A.; Tramontano, E.; Fiorentino, A.; Esposito, F.; D’abrosca, B. Chemical Characterization and Anti-HIV-1 Activity Assessment of Iridoids and Flavonols from Scrophularia trifoliata. Molecules 2021, 26, 4777. [Google Scholar] [CrossRef] [PubMed]
- Sanna, C.; Scognamiglio, M.; Fiorentino, A.; Corona, A.; Graziani, V.; Caredda, A.; Cortis, P.; Montisci, M.; Ceresola, E.R.; Canducci, F.; et al. Prenylated Phloroglucinols from Hypericum scruglii, an Endemic Species of Sardinia (Italy), as New Dual HIV-1 Inhibitors Effective on HIV-1 Replication. PLoS ONE 2018, 12, e0195168. [Google Scholar] [CrossRef] [PubMed]
- Fois, M.; Farris, E.; Calvia, G.; Campus, G.; Fenu, G.; Porceddu, M.; Bacchetta, G. The Endemic Vascular Flora of Sardinia: A Dynamic Checklist with an Overview of Biogeography and Conservation Status. Plants 2022, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Marongiu, B.; Piras, A.; Porcedda, S.; Tuveri, E.; Laconi, S.; Deidda, D.; Maxia, A. Chemical and Biological Comparisons on Supercritical Extracts of Tanacetum cinerariifolium (Trevir) Sch. Bip. with Three Related Species of Chrysanthemums of Sardinia (Italy). Nat. Prod. Res. 2009, 23, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Casu, L.; Bonsignore, L.; Pinna, M.; Casu, M.; Floris, C.; Gertsch, J.; Cottiglia, F. Cytotoxic Diacetylenic Spiroketal Enol Ethers from Plagius flosculosus. J. Nat. Prod. 2006, 69, 295–298. [Google Scholar] [CrossRef]
- Xiao, C.Y.; Lan, J.E.; Liu, X.; Sun, Z.L.; Li, X.J.; Yin, Y.H.; Gibbons, S.; Mu, Q. Acetylenic spiroketal enol ethers from Artemisia rupestris and their synergistic antibacterial effects on methicillin-resistant Staphylococcus aureus. Nat. Prod. Res. 2023, 1–5. [Google Scholar] [CrossRef]
- Nakamura, Y.; Ohto, Y.; Murakami, A.; Jiwajinda, S.; Ohigashi, H. Isolation and Identification of Acetylenic Spiroketal Enol Ethers from Artemisia lactiflora as Inhibitors of Superoxide Generation Induced by a Tumor Promoter in Differentiated HL-60 Cells. J. Agric. Food Chem. 1998, 46, 5031–5036. [Google Scholar] [CrossRef]
- Calzado, M.A.; Lüdi, K.S.; Fiebich, B.; Ben-Neriah, Y.; Bacher, S.; Munoz, E.; Ballero, M.; Prosperini, S.; Appendino, G.; Schmitz, M.L. Inhibition of NF-KappaB Activation and Expression of Inflammatory Mediators by Polyacetylene Spiroketals from Plagius flosculosus. Biochim. Biophys. Acta 2005, 1729, 88–93. [Google Scholar] [CrossRef]
- Álvarez, Á.L.; Habtemariam, S.; Abdel Moneim, A.E.; Melón, S.; Dalton, K.P.; Parra, F. A spiroketal-enol ether derivative from Tanacetum vulgare selectively inhibits HSV-1 and HSV-2 glycoprotein accumulation in Vero cells. Antivir. Res. 2015, 119, 8–18. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-Infective Potential of Natural Products: How to Develop a Stronger in Vitro “Proof-of-Concept”. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Sanna, C.; Rigano, D.; Corona, A.; Piano, D.; Formisano, C.; Farci, D.; Franzini, G.; Ballero, M.; Chianese, G.; Tramontano, E.; et al. Dual HIV-1 Reverse Transcriptase and Integrase Inhibitors from Limonium morisianum Arrigoni, an endemic species of Sardinia (Italy). Nat. Prod. Res. 2019, 33, 1798–1803. [Google Scholar] [CrossRef] [PubMed]
- Hnatyszyn, O.; Broussalis, A.; Herrera, G.; Muschietti, L.; Coussio, J.; Martino, V.; Ferraro, G.; Font, M.; Monge, A.; Martínez-Irujo, J.J.; et al. Argentine Plant Extracts Active against Polymerase and Ribonuclease H Activities of HIV-1 Reverse Transcriptase. Phytother. Res. 1999, 13, 206–209. [Google Scholar] [CrossRef]
- Buono-Core, G.E.; Nunez, M.V.; Lucero, A.; Vargas, M.R.; Jullian, C. Structural elucidation of bioactive principles in floral extracts of German chamomille (Matricaria recutita L.). J. Chil. Chem. Soc. 2011, 56, 549–553. [Google Scholar] [CrossRef]
- Maqua, M.P.; Vines, A.C.G.; Caballero, E.; Grande, M.C.; Medarde, M.; Bellido, I.S. Components from Santolina rosmarinifolia, subspecies rosmarinifolia and canescens. Phytochemistry 1988, 27, 3664–3667. [Google Scholar] [CrossRef]
- Konovalov, D.A. Polyacetylene Compounds of Plants of the Asteraceae Family (Review). Pharm. Chem. J. 2014, 48, 613–631. [Google Scholar] [CrossRef]
- Yin, B.L.; Fan, J.F.; Gao, Y.; Wu, Y.L. Progress in Molecular Diversity of Tonghaosu and Its Analogs. ARKIVOC 2003, ii, 70–83. [Google Scholar] [CrossRef]
- Amoros, M.; Simões, C.M.; Girre, L.; Sauvager, F.; Cormier, M. Synergistic effect of flavones and flavonols against herpes simplex virus type 1 in cell culture. Comparison with the antiviral activity of propolis. J. Nat. Prod. 1992, 55, 1732–1740. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, H.; Lyu, L.; Huang, Z.; Fan, S.; Wu, W.; Li, W. Synergistic effect of natural antifungal agents for postharvest diseases of blackberry fruits. J. Sci. Food Agric. 2019, 9, 3343–3349. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef]
- Corona, A.; Di Leva, F.S.; Thierry, S.; Pescatori, L.; Cuzzucoli Crucitti, G.; Subra, F.; Delelis, O.; Esposito, F.; Rigogliuso, G.; Costi, R.; et al. Identification of highly conserved residues involved in inhibition of HIV-1 RNase H function by Diketo acid derivatives. Antimicrob. Agents Chemother. 2014, 58, 6101–6110. [Google Scholar] [CrossRef]
- Summa, V.; Petrocchi, A.; Bonelli, F.; Crescenzi, B.; Donghi, M.; Ferrara, M.; Fiore, F.; Gardelli, C.; Gonzalez Paz, O.; Hazuda, D.J.; et al. Discovery of raltegravir, a potent, selective orally bioavailable HIV-integrase inhibitor for the treatment of HIV-AIDS infection. J. Med. Chem. 2008, 51, 5843–5855. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, E.; Esposito, F.; Badas, R.; Di Santo, R.; Costi, R.; La Colla, P. 6-[1-(4-Fluorophenyl)Methyl-1H-Pyrrol-2-Yl)]-2,4-Dioxo-5-Hexenoic Acid Ethyl Ester a Novel Diketo Acid Derivative Which Selectively Inhibits the HIV-1 Viral Replication in Cell Culture and the Ribonuclease H Activity in Vitro. Antivir. Res. 2005, 65, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; McKee, C.J.; Kessl, J.J.; Santos, W.L.; Daigle, J.E.; Engelman, A.; Verdine, G.; Kvaratskhelia, M. Subunit-specific protein footprinting reveals significant structural rearrangements and a role for N-terminal Lys-14 of HIV-1 Integrase during viral DNA binding. J. Biol. Chem. 2008, 283, 5632–5641. [Google Scholar] [CrossRef] [PubMed]
- Kessl, J.J.; Li, M.; Ignatov, M.; Shkriabai, N.; Eidahl, J.O.; Feng, L.; Musier-Forsyth, K.; Craigie, R.; Kvaratskhelia, M. FRET analysis reveals distinct conformations of IN tetramers in the presence of viral DNA or LEDGF/p75. Nucleic Acids Res. 2011, 39, 9009–9022. [Google Scholar] [CrossRef]
- Saladini, F.; Giannini, A.; Boccuto, A.; Vicenti, I.; Zazzi, M. Agreement between an In-House Replication Competent and a Reference Replication Defective Recombinant Virus Assay for Measuring Phenottypic Resistance to HIV-1 Protease, Reverse Transcriptase, and Integrase Inhibitors. J. Clin. Lab. Anal. 2018, 32, e22206. [Google Scholar] [CrossRef]
- Guzzo, F.; Buommino, E.; Landrum, L.; Russo, R.; Lembo, F.; Fiorentino, A.; D’Abrosca, B. Phytochemical Investigation of Myrcianthes cisplatensis: Structural Characterization of New p-Coumaroyl Alkylphloroglucinols and Antimicrobial Evaluation against Staphylococcus aureus. Plants 2023, 12, 1046. [Google Scholar] [CrossRef]
- Esposito, F.; Tintori, C.; Martini, R.; Christ, F.; Debyser, Z.; Ferrarese, R.; Cabiddu, G.; Corona, A.; Ceresola, E.R.; Calcaterra, A.; et al. Kuwanon-L as a New Allosteric HIV-1 Integrase Inhibitor: Molecular Modeling and Biological Evaluation. ChemBioChem 2015, 16, 2507–2512. [Google Scholar] [CrossRef]


| Compound | IN-LEDGF-Dependent 1 IC50 (µM) | RNase H 1 IC50 (µM) |
|---|---|---|
| SPK1 | 1.69 ± 0.38 | 10.02 ± 5.40 |
| SPK2 | 14.70 ± 0.30 | 20.0 ± 6.10 |
| SPK3 | 1.46 ± 0.16 | 3.60 ± 0.60 |
| Raltegravir | 0.05 ± 0.02 | - |
| RDS1759 | - | 0.0067 ± 0.90 |
| Code | IN–LEDGF Binding 1 IC50(µM) | IN–IN Subunit Exchange 2 IC50(µM) | IN Multimerization 3 MI50(µM) | LEDGF-Independent IN Activity 4 IC50(µM) |
|---|---|---|---|---|
| SPK3 | >100 | >100 | 1.00 ± 0.01 | 27.3 ± 2.3 |
| Kuwanon-L | 22.0 ± 0.5 | >100 | 38.0 ± 0.02 | 34.0 ± 0.5 |
| Compound | 1 EC50 (µM) | 2 CC50 (µM) |
|---|---|---|
| SPK3 | NA | 50.1 ± 4.3 |
| Raltegravir | 0.0087 ± 0.059 | >100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanna, C.; D’Abrosca, B.; Fiorentino, A.; Giammarino, F.; Vicenti, I.; Corona, A.; Caredda, A.; Tramontano, E.; Esposito, F. HIV-1 Integrase Inhibition Activity by Spiroketals Derived from Plagius flosculosus, an Endemic Plant of Sardinia (Italy) and Corsica (France). Pharmaceuticals 2023, 16, 1118. https://doi.org/10.3390/ph16081118
Sanna C, D’Abrosca B, Fiorentino A, Giammarino F, Vicenti I, Corona A, Caredda A, Tramontano E, Esposito F. HIV-1 Integrase Inhibition Activity by Spiroketals Derived from Plagius flosculosus, an Endemic Plant of Sardinia (Italy) and Corsica (France). Pharmaceuticals. 2023; 16(8):1118. https://doi.org/10.3390/ph16081118
Chicago/Turabian StyleSanna, Cinzia, Brigida D’Abrosca, Antonio Fiorentino, Federica Giammarino, Ilaria Vicenti, Angela Corona, Alessia Caredda, Enzo Tramontano, and Francesca Esposito. 2023. "HIV-1 Integrase Inhibition Activity by Spiroketals Derived from Plagius flosculosus, an Endemic Plant of Sardinia (Italy) and Corsica (France)" Pharmaceuticals 16, no. 8: 1118. https://doi.org/10.3390/ph16081118
APA StyleSanna, C., D’Abrosca, B., Fiorentino, A., Giammarino, F., Vicenti, I., Corona, A., Caredda, A., Tramontano, E., & Esposito, F. (2023). HIV-1 Integrase Inhibition Activity by Spiroketals Derived from Plagius flosculosus, an Endemic Plant of Sardinia (Italy) and Corsica (France). Pharmaceuticals, 16(8), 1118. https://doi.org/10.3390/ph16081118












