Anethole Pretreatment Modulates Cerebral Ischemia/Reperfusion: The Role of JNK, p38, MMP-2 and MMP-9 Pathways
Abstract
1. Introduction
2. Results
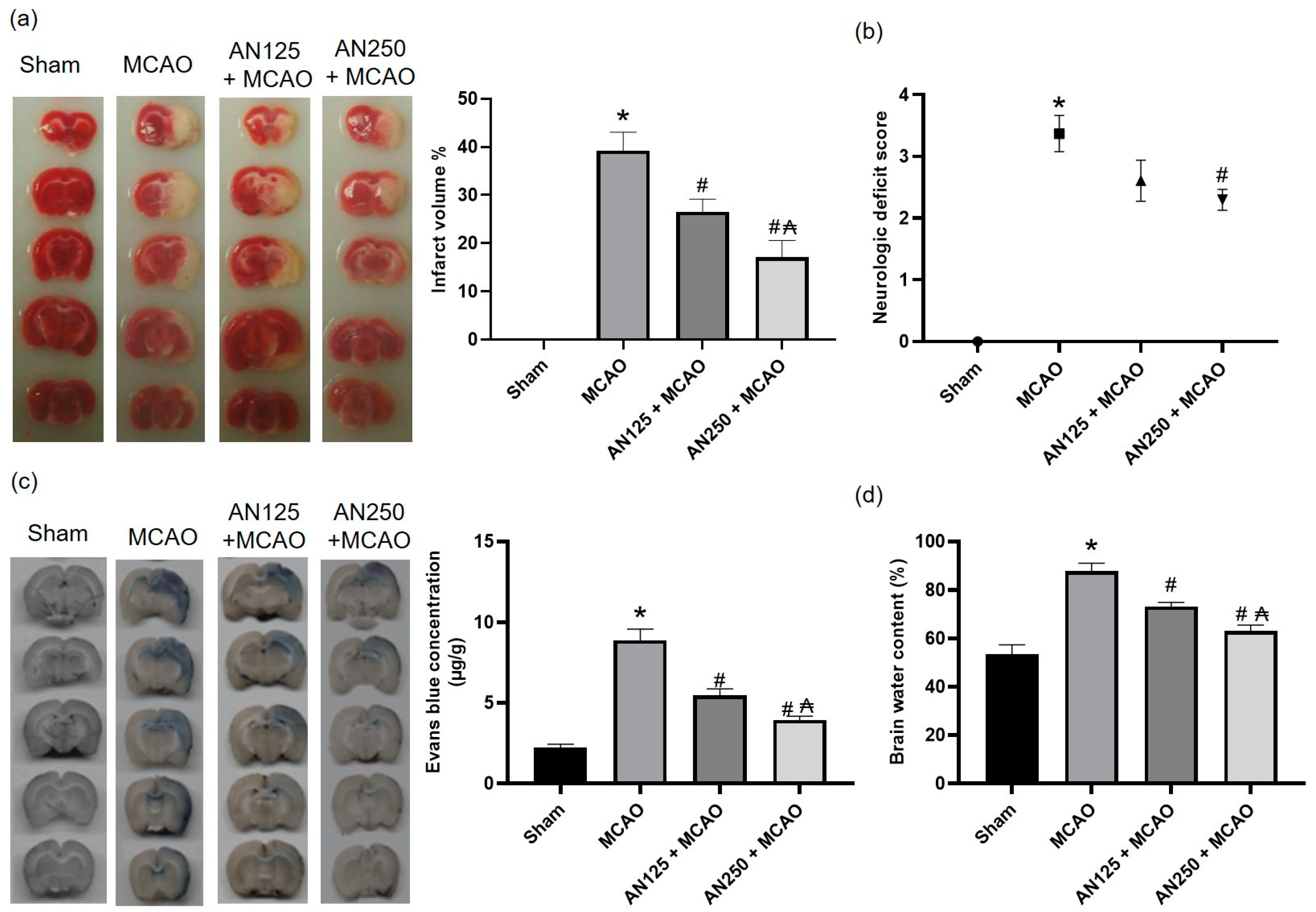
2.1. AN Alleviated Brain Injury Caused by Middle Cerebral Artery Occlusion (MCAO)-Induced C I/R
2.2. AN Alleviated Behavioral Changes Caused by MCAO-Induced C I/R
2.3. AN Improved BBB Integrity and Reduced Brain Edema Caused by MCAO-Induced C I/R
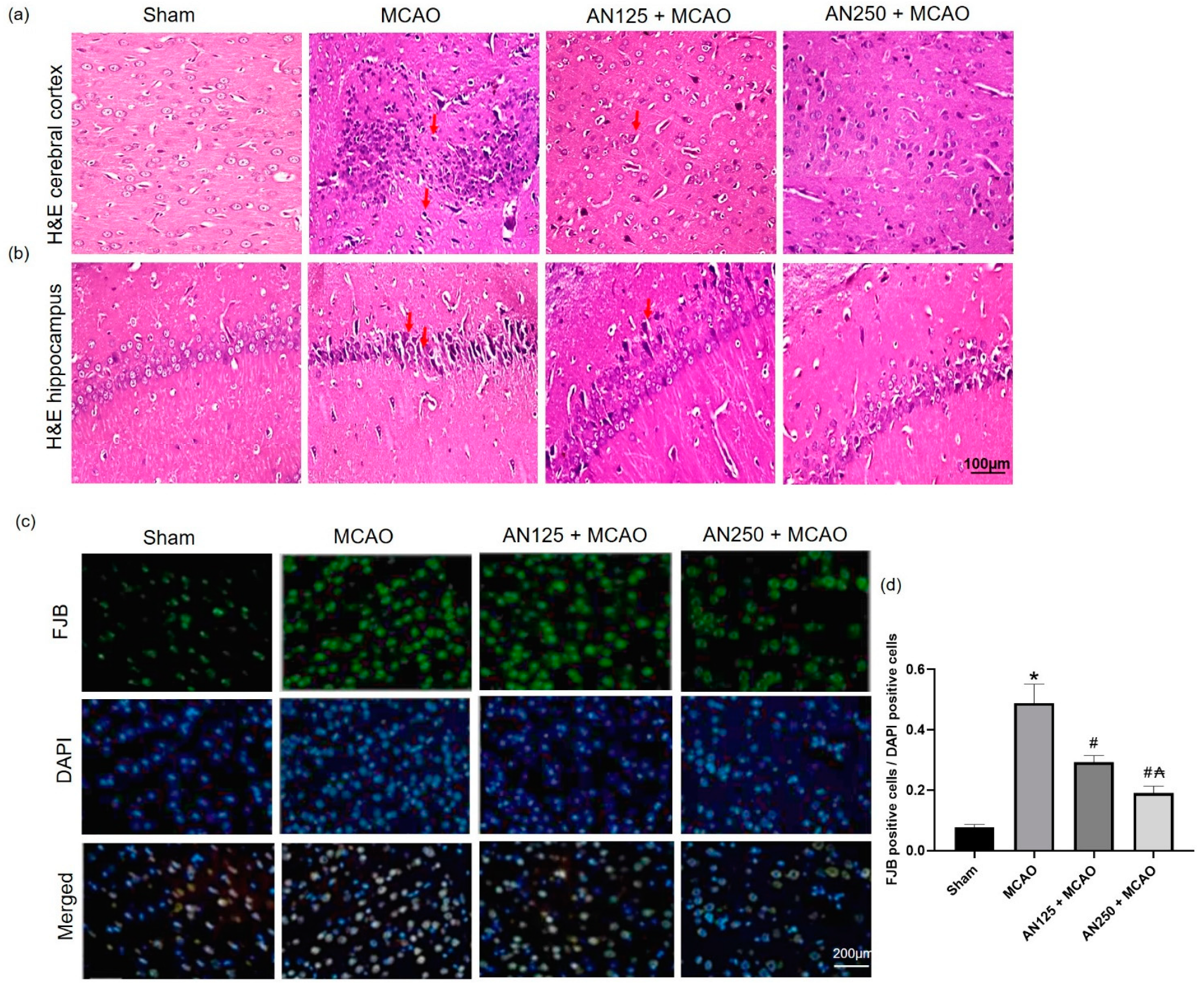
2.4. AN Amended Histopathological Alteration Caused by MCAO-Induced C I/R
2.5. AN Alleviated Neuronal Degeneration Caused by MCAO-Induced C I/R
2.6. AN Reduced MMP-9 and MMP-2 Caused by MCAO-Induced C I/R
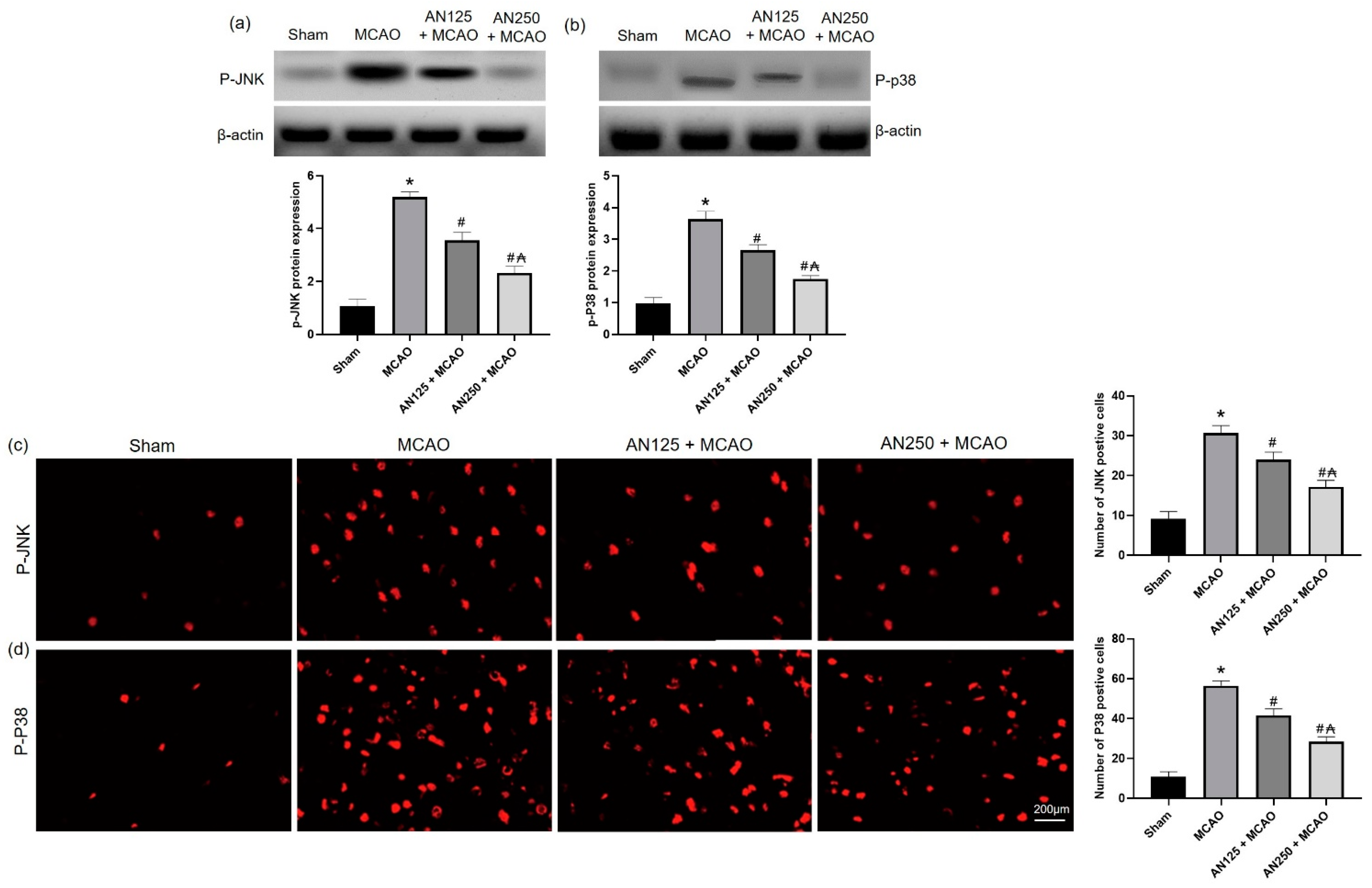
2.7. AN Reduces Phosphorylation of JNK/P38 Caused by MCAO-Induced C I/R
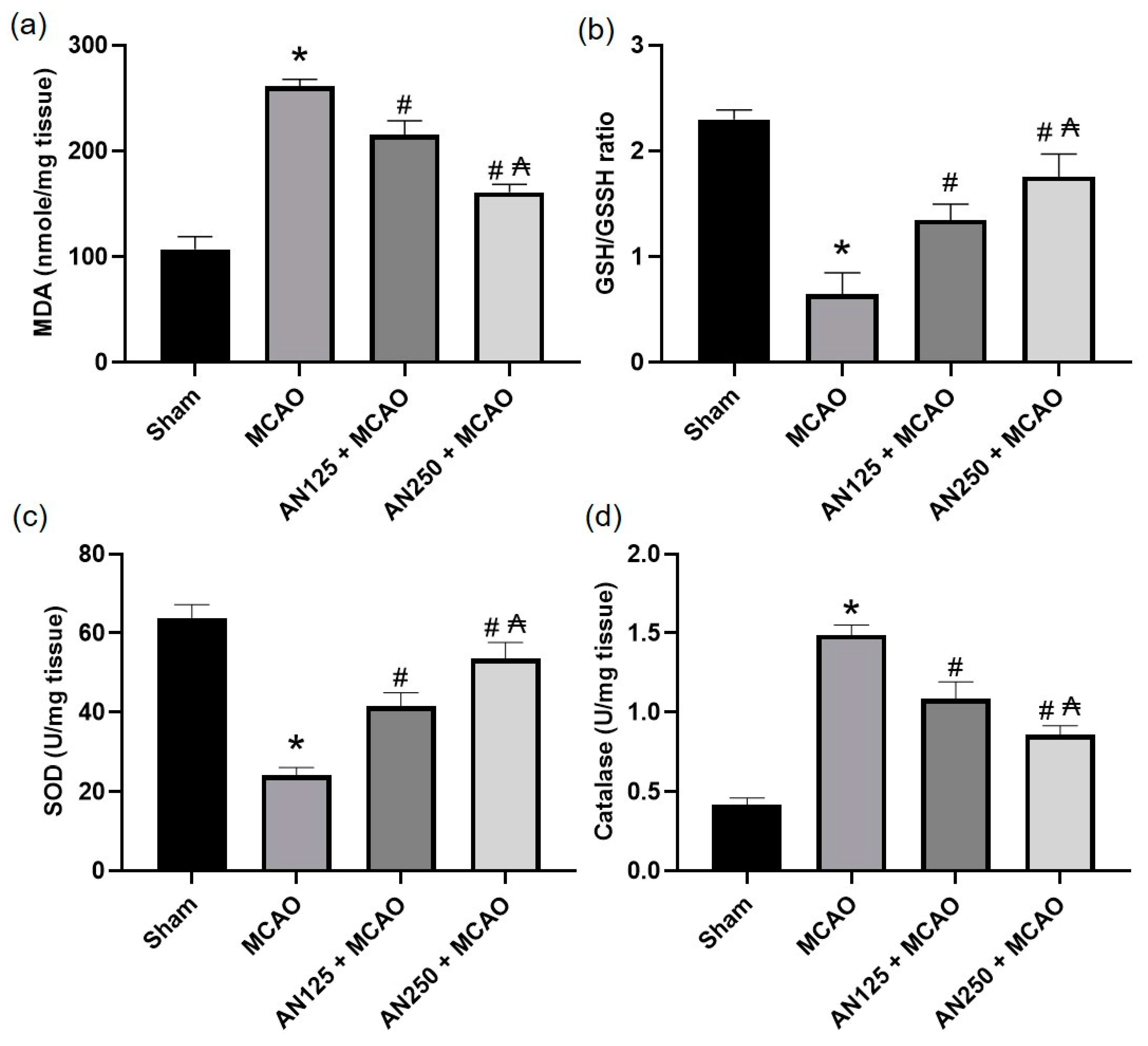
2.8. AN Alleviated Oxidative Stress Caused by MCAO-Induced C I/R
2.9. AN Alleviated NO Amplification Caused by MCAO-Induced C I/R
2.10. AN Reduces NFKB Activation Caused by MCAO-Induced C I/R
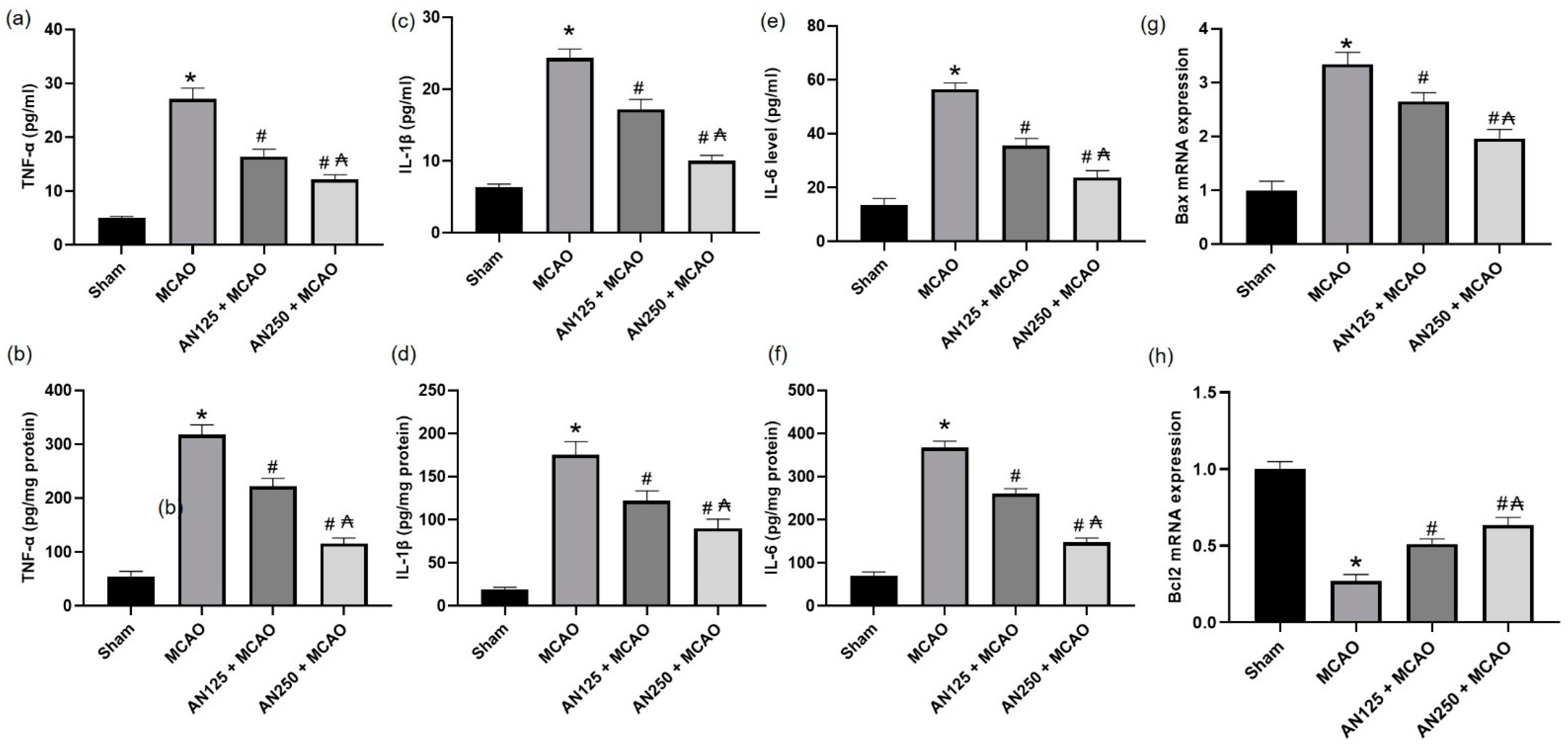
2.11. AN Alleviated the Inflammation and Apoptosis Caused by MCAO-Induced C I/R
3. Discussion
4. Materials and Methods
4.1. Middle Cerebral Artery Occlusion (MCAO)-Induced C I/R
4.2. Animals Attaining and Ethical Code Approval
4.3. Experimental Design
4.4. Behavioral Tests
4.4.1. Neurologic Deficit Assessment
4.4.2. Assessment of Spontaneous Locomotor Movement
4.4.3. Assessment of Motor Coordination
4.5. Euthanasia, Blood and Brain Tissue Samplings
4.6. Infarct Volume Assessment
4.7. Brain Water Content Assessment
4.8. Blood–Brain Barrier Integrity Assessment
4.9. Determination Using Ischemic Brain Sections
4.9.1. Neuronal Degeneration Assessment
4.9.2. Histopathological Staining
4.9.3. Immunohistochemical Staining
4.9.4. Immunofluorescence Analyses
4.10. Ischemic Brain Hemispheres
4.10.1. RT-PCR Detection
4.10.2. Gelatin Zymography Assay
4.10.3. Western Blot Analysis
4.10.4. Determination of NO Content and the Activities of the Total NOS (TNOS), Induced NOS (iNOS), and Constitutive NOS (cNOS) in Brain Homogenate
4.10.5. Determination of Oxidative Stress
4.10.6. Measurement of Inflammatory Mediators
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aprotosoaie, A.C.; Costache, I.-I.; Miron, A. Anethole and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.M.; Domiciano, T.P.; Verri, W.A., Jr.; Zarpelon, A.C.; da Silva, L.G.; Barbosa, C.P.; Natali, M.R.; Cuman, R.K.; Bersani-Amado, C.A. Antihypernociceptive activity of anethole in experimental inflammatory pain. Inflammopharmacology 2013, 21, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Kim, K.Y.; Lee, H.S.; Min, S.S.; Seol, G.H. Anti-inflammatory effects of anethole in lipopolysaccharide-induced acute lung injury in mice. Life Sci. 2013, 93, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Wlodarska, M.; Willing, B.P.; Bravo, D.M.; Finlay, B.B. Phytonutrient diet supplementation promotes beneficial Clostridia species and intestinal mucus secretion resulting in protection against enteric infection. Sci. Rep. 2015, 5, 9253. [Google Scholar] [CrossRef] [PubMed]
- Freire, R.S.; Morais, S.M.; Catunda-Junior, F.E.A.; Pinheiro, D.C. Synthesis and antioxidant, anti-inflammatory and gastroprotector activities of anethole and related compounds. Bioorg. Med. Chem. 2005, 13, 4353–4358. [Google Scholar] [CrossRef]
- De Siqueira, R.; Leal-Cardoso, J.; Couture, R.; Lahlou, S. Role of capsaicin-sensitive sensory nerves in mediation of the cardiovascular effects of the essential oil of Croton zehntneri leaves in anaesthetized rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 238–247. [Google Scholar] [CrossRef]
- Tognolini, M.; Ballabeni, V.; Bertoni, S.; Bruni, R.; Impicciatore, M.; Barocelli, E. Protective effect of Foeniculum vulgare essential oil and anethole in an experimental model of thrombosis. Pharmacol. Res. 2007, 56, 254–260. [Google Scholar] [CrossRef]
- Domiciano, T.P.; Dalalio, M.M.d.O.; Silva, E.L.; Ritter, A.M.V.; Estevão-Silva, C.F.; Ramos, F.S.; Caparroz-Assef, S.M.; Cuman, R.K.N.; Bersani-Amado, C.A. Inhibitory effect of anethole in nonimmune acute inflammation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2013, 386, 331–338. [Google Scholar] [CrossRef]
- Moradi, J.; Abbasipour, F.; Zaringhalam, J.; Maleki, B.; Ziaee, N.; Khodadoustan, A.; Janahmadi, M. Anethole, a Medicinal Plant Compound, Decreases the Production of Pro-Inflammatory TNF-α and IL-1β in a Rat Model of LPS-Induced Periodontitis. Iran. J. Pharm. Res. 2014, 13, 1319–1325. [Google Scholar]
- Chen, C.H.; deGraffenried, L.A. Anethole suppressed cell survival and induced apoptosis in human breast cancer cells independent of estrogen receptor status. Phytomedicine 2012, 19, 763–767. [Google Scholar] [CrossRef]
- Carvalho, A.A.; Andrade, L.N.; de Sousa, É.B.V.; de Sousa, D.P. Antitumor phenylpropanoids found in essential oils. BioMed Res. Int. 2015, 2015, 392674. [Google Scholar] [CrossRef]
- Rhee, Y.H.; Chung, P.S.; Kim, S.H.; Ahn, J.C. CXCR4 and PTEN are involved in the anti-metastatic regulation of anethole in DU145 prostate cancer cells. Biochem. Biophys. Res. Commun. 2014, 447, 557–562. [Google Scholar] [CrossRef]
- al-Harbi, M.M.; Qureshi, S.; Raza, M.; Ahmed, M.M.; Giangreco, A.B.; Shah, A.H. Influence of anethole treatment on the tumour induced by Ehrlich ascites carcinoma cells in paw of Swiss albino mice. Eur. J. Cancer Prev. 1995, 4, 307–318. [Google Scholar] [CrossRef]
- Chen, C.; De Gasperi, M.; Salcedo, R.; Cavazos, D.; deGraffenried, L. Evaluation of the Phytochemical Anethole as an Anti-Tumor Agent in MCF-7 Cells. Cancer Res. 2009, 69, 3100. [Google Scholar] [CrossRef]
- Jana, S.; Patra, K.; Mukherjee, G.; Bhattacharjee, S.; Mandal, D.P. Antitumor potential of anethole singly and in combination with cyclophosphamide in murine Sarcoma-180 transplantable tumor model. RSC Adv. 2015, 5, 56549–56559. [Google Scholar] [CrossRef]
- Ryu, S.; Seol, G.H.; Park, H.; Choi, I.-Y. Trans-anethole protects cortical neuronal cells against oxygen–glucose deprivation/reoxygenation. Neurol. Sci. 2014, 35, 1541–1547. [Google Scholar] [CrossRef]
- Bhadra, S.; Mukherjee, P.K.; Kumar, N.S.; Bandyopadhyay, A. Anticholinesterase activity of standardized extract of Illicium verum Hook. f. fruits. Fitoterapia 2011, 82, 342–346. [Google Scholar] [CrossRef]
- Chainy, G.B.N.; Manna, S.K.; Chaturvedi, M.M.; Aggarwal, B.B. Anethole blocks both early and late cellular responses transduced by tumor necrosis factor: Effect on NF-κB, AP-1, JNK, MAPKK and apoptosis. Oncogene 2000, 19, 2943–2950. [Google Scholar] [CrossRef]
- Sung, Y.-Y.; Kim, H.K. Illicium verum extract suppresses IFN-γ-induced ICAM-1 expression via blockade of JAK/STAT pathway in HaCaT human keratinocytes. J. Ethnopharmacol. 2013, 149, 626–632. [Google Scholar] [CrossRef]
- Sung, Y.-Y.; Kim, Y.S.; Kim, H.K. Illicium verum extract inhibits TNF-α-and IFN-γ-induced expression of chemokines and cytokines in human keratinocytes. J. Ethnopharmacol. 2012, 144, 182–189. [Google Scholar] [CrossRef]
- Galicka, A.; Krętowski, R.; Nazaruk, J.; Cechowska-Pasko, M. Anethole prevents hydrogen peroxide-induced apoptosis and collagen metabolism alterations in human skin fibroblasts. Mol. Cell. Biochem. 2014, 394, 217–224. [Google Scholar] [CrossRef]
- Zemgulyte, G.; Tanaka, S.; Hide, I.; Sakai, N.; Pampuscenko, K.; Borutaite, V.; Rastenyte, D. Evaluation of the Effectiveness of Post-Stroke Metformin Treatment Using Permanent Middle Cerebral Artery Occlusion in Rats. Pharmaceuticals 2021, 14, 312. [Google Scholar] [CrossRef]
- Rosenberg, G.A.; Estrada, E.Y.; Dencoff, J.E. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke 1998, 29, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ren, Q.; Shi, M.; Liu, Y.; Bai, H.; Chang, Y.-Z. Overexpression of Mitochondrial Ferritin Enhances Blood–Brain Barrier Integrity Following Ischemic Stroke in Mice by Maintaining Iron Homeostasis in Endothelial Cells. Antioxidants 2022, 11, 1257. [Google Scholar] [PubMed]
- Guo, P.; Jin, Z.; Wu, H.; Li, X.; Ke, J.; Zhang, Z.; Zhao, Q. Effects of irisin on the dysfunction of blood-brain barrier in rats after focal cerebral ischemia/reperfusion. Brain Behav. 2019, 9, e01425. [Google Scholar] [CrossRef]
- Kamada, H.; Yu, F.; Nito, C.; Chan, P.H. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: Relation to blood-brain barrier dysfunction. Stroke 2007, 38, 1044–1049. [Google Scholar] [CrossRef]
- Clark, A.W.; Krekoski, C.A.; Bou, S.-S.; Chapman, K.R.; Edwards, D.R. Increased gelatinase A (MMP-2) and gelatinase B (MMP-9) activities in human brain after focal ischemia. Neurosci. Lett. 1997, 238, 53–56. [Google Scholar] [CrossRef]
- Kim, E.-H.; Kim, E.-S.; Shin, D.; Kim, D.; Choi, S.; Shin, Y.-J.; Kim, K.-A.; Noh, D.; Caglayan, A.B.; Rajanikant, G.K.; et al. Carnosine Protects against Cerebral Ischemic Injury by Inhibiting Matrix-Metalloproteinases. Int. J. Mol. Sci. 2021, 22, 7495. [Google Scholar] [CrossRef]
- Jian Liu, K.; Rosenberg, G.A. Matrix metalloproteinases and free radicals in cerebral ischemia. Free Radic. Biol. Med. 2005, 39, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Muraleva, N.A.; Stefanova, N.A.; Kolosova, N.G. SkQ1 Suppresses the p38 MAPK Signaling Pathway Involved in Alzheimer’s Disease-Like Pathology in OXYS Rats. Antioxidants 2020, 9, 676. [Google Scholar] [CrossRef]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef]
- Meanti, R.; Rizzi, L.; Bresciani, E.; Molteni, L.; Locatelli, V.; Coco, S.; Omeljaniuk, R.J.; Torsello, A. Hexarelin Modulation of MAPK and PI3K/Akt Pathways in Neuro-2A Cells Inhibits Hydrogen Peroxide-Induced Apoptotic Toxicity. Pharmaceuticals 2021, 14, 444. [Google Scholar] [CrossRef]
- Nadeem, M.S.; Kazmi, I.; Ullah, I.; Muhammad, K.; Anwar, F. Allicin, an Antioxidant and Neuroprotective Agent, Ameliorates Cognitive Impairment. Antioxidants 2021, 11, 87. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Xiong, X.; Zhu, H.; Chen, R.; Zhang, S.; Chen, G.; Jian, Z. Nrf2 Regulates Oxidative Stress and Its Role in Cerebral Ischemic Stroke. Antioxidants 2022, 11, 2377. [Google Scholar] [CrossRef] [PubMed]
- Althurwi, H.N.; Abdel-Rahman, R.F.; Soliman, G.A.; Ogaly, H.A.; Alkholifi, F.K.; Abd-Elsalam, R.M.; Alqasoumi, S.I.; Abdel-Kader, M.S. Protective Effect of Beta-Carotene against Myeloperoxidase- Mediated Oxidative Stress and Inflammation in Rat Ischemic Brain Injury. Antioxidants 2022, 11, 2344. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Liu, C.C.; Lin, Y.C.; Liao, C.S. Resveratrol Protects against Cerebral Ischemic Injury via Restraining Lipid Peroxidation, Transition Elements, and Toxic Metal Levels, but Enhancing Anti-Oxidant Activity. Antioxidants 2021, 10, 1515. [Google Scholar] [CrossRef]
- Fang, X.; Li, Y.; Qiao, J.; Guo, Y.; Miao, M. Neuroprotective effect of total flavonoids from Ilex pubescens against focal cerebral ischemia/reperfusion injury in rats. Mol. Med. Rep. 2017, 16, 7439–7449. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, Z.; Zhang, Z.; Zhu, P.; Jiang, X.; Wang, Y.; Deng, Q.; Lam Yung, K.K.; Zhang, S. Oleanolic Acid Alleviates Cerebral Ischemia/Reperfusion Injury via Regulation of the GSK-3β/HO-1 Signaling Pathway. Pharmaceuticals 2021, 15, 1. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Y.; Liu, Y.; Chen, X.; Huang, Z.; Zhao, X.; He, H.; Deng, Y. Melibiose Confers a Neuroprotection against Cerebral Ischemia/Reperfusion Injury by Ameliorating Autophagy Flux via Facilitation of TFEB Nuclear Translocation in Neurons. Life 2021, 11, 948. [Google Scholar] [CrossRef] [PubMed]
- Vastegani, S.M.; Khoshnam, S.E.; Mansouri, E.; Hajipour, S.; Ghafouri, S.; Bakhtiari, N.; Sarkaki, A.; Farbood, Y. Neuroprotective effect of anethole against rotenone induced non-motor deficits and oxidative stress in rat model of Parkinson’s disease. Behav. Brain Res. 2022, 437, 114100. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Wang, Z.J.; Miao, L.; Wang, Y.; Chang, L.L.; Guo, W.; Zhu, Y.Z. Neuroprotective Effect of SCM-198 through Stabilizing Endothelial Cell Function. Oxid. Med. Cell. Longev. 2019, 2019, 7850154. [Google Scholar] [CrossRef]
- Kim, H.Y.; Han, S.H. Matrix metalloproteinases in cerebral ischemia. J. Clin. Neurol. 2006, 2, 163–170. [Google Scholar] [CrossRef]
- Ha, B.; Ko, H.; Kim, B.; Sohn, E.J.; Jung, J.H.; Kim, J.S.; Yoon, J.J.; Won, G.; Kim, J.H.; Jung, D.B.; et al. Regulation of crosstalk between epithelial to mesenchymal transition molecules and MMP-9 mediates the antimetastatic activity of anethole in DU145 prostate cancer cells. J. Nat. Prod. 2014, 77, 63–69. [Google Scholar] [CrossRef]
- Li, M.; Tian, X.; An, R.; Yang, M.; Zhang, Q.; Xiang, F.; Liu, H.; Wang, Y.; Xu, L.; Dong, Z. All-Trans Retinoic Acid Ameliorates the Early Experimental Cerebral Ischemia-Reperfusion Injury in Rats by Inhibiting the Loss of the Blood-Brain Barrier via the JNK/P38MAPK Signaling Pathway. Neurochem. Res. 2018, 43, 1283–1296. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Morón-Oset, J.; Díaz-Castroverde, S.; García-Font, N.; Roncero, C.; López-Muñoz, F.; Marco Contelles, J.L.; Oset-Gasque, M.J. Neuroprotection by Phytoestrogens in the Model of Deprivation and Resupply of Oxygen and Glucose In Vitro: The Contribution of Autophagy and Related Signaling Mechanisms. Antioxidants 2020, 9, 545. [Google Scholar] [CrossRef]
- Cho, H.I.; Kim, K.M.; Kwak, J.H.; Lee, S.K.; Lee, S.M. Protective mechanism of anethole on hepatic ischemia/reperfusion injury in mice. J. Nat. Prod. 2013, 76, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Kirchgessner, A.; Hofer, M. Inflammatory mechanisms in ischemic stroke: Therapeutic approaches. J. Transl. Med. 2009, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Cazevielle, C.; Muller, A.; Meynier, F.; Bonne, C. Superoxide and nitric oxide cooperation in hypoxia/reoxygenation-induced neuron injury. Free. Radic. Biol. Med. 1993, 14, 389–395. [Google Scholar] [CrossRef] [PubMed]
- ArunaDevi, R.; Ramteke, V.D.; Kumar, S.; Shukla, M.K.; Jaganathan, S.; Kumar, D.; Sharma, A.K.; Tandan, S.K. Neuroprotective effect of s-methylisothiourea in transient focal cerebral ischemia in rat. Nitric Oxide 2010, 22, 1–10. [Google Scholar] [CrossRef]
- Ritter, A.M.V.; Hernandes, L.; da Rocha, B.A.; Estevão-Silva, C.F.; Wisniewski-Rebecca, E.S.; Cezar, J.D.S.; Caparroz-Assef, S.M.; Cuman, R.K.N.; Bersani-Amado, C.A. Anethole reduces inflammation and joint damage in rats with adjuvant-induced arthritis. Inflamm. Res. 2017, 66, 725–737. [Google Scholar] [CrossRef]
- Conforti, F.; Tundis, R.; Marrelli, M.; Menichini, F.; Statti, G.A.; De Cindio, B.; Menichini, F.; Houghton, P.J. Protective effect of Pimpinella anisoides ethanolic extract and its constituents on oxidative damage and its inhibition of nitric oxide in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Med. Food 2010, 13, 137–141. [Google Scholar] [CrossRef]
- Jelinek, M.; Jurajda, M.; Duris, K. Oxidative Stress in the Brain: Basic Concepts and Treatment Strategies in Stroke. Antioxidants 2021, 10, 1886. [Google Scholar] [CrossRef]
- Espinós, C.; Galindo, M.I.; García-Gimeno, M.A.; Ibáñez-Cabellos, J.S.; Martínez-Rubio, D.; Millán, J.M.; Rodrigo, R.; Sanz, P.; Seco-Cervera, M.; Sevilla, T.; et al. Oxidative Stress, a Crossroad Between Rare Diseases and Neurodegeneration. Antioxidants 2020, 9, 313. [Google Scholar] [CrossRef]
- El-Marasy, S.A.; Abdel-Rahman, R.F.; Abd-Elsalam, R.M. Neuroprotective effect of vildagliptin against cerebral ischemia in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 1133–1145. [Google Scholar] [CrossRef]
- Silva-Islas, C.A.; Chánez-Cárdenas, M.E.; Barrera-Oviedo, D.; Ortiz-Plata, A.; Pedraza-Chaverri, J.; Maldonado, P.D. Diallyl Trisulfide Protects Rat Brain Tissue against the Damage Induced by Ischemia-Reperfusion through the Nrf2 Pathway. Antioxidants 2019, 8, 410. [Google Scholar] [CrossRef]
- Ghasemi-Dehnoo, M.; Safari, A.A.; Rahimi-Madiseh, M.; Lorigooini, Z.; Moradi, M.T.; Amini-Khoei, H. Anethole Ameliorates Acetic Acid-Induced Colitis in Mice: Anti-Inflammatory and Antioxidant Effects. Evid. Based Complement. Altern. Med. 2022, 2022, 9057451. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Kandeel, M.; Abd El-Lateef, H.M.; El-Beltagi, H.S.; Younis, N.S. The Protective Effect of Anethole against Renal Ischemia/Reperfusion: The Role of the TLR2,4/MYD88/NFκB Pathway. Antioxidants 2022, 11, 535. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.W.; Tyrrell, P.; Sattar, N.; Warburton, E. Inflammation and ischaemic stroke. Curr. Opin. Neurol. 2007, 20, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhu, L.; Tang, P.; Chen, D.; Li, Y.; Li, J.; Bao, C. Carthamin yellow improves cerebral ischemia-reperfusion injury by attenuating inflammation and ferroptosis in rats. Int. J. Mol. Med. 2021, 47, 52. [Google Scholar] [CrossRef]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.M.V.; Ames, F.Q.; Otani, F.; de Oliveira, R.M.W.; Cuman, R.K.N.; Bersani-Amado, C.A. Effects of anethole in nociception experimental models. Evid. Based Complement. Altern. Med. 2014, 2014, 345829. [Google Scholar] [CrossRef]
- Wang, Z.; Kou, D.; Li, Z.; He, Y.; Yu, W.; Du, H. Effects of propofol-dexmedetomidine combination on ischemia reperfusion-induced cerebral injury. NeuroRehabilitation 2014, 35, 825–834. [Google Scholar] [CrossRef]
- Gerriets, T.; Stolz, E.; Walberer, M.; Müller, C.; Rottger, C.; Kluge, A.; Kaps, M.; Fisher, M.; Bachmann, G. Complications and pitfalls in rat stroke models for middle cerebral artery occlusion: A comparison between the suture and the macrosphere model using magnetic resonance angiography. Stroke 2004, 35, 2372–2377. [Google Scholar] [CrossRef]
- Pavić, R.; Tvrdeić, A.; Tot, O.K.; Heffer-Lauc, M. Activity cage as a method to analyze functional recovery after sciatic nerve injury in mice. Somatosens. Mot. Res. 2007, 24, 213–219. [Google Scholar] [CrossRef]
- Vijitruth, R.; Liu, M.; Choi, D.Y.; Nguyen, X.V.; Hunter, R.L.; Bing, G. Cyclooxygenase-2 mediates microglial activation and secondary dopaminergic cell death in the mouse MPTP model of Parkinson’s disease. J. Neuroinflamm. 2006, 3, 6. [Google Scholar] [CrossRef]
- Ahishali, B.; Kaya, M. Evaluation of Blood-Brain Barrier Integrity Using Vascular Permeability Markers: Evans Blue, Sodium Fluorescein, Albumin-Alexa Fluor Conjugates, and Horseradish Peroxidase. Methods Mol. Biol. 2021, 2367, 87–103. [Google Scholar] [CrossRef]
- Li, Y.; Lein, P.J.; Liu, C.; Bruun, D.A.; Tewolde, T.; Ford, G.; Ford, B.D. Spatiotemporal pattern of neuronal injury induced by DFP in rats: A model for delayed neuronal cell death following acute OP intoxication. Toxicol. Appl. Pharmacol. 2011, 253, 261–269. [Google Scholar] [CrossRef]
- Barakat, W.; Safwet, N.; El-Maraghy, N.N.; Zakaria, M.N. Candesartan and glycyrrhizin ameliorate ischemic brain damage through downregulation of the TLR signaling cascade. Eur. J. Pharmacol. 2014, 724, 43–50. [Google Scholar] [CrossRef]
- You, W.C.; Wang, C.X.; Pan, Y.X.; Zhang, X.; Zhou, X.M.; Zhang, X.S.; Shi, J.X.; Zhou, M.L. Activation of nuclear factor-κB in the brain after experimental subarachnoid hemorrhage and its potential role in delayed brain injury. PLoS ONE 2013, 8, e60290. [Google Scholar] [CrossRef]
- Zhang, B.; Zhong, Q.; Chen, X.; Wu, X.; Sha, R.; Song, G.; Zhang, C.; Chen, X. Neuroprotective Effects of Celastrol on Transient Global Cerebral Ischemia Rats via Regulating HMGB1/NF-κB Signaling Pathway. Front. Neurosci. 2020, 14, 847. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xu, P.; Fu, T.; Huang, X.; Song, J.; Chen, M.; Tian, X.; Yin, H.; Han, J. Myricetin against ischemic cerebral injury in rat middle cerebral artery occlusion model. Mol. Med. Rep. 2018, 17, 3274–3280. [Google Scholar] [CrossRef]



| Groups | Locomotor Activity (Counts/5 min) | Falling Latency Time (min) | ||
|---|---|---|---|---|
| Basal | Final | Basal | Final | |
| Sham | 150.00 ± 20.47 | 80.00 ± 4.57 | 5.01 ± 0.41 | 4.74 ± 0.35 |
| MCAO | 142.75 ± 12.93 | 27.92 ± 1.63 * | 4.95 ± 0.54 | 2.19 ± 0.36 * |
| AN125 + MCAO | 146.75 ± 15.42 | 56.38 ± 8.32 # | 4.76 ± 0.36 | 3.57 ± 0.52 # |
| AN250 + MCAO | 125.60 ± 16.35 | 59.25 ± 7.40 # | 4.85 ± 0.44 | 3.86 ± 0.47 # |
| Group | tNOS (U/mg Tissue Protein) | iNOS (U/mg Tissue Protein) | cNOS (U/mg Tissue Protein) | NO (µmol/g Tissue Protein) |
|---|---|---|---|---|
| Sham | 2.13 ± 0.53 | 0.39 ± 0.13 | 0.92 ± 0.12 | 6.34 ± 2.21 |
| Model | 3.47 ± 0.42 * | 0.96 ± 0.21 * | 2.45 ± 0.22 * | 19.26 ± 2.6 * |
| AN 125 + MCAO | 2.86 ± 0.34 # | 0.61 ± 0.17 # | 1.59 ± 0.28 # | 12.67 ± 2.30 # |
| AN 250 + MCAO | 2.47 ± 0.23 #₳ | 0.46 ± 0.14 #₳ | 1.08 ± 0.13 #₳ | 9.57 ± 3.2 #₳ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Younis, N.S.; Mohamed, M.E. Anethole Pretreatment Modulates Cerebral Ischemia/Reperfusion: The Role of JNK, p38, MMP-2 and MMP-9 Pathways. Pharmaceuticals 2023, 16, 442. https://doi.org/10.3390/ph16030442
Younis NS, Mohamed ME. Anethole Pretreatment Modulates Cerebral Ischemia/Reperfusion: The Role of JNK, p38, MMP-2 and MMP-9 Pathways. Pharmaceuticals. 2023; 16(3):442. https://doi.org/10.3390/ph16030442
Chicago/Turabian StyleYounis, Nancy S., and Maged E. Mohamed. 2023. "Anethole Pretreatment Modulates Cerebral Ischemia/Reperfusion: The Role of JNK, p38, MMP-2 and MMP-9 Pathways" Pharmaceuticals 16, no. 3: 442. https://doi.org/10.3390/ph16030442
APA StyleYounis, N. S., & Mohamed, M. E. (2023). Anethole Pretreatment Modulates Cerebral Ischemia/Reperfusion: The Role of JNK, p38, MMP-2 and MMP-9 Pathways. Pharmaceuticals, 16(3), 442. https://doi.org/10.3390/ph16030442







