The Potential Effect of Polysaccharides Extracted from Red Alga Gelidium spinosum against Intestinal Epithelial Cell Apoptosis
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Yield
2.2. Chemical Analysis
2.3. Structural Characterization
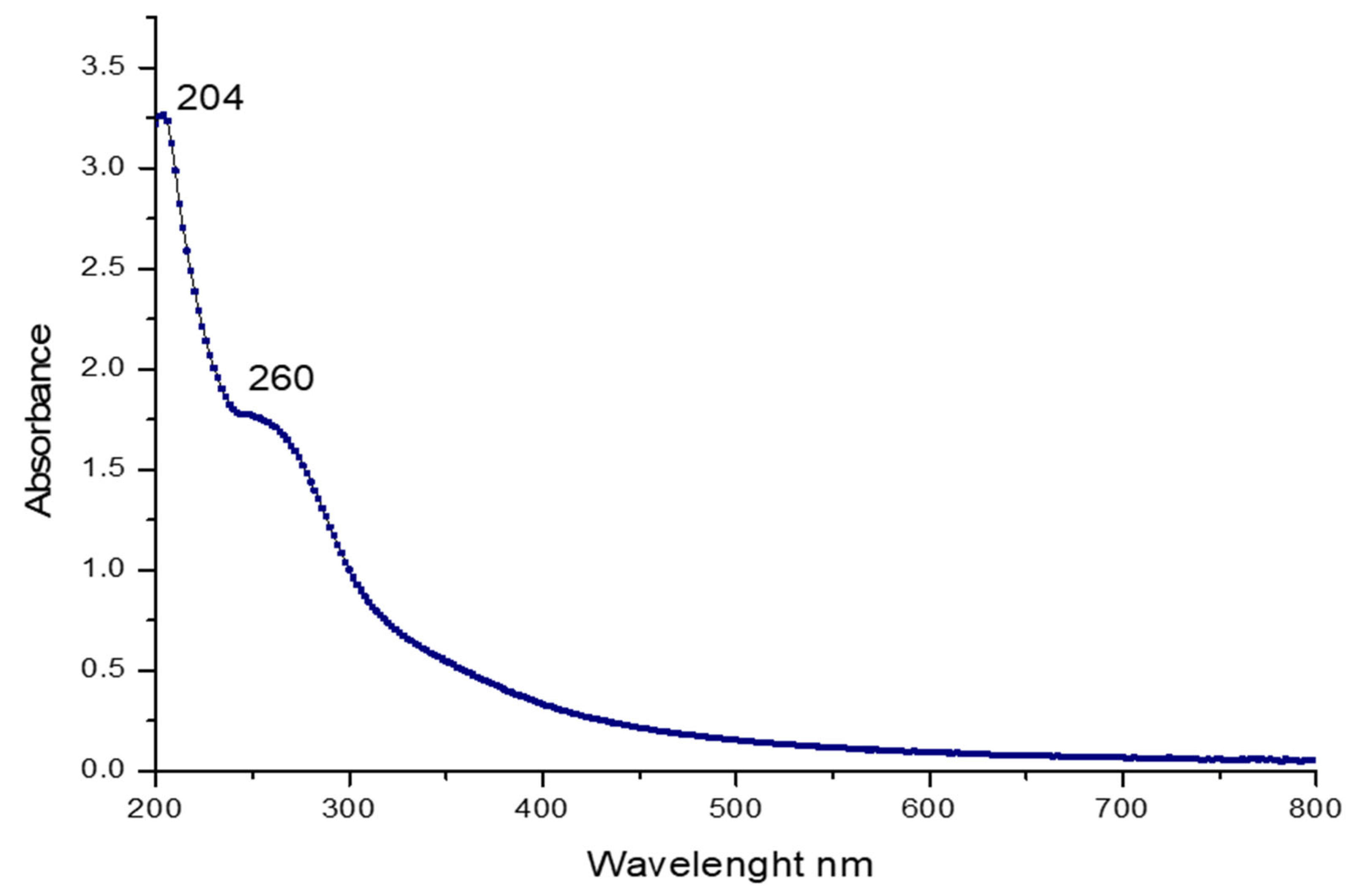
2.3.1. UV and FT-IR Spectroscopy Analysis
2.3.2. X-ray Diffractometry (XRD) Analysis
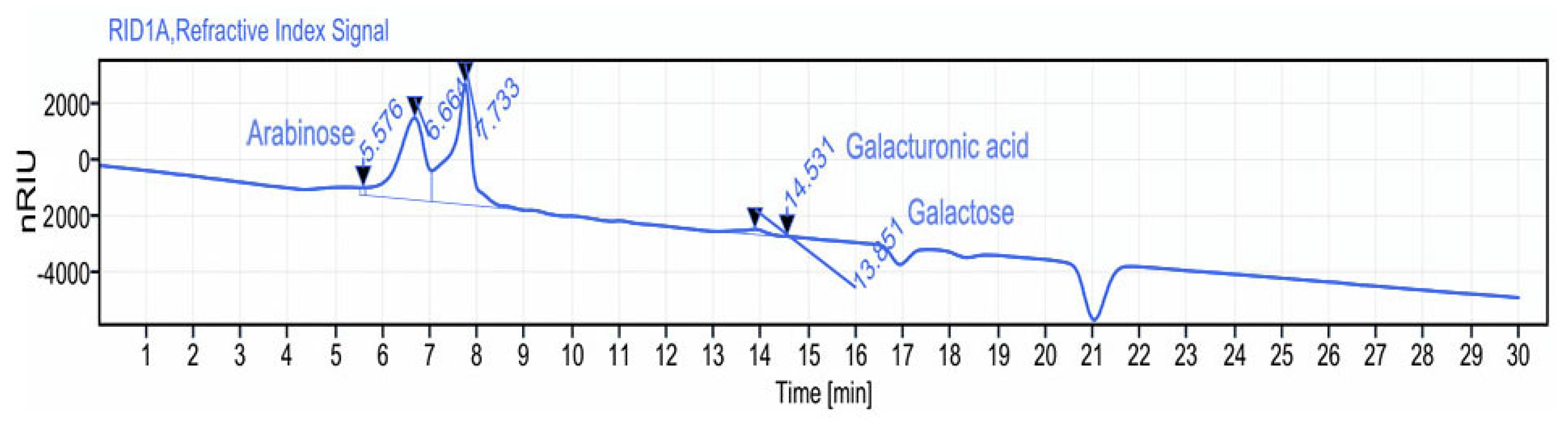
2.3.3. Monosaccharide Composition Analysis
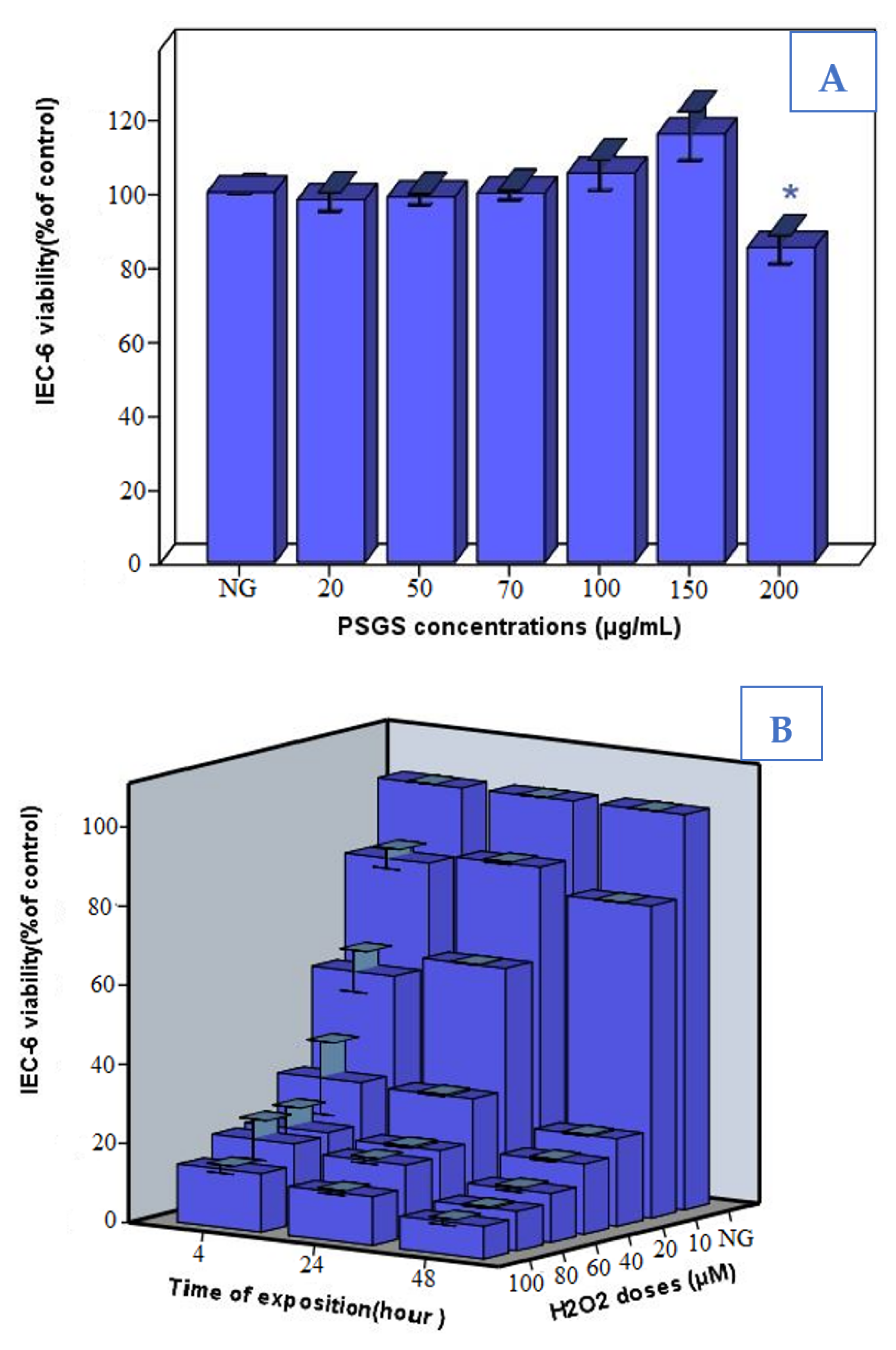
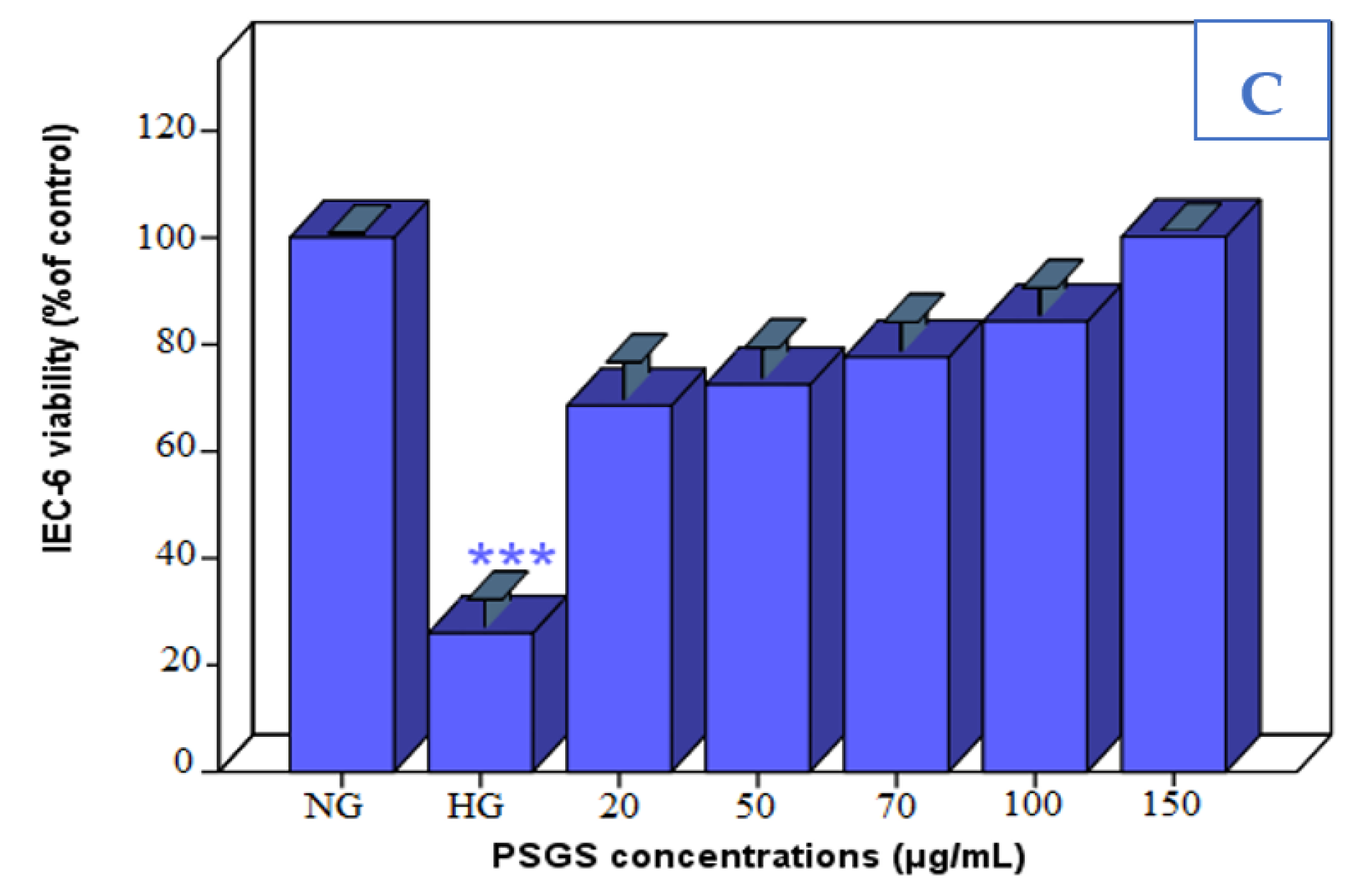
2.4. Effect of PSGS and H2O2 on IEC-6 Cells Viability
2.5. Effect of PSGS and H2O2 on the Morphological Aspect
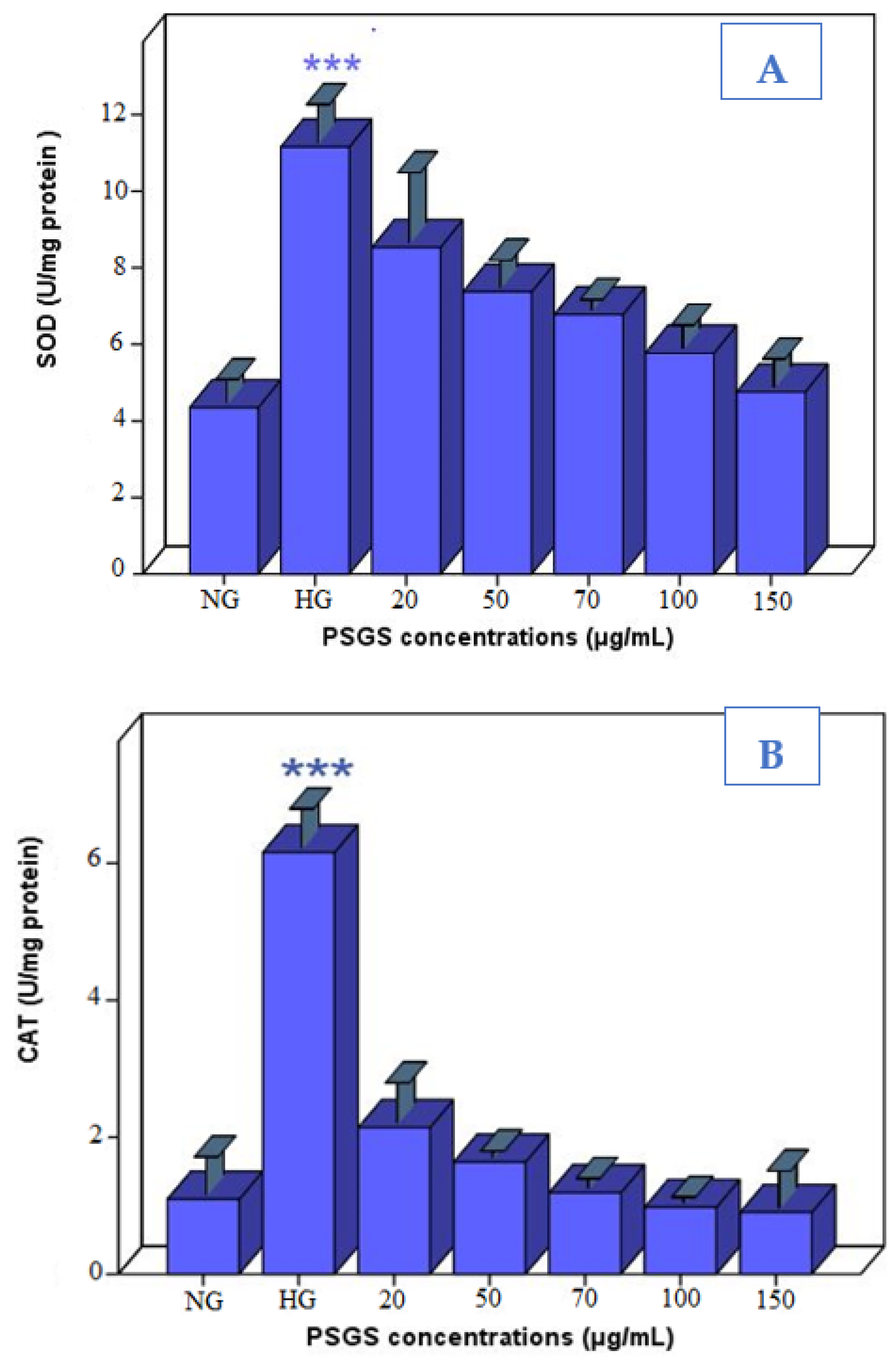
2.6. PSGS Supported Enzymatic Defense against H2O2 Toxicity
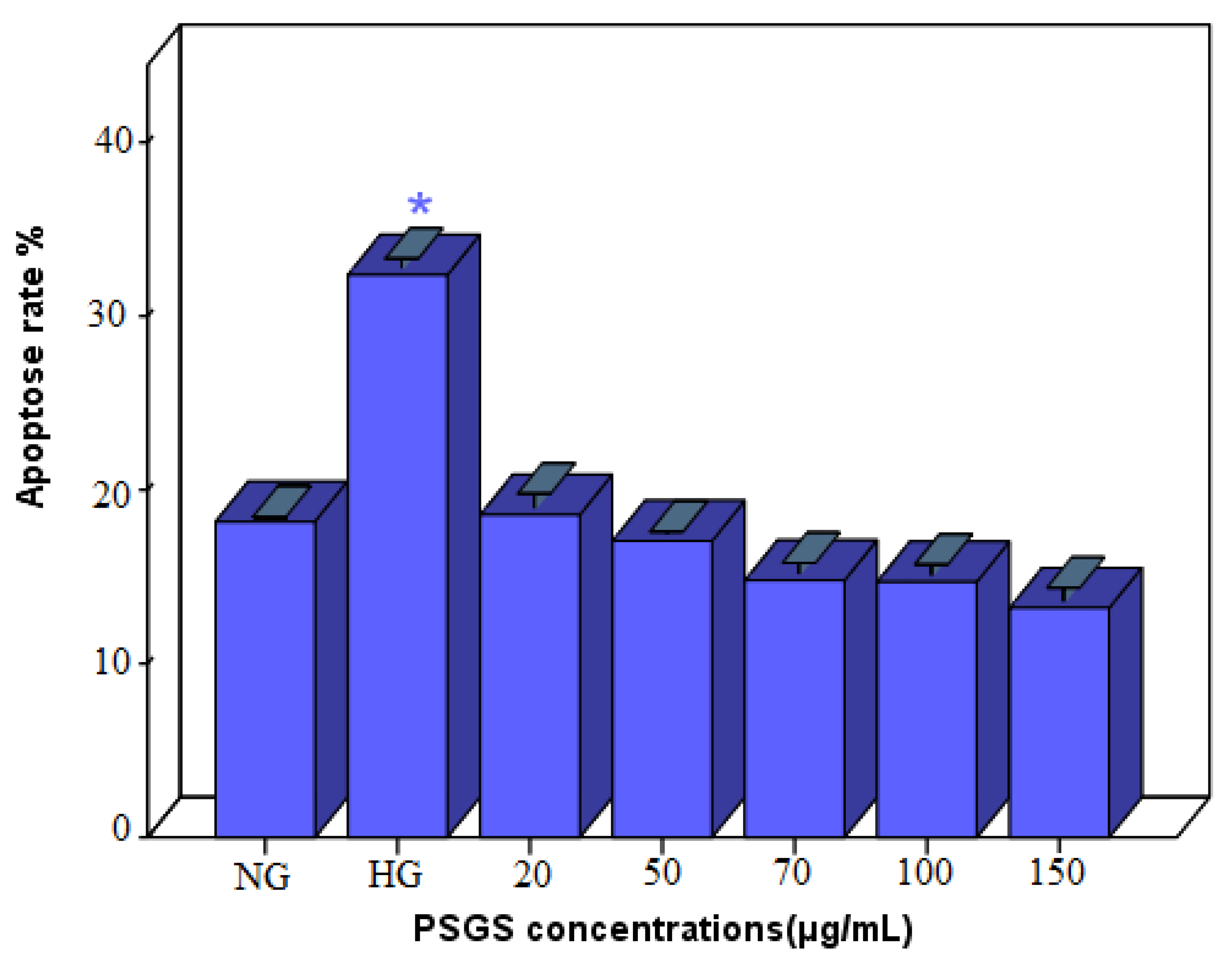
2.7. Effect of PSGS on H2O2 Induced Apoptosis in IEC-6 Cells
3. Materials and Methods
3.1. Chemicals and Reagents
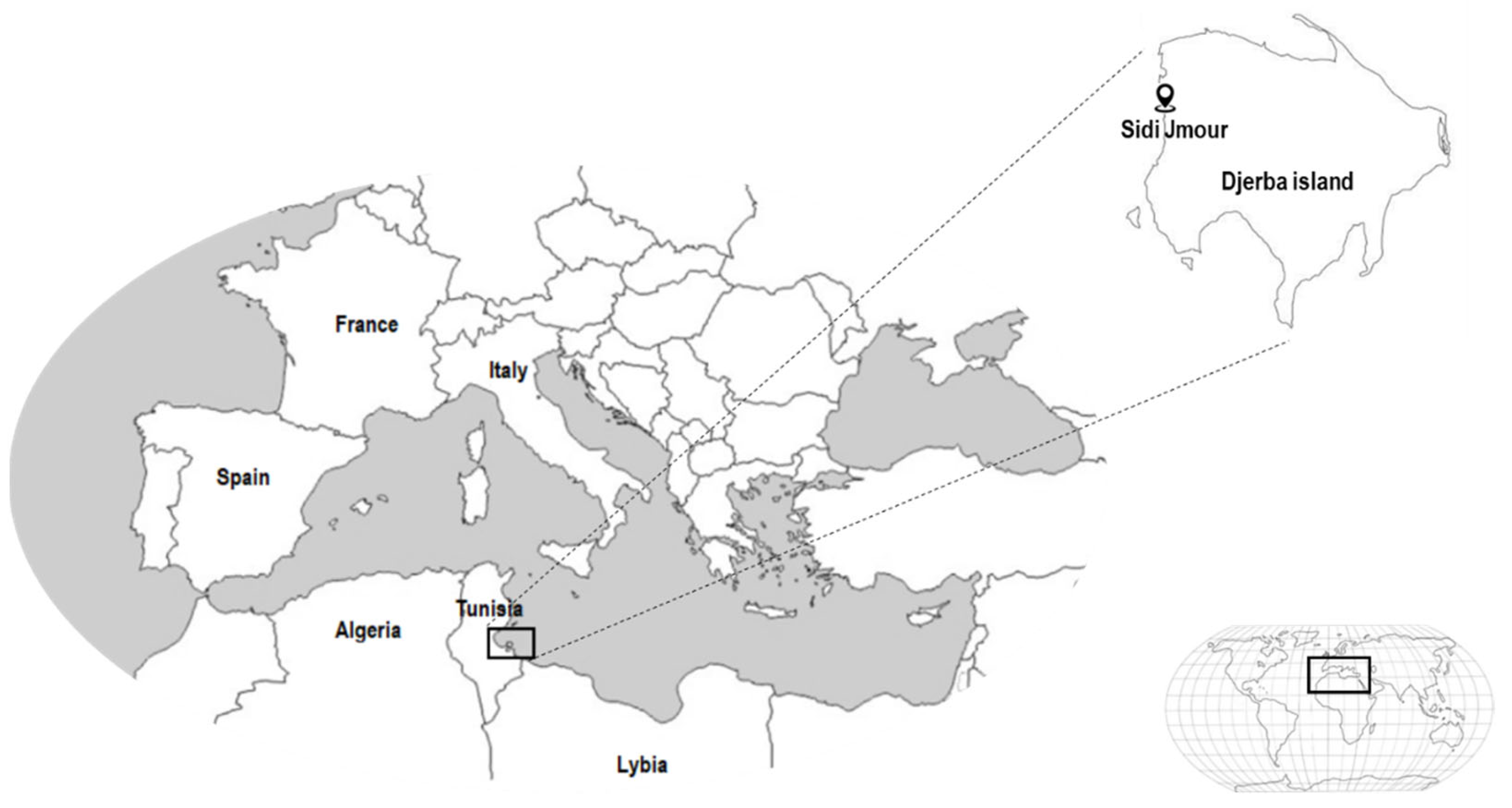
3.2. Seaweed Collection and Processing
3.3. Polysaccharides Extraction
3.4. Chemical Characterization of PSGS
Determination of Total Carbohydrate, Protein, Uronic Acid, Sulfate, and Ash Content
3.5. Structural Characterization of PSGS
3.5.1. Ultraviolet and Fourier Transform Infrared (FT-IR) Spectroscopic Analysis
3.5.2. X-ray Diffractometry (XRD) Analysis
3.5.3. Monosaccharide Composition Analysis
3.6. Cytoprotective Activity of PSGS on IEC-6 Cells
3.6.1. Measurement of IEC-6 Cells Viability
3.6.2. Cell Morphology Observation
3.7. Determination of Antioxidant Enzymes Activity
3.7.1. Superoxide DISMUTASE Activity
3.7.2. Catalase Activity
3.8. Apoptosis Rate Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The epidemiology of inflammatory bowel disease: East meets west. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Li, C.; Wu, G.; Zhao, H.; Dong, N.; Wu, B.; Chen, Y.; Lu, Q. Natural-Derived Polysaccharides From Plants, Mushrooms, and Seaweeds for the Treatment of Inflammatory Bowel Disease. Front. Pharmacol. 2021, 12, 651813. [Google Scholar] [CrossRef]
- Raoul, P.; Cintoni, M.; Palombaro, M.; Basso, L.; Rinninella, E.; Gasbarrini, A.; Mele, M.C. Food Additives, a Key Environmental Factor in the Development of IBD through Gut Dysbiosis. Microorganisms 2022, 10, 167. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Bernstein, C.N.; Iliopoulos, D.; Macpherson, A.; Neurath, M.F.; Ali, R.A.R.; Vavricka, S.R.; Fiocchi, C. Environmental triggers in IBD: A review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 39–49. [Google Scholar] [CrossRef]
- Kong, S.; Zhang, Y.H.; Zhang, W. Regulation of Intestinal Epithelial Cells Properties and Functions by Amino Acids. Biomed. Res. Int. 2018, 2018, 2819154. [Google Scholar] [CrossRef]
- Flynn, S.; Eisenstein, S. Inflammatory Bowel Disease Presentation and Diagnosis. Surg. Clin. N. Am. 2019, 99, 1051–1062. [Google Scholar] [CrossRef]
- Sairenji, T.; Collins, K.L.; Evans, D.V. An Update on Inflammatory Bowel Disease. Prim. Care 2017, 44, 673–692. [Google Scholar] [CrossRef]
- Kim, D.H.; Cheon, J.H. Pathogenesis of Inflammatory Bowel Disease and Recent Advances in Biologic Therapies. Immune Netw. 2017, 17, 25–40. [Google Scholar] [CrossRef]
- Salice, M.; Rizzello, F.; Calabrese, C.; Calandrini, L.; Gionchetti, P. A current overview of corticosteroid use in active ulcerative colitis. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 557–561. [Google Scholar] [CrossRef]
- Shukla, M.K.; Singh, S.K.; Pandey, S.; Gupta, P.K.; Choudhary, A.; Jindal, D.K.; Dua, K.; Kumar, D. Potential Immunomodulatory Activities of Plant Products. S. Afr. J. Bot. 2022, 149, 937–943. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B. Chapter One—Natural Antioxidants of Plant Origin. In Advances in Food and Nutrition Research; Ferreira, I.C.F.R., Barros, L., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 90, pp. 1–81. [Google Scholar]
- Maione, F.; Russo, R.; Khan, H.; Mascolo, N. Medicinal plants with anti-inflammatory activities. Nat. Prod. Res. 2016, 30, 1343–1352. [Google Scholar] [CrossRef]
- Sharma, S.; Shukla, M.K.; Sharma, K.C.; Tirath; Kumar, L.; Anal, J.M.H.; Upadhyay, S.K.; Bhattacharyya, S.; Kumar, D. Revisiting the therapeutic potential of gingerols against different pharmacological activities. Naunyn-Schmiedeberg’s Arch. Pharm. 2022, 31, 1–15. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Ma, R.; Li, L.; Wu, W.; Cai, D.; Lu, Q. Natural-derived alkaloids exhibit great potential in the treatment of ulcerative colitis. Pharmacol. Res. 2022, 175, 105972. [Google Scholar] [CrossRef]
- Arokiarajan, M.S.; Thirunavukkarasu, R.; Joseph, J.; Ekaterina, O.; Aruni, W. Advance research in biomedical applications on marine sulfated polysaccharide. Int. J. Biol. Macromol. 2022, 194, 870–881. [Google Scholar] [CrossRef]
- Pengzhan, Y.; Ning, L.; Xiguang, L.; Gefei, Z.; Quanbin, Z.; Pengcheng, L. Antihyperlipidemic effects of different molecular weight sulfated polysaccharides from Ulva pertusa (Chlorophyta). Pharmacol. Res. 2003, 48, 543–549. [Google Scholar] [CrossRef]
- Talarico, L.B.; Zibetti, R.G.M.; Faria, P.C.S.; Scolaro, L.A.; Duarte, M.E.R.; Noseda, M.D.; Pujol, C.A.; Damonte, E.B. Anti-herpes simplex virus activity of sulfated galactans from the red seaweeds Gymnogongrus griffithsiae and Cryptonemia crenulata. Int. J. Biol. Macromol. 2004, 34, 63–71. [Google Scholar] [CrossRef]
- Shi, J.; Cheng, C.; Zhao, H.; Jing, J.; Gong, N.; Lu, W. In vivo anti-radiation activities of the Ulva pertusa polysaccharides and polysaccharide–iron(III) complex. Int. J. Biol. Macromol. 2013, 60, 341–346. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.A.; Câmara, R.B.G.; Nobre, L.T.D.B.; Costa, M.S.S.P.; Almeida-Lima, J.; Farias, E.H.C.; et al. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharm. 2010, 64, 21–28. [Google Scholar] [CrossRef]
- Chen, L.; Hu, M.-B.; Chen, Z.-Y.; Wang, G.; Su, Q.; Liu, Y.-J. Preparation, structural characterization and neuroprotective effects of polysaccharides from the pericarp of Zanthoxylum bungeanum Maxim against H2O2-induced oxidative damage in PC12 cells. S. Afr. J. Bot. 2021, 142, 165–174. [Google Scholar] [CrossRef]
- Zaitseva, O.O.; Sergushkina, M.I.; Khudyakov, A.N.; Polezhaeva, T.V.; Solomina, O.N. Seaweed sulfated polysaccharides and their medicinal properties. Algal Res. 2022, 68, 102885. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, T.; Yang, Y.; Meng, F.; Zhan, F.; Jiang, Q.; Sun, X. Anti-Cancer Activity of Porphyran and Carrageenan from Red Seaweeds. Molecules 2019, 24, 4286. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.-K. Biological Activities of Carrageenan. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 72, pp. 113–124. ISBN 978-0-12-800269-8. [Google Scholar]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Veiga, M.-D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef]
- Rupert, R.; Rodrigues, K.F.; Thien, V.Y.; Yong, W.T.L. Carrageenan From Kappaphycus alvarezii (Rhodophyta, Solieriaceae): Metabolism, Structure, Production, and Application. Front. Plant Sci. 2022, 13, 859635. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Ardiana, N.; Padmi, H.; Ilhami, B.T.K.; Martyasari, N.W.R.; Sunarwidhi, A.L.; Nikmatullah, A.; Widyastuti, S.; Sunarpi, H.; Frediansyah, A. The Antiproliferative and Apoptosis-Inducing Effects of the Red Macroalgae Gelidium latifolium Extract against Melanoma Cells. Molecules 2021, 26, 6568. [Google Scholar] [CrossRef]
- Said, R.B.; Romdhane, M.S.; Abed, A.E.; M’rabet, R. La Rhodophycée Gelidium Spinosum (S.G. Gmelin) P.C. Silva, des Côtes de Monastir, en Tunisie: Quelques Éléments Hydro Biologiques et Potentialités en Agar-Agar. Available online: http://revues.refer.org/document.php?id=1344 (accessed on 14 February 2023).
- Nai, J.; Zhang, C.; Shao, H.; Li, B.; Li, H.; Gao, L.; Dai, M.; Zhu, L.; Sheng, H. Extraction, structure, pharmacological activities and drug carrier applications of Angelica sinensis polysaccharide. Int. J. Biol. Macromol. 2021, 183, 2337–2353. [Google Scholar] [CrossRef]
- Pereira, M.G.; Benevides, N.M.B.; Melo, M.R.S.; Valente, A.P.; Melo, F.R.; Mourão, P.A.S. Structure and anticoagulant activity of a sulfated galactan from the red alga, Gelidium crinale. Is there a specific structural requirement for the anticoagulant action? Carbohydr. Res. 2005, 340, 2015–2023. [Google Scholar] [CrossRef]
- Jaballi, I.; Sallem, I.; Feki, A.; Cherif, B.; Kallel, C.; Boudawara, O.; Jamoussi, K.; Mellouli, L.; Nasri, M.; Amara, I.B. Polysaccharide from a Tunisian red seaweed Chondrus canaliculatus: Structural characteristics, antioxidant activity and in vivo hemato-nephroprotective properties on maneb induced toxicity. Int. J. Biol. Macromol. 2019, 123, 1267–1277. [Google Scholar] [CrossRef]
- Marinho-Soriano, E. Agar polysaccharides from Gracilaria species (Rhodophyta, Gracilariaceae). J. Biotechnol. 2001, 89, 81–84. [Google Scholar] [CrossRef]
- Hao, H.; Han, Y.; Yang, L.; Hu, L.; Duan, X.; Yang, X.; Huang, R. Structural characterization and immunostimulatory activity of a novel polysaccharide from green alga Caulerpa racemosa var peltata. Int. J. Biol. Macromol. 2019, 134, 891–900. [Google Scholar] [CrossRef]
- Dore, C.M.P.G.; Alves, M.G.d.C.F.; Will, L.S.E.P.; Costa, T.G.; Sabry, D.A.; de Souza Rêgo, L.A.R.; Accardo, C.M.; Rocha, H.A.; Filgueira, L.G.A.; Leite, E.L. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr. Polym. 2013, 91, 467–475. [Google Scholar] [CrossRef]
- Jouki, M.; Mortazavi, S.A.; Yazdi, F.T.; Koocheki, A. Optimization of extraction, antioxidant activity and functional properties of quince seed mucilage by RSM. Int. J. Biol. Macromol. 2014, 66, 113–124. [Google Scholar] [CrossRef]
- Fleury, N.; Lahaye, M. Chemical and physico-chemical characterisation of fibres from Laminaria digitata (kombu breton): A physiological approach. J. Sci. Food Agric. 1991, 55, 389–400. [Google Scholar] [CrossRef]
- Liu, G.; Xu, S.; Chen, L. Chemical composition and bioactivities of a water-soluble polysaccharide from the endodermis of shaddock. Int. J. Biol. Macromol. 2012, 51, 763–766. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Kang, N.; Ahn, G.; Jee, Y.; Kim, Y.-T.; Jeon, Y.-J. Bioactive potentials of sulfated polysaccharides isolated from brown seaweed Sargassum spp in related to human health applications: A review. Food Hydrocoll. 2018, 81, 200–208. [Google Scholar] [CrossRef]
- Rioux, L.-E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Cui, M.; Zhou, R.; Wang, Y.; Zhang, M.; Liu, K.; Ma, C. Beneficial effects of sulfated polysaccharides from the red seaweed Gelidium pacificum Okamura on mice with antibiotic-associated diarrhea. Food Funct. 2020, 11, 4625–4637. [Google Scholar] [CrossRef]
- Jose, G.M.; Raghavankutty, M.; Kurup, G.M. Sulfated polysaccharides from Padina tetrastromatica induce apoptosis in HeLa cells through ROS triggered mitochondrial pathway. Process Biochem. 2018, 68, 197–204. [Google Scholar] [CrossRef]
- Shi, J.-J.; Zhang, J.-G.; Sun, Y.-H.; Qu, J.; Li, L.; Prasad, C.; Wei, Z.-J. Physicochemical properties and antioxidant activities of polysaccharides sequentially extracted from peony seed dreg. Int. J. Biol. Macromol. 2016, 91, 23–30. [Google Scholar] [CrossRef]
- Luo, D. Identification of structure and antioxidant activity of a fraction of polysaccharide purified from Dioscorea nipponica Makino. Carbohydr. Polym. 2008, 71, 544–549. [Google Scholar] [CrossRef]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.-R.; Liu, W.; Su, Y.; Han, Q.-H.; Zhao, L.; Zhang, Q.; Lin, D.-R.; et al. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2019, 127, 178–186. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Gnanakan, A.; Lakshmana, S.; Meivelu, M.; Jeganathan, A. Structural characterization and anticancer activity of extracellular polysaccharides from ascidian symbiotic bacterium Bacillus thuringiensis. Carbohydr. Polym. 2018, 190, 113–120. [Google Scholar] [CrossRef]
- Jing, L.; Sheng, J.; Jiang, J.; Wang, Y.; Shen, X.; Liu, D.; Zhang, W.; Mao, S. Chemical characteristics and cytoprotective activities of polysaccharide fractions from Athyrium Multidentatum (Doll.) Ching. Int. J. Biol. Macromol. 2020, 158, 1227–1238. [Google Scholar] [CrossRef]
- Pei, Y.; Yang, S.; Xiao, Z.; Zhou, C.; Hong, P.; Qian, Z.-J. Structural Characterization of Sulfated Polysaccharide Isolated From Red Algae (Gelidium crinale) and Antioxidant and Anti-Inflammatory Effects in Macrophage Cells. Front. Bioeng. Biotechnol. 2021, 9, 794818. [Google Scholar] [CrossRef]
- Volery, P.; Besson, R.; Schaffer-Lequart, C. Characterization of commercial carrageenans by Fourier transform infrared spectroscopy using single-reflection attenuated total reflection. J. Agric. Food Chem. 2004, 52, 7457–7463. [Google Scholar] [CrossRef]
- Barker, S.A.; Bourne, E.J.; Stacey, M.; Whiffen, D.H. Infra-red spectra of carbohydrates. Part I. Some derivatives of D-glucopyranose. J. Chem. Soc. 1954, 171–176. [Google Scholar] [CrossRef]
- Qian, J.-Y.; Chen, W.; Zhang, W.-M.; Zhang, H. Adulteration identification of some fungal polysaccharides with SEM, XRD, IR and optical rotation: A primary approach. Carbohydr. Polym. 2009, 78, 620–625. [Google Scholar] [CrossRef]
- Phyo, P.; Wang, T.; Xiao, C.; Anderson, C.T.; Hong, M. Effects of Pectin Molecular Weight Changes on the Structure, Dynamics, and Polysaccharide Interactions of Primary Cell Walls of Arabidopsis thaliana: Insights from Solid-State NMR. Biomacromolecules 2017, 18, 2937–2950. [Google Scholar] [CrossRef]
- Van de Velde, F.; Knutsen, S.H.; Usov, A.I.; Rollema, H.S.; Cerezo, A.S. 1H and 13C high resolution NMR spectroscopy of carrageenans: Application in research and industry. Trends Food Sci. Technol. 2002, 13, 73–92. [Google Scholar] [CrossRef]
- Hamzaoui, A.; Ghariani, M.; Sellem, I.; Hamdi, M.; Feki, A.; Jaballi, I.; Nasri, M.; Amara, I.B. Extraction, characterization and biological properties of polysaccharide derived from green seaweed “Chaetomorpha linum” and its potential application in Tunisian beef sausages. Int. J. Biol. Macromol. 2020, 148, 1156–1168. [Google Scholar] [CrossRef]
- Ktari, N.; Feki, A.; Trabelsi, I.; Triki, M.; Maalej, H.; Slima, S.B.; Nasri, M.; Amara, I.B.; Salah, R.B. Structure, functional and antioxidant properties in Tunisian beef sausage of a novel polysaccharide from Trigonella foenum-graecum seeds. Int. J. Biol. Macromol. 2017, 98, 169–181. [Google Scholar] [CrossRef]
- Qiu, H.-M.; Veeraperumal, S.; Lv, J.-H.; Wu, T.-C.; Zhang, Z.-P.; Zeng, Q.-K.; Liu, Y.; Chen, X.-Q.; Aweya, J.J.; Cheong, K.-L. Physicochemical properties and potential beneficial effects of porphyran from Porphyra haitanensis on intestinal epithelial cells. Carbohydr. Polym. 2020, 246, 116626. [Google Scholar] [CrossRef]
- McGaw, L.J.; Elgorashi, E.E.; Eloff, J.N. 8—Cytotoxicity of African Medicinal Plants Against Normal Animal and Human Cells. In Toxicological Survey of African Medicinal Plants; Kuete, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 181–233. ISBN 978-0-12-800018-2. [Google Scholar]
- Bettaib, J.; Talarmin, H.; Droguet, M.; Magné, C.; Boulaaba, M.; Giroux-Metges, M.-A.; Ksouri, R. Tamarix gallica phenolics protect IEC-6 cells against H2O2 induced stress by restricting oxidative injuries and MAPKs signaling pathways. Biomed. Pharm. 2017, 89, 490–498. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Xie, J.-H.; Kan, L.-J.; Wang, J.-Q.; Shen, M.-Y.; Li, W.-J.; Nie, S.-P.; Xie, M.-Y. Sulfated polysaccharides from Cyclocarya paliurus reduce H2O2-induced oxidative stress in RAW264.7 cells. Int. J. Biol. Macromol. 2015, 80, 410–417. [Google Scholar] [CrossRef]
- Whitehouse, S.; Chen, P.-L.; Greenshields, A.L.; Nightingale, M.; Hoskin, D.W.; Bedard, K. Resveratrol, piperine and apigenin differ in their NADPH-oxidase inhibitory and reactive oxygen species-scavenging properties. Phytomedicine 2016, 23, 1494–1503. [Google Scholar] [CrossRef]
- Gülden, M.; Jess, A.; Kammann, J.; Maser, E.; Seibert, H. Cytotoxic potency of H2O2 in cell cultures: Impact of cell concentration and exposure time. Free Radic. Biol. Med. 2010, 49, 1298–1305. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Salla, S.; Sunkara, R.; Ogutu, S.; Walker, L.T.; Verghese, M. Antioxidant activity of papaya seed extracts against H2O2 induced oxidative stress in HepG2 cells. LWT—Food Sci. Technol. 2016, 66, 293–297. [Google Scholar] [CrossRef]
- De Souza, M.C.R.; Marques, C.T.; Dore, C.M.G.; da Silva, F.R.F.; Rocha, H.A.O.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef]
- Jing, L.; Jiang, J.-R.; Liu, D.-M.; Sheng, J.-W.; Zhang, W.-F.; Li, Z.-J.; Wei, L.-Y. Structural Characterization and Antioxidant Activity of Polysaccharides from Athyrium multidentatum (Doll.) Ching in d-Galactose-Induced Aging Mice via PI3K/AKT Pathway. Molecules 2019, 24, 3364. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, X.; Zheng, B.; Chen, Y.; Xie, J.; Shan, J.; Hu, X.; Ding, X.; Hu, X.; Yu, Q. Protective Effect of Ganoderma atrum Polysaccharide on Acrolein-Induced Apoptosis and Autophagic Flux in IEC-6 Cells. Foods 2022, 11, 240. [Google Scholar] [CrossRef]
- Subramanian, S.; Geng, H.; Tan, X.-D. Cell death of intestinal epithelial cells in intestinal diseases. Sheng Li Xue Bao Acta Physiol. Sin. 2020, 72, 308–324. [Google Scholar]
- Ma, L.; Liu, J.; Liu, A.; Wang, Y. Cytoprotective effect of selenium polysaccharide from Pleurotus ostreatus against H2O2-induced oxidative stress and apoptosis in PC12 cells. Arab. J. Chem. 2022, 15, 103686. [Google Scholar] [CrossRef]
- Zhuang, C.; Xu, N.-W.; Gao, G.-M.; Ni, S.; Miao, K.-S.; Li, C.-K.; Wang, L.-M.; Xie, H.-G. Polysaccharide from Angelica sinensis protects chondrocytes from H2O2-induced apoptosis through its antioxidant effects in vitro. Int. J. Biol. Macromol. 2016, 87, 322–328. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Q.; Wang, C.; Shi, J.; Zhang, J. A polysaccharide isolated from Sphallerocarpus gracilis protects PC12 cells against hydrogen peroxide-induced injury. Int. J. Biol. Macromol. 2019, 129, 1133–1139. [Google Scholar] [CrossRef]
- Gong, Y.; Ma, Y.; Cheung, P.C.-K.; You, L.; Liao, L.; Pedisić, S.; Kulikouskaya, V. Structural characteristics and anti-inflammatory activity of UV/H2O2-treated algal sulfated polysaccharide from Gracilaria lemaneiformis. Food Chem. Toxicol. 2021, 152, 112157. [Google Scholar] [CrossRef]
- Huang, L.; Shen, M.; Zhang, X.; Jiang, L.; Song, Q.; Xie, J. Effect of high-pressure microfluidization treatment on the physicochemical properties and antioxidant activities of polysaccharide from Mesona chinensis Benth. Carbohydr. Polym. 2018, 200, 191–199. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Liu, H.; Chen, F.; Yang, H.; Yao, Y.; Gong, X.; Xin, Y.; Ding, C. Effect of calcium treatment on nanostructure of chelate-soluble pectin and physicochemical and textural properties of apricot fruits. Food Res. Int. 2009, 42, 1131–1140. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef]
- Seedevi, P.; Moovendhan, M.; Sudharsan, S.; Vasanthkumar, S.; Srinivasan, A.; Vairamani, S.; Shanmugam, A. Structural characterization and bioactivities of sulfated polysaccharide from Monostroma oxyspermum. Int. J. Biol. Macromol. 2015, 72, 1459–1465. [Google Scholar] [CrossRef]
- Xie, J.-H.; Xie, M.-Y.; Nie, S.-P.; Shen, M.-Y.; Wang, Y.-X.; Li, C. Isolation, chemical composition and antioxidant activities of a water-soluble polysaccharide from Cyclocarya paliurus (Batal.) Iljinskaja. Food Chem. 2010, 119, 1626–1632. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford method for protein quantitation. Methods Mol. Biol. 1994, 32, 9–15. [Google Scholar] [CrossRef]



| Content % | PSGs |
|---|---|
| Total sugars | 67.28 ± 0.08 |
| Proteins | 4.81 ± 0.06 |
| Uronic acids | 14.30 ± 0.13 |
| Sulfate | 17.30 ± 0.77 |
| Ash | 2.64 ± 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajala, M.; Droguet, M.; Kraiem, M.; Ben Saad, H.; Boujhoud, Z.; Hilali, A.; Kallel, H.; Pujo, J.M.; Ben Amara, I. The Potential Effect of Polysaccharides Extracted from Red Alga Gelidium spinosum against Intestinal Epithelial Cell Apoptosis. Pharmaceuticals 2023, 16, 444. https://doi.org/10.3390/ph16030444
Ajala M, Droguet M, Kraiem M, Ben Saad H, Boujhoud Z, Hilali A, Kallel H, Pujo JM, Ben Amara I. The Potential Effect of Polysaccharides Extracted from Red Alga Gelidium spinosum against Intestinal Epithelial Cell Apoptosis. Pharmaceuticals. 2023; 16(3):444. https://doi.org/10.3390/ph16030444
Chicago/Turabian StyleAjala, Marwa, Mickael Droguet, Marwa Kraiem, Hajer Ben Saad, Zakaria Boujhoud, Abderraouf Hilali, Hatem Kallel, Jean Marc Pujo, and Ibtissem Ben Amara. 2023. "The Potential Effect of Polysaccharides Extracted from Red Alga Gelidium spinosum against Intestinal Epithelial Cell Apoptosis" Pharmaceuticals 16, no. 3: 444. https://doi.org/10.3390/ph16030444
APA StyleAjala, M., Droguet, M., Kraiem, M., Ben Saad, H., Boujhoud, Z., Hilali, A., Kallel, H., Pujo, J. M., & Ben Amara, I. (2023). The Potential Effect of Polysaccharides Extracted from Red Alga Gelidium spinosum against Intestinal Epithelial Cell Apoptosis. Pharmaceuticals, 16(3), 444. https://doi.org/10.3390/ph16030444





