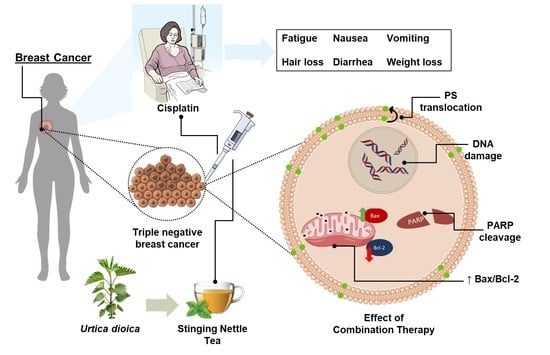
Urtica dioica Leaf Infusion Enhances the Sensitivity of Triple-Negative Breast Cancer Cells to Cisplatin Treatment
Abstract
1. Introduction
2. Results
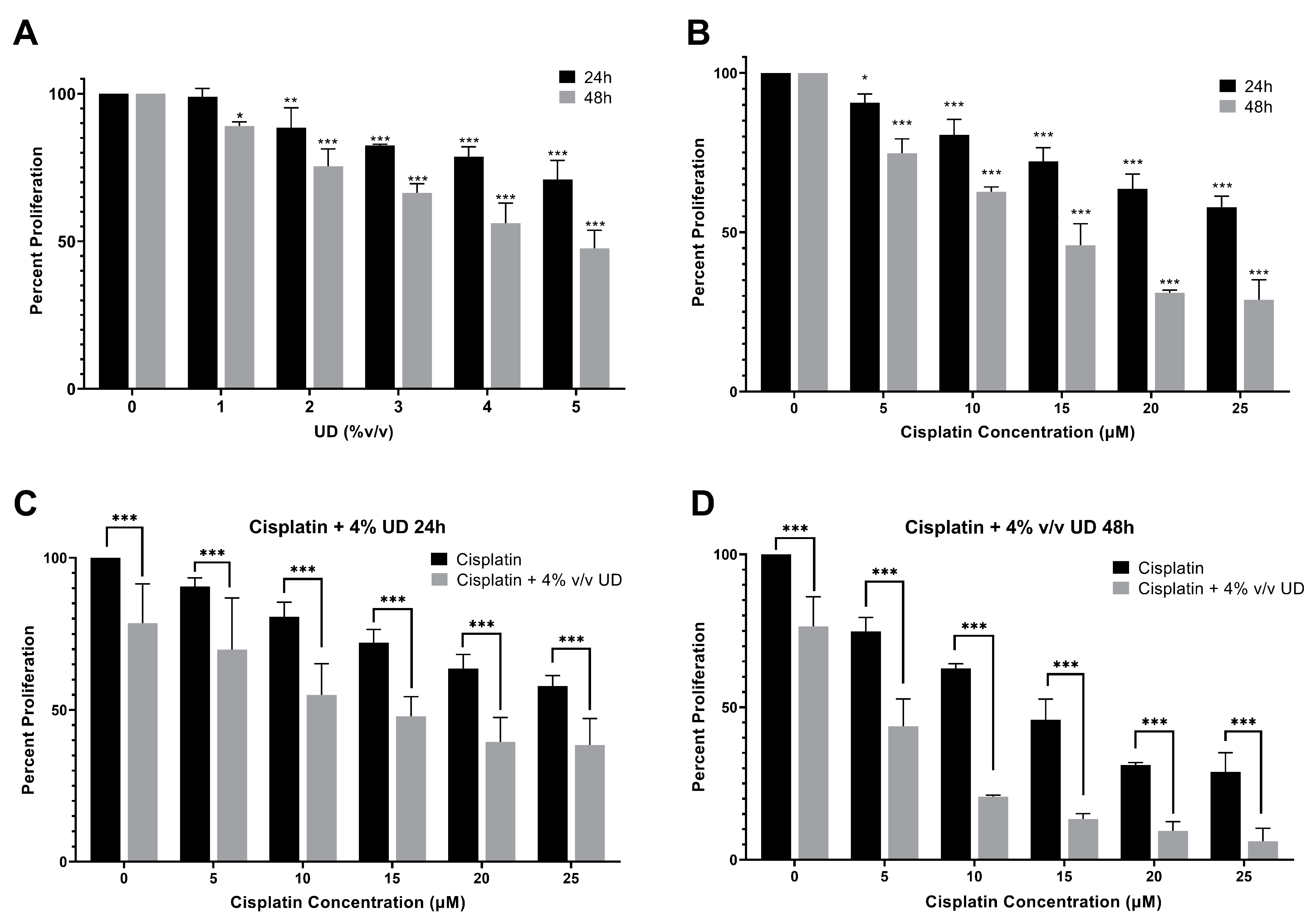
2.1. Antiproliferative Effect of Cisplatin and UD on Cell Proliferation
2.2. UD Enhances the Sensitivity of the MDA-MB-231 Cells to Cisplatin
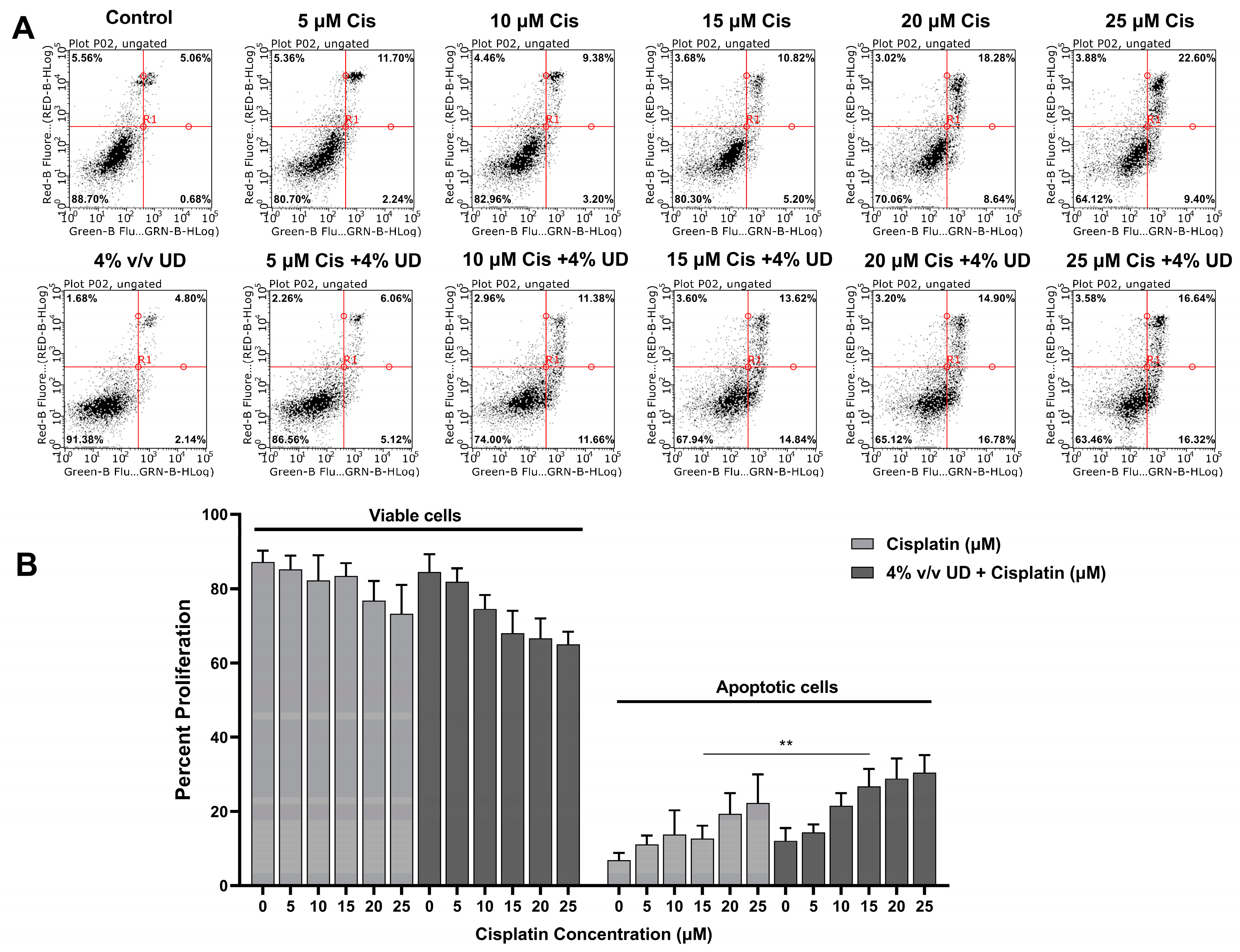
2.3. The Combination of UD and Cisplatin Promotes Apoptosis in the MDA-MB-231 Cells
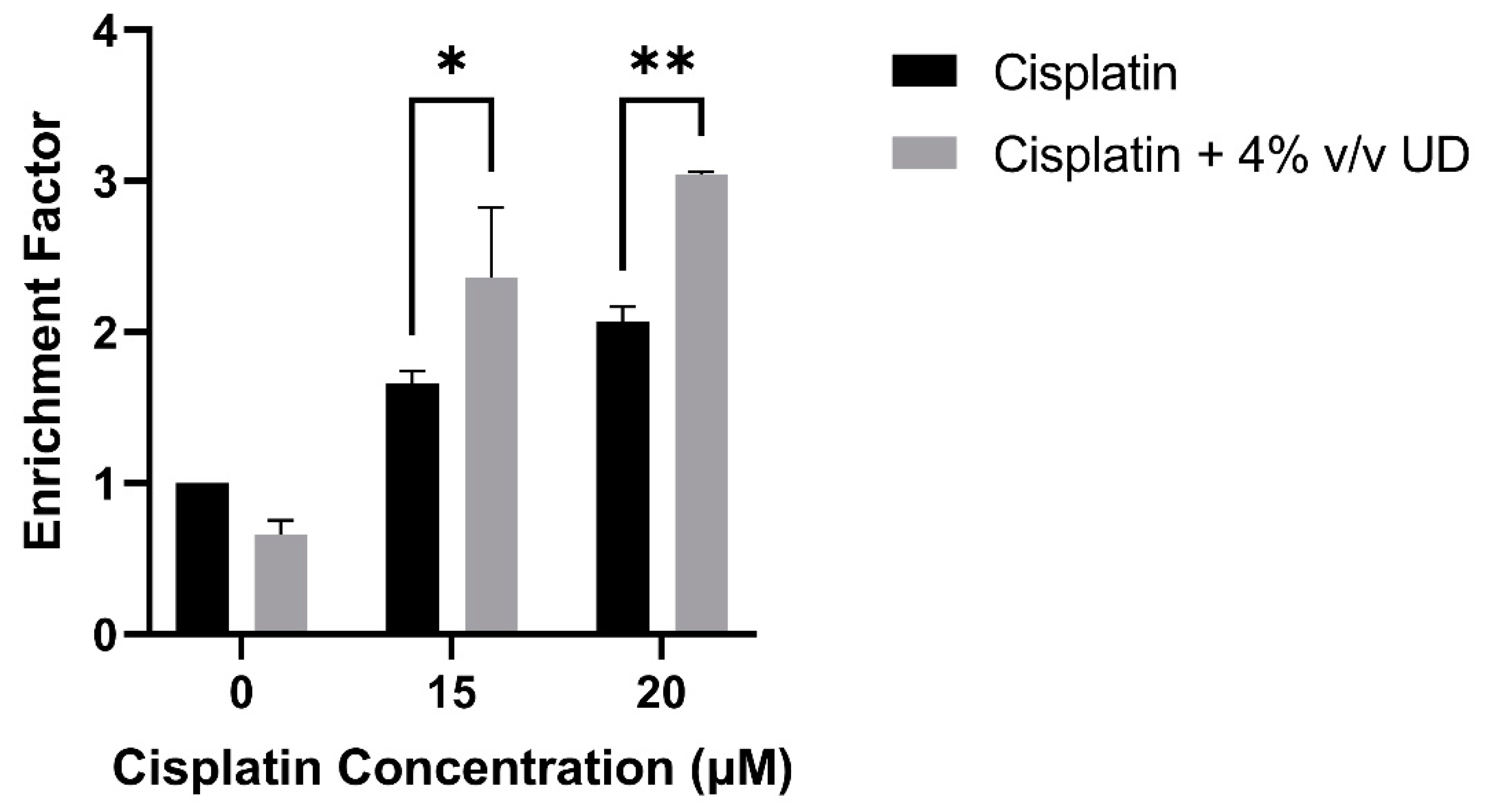
2.4. The Combination of UD and Cisplatin Increases DNA Fragmentation in the MDA-MB-231 Cells
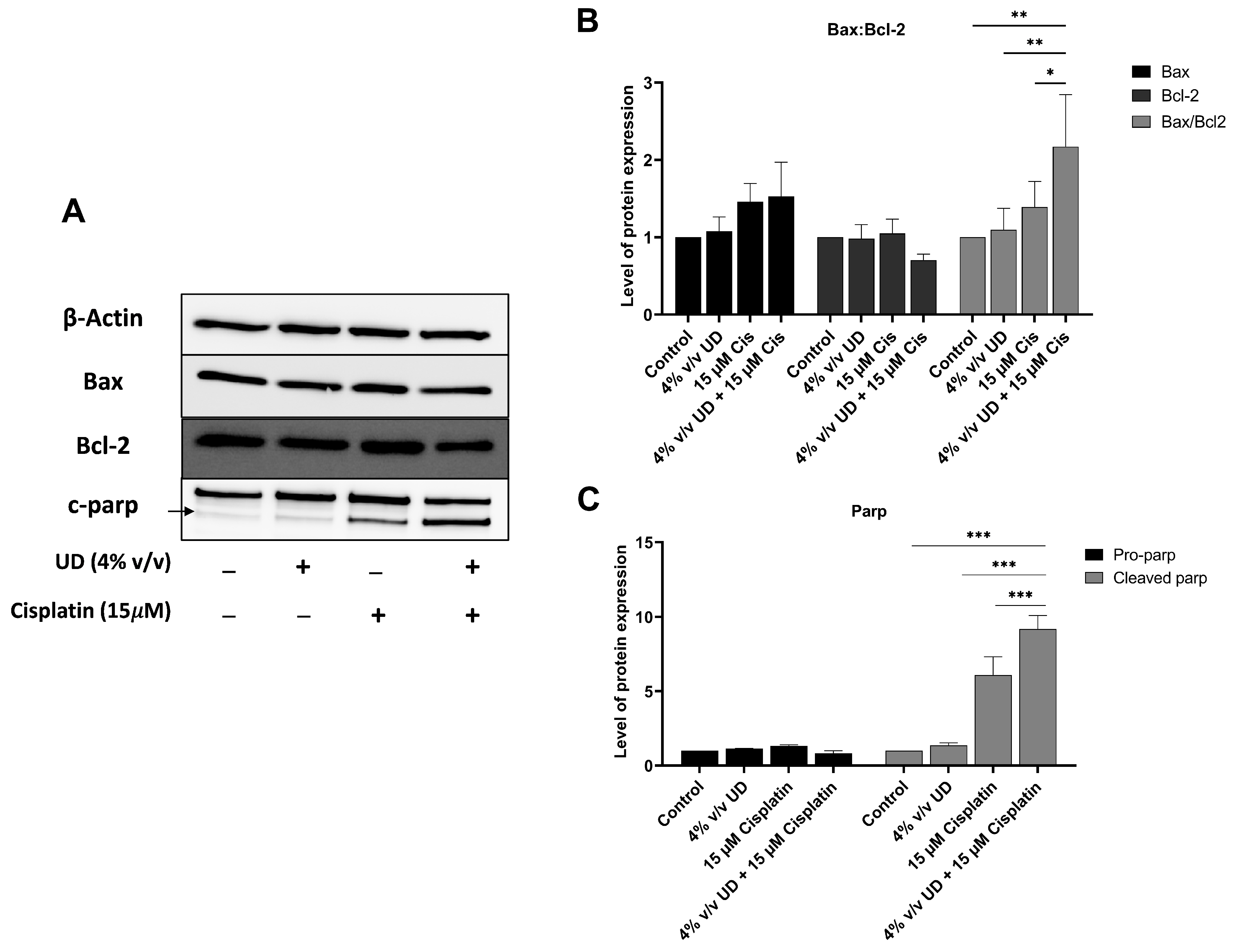
2.5. The Combination of UD and Cisplatin Upregulates the Expression of Apoptotic Proteins
3. Discussion
4. Methods
4.1. Urtica Dioica Aqueous Extract Preparation
4.2. Cell Culture
4.3. MTS Cytotoxicity Assay
4.4. Annexin V/Propidium Iodide Dual Staining
4.5. Cell Death ELISA
4.6. Western Blot
4.7. Statistical Analysis
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AML | Acute Myeloid Leukemia |
| DMEM | Dulbecco’s Modified Eagle Medium |
| ELISA | Enzyme Linked Immunostaining Assay |
| FBS | Fetal Bovine Serum |
| HER2 | Human Epidermal Growth Factors Receptor 2 |
| IC50 | Inhibitory Concentration |
| LC-MS | Liquid Chromatography/Mass Spectrometry |
| PARP | Poly-ADP ribose polymerase |
| PBS | Phosphate Buffered Saline |
| PI | Propidium Iodide |
| PVDF | Polyvinylidene fluoride |
| RIPA | Radio-Immunoprecipitation assay |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Arafa, M.A.; Farhat, K.H. Why cancer incidence in the Arab counties is much lower than other parts of the world? J. Egypt. Natl. Cancer Inst. 2022, 34, 41. [Google Scholar] [CrossRef] [PubMed]
- Elias, F.; Rabah, H.; Saleh, M.; Boushnak, M.; Said, C. Effectiveness of breast cancer screening campaigns from 2012 to 2017 by analysis of stage at diagnosis, Lebanon. East. Mediterr. Health J. 2021, 27, 580–586. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J. Animal Models for Studying Prevention and Treatment of Breast Cancer. In Animal Models for the Study of Human Disease; Academic Press: Cambridge, MA, USA, 2013; pp. 997–1018. [Google Scholar]
- Mansour, N.; Bodman-Smith, K.; Khnayzer, R.S.; Daher, C.F. A photoactivatable Ru (II) complex bearing 2,9-diphenyl-1,10-phenanthroline: A potent chemotherapeutic drug inducing apoptosis in triple negative human breast adenocarcinoma cells. Chem. Interact. 2020, 336, 109317. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Loerzel, V.W. Symptom Experience in Older Adults Undergoing Treatment for Cancer. Oncol. Nurs. Forum 2015, 42, E269–E278. [Google Scholar] [CrossRef]
- Altun, I.; Sonkaya, A. The Most Common Side Effects Experienced by Patients Were Receiving First Cycle of Chemotherapy. Iran. J. Public. Health 2018, 47, 1218–1219. [Google Scholar]
- Dhouibi, R.; Affes, H.; Ben Salem, M.; Hammami, S.; Sahnoun, Z.; Zeghal, K.M.; Ksouda, K. Screening of pharmacological uses of Urtica dioica and others benefits. Prog. Biophys. Mol. Biol. 2020, 150, 67–77. [Google Scholar] [CrossRef]
- Weigend, M. Urtica dioica subsp. cypria, with a Re-Evaluation of the U. dioica Group (Urticaceae) in Western Asia. Willdenowia 2006, 36, 811. [Google Scholar] [CrossRef]
- Grauso, L.; de Falco, B.; Lanzotti, V.; Motti, R. Stinging nettle, Urtica dioica L.: Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2020, 19, 1341–1377. [Google Scholar] [CrossRef]
- Adhikari, B.M.; Bajracharya, A.; Shrestha, A.K. Comparison of nutritional properties of Stinging nettle (Urtica dioica) flour with wheat and barley flours. Food Sci. Nutr. 2016, 4, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica spp.: Ordinary Plants with Extraordinary Properties. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, K.K.; Magar, S.K.; Thapa, R.; Lamsal, A.; Bhandari, S.; Maharjan, R.; Shrestha, S.; Shrestha, J. Nutritional and pharmacological importance of stinging nettle (Urtica dioica L.): A review. Heliyon 2022, 8, e09717. [Google Scholar] [CrossRef] [PubMed]
- Paulauskienė, A.; Tarasevičienė, Ž.; Laukagalis, V. Influence of Harvesting Time on the Chemical Composition of Wild Stinging Nettle (Urtica dioica L. ) Plants 2021, 10, 686. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.R.; DAR, S.A.; Sharma, D.P. Antibacterial Activity and Toxicological Evaluation of Semi Purified Hexane Extract of Urtica dioica Leaves. Res. J. Med. Plants 2012, 6, 123–135. [Google Scholar]
- Janghel, V.; Patel, P.; Chandel, S.S. Plants used for the treatment of icterus (jaundice) in Central India: A review. Ann. Hepatol. 2019, 18, 658–672. [Google Scholar] [CrossRef]
- Bouassida, K.Z.; Bardaa, S.; Khimiri, M.; Rebaii, T.; Tounsi, S.; Jlaiel, L.; Trigui, M. Exploring the Urtica dioica Leaves Hemostatic and Wound-Healing Potential. BioMed Res. Int. 2017, 2017, 1047523. [Google Scholar]
- Kültür, Ş. Medicinal plants used in Kırklareli Province (Turkey). J. Ethnopharmacol. 2007, 111, 341–364. [Google Scholar] [CrossRef]
- Kav, S.; Hanoğlu, Z.; Algier, L. Use of Complementary and Alternative Medicine by Cancer Patients in Turkey: A Literature Review. Int. J. Hematol. Oncol. 2008, 33, 032–038. [Google Scholar]
- Abu-Darwish, M.S.; Efferth, T. Medicinal Plants from Near East for Cancer Therapy. Front. Pharmacol. 2018, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Malak, A.T.; Karayurt, O.; Demir, E.; Yümer, A.S. Complementary and alternative medicine in cancer patients—Analysis of influencing factors in Turkey. Asian Pac. J. Cancer Prev. 2009, 10, 1083–1087. [Google Scholar] [PubMed]
- Yildiz, I.; Ozguroglu, M.; Toptas, T.; Turna, H.; Sen, F.; Yildiz, M. Patterns of Complementary and Alternative Medicine Use among Turkish Cancer Patients. J. Palliat. Med. 2013, 16, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Taheri, Y.; Quispe, C.; Herrera-Bravo, J.; Sharifi-Rad, J.; Ezzat, S.M.; Merghany, R.M.; Shaheen, S.; Azmi, L.; Mishra, A.P.; Sener, B.; et al. Urtica dioica-Derived Phytochemicals for Pharmacological and Therapeutic Applications. Evidence-Based Complement. Altern. Med. 2022, 2022, 4024331. [Google Scholar] [CrossRef]
- Hodroj, M.H.; Al Bast N al, H.; Taleb, R.I.; Borjac, J.; Rizk, S. Nettle Tea Inhibits Growth of Acute Myeloid Leukemia Cells In Vitro by Promoting Apoptosis. Nutrients 2020, 12, 2629. [Google Scholar] [CrossRef]
- Devkota, H.P.; Paudel, K.R.; Khanal, S.; Baral, A.; Panth, N.; Adhikari-Devkota, A.; Jha, N.K.; Das, N.; Singh, S.K.; Chellappan, D.K.; et al. Stinging Nettle (Urtica dioica L.): Nutritional Composition, Bioactive Compounds, and Food Functional Properties. Molecules 2022, 27, 5219. [Google Scholar] [CrossRef]
- Dar, S.A.; Ganai, F.A.; Yousuf, A.R.; Balkhi, M.-U.; Bhat, T.M.; Sharma, P. Pharmacological and toxicological evaluation of Urtica dioica. Pharm. Biol. 2013, 51, 170–180. [Google Scholar] [CrossRef]
- Fattahi, S.; Ardekani, A.M.; Zabihi, E.; Abedian, Z.; Mostafazadeh, A.; Pourbagher, R.; Akhavan-Niaki, H. Antioxidant and Apoptotic Effects of an Aqueous Extract of Urtica dioica on the MCF-7 Human Breast Cancer Cell Line. Asian Pac. J. Cancer Prev. 2013, 14, 5317–5323. [Google Scholar] [CrossRef]
- Levy, A.; Sivanesan, D.; Murugan, R.; Jornadal, J.; Quinonez, Y.; Jaffe, M.; Rathinavelu, A. Urtica dioica Induces Cytotoxicity in Human Prostate Carcinoma LNCaP Cells: Involvement of Oxidative Stress, Mitochondrial Depolarization and Apoptosis. Trop. J. Pharm. Res. 2014, 13, 711. [Google Scholar] [CrossRef]
- Lewandowska, U.; Gorlach, S.; Owczarek, K.; Hrabec, E.; Szewczyk, K. Synergistic interactions between anticancer chemotherapeutics and phenolic compounds and anticancer synergy between polyphenols. Postep. Hig. Med. Dosw. 2014, 68, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Okem, A.; Henstra, C.; Lambert, M.; Hayeshi, R. A review of the pharmacodynamic effect of chemo-herbal drug combinations therapy for cancer treatment. Med. Drug Discov. 2023, 17, 100147. [Google Scholar] [CrossRef]
- Yin, S.Y.; Wei, W.C.; Jian, F.Y.; Yang, N.S. Therapeutic Applications of Herbal Medicines for Cancer Patients. Evidence-Based Complement. Altern. Med. 2013, 2013, e302426. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Mansoori, B.; Aghapour, M.; Shirjang, S.; Nami, S.; Baradaran, B. The Urtica dioica extract enhances sensitivity of paclitaxel drug to MDA-MB-468 breast cancer cells. Biomed. Pharmacother. 2016, 83, 835–842. [Google Scholar] [CrossRef]
- D’Abrosca, B.; Ciaramella, V.; Graziani, V.; Papaccio, F.; Della Corte, C.M.; Potenza, N.; Fiorentino, A.; Ciardiello, F.; Morgillo, F. Urtica dioica L. inhibits proliferation and enhances cisplatin cytotoxicity in NSCLC cells via Endoplasmic Reticulum-stress mediated apoptosis. Sci. Rep. 2019, 9, 4986. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Langner, E.; Rzeski, W. Dietary derived compounds in cancer chemoprevention. Contemp. Oncol. 2012, 16, 394–400. [Google Scholar] [CrossRef]
- Sun, Y.-S.; Zhao, Z.; Yang, Z.-N.; Xu, F.; Lu, H.-J.; Zhu, Z.-Y.; Shi, W.; Jiang, J.; Yao, P.-P.; Zhu, H.-P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef]
- Mohammadi, A.; Mansoori, B.; Goldar, S.; Shanehbandi, D.; Khaze, V.; Mohammadnejad, L.; Baghbani, E.; Baradaran, B. Effects of Urtica dioica dichloromethane extract on cell apoptosis and related gene expression in human breast cancer cell line (MDA-MB-468). Cell. Mol. Biol. 2016, 62, 62–67. [Google Scholar]
- Zhang, J.H.; Xu, M. DNA fragmentation in apoptosis. Cell Res. 2000, 10, 205–211. [Google Scholar] [CrossRef]
- Brunelle, J.K.; Letai, A. Control of mitochondrial apoptosis by the Bcl-2 family. J. Cell Sci. 2009, 122, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Brady, H.J.M.; Gil-Gómez, G. Molecules in focus Bax. The pro-apoptotic Bcl-2 family member, Bax. Int. J. Biochem. Cell Biol. 1998, 30, 647–650. [Google Scholar] [CrossRef]
- Del Poeta, G.; Venditti, A.; Del Principe, M.I.; Maurillo, L.; Buccisano, F.; Tamburini, A.; Cox, M.C.; Franchi, A.; Bruno, A.; Mazzone, C.; et al. Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML): Presented in part at the 42nd annual meeting of the American Society of Hematology, San Francisco, CA, 1–5 December 2000. J. Am. Soc. Hematol. 2003, 101, 2125–2131. [Google Scholar]
- Raisova, M.; Hossini, A.M.; Eberle, J.; Riebeling, C.; Orfanos, C.E.; Geilen, C.C.; Wieder, T.; Sturm, I.; Daniel, P.T. The Bax/Bcl-2 Ratio Determines the Susceptibility of Human Melanoma Cells to CD95/Fas-Mediated Apoptosis. J. Investig. Dermatol. 2001, 117, 333–340. [Google Scholar] [CrossRef]
- Zhu, W.; Lv, C.; Wang, J.; Gao, Q.; Zhu, H.; Wen, H. Patuletin induces apoptosis of human breast cancer SK-BR-3 cell line via inhibiting fatty acid synthase gene expression and activity. Oncol. Lett. 2017, 14, 7449–7454. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Shen, H.; Xi, W.; Wang, Q.; Yin, L.; Zhang, Y.; Yu, Y.; Yang, Q.; Wang, Z.-N. Synergistic Anticancer Activity of Combined Use of Caffeic Acid with Paclitaxel Enhances Apoptosis of Non-Small-Cell Lung Cancer H1299 Cells in Vivo and in Vitro. Cell. Physiol. Biochem. 2018, 48, 1433–1442. [Google Scholar] [CrossRef]
- Koraneekit, A.; Limpaiboon, T.; Sangka, A.; Boonsiri, P.; Daduang, S.; Daduang, J. Synergistic effects of cisplatin-caffeic acid induces apoptosis in human cervical cancer cells via the mitochondrial pathways. Oncol. Lett. 2018, 15, 7397–7402. [Google Scholar] [CrossRef]
- Younes, M.; Ammoury, C.; Haykal, T.; Nasr, L.; Sarkis, R.; Rizk, S. The selective anti-proliferative and pro-apoptotic effect of A. cherimola on MDA-MB-231 breast cancer cell line. BMC Complement. Med. Ther. 2020, 20, 343. [Google Scholar] [CrossRef]
- Khalil, C. In Vitro UVB induced Cellular Damage Assessment Using Primary Human Skin Derived Fibroblasts. MOJ Toxicol. 2015, 1, 138–143. [Google Scholar] [CrossRef]
- Idriss, M.; Younes, M.; Abou Najem, S.; Hodroj, M.H.; Fakhoury, R.; Rizk, S. Gamma-Tocotrienol Synergistically Promotes the Anti-proliferative and Pro-apoptotic Effects of Etoposide on Breast Cancer Cell Lines. Curr. Mol. Pharmacol. 2022, 15, 980–986. [Google Scholar]
- Ramaraju, H.; McAtee, A.M.; Akman, R.E.; Verga, A.S.; Bocks, M.L.; Hollister, S.J. Sterilization effects on poly(glycerol dodecanedioate): A biodegradable shape memory elastomer for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.S.M.; Ho, J.; Wills, S.; Kawall, A.; Sharma, A.; Chavada, K.; Ebert, M.C.C.J.C.; Evoli, S.; Singh, A.; Rayalam, S.; et al. Aloin isoforms (A and B) selectively inhibits proteolytic and deubiquitinating activity of papain like protease (PLpro) of SARS-CoV-2 in vitro. Sci. Rep. 2022, 12, 2145. [Google Scholar] [CrossRef] [PubMed]
- ElNaggar, M.H.; Abdelwahab, G.M.; Kutkat, O.; GabAllah, M.; Ali, M.A.; El-Metwally, M.E.A.; Sayed, A.M.; Abdelmohsen, U.R.; Khalil, A.T. Aurasperone A Inhibits SARS CoV-2 In Vitro: An Integrated In Vitro and In Silico Study. Mar. Drugs 2022, 20, 179. [Google Scholar] [CrossRef] [PubMed]
- Khalil, C.; Chahine, J.B.; Haykal, T.; Al Hageh, C.; Rizk, S.; Khnayzer, R.S. E-cigarette aerosol induced cytotoxicity, DNA damages and late apoptosis in dynamically exposed A549 cells. Chemosphere 2021, 263, 127874. [Google Scholar] [CrossRef] [PubMed]
- El Samarji, M.; Younes, M.; El Khoury, M.; Haykal, T.; Elias, N.; Gasilova, N.; Menin, L.; Houri, A.; Machaka-Houri, N.; Rizk, S. The Antioxidant and Proapoptotic Effects of Sternbergia clusiana Bulb Ethanolic Extract on Triple-Negative and Estrogen-Dependent Breast Cancer Cells In Vitro. Plants 2023, 12, 529. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, S.; Mansour, N.; Daher, C.F.; Elias, M.G.; Dagher, C.; Khnayzer, R.S. Drug-free phototherapy of superficial tumors: White light at the end of the tunnel. J. Photochem. Photobiol. B Biol. 2021, 224, 112324. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nafeh, G.; Abi Akl, M.; Samarani, J.; Bahous, R.; Al Kari, G.; Younes, M.; Sarkis, R.; Rizk, S. Urtica dioica Leaf Infusion Enhances the Sensitivity of Triple-Negative Breast Cancer Cells to Cisplatin Treatment. Pharmaceuticals 2023, 16, 780. https://doi.org/10.3390/ph16060780
Nafeh G, Abi Akl M, Samarani J, Bahous R, Al Kari G, Younes M, Sarkis R, Rizk S. Urtica dioica Leaf Infusion Enhances the Sensitivity of Triple-Negative Breast Cancer Cells to Cisplatin Treatment. Pharmaceuticals. 2023; 16(6):780. https://doi.org/10.3390/ph16060780
Chicago/Turabian StyleNafeh, Guy, Maria Abi Akl, Jad Samarani, Rawane Bahous, Georges Al Kari, Maria Younes, Rita Sarkis, and Sandra Rizk. 2023. "Urtica dioica Leaf Infusion Enhances the Sensitivity of Triple-Negative Breast Cancer Cells to Cisplatin Treatment" Pharmaceuticals 16, no. 6: 780. https://doi.org/10.3390/ph16060780
APA StyleNafeh, G., Abi Akl, M., Samarani, J., Bahous, R., Al Kari, G., Younes, M., Sarkis, R., & Rizk, S. (2023). Urtica dioica Leaf Infusion Enhances the Sensitivity of Triple-Negative Breast Cancer Cells to Cisplatin Treatment. Pharmaceuticals, 16(6), 780. https://doi.org/10.3390/ph16060780