Development of Novel Fluorinated Polyphenols as Selective Inhibitors of DYRK1A/B Kinase for Treatment of Neuroinflammatory Diseases including Parkinson’s Disease
Abstract
1. Introduction
2. Results
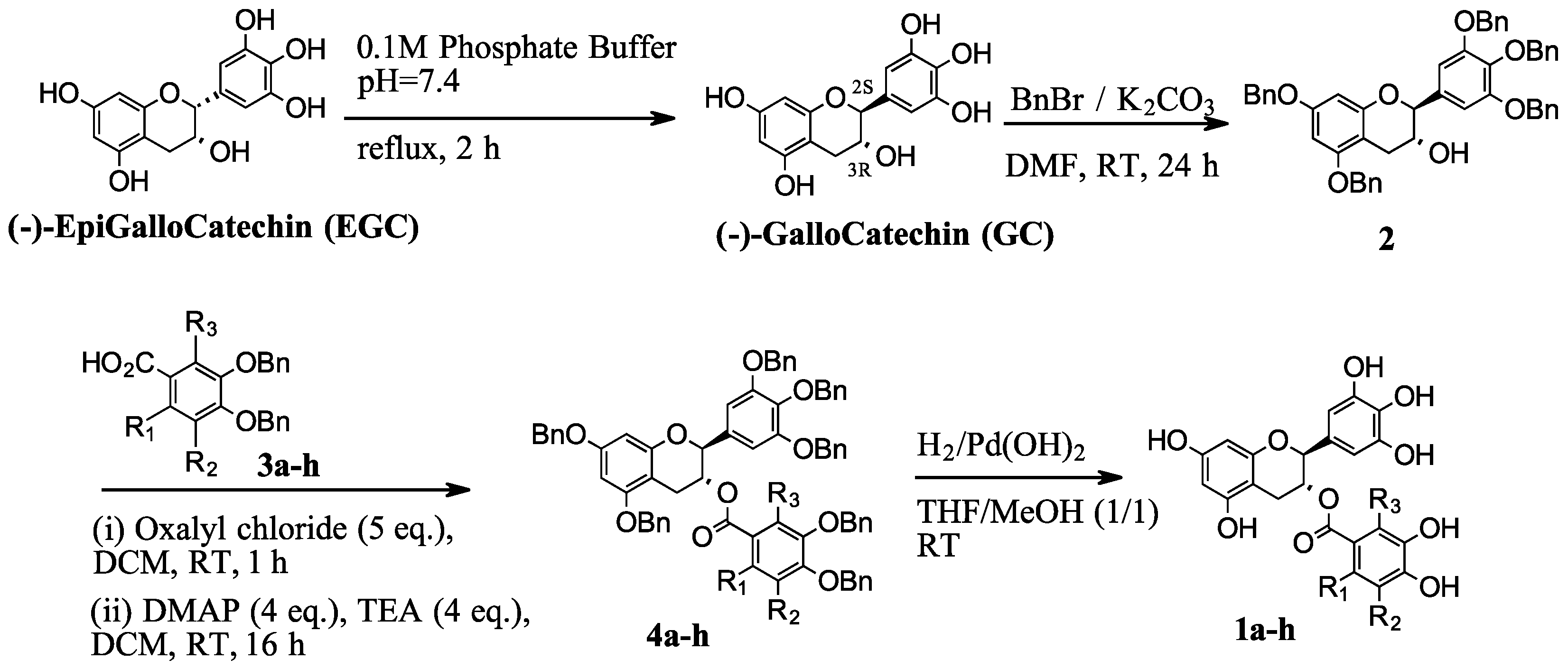
2.1. Synthesis of Compounds
2.2. Overview of Activities and SAR
2.3. Pharmacokinetic Studies
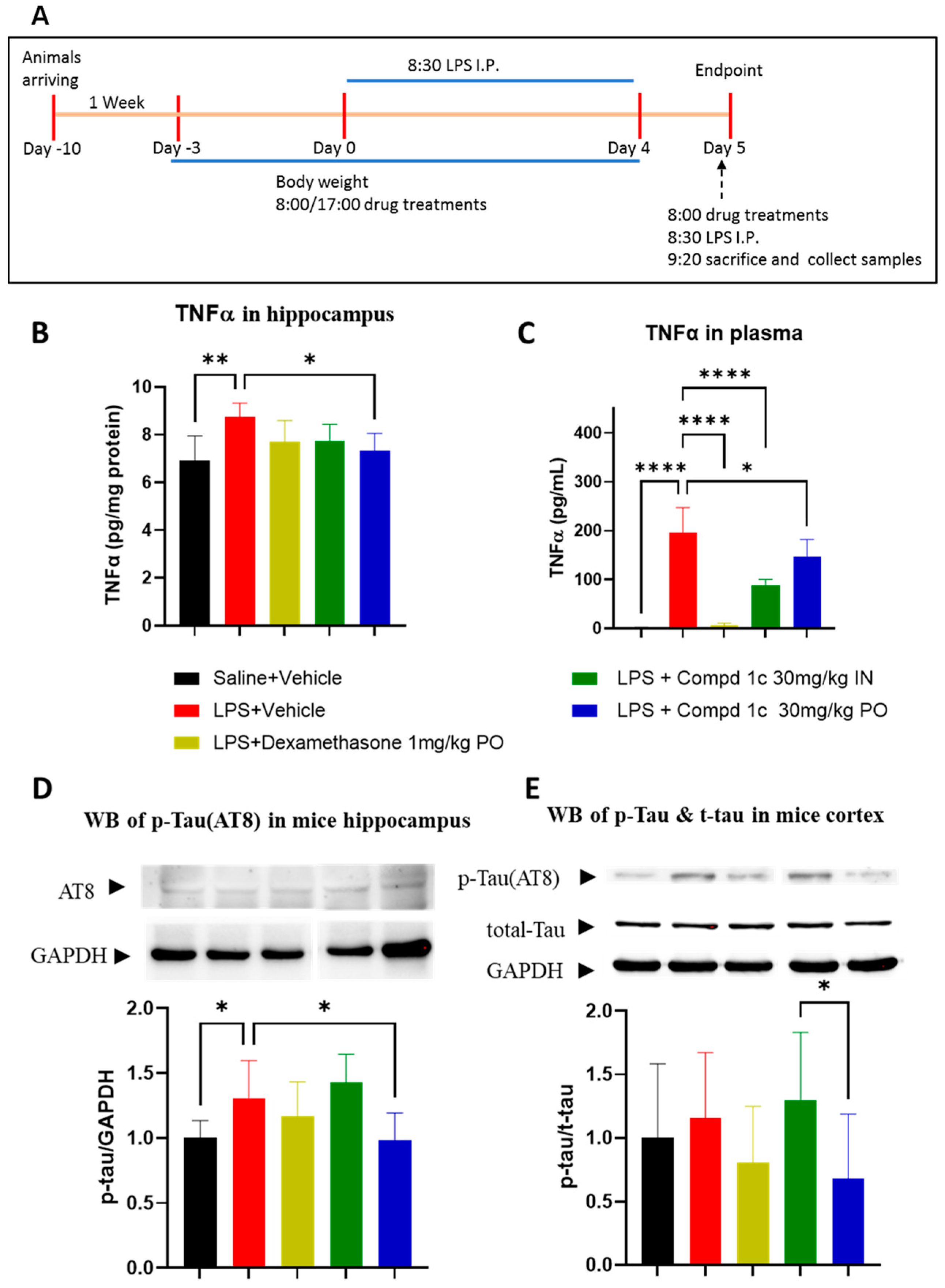
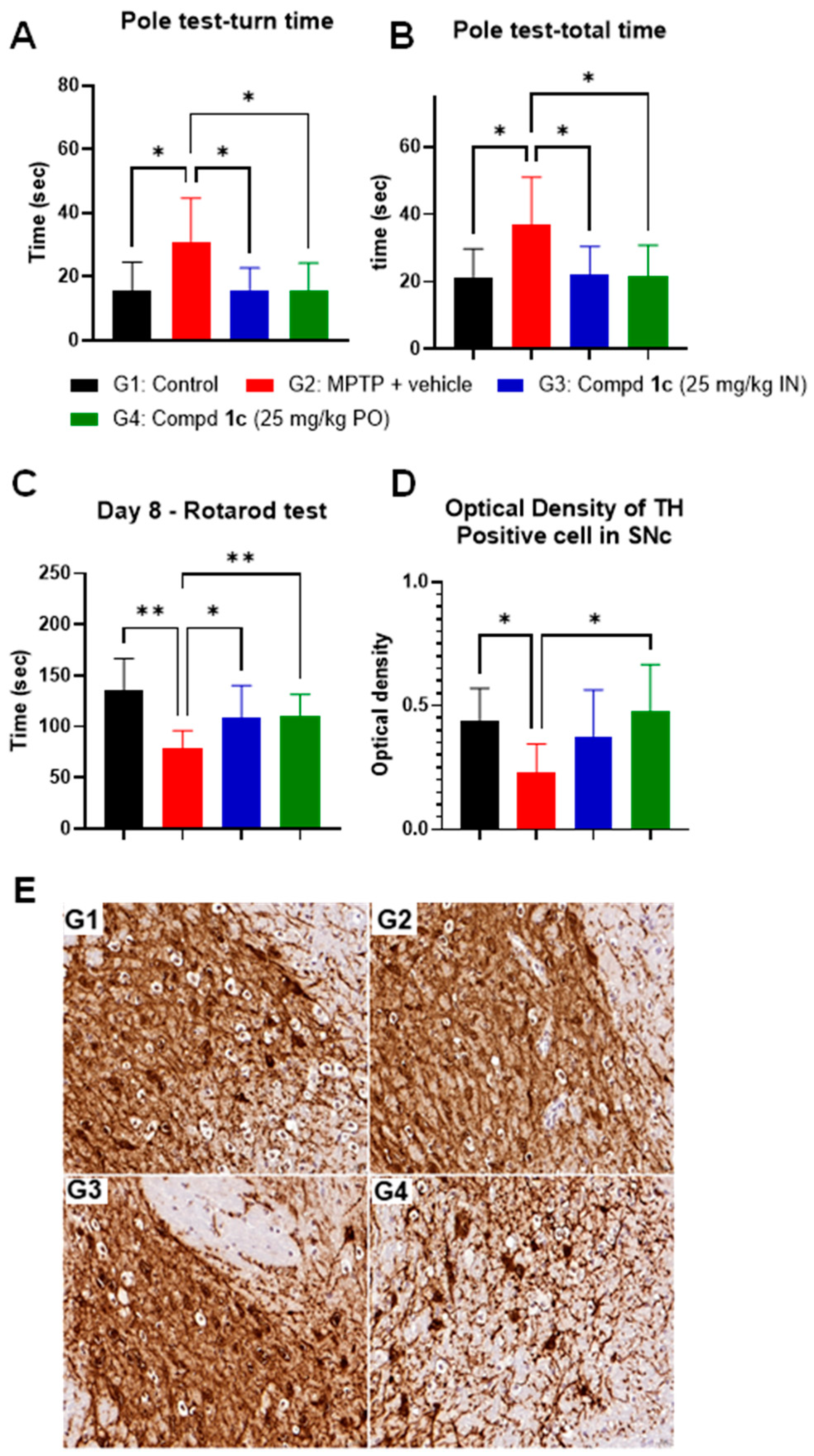
2.4. Pharmacological Studies
2.4.1. LPS-Induced Inflammation Studies
2.4.2. MPTP Model
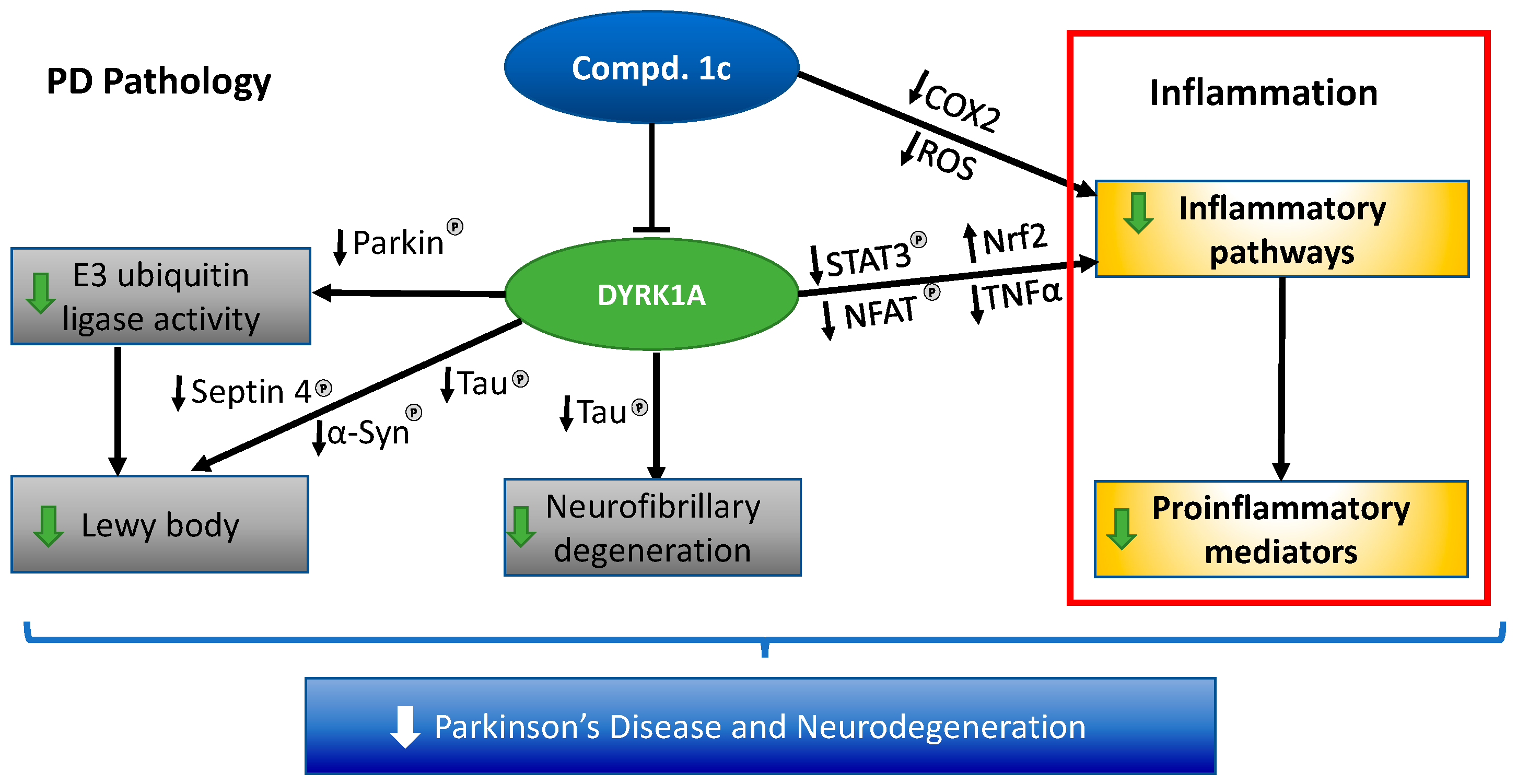
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. Synthesis of (2S,3R)-2-(3,4,5-trihydroxyphenyl)-3,5,7-chromantriol ((−)-gallocatechin, GC)
4.1.2. Synthesis of (2S,3R)-5,7-bis(benzyloxy)-2-(3,4,5-tris(benzyloxy)phenyl)chroman-3-ol (2)
4.1.3. Synthesis of 3,4,5-tris(benzyloxy)-2-fluorobenzoic acid (3a)
4.1.4. Synthesis of 3,4,5-tris(benzyloxy)-2,6-difluorobenzoic acid (3b)
4.1.5. Synthesis of 3, 4-bis(benzyloxy)-2-fluoro-5-methoxybenzoic acid (3c)
4.1.6. Synthesis of benzyl 4,5-bis(benzyloxy)-2-fluoro-3-methoxybenzoate (3d)
4.1.7. Synthesis of 3,4-bis(benzyloxy)-2,6-difluoro-5-methoxybenzoic acid (3e)
4.1.8. Synthesis of 3,4-bis(benzyloxy)-5-(difluoromethoxy)benzoic acid (3f)
4.1.9. Synthesis of 3,4-bis(benzyloxy)-5-(difluoromethoxy)-2-fluorobenzoic acid (3g)
4.1.10. Synthesis of 3,4-bis(benzyloxy)-2,6-difluoro-5-isopropoxybenzoic acid (3h)
4.1.11. General Procedure for Synthesis of Compounds 4a–h
4.1.12. General Procedure for Synthesis of Compounds 1a–h
4.2. Kinase Assays
4.3. Pharmacokinetic Assays
4.4. Pharmacological Studies
4.4.1. LPS-Induced Inflammation Model
4.4.2. MPTP Model
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Dowjat, W.K.; Adayev, T.; Kuchna, I.; Nowicki, K.; Palminiello, S.; Hwang, Y.W.; Wegiel, J. Trisomy-driven overexpression of DYRK1A kinase in the brain of subjects with Down syndrome. Neurosci. Lett. 2007, 413, 77–81. [Google Scholar] [CrossRef]
- Nalls, M.A.; Blauwendraat, C.; Vallerga, C.L.; Heilbron, K.; Bandres-Ciga, S.; Chang, D.; Tan, M.; Kia, D.A.; Noyce, A.J.; Xue, A.; et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 2019, 18, 1091–1102. [Google Scholar] [CrossRef]
- Fan, K.; Tang, B.S.; Wang, Y.Q.; Kang, J.F.; Li, K.; Liu, Z.H.; Sun, Q.Y.; Xu, Q.; Yan, X.X.; Guo, J.F. The GBA, DYRK1A and MS4A6A polymorphisms influence the age at onset of Chinese Parkinson patients. Neurosci. Lett. 2016, 621, 133–136. [Google Scholar] [CrossRef]
- Cen, L.; Xiao, Y.; Wei, L.; Mo, M.; Chen, X.; Li, S.; Yang, X.; Huang, Q.; Qu, S.; Pei, Z.; et al. Association of DYRK1A polymorphisms with sporadic Parkinson’s disease in Chinese Han population. Neurosci. Lett. 2016, 632, 39–43. [Google Scholar] [CrossRef]
- Kitada, T.; Asakawa, S.; Hattori, N.; Matsumine, H.; Yamamura, Y.; Minoshima, S.; Yokochi, M.; Mizuno, Y.; Shimizu, N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 1998, 392, 605–608. [Google Scholar] [CrossRef]
- Im, E.; Chung, K.C. Dyrk1A phosphorylates parkin at Ser-131 and negatively regulates its ubiquitin E3 ligase activity. J. Neurochem. 2015, 134, 756–768. [Google Scholar] [CrossRef]
- Sitz, J.H.; Baumgartel, K.; Hammerle, B.; Papadopoulos, C.; Hekerman, P.; Tejedor, F.J.; Becker, W.; Lutz, B. The Down syndrome candidate dual-specificity tyrosine phosphorylation-regulated kinase 1A phosphorylates the neurodegeneration-related septin 4. Neuroscience 2008, 157, 596–605. [Google Scholar] [CrossRef]
- Arbones, M.L.; Thomazeau, A.; Nakano-Kobayashi, A.; Hagiwara, M.; Delabar, J.M. DYRK1A and cognition: A lifelong relationship. Pharmacol. Ther. 2019, 194, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Barrachina, M.; Puig, B.; Martinez de Lagran, M.; Marti, E.; Avila, J.; Dierssen, M. Constitutive Dyrk1A is abnormally expressed in Alzheimer disease, Down syndrome, Pick disease, and related transgenic models. Neurobiol. Dis. 2005, 20, 392–400. [Google Scholar] [CrossRef]
- Khor, B.; Gagnon, J.D.; Goel, G.; Roche, M.I.; Conway, K.L.; Tran, K.; Aldrich, L.N.; Sundberg, T.B.; Paterson, A.M.; Mordecai, S.; et al. The kinase DYRK1A reciprocally regulates the differentiation of Th17 and regulatory T cells. eLife 2015, 4, e05920. [Google Scholar] [CrossRef] [PubMed]
- Latour, A.; Gu, Y.; Kassis, N.; Daubigney, F.; Colin, C.; Gausseres, B.; Middendorp, S.; Paul, J.L.; Hindie, V.; Rain, J.C.; et al. LPS-Induced Inflammation Abolishes the Effect of DYRK1A on IkB Stability in the Brain of Mice. Mol. Neurobiol. 2019, 56, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Wang, Y.; Zang, C.; Liu, H.; Yuan, F.; Ning, J.; Shang, M.; Ma, J.; Li, G.; Yang, Y.; et al. Inhibition of Dyrk1A Attenuates LPS-Induced Neuroinflammation via the TLR4/NF-kappaB P65 Signaling Pathway. Inflammation 2022, 45, 2375–2387. [Google Scholar] [CrossRef] [PubMed]
- Nakano-Kobayashi, A.; Fukumoto, A.; Morizane, A.; Nguyen, D.T.; Le, T.M.; Hashida, K.; Hosoya, T.; Takahashi, R.; Takahashi, J.; Hori, O.; et al. Therapeutics potentiating microglial p21-Nrf2 axis can rescue neurodegeneration caused by neuroinflammation. Sci. Adv. 2020, 6, eabc1428. [Google Scholar] [CrossRef]
- Lochhead, P.A.; Sibbet, G.; Morrice, N.; Cleghon, V. Activation-loop autophosphorylation is mediated by a novel transitional intermediate form of DYRKs. Cell 2005, 121, 925–936. [Google Scholar] [CrossRef]
- Aranda, S.; Laguna, A.; de la Luna, S. DYRK family of protein kinases: Evolutionary relationships, biochemical properties, and functional roles. FASEB J. 2011, 25, 449–462. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Fruit, C.; Herault, Y.; Meijer, L.; Besson, T. Dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) inhibitors: A survey of recent patent literature. Expert. Opin. Ther. Pat. 2017, 27, 1183–1199. [Google Scholar] [CrossRef]
- Ashford, A.L.; Oxley, D.; Kettle, J.; Hudson, K.; Guichard, S.; Cook, S.J.; Lochhead, P.A. A novel DYRK1B inhibitor AZ191 demonstrates that DYRK1B acts independently of GSK3beta to phosphorylate cyclin D1 at Thr(286), not Thr(288). Biochem. J. 2014, 457, 43–56. [Google Scholar] [CrossRef]
- Bain, J.; McLauchlan, H.; Elliott, M.; Cohen, P. The specificities of protein kinase inhibitors: An update. Biochem. J. 2003, 371, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Adayev, T.; Chen-Hwang, M.C.; Murakami, N.; Wegiel, J.; Hwang, Y.W. Kinetic properties of a MNB/DYRK1A mutant suitable for the elucidation of biochemical pathways. Biochemistry 2006, 45, 12011–12019. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Park, C.S.; Kim, D.J.; Cho, M.H.; Jin, B.K.; Pie, J.E.; Chung, W.G. Prevention of nitric oxide-mediated 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease in mice by tea phenolic epigallocatechin 3-gallate. Neurotoxicology 2002, 23, 367–374. [Google Scholar] [CrossRef]
- Zhou, T.; Zhu, M.; Liang, Z. (-)-Epigallocatechin-3-gallate modulates peripheral immunity in the MPTP-induced mouse model of Parkinson’s disease. Mol. Med. Rep. 2018, 17, 4883–4888. [Google Scholar] [CrossRef]
- Araldi, G.L. Methods of treating cognitive and behavioral impairment in down syndrome and Alzheimer’s disease. Patent US 2018/0000774 A1, 4 January 2018. [Google Scholar]
- Araldi, G.L.; Hwang, Y.W. Design, synthesis, and biological evaluation of polyphenol derivatives as DYRK1A inhibitors. The discovery of a potentially promising treatment for Multiple Sclerosis. Bioorg. Med. Chem. Lett. 2022, 64, 128675. [Google Scholar] [CrossRef] [PubMed]
- Maeda-Yamamoto, M.; Ema, K.; Shibuichi, I. In vitro and in vivo anti-allergic effects of ’benifuuki’ green tea containing O-methylated catechin and ginger extract enhancement. Cytotechnology 2007, 55, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Adayev, T.; Hwang, Y.W. An ELISA DYRK1A non-radioactive kinase assay suitable for the characterization of inhibitors. F1000Research 2017, 6, 42. [Google Scholar] [CrossRef]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Assessment of plasma concentrations of (-)-epigallocatechin gallate in mice following administration of a dose reflecting consumption of a standard green tea beverage. Food Chem. 2011, 128, 7–13. [Google Scholar] [CrossRef]
- Tu, Y.; Wang, L.; Rong, Y.; Tam, V.; Yin, T.; Gao, S.; Singh, R.; Hu, M. Hepatoenteric recycling is a new disposition mechanism for orally administered phenolic drugs and phytochemicals in rats. eLife 2021, 10, e58820. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Duarte, J.; Santos-Buelga, C. The flavonoid paradox: Conjugation and deconjugation as key steps for the biological activity of flavonoids. J. Sci. Food Agric. 2012, 92, 1822–1825. [Google Scholar] [CrossRef]
- Sarlus, H.; Heneka, M.T. Microglia in Alzheimer’s disease. J. Clin. Investig. 2017, 127, 3240–3249. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, Y.K.; Yuk, D.Y.; Choi, D.Y.; Ban, S.B.; Oh, K.W.; Hong, J.T. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J. Neuroinflammation 2008, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Woo, H.; Lee, H.E.; Jeon, H.; Ryu, K.Y.; Nam, J.H.; Jeon, S.G.; Park, H.; Lee, J.S.; Han, K.M.; et al. The novel DYRK1A inhibitor KVN93 regulates cognitive function, amyloid-beta pathology, and neuroinflammation. Free Radic. Biol. Med. 2020, 160, 575–595. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Hong, J.S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef]
- Tiraboschi, P.; Hansen, L.A.; Thal, L.J.; Corey-Bloom, J. The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology 2004, 62, 1984–1989. [Google Scholar] [CrossRef]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef]
- Masters, C.L.; Simms, G.; Weinman, N.A.; Multhaup, G.; McDonald, B.L.; Beyreuther, K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. USA 1985, 82, 4245–4249. [Google Scholar] [CrossRef] [PubMed]
- Iwatsubo, T.; Saido, T.C.; Mann, D.M.; Lee, V.M.; Trojanowski, J.Q. Full-length amyloid-beta (1-42(43)) and amino-terminally modified and truncated amyloid-beta 42(43) deposit in diffuse plaques. Am. J. Pathol. 1996, 149, 1823–1830. [Google Scholar]
- Dickson, D.W. The pathogenesis of senile plaques. J. Neuropathol. Exp. Neurol. 1997, 56, 321–339. [Google Scholar] [CrossRef]
- Liao, L.; Cheng, D.; Wang, J.; Duong, D.M.; Losik, T.G.; Gearing, M.; Rees, H.D.; Lah, J.J.; Levey, A.I.; Peng, J. Proteomic characterization of postmortem amyloid plaques isolated by laser capture microdissection. J. Biol. Chem. 2004, 279, 37061–37068. [Google Scholar] [CrossRef]
- Marti, E.; Altafaj, X.; Dierssen, M.; de la Luna, S.; Fotaki, V.; Alvarez, M.; Perez-Riba, M.; Ferrer, I.; Estivill, X. Dyrk1A expression pattern supports specific roles of this kinase in the adult central nervous system. Brain Res. 2003, 964, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Souchet, B.; Audrain, M.; Billard, J.M.; Dairou, J.; Fol, R.; Orefice, N.S.; Tada, S.; Gu, Y.; Dufayet-Chaffaud, G.; Limanton, E.; et al. Inhibition of DYRK1A proteolysis modifies its kinase specificity and rescues Alzheimer phenotype in APP/PS1 mice. Acta Neuropathol. Commun. 2019, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, S.R.; Cho, H.J.; Lee, H.W.; Jeong, H.K.; Radnaabazar, C.; Kim, Y.S.; Kim, M.J.; Son, M.Y.; Seo, H.; Chung, S.H.; et al. Dual-specificity tyrosine(Y)-phosphorylation regulated kinase 1A-mediated phosphorylation of amyloid precursor protein: Evidence for a functional link between Down syndrome and Alzheimer’s disease. J. Neurochem. 2008, 104, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Kamino, K.; Yamamoto, M.; Nuripa, A.; Kida, T.; Kazui, H.; Hashimoto, R.; Tanaka, T.; Kudo, T.; Yamagata, H.; et al. The DYRK1A gene, encoded in chromosome 21 Down syndrome critical region, bridges between beta-amyloid production and tau phosphorylation in Alzheimer disease. Hum. Mol. Genet. 2007, 16, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Woods, Y.L.; Cohen, P.; Becker, W.; Jakes, R.; Goedert, M.; Wang, X.; Proud, C.G. The kinase DYRK phosphorylates protein-synthesis initiation factor eIF2Bepsilon at Ser539 and the microtubule-associated protein tau at Thr212: Potential role for DYRK as a glycogen synthase kinase 3-priming kinase. Biochem. J. 2001, 355, 609–615. [Google Scholar] [CrossRef]
- Liu, F.; Liang, Z.; Wegiel, J.; Hwang, Y.W.; Iqbal, K.; Grundke-Iqbal, I.; Ramakrishna, N.; Gong, C.X. Overexpression of Dyrk1A contributes to neurofibrillary degeneration in Down syndrome. FASEB J. 2008, 22, 3224–3233. [Google Scholar] [CrossRef]
- Araldi, G.L. Composition and methods for antioxidant and anti-inflammatory therapeutics. Patent WO/2023/288020 A1, 19 January 2023. [Google Scholar]
- Ehrnhoefer, D.E.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; Wanker, E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566. [Google Scholar] [CrossRef]
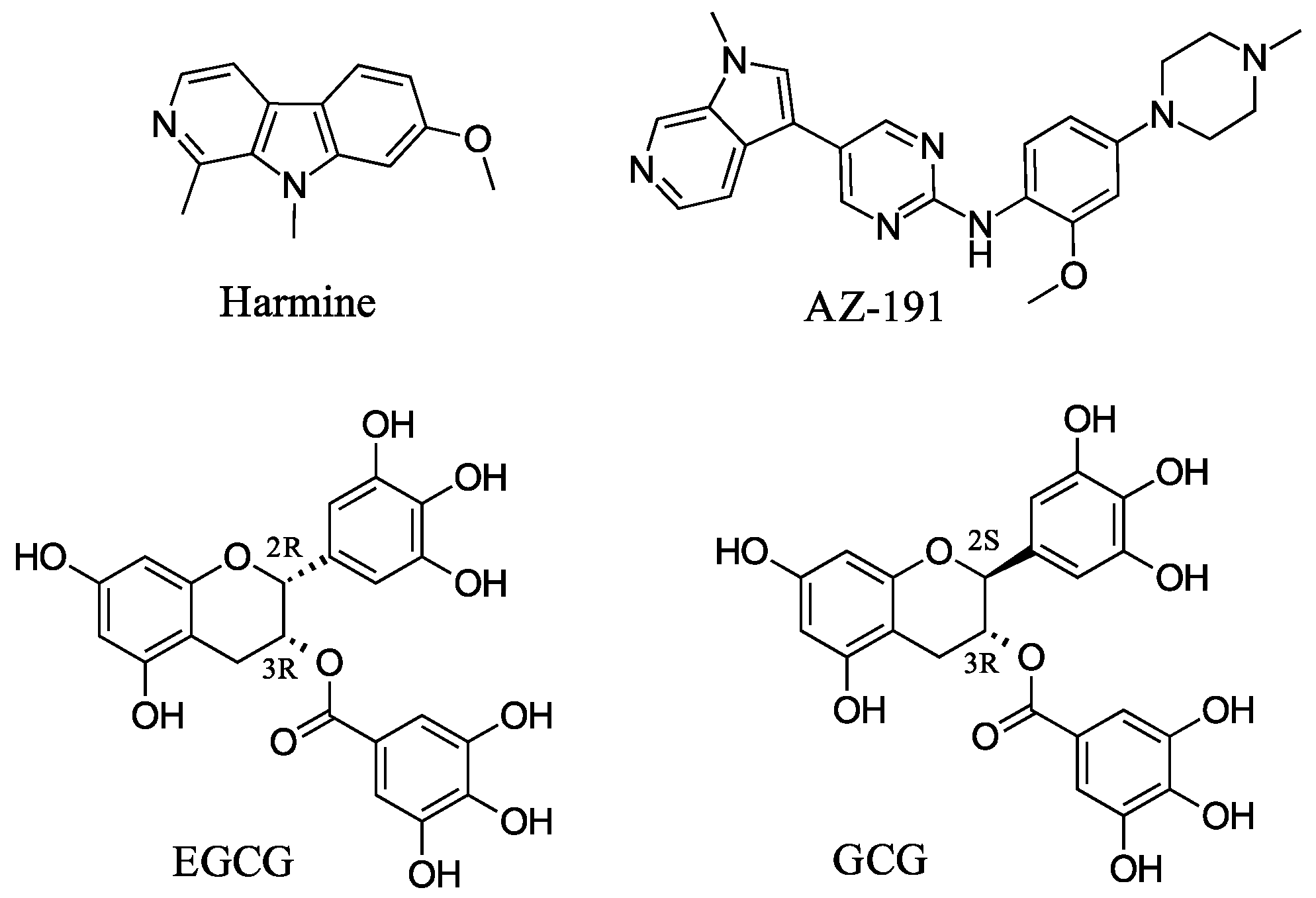
| Compound | R1 | R2 | R3 | DYRK1A IC50 (nM) | DYRK1B IC50 (nM) | DYRK2 IC50 (nM) |
|---|---|---|---|---|---|---|
| EGCG | H | OH | H | 220 | 257 | 1488 |
| AZ-191 | 98 | 27 | 1720 | |||
| GCG | H | OH | H | 121 | 132 | 574 |
| GCG-3”OMe | H | OMe | H | 206 | ||
| 1a | H | OH | F | 35 | 42 | 258 |
| 1b | F | OH | F | 28 | 45 | 181 |
| 1c | H | OMe | F | 73 | 79 | 331 |
| 1d | F | OMe | H | 152 | ||
| 1e | F | OMe | F | 125 | ||
| 1f | H | OCHF2 | H | 252 | ||
| 1g | H | OCHF2 | F | 136 | ||
| 1h | F | OiPr | F | 356 |
| 1c | 1b | EGCG | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Unit | i.v. (5mg/kg) | IN (60 mg/kg) | PO (100 mg/kg) | PO (100 mg/kg) | PO (100 mg/kg) | ||
| Plasma | Brain | Plasma | Brain | Plasma | Plasma | |||
| T1/2 | h | 3.2 | 4.8 | NRV | 4.7 | NRV | 12.7 | 5.02 |
| Tmax | h | 0 | 2 | 0.5 | 1 | 2 | 0.5 | |
| Tlast | h | 10 | 24 | 8 | 24 | 24 | 24 | |
| Cmax | ng/mL | 657 | 2353 | 18 | 684 | 110 | 131 | 55 |
| AUC0-t | ng h/mL | 1276 | 15763 | 116 | 4174 | 2392 | 379 | 360 |
| AUC (0-inf) | ng h/mL | 1393 | 16079 | NRV | 4275 | NRV | 472 | 395 |
| F | % | NA | >100 | 16 | N/A | 2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araldi, G.L.; Hwang, Y.-W. Development of Novel Fluorinated Polyphenols as Selective Inhibitors of DYRK1A/B Kinase for Treatment of Neuroinflammatory Diseases including Parkinson’s Disease. Pharmaceuticals 2023, 16, 443. https://doi.org/10.3390/ph16030443
Araldi GL, Hwang Y-W. Development of Novel Fluorinated Polyphenols as Selective Inhibitors of DYRK1A/B Kinase for Treatment of Neuroinflammatory Diseases including Parkinson’s Disease. Pharmaceuticals. 2023; 16(3):443. https://doi.org/10.3390/ph16030443
Chicago/Turabian StyleAraldi, Gian Luca, and Yu-Wen Hwang. 2023. "Development of Novel Fluorinated Polyphenols as Selective Inhibitors of DYRK1A/B Kinase for Treatment of Neuroinflammatory Diseases including Parkinson’s Disease" Pharmaceuticals 16, no. 3: 443. https://doi.org/10.3390/ph16030443
APA StyleAraldi, G. L., & Hwang, Y.-W. (2023). Development of Novel Fluorinated Polyphenols as Selective Inhibitors of DYRK1A/B Kinase for Treatment of Neuroinflammatory Diseases including Parkinson’s Disease. Pharmaceuticals, 16(3), 443. https://doi.org/10.3390/ph16030443







