Nanogel Containing Gamma-Oryzanol-Loaded Nanostructured Lipid Carriers and TiO2/MBBT: A Synergistic Nanotechnological Approach of Potent Natural Antioxidants and Nanosized UV Filters for Skin Protection
Abstract
1. Introduction
2. Results and Discussion
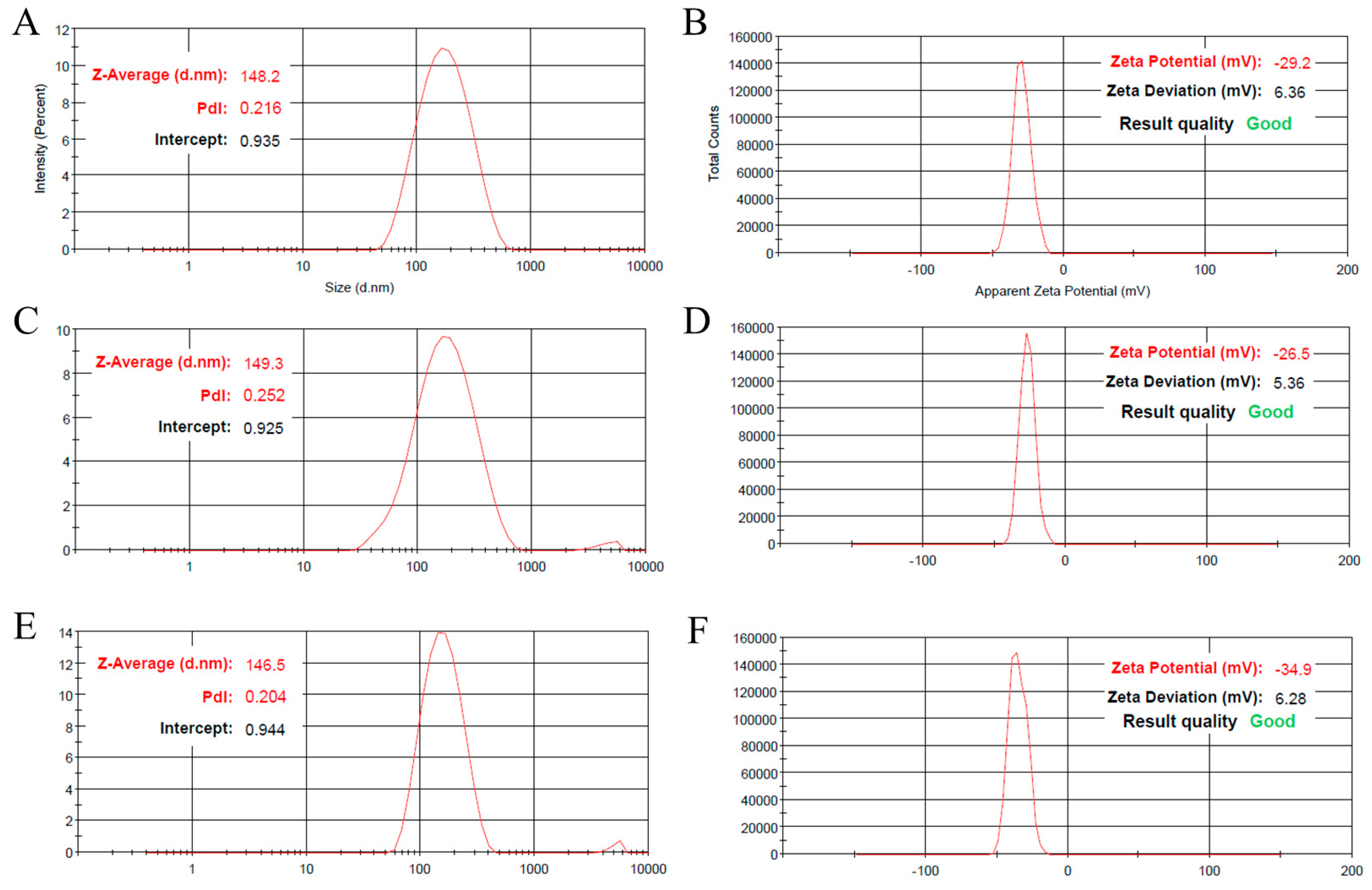
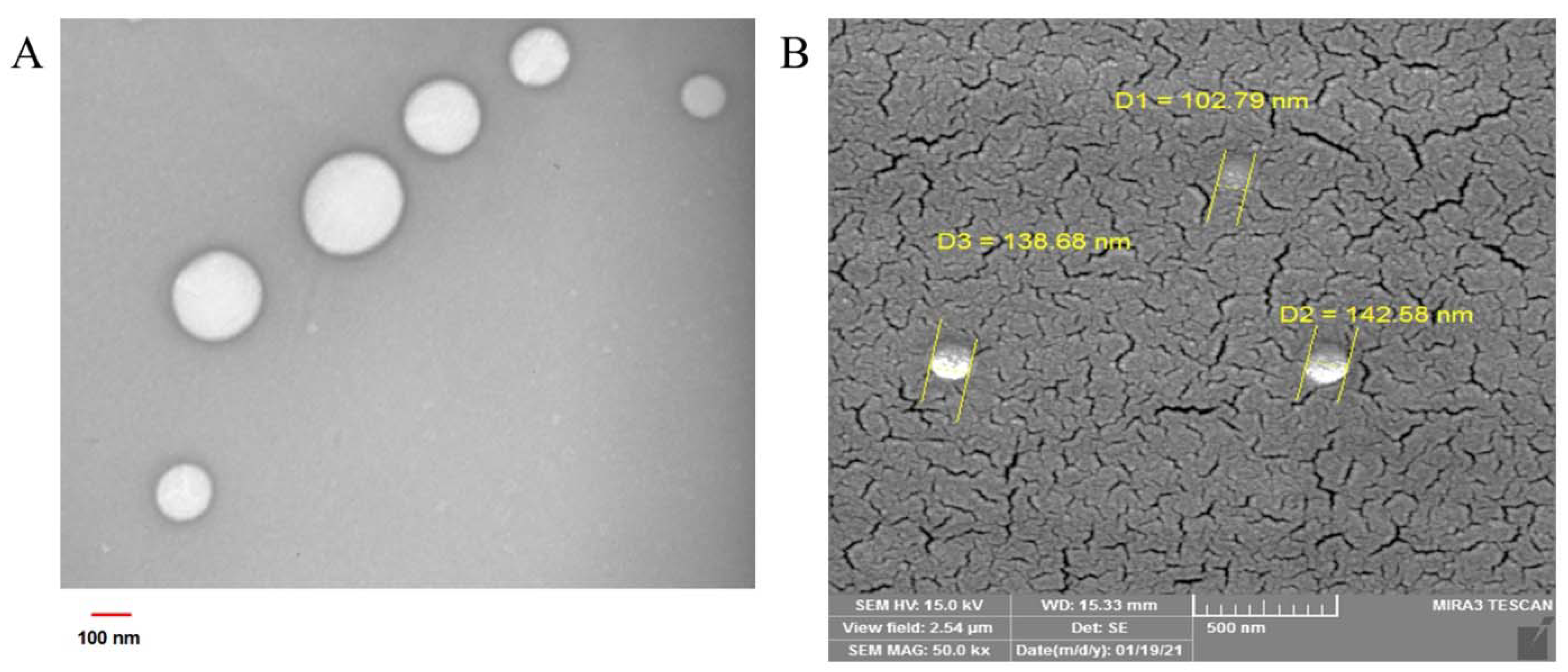
2.1. GO-NLC Formulation Development, Characterization, and Stability
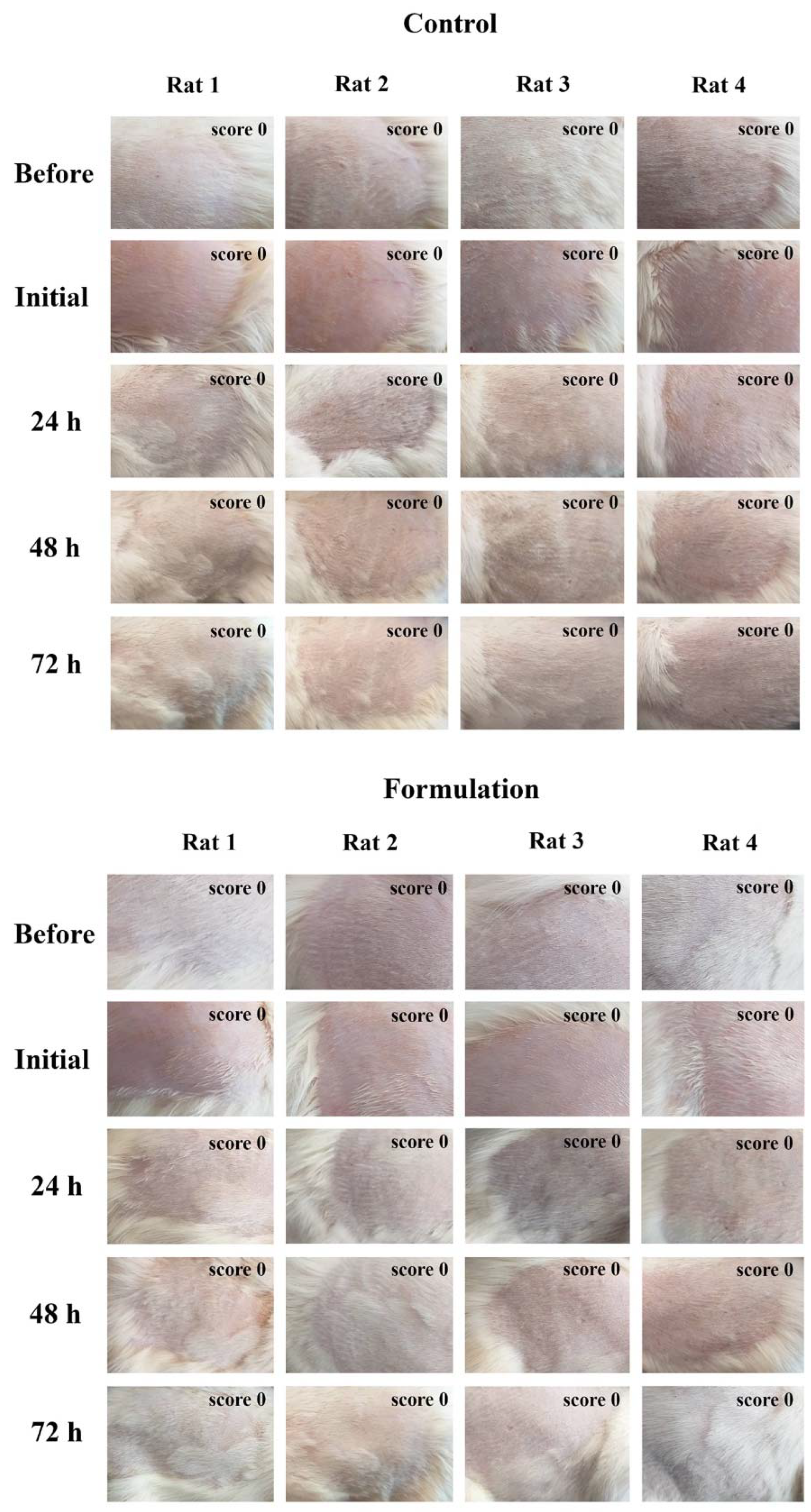
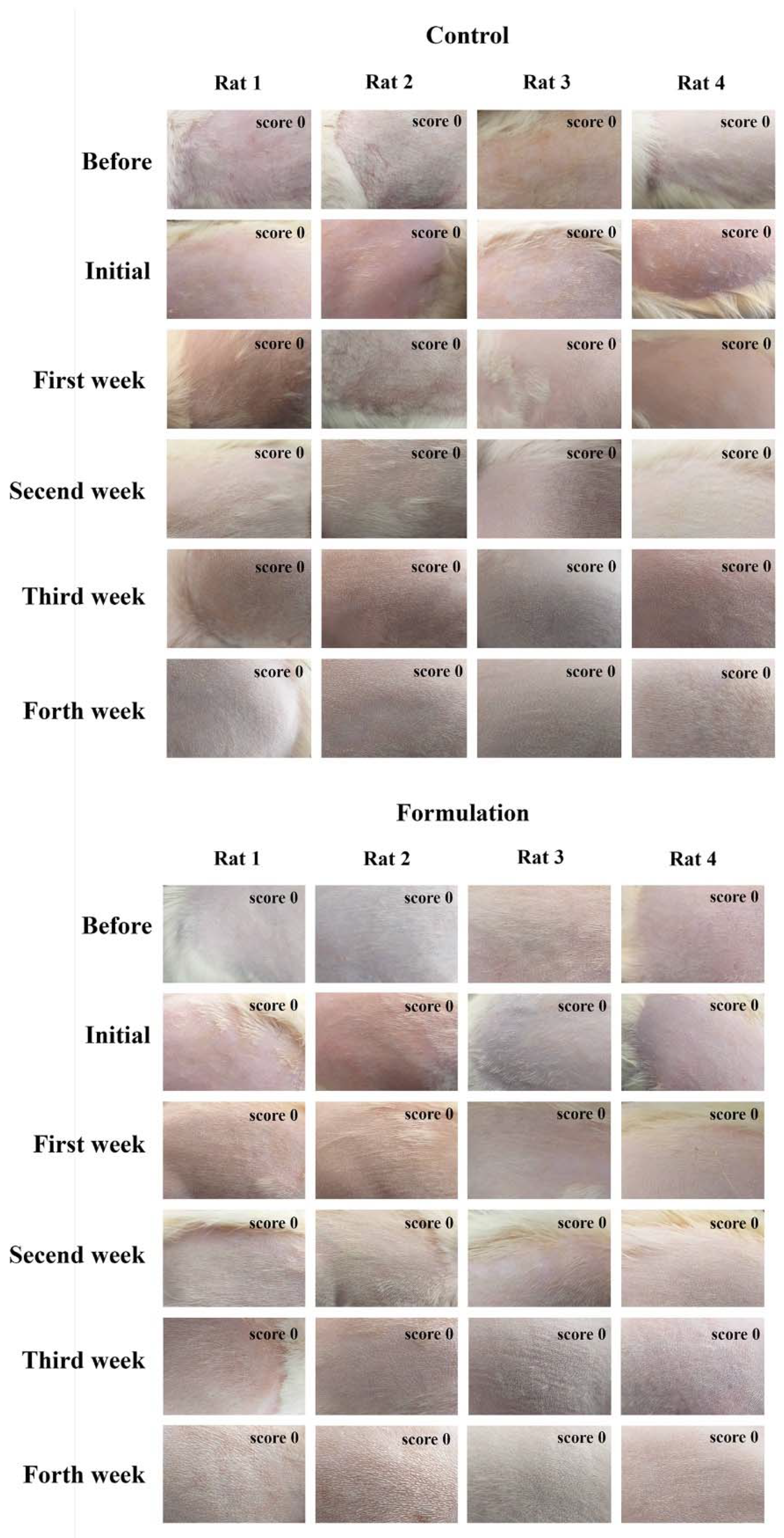
2.2. Determination of Formulation Adequacy for Skin Application and Protection: In Vitro SPF Assessment, pH, and In Vivo Skin Irritation Sensitization Study
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. GO-Loaded NLC Preparation
3.2.2. Particle Size and Surface Charge Measurement
3.2.3. Encapsulation Efficiency Determination
3.2.4. In Vitro Release Study
3.2.5. Real-Time and Accelerated Stability Studies
3.2.6. Topical Nanogel Development
3.2.7. In Vitro SPF Assessment
3.2.8. Skin Irritation and Sensitization Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Baron, E.D.; Suggs, A.K. Introduction to Photobiology. Dermatol. Clin. 2014, 32, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, C.; Jiang, G. Research Progress on Skin Photoaging and Oxidative Stress. Adv. Dermatol. Allergol. 2021, 38, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Krambeck, K.; Santos, D.; Sousa Lobo, J.M.; Amaral, M.H. Benefits of Skin Application of Piceatannol—A Minireview. Australas. J. Dermatol. 2023, 64, e21–e25. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Gullì, M.; Percaccio, E.; Vitalone, A.; Mazzanti, G.; Di Giacomo, S. Efficacy and Safety of Oral Green Tea Preparations in Skin Ailments: A Systematic Review of Clinical Studies. Nutrients 2022, 14, 3149. [Google Scholar] [CrossRef] [PubMed]
- García-Villegas, A.; Rojas-García, A.; Villegas-Aguilar, M.d.C.; Fernández-Moreno, P.; Fernández-Ochoa, Á.; Cádiz-Gurrea, M.d.l.L.; Arráez-Román, D.; Segura-Carretero, A. Cosmeceutical Potential of Major Tropical and Subtropical Fruit By-Products for a Sustainable Revalorization. Antioxidants 2022, 11, 203. [Google Scholar] [CrossRef]
- Frantz, M.; Rozot, R.; Marrot, L. NRF2 in Dermo-cosmetic: From Scientific Knowledge to Skin Care Products. BioFactors 2023, 49, 32–61. [Google Scholar] [CrossRef]
- Ramer, R.; Hinz, B. Cannabinoid Compounds as a Pharmacotherapeutic Option for the Treatment of Non-Cancer Skin Diseases. Cells 2022, 11, 4102. [Google Scholar] [CrossRef]
- Cruz, A.M.; Gonçalves, M.C.; Marques, M.S.; Veiga, F.; Paiva-Santos, A.C.; Pires, P.C. In Vitro Models for Anti-Aging Efficacy Assessment: A Critical Update in Dermocosmetic Research. Cosmetics 2023, 10, 66. [Google Scholar] [CrossRef]
- Young, A.R.; Claveau, J.; Rossi, A.B. Ultraviolet Radiation and the Skin: Photobiology and Sunscreen Photoprotection. J. Am. Acad. Dermatol. 2017, 76, S100–S109. [Google Scholar] [CrossRef]
- Guan, L.L.; Lim, H.W.; Mohammad, T.F. Sunscreens and Photoaging: A Review of Current Literature. Am. J. Clin. Dermatol. 2021, 22, 819–828. [Google Scholar] [CrossRef]
- Alexander, A.; Dwivedi, S.; Ajazuddin; Giri, T.K.; Saraf, S.; Saraf, S.; Tripathi, D.K. Approaches for Breaking the Barriers of Drug Permeation through Transdermal Drug Delivery. J. Control. Release 2012, 164, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Cho, H.-E.; Moon, S.H.; Ahn, H.-J.; Bae, S.; Cho, H.-D.; An, S. Transdermal Delivery Systems in Cosmetics. Biomed. Dermatol. 2020, 4, 10. [Google Scholar] [CrossRef]
- Martínez-Razo, G.; Pires, P.C.; Domínguez-López, M.L.; Veiga, F.; Vega-López, A.; Paiva-Santos, A.C. Norcantharidin Nanoemulsion Development, Characterization, and In Vitro Antiproliferation Effect on B16F1 Melanoma Cells. Pharmaceuticals 2023, 16, 501. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Coelho, C.; Teixeira, J.A.; Ferreira-Santos, P.; Botelho, C.M. Nanocarriers as Active Ingredients Enhancers in the Cosmetic Industry—The European and North America Regulation Challenges. Molecules 2022, 27, 1669. [Google Scholar] [CrossRef]
- Souto, E.B.; Müller, R.H. Cosmetic Features and Applications of Lipid Nanoparticles. Int. J. Cosmet. Sci. 2008, 30, 157–165. [Google Scholar] [CrossRef]
- Khater, D.; Nsairat, H.; Odeh, F.; Saleh, M.; Jaber, A.; Alshaer, W.; Al Bawab, A.; Mubarak, M.S. Design, Preparation, and Characterization of Effective Dermal and Transdermal Lipid Nanoparticles: A Review. Cosmetics 2021, 8, 39. [Google Scholar] [CrossRef]
- Muller, R.; Petersen, R.; Hommoss, A.; Pardeike, J. Nanostructured Lipid Carriers (NLC) in Cosmetic Dermal Products. Adv. Drug Deliv. Rev. 2007, 59, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Lautenschlager, S.; Wulf, H.C.; Pittelkow, M.R. Photoprotection. Lancet 2007, 370, 528–537. [Google Scholar] [CrossRef]
- Antoniou, C.; Kosmadaki, M.; Stratigos, A.; Katsambas, A. Sunscreens—What’s Important to Know. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1110–1119. [Google Scholar] [CrossRef]
- Manaia, E.B.; Kaminski, R.C.K.; Corrêa, M.A.; Chiavacci, L.A. Inorganic UV Filters. Braz. J. Pharm. Sci. 2013, 49, 201–209. [Google Scholar] [CrossRef]
- Serpone, N.; Dondi, D.; Albini, A. Inorganic and Organic UV Filters: Their Role and Efficacy in Sunscreens and Suncare Products. Inorg. Chim. Acta 2007, 360, 794–802. [Google Scholar] [CrossRef]
- Lu, P.-J.; Huang, S.-C.; Chen, Y.-P.; Chiueh, L.-C.; Shih, D.Y.-C. Analysis of Titanium Dioxide and Zinc Oxide Nanoparticles in Cosmetics. J. Food Drug Anal. 2015, 23, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Coiffard, L. The Interest in Nanomaterials for Topical Photoprotection. Cosmetics 2015, 2, 394–408. [Google Scholar] [CrossRef]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.; Jose, S.; Sumod, U.; Sabitha, M. Nanotechnology in Cosmetics: Opportunities and Challenges. J. Pharm. Bioallied Sci. 2012, 4, 186. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A.; Sousa, E.; Cruz, M.; Cidade, H.; Lobo, J.; Almeida, I. UV Filters: Challenges and Prospects. Pharmaceuticals 2022, 15, 263. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, A.; Li, S.; Tang, J.; Li, L.; Xiong, L. Natural Components in Sunscreens: Topical Formulations with Sun Protection Factor (SPF). Biomed. Pharmacother. 2021, 134, 111161. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Jose, J. Current Developments in the Nanomediated Delivery of Photoprotective Phytochemicals. Environ. Sci. Pollut. Res. 2020, 27, 38446–38471. [Google Scholar] [CrossRef]
- Xu, Z.; Godber, J.S. Purification and Identification of Components of γ-Oryzanol in Rice Bran Oil. J. Agric. Food Chem. 1999, 47, 2724–2728. [Google Scholar] [CrossRef]
- Juliano, C.; Cossu, M.; Alamanni, M.C.; Piu, L. Antioxidant Activity of Gamma-Oryzanol: Mechanism of Action and Its Effect on Oxidative Stability of Pharmaceutical Oils. Int. J. Pharm. 2005, 299, 146–154. [Google Scholar] [CrossRef]
- Heydari, S.; Ghanbarzadeh, S.; Anoush, B.; Ranjkesh, M.; Javadzadeh, Y.; Kouhsoltani, M.; Hamishehkar, H. Nanoethosomal Formulation of Gammaoryzanol for Skin-Aging Protection and Wrinkle Improvement: A Histopathological Study. Drug Dev. Ind. Pharm. 2017, 43, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, M.; Abbaspour-Ravasjani, S.; Soltanfam, T.; Paiva-Santos, A.C.; Babaei, H.; Veiga, F.; Hamishehkar, H. Prevention of UV-Induced Skin Cancer in Mice by Gamma Oryzanol-Loaded Nanoethosomes. Life Sci. 2021, 283, 119759. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Lohani, A.; Mishra, A.K.; Verma, A. Formulation and Evaluation of Carrot Seed Oil-Based Cosmetic Emulsions. J. Cosmet. Laser Ther. 2019, 21, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.J.; Al-Ghamdi, A.; Usmani, S.; Khan, K.A.; Alqarni, A.S.; Kaur, M.; Al-Waili, N. In Vitro Evaluation of the Effects of Some Plant Essential Oils on Ascosphaera Apis, the Causative Agent of Chalkbrood Disease. Saudi J. Biol. Sci. 2017, 24, 1001–1006. [Google Scholar] [CrossRef]
- Jasicka-Misiak, I.; Lipok, J.; Nowakowska, E.M.; Wieczorek, P.P.; Młynarz, P.; Kafarski, P. Antifungal Activity of the Carrot Seed Oil and Its Major Sesquiterpene Compounds. Z. Nat. C 2004, 59, 791–796. [Google Scholar] [CrossRef]
- Bergonzelli, G.E.; Donnicola, D.; Porta, N.; Corthésy-Theulaz, I.E. Essential Oils as Components of a Diet-Based Approach to Management of Helicobacter Infection. Antimicrob. Agents Chemother. 2003, 47, 3240–3246. [Google Scholar] [CrossRef]
- Lin, T.-K.; Zhong, L.; Santiago, J. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef]
- Zhang, J.; Kurita, M.; Shinozaki, T.; Ukiya, M.; Yasukawa, K.; Shimizu, N.; Tokuda, H.; Masters, E.T.; Akihisa, M.; Akihisa, T. Triterpene Glycosides and Other Polar Constituents of Shea (Vitellaria paradoxa) Kernels and Their Bioactivities. Phytochemistry 2014, 108, 157–170. [Google Scholar] [CrossRef]
- Ugwu-Dike, P.; Nambudiri, V.E. A Review of Ethnomedicinal Uses of Shea Butter for Dermatoses in Sub-Saharan Africa. Dermatol. Ther. 2022, 35, e14786. [Google Scholar] [CrossRef]
- Nainu, F.; Masyita, A.; Bahar, M.A.; Raihan, M.; Prova, S.R.; Mitra, S.; Bin Emran, T.; Simal-Gandara, J. Pharmaceutical Prospects of Bee Products: Special Focus on Anticancer, Antibacterial, Antiviral, and Antiparasitic Properties. Antibiotics 2021, 10, 822. [Google Scholar] [CrossRef]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.D.; Neacsu, I.A.; Grumezescu, A.M.; Andronescu, E. Bee-Derived Products: Chemical Composition and Applications in Skin Tissue Engineering. Pharmaceutics 2022, 14, 750. [Google Scholar] [CrossRef] [PubMed]
- Veronovski, N.; Lešnik, M.; Lubej, A.; Verhovšek, D. Surface Treated Titanium Dioxide Nanoparticles as Inorganic UV Filters in Sunscreen Products. Acta Chim. Slov. 2014, 61, 595–600. [Google Scholar] [PubMed]
- Pornputtapitak, W.; Pantakitcharoenkul, J.; Panpakdee, R.; Teeranachaideekul, V.; Sinchaipanid, N. Development of γ-Oryzanol Rich Extract from Leum Pua Glutinous Rice Bran Loaded Nanostructured Lipid Carriers for Topical Delivery. J. Oleo Sci. 2018, 67, 125–133. [Google Scholar] [CrossRef]
- Smijs, T.; Pavel, S. Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreens: Focus on Their Safety and Effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Baharifar, H.; Amani, A. Efficacy of a Model Nano-TiO2 Sunscreen Preparation as a Function of Ingredients Concentration and Ultrasonication Treatment. Pharm. Sci. 2017, 23, 129–135. [Google Scholar] [CrossRef]
- Elboury, S.; Couteau, C.; Boulande, L.; Paparis, E.; Coiffard, L. Effect of the Combination of Organic and Inorganic Filters on the Sun Protection Factor (SPF) Determined by in Vitro Method. Int. J. Pharm. 2007, 340, 1–5. [Google Scholar] [CrossRef]
- Nikolić, S.; Keck, C.M.; Anselmi, C.; Müller, R.H. Skin Photoprotection Improvement: Synergistic Interaction between Lipid Nanoparticles and Organic UV Filters. Int. J. Pharm. 2011, 414, 276–284. [Google Scholar] [CrossRef]
- Reis, S.; Gomes, M.J.; Martins, S.; Ferreira, D.; Segundo, M.A. Lipid Nanoparticles for Topical and Transdermal Application for Alopecia Treatment: Development, Physicochemical Characterization, and in Vitro Release and Penetration Studies. Int. J. Nanomed. 2014, 9, 1231–1242. [Google Scholar] [CrossRef]
- Mardhiah Adib, Z.; Ghanbarzadeh, S.; Kouhsoltani, M.; Yari Khosroshahi, A.; Hamishehkar, H. The Effect of Particle Size on the Deposition of Solid Lipid Nanoparticles in Different Skin Layers: A Histological Study. Adv. Pharm. Bull. 2016, 6, 31–36. [Google Scholar] [CrossRef]
- Torrisi, C.; Cardullo, N.; Russo, S.; La Mantia, A.; Acquaviva, R.; Muccilli, V.; Castelli, F.; Sarpietro, M.G. Benzo[k,l]Xanthene Lignan-Loaded Solid Lipid Nanoparticles for Topical Application: A Preliminary Study. Molecules 2022, 27, 5887. [Google Scholar] [CrossRef] [PubMed]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lipid Nanocarriers for Dermal Delivery of Lutein: Preparation, Characterization, Stability and Performance. Int. J. Pharm. 2011, 414, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.C.; Tan, C.P.; Nyam, K.L. Development of Nanostructured Lipid Carriers (NLCs) Using Pumpkin and Kenaf Seed Oils with Potential Photoprotective and Antioxidative Properties. Eur. J. Lipid Sci. Technol. 2019, 121, 1900082. [Google Scholar] [CrossRef]
- Lacatusu, I.; Arsenie, L.V.; Badea, G.; Popa, O.; Oprea, O.; Badea, N. New Cosmetic Formulations with Broad Photoprotective and Antioxidative Activities Designed by Amaranth and Pumpkin Seed Oils Nanocarriers. Ind. Crops Prod. 2018, 123, 424–433. [Google Scholar] [CrossRef]
- Sanad, R.A.; AbdelMalak, N.S.; ElBayoomy, T.S.; Badawi, A.A. Formulation of a Novel Oxybenzone-Loaded Nanostructured Lipid Carriers (NLCs). AAPS PharmSciTech 2010, 11, 1684–1694. [Google Scholar] [CrossRef] [PubMed]
- Niculae, G.; Lacatusu, I.; Badea, N.; Meghea, A. Lipid Nanoparticles Based on Butyl-Methoxydibenzoylmethane: In Vitro UVA Blocking Effect. Nanotechnology 2012, 23, 315704. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E. PH in Nature, Humans and Skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Ali, S.; Yosipovitch, G. Skin PH: From Basic Science to Basic Skin Care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.-H.; Korting, H.C. The PH of the Skin Surface and Its Impact on the Barrier Function. Ski. Pharm. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef]
- Fischer, P.; Eugster, A.; Windhab, E.J.; Schuleit, M. Predictive Stress Tests to Study the Influence of Processing Procedures on Long Term Stability of Supersaturated Pharmaceutical o/w Creams. Int. J. Pharm. 2007, 339, 189–196. [Google Scholar] [CrossRef]
- Tadros, T. Application of Rheology for Assessment and Prediction of the Long-Term Physical Stability of Emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 227–258. [Google Scholar] [CrossRef] [PubMed]
- Diffey, B.L.; Robson, J. The Influence of Pigmentation and Illumination on the Perception of Erythema. Photodermatol. Photoimmunol. Photomed. 1992, 9, 45–47. [Google Scholar] [PubMed]
- Draize, J.; Woodard, G.; Calvary, H. Methods for the Study of Irritation and Toxicity of Substances Applied Topically to the Skin and Mucus Membranes. J. Pharmacol. Exp. Ther. 1944, 1, 377–390. [Google Scholar]
- Pokharkar, V.B.; Mendiratta, C.; Kyadarkunte, A.Y.; Bhosale, S.H.; Barhate, G.A. Skin Delivery Aspects of Benzoyl Peroxide-Loaded Solid Lipid Nanoparticles for Acne Treatment. Ther. Deliv. 2014, 5, 635–652. [Google Scholar] [CrossRef] [PubMed]


| NLC Formulation Code | Coemulsifier (Lipid Phase) | Surfactant (Aqueous Phase) | Oil–Lipid | Results | |||
|---|---|---|---|---|---|---|---|
| Size a | PDI b | ||||||
| Type | Percent | Type | Percent | Percent | (nm) | ||
| F1 | - | - | Poloxamer 407 | 3 | 7.6 | 280 | 0.29 |
| F2 | - | - | 2.5 | 7.6 | 195 | 0.28 | |
| F3 | - | - | 2 | 7.6 | 195 | 0.17 | |
| F4 | PGPR c | 1 | 2.5 | 7.6 | 286 | 0.39 | |
| F5 | 1 | 2 | 7.6 | 190 | 0.15 | ||
| F6 | 1 | 1 | 7.6 | 380 | 0.34 | ||
| F7 | 1.1 | 2.3 | 8.6 | 181 | 0.28 | ||
| F8 | 1.2 | 2.4 | 9 | 189 | 0.22 | ||
| F9 | 1 | Tween® 80 | 2 | 7.6 | 241 | 0.26 | |
| F10 | PGE d | 1 | 2 | 7.6 | 209 | 0.37 | |
| F11 | 1.5 | 2 | 7.6 | 189 | 0.34 | ||
| F12 | 1 | 2.5 | 7.6 | 208 | 0.41 | ||
| F13 | 1 | 2.5 | 8 | 215 | 0.46 | ||
| F14 | 1.6 | 2.1 | 8 | 151 | 0.28 | ||
| F15 | 1.8 | 2.4 | 9 | 148 | 0.21 | ||
| F15 after 1 month storage | 1.8 | 2.4 | 9 | 149 | 0.25 | ||
| F16 (loaded with GO) | 1.8 | 2.4 | 9 | 146 | 0.20 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badalkhani, O.; Pires, P.C.; Mohammadi, M.; Babaie, S.; Paiva-Santos, A.C.; Hamishehkar, H. Nanogel Containing Gamma-Oryzanol-Loaded Nanostructured Lipid Carriers and TiO2/MBBT: A Synergistic Nanotechnological Approach of Potent Natural Antioxidants and Nanosized UV Filters for Skin Protection. Pharmaceuticals 2023, 16, 670. https://doi.org/10.3390/ph16050670
Badalkhani O, Pires PC, Mohammadi M, Babaie S, Paiva-Santos AC, Hamishehkar H. Nanogel Containing Gamma-Oryzanol-Loaded Nanostructured Lipid Carriers and TiO2/MBBT: A Synergistic Nanotechnological Approach of Potent Natural Antioxidants and Nanosized UV Filters for Skin Protection. Pharmaceuticals. 2023; 16(5):670. https://doi.org/10.3390/ph16050670
Chicago/Turabian StyleBadalkhani, Omolbanin, Patrícia C. Pires, Maryam Mohammadi, Soraya Babaie, Ana Cláudia Paiva-Santos, and Hamed Hamishehkar. 2023. "Nanogel Containing Gamma-Oryzanol-Loaded Nanostructured Lipid Carriers and TiO2/MBBT: A Synergistic Nanotechnological Approach of Potent Natural Antioxidants and Nanosized UV Filters for Skin Protection" Pharmaceuticals 16, no. 5: 670. https://doi.org/10.3390/ph16050670
APA StyleBadalkhani, O., Pires, P. C., Mohammadi, M., Babaie, S., Paiva-Santos, A. C., & Hamishehkar, H. (2023). Nanogel Containing Gamma-Oryzanol-Loaded Nanostructured Lipid Carriers and TiO2/MBBT: A Synergistic Nanotechnological Approach of Potent Natural Antioxidants and Nanosized UV Filters for Skin Protection. Pharmaceuticals, 16(5), 670. https://doi.org/10.3390/ph16050670










