Cannabidiol-Loaded Nanocarriers and Their Therapeutic Applications
Abstract
1. Introduction
2. Cannabidiol (CBD): An Overview
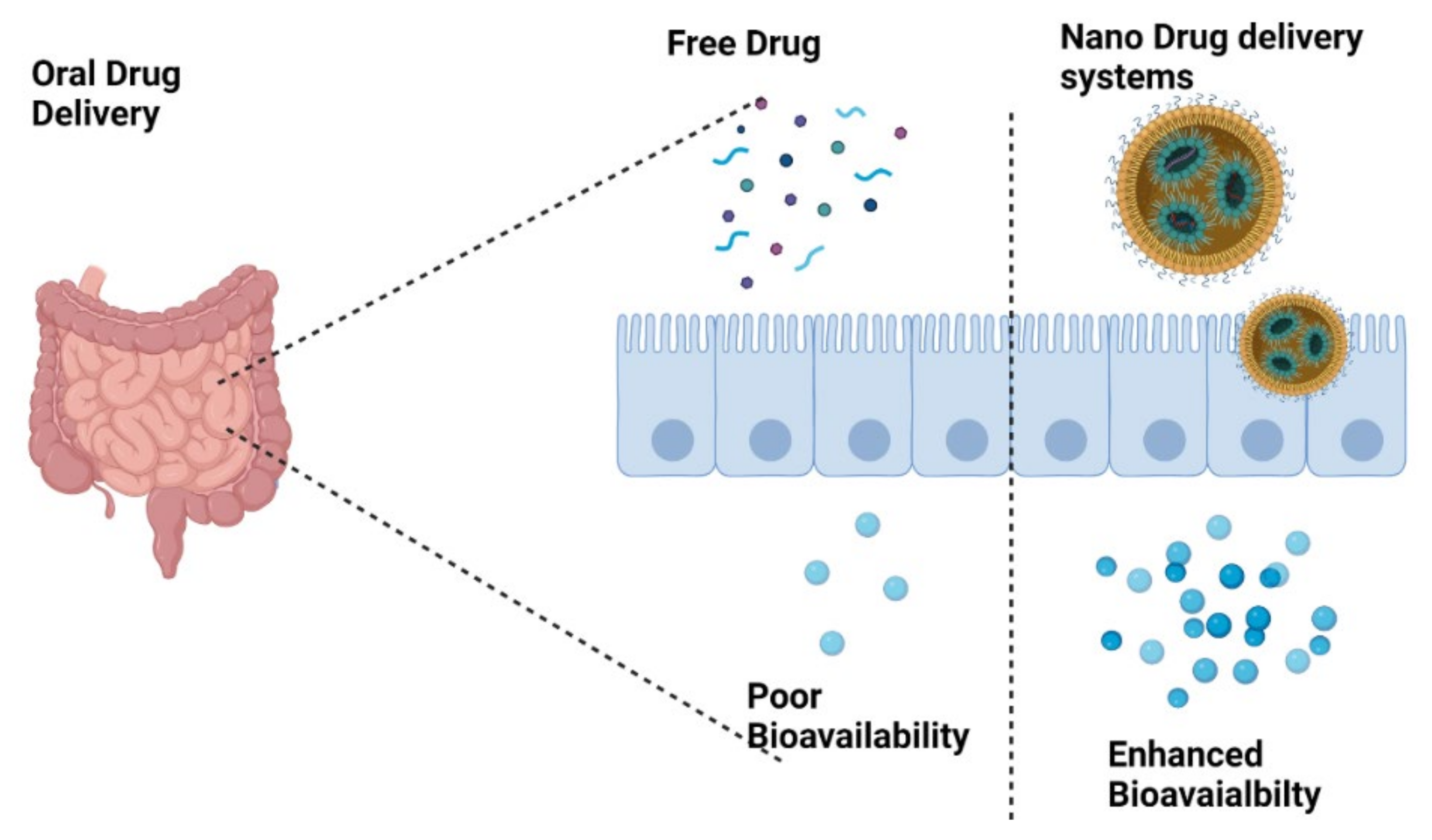
2.1. Bioavailability and Safety of CBD and Associated Derivatives
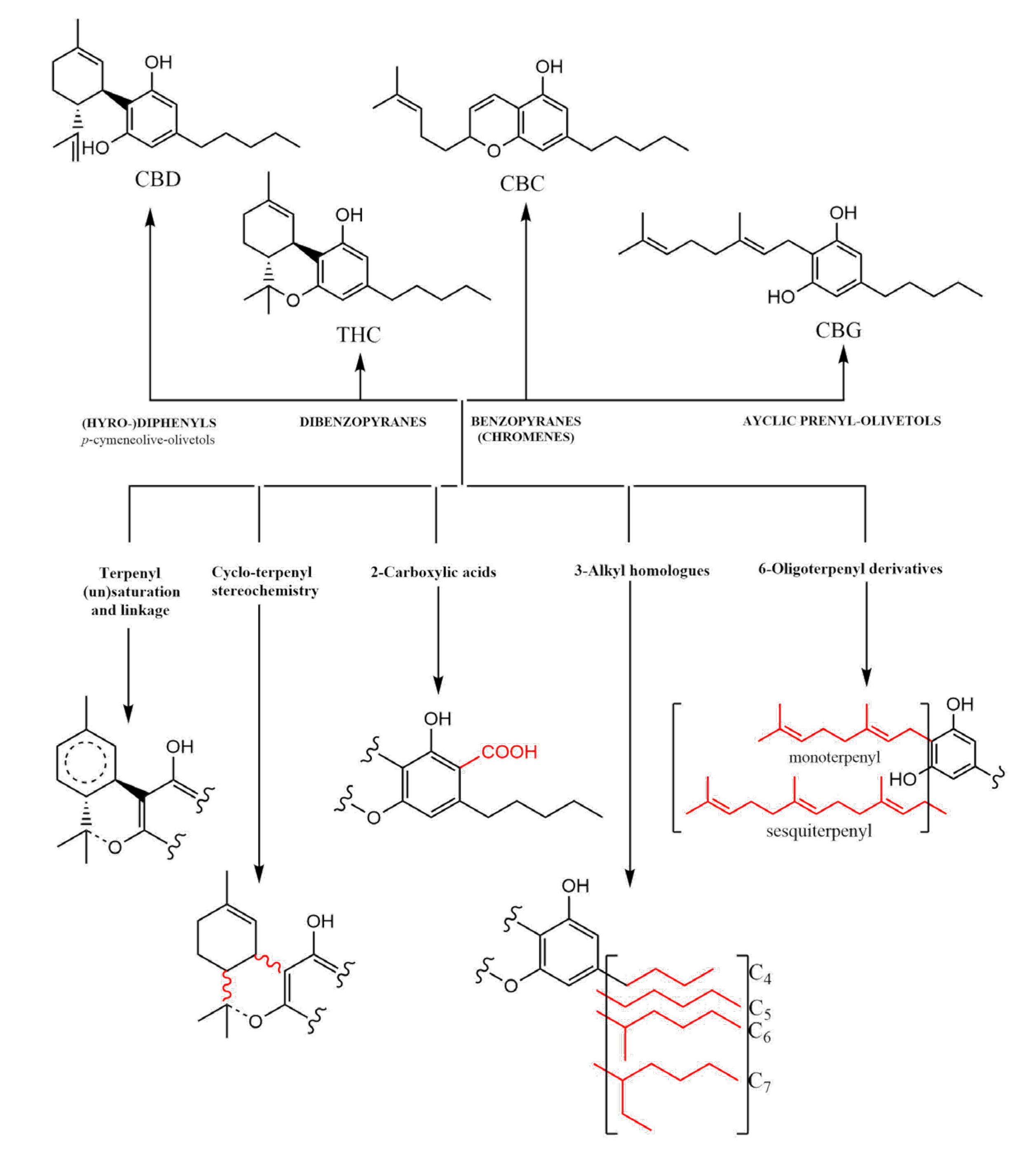
2.2. Extraction
2.3. Biological Effects of CBD
2.3.1. Roles of CBD in the Immune System
2.3.2. Roles of CBD in the Nervous System
2.3.3. Roles of CBD in the Cardiovascular System
2.4. Therapeutic Potential of CBD and Its Derivatives in Various Diseases and Disorders
2.4.1. Addition Disorder
2.4.2. Epilepsy
2.4.3. Anxiolytic
2.4.4. Cancer
2.4.5. Chronic Pain
2.4.6. Neuroprotection
2.4.7. Spasticity
2.4.8. Anti-Psychotic
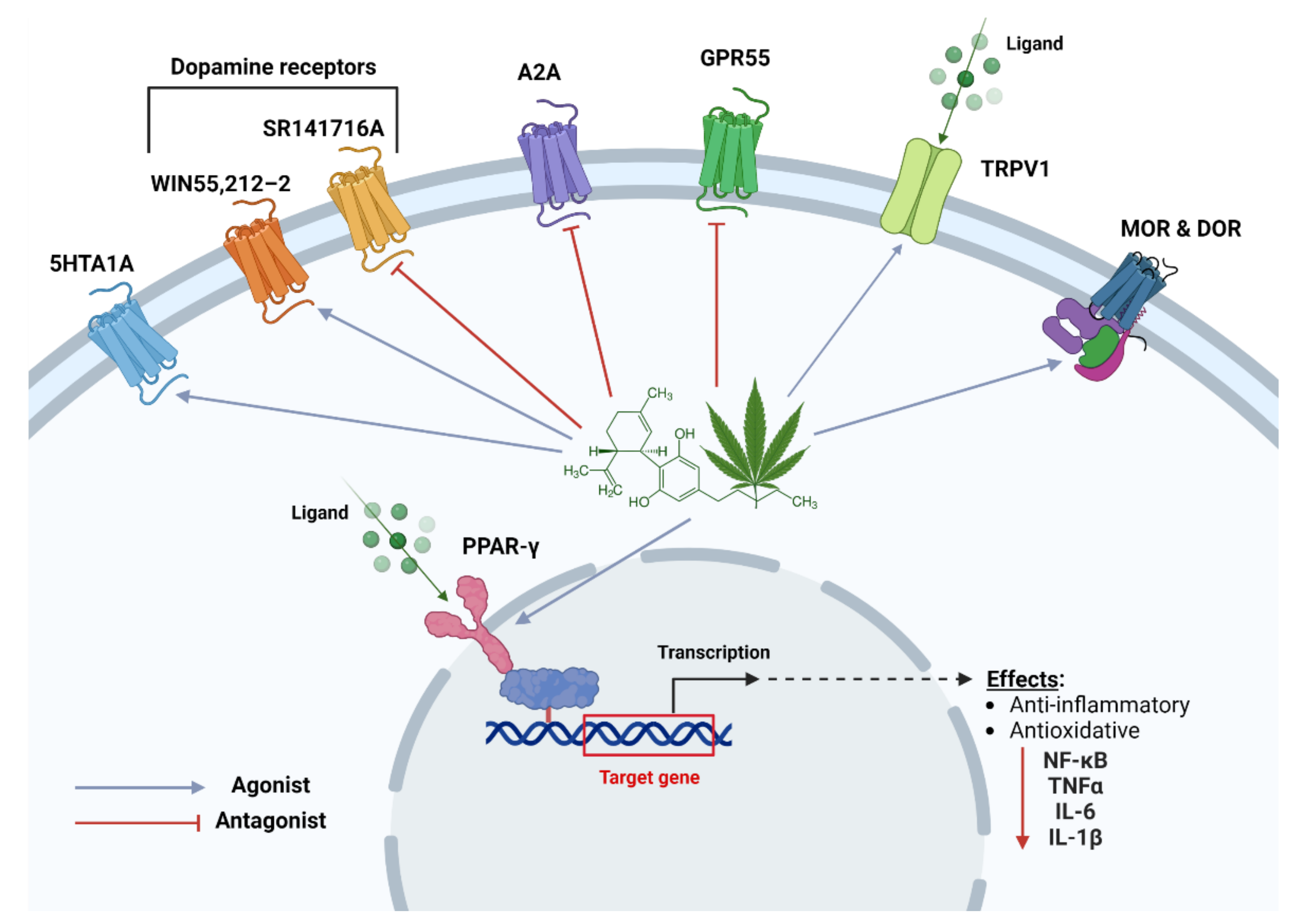
3. Pharmacological Mechanisms of CBD; Various Molecular Pathways/Targets
3.1. 5-HT1A Receptors
3.2. Adenosine Receptors
3.3. Dopamine Receptors
3.4. GPR55
3.5. Ion Channels
3.6. Opioid Receptors
3.7. PPARγ Receptors
4. Nanocarriers: A Potential Platform for Targeted Delivery of CBD
4.1. Lipid-Based Nanocarriers for CBD
4.1.1. Pro-Nanolipospheres (PNLs)
4.1.2. Nanoliposomes
4.1.3. Transferosomes
4.1.4. Solid Lipid Nanoparticles (SLNs)
4.1.5. Nanostructured Lipid Carriers (NLCs)
4.1.6. Nanoemulsions
4.2. Polymeric/Biopolymeric Nanocarriers for CBD
4.3. Discussion of Nanocarrier Systems Available: Toxicity Concerns
5. Clinical Significance of CBD-Loaded Nanodelivery Systems (In Vitro/In Vivo Studies)
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valizadehderakhshan, M.; Shahbazi, A.; Kazem-Rostami, M.; Todd, M.S.; Bhowmik, A.; Wang, L. Extraction of Cannabinoids from Cannabis sativa L. (Hemp)—Review. Agriculture 2021, 11, 384. [Google Scholar] [CrossRef]
- Touw, M. The Religious and Medicinal Uses of Cannabis in China, India and Tibet. J. Psychoact. Drugs 1981, 13, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial Cannabinoids from Cannabis sativa: A Structure−Activity Study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef]
- Nelson, K.M.; Bisson, J.; Singh, G.; Graham, J.G.; Chen, S.-N.; Friesen, J.B.; Dahlin, J.L.; Niemitz, M.; Walters, M.A.; Pauli, G.F. The Essential Medicinal Chemistry of Cannabidiol (CBD). J. Med. Chem. 2020, 63, 12137–12155. [Google Scholar] [CrossRef]
- Basas-Jaumandreu, J.; de Las Heras, F.X.C. GC-MS Metabolite Profile and Identification of Unusual Homologous Cannabinoids in High Potency Cannabis sativa. Planta Med. 2020, 86, 338–347. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Soydaner, U.; Öztürk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes. J. Nat. Prod. 2016, 79, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Blanco, J.; Romero, I.A.; Male, D.K.; Slowing, K.; García-García, L.; Torres-Suárez, A.I. Cannabidiol Enhances the Passage of Lipid Nanocapsules across the Blood–Brain Barrier Both in Vitro and in Vivo. Mol. Pharm. 2019, 16, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Blanco, J.; Sebastián, V.; Benoit, J.P.; Torres-Suárez, A.I. Lipid nanocapsules decorated and loaded with cannabidiol as targeted prolonged release carriers for glioma therapy: In vitro screening of critical parameters. Eur. J. Pharm. Biopharm. 2018, 134, 126–137. [Google Scholar] [CrossRef]
- Cherniakov, I.; Izgelov, D.; Barasch, D.; Davidson, E.; Domb, A.J.; Hoffman, A. Piperine-pro-nanolipospheres as a novel oral delivery system of cannabinoids: Pharmacokinetic evaluation in healthy volunteers in comparison to buccal spray administration. J. Control. Release 2017, 266, 1–7. [Google Scholar] [CrossRef]
- Atsmon, J.; Cherniakov, I.; Izgelov, D.; Hoffman, A.; Domb, A.J.; Deutsch, L.; Deutsch, F.; Heffetz, D.; Sacks, H. PTL401, a New Formulation Based on Pro-Nano Dispersion Technology, Improves Oral Cannabinoids Bioavailability in Healthy Volunteers. J. Pharm. Sci. 2018, 107, 1423–1429. [Google Scholar] [CrossRef]
- Atsmon, J.; Heffetz, D.; Deutsch, L.; Deutsch, F.; Sacks, H. Single-Dose Pharmacokinetics of Oral Cannabidiol Following Administration of PTL101: A New Formulation Based on Gelatin Matrix Pellets Technology. Clin. Pharmacol. Drug Dev. 2017, 7, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Mitelpunkt, A.; Kramer, U.; Kedem, M.H.; Fink, E.Z.; Orbach, R.; Chernuha, V.; Fattal-Valevski, A.; Deutsch, L.; Heffetz, D.; Sacks, H. The safety, tolerability, and effectiveness of PTL-101, an oral cannabidiol formulation, in pediatric intractable epilepsy: A phase II, open-label, single-center study. Epilepsy Behav. 2019, 98, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Di Bello, M.P.; Bloise, E.; Mazzetto, S.E.; Mele, G. Formulation and Chemical Stability in Aqueous Media of Cannabidiol Embedded in Cardanol-Based Nanovesicles. ACS Sustain. Chem. Eng. 2017, 5, 8870–8875. [Google Scholar] [CrossRef]
- Lodzki, M.; Godin, B.; Rakou, L.; Mechoulam, R.; Gallily, R.; Touitou, E. Cannabidiol—Transdermal delivery and anti-inflammatory effect in a murine model. J. Control. Release 2003, 93, 377–387. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.; Fernández-Carballido, A.; Simancas-Herbada, R.; Martin-Sabroso, C.; Torres-Suárez, A. CBD loaded microparticles as a potential formulation to improve paclitaxel and doxorubicin-based chemotherapy in breast cancer. Int. J. Pharm. 2019, 574, 118916. [Google Scholar] [CrossRef] [PubMed]
- De La Ossa, D.H.P.; Ligresti, A.; Gil-Alegre, M.; Aberturas, M.; Molpeceres, J.; Di Marzo, V.; Suárez, A.T. Poly-ε-caprolactone microspheres as a drug delivery system for cannabinoid administration: Development, characterization and in vitro evaluation of their antitumoral efficacy. J. Control. Release 2012, 161, 927–932. [Google Scholar] [CrossRef]
- De La Ossa, D.H.P.; Lorente, M.; Gil-Alegre, M.E.; Torres, S.; García-Taboada, E.; Aberturas, M.D.R.; Molpeceres, J.; Velasco, G.; Torres-Suárez, A.I. Local Delivery of Cannabinoid-Loaded Microparticles Inhibits Tumor Growth in a Murine Xenograft Model of Glioblastoma Multiforme. PLoS ONE 2013, 8, e54795. [Google Scholar] [CrossRef]
- Lv, P.; Zhang, D.; Guo, M.; Liu, J.; Chen, X.; Guo, R.; Xu, Y.; Zhang, Q.; Liu, Y.; Guo, H.; et al. Structural analysis and cytotoxicity of host-guest inclusion complexes of cannabidiol with three native cyclodextrins. J. Drug Deliv. Sci. Technol. 2019, 51, 337–344. [Google Scholar] [CrossRef]
- Mannila, J.; Järvinen, T.; Järvinen, K.; Tarvainen, M.; Jarho, P. Effects of RM-β-CD on sublingual bioavailability of Δ9-tetrahydrocannabinol in rabbits. Eur. J. Pharm. Sci. 2005, 26, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Cortés, F.B.; Zapata, K.; Rojano, B.A.; Carrasco-Marín, F.; Gallego, J.; Hernández, M.A.; Franco, C.A. Dual-Purpose Materials Based on Carbon Xerogel Microspheres (CXMs) for Delayed Release of Cannabidiol (CBD) and Subsequent Aflatoxin Removal. Molecules 2019, 24, 3398. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M.; Bentley, M.D.; Moreadith, R.W.; Viegas, T.X.; Fang, Z.; Yoon, K.; Weimer, R.; Dizman, B.; Nordstierna, L. Tuning drug release from polyoxazoline-drug conjugates. Eur. Polym. J. 2019, 120, 109241. [Google Scholar] [CrossRef]
- Štukelj, R.; Benčina, M.; Fanetti, M.; Valant, M.; Drab, M.; Iglič, A.; Kralj-Iglič, V. Synthesis of stable cannabidiol (CBD) nanoparticles in suspension. Mater. Teh. 2019, 53, 543–549. [Google Scholar] [CrossRef]
- Huestis, M.A. Human Cannabinoid Pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H.; Schneider, J.; Lucas, C.J.; Galettis, P. Exogenous Cannabinoid Efficacy: Merely a Pharmacokinetic Interaction? Clin. Pharmacokinet. 2017, 57, 539–545. [Google Scholar] [CrossRef]
- Harshita; Barkat, M.A.; Das, S.S.; Pottoo, F.H.; Beg, S.; Rahman, Z. Lipid- Based Nanosystem As Intelligent Carriers for Versatile Drug Delivery Applications. Curr. Pharm. Des. 2020, 26, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.A.; Maguire, R.F.; Yates, A.S.; O’Sullivan, S.E. Towards Better Delivery of Cannabidiol (CBD). Pharmaceuticals 2020, 13, 219. [Google Scholar] [CrossRef]
- Aladić, K. Cold Pressing and Supercritical CO2 Extraction of Hemp (Cannabis sativa) Seed Oil. Chem. Biochem. Eng. Q. 2015, 28, 481–490. [Google Scholar] [CrossRef]
- De Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Nichols, J.M.; Kaplan, B.L.F. Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid Res. 2020, 5, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Fogaça, M.V.; Sonego, A.B.; Guimarães, F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res. 2016, 112, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Tian, D.; Tian, L.; Ju, X.; Qi, L.; Wang, Y.; Liang, C. Overview of cannabidiol (CBD) and its analogues: Structures, biological activities, and neuroprotective mechanisms in epilepsy and Alzheimer’s disease. Eur. J. Med. Chem. 2020, 192, 112163. [Google Scholar] [CrossRef] [PubMed]
- Sherman, J.J.; Riche, D.M.; Warren, C.P. Cannabidiol Oral Solution: Challenges as a Treatment for Seizure Syndromes. The J Nurse Pract. 2020, 16, 210–212. [Google Scholar] [CrossRef]
- Leo, A.; Russo, E.; Elia, M. Cannabidiol and epilepsy: Rationale and therapeutic potential. Pharmacol. Res. 2016, 107, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Rabino, M.; Mallia, S.; Castiglioni, E.; Rovina, D.; Pompilio, G.; Gowran, A. The Endocannabinoid System and Cannabidiol: Past, Present, and Prospective for Cardiovascular Diseases. Pharmaceuticals 2021, 14, 936. [Google Scholar] [CrossRef]
- Stanley, C.P.; Hind, W.H.; O’Sullivan, S.E. Is the cardiovascular system a therapeutic target for cannabidiol? Br. J. Clin. Pharmacol. 2013, 75, 313–322. [Google Scholar] [CrossRef]
- Kicman, A.; Toczek, M. The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease. Int. J. Mol. Sci. 2020, 21, 6740. [Google Scholar] [CrossRef]
- Solowij, N.; Broyd, S.J.; Beale, C.; Prick, J.-A.; Greenwood, L.-M.; van Hell, H.; Suo, C.; Galettis, P.; Pai, N.; Fu, S.; et al. Therapeutic Effects of Prolonged Cannabidiol Treatment on Psychological Symptoms and Cognitive Function in Regular Cannabis Users: A Pragmatic Open-Label Clinical Trial. Cannabis Cannabinoid Res. 2018, 3, 21–34. [Google Scholar] [CrossRef]
- Beale, C.; Broyd, S.J.; Chye, Y.; Suo, C.; Schira, M.; Galettis, P.; Martin, J.H.; Yücel, M.; Solowij, N. Prolonged Cannabidiol Treatment Effects on Hippocampal Subfield Volumes in Current Cannabis Users. Cannabis Cannabinoid Res. 2018, 3, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Ferraz-De-Paula, V.; Pinheiro, M.L.; Vitoretti, L.B.; Mariano-Souza, D.P.; Quinteiro-Filho, W.M.; Akamine, A.T.; Almeida, V.I.; Quevedo, J.; Dal-Pizzol, F.; et al. Cannabidiol, a non-psychotropic plant-derived cannabinoid, decreases inflammation in a murine model of acute lung injury: Role for the adenosine A2A receptor. Eur. J. Pharmacol. 2012, 678, 78–85. [Google Scholar] [CrossRef]
- Karimi-Haghighi, S.; Razavi, Y.; Iezzi, D.; Scheyer, A.F.; Manzoni, O.; Haghparast, A. Cannabidiol and substance use disorder: Dream or reality. Neuropharmacology 2022, 207, 108948. [Google Scholar] [CrossRef] [PubMed]
- Cunha, J.M.; Carlini, E.; Pereira, A.E.; Ramos, O.L.; Pimentel, C.; Gagliardi, R.; Sanvito, W.L.; Lander, N.; Mechoulam, R. Chronic Administration of Cannabidiol to Healthy Volunteers and Epileptic Patients. Pharmacology 1980, 21, 175–185. [Google Scholar] [CrossRef]
- Lattanzi, S.; Brigo, F.; Trinka, E.; Zaccara, G.; Cagnetti, C.; Del Giovane, C.; Silvestrini, M. Efficacy and Safety of Cannabidiol in Epilepsy: A Systematic Review and Meta-Analysis. Drugs 2018, 78, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.; Devinsky, O. Cannabinoids in the Treatment of Epilepsy. N. Engl. J. Med. 2015, 373, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Morrison, P.D.; Fusar-Poli, P.; Martin-Santos, R.; Borgwardt, S.; Wintonbrown, T.T.; Nosarti, C.; Carroll, C.M.O.; Seal, M.L.; Allen, P.; et al. Opposite Effects of Δ-9-Tetrahydrocannabinol and Cannabidiol on Human Brain Function and Psychopathology. Neuropsychopharmacology 2010, 35, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, A.P.; Bonato, J.M.; Milani, H.; Guimarães, F.S.; de Oliveira, R.M.W. Influence of single and repeated cannabidiol administration on emotional behavior and markers of cell proliferation and neurogenesis in non-stressed mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Aviello, G.; Romano, B.; Borrelli, F.; Capasso, R.; Gallo, L.; Piscitelli, F.; Di Marzo, V.; Izzo, A.A. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J. Mol. Med. 2012, 90, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Gonca, E.; Darıcı, F. The Effect of Cannabidiol on Ischemia/Reperfusion-Induced Ventricular Arrhythmias. J. Cardiovasc. Pharmacol. Ther. 2014, 20, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Louis-Gray, K.; Tupal, S.; Premkumar, L.S. TRPV1: A Common Denominator Mediating Antinociceptive and Antiemetic Effects of Cannabinoids. Int. J. Mol. Sci. 2022, 23, 10016. [Google Scholar] [CrossRef]
- Urits, I.; Borchart, M.; Hasegawa, M.; Kochanski, J.; Orhurhu, V.; Viswanath, O. An Update of Current Cannabis-Based Pharmaceuticals in Pain Medicine. Pain Ther. 2019, 8, 41–51. [Google Scholar] [CrossRef]
- Greish, K.; Mathur, A.; Al Zahrani, R.; Elkaissi, S.; Al Jishi, M.; Nazzal, O.; Taha, S.; Pittalà, V.; Taurin, S. Synthetic cannabinoids nano-micelles for the management of triple negative breast cancer. J. Control. Release 2018, 291, 184–195. [Google Scholar] [CrossRef]
- Yücel, M.; Lorenzetti, V.; Suo, C.; Zalesky, A.A.; Fornito, A.A.; Takagi, M.J.; Lubman, D.I.; Solowij, N. Hippocampal harms, protection and recovery following regular cannabis use. Transl. Psychiatry 2016, 6, e710. [Google Scholar] [CrossRef] [PubMed]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef] [PubMed]
- McGuire, P.; Robson, P.; Cubala, W.J.; Vasile, D.; Morrison, P.D.; Barron, R.; Taylor, A.; Wright, S. Cannabidiol (CBD) as an Adjunctive Therapy in Schizophrenia: A Multicenter Randomized Controlled Trial. Am. J. Psychiatry 2018, 175, 225–231. [Google Scholar] [CrossRef]
- Chagas, M.H.N.; Zuardi, A.W.; Tumas, V.; Pena-Pereira, M.A.; Sobreira, E.T.; Bergamaschi, M.M.; Dos Santos, A.C.; Teixeira, A.L.; Hallak, J.E.C.; Crippa, J.A.S. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: An exploratory double-blind trial. J. Psychopharmacol. 2014, 28, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Spiro, A.S.; Jenner, A.M.; Garner, B.; Karl, T. Long-Term Cannabidiol Treatment Prevents the Development of Social Recognition Memory Deficits in Alzheimer’s Disease Transgenic Mice. J. Alzheimer’s Dis. 2014, 42, 1383–1396. [Google Scholar] [CrossRef]
- Wade, D.T.; Makela, P.; Robson, P.; House, H.; Bateman, C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult. Scler. J. 2004, 10, 434–441. [Google Scholar] [CrossRef]
- Russo, E.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic Properties of Cannabidiol at 5-HT1a Receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef]
- De Almeida, D.L.; Devi, L.A. Diversity of molecular targets and signaling pathways for CBD. Pharmacol. Res. Perspect. 2020, 8, e00682. [Google Scholar] [CrossRef]
- Seeman, P. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl. Psychiatry 2016, 6, e920. [Google Scholar] [CrossRef] [PubMed]
- Stark, T.; Di Bartolomeo, M.; Di Marco, R.; Drazanova, E.; Platania, C.B.M.; Iannotti, F.A.; Ruda-Kucerova, J.; D’Addario, C.; Kratka, L.; Pekarik, V.; et al. Altered dopamine D3 receptor gene expression in MAM model of schizophrenia is reversed by peripubertal cannabidiol treatment. Biochem. Pharmacol. 2020, 177, 114004. [Google Scholar] [CrossRef]
- Hudson, R.; Rushlow, W.; LaViolette, S.R. Phytocannabinoids modulate emotional memory processing through interactions with the ventral hippocampus and mesolimbic dopamine system: Implications for neuropsychiatric pathology. Psychopharmacology 2017, 235, 447–458. [Google Scholar] [CrossRef]
- Ross, R.A. The enigmatic pharmacology of GPR55. Trends Pharmacol. Sci. 2009, 30, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Celorrio, M.; Rojo-Bustamante, E.; Fernández-Suárez, D.; Sáez, E.; de Mendoza, A.E.-H.; Müller, C.E.; Ramírez, M.J.; Oyarzábal, J.; Franco, R.; Aymerich, M.S. GPR55: A therapeutic target for Parkinson’s disease? Neuropharmacology 2017, 125, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Hanuš, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef]
- Vilela, L.R.; Lima, I.V.; Kunsch, B.; Pinto, H.P.P.; de Miranda, A.S.; Vieira, L.M.; de Oliveira, A.C.P.; Moraes, M.F.D.; Teixeira, A.L.; Moreira, F.A. Anticonvulsant effect of cannabidiol in the pentylenetetrazole model: Pharmacological mechanisms, electroencephalographic profile, and brain cytokine levels. Epilepsy Behav. 2017, 75, 29–35. [Google Scholar] [CrossRef]
- Vaysse, P.J.; Gardner, E.L.; Zukin, R.S. Modulation of rat brain opioid receptors by cannabinoids. J. Pharmacol. Exp. Ther. 1987, 241, 534–539. [Google Scholar]
- Kathmann, M.; Flau, K.; Redmer, A.; Tränkle, C.; Schlicker, E. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2006, 372, 354–361. [Google Scholar] [CrossRef]
- Janani, C.; Kumari, B.R. PPAR gamma gene—A review. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 46–50. [Google Scholar] [CrossRef]
- Moore, G.B.; Chapman, H.; Holder, J.C.; Lister, C.A.; Piercy, V.; Smith, S.A.; Clapham, J.C. Differential Regulation of Adipocytokine mRNAs by Rosiglitazone in db/db Mice. Biochem. Biophys. Res. Commun. 2001, 286, 735–741. [Google Scholar] [CrossRef]
- Esposito, G.; Scuderi, C.; Valenza, M.; Togna, G.I.; Latina, V.; De Filippis, D.; Cipriano, M.; Carratù, M.R.; Iuvone, T.; Steardo, L. Cannabidiol Reduces Aβ-Induced Neuroinflammation and Promotes Hippocampal Neurogenesis through PPARγ Involvement. PLoS ONE 2011, 6, e28668. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Bhattacharjee, A.; Das, S.S.; Srivastava, A.; Choudhury, A.; Bhattacharjee, R.; De, S.; Perveen, A.; Iqbal, D.; Gupta, P.K.; et al. Molecular Insights into Therapeutic Potentials of Hybrid Compounds Targeting Alzheimer’s Disease. Mol Neurobiol. 2022, 59, 3512–3528. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. Cannabinoids go nuclear: Evidence for activation of peroxisome proliferator-activated receptors. Br. J. Pharmacol. 2007, 152, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Jha, N.K.; Arfin, S.; Jha, S.K.; Kar, R.; Dey, A.; Gundamaraju, R.; Ashraf, G.M.; Gupta, P.K.; Dhanasekaran, S.; Abomughaid, M.M.; et al. Re-establishing the comprehension of phytomedicine and nanomedicine in inflammation mediated cancer signaling. Semin Cancer Biol. 2022, 86, 1086–1104. [Google Scholar] [CrossRef]
- Das, S.S.; Hussain, A.; Verma, P.R.P.; Imam, S.S.; Altamimi, M.A.; Alshehri, S.; Singh, S.K. Recent Advances in Liposomal Drug Delivery System of Quercetin for Cancer Targeting: A Mechanistic Approach. Curr Drug Deliv. 2020, 17, 845–860. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.R. Nanoliposomes: Preparation and analysis. Methods Mol. Boil. 2010, 605, 29–50. [Google Scholar] [CrossRef]
- Verrico, C.D.; Wesson, S.; Konduri, V.; Hofferek, C.J.; Vazquez-Perez, J.; Blair, E.; Dunner, K.; Salimpour, P.; Decker, W.K.; Halpert, M.M. A randomized, double-blind, placebo-controlled study of daily cannabidiol for the treatment of canine osteoarthritis pain. Pain Pi 2020, 161, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Mozafari, M.R.; Mohebbi, M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci. Technol. 2012, 23, 13–27. [Google Scholar] [CrossRef]
- Shilo-Benjamini, Y.; Cern, A.; Zilbersheid, D.; Hod, A.; Lavy, E.; Barasch, D.; Barenholz, Y. A Case Report of Subcutaneously Injected Liposomal Cannabidiol Formulation Used as a Compassion Therapy for Pain Management in a Dog. Front. Vet. Sci. 2022, 9, 550. [Google Scholar] [CrossRef]
- Benson, H.A. Transfersomes for transdermal drug delivery. Expert Opin. Drug Deliv. 2006, 3, 727–737. [Google Scholar] [CrossRef]
- Moqejwa, T.; Marimuthu, T.; Kondiah, P.P.D.; Choonara, Y.E. Development of Stable Nano-Sized Transfersomes as a Rectal Colloid for Enhanced Delivery of Cannabidiol. Pharmaceutics 2022, 14, 703. [Google Scholar] [CrossRef]
- Balaga, V.K.R.; Pradhan, A.; Singh, S.; Dev, A. An overview on nanoparticulate drug delivery system for its specific and targeted effects in various diseases. In Nanoparticle and Nanocarrier Based Pharmaceutical Formulations; Bentham Science Publishers Pte. Ltd.: Singapore, 2022; pp. 55–92. [Google Scholar] [CrossRef]
- Müller, R.; Radtke, M.; Wissing, S. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- He, X.; Yang, L.; Wang, M.; Zhuang, X.; Huang, R.; Zhu, R.; Wang, S. Targeting the Endocannabinoid/CB1 Receptor System for Treating Major Depression Through Antidepressant Activities of Curcumin and Dexanabinol-Loaded Solid Lipid Nanoparticles. Cell. Physiol. Biochem. 2017, 42, 2281–2294. [Google Scholar] [CrossRef]
- Punyamurthula, N.S.; Adelli, G.R.; Gul, W.; Repka, M.A.; ElSohly, M.A.; Majumdar, S. Ocular Disposition of ∆8-Tetrahydrocannabinol from Various Topical Ophthalmic Formulations. AAPS PharmSciTech 2016, 18, 1936–1945. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.-Y.; Fang, C.-L.; Liu, C.-H.; Su, Y.-H. Lipid nanoparticles as vehicles for topical psoralen delivery: Solid lipid nanopar-ticles (SLN) versus nanostructured lipid carriers (NLC). Eur. J. Pharm. Biopharm. 2008, 70, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Matarazzo, A.P.; Elisei, L.M.S.; Carvalho, F.C.; Bonfílio, R.; Ruela, A.L.M.; Galdino, G.; Pereira, G.R. Mucoadhesive nanostructured lipid carriers as a cannabidiol nasal delivery system for the treatment of neuropathic pain. Eur. J. Pharm. Sci. 2021, 159, 105698. [Google Scholar] [CrossRef] [PubMed]
- Das, S.S.; Sarkar, A.; Chabattula, S.C.; Verma, P.R.P.; Nazir, A.; Gupta, P.K.; Ruokolainen, J.; Kesari, K.K.; Singh, S.K. Food-Grade Quercetin-Loaded Nanoemulsion Ameliorates Effects Associated with Parkinson’s Disease and Cancer: Studies Employing a Transgenic C. elegans Model and Human Cancer Cell Lines. Antioxidants 2022, 11, 1378. [Google Scholar] [CrossRef]
- Taskar, P.S.; Patil, A.; Lakhani, P.; Ashour, E.; Gul, W.; ElSohly, M.A.; Murphy, B.; Majumdar, S. Δ9-Tetrahydrocannabinol Derivative-Loaded Nanoformulation Lowers Intraocular Pressure in Normotensive Rabbits. Transl. Vis. Sci. Technol. 2019, 8, 15. [Google Scholar] [CrossRef]
- Chaudhari, V.S.; Gawali, B.; Saha, P.; Naidu, V.; Murty, U.S.; Banerjee, S. Quercetin and piperine enriched nanostructured lipid carriers (NLCs) to improve apoptosis in oral squamous cellular carcinoma (FaDu cells) with improved biodistribution profile. Eur. J. Pharmacol. 2021, 909, 174400. [Google Scholar] [CrossRef] [PubMed]
- Erfle, P.; Riewe, J.; Bunjes, H.; Dietzel, A. Goodbye fouling: A unique coaxial lamination mixer (CLM) enabled by two-photon polymerization for the stable production of monodisperse drug carrier nanoparticles. Lab Chip 2021, 21, 2178–2193. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Tajima, M.; Sugiyama, E.; Sato, V.H.; Sato, H. Development of a Novel Nano-emulsion Formulation to Improve Intestinal Absorption of Cannabidiol. Med. Cannabis Cannabinoids 2019, 2, 35–42. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, B.; Rao, J. Nutraceutical potential of industrial hemp (Cannabis sativa L.) extracts: Physicochemical stability and bioaccessibility of cannabidiol (CBD) nanoemulsions. Food Funct. 2022, 13, 4502–4512. [Google Scholar] [CrossRef]
- Francke, N.; Schneider, F.; Baumann, K.; Bunjes, H. Formulation of Cannabidiol in Colloidal Lipid Carriers. Molecules 2021, 26, 1469. [Google Scholar] [CrossRef]
- Das, S.S.; Singh, S.K.; Verma, P.R.P.; Gahtori, R.; Sibuh, B.Z.; Kesari, K.K.; Jha, N.K.; Dhanasekaran, S.; Thakur, V.K.; Wong, L.S.; et al. Polyester nanomedicines targeting inflammatory signaling pathways for cancer therapy. Biomed Pharmacother. 2022, 154, 113654. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I.; Cohen, M.; Delie, F.; Bastida-Ruiz, D.; Yart, L.; Martin-Sabroso, C.; Fernández-Carballido, A. PLGA Nanoparticles for the Intraperitoneal Administration of CBD in the Treatment of Ovarian Cancer: In Vitro and In Ovo Assessment. Pharmaceutics 2020, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- Durán-Lobato, M.; Álvarez-Fuentes, J.; Fernández-Arévalo, M.; Martín-Banderas, L. Receptor-targeted nanoparticles modulate cannabinoid anticancer activity through delayed cell internalization. Sci. Rep. 2022, 12, 1297. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Li, R.; Liu, S.; Meng, L.; Wu, Q.; Yuan, Q.; Liang, H.; Qin, M. Enhanced bioavailability and biosafety of cannabidiol nanomicelles for effective anti-inflammatory therapy. Particuology 2021, 69, 1–9. [Google Scholar] [CrossRef]
- Wang, C.; Cui, B.; Sun, Y.; Wang, C.; Guo, M. Preparation, stability, antioxidative property and in vitro release of cannabidiol (CBD) in zein-whey protein composite nanoparticles. LWT 2022, 162, 113466. [Google Scholar] [CrossRef]
- Sosnik, A.; Ben Shabo, R.; Halamish, H.M. Cannabidiol-Loaded Mixed Polymeric Micelles of Chitosan/Poly(Vinyl Alcohol) and Poly(Methyl Methacrylate) for Trans-Corneal Delivery. Pharmaceutics 2021, 13, 2142. [Google Scholar] [CrossRef]
- Momekova, D.; Danov, Y.; Momekov, G.; Ivanov, E.; Petrov, P. Polysaccharide Cryogels Containing β-Cyclodextrin for the Delivery of Cannabidiol. Pharmaceutics 2021, 13, 1774. [Google Scholar] [CrossRef]
- Momekova, D.; Ivanov, E.; Konstantinov, S.; Ublekov, F.; Petrov, P.D. Nanocomposite Cryogel Carriers from 2-Hydroxyethyl Cellulose Network and Cannabidiol-Loaded Polymeric Micelles for Sustained Topical Delivery. Polymers 2020, 12, 1172. [Google Scholar] [CrossRef]
- Sharkawy, A.; Barreiro, F.; Rodrigues, A. Pickering emulsions stabilized with chitosan/gum Arabic particles: Effect of chitosan degree of deacetylation on the physicochemical properties and cannabidiol (CBD) topical delivery. J. Mol. Liq. 2022, 355, 118993. [Google Scholar] [CrossRef]
- Kamali, A.; Oryan, A.; Hosseini, S.; Ghanian, M.H.; Alizadeh, M.; Eslaminejad, M.B.; Baharvand, H. Cannabidiol-loaded microspheres incorporated into osteoconductive scaffold enhance mesenchymal stem cell recruitment and regeneration of critical-sized bone defects. Mater. Sci. Eng. C 2019, 101, 64–75. [Google Scholar] [CrossRef]
- Grifoni, L.; Vanti, G.; Donato, R.; Sacco, C.; Bilia, A.R. Promising Nanocarriers to Enhance Solubility and Bioavailability of Cannabidiol for a Plethora of Therapeutic Opportunities. Molecules. 2022, 27, 6070. [Google Scholar] [CrossRef]
- Kolesarova, M.; Simko, P.; Urbanska, N.; Kiskova, T. Exploring the Potential of Cannabinoid Nanodelivery Systems for CNS Disorders. Pharmaceutics 2023, 15, 204. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A.; Solimini, R.; Pichini, S.; Pacifici, R.; Carlier, J.; Busardò, F.P. Cannabidiol Adverse Effects and Toxicity. Curr. Neuropharmacol. 2019, 17, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, H.; Kang, E.K.; Ji, G.Y.; Kim, Y.; Choi, I.S. In Vitro Studies on Therapeutic Effects of Cannabidiol in Neural Cells: Neurons, Glia, and Neural Stem Cells. Molecules 2021, 26, 6077. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.M.; Mohammadi, E.; Askarian-Amiri, S.; Azizi, Y.; Shakeri-Zadeh, A.; Neshastehriz, A. Investigating the in vitro photothermal effect of green synthesized apigenin-coated gold nanoparticle on colorectal carcinoma. IET Nanobiotechnol. 2021, 15, 329–337. [Google Scholar] [CrossRef] [PubMed]

| Delivery System | Route of Administration | Pharmacological Activity | Application | Ref. | |
|---|---|---|---|---|---|
| In Vitro | In Vivo | ||||
| Lipid nanocarriers | Intravenous | Transport across BBB | Biodistribution in mice | Central nervous system diseases | [8] |
| Lipid nanocarriers | Intravenous | Cellular uptake | - | Glioblastoma | [9] |
| Pro-liponanospheres | Oral | - | Bioavailability in rats | - | [10] |
| PK in healthy males | |||||
| Pro nano dispersions | Oral | - | PK profile in healthy males | - | [11] |
| Gelatin matrix pellets | Oral | - | Safety, PK profiles and relative bioavailability in healthy male | - | [12] |
| Gelatin matrix pellets | Oral | - | Safety, tolerability, and effectiveness in pediatric patients | - | [13] |
| Nanovesicles | - | - | - | - | [14] |
| Ethosomes | Transdermal | - | Anti-inflammatory and permeation studies in mice | Rheumatoid arthritis | [15] |
| Encapsulated Oil beads | Oral and Transdermal | - | Pharmacokinetic study | - | |
| Polymeric microparticles | - | Antitumor activity | - | Breast cancer | [16] |
| Poly-ε-caprolactone microparticles | - | Antitumor activity | - | Breast cancer | [17] |
| Poly-ε-caprolactone microparticles | Parenteral | Antitumor activity | - | Glioblastoma | [18] |
| α, β and γ-Cyclodextrin inclusion complexes | - | Antitumor activity | Enhanced aqueous solubility | Hepatoma/Lung adenocarcinoma | [19] |
| β-Cyclodextrin Inclusion complexes | Sublingual | - | Enhanced aqueous solubility and dissolution rate | - | [20] |
| Enhanced dissolution and absorption | |||||
| Carbon Xerogel microspheres | - | - | Delays drug release | - | [21] |
| Polyoxazoline-drug conjugates | Subcutaneous | Delays drug release | Extended in vivo kinetic profile | - | [22] |
| Nanocrystals | - | - | Enhanced bioavailability | - | [23] |
| Therapeutic Application | Carrier | In Vivo or In Vitro System | Results | Ref. |
|---|---|---|---|---|
| Bone healing and regeneration of critical-sized bone defects | Poly (lactic-coglycolic acid) (PLGA) microspheres | In vivo: a rat model |
| [105] |
| Glioma therapy | Lipid nanocapsules (LNCs) | In vitro: the human glioblastoma cell line U373MG |
| [9] |
| Treatment of neuropathic pain | Nanostructured lipid carriers (NLCs) | In vivo: male Swiss mice |
| [88] |
| Treatment of ovarian cancer | Poly-lactic-co-glycolic acid (PLGA) NPs | In vitro: ovarian cancer cells (SKOV-3, OAW-42, IGROV-1) The CAM model was used for in vivo assay |
| [97] |
| Anti-inflammatory | Nanomicelles of Poloxamer 407 (P407) | In vitro In vivo: a mouse model |
| [99] |
| Anti-inflammatory and treatment of canine osteoarthritis pain | Liposomes | In vitro: Mouse RAW267.4 macrophage cells, primary mouse splenocytes, human monocytic THP-1 cells, and human PBMC In vivo: C57BL/6J mice |
| [79] |
| Treatment of Alzheimer’s disease | Nano chitosan | In vivo: Rat model male Wistar rats |
| [110] |
| Biocompatibility with human skin cell lines | Nanoemulsions | In vitro: human skin cell lines HaCaT keratinocytes and NHDF normal human dermal fibroblasts |
| [89] |
| Rectal tissue permeation | Transfersomes | In vivo: Sprague Dawley rat |
| [82] |
| Anticancer activity against triple-negative breast cancer (TNBC) | Nanomicelles | In vitro: Triple-negative breast cancer (TNBC) namely MDA-MB-231, 4 T1, and MCF-7 In vivo: Female Balb/c mice |
| [52] |
| Analgesic treatment | Liposomes | In vivo: in dog |
| [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assadpour, E.; Rezaei, A.; Das, S.S.; Krishna Rao, B.V.; Singh, S.K.; Kharazmi, M.S.; Jha, N.K.; Jha, S.K.; Prieto, M.A.; Jafari, S.M. Cannabidiol-Loaded Nanocarriers and Their Therapeutic Applications. Pharmaceuticals 2023, 16, 487. https://doi.org/10.3390/ph16040487
Assadpour E, Rezaei A, Das SS, Krishna Rao BV, Singh SK, Kharazmi MS, Jha NK, Jha SK, Prieto MA, Jafari SM. Cannabidiol-Loaded Nanocarriers and Their Therapeutic Applications. Pharmaceuticals. 2023; 16(4):487. https://doi.org/10.3390/ph16040487
Chicago/Turabian StyleAssadpour, Elham, Atefe Rezaei, Sabya Sachi Das, Balaga Venkata Krishna Rao, Sandeep Kumar Singh, Mohammad Saeed Kharazmi, Niraj Kumar Jha, Saurabh Kumar Jha, Miguel A. Prieto, and Seid Mahdi Jafari. 2023. "Cannabidiol-Loaded Nanocarriers and Their Therapeutic Applications" Pharmaceuticals 16, no. 4: 487. https://doi.org/10.3390/ph16040487
APA StyleAssadpour, E., Rezaei, A., Das, S. S., Krishna Rao, B. V., Singh, S. K., Kharazmi, M. S., Jha, N. K., Jha, S. K., Prieto, M. A., & Jafari, S. M. (2023). Cannabidiol-Loaded Nanocarriers and Their Therapeutic Applications. Pharmaceuticals, 16(4), 487. https://doi.org/10.3390/ph16040487










