Identification of the Hepatic Metabolites of Flumazenil and their Kinetic Application in Neuroimaging
Abstract
1. Introduction
2. Results
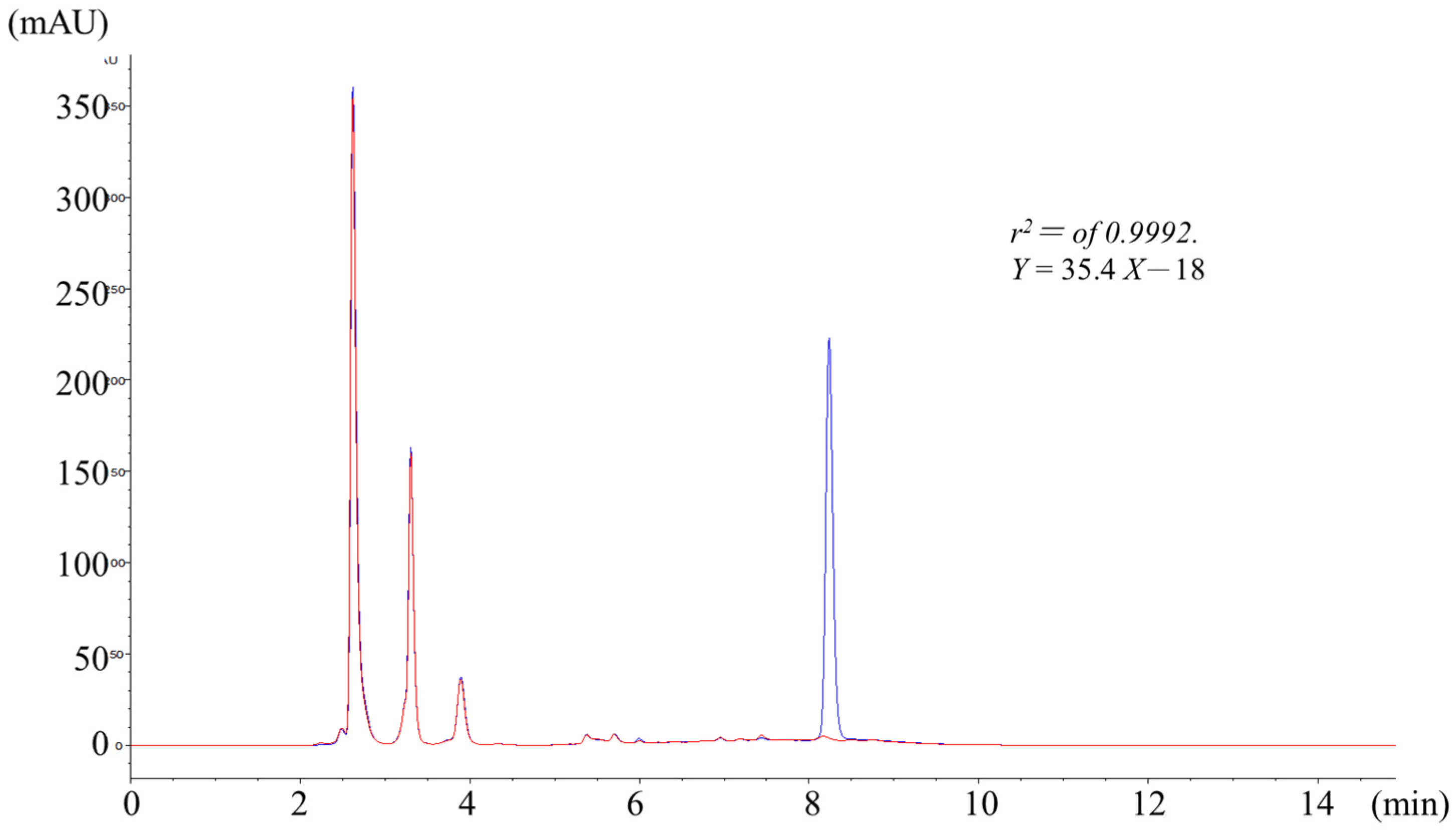
2.1. High Performance Liquid Chromatography Analysis of Flumazenil
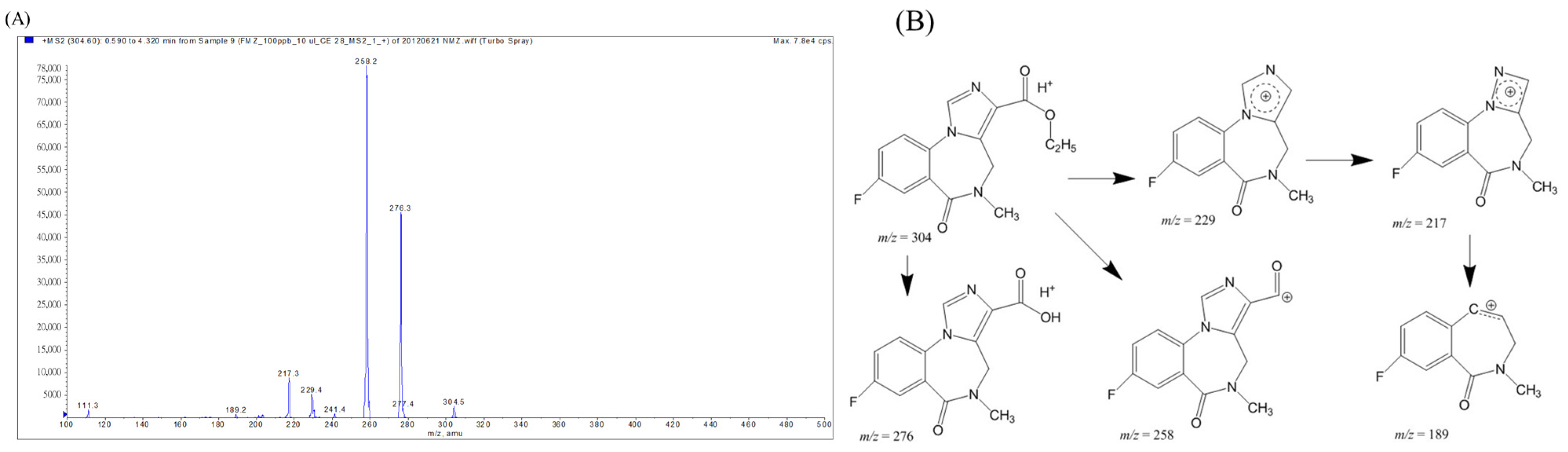
2.2. Mass Spectrometric Analysis of Flumazenil
2.3. Study of Flumazenil Biotransformation by Hepatic Enzyme Systems
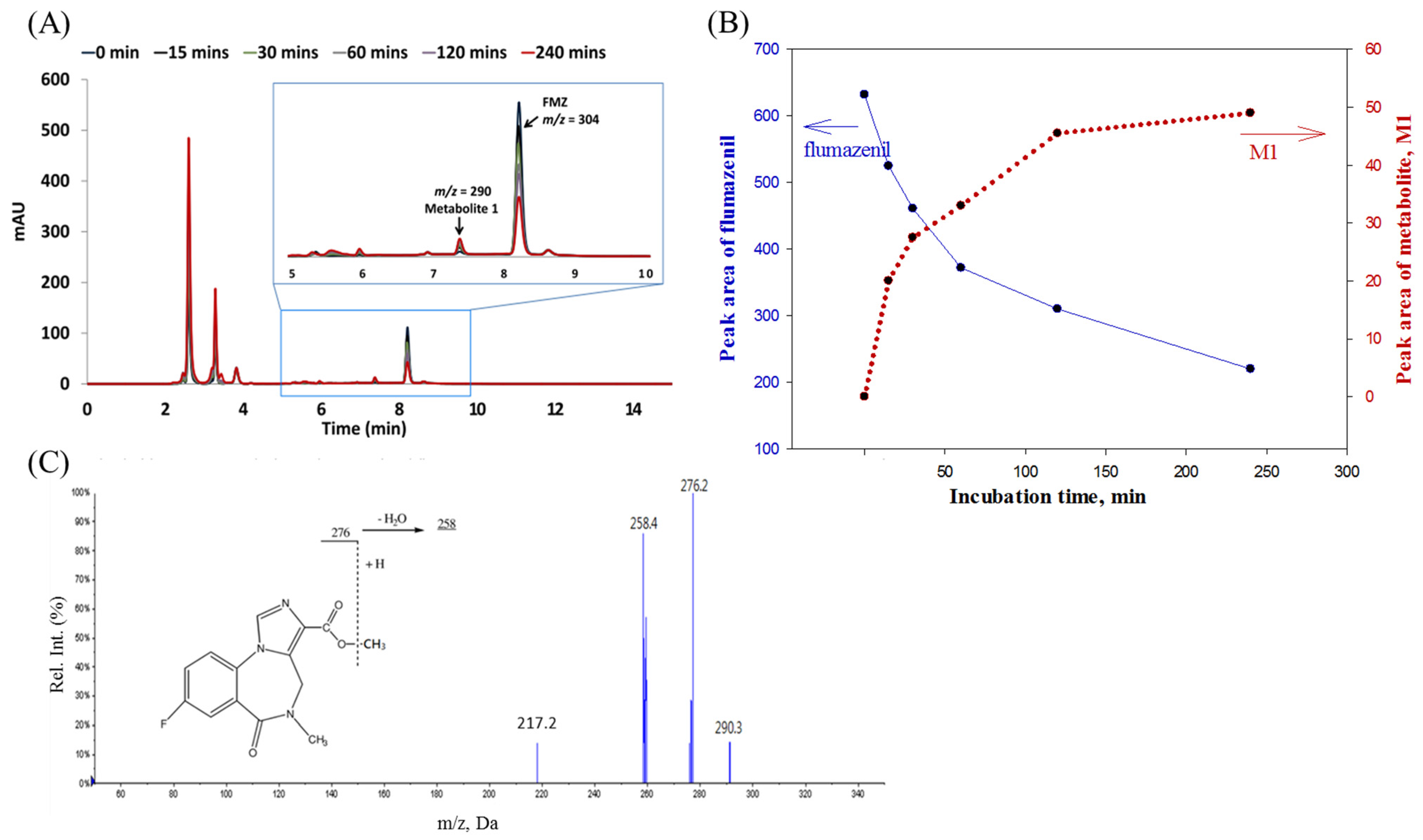
2.3.1. Flumazenil Metabolism Study in Rat Liver Homogenate
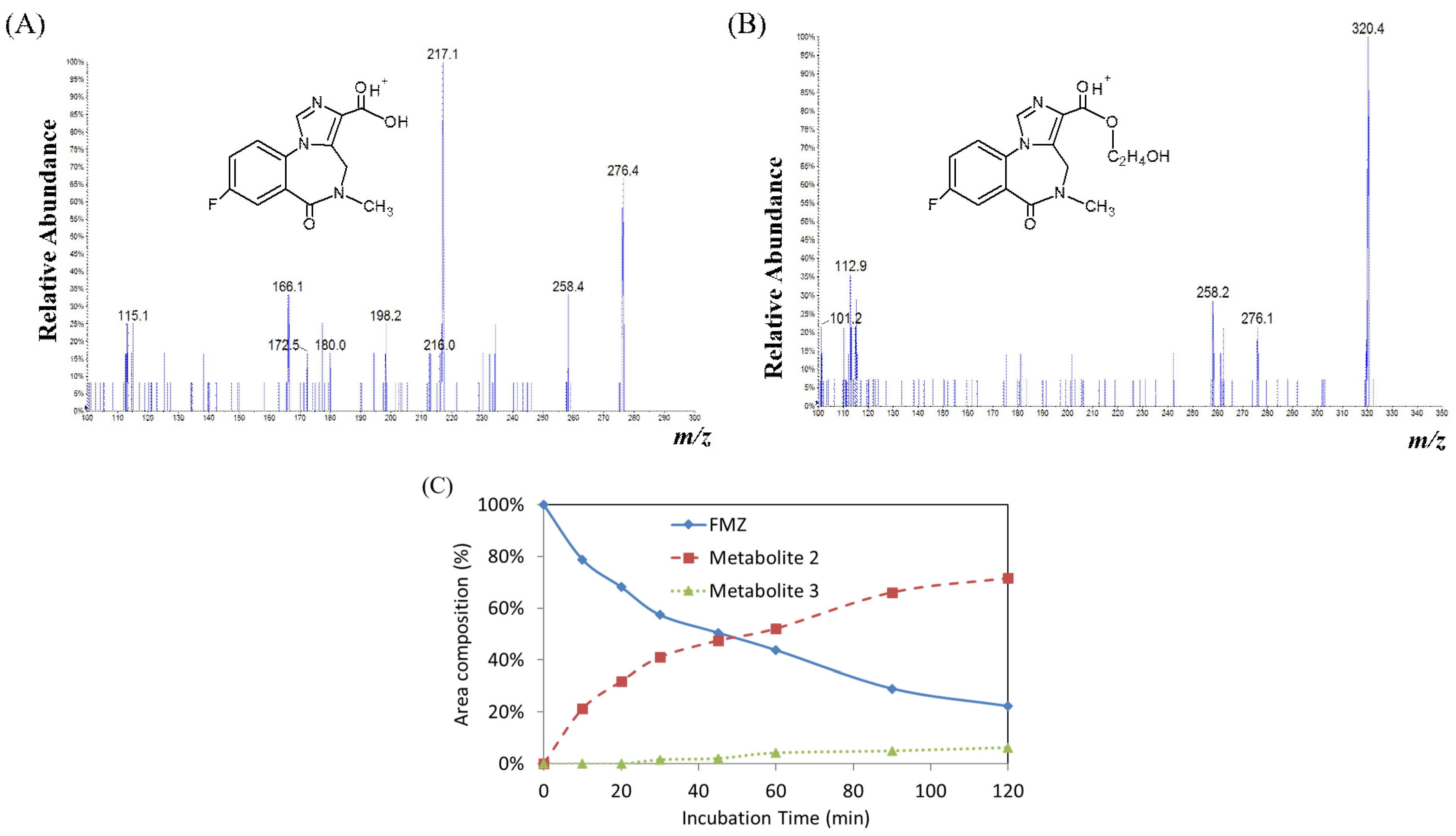
2.3.2. Flumazenil Metabolism Study in Human Liver Microsomes
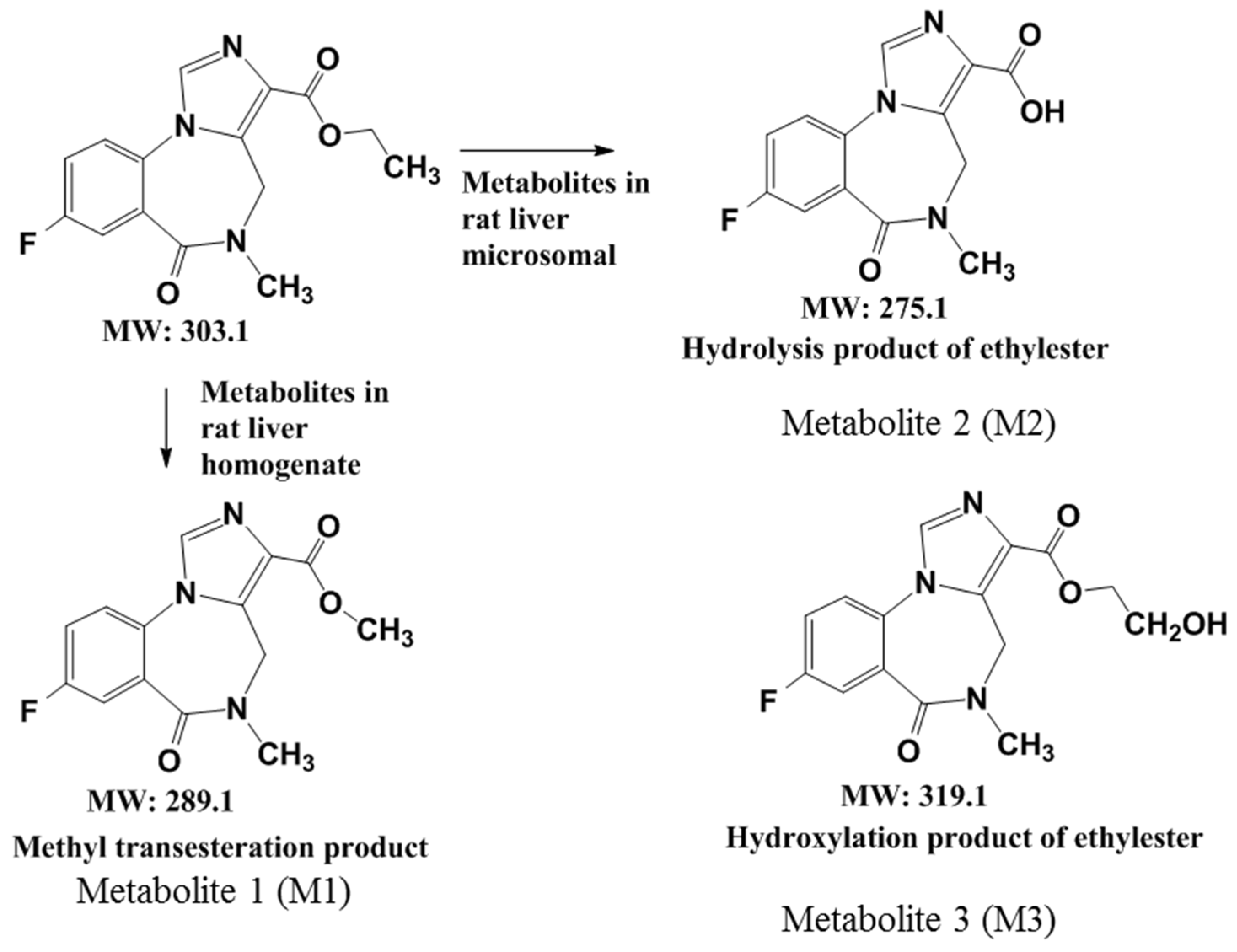
2.4. A Summary of the Metabolic Pathways of Flumazenil
2.5. In Vitro Serum Stability of [18F]flumazenil
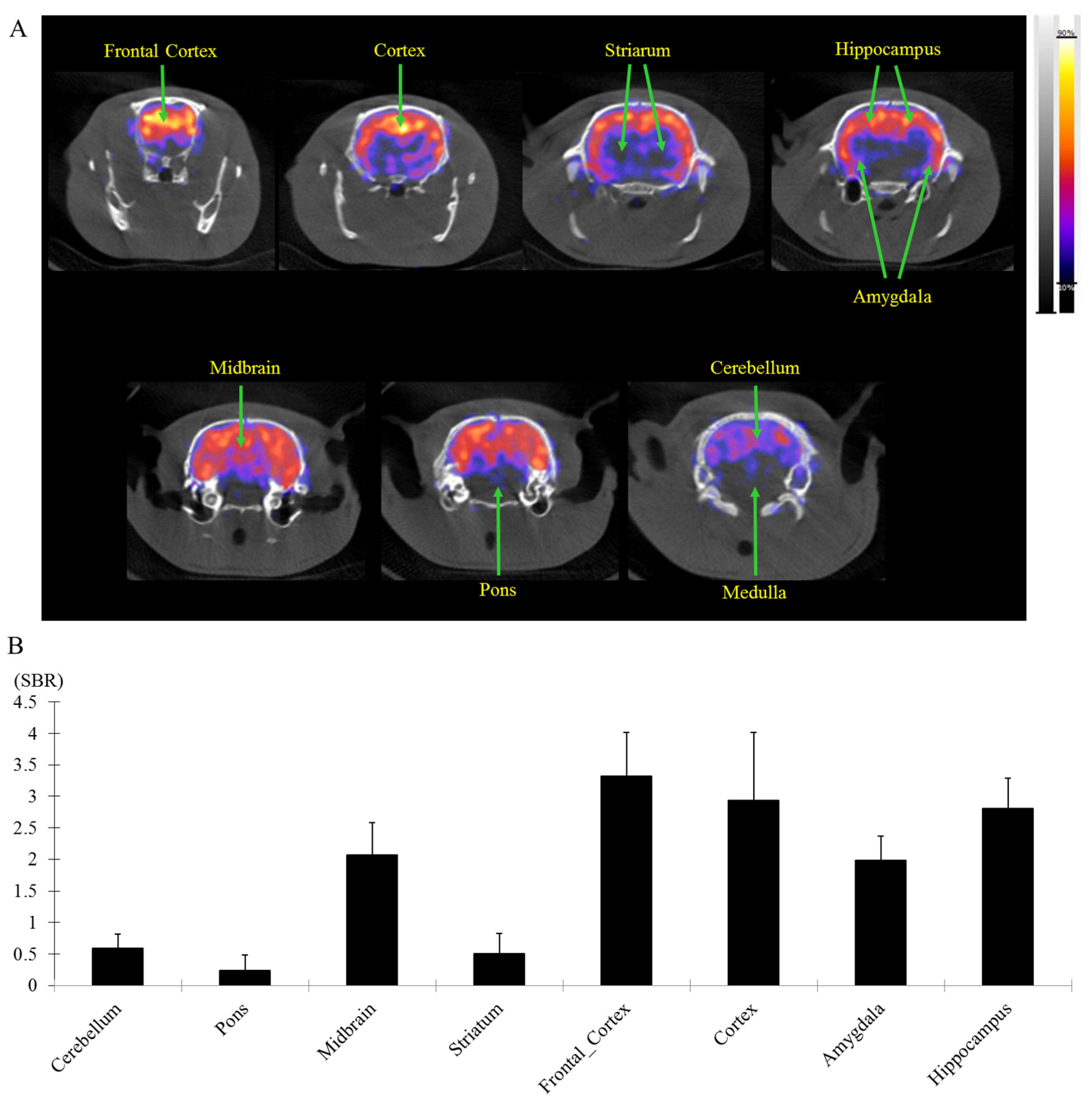
2.6. In Vivo NanoPET/CT Assay of [18F]flumazenil
2.7. Ex Vivo Biodistribution Assay with [18F]flumazenil
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Animals
4.3. Apparatus and Equipment
4.3.1. High Performance Liquid Chromatography Instrumentation
4.3.2. Liquid Chromatography-Mass Spectrometry/Mass Spectrometry Instrumentation
4.4. Procedures for Metabolism Study
4.4.1. Biotransformation and Pretreatment for High Performance Liquid Chromatography of Flumazenil in Rat Liver Homogenate
4.4.2. Flumazenil Metabolization in Rat Liver Microsomes
4.5. Synthesis of [18F]flumazenil
4.6. In Vitro Serum Stability
4.7. In Vivo NanoPET/CT Neuroimaging Studies
4.8. Ex Vivo Bio-Distribution Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, W.; Park, H.S.; Moon, B.S.; Lee, B.C.; Kim, S.E. PET measurement of “GABA shift” in the rat brain: A preclinical application of bolus plus constant infusion paradigm of [(18)F]flumazenil. Nucl. Med. Biol. 2017, 45, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, J. [Flumazenil]. Masui 2013, 62, 10–18. [Google Scholar] [PubMed]
- Ghosh, K.K.; Padmanabhan, P.; Yang, C.T.; Wang, Z.; Palanivel, M.; Ng, K.C.; Lu, J.; Carlstedt-Duke, J.; Halldin, C.; Gulyás, B. An In Vivo Study of a Rat Fluid-Percussion-Induced Traumatic Brain Injury Model with [(11)C]PBR28 and [(18)F]flumazenil PET Imaging. Int. J. Mol. Sci. 2021, 22, 951. [Google Scholar] [CrossRef] [PubMed]
- Rodnick, M.E.; Hockley, B.G.; Sherman, P.; Quesada, C.; Battle, M.R.; Jackson, A.; Linder, K.E.; Macholl, S.; Trigg, W.J.; Kilbourn, M.R.; et al. Novel fluorine-18 PET radiotracers based on flumazenil for GABAA imaging in the brain. Nucl. Med. Biol. 2013, 40, 901–905. [Google Scholar] [CrossRef]
- Moon, B.S.; Park, J.H.; Lee, H.J.; Lee, B.C.; Kim, S.E. Routine production of [(18)F]flumazenil from iodonium tosylate using a sample pretreatment method: A 2.5-year production report. Mol. Imaging Biol. 2014, 16, 619–625. [Google Scholar] [CrossRef]
- Vivash, L.; Gregoire, M.C.; Bouilleret, V.; Berard, A.; Wimberley, C.; Binns, D.; Roselt, P.; Katsifis, A.; Myers, D.E.; Hicks, R.J.; et al. In vivo measurement of hippocampal GABAA/cBZR density with [18F]flumazenil PET for the study of disease progression in an animal model of temporal lobe epilepsy. PLoS ONE 2014, 9, e86722. [Google Scholar] [CrossRef]
- Vivash, L.; Gregoire, M.C.; Lau, E.W.; Ware, R.E.; Binns, D.; Roselt, P.; Bouilleret, V.; Myers, D.E.; Cook, M.J.; Hicks, R.J.; et al. 18F-flumazenil: A γ-aminobutyric acid A-specific PET radiotracer for the localization of drug-resistant temporal lobe epilepsy. J. Nucl. Med. 2013, 54, 1270–1277. [Google Scholar] [CrossRef]
- Kaplan, M.M.; Utiger, R.D. Iodothyronine metabolism in rat liver homogenates. J. Clin. Investig. 1978, 61, 459–471. [Google Scholar] [CrossRef]
- Dunn, W.B.; Ellis, D.I. Metabolomics: Current analytical platforms and methodologies. TrAC Trends Anal. Chem. 2005, 24, 285–294. [Google Scholar]
- Lavén, M.; Markides, K.; Långström, B. Analysis of microsomal metabolic stability using high-flow-rate extraction coupled to capillary liquid chromatography-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 806, 119–126. [Google Scholar] [CrossRef]
- Amini, N.; Nakao, R.; Schou, M.; Halldin, C. Identification of PET radiometabolites by cytochrome P450, UHPLC/Q-ToF-MS and fast radio-LC: Applied to the PET radioligands [11C]flumazenil, [18F]FE-PE2I, and [11C]PBR28. Anal. Bioanal. Chem. 2013, 405, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Levêque, P.; de Hoffmann, E.; Labar, D.; Gallez, B. Assessment of [18F]fluoroethylflumazenil metabolites using high-performance liquid chromatography and tandem mass spectrometry. J. Chromatogr. B. Biomed. Sci. Appl. 2001, 754, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Nutt, D.J.; Malizia, A.L. New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. Br. J. Psychiatry 2001, 179, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Kleingeist, B.; Böcker, R.; Geisslinger, G.; Brugger, R. Isolation and pharmacological characterization of microsomal human liver flumazenil carboxylesterase. J. Pharm. Pharm. Sci. 1998, 1, 38–46. [Google Scholar] [PubMed]
- Chen, W.H.; Liao, C.W.; Luo, T.Y.; Chang, Y.; Men, L.C.; Hsieh, Y.C. High performance liquid chromatography-tandem mass spectrometry method for ex vivo metabolic studies of a rhenium-labeled radiopharmaceutical for liver cancer. Eur. J. Mass. Spectrom. 2014, 20, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Marwah, A.; Marwah, P.; Lardy, H. Ergosteroids: VI. Metabolism of dehydroepiandrosterone by rat liver in vitro: A liquid chromatographic-mass spectrometric study. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2002, 767, 285–299. [Google Scholar] [CrossRef]
- Lavén, M.; Appel, L.; Moulder, R.; Tyrefors, N.; Markides, K.; Långström, B. Determination of flumazenil in human plasma by liquid chromatography-electrospray ionisation tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 808, 221–227. [Google Scholar] [CrossRef]
- Pascual, B.; Prieto, E.; Arbizu, J.; Marti-Climent, J.M.; Peñuelas, I.; Quincoces, G.; Zarauza, R.; Pappatà, S.; Masdeu, J.C. Decreased carbon-11-flumazenil binding in early Alzheimer’s disease. Brain 2012, 135, 2817–2825. [Google Scholar] [CrossRef]
- Brambilla, P.; Perez, J.; Barale, F.; Schettini, G.; Soares, J.C. GABAergic dysfunction in mood disorders. Mol. Psychiatry 2003, 8, 721–737, 715. [Google Scholar] [CrossRef]
- Müller Herde, A.; Benke, D.; Ralvenius, W.T.; Mu, L.; Schibli, R.; Zeilhofer, H.U.; Krämer, S.D. GABA(A) receptor subtypes in the mouse brain: Regional mapping and diazepam receptor occupancy by in vivo [(18)F]flumazenil PET. Neuroimage 2017, 150, 279–291. [Google Scholar] [CrossRef]
- Valentine, K.E.; Milling, L.S.; Clark, L.J.; Moriarty, C.L. The Efficacy of Hypnosis as a Treatment for Anxiety: A Meta-Analysis. Int. J. Clin. Exp. Hypn. 2019, 67, 336–363. [Google Scholar] [CrossRef] [PubMed]
- Nutt, D.J. Overview of diagnosis and drug treatments of anxiety disorders. CNS Spectr. 2005, 10, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Babaev, O.; Piletti Chatain, C.; Krueger-Burg, D. Inhibition in the amygdala anxiety circuitry. Exp. Mol. Med. 2018, 50, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Nutt, D.J. Role of GABA in anxiety and depression. Depress. Anxiety 2007, 24, 495–517. [Google Scholar] [CrossRef]
- Collins, S.A.; Ninan, I. Development-Dependent Plasticity in Vasoactive Intestinal Polypeptide Neurons in the Infralimbic Cortex. Cereb. Cortex Commun. 2021, 2, tgab007. [Google Scholar] [CrossRef]
- Kraguljac, N.V.; McDonald, W.M.; Widge, A.S.; Rodriguez, C.I.; Tohen, M.; Nemeroff, C.B. Neuroimaging Biomarkers in Schizophrenia. Am. J. Psychiatry 2021, 178, 509–521. [Google Scholar] [CrossRef]
- Heiss, W.D. Radionuclide imaging in ischemic stroke. J. Nucl. Med. 2014, 55, 1831–1841. [Google Scholar] [CrossRef]
- Ishibashi, K.; Miura, Y.; Matsumura, K.; Kanemasa, Y.; Nakamichi, K.; Saijo, M.; Toyohara, J.; Ishii, K. PET Imaging of 18F-FDG, 11C-methionine, 11C-flumazenil, and 11C-4DST in Progressive Multifocal Leukoencephalopathy. Intern. Med. 2017, 56, 1219–1223. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, J.S.; Moon, C.; Son, Y. Profiling of gene expression in the brain associated with anxiety-related behaviors in the chronic phase following cranial irradiation. Sci. Rep. 2022, 12, 13162. [Google Scholar] [CrossRef]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain 2014, 137, 12–32. [Google Scholar] [CrossRef]
- Palner, M.; Beinat, C.; Banister, S.; Zanderigo, F.; Park, J.H.; Shen, B.; Hjoernevik, T.; Jung, J.H.; Lee, B.C.; Kim, S.E.; et al. Effects of common anesthetic agents on [(18)F]flumazenil binding to the GABA(A) receptor. EJNMMI Res. 2016, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Toth, I.; Neumann, I.D. Animal models of social avoidance and social fear. Cell Tissue Res. 2013, 354, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Farn, S.-S.; Cheng, K.-H.; Huang, Y.-R.; Lee, S.-Y.; Chen, J.-T.; Chang, K.-W. Automated Synthesis of [18F]flumazenil Application in GABAA Receptor Neuroimaging Availability for Rat Model of Anxiety. Pharmaceuticals 2023, 16, 417. [Google Scholar] [CrossRef] [PubMed]

| Parameters | |
|---|---|
| HPLC | |
| Stationary phase | ZORBAX Eclipse XDB-C18 4.6 ID × 100 mm, 5 μm thermostat at 25 °C |
| Mobile phase | |
| Flow rate | 0.6 mL min−1 |
| Composition | A: ammonium acetate 5 mM aqueous pH 7.0 with 1% CH3CN |
| B: only CH3CN | |
| Gradient program | 0–3.5 min, 10% → 40% B |
| 3.5–10.0 min, 40% B isocratic | |
| 10.0–10.1 min, 40% → 10% B | |
| 10.1–15.0 min, 10% B isocratic | |
| Detector | DAD at 250 nm |
| Turnaround time | 17 min |
| Mass Spectrometry | |
| Source temperature (°C) | 350 |
| Polarity | Positive |
| Resolution, Q1 and Q3 | Unit |
| Nebulizer gas, NEB (psi) | 40 |
| Curtain gas, CUR (psi) | 10 |
| Turbo gas | 15 |
| Collision gas, CAD (psi) | Medium |
| Ion spray voltage, IS (V) | 5000 |
| Ion energy 1, IE1 (V) | 0.4 |
| Ion energy 3, IE3 (V) | 0.3 |
| Declustering potential, DP (V) | 70 |
| Entrance potential, EP (V) | 10 |
| Detector parameter | Positive |
| -Channel electron multiplier, CEM (V) | 1950 |
| Multiple reaction monitoring (MRM) transition pair | 304 > 258 and 304 > 276 |
| Time (Min) | Radiochemical Purity (RCP, %) |
|---|---|
| 0 | 95.76 |
| 5 | 93.28 |
| 10 | 91.07 |
| 30 | 87.46 |
| 60 | 85.68 |
| 120 | 79.95 |
| 240 | 72.13 |
| %ID/g | 5 min | 10 min | 30 min | 60 min | 120 min | 240 min | |
|---|---|---|---|---|---|---|---|
| Cerebellum | 2.22 ± 0.12 | 0.74 ± 0.04 | 0.07 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | |
| Pons/Medulla | 1.80 ± 0.19 | 0.60 ± 0.06 | 0.06 ± 0.01 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | |
| Striatum | 3.27 ± 0.32 | 1.09 ± 0.11 | 0.11 ±0.01 | 0.04 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | |
| Cortex (Amyglada) | 5.28 ± 0.60 | 2.16 ± 0.39 | 0.38 ± 0.02 | 0.06 ± 0.01 | 0.03 ± 0.00 | 0.01 ± 0.00 | |
| Hippocampus | 4.57 ± 0.56 | 1.82 ± 0.19 | 0.25 ± 0.01 | 0.05 ± 0.00 | 0.03 ± 0.00 | 0.01 ± 0.00 | |
| Thalamus | 4.22 ± 0.41 | 1.61 ± 0.14 | 0.14 ± 0.00 | 0.05 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | |
| Hypothalamus | 3.71 ± 0.18 | 1.34 ± 0.06 | 0.12 ± 0.00 | 0.04 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | |
| SBR | 5 min | 10 min | 30 min | 60 min | 120 min | 240 min | |
| Cerebellum | 0.25 ± 0.18 | 0.36 ± 0.26 | 0.16 ± 0.15 | 0.36 ± 0.14 | 0.32 ± 0.07 | 0.71 ± 0.32 | |
| Striatum | 0.84 ± 0.37 | 0.92 ± 0.34 | 0.91 ±0.29 | 1.00 ± 0.36 | 0.94 ± 0.24 | 0.98 ± 0.33 | |
| Cortex (Amyglada) | 1.96 ± 0.49 | 2.65 ± 0.85 | 5.32 ± 0.82 | 2.22 ± 0.42 | 2.14 ± 0.43 | 1.71 ± 0.51 | |
| Hippocampus | 1.55 ± 0.37 | 2.06 ± 0.41 | 3.24 ± 0.52 | 1.78 ± 0.32 | 1.71 ± 0.41 | 1.73 ± 0.87 | |
| Thalamus | 1.37 ± 0.37 | 1.70 ± 0.40 | 1.37 ± 0.36 | 1.57 ± 0.31 | 1.51 ± 0.30 | 1.53 ± 0.58 | |
| Hypothalamus | 1.08 ± 0.26 | 1.25 ± 0.28 | 1.15 ± 0.14 | 1.26 ± 0.21 | 1.20 ± 0.19 | 1.02 ± 0.42 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-H.; Chiu, C.-H.; Farn, S.-S.; Cheng, K.-H.; Huang, Y.-R.; Lee, S.-Y.; Fang, Y.-C.; Lin, Y.-H.; Chang, K.-W. Identification of the Hepatic Metabolites of Flumazenil and their Kinetic Application in Neuroimaging. Pharmaceuticals 2023, 16, 764. https://doi.org/10.3390/ph16050764
Chen W-H, Chiu C-H, Farn S-S, Cheng K-H, Huang Y-R, Lee S-Y, Fang Y-C, Lin Y-H, Chang K-W. Identification of the Hepatic Metabolites of Flumazenil and their Kinetic Application in Neuroimaging. Pharmaceuticals. 2023; 16(5):764. https://doi.org/10.3390/ph16050764
Chicago/Turabian StyleChen, Wei-Hsi, Chuang-Hsin Chiu, Shiou-Shiow Farn, Kai-Hung Cheng, Yuan-Ruei Huang, Shih-Ying Lee, Yao-Ching Fang, Yu-Hua Lin, and Kang-Wei Chang. 2023. "Identification of the Hepatic Metabolites of Flumazenil and their Kinetic Application in Neuroimaging" Pharmaceuticals 16, no. 5: 764. https://doi.org/10.3390/ph16050764
APA StyleChen, W.-H., Chiu, C.-H., Farn, S.-S., Cheng, K.-H., Huang, Y.-R., Lee, S.-Y., Fang, Y.-C., Lin, Y.-H., & Chang, K.-W. (2023). Identification of the Hepatic Metabolites of Flumazenil and their Kinetic Application in Neuroimaging. Pharmaceuticals, 16(5), 764. https://doi.org/10.3390/ph16050764







