Alkaloid from Geissospermum sericeum Benth. & Hook.f. ex Miers (Apocynaceae) Induce Apoptosis by Caspase Pathway in Human Gastric Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Phytochemical Analysis
2.2. Antitumor Activity, Cytotoxicity, and Selectivity Index
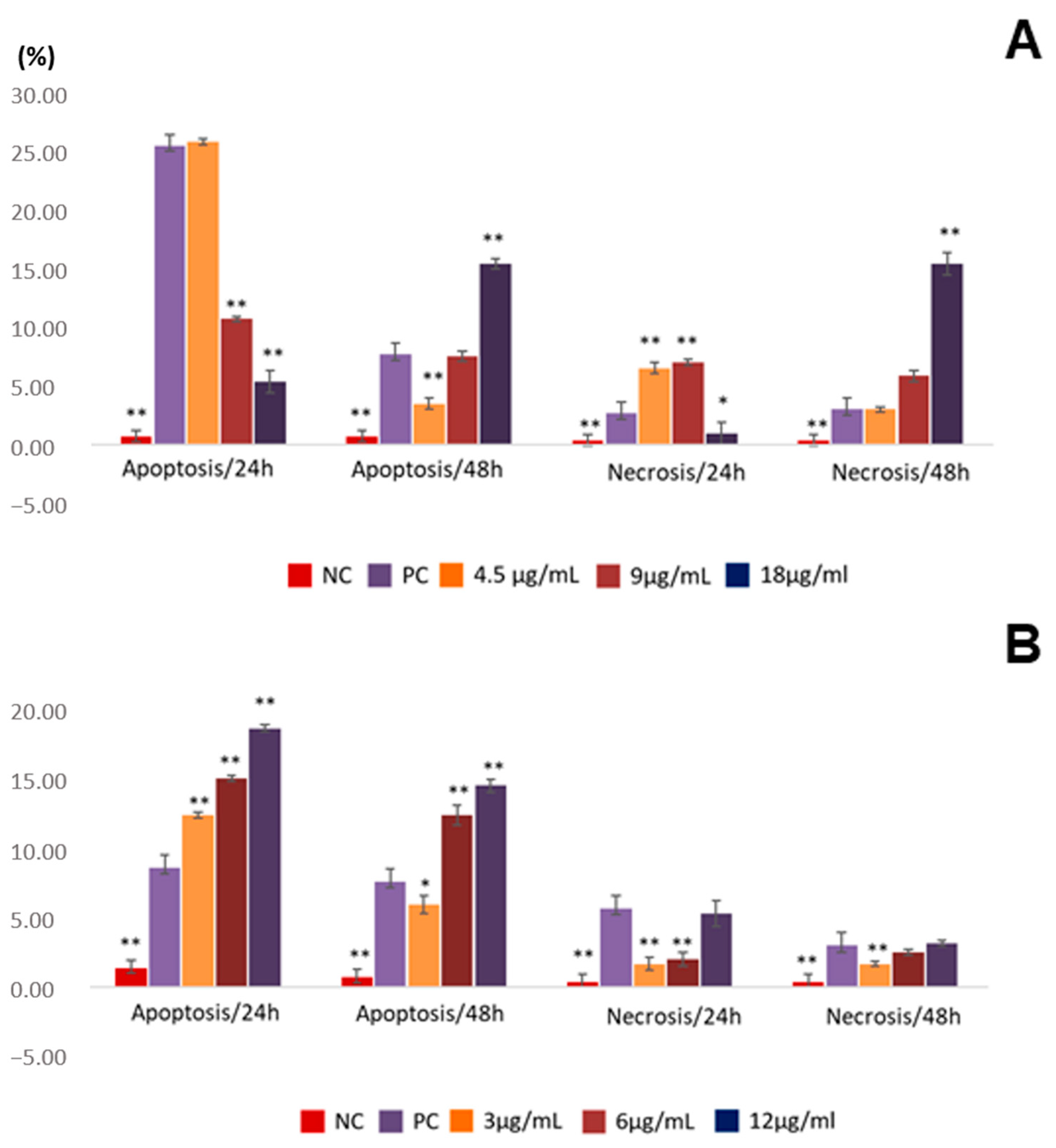
2.3. Assessment of Apoptosis Induction
2.4. In Silico
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Equipment
4.3. Plant Material, Extraction Procedure and Isolation
4.4. NMR and MS Analysis
4.5. Cells
4.6. Antitumor Activity and Cytotoxicity through the Cell Viability Assay (MTT)
4.7. Investigation of Necrosis and Apoptosis by Quantifying Cell Death Patterns
4.8. Molecular Docking
4.9. Molecular Dynamics (MD) Simulations
4.10. Binding Affinities Calculations
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Cancer—Key Facts. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 9 April 2022).
- World Health Organisation. Stomach—Global Cancer Observatory. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/7-Stomach-fact-sheet.pdf (accessed on 27 April 2023).
- Ajani, J.A.; D’Amico, T.A.; Almhanna, K.; Bentrem, D.J.; Chao, J.; Das, P.; Denlinger, C.S.; Fanta, P.; Farjah, F.; Fuchs, C.S.; et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2016, 14, 1286–1312. [Google Scholar] [CrossRef] [PubMed]
- Barati, N.; Momtazi-Borojeni, A.A.; Majeed, M.; Sahebkar, A. Potential Therapeutic Effects of Curcumin in Gastric Cancer. J. Cell. Physiol. 2019, 234, 2317–2328. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. From Chemotherapy to Biological Therapy: A Review of Novel Concepts to Reduce the Side Effects of Systemic Cancer Treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.; Rajkumar, S.V. The High Cost of Cancer Drugs and What We Can Do about It. Mayo Clin. Proc. 2012, 87, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Habli, Z.; Toumieh, G.; Fatfat, M.; Rahal, O.N.; Gali-Muhtasib, H. Emerging Cytotoxic Alkaloids in the Battle against Cancer: Overview of Molecular Mechanisms. Molecules 2017, 22, 250. [Google Scholar] [CrossRef]
- Kaur, R.; Arora, S. Alkaloids—Important Therapeutic Secondary Metabolites of Plant Origin. J. Crit. Rev. 2015, 2, 1–8. [Google Scholar]
- Mbeunkui, F.; Grace, M.H.; Lategan, C.; Smith, P.J.; Raskin, I.; Lila, M.A. In Vitro Antiplasmodial Activity of Indole Alkaloids from the Stem Bark of Geissospermum Vellosii. J. Ethnopharmacol. 2012, 139, 471–477. [Google Scholar] [CrossRef]
- Silva, J.V.d.S.e.; Brigido, H.P.C.; de Albuquerque, K.C.O.; Carvalho, J.M.; Reis, J.F.; Faria, L.V.; Coelho-Ferreira, M.R.; Silveira, F.T.; Carneiro, A.d.S.; Percário, S.; et al. Flavopereirine—An Alkaloid Derived from Geissospermum Vellosii—Presents Leishmanicidal Activity In Vitro. Molecules 2019, 24, 785. [Google Scholar] [CrossRef]
- Steele, J.C.P.; Veitch, N.C.; Kite, G.C.; Simmonds, M.S.J.; Warhurst, D.C. Indole and β-Carboline Alkaloids from Geissospermum s Ericeum. J. Nat. Prod. 2002, 65, 85–88. [Google Scholar] [CrossRef]
- Yu, J.; Chen, Q. The Plant Extract of Pao Pereira Potentiates Carboplatin Effects against Ovarian Cancer. Pharm. Biol. 2014, 52, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Beljanski, M. The Anticancer Agent PB-100, Selectively Active on Malignant Cells, Inhibits Multiplication of Sixteen Malignant Cell Lines, Even Multidrug Resistant. Genet. Mol. Biol. 2000, 23, 29–33. [Google Scholar] [CrossRef]
- Beljanski, M.; Beljanski, M.S. Selective Inhibition of in Vitro Synthesis of Cancer DNA by Alkaloids of β-Carboline Class. Pathobiology 1982, 50, 79–87. [Google Scholar] [CrossRef]
- Slaninová, I.; Pěnčíková, K.; Urbanová, J.; Slanina, J.; Táborská, E. Antitumour Activities of Sanguinarine and Related Alkaloids. Phytochem. Rev. 2014, 13, 51–68. [Google Scholar] [CrossRef]
- Kim, S.; Lee, T.-J.; Leem, J.; Choi, K.S.; Park, J.-W.; Kwon, T.K. Sanguinarine-Induced Apoptosis: Generation of ROS, down-Regulation of Bcl-2, c-FLIP, and Synergy with TRAIL. J. Cell. Biochem. 2008, 104, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H.; Kondo, S.; Mukudai, Y.; Nagumo, T.; Yasuda, A.; Kurihara, Y.; Kamatani, T.; Shintani, S. Evaluation of Anticancer Activities of Benzo[c]Phenanthridine Alkaloid Sanguinarine in Oral Squamous Cell Carcinoma Cell Line. Anticancer Res. 2011, 31, 2841–2846. [Google Scholar] [PubMed]
- Luqmani, Y.A. Mechanisms of Drug Resistance in Cancer Chemotherapy. Med. Princ. Pract. 2005, 14, 35–48. [Google Scholar] [CrossRef]
- Abdo, N.Y.M.; Kamel, M.M. Synthesis and Anticancer Evaluation of 1,3,4-Oxadiazoles, 1,3,4-Thiadiazoles, 1,2,4-Triazoles and Mannich Bases. Chem. Pharm. Bull 2015, 63, 369–376. [Google Scholar] [CrossRef]
- Yar, M.S.; Akhter, M.W. Synthesis and Anticonvulsant Activity of Substituted Oxadiazole and Thiadiazole Derivatives. Acta Pol. Pharm. 2009, 66, 393–397. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Hu, W.-P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2009, 26, 170–244. [Google Scholar] [CrossRef]
- Chang, C.; Zhao, W.; Xie, B.; Deng, Y.; Han, T.; Cui, Y.; Dai, Y.; Zhang, Z.; Gao, J.; Guo, H.; et al. Pao Pereira Extract Suppresses Castration-Resistant Prostate Cancer Cell Growth, Survival, and Invasion through Inhibition of NFκB Signaling. Integr. Cancer Ther. 2014, 13, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Goodwin, M.E.; Birrer, M.J.; Chambers, T.C. The C-Jun NH(2)-Terminal Protein Kinase/AP-1 Pathway Is Required for Efficient Apoptosis Induced by Vinblastine. Cancer Res. 2001, 61, 4450–4458. [Google Scholar] [PubMed]
- Bianchi, J.J.; Zhao, X.; Mays, J.C.; Davoli, T. Not All Cancers Are Created Equal: Tissue Specificity in Cancer Genes and Pathways. Curr. Opin. Cell Biol. 2020, 63, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins y Cotran. Patología Estructural y Funcional; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021; ISBN 84-13-82111-8. [Google Scholar]
- Igney, F.H.; Krammer, P.H. Death and Anti-Death: Tumour Resistance to Apoptosis. Nat. Rev. Cancer 2002, 2, 277–288. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell Death: A Review of the Major Forms of Apoptosis, Necrosis and Autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, New and Emerging Functions of Caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef]
- Gu, Q.; De Wang, J.; Xia, H.H.; Lin, M.C.; He, H.; Zou, B.; Tu, S.P.; Yang, Y.; Liu, X.G.; Lam, S.K.; et al. Activation of the Caspase-8/Bid and Bax Pathways in Aspirin-Induced Apoptosis in Gastric Cancer. Carcinogenesis 2005, 26, 541–546. [Google Scholar] [CrossRef]
- Desai, T.H.; Joshi, S.V. In Silico Evaluation of Apoptogenic Potential and Toxicological Profile of Triterpenoids. Indian J. Pharmacol. 2019, 51, 181–207. [Google Scholar] [CrossRef]
- Daniel, A.G.; Peterson, E.J.; Farrell, N.P. The Bioinorganic Chemistry of Apoptosis: Potential Inhibitory Zinc Binding Sites in Caspase-3. Angew. Chem. Int. Ed. 2014, 53, 4098–4101. [Google Scholar] [CrossRef]
- Lee, D.; Long, S.A.; Adams, J.L.; Chan, G.; Vaidya, K.S.; Francis, T.A.; Kikly, K.; Winkler, J.D.; Sung, C.-M.; Debouck, C.; et al. Potent and Selective Nonpeptide Inhibitors of Caspases 3 and 7 Inhibit Apoptosis and Maintain Cell Functionality. J. Biol. Chem. 2000, 275, 16007–16014. [Google Scholar] [CrossRef]
- Jamal, S.; Grover, A.; Grover, S. Machine Learning from Molecular Dynamics Trajectories to Predict Caspase-8 Inhibitors against Alzheimer’s Disease. Front. Pharmacol. 2019, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Watt, W.; Koeplinger, K.A.; Mildner, A.M.; Heinrikson, R.L.; Tomasselli, A.G.; Watenpaugh, K.D. The Atomic-Resolution Structure of Human Caspase-8, a Key Activator of Apoptosis. Structure 1999, 7, 1135–1143. [Google Scholar] [CrossRef]
- Carter, P.; Wells, J.A. Dissecting the Catalytic Triad of a Serine Protease. Nature 1988, 332, 564–568. [Google Scholar] [CrossRef]
- Cole, E.R.; de Andrade, J.P.; Filho, J.F.A.; Schmitt, E.F.P.; Alves-Araújo, A.; Bastida, J.; Endringer, D.C.; de S. Borges, W.; Lacerda, V. Cytotoxic and Genotoxic Activities of Alkaloids from the Bulbs of Griffinia gardneriana and Habranthus itaobinus (Amaryllidaceae). Anticancer Agents Med. Chem. 2019, 19, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Rawat, A.; Keshari, A.K.; Singh, A.K.; Maity, S.; De, A.; Samanta, A.; Saha, S. Antiproliferative Effect of Isolated Isoquinoline Alkaloid from Mucuna pruriens Seeds in Hepatic Carcinoma Cells. Nat. Prod. Res. 2016, 30, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Fadaeinasab, M.; Karimian, H.; Omar, H.; Taha, H.; Khorasani, A.; Banisalam, B.; Ketuly, K.A.; Abdullah, Z. Reflexin A, a New Indole Alkaloid from Rauvolfia reflexa Induces Apoptosis against Colon Cancer Cells. J. Asian Nat. Prod. Res. 2020, 22, 474–488. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Thomsen, R.; Christensen, M.H. MolDock: A New Technique for High-Accuracy Molecular Docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef]
- Ganesan, R.; Jelakovic, S.; Mittl, P.R.E.; Caflisch, A.; Grütter, M.G. In Silico Identification and Crystal Structure Validation of Caspase-3 Inhibitors without a P1 Aspartic Acid Moiety. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2011, 67, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Watt, W.; Brooks, N.A.; Harris, M.S.; Urban, J.; Boatman, D.; McMillan, M.; Kahn, M.; Heinrikson, R.L.; Finzel, B.C.; et al. Kinetic and Structural Characterization of Caspase-3 and Caspase-8 Inhibition by a Novel Class of Irreversible Inhibitors. Biochim. Et Biophys. Acta (BBA) Proteins Proteom. 2010, 1804, 1817–1831. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.N.; Silva, S.G.; Pereira, D.S.; Filho, A.P.d.S.S.; de Oliveira, M.S.; Lima, R.R.; Andrade, E.H.d.A. In Silico Evaluation of the Antimicrobial Activity of Thymol—Major Compounds in the Essential Oil of Lippia thymoides Mart. & Schauer (Verbenaceae). Molecules 2022, 27, 4768. [Google Scholar] [CrossRef] [PubMed]
- Cornell, W.D.; Cieplak, P.; Bayly, C.I.; Kollman, P.A. Application of RESP Charges to Calculate Conformational Energies, Hydrogen Bond Energies, and Free Energies of Solvation. J. Am. Chem. Soc. 1993, 115, 9620–9631. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic Atom Type and Bond Type Perception in Molecular Mechanical Calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General Amber Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Araújo, P.H.F.; Ramos, R.S.; da Cruz, J.N.; Silva, S.G.; Ferreira, E.F.B.; de Lima, L.R.; Macêdo, W.J.C.; Espejo-Román, J.M.; Campos, J.M.; Santos, C.B.R. Identification of Potential COX-2 Inhibitors for the Treatment of Inflammatory Diseases Using Molecular Modeling Approaches. Molecules 2020, 25, 4183. [Google Scholar] [CrossRef]
- Neto, R.d.A.M.; Santos, C.B.R.; Henriques, S.V.C.; Machado, L.d.O.; Cruz, J.N.; da Silva, C.H.T.d.P.; Federico, L.B.; Oliveira, E.H.C.d.; de Souza, M.P.C.; da Silva, P.N.B.; et al. Novel Chalcones Derivatives with Potential Antineoplastic Activity Investigated by Docking and Molecular Dynamics Simulations. J. Biomol. Struct. Dyn. 2022, 40, 2204–2216. [Google Scholar] [CrossRef]
- Lima, A.d.M.; Siqueira, A.S.; Möller, M.L.S.; Souza, R.C.d.; Cruz, J.N.; Lima, A.R.J.; Silva, R.C.d.; Aguiar, D.C.F.; Junior, J.L.d.S.G.V.; Gonçalves, E.C. In Silico Improvement of the Cyanobacterial Lectin Microvirin and Mannose Interaction. J. Biomol. Struct. Dyn. 2022, 40, 1064–1073. [Google Scholar] [CrossRef]
- de Oliveira, M.S.; da Cruz, J.N.; da Costa, W.A.; Silva, S.G.; Brito, M.d.P.; de Menezes, S.A.F.; Neto, A.M.d.J.C.; Andrade, E.H.d.A.; Junior, R.N.d.C. Chemical Composition, Antimicrobial Properties of Siparuna Guianensis Essential Oil and a Molecular Docking and Dynamics Molecular Study of Its Major Chemical Constituent. Molecules 2020, 25, 3852. [Google Scholar] [CrossRef]
- Dolinsky, T.J.; Nielsen, J.E.; McCammon, J.A.; Baker, N.A. PDB2PQR: An Automated Pipeline for the Setup of Poisson-Boltzmann Electrostatics Calculations. Nucleic Acids Res. 2004, 32, W665–W667. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. Ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from Ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Cruz, J.N.; De Oliveira, M.S.; Silva, S.G.; Filho, A.P.d.S.S.; Pereira, D.S.; Lima, E.A.H.L.; Andrade, E.H.d.A. Insight into the Interaction Mechanism of Nicotine, NNK, and NNN with Cytochrome P450 2A13 Based on Molecular Dynamics Simulation. J. Chem. Inf. Model. 2020, 60, 766–776. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle Mesh Ewald: An N⋅log(N) Method for Ewald Sums in Large Systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J.C. Numerical Integration of the Cartesian Equations of Motion of a System with Constraints: Molecular Dynamics of n-Alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Izaguirre, J.A.; Catarello, D.P.; Wozniak, J.M.; Skeel, R.D. Langevin Stabilization of Molecular Dynamics. J. Chem. Phys. 2001, 114, 2090–2098. [Google Scholar] [CrossRef]
- Almeida, V.M.; Dias, Ê.R.; Souza, B.C.; Cruz, J.N.; Santos, C.B.R.; Leite, F.H.A.; Queiroz, R.F.; Branco, A. Methoxylated Flavonols from Vellozia dasypus Seub Ethyl Acetate Active Myeloperoxidase Extract: In Vitro and in Silico Assays. J. Biomol. Struct. Dyn. 2021, 40, 7574–7583. [Google Scholar] [CrossRef]
- Castro, A.L.G.; Cruz, J.N.; Sodré, D.F.; Correa-Barbosa, J.; Azonsivo, R.; de Oliveira, M.S.; Siqueira, J.E.d.S.; Galucio, N.C.D.R.; Bahia, M.D.O.; Burbano, R.M.R.; et al. Evaluation of the Genotoxicity and Mutagenicity of Isoeleutherin and Eleutherin Isolated from Eleutherine Plicata Herb. Using Bioassays and in Silico Approaches. Arab. J. Chem. 2021, 14, 103084. [Google Scholar] [CrossRef]
- Mascarenhas, A.M.S.; de Almeida, R.B.M.; Neto, M.F.d.A.; Mendes, G.O.; da Cruz, J.N.; dos Santos, C.B.R.; Botura, M.B.; Leite, F.H.A. Pharmacophore-Based Virtual Screening and Molecular Docking to Identify Promising Dual Inhibitors of Human Acetylcholinesterase and Butyrylcholinesterase. J. Biomol. Struct. Dyn. 2021, 39, 6021–6030. [Google Scholar] [CrossRef]
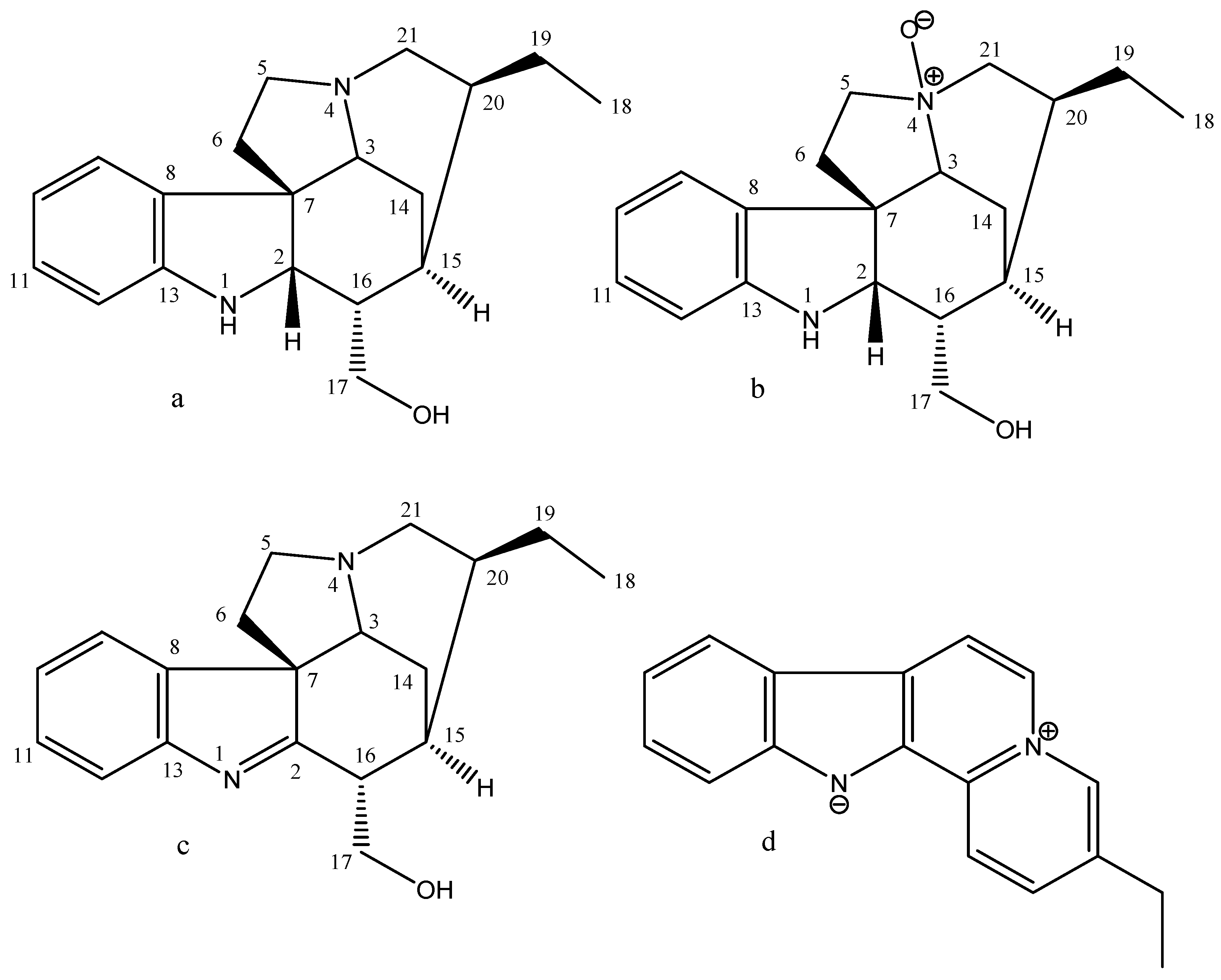

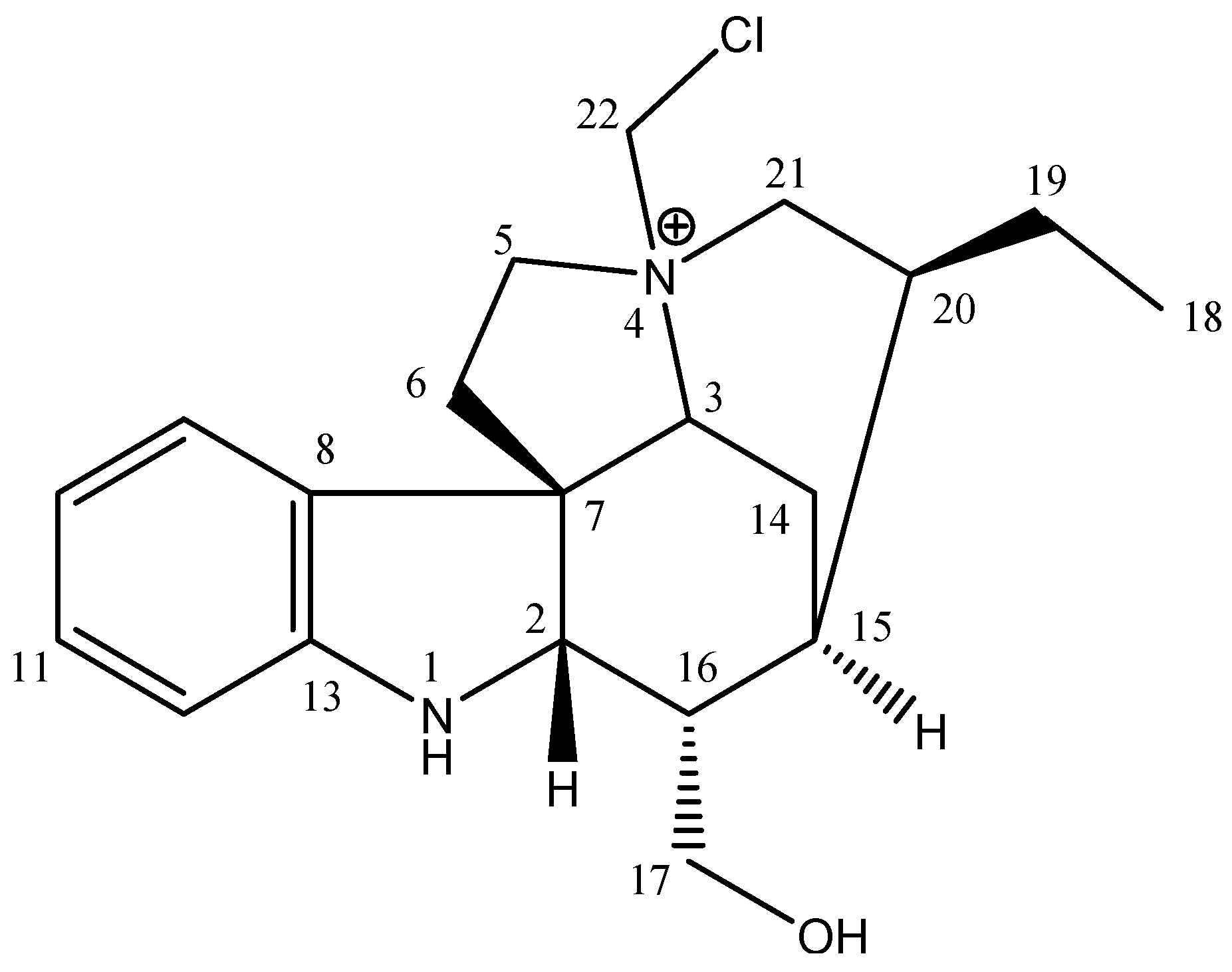



| Samples | Yield (%) | TLC | |
|---|---|---|---|
| UV Light | Dragendorff | ||
| EE | 2.2 | + | + |
| NF | 17.3 | + | + |
| AF | 10.7 | + | + |
| Position | 1H-NMR | 13C-NMR | HMBC |
|---|---|---|---|
| 1 | |||
| 2 | 4.03 (d, 5.5) | 64.3 | C-3, C-7, C-15, C-16 |
| 3 | 3.72 (dd, 14.5, 4.2) | 77.0 | C-8, C-15, C-22 |
| 4 | |||
| 5 | 3.85 (dd, 12.0, 6.8) 3.96 (ddd, 12.0, 11.8, 8.1) | 64.2 | C-3, C-7 |
| 6 | 2.66 (m) | 35.1 | C-7, C-8 |
| 7 | 53.4 | ||
| 8 | 134.2 | ||
| 9 | 7.29 (d, 7.5) | 123.7 | C-7, C-11, C-13 |
| 10 | 6.80 (t, 7.5) | 121.0 | C-8, C-12 |
| 11 | 7.08 (t, 7.5) | 130.4 | C-9, C-13 |
| 12 | 6.65 (d, 7.7) | 111.4 | C-8, C-10 |
| 13 | 151.0 | ||
| 14 | 2.43 (dd, 15.0, 2.6) 1.87 (dt, 15.0, 3.1) | 23.3 | C-15 |
| 15 | 1.74 (br) | 28.7 | |
| 16 | 2.16 (m) | 34.8 | C-17, C-20 |
| 17 | 3.64 (dd, 10.5, 6.8) 3.79 (dd, 10.5, 9,7) | 65.6 | C-2, C-15, C-16 |
| 18 | 1.02 (t, 7.4) | 11.5 | C-19, C-20 |
| 19 | 1.33 (m) 1.46 (m) | 23.6 | C-15, C-18, C-20, C-21 |
| 20 | 1.96 (m) | 39.2 | C-16 |
| 21 | 3.42 (t, 14.0) 3.71 (m) | 54.2 | C-20, C-22 |
| 22 | 5.40 (d, 10.0) 5.59 (d, 10.0) | 67.1 | C-3, C-5, C-21 |
| Samples | ACP02 | Vero Cells | HepG2 Cells | ||
|---|---|---|---|---|---|
| IC50 (µg/mL) | CC50 (µg/mL) | SIACP02 | CC50 (µg/mL) | SIACP02 | |
| EE | 131.55 ± 0.49 | 113.65 ± 0.35 | 0.86 | 472.55 ± 0.63 | 3.59 |
| NF | 114.45 ± 0.21 | 130.10 ± 0.42 | 1.13 | 259.30 ± 0.42 | 2.27 |
| AF | 18.29 ± 0.02 | 173.3 ± 0.32 | 9.48 | 299.45 ± 0.35 | 16.37 |
| Geissoschizoline N4-methylchlorine | 12.06 ± 0.04 | 476.0 ± 0.54 | 39.47 | 50.5 ± 0.28 | 41.75 |
| Compound | Molecular Target | |
|---|---|---|
| Caspase-3 | Caspase-8 | |
| Geissoschizoline N4-methylchlorine | −49.72 | −56.31 |
| Complex | ΔEvdW | ΔEele | ΔGGB | ΔGNP | ΔGbind |
|---|---|---|---|---|---|
| Caspase-3 | −32.15 | −9.34 | 10.38 | −4.51 | −35.62 |
| Caspase-8 | −30.64 | −8.84 | 12.42 | −3.63 | −30.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmo Bastos, M.L.; Silva-Silva, J.V.; Neves Cruz, J.; Palheta da Silva, A.R.; Bentaberry-Rosa, A.A.; da Costa Ramos, G.; de Sousa Siqueira, J.E.; Coelho-Ferreira, M.R.; Percário, S.; Santana Barbosa Marinho, P.; et al. Alkaloid from Geissospermum sericeum Benth. & Hook.f. ex Miers (Apocynaceae) Induce Apoptosis by Caspase Pathway in Human Gastric Cancer Cells. Pharmaceuticals 2023, 16, 765. https://doi.org/10.3390/ph16050765
Carmo Bastos ML, Silva-Silva JV, Neves Cruz J, Palheta da Silva AR, Bentaberry-Rosa AA, da Costa Ramos G, de Sousa Siqueira JE, Coelho-Ferreira MR, Percário S, Santana Barbosa Marinho P, et al. Alkaloid from Geissospermum sericeum Benth. & Hook.f. ex Miers (Apocynaceae) Induce Apoptosis by Caspase Pathway in Human Gastric Cancer Cells. Pharmaceuticals. 2023; 16(5):765. https://doi.org/10.3390/ph16050765
Chicago/Turabian StyleCarmo Bastos, Mirian Letícia, João Victor Silva-Silva, Jorddy Neves Cruz, Amanda Roberta Palheta da Silva, Alexandre Augusto Bentaberry-Rosa, Gisele da Costa Ramos, José Edson de Sousa Siqueira, Márlia Regina Coelho-Ferreira, Sandro Percário, Patrícia Santana Barbosa Marinho, and et al. 2023. "Alkaloid from Geissospermum sericeum Benth. & Hook.f. ex Miers (Apocynaceae) Induce Apoptosis by Caspase Pathway in Human Gastric Cancer Cells" Pharmaceuticals 16, no. 5: 765. https://doi.org/10.3390/ph16050765
APA StyleCarmo Bastos, M. L., Silva-Silva, J. V., Neves Cruz, J., Palheta da Silva, A. R., Bentaberry-Rosa, A. A., da Costa Ramos, G., de Sousa Siqueira, J. E., Coelho-Ferreira, M. R., Percário, S., Santana Barbosa Marinho, P., Marinho, A. M. d. R., de Oliveira Bahia, M., & Dolabela, M. F. (2023). Alkaloid from Geissospermum sericeum Benth. & Hook.f. ex Miers (Apocynaceae) Induce Apoptosis by Caspase Pathway in Human Gastric Cancer Cells. Pharmaceuticals, 16(5), 765. https://doi.org/10.3390/ph16050765










