Tris(aminomethyl)phosphines and Their Copper(I) (Pseudo)halide Complexes with Aromatic Diimines—A Critical Retrospection
Abstract
1. Introduction
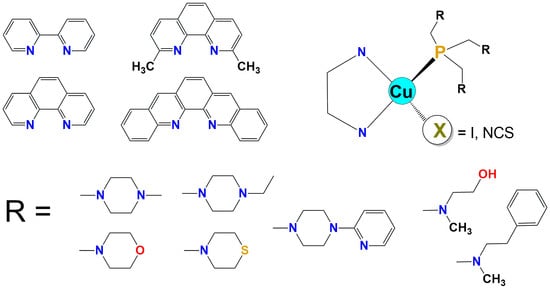
2. Synthesis and the Characteristics of Tris(aminomethyl)phosphines
3. [CuX(NN)PR3] Complexes
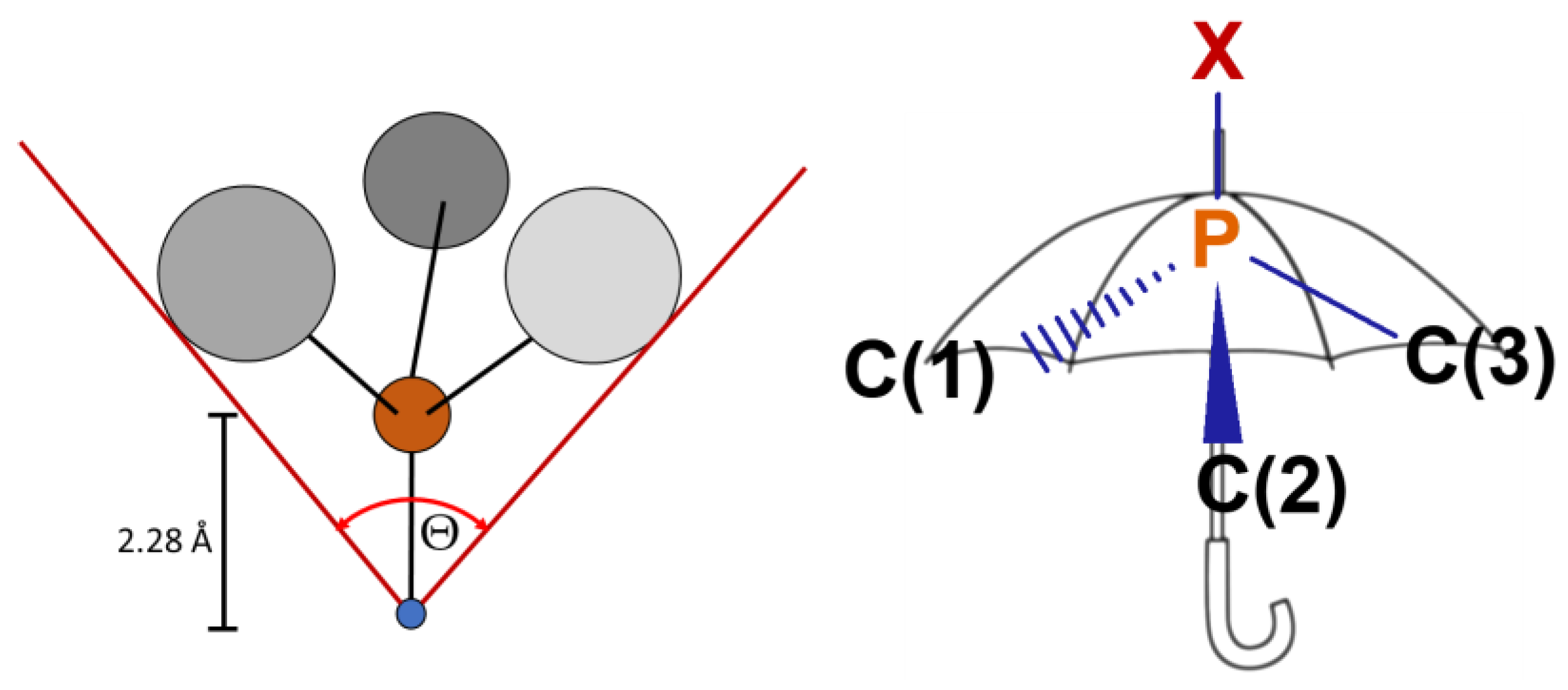
3.1. X-ray Structures of the Complexes
3.2. Solid-State Luminescence
4. Antimicrobial Activity
5. In Vitro Cytotoxicity
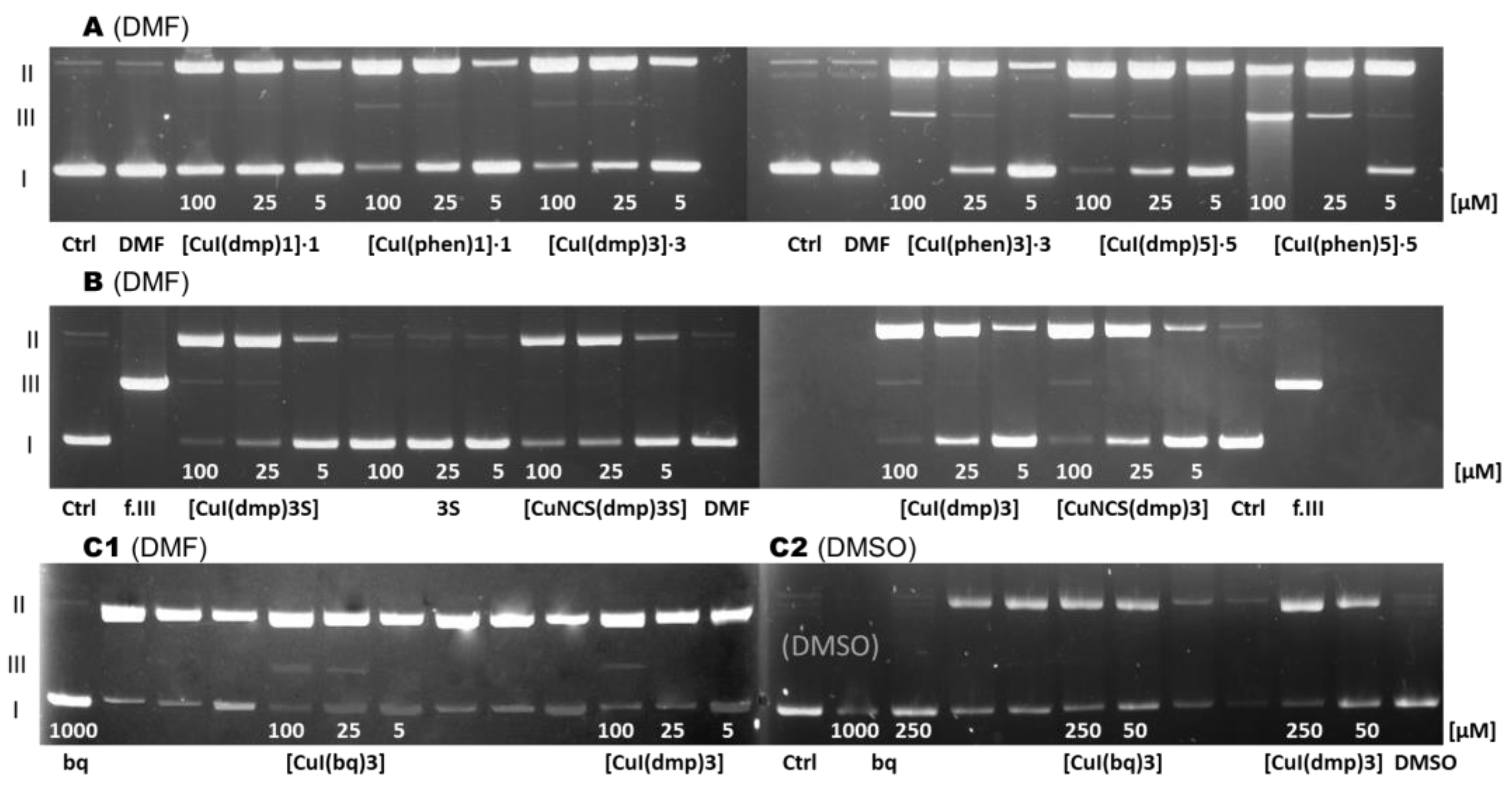
6. Interactions with Plasmid DNA and Serum Albumins
7. Conclusions
- (1)
- Tris(aminomethyl)phosphines are characterized by extremely low antimicrobial activity, negligible in vitro cytotoxicity, and low activity in inducing DNA damage. This finding is particularly important, because phosphines are commonly considered to be a toxic class of compounds. There is a strong certainty among the community of chemists that phosphines belong to a very poisonous class of compounds, which probably originates from a high toxicity of phosphorus trihydride PH3 [62,63]. Sometimes it is connected to the fact that some of the molecules of the chemical warfare gases (Tabun, Sarin, Soman, VX [64]) contain phosphorus atoms. Admittedly, when starting this project, we expected a high biological activity of these ligands; however, our studies showed that phosphines, at least tris(aminomethyl)phosphines, were not active in most of the in vitro tests.
- (2)
- Regarding the structural aspects, the presented phosphines are characterized by very large Tolman’s cone angles, which indicates their high steric demands. On the other hand, the structural S4′ and S4 parameters of the tris(aminomethyl) phosphines are also high, which indicates a low bulkiness of these compounds in the direct vicinity of the phosphorus atom and makes them convenient ligands in the synthesis of transition metal complexes. It should be noted that tris(aminomethyl)phosphines may be a good alternative for commonly used triphenylphosphine (PPh3) in the complexes of different transition metals. A great example of successful usage of a less popular phosphine ligand are two ruthenium(II) complexes, RAPTA-C and RAPTA-T [2], bearing a molecule of 1,3,5-triaza-7-phosphaadamantane, which also can be regarded as a tris(aminomethyl)phosphine.
- (3)
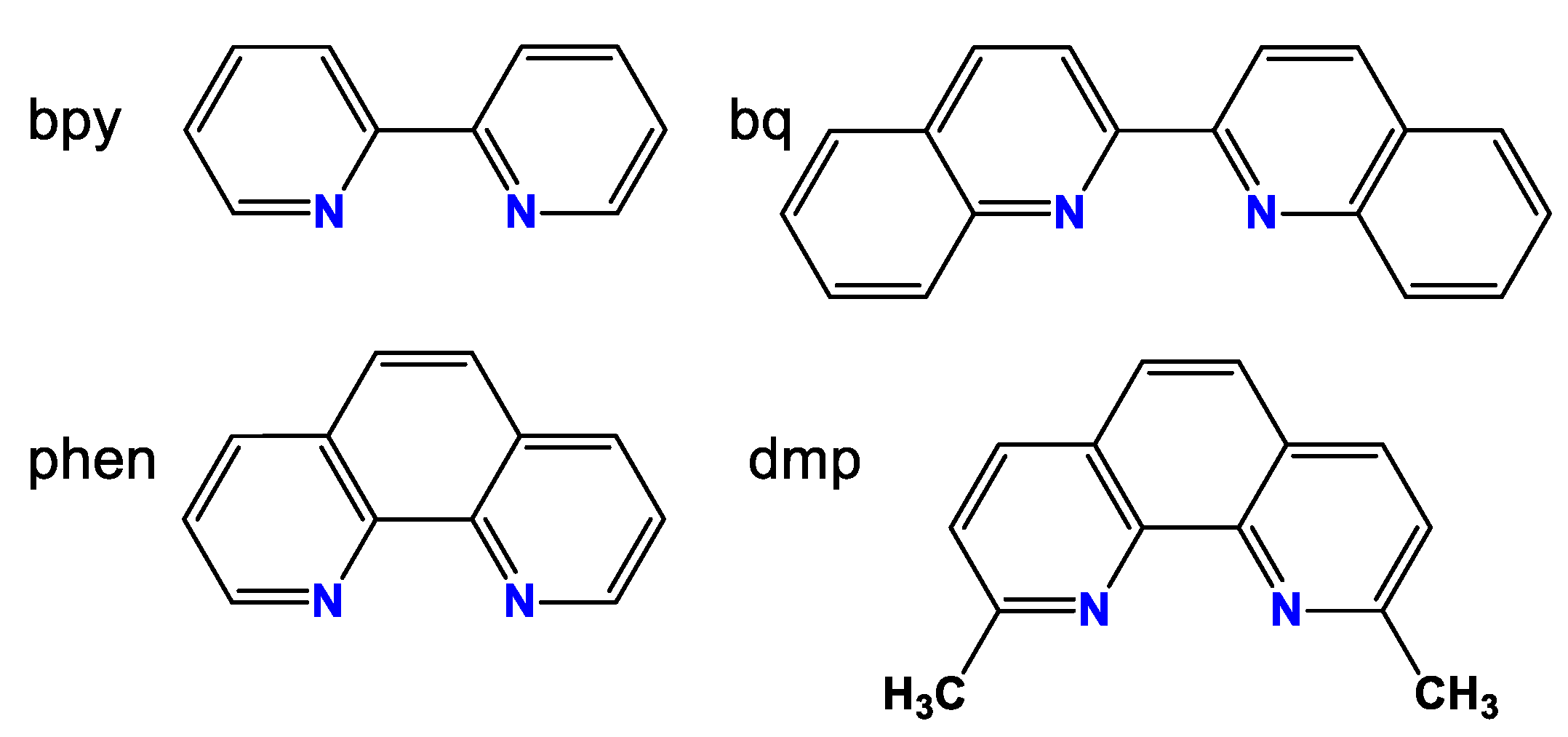
- Stability of the Cu(I) (pseudo)halogenide complexes with phosphines and diimines depends mainly on the diimine ligand. Complexes with 2,2 ‘-bipyridyl (bpy) are the least stable, complexes with 1,10-phenanthroline (phen) proved to be much more stable, and the stability of complexes of 2,9-dimethyl-1,10-phenanthroline (dmp) and 2,2 ‘-quinoline (bq) ligands is very high if not unlimited.
- (4)
- TDDFT theoretical studies showed that MLCT absorption and emission bands observed for the studied complexes may be more accurately defined as (MX,MPR3)LCT bands resulting from the electron transfer from the copper–(pseudo)halide bond with a small admixture of copper–phosphorus bond to the π* orbitals of diimine ligands.
- (5)
- Significant differences in the position of the emission bands maxima observed for the complexes with various anions and phosphine ligands have led us to a rather unexpected conclusion. Namely, the position of the luminescence bands depends mostly on the orientation of molecules in the unit cell, which directly affects the type of the π-stacking interactions between the diimine rings. The role of the other ligands is reduced to modifications of the molecular packing in a crystal cell.
- (6)
- The complexes with highly sterically demanding dmp are characterized by high solid-state photoluminescence intensity and relatively long lifetimes in contrast to complexes with bpy and phen. The luminescence intensity also depends on other ligands and is higher for the iodide complexes than the thiocyanate ones, what is reflected in the significantly shorter luminescence lifetimes of the latter complexes. Structural analysis with DFT methods showed that the main factor determining the luminescence intensity is the rigidity of the immediate surroundings of the copper ion. For complexes with the ligands with high steric requirements, the possibility of deformation of the molecule in an excited state is minimal, therefore the probability of nonradiative transfer to the ground state is also much lower, resulting in elongation of the MLCT excited state lifetimes and more intense luminescence.
- (7)
- The copper complexes presented herein are interesting as potential antibacterial and antifungal agents, as well as potential cytostatics, as demonstrated by the in vitro studies on selected cell lines. A relatively high selectivity of cytostatic (or cytotoxic) properties makes these compounds promising potential therapeutics.
- (8)
- The biological properties of the complexes are most probably a function of the mixed molecular structure. On one hand, we have a diimine molecule capable of π-stacking interactions with rings of tryptophan, tyrosine, and phenylalanine and partial intercalation to the DNA or RNA chain; on the other hand, we have a phosphine ligand with easily modifiable steric properties, hydrophilicity, and a capacity of potential specific interactions. The type of the (pseudo)halide and phosphine used affects the biological activity of the complexes much less, but it is not negligible.
- (9)
- Complexes with dmp showed the greatest activity. The complexes with bq and phen were less active, although in the latter case this is most likely caused by the relatively low stability.
- (10)
- The interactions between the tested complexes and biomolecules confirm the promising properties of these compounds. Importantly, they bind to serum albumins without changing their tertiary structure and without oxidation and decomposition. Most of the complexes induce DNA degradation through a free radical mechanism; however, they cause a double cut of plasmids only at high concentrations.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, S.; Guo, Y.; Guo, Z.; Wang, X. Monofunctional Platinum(II) Anticancer Agents. Pharmaceuticals 2021, 14, 133. [Google Scholar] [CrossRef]
- Allardycea, C.S.; Dyson, P.J. Metal-based drugs that break the rules. Dalton Trans. 2016, 45, 3201–3209. [Google Scholar] [CrossRef]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs are unique: Opportunities and challenges of discovery and development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef]
- Karges, J.; Stokes, R.W.; Cohen, S.M. Metal Complexes for Therapeutic Applications. Trends Chem. 2021, 3, 523–534. [Google Scholar] [CrossRef]
- Franz, K.J.; Metzler-Nolte, N. Introduction: Metals in Medicine. Chem. Rev. 2019, 119, 727–772. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Wang, P.; Chen, H.; Xu, Y.; Ge, J.; Tian, Z.; Yan, Z. Potential of Copper and Copper Compounds for Anticancer Applications. Pharmaceuticals 2023, 16, 234. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in Copper Complexes as Anticancer Agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef]
- Starosta, R.; Florek, M.; Król, J.; Puchalska, M.; Kochel, A. Copper(I) iodide complexes containing new aliphatic aminophosphine ligands and diimines—Luminescent properties and antibacterial activity. New J. Chem. 2010, 34, 1441–1449. [Google Scholar] [CrossRef]
- Starosta, R.; Bażanów, B.; Barszczewski, W. Chalcogenides of the aminomethylphosphines derived from 1-methylpiperazine, 1-ethylpiperazine and morpholine: NMR, DFT and structural studies for determination of electronic and steric properties of the phosphines. Dalton Trans. 2010, 39, 7547–7555. [Google Scholar] [CrossRef]
- Starosta, R.; Puchalska, M.; Cybińska, J.; Barys, M.; Mudring, A.-V. Structures, electronic properties and solid state luminescence of Cu(I) iodide complexes with 2,9-dimethyl-1,10-phenanthroline and aliphatic aminomethylphosphines or triphenylphosphine. Dalton Trans. 2011, 40, 2459–2468. [Google Scholar] [CrossRef]
- Starosta, R.; Stokowa, K.; Florek, M.; Król, J.; Chwiłkowska, A.; Kulbacka, J.; Saczko, J.; Skała, J.; Jeżowska-Bojczuk, M. Biological activity and structure dependent properties of cuprous iodide complexes with phenanthrolines and water soluble tris(aminomethyl)phosphanes. J. Inorg. Biochem. 2011, 105, 1102–1108. [Google Scholar] [CrossRef]
- Starosta, R.; Komarnicka, U.K.; Sobczyk, M.; Barys, M. Laser induced multi-component luminescence of [CuNCS(1,10-phen)P(CH2N(CH2CH2)2O)3]–the first example of CuNCS complexes with diimines and tris(aminomethyl)phosphanes. J. Lumin. 2012, 132, 1842–1847. [Google Scholar] [CrossRef]
- Starosta, R.; Komarnicka, U.K.; Puchalska, M.; Barys, M. Solid state luminescence of copper(I) (pseudo)halide complexes with neocuproine and aminomethylphosphanes derived from morpholine and thiomorpholine. New J. Chem. 2012, 36, 1673–1683. [Google Scholar] [CrossRef]
- Starosta, R.; Bykowska, A.; Kyzioł, A.; Płotek, M.; Florek, M.; Król, J.; Jezowska-Bojczuk, M. Copper(I) (pseudo)halide complexes with neocuproine and aminomethylphosphines derived from morpholine and thiomorpholine—In vitro cytotoxic and antimicrobial activity and the interactions with DNA and Serum Albumins. Chem. Biol. Drug Des. 2013, 82, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Starosta, R.; Brzuszkiewicz, A.; Bykowska, A.; Komarnicka, U.K.; Bażanów, B.; Florek, M.; Gadzała, Ł.; Jackulak, N.; Król, J.; Marycz, K. A novel copper(I) complex, [CuI(2,2′-biquinoline)P(CH2N(CH2CH2)2O)3]—Synthesis, characterisation and comparative studies on biological activity. Polyhedron 2013, 50, 481–489. [Google Scholar] [CrossRef]
- Starosta, R.; Komarnicka, U.K.; Puchalska, M. Luminescent copper(I) (pseudo)halide complexes with neocuproine and a novel bulky tris(aminomethyl)phosphine derived from 2-piperazinopyridine. J. Lumin. 2013, 143, 137–144. [Google Scholar] [CrossRef]
- Starosta, R.; Komarnicka, U.K.; Puchalska, M. Solid state luminescence of CuI and CuNCS complexes with phenanthrolines and a new tris(aminomethyl)phosphine derived from N-methyl-2-phenylethanamine. J. Lumin. 2014, 145, 430–437. [Google Scholar] [CrossRef]
- Coates, H.; Hoye, P.A.T. Tris-Aminomethylphosphines. U.S. Patent 3,035,053, 15 May 1962. [Google Scholar]
- Moiseev, D.V.; James, B.R. Phospha-Mannich reactions of PH3 and its analogs. Phosphorus Sulfur Silicon Relat. Elem. 2022, 197, 277–326. [Google Scholar] [CrossRef]
- Smith, M.B. The Backbone of Success of P,N-Hybrid Ligands: Some Recent Developments. Molecules 2022, 27, 6293. [Google Scholar] [CrossRef]
- Keen, A.L.; Doster, M.; Han, H.; Johnson, S.A. Facile assembly of a Cu9 amido complex: A new tripodal ligand design that promotes transition metal cluster formation. Chem. Commun. 2006, 2006, 1221–1223. [Google Scholar] [CrossRef]
- Hatnean, J.A.; Raturi, R.; Lefebvre, J.; Leznoff, D.B.; Lawes, G.; Johnson, S.A. Assembly of Triangular Trimetallic Complexes by Triamidophosphine Ligands: Spin-Frustrated Mn2+ Plaquettes and Diamagnetic Mg2+ Analogues with a Combined Through-Space, Through-Bond Pathway for 31P-31P Spin−Spin Coupling. J. Am. Chem. Soc. 2006, 128, 14992–14999. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Elsmaili, M.; Johnson, S.A. Diligating Tripodal Amido-Phosphine Ligands: the Effect of a Proximal Antipodal Early Transition Metal on Phosphine Donor Ability in a Building Block for Heterometallic Complexes. Inorg. Chem. 2006, 45, 7435–7445. [Google Scholar] [CrossRef]
- Marzano, C.; Gandin, V.; Pellei, M.; Colavito, D.; Papini, G.; Lobbia, G.G.; Del Giudice, E.; Porchia, M.; Tisato, F.; Santini, C. In Vitro Antitumor Activity of the Water Soluble Copper(I) Complexes Bearing the Tris(hydroxymethyl)phosphine Ligand. J. Med. Chem. 2008, 51, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Marzano, C.; Pellei, M.; Alidori, S.; Brossa, A.; Lobbia, G.G.; Tisato, F.; Santini, C. New copper(I) phosphane complexes of dihydridobis(3-nitro-1,2,4-triazolyl)borate ligand showing cytotoxic activity. J. Inorg. Biochem. 2006, 100, 299–304. [Google Scholar] [CrossRef]
- Rauf, M.K.; Imtiaz-ud-Din; Badshah, A.; Gielen, M.; Ebihara, M.; de Vos, D.; Ahmed, S. Synthesis, structural characterization and in vitro cytotoxicity and anti-bacterial activity of some copper(I) complexes with N,N’-disubstituted thioureas. J. Inorg. Biochem. 2009, 103, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Sanghamitra, N.J.; Phatak, P.; Das, S.; Samuelson, A.G.; Somasusundaram, K. Mechanism of cytotoxicity of copper(I) complexes of 1,2-bis(diphenylphosphino)ethane. J. Med. Chem. 2005, 48, 977–985. [Google Scholar] [CrossRef] [PubMed]
- McKeage, M.J.; Papathanasiou, P.; Salem, G.; Sjaarda, A.; Swiegers, G.F.; Waring, P.; Wild, S.B. Antitumor activity of gold(i), silver(i) and copper(i) complexes containing chiral tertiary phosphines. Met.-Based Drugs 1998, 5, 217–223. [Google Scholar] [CrossRef]
- Balakrishna, M.S.; Suresh, D.; Rai, A.; Mague, J.T.; Panda, D. Dinuclear copper(I) complexes containing cyclodiphosphazane derivatives and pyridyl ligands: Synthesis, structural studies, and antiproliferative activity toward human cervical and breast cancer cells. Inorg. Chem. 2010, 49, 8790–87801. [Google Scholar] [CrossRef]
- Lazarou, K.; Bednarz, B.; Kubicki, M.; Verginadis, I.I.; Charalabopoulos, K.; Kourkoumelis, N.; Hadjikakou, S.K. Structural photolysis and biological studies of the bis(μ2-chloro)-tris(triphenylphosphine)-di-copper(I) and chloro-tris(triphenylphosphine)-copper(I) complexes. Study of copper(I)-copper(I) interactions. Inorg. Chim. Acta 2010, 363, 763–772. [Google Scholar] [CrossRef]
- Marzano, C.; Pellei, M.; Colavito, D.; Alidori, S.; Lobbia, G.G.; Gandin, V.; Tisato, F.; Santini, C. Synthesis, Characterization, and in Vitro Antitumor Properties of Tris(hydroxymethyl)phosphine Copper(I) Complexes Containing the New Bis(1,2,4-triazol-1-yl)acetate Ligand. J. Med. Chem. 2006, 49, 7317–7324. [Google Scholar] [CrossRef]
- Marzano, C.; Pellei, M.; Tisato, F.; Santini, C. Copper complexes as anticancer agents. Anti-Cancer Agents Med. Chem. 2009, 9, 185–211. [Google Scholar] [CrossRef] [PubMed]
- Vorontsov, I.I.; Graber, T.; Kovalevsky, A.Y.; Novozhilova, I.V.; Gembicky, M.; Chen, Y.-S.; Coppens, P. Capturing and Analyzing the Excited-State Structure of a Cu(I) Phenanthroline Complex by Time-Resolved Diffraction and Theoretical Calculations. J. Am. Chem. Soc. 2009, 131, 6566–6573. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, J.R.; McMillin, D.R.; Robinson, W.R.; Powell, D.R.; McKenzie, A.T.; Chen, S. Steric effects and the behavior of Cu(NN)(PPh3)2+ systems in fluid solution. Crystal and molecular structures of [Cu(dmp)(PPh3)2]NO3 and [Cu(phen)(PPh3)2]NO3∙1.5EtOH. Inorg. Chem. 1985, 24, 3928–3933. [Google Scholar] [CrossRef]
- Sakaki, S.; Mizutani, H.; Kase, Y.; Inokuchi, K.; Arai, T.; Hamada, T. Photoinduced electron transfer between [Cu(dmphen)L2]+ [dmphen = 2,9-dimethyl-1,10-phenanthroline, L = PPhn(C6H4OMe-p)3–n (n = 0–3)] and methyl viologen. J. Chem. Soc. Dalton Trans. 1996, 1996, 1909–1914. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Li, G.; Xie, Y.; Zhao, D. Crystallographic and spectroscopic studies of a luminescent binuclear copper(I) complex containing mixed-ligands. Trans. Met. Chem. 2003, 28, 772–776. [Google Scholar] [CrossRef]
- Li, D.; Li, R.-Z.; Ni, Z.; Qi, Z.-Y.; Feng, X.-L.; Cai, J.-W. Synthesis and crystal structure of photoluminescent copper(I)–phosphine complexes with oxygen and nitrogen donor ligands. Inorg. Chem. Commun. 2003, 6, 469–473. [Google Scholar] [CrossRef]
- Yang, L.; Feng, J.-K.; Ren, A.-M.; Zhang, M.; Ma, Y.-G.; Liu, X.-D. Structures, Electronic States and Electroluminescent Properties of a Series of CuI Complexes. Eur. J. Inorg. Chem. 2005, 2005, 1867–1879. [Google Scholar] [CrossRef]
- McCormic, T.; Jia, W.-L.; Wang, S. Phosphorescent Cu(I) Complexes of 2-(2‘-pyridylbenzimidazolyl)benzene: Impact of Phosphine Ancillary Ligands on Electronic and Photophysical Properties of the Cu(I) Complexes. Inorg. Chem. 2006, 45, 147–155. [Google Scholar] [CrossRef]
- Moudam, O.; Kaeser, A.; Dalavoux-Nicot, B.; Duhayon, C.; Holler, M.; Accorsi, G.; Séguy, I.; Destruel, P.; Nierengarten, J.-F. Electrophosphorescent homo- and heteroleptic copper(i) complexes prepared from various bis-phosphine ligands. Chem. Commun. 2007, 2007, 3077–3079. [Google Scholar] [CrossRef]
- Fu, W.F.; Gan, X.; Jiao, J.; Chen, Y.; Yuan, M.; Chi, S.-M.; Yu, M.-M.; Xiong, S.-X. Synthesis, structural and spectroscopic characterization of mono- and binuclear copper(I) complexes with substituted diimine and phosphine ligands. Inorg. Chim. Acta 2007, 360, 2758–2766. [Google Scholar] [CrossRef]
- Listorti, A.; Accorsi, G.; Rio, Y.; Armaroli, N.; Moudam, O.; Gégout, A.; Delavaux-Nicot, B.; Holler, M.; Nierengarten, J.F. Heteroleptic Copper(I) Complexes Coupled with Methano[60]fullerene: Synthesis, Electrochemistry, and Photophysics. Inorg. Chem. 2008, 47, 6254–6262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, B.; Su, Z. Realization of High-Energy Emission from [Cu(N−N)(P−P)]+ Complexes for Organic Light-Emitting Diode Applications. J. Phys. Chem. C 2009, 113, 13968–13973. [Google Scholar] [CrossRef]
- Zhang, L.; Li, B.; Su, Z. Phosphorescence Enhancement Triggered by Π Stacking in Solid-State [Cu(N−N)(P−P)]BF4 Complexes. Langmuir 2009, 25, 2068–2074. [Google Scholar] [CrossRef]
- Shi, Y.-J.; Chen, S.-J.; Huang, B.; Chen, X.-T.; Zhang, Y.; You, X.-Z. The preparation, crystal structure and properties of a mixed-ligand copper(I) complex [CuI(PPh3)(DPPZ)]·DMF. J. Mol. Struct. 2003, 650, 27–32. [Google Scholar] [CrossRef]
- Gan, X.; Fu, W.-F.; Lin, Y.-Y.; Yuan, M.; Che, C.-M.; Chi, S.-M.; Li, H.-F.J.; Chen, J.-H.; Zhou, Z.-Y. Synthesis, structures and photophysical properties of polynuclear copper(I) iodide complexes containing phosphine and 4,4′-bipyridine ligands. Polyhedron 2008, 27, 2202–2208. [Google Scholar] [CrossRef]
- Pettinari, C.; di Nicola, C.; Marchetti, F.; Pettinari, R.; Skelton, B.W.; Somers, N.; White, A.H.; Robinson, W.T.; Chierotti, M.R.; Gobetto, R.; et al. Synthesis, characterization, spectroscopic and photophysical properties of new [Cu(NCS){(L-N)2 or (L′-N∧N)}(PPh3)] complexes (L-N, L′-N∧N = aromatic nitrogen base). Eur. J. Inorg. Chem. 2008, 2008, 1974–1984. [Google Scholar] [CrossRef]
- Krauter, J.G.E.; Beller, M. An Easy and Practical Synthetic Route to Electron Rich Water Soluble Ligands: α-Aminomethylation of Trishydroxymethylphosphine. Tetrahedron 2000, 56, 771–774. [Google Scholar] [CrossRef]
- Tolman, C.A. Electron donor-acceptor properties of phosphorus ligands. Substituent additivity. J. Am. Chem. Soc. 1970, 92, 2953–2956. [Google Scholar] [CrossRef]
- Dunne, B.J.; Morris, R.B.; Orpen, A.G.J. Structural systematics. Part 3. Geometry deformations in triphenylphosphine fragments: A test of bonding theories in phosphine complexes. J. Chem. Soc. Dalton Trans. 1991, 1991, 653–661. [Google Scholar] [CrossRef]
- Suresh, C.H. Molecular electrostatic potential approach to determining the steric effect of phosphine ligands in organometallic chemistry. Inorg. Chem. 2006, 45, 4982–4986. [Google Scholar] [CrossRef]
- Dunne, B.J.; Orpen, A.G. Triphenylphosphine: A redetermination. Acta Cryst. 1991, C47, 345–347. [Google Scholar] [CrossRef]
- Suresh, C.H.; Koga, N. Quantifying the Electronic Effect of Substituted Phosphine Ligands via Molecular Electrostatic Potential. Inorg. Chem. 2002, 41, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Mathew, J.; Thomas, T.; Suresh, C.H. Quantitative Assessment of the Stereoelectronic Profile of Phosphine Ligands. Inorg. Chem. 2007, 46, 10800–10809. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Tsuboyama, A.; Kuge, K.; Furugori, M.; Okada, S.; Hoshino, M.; Ueno, K. Photophysical properties of highly luminescent copper(I) halide complexes chelated with 1,2-bis(diphenylphosphino)benzene. Inorg. Chem. 2007, 46, 1992–2001. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-L.; Song, P.; Liao, J.; Wen, H.-R.; Hong, R.; Chen, Z.-N.; Chi, Y. Luminescent homodinuclear copper(I) halide complexes based on the 3,5-bis{6-(2,2′-dipyridyl)}pyrazole ligand. Inorg. Chem. Commun. 2010, 13, 1057–1060. [Google Scholar] [CrossRef]
- Du, L.; Lan, Z. Ultrafast structural flattening motion in photoinduced excited state dynamics of a bis(diimine) copper(i) complex. Phys. Chem. Chem. Phys. 2016, 18, 7641–7650. [Google Scholar] [CrossRef]
- Viganor, L.; Howe, O.; McCarron, P.; McCann, M.; Devereux, M. The Antibacterial Activity of Metal Complexes Containing 1,10- phenanthroline: Potential as Alternative Therapeutics in the Era of Antibiotic Resistance. Curr. Top. Med. Chem. 2017, 17, 1280–1302. [Google Scholar] [CrossRef]
- Zwicker, F.; Hauswald, H.; Debus, J.; Huber, P.E.; Weber, K.J. Impact of dimethyl sulfoxide on irradiation-related DNA double-strand-break induction, -repair and cell survival. Radiat. Eenviron. Biophys. 2019, 58, 417–424. [Google Scholar] [CrossRef]
- Petitpas, I.; Bhattacharya, A.A.; Twine, S.; East, M.; Curry, S. Crystal Structure Analysis of Warfarin Binding to Human Serum Albumin: Anatomy of Drug Site, I.J. Biol. Chem. 2001, 276, 22804–22809. [Google Scholar] [CrossRef]
- Phosphine: Lung Damaging Agent. Available online: https://www.cdc.gov/niosh/ershdb/emergencyresponsecard_29750035.html (accessed on 26 April 2023).
- Phosphine. Toxicological Review. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/338253/HPA_Phosphine_toxicological_overview_v1.pdf (accessed on 26 April 2023).
- Ganesan, K.; Raza, S.K.; Vijayaraghavan, R. Chemical warfare agents. J. Pharm. Bioallied. Sci. 2010, 2, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Rosko, M.C.; Espinoza, E.M.; Arteta, S.; Kromer, S.; Wheeler, J.P.; Castellano, F.N. Castellano Employing Long-Range Inductive Effects to Modulate Metal-to-Ligand Charge Transfer Photoluminescence in Homoleptic Cu(I) Complexes. Inorg. Chem. 2023, 62, 3248–3259. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Zhu, C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: A Trailblazing Journey to the Field of Biomedicine. ACS Appl. Biol. Mater. 2018, 1, 1768–1786. [Google Scholar] [CrossRef]
- Duo, Y.; Luo, G.; Zhang, W.; Wang, R.; Xiao, G.G.; Li, Z.; Li, X.; Chen, M.; Yoon, J.; Tang, B.Z. Noncancerous disease-targeting AIEgens. Chem. Soc. Rev. 2023, 52, 1024–1067. [Google Scholar] [CrossRef]
- Kaim, W.; Schwederski, B. Non-innocent ligands in bioinorganic chemistry—An overview. Coord. Chem. Rev. 2010, 254, 1580–1588. [Google Scholar] [CrossRef]




| 1 | 2 | 3 | 3S | 5 | 6 | 7 | PPh3 | |
|---|---|---|---|---|---|---|---|---|
| XRD | ||||||||
| av. d(P-C) [Å] | 1.855 | 1.856 | 1.845 | 1.830 [52] | ||||
| av. α(C-P-C) [deg] | 97.89 | 97.91 | 98.8 | 102.7 [52] | ||||
| S4′ | 64.8 | 64.6 | 59.8 | 8.5 [52] | ||||
| DFT | ||||||||
| Θ [deg] | 210.2 | 218.9 | 206.7 | 213.5 | 194.8 | 194.7 | 207.7 | 145 |
| S4 | 63.7 | 63.7 | 63.4 | 63.5 | 63.4 | 63.2 | 64.2 | 40.2 |
| Vmin [kcal/mol] | −35.74 | −36.06 | −30.38 | −27.95 | −31.71 | −35.10 | −32.22 | −34.67 |
| Seff | 2.70 | 2.73 | 2.66 | 2.74 | 2.74 | 2.67 | 2.67 | 6.41 |
| Eeff | 4.92 | 5.21 | −0.40 | −2.91 | 0.85 | 4.51 | 1.43 | 0.15 |
| Compound | Ref. | NMR | X-ray | DFT | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| δ(P) | δ(C1) | 1J(PC1) | δ(H1) | 2J(PH1) | S4′ | Average r(P–C) | S4 | Average r(P–C1) | ||
| 3 | [8,9] | −62.8 | 59.3 | 4.3 | 2.64 | 2.9 | 59.8 | 1.845(3) | 63.4 | 1.8820 |
| O = 3 | [9] | 44.2 | 55.0 | 81.2 | 2.82 | 7.2 | 34.5 | 1.821(2) | 32.5 | 1.8532 |
| S = 3 | [9] | 42.4 | 58.0 | 67.0 | 3.03 | 5.2 | 35.1 | 1.822(2) | 39.3 | 1.8694 |
| Se = 3 | [9] | 26.3 | 57.8 | 59.8 | 3.18 | 4.6 | 31.4 | 1.825(3) | 39.9 | 1.8688 |
| 33.3 | 1.829(3) | |||||||||
| [CuI(bpy)3] | [8] | −35 * | 55.8 | 26.0 | 2.88 * | - | ||||
| [CuI(phen)3] | [8] | −35 * | 56.4 | 24.4 | 2.87 * | - | 50.7 | 1.837(6) | 49.6 | 1.8750 |
| [CuNCS(phen)3] | [12] | −32 * | 55.5 | 25.9 | 2.76 * | - | 53.7 | 1.843(1) | 48.8 | |
| [CuI(dmp)3]3 | [10] | −29 *; −60 * | 56.6 * | - | 2.77 * | - | ||||
| [CuI(dmp)3] | [10,13] | −28 * | 55.8 * | - | 2.88 * | - | 58.1 | 1.855(2) | 57.6 | 1.8844 |
| [CuNCS(dmp)3] | [13] | −30 * | 56.5 * | - | 2.74 * | - | 62.0 | 1.853(2) | 56.5 | 1.8819 |
| [CuI(bq)3] | [15] | −28 * | 55.2 * | - | 2.78 * | - | 44.0 | 1.844(3) | 54.1 | 1.8421 |
| Ref. | Compound | Cu1–I1 | Cu1–N1 | Cu1–N1–C1 | Cu1–P1 | Average (P1–C) | α | | β − γ | | S4′ |
|---|---|---|---|---|---|---|---|---|---|
| [8] | 3 | 1.845(3) | 59.8 | ||||||
| [8] | [CuI(phen)3] | 2.631(1) | 2.193(2) | 1.837(6) | 79.8 | 12.0 | 50.7 | ||
| [12] | [CuNCS(phen)3] | 1.989(1) | 164.81(9) | 2.1885(3) | 1.843(1) | 89.2 | 10.2 | 53.7 | |
| [10] | [CuI(dmp)3] | 2.674(1) | 2.206(1) | 1.855(2) | 85.6 | 33.3 | 58.1 | ||
| [13] | [CuNCS(dmp)3] | 1.969(1) | 175.27(12) | 2.197(1) | 1.853(2) | 89.2 | 20.2 | 62.0 | |
| [15] | [CuI(bq)3] | 2.609(2) | 2.216(2) | 1.844(3) | 80.1 | 13.8 | 44.7 |
| Ref. | Compound | E. coli | P. aeruginosa | S. aureus | C. albicans |
|---|---|---|---|---|---|
| [8] | 1 | >2560 | >2560 | >2560 | 640 |
| 2 | >2560 | >2560 | >2560 | >2560 | |
| 3 | 1280 | 1280 | 640 | 1280 | |
| [CuI(bpy)1] | 2560 | 2560 | 320 | 1280 | |
| [CuI(bpy)2] | 1280 | 2560 | 320 | 2560 | |
| [CuI(bpy)3] | 640 | 2560 | 320 | 2560 | |
| [CuI(phen)1] | 320 | 2560 | 80 | 160 | |
| [CuI(phen)2] | 320 | 1280 | 80 | 160 | |
| [CuI(phen)3] | 320 | 2560 | 80 | 160 | |
| [11] | [CuI(phen)1]·1 | 160 | 1280 | 40 | 160 |
| [CuI(phen)3]·3 | 160 | 2560 | 20 | 160 | |
| [CuI(phen)5]·5 | 160 | * | 20 | 40 | |
| [CuI(dmp)1] | 80 | * | 5.0 | 2.5 | |
| [CuI(dmp)1]·1 | 80 | 2560 | 2.5 | 2.5 | |
| [CuI(dmp)3] | 80 | * | 2.5 | 2.5 | |
| [CuI(dmp)3]·3 | 80 | 2560 | 2.5 | 2.5 | |
| [CuI(dmp)5]·5 | 80 | * | 2.5 | 1.25 | |
| [14] | [CuNCS(dmp)3] | 250 | * | 2 | 1 |
| [CuI(dmp)3S] | 200 | * | 2 | 1 | |
| [CuNCS(dmp)3S] | 250 | * | 2 | 2 | |
| ciprofloxacin | 0.1 | * | 0.5 | >300 | |
| gentamycin | 10 | * | 5 | >300 | |
| ampicillin | 1 | * | 0.2 | >300 | |
| [15] | [CuI(bq)3] | >300 | * | 20 | 100 |
| Ref. | Compound | SKOV 3 | MDAH 2774 |
|---|---|---|---|
| [11] | [CuI(phen)1]·1 | 3.2 ± 0.3 | 7.0 ± 0.7 |
| [CuI(phen)3]·3 | 1.9 ± 0.1 | 6.5 ± 0.2 | |
| [CuI(phen)5]·5 | 2.2 ± 0.3 | 4.2 ± 0.2 | |
| [CuI(dmp)1]·1 | 1.8 ± 0.1 | 2.0 ± 0.5 | |
| [CuI(dmp)3]·3 | 2.2 ± 0.1 | 3.0 ± 0.1 | |
| [CuI(dmp)5]·5 | 2.0 ± 0.4 | 4.0 ± 0.4 | |
| cisplatin | 180.5 ± 9.3 | 77.2 ± 7.6 |
| 4 h | 24 h | 48 h | |||||
|---|---|---|---|---|---|---|---|
| Ref. | Compound | CT26 | A549 | CT26 | A549 | CT26 | A549 |
| [14] | [CuI(dmp)3] | 9.06 ± 0.48 | 1.56 ± 0.39 | 14,650 ± 340 | 280 ± 60 | 193.53 ± 11.31 | 35.03 ± 6.26 |
| [CuNCS(dmp)3] | 6.78 ± 0.47 | 4.99 ± 0.40 | 1200 ± 560 | 80 ± 10 | 65.38 ± 5.68 | 21.23 ± 2.82 | |
| [CuI(dmp)3S] | 5.37 ± 0.65 | 4.04 ± 0.38 | 2330 ± 430 | 180 ± 80 | 86.49 ± 4.95 | 27.87 ± 4.24 | |
| [CuNCS(dmp)3S] | 2.12 ± 0.29 | 6.10 ± 0.73 | 5750 ± 320 | 140 ± 30 | 611.31 ± 17.67 | 45.28 ± 4.64 | |
| cisplatin | 2200 ± 820 | 3150 ± 450 | 4990 ± 670 | 3850 ± 430 | 39,040 ± 5450 | 43,310 ± 7210 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starosta, R. Tris(aminomethyl)phosphines and Their Copper(I) (Pseudo)halide Complexes with Aromatic Diimines—A Critical Retrospection. Pharmaceuticals 2023, 16, 766. https://doi.org/10.3390/ph16050766
Starosta R. Tris(aminomethyl)phosphines and Their Copper(I) (Pseudo)halide Complexes with Aromatic Diimines—A Critical Retrospection. Pharmaceuticals. 2023; 16(5):766. https://doi.org/10.3390/ph16050766
Chicago/Turabian StyleStarosta, Radosław. 2023. "Tris(aminomethyl)phosphines and Their Copper(I) (Pseudo)halide Complexes with Aromatic Diimines—A Critical Retrospection" Pharmaceuticals 16, no. 5: 766. https://doi.org/10.3390/ph16050766
APA StyleStarosta, R. (2023). Tris(aminomethyl)phosphines and Their Copper(I) (Pseudo)halide Complexes with Aromatic Diimines—A Critical Retrospection. Pharmaceuticals, 16(5), 766. https://doi.org/10.3390/ph16050766