α-Hederin Saponin Augments the Chemopreventive Effect of Cisplatin against Ehrlich Tumors and Bioinformatic Approach Identifying the Role of SDF1/CXCR4/p-AKT-1/NFκB Signaling
Abstract
1. Introduction
2. Results
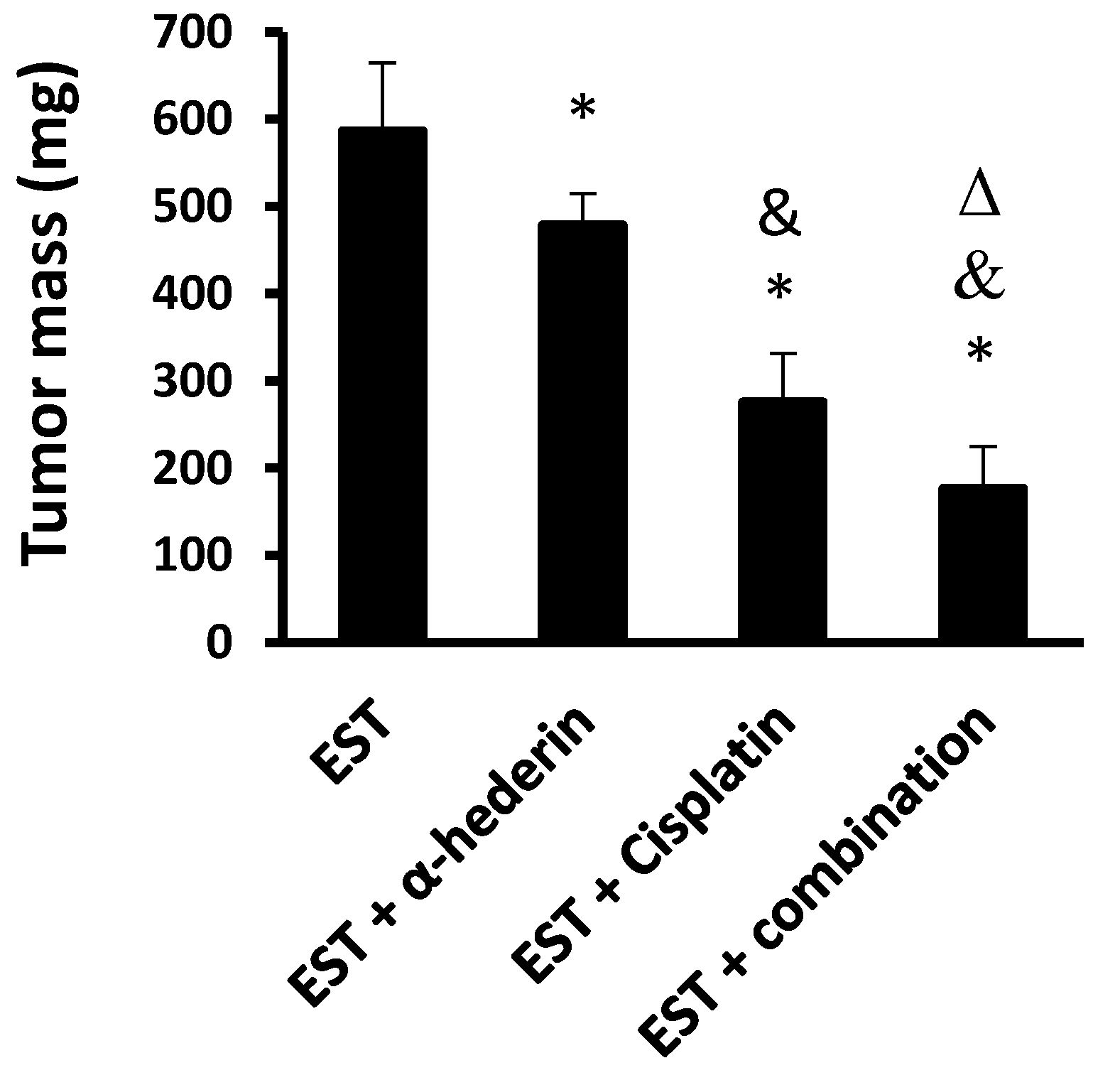
2.1. Effect of α-Hederin and Cisplatin in Reducing the Solid Tumor Masses
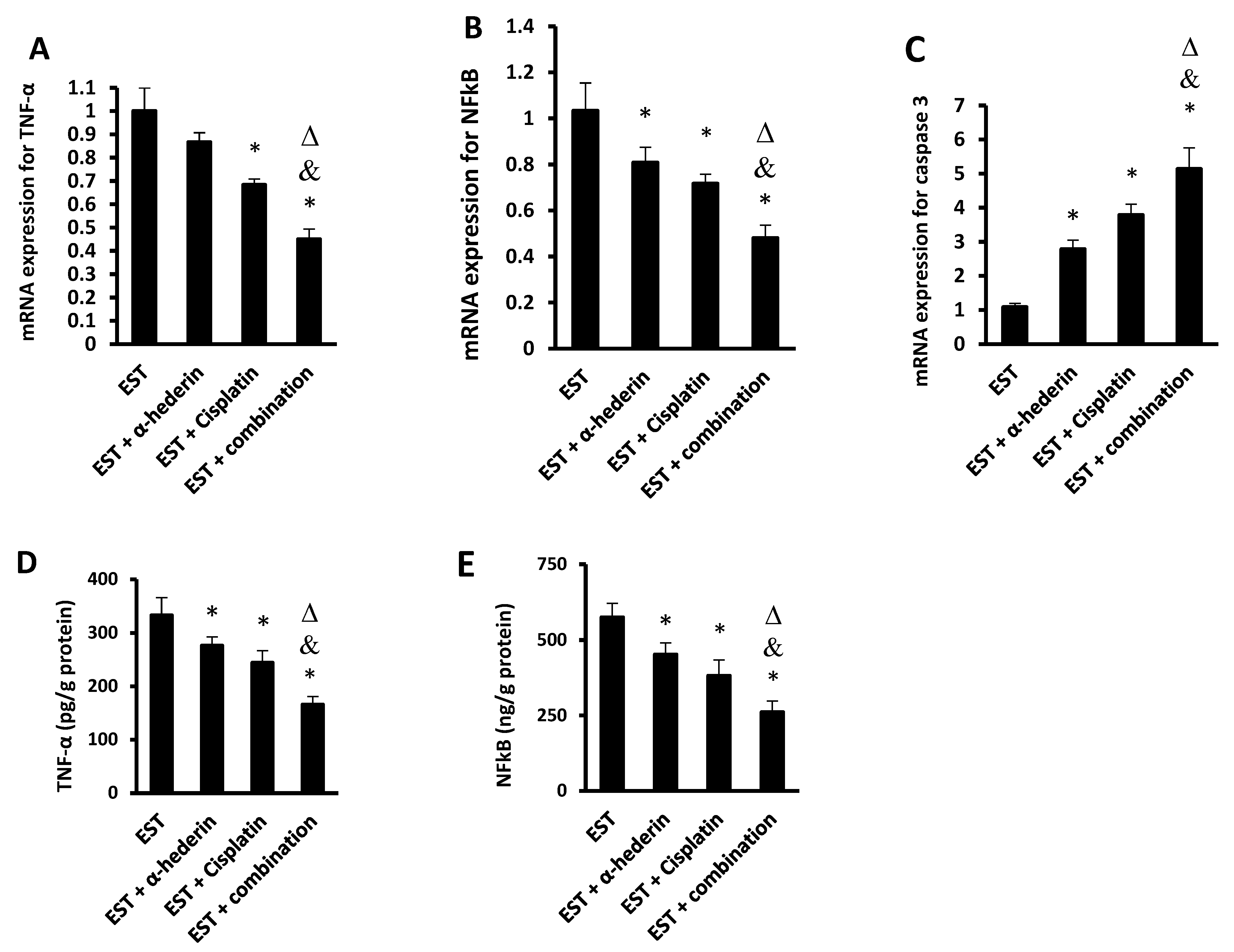
2.2. Effect of α-Hederin and Cisplatin on the Level of Inflammatory Factors in ESTs
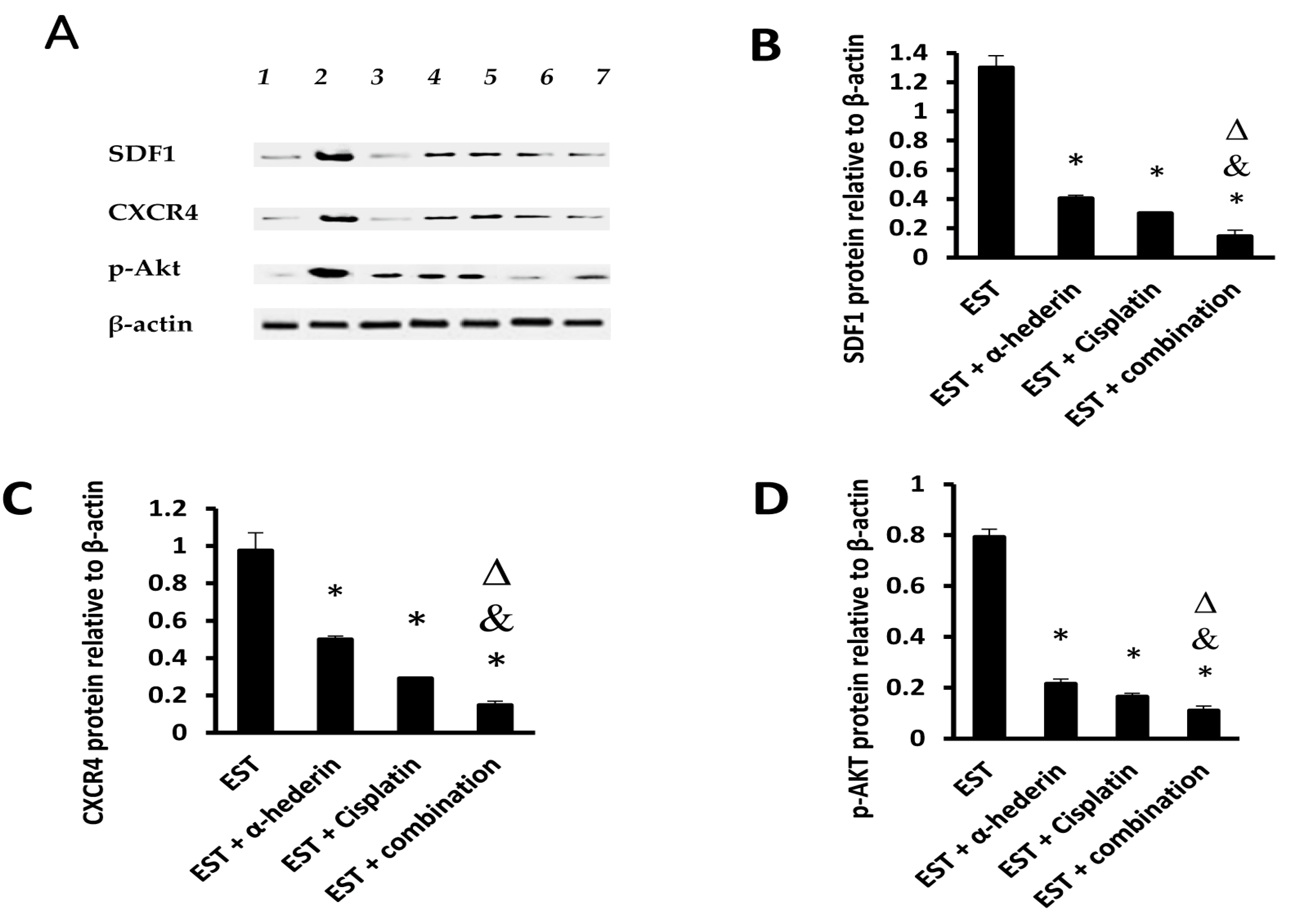
2.3. Effect of α-Hederin and Cisplatin on Tumoral Level of SDF1/CXCR4/p-AKT Proteins
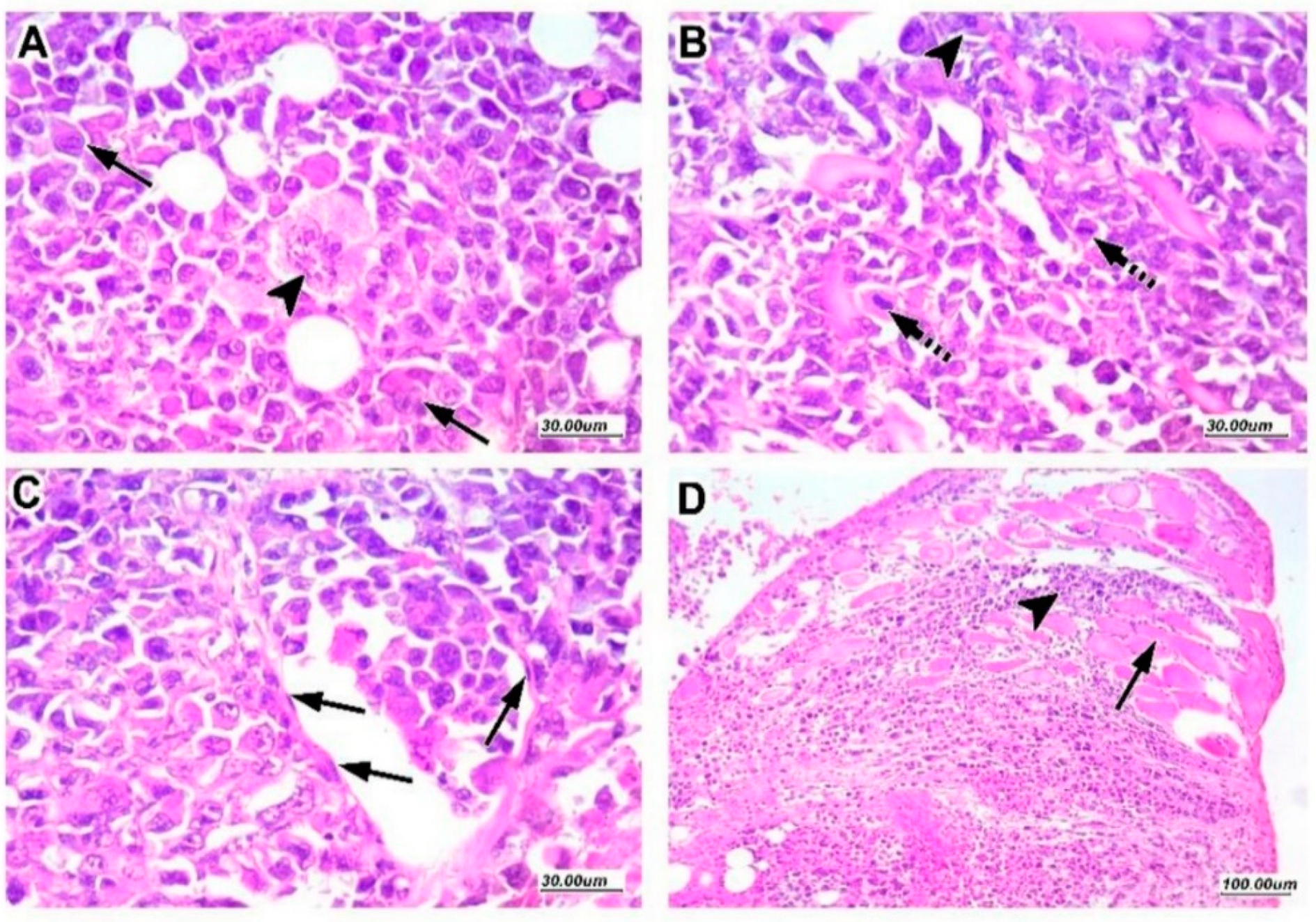
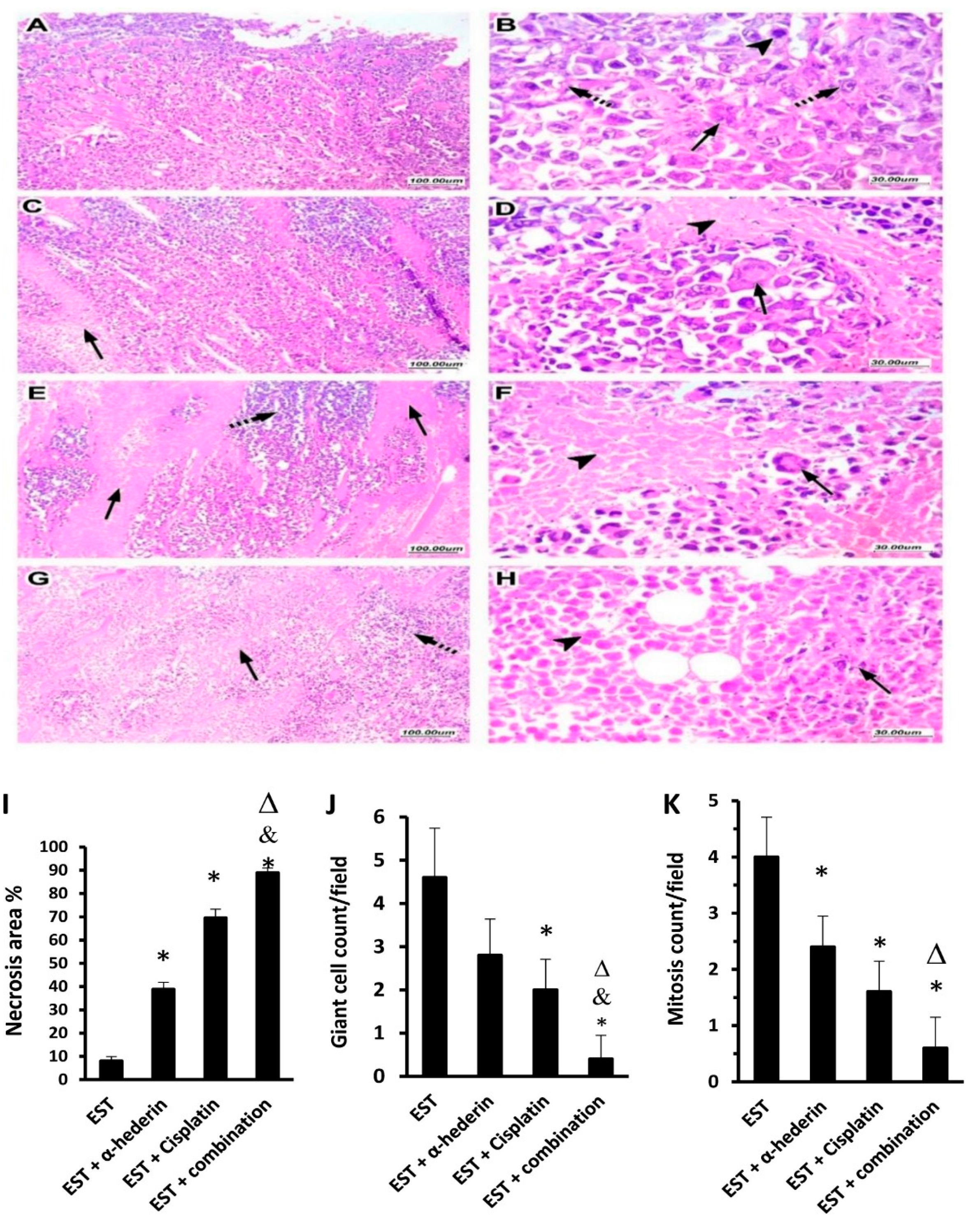
2.4. Tumor Characteristics in Sections Stained with Hematoxylin and Eosin
2.5. α-Hederin Regressed the Viable Tumor Area in Combination with Cisplatin
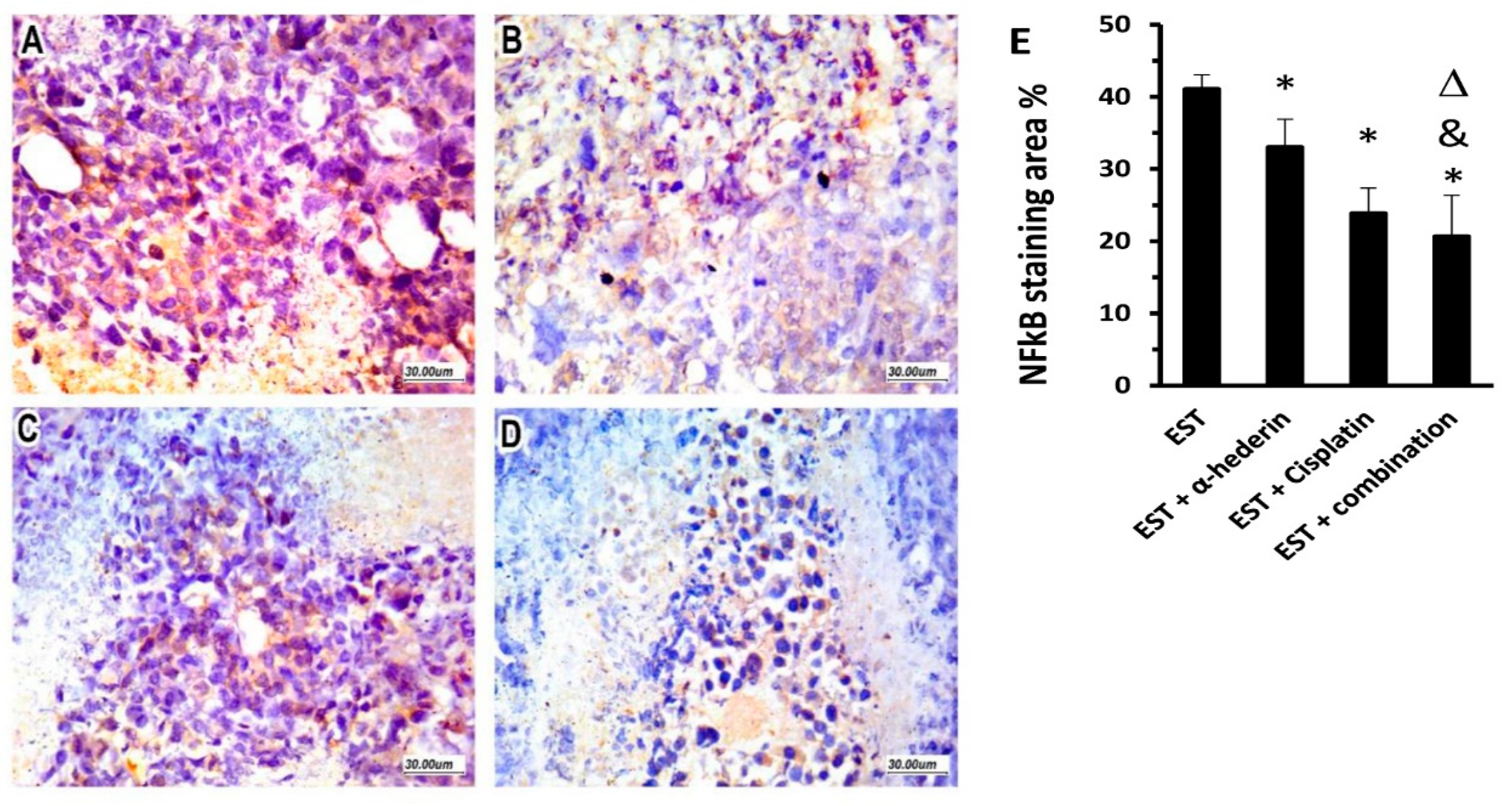
2.6. Effect of α-Hederin and Cisplatin on Immunostaining for NFκB in ESTs
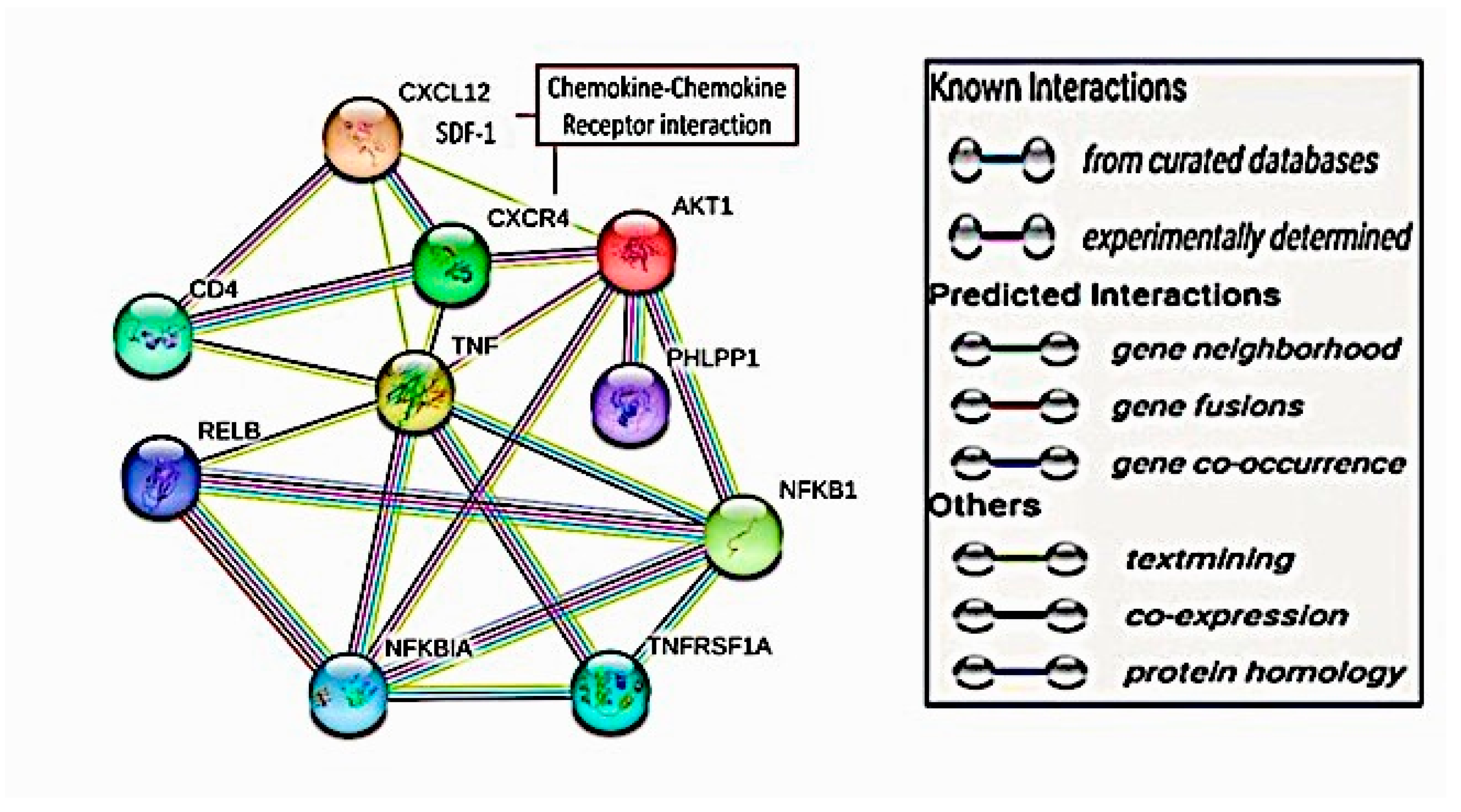
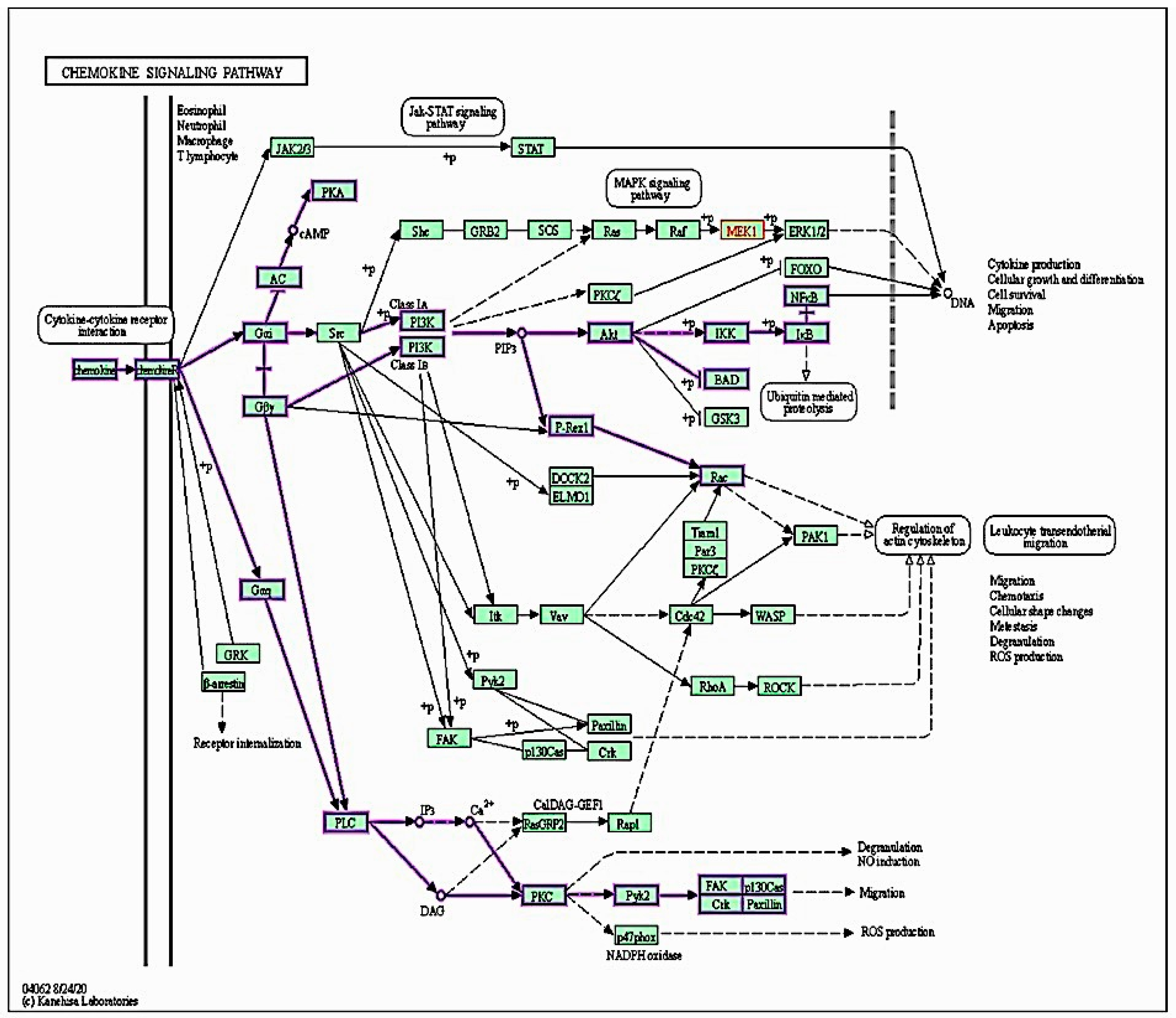
2.7. Targeted Protein–Protein Interactions and Analysis of Pathway Enrichment
3. Discussion
4. Materials and Methods
4.1. Computational Interaction Analysis for the Target Proteins
4.2. Signaling Pathway Enrichment Analysis
4.3. Experimental Animals
4.4. Drugs and Reagents
4.5. Tumor Cell Preparation and Intradermal Inoculation
4.6. Study Groups
4.7. Harvesting the Solid Tumors
4.8. Western Blotting for p-Akt, SDF1, and CXCR4 Proteins
4.9. RT-PCR Quantification of Cytokines and Factors in ESTs
4.10. ELISA Quantification of Cytokines in EST Homogenates
4.11. Tumor Histopathology and Assessment
4.12. Immunohistochemical Imaging and Quantification of NFκB in Tumor Tissue
4.13. Data Handling and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.T.; Macedo, L.T.; Curigliano, G.; Fumagalli, L.; Locatelli, M.; Dalton, M.; Quintela, A.; Carvalheira, J.B.C.; Manunta, S.; Mazzarella, L.; et al. Cytotoxic Drugs for Patients with Breast Cancer in the Era of Targeted Treatment: Back to the Future? Ann. Oncol. 2012, 23, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Mazumder, K.; Haldar, P.; Naskar, S.; Kundusen, S.; Bala, A.; Kar, B. Anticancer Activity of Methanol Extract of Cucurbita Maxima against Ehrlich As-Cites Carcinoma. Int. J. Res. Pharm. Sci. 2011, 2, 52–59. [Google Scholar]
- Bom, J.; Gunutzmann, P.; Hurtado, E.C.P.; Maragno-Correa, J.M.R.; Kleeb, S.R.; Lallo, M.A. Long-Term Treatment with Aqueous Garlic and/or Tomato Suspensions Decreases Ehrlich Ascites Tumors. Evid. Based Complement. Altern. Med. 2014, 2014, 381649. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.; Islam, M.; Karim, R. Antitumour Activity of Amoora Rohituka Roxb. Stem against Ehrlich Ascites Carcinoma in Mice. Biharean Biol. 2011, 5, 109–112. [Google Scholar]
- Ozaslan, M.; Karagoz, I.D.; Kilic, I.H.; Guldur, M.E. Ehrlich Ascites Carcinoma. Afr. J. Biotechnol. 2011, 10, 2375–2378. [Google Scholar] [CrossRef]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the Cancer Microenvironment and Their Relevance in Cancer Immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef]
- Meng, W.; Xue, S.; Chen, Y. The Role of CXCL12 in Tumor Microenvironment. Gene 2018, 641, 105–110. [Google Scholar] [CrossRef]
- Lecavalier-Barsoum, M.; Chaudary, N.; Han, K.; Koritzinsky, M.; Hill, R.; Milosevic, M. Targeting the CXCL12/CXCR4 Pathway and Myeloid Cells to Improve Radiation Treatment of Locally Advanced Cervical Cancer. Int. J. Cancer 2018, 143, 1017–1028. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, X.; Ye, X.; Feng, C.; Yang, G.; Lu, Y.; Lin, Y.; Dong, C. High Expression of Stromal Cell-Derived Factor 1 (SDF-1) and NF-ΚB Predicts Poor Prognosis in Cervical Cancer. Med. Sci. Monit. 2017, 23, 151–157. [Google Scholar] [CrossRef]
- Mosley, L.D.; Singh, R.; Sharma, P.K.; Singh, S.; Lillard, J.W., Jr. CXCL11 and CXCL12 Activates ERK1/2 and NF-KB in Breast Cancer Cells via CXCR3, CXCR4 and CXCR7 (98.3). J. Immunol. 2009, 182, 98.3. [Google Scholar] [CrossRef]
- Ourique, F.; Kviecinski, M.R.; Felipe, K.B.; Correia, J.F.G.; Farias, M.S.; Castro, L.S.E.P.W.; Grinevicius, V.M.A.S.; Valderrama, J.; Rios, D.; Benites, J.; et al. DNA Damage and Inhibition of Akt Pathway in MCF-7 Cells and Ehrlich Tumor in Mice Treated with 1,4-Naphthoquinones in Combination with Ascorbate. Oxid. Med. Cell. Longev. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.M.; Hemmings, B.A. Inhibition of Protein Kinase B/Akt: Implications for Cancer Therapy. Pharmacol. Ther. 2002, 93, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-ΚB Signaling Pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef]
- Ghosh, S.; Hayden, M.S. New Regulators of NF-ΚB in Inflammation. Nat. Rev. Immunol. 2008, 8, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; de Fougerolles, A.R.; Blaikie, P.; Khan, L.; Pepe, A.; Green, C.D.; Koteliansky, V.; Giancotti, F.G. A5β1 Integrin Activates an NF-ΚB-Dependent Program of Gene Expression Important for Angiogenesis and Inflammation. Mol. Cell Biol. 2002, 22, 5912–5922. [Google Scholar] [CrossRef]
- Schulte-Michels, J.; Keksel, C.; Häberlein, H.; Franken, S. Anti-Inflammatory Effects of Ivy Leaves Dry Extract: Influence on Transcriptional Activity of NFκB. Inflammopharmacology 2019, 27, 339–347. [Google Scholar] [CrossRef]
- Sakamoto, K.; Maeda, S.; Hikiba, Y.; Nakagawa, H.; Hayakawa, Y.; Shibata, W.; Yanai, A.; Ogura, K.; Omata, M. Constitutive NF-KappaB Activation in Colorectal Carcinoma Plays a Key Role in Angiogenesis, Promoting Tumor Growth. Clin. Cancer Res. 2009, 15, 2248–2258. [Google Scholar] [CrossRef]
- Peng, L.; Liu, A.; Shen, Y.; Xu, H.-Z.; Yang, S.-Z.; Ying, X.-Z.; Liao, W.; Liu, H.-X.; Lin, Z.-Q.; Chen, Q.-Y.; et al. Antitumor and Anti-Angiogenesis Effects of Thymoquinone on Osteosarcoma through the NF-ΚB Pathway. Oncol. Rep. 2013, 29, 571–578. [Google Scholar] [CrossRef]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.-H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Al-Bazz, Y.O.; Underwood, J.C.E.; Brown, B.L.; Dobson, P.R.M. Prognostic Significance of Akt, Phospho-Akt and BAD Expression in Primary Breast Cancer. Eur. J. Cancer 2009, 45, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Hemmings, B.A. PKB/Akt–Dependent Regulation of Cell Motility. JNCI J. Natl. Cancer Inst. 2013, 105, 393–404. [Google Scholar] [CrossRef]
- Cheng, L.; Xia, T.-S.; Wang, Y.-F.; Zhou, W.; Liang, X.-Q.; Xue, J.-Q.; Shi, L.; Wang, Y.; Ding, Q.; Wang, M. The Anticancer Effect and Mechanism of α-Hederin on Breast Cancer Cells. Int. J. Oncol. 2014, 45, 757–763. [Google Scholar] [CrossRef]
- Sun, J.; Feng, Y.; Wang, Y.; Ji, Q.; Cai, G.; Shi, L.; Wang, Y.; Huang, Y.; Zhang, J.; Li, Q. α-Hederin Induces Autophagic Cell Death in Colorectal Cancer Cells through Reactive Oxygen Species Dependent AMPK/MTOR Signaling Pathway Activation. Int. J. Oncol. 2019, 54, 1601–1612. [Google Scholar] [CrossRef]
- Wang, J.; Deng, H.; Zhang, J.; Wu, D.; Li, J.; Ma, J.; Dong, W. α-Hederin Induces the Apoptosis of Gastric Cancer Cells Accompanied by Glutathione Decrement and Reactive Oxygen Species Generation via Activating Mitochondrial Dependent Pathway. Phytother. Res. 2020, 34, 601–611. [Google Scholar] [CrossRef]
- Jeong, K.-B.; Luo, K.; Lee, H.; Lim, M.-C.; Yu, J.; Choi, S.-J.; Kim, K.-B.; Jeon, T.-J.; Kim, Y.-R. Alpha-Hederin Nanopore for Single Nucleotide Discrimination. ACS Nano 2019, 13, 1719–1727. [Google Scholar] [CrossRef]
- Osman, A.E.M.M.; Ahmed, M.M.S.; Khayyal, M.T.E.D.; El-Merzabani, M.M. Hyperthermic Potentiation of Cisplatin Cytotoxicity on Solid Ehrlich Carcinoma. Tumori 1993, 79, 268–272. [Google Scholar] [CrossRef]
- Reedijk, J.; Lohman, P.H. Cisplatin: Synthesis, Antitumour Activity and Mechanism of Action. Pharm. Weekbl. Sci. 1985, 7, 173–180. [Google Scholar] [CrossRef]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin Nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef]
- Zamble, D.B.; Lippard, S.J. Cisplatin and DNA Repair in Cancer Chemotherapy. Trends. Biochem. Sci. 1995, 20, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Panichpisal, K.; Kurtzman, N.; Nugent, K. Cisplatin Nephrotoxicity: A Review. Am. J. Med. Sci. 2007, 334, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.K.; El-Boghdady, N.A.; El-Sayed, A.M.; Helmy, H.S. Comparative Study of the Effects of PEGylated Interferon-A2a versus 5-Fluorouracil on Cancer Stem Cells in a Rat Model of Hepatocellular Carcinoma. Tumor Biol. 2016, 37, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Lin, H.; Li, G.; Sun, Y.; Shen, J.; Xu, J.; Lin, C.; Yeh, S.; Cai, X.; Chang, C. Cisplatin Enhances NK Cells Immunotherapy Efficacy to Suppress HCC Progression via Altering the Androgen Receptor (AR)-ULBP2 Signals. Cancer Lett. 2016, 373, 45–56. [Google Scholar] [CrossRef]
- Yin, W.; Nie, Y.; Zhang, Z.; Xie, L.; He, X. MiR-193b Acts as a Cisplatin Sensitizer via the Caspase-3-Dependent Pathway in HCC Chemotherapy. Oncol. Rep. 2015, 34, 368–374. [Google Scholar] [CrossRef]
- Adamska, A.; Stefanowicz-Hajduk, J.; Ochocka, J.R. Alpha-Hederin, the Active Saponin of Nigella Sativa, as an Anticancer Agent Inducing Apoptosis in the SKOV-3 Cell Line. Molecules 2019, 24, 2958. [Google Scholar] [CrossRef]
- Deng, H.; Ma, J.; Liu, Y.; He, P.; Dong, W. Combining α-Hederin with Cisplatin Increases the Apoptosis of Gastric Cancer in Vivo and in Vitro via Mitochondrial Related Apoptosis Pathway. Biomed. Pharmacother. 2019, 120, 109477. [Google Scholar] [CrossRef]
- Sun, D.; Shen, W.; Zhang, F.; Fan, H.; Tan, J.; Li, L.; Xu, C.; Zhang, H.; Yang, Y.; Cheng, H. α-Hederin Arrests Cell Cycle at G2/M Checkpoint and Promotes Mitochondrial Apoptosis by Blocking Nuclear Factor-ΚB Signaling in Colon Cancer Cells. Biomed. Res. Int. 2018, 2018, 2548378. [Google Scholar] [CrossRef]
- Gülçin, I.; Mshvildadze, V.; Gepdiremen, A.; Elias, R. Antioxidant Activity of Saponins Isolated from Ivy: Alpha-Hederin, Hederasaponin-C, Hederacolchiside-E and Hederacolchiside-F. Planta Med. 2004, 70, 561–563. [Google Scholar] [CrossRef]
- Gardouh, A.R.; Attia, M.A.; Enan, E.T.; Elbahaie, A.M.; Fouad, R.A.; El-Shafey, M.; Youssef, A.M.; Alomar, S.Y.; Ali, Z.A.-E.; Zaitone, S.A.; et al. Synthesis and Antitumor Activity of Doxycycline Polymeric Nanoparticles: Effect on Tumor Apoptosis in Solid Ehrlich Carcinoma. Molecules 2020, 25, 3230. [Google Scholar] [CrossRef]
- Elsherbiny, N.M.; El-Sherbiny, M.; Zaitone, S.A. Diallyl Trisulfide Potentiates Chemotherapeutic Efficacy of Doxorubicin in Experimentally Induced Mammary Carcinoma: Role of Notch Signaling. Pathol. Res. Pract. 2020, 216, 153139. [Google Scholar] [CrossRef] [PubMed]
- Elmeligie, S.; Ahmed, E.M.; Abuel-Maaty, S.M.; Zaitone, S.A.-B.; Mikhail, D.S. Design and Synthesis of Pyridazine Containing Compounds with Promising Anticancer Activity. Chem. Pharm. Bull. 2017, 65, 236–247. [Google Scholar] [CrossRef]
- Saini, V.; Staren, D.M.; Ziarek, J.J.; Nashaat, Z.N.; Campbell, E.M.; Volkman, B.F.; Marchese, A.; Majetschak, M. The CXC Chemokine Receptor 4 Ligands Ubiquitin and Stromal Cell-Derived Factor-1α Function through Distinct Receptor Interactions. J. Biol. Chem. 2011, 286, 33466–33477. [Google Scholar] [CrossRef]
- Bi, Z.; Zhang, Q.; Fu, Y.; Wadgaonkar, P.; Zhang, W.; Almutairy, B.; Xu, L.; Rice, M.; Qiu, Y.; Thakur, C.; et al. Nrf2 and HIF1α Converge to Arsenic-Induced Metabolic Reprogramming and the Formation of the Cancer Stem-like Cells. Theranostics 2020, 10, 4134–4149. [Google Scholar] [CrossRef]
- Pathak, S.; Wilczyński, J.R.; Paradowska, E. Factors in Oncogenesis: Viral Infections in Ovarian Cancer. Cancers 2020, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.N.; Rodriguez, C.; Hubert, M.; Ardin, M.; Treilleux, I.; Ries, C.H.; Lavergne, E.; Chabaud, S.; Colombe, A.; Trédan, O.; et al. CD163+ Tumor-Associated Macrophage Accumulation in Breast Cancer Patients Reflects Both Local Differentiation Signals and Systemic Skewing of Monocytes. Clin. Transl. Immunol. 2020, 9, e1108. [Google Scholar] [CrossRef]
- Kariri, Y.A.; Joseph, C.; Kurozumi, S.; Toss, M.S.; Alsaleem, M.; Raafat, S.; Mongan, N.P.; Aleskandarany, M.A.; Green, A.R.; Rakha, E.A. Prognostic Significance of KN Motif and Ankyrin Repeat Domains 1 (KANK1) in Invasive Breast Cancer. Breast Cancer Res. Treat. 2020, 179, 349–357. [Google Scholar] [CrossRef]
- Folschette, M.; Legagneux, V.; Poret, A.; Chebouba, L.; Guziolowski, C.; Théret, N. A Pipeline to Create Predictive Functional Networks: Application to the Tumor Progression of Hepatocellular Carcinoma. BMC Bioinform. 2020, 21, 18. [Google Scholar] [CrossRef]
- Lajkó, E.; Hegedüs, R.; Mező, G.; Kőhidai, L. Apoptotic Effects of Drug Targeting Conjugates Containing Different GnRH Analogs on Colon Carcinoma Cells. Int. J. Mol. Sci. 2019, 20, 4421. [Google Scholar] [CrossRef]
- Bahr, H.I.; Ibrahiem, A.T.; Gabr, A.M.; Elbahaie, A.M.; Elmahdi, H.S.; Soliman, N.; Youssef, A.M.; El-Sherbiny, M.; Zaitone, S.A. Chemopreventive Effect of α-Hederin/Carboplatin Combination against Experimental Colon Hyperplasia and Impact on JNK Signaling. Toxicol. Mech. Methods 2021, 31, 138–149. [Google Scholar] [CrossRef]
- Salomonnson, E.; Stacer, A.C.; Ehrlich, A.; Luker, K.E.; Luker, G.D. Imaging CXCL12-CXCR4 Signaling in Ovarian Cancer Therapy. PLoS ONE 2013, 8, e51500. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-X.; Wang, J.; Shelburne, C.E.; Lopatin, D.E.; Chinnaiyan, A.M.; Rubin, M.A.; Pienta, K.J.; Taichman, R.S. Expression of CXCR4 and CXCL12 (SDF-1) in Human Prostate Cancers (PCa) in Vivo. J. Cell. Biochem. 2003, 89, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Q.; Zhao, B.-X.; Liu, Y.; Wang, Y.-T.; Liang, Q.-Y.; Cai, Y.; Zhang, Y.-Q.; Yang, J.-H.; Song, Z.-H.; Li, G.-F. New Application of an Old Drug: Antitumor Activity and Mechanisms of Doxycycline in Small Cell Lung Cancer. Int. J. Oncol. 2016, 48, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.N.; Zamler, D.; Baker, G.J.; Kadiyala, P.; Erdreich-Epstein, A.; DeCarvalho, A.C.; Mikkelsen, T.; Castro, M.G.; Lowenstein, P.R. CXCR4 Increases In-Vivo Glioma Perivascular Invasion, and Reduces Radiation Induced Apoptosis: A Genetic Knockdown Study. Oncotarget 2016, 7, 83701–83719. [Google Scholar] [CrossRef]
- Wang, D.; Jiao, C.; Zhu, Y.; Liang, D.; Zao, M.; Meng, X.; Gao, J.; He, Y.; Liu, W.; Hou, J.; et al. Activation of CXCL12/CXCR4 Renders Colorectal Cancer Cells Less Sensitive to Radiotherapy via up-Regulating the Expression of Survivin. Exp. Biol. Med. 2017, 242, 429–435. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, T.; Zhao, Y.; Huang, Y.; Gao, Y. Cisplatin Targets the Stromal Cell-Derived Factor-1-CXC Chemokine Receptor Type 4 Axis to Suppress Metastasis and Invasion of Ovarian Cancer-Initiating Cells. Tumor. Biol. 2014, 35, 4637–4644. [Google Scholar] [CrossRef]
- Wang, H.; Wu, B.; Wang, H. Alpha-Hederin Induces the Apoptosis of Oral Cancer SCC-25 Cells by Regulating PI3K/Akt/MTOR Signaling Pathway. Electron. J. Biotechnol. 2019, 38, 27–31. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Sig. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Shishodia, S.; Aggarwal, B.B. Nuclear Factor-KappaB Activation Mediates Cellular Transformation, Proliferation, Invasion Angiogenesis and Metastasis of Cancer. Cancer Treat. Res. 2004, 119, 139–173. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-ΚB, Inflammation, Immunity and Cancer: Coming of Age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Abouzed, T.K.; Beltagy, E.-E.R.; Kahilo, K.A.; Ibrahim, W.M. Molecular Changes Associated with the Anticancer Effect of Sulforaphane against Ehrlich Solid Tumour in Mice. J. Biochem. Mol. Toxicol. 2021, 35, e22655. [Google Scholar] [CrossRef]
- Aldubayan, M.A.; Elgharabawy, R.M.; Ahmed, A.S.; Tousson, E. Antineoplastic Activity and Curative Role of Avenanthramides against the Growth of Ehrlich Solid Tumors in Mice. Oxid. Med. Cell. Longev. 2019, 2019, e5162687. [Google Scholar] [CrossRef] [PubMed]
- Salah, R.; Salama, M.F.; Mahgoub, H.A.; El-Sherbini, E.-S. Antitumor Activity of Sitagliptin and Vitamin B12 on Ehrlich Ascites Carcinoma Solid Tumor in Mice. J. Biochem. Mol. Toxicol. 2021, 35, e22645. [Google Scholar] [CrossRef] [PubMed]
- Haffner, M.C.; Berlato, C.; Doppler, W. Exploiting Our Knowledge of NF-ΚB Signaling for the Treatment of Mammary Cancer. J. Mammary Gland Biol. Neoplasia 2006, 11, 63–73. [Google Scholar] [CrossRef]
- Kisseleva, T.; Song, L.; Vorontchikhina, M.; Feirt, N.; Kitajewski, J.; Schindler, C. NF-ΚB Regulation of Endothelial Cell Function during LPS-Induced Toxemia and Cancer. J. Clin. Investig. 2006, 116, 2955–2963. [Google Scholar] [CrossRef]
- Chen, S.-H.; Chang, J.-Y. New Insights into Mechanisms of Cisplatin Resistance: From Tumor Cell to Microenvironment. Int. J. Mol. Sci. 2019, 20, 4136. [Google Scholar] [CrossRef]
- Shen, D.-W.; Pouliot, L.M.; Hall, M.D.; Gottesman, M.M. Cisplatin Resistance: A Cellular Self-Defense Mechanism Resulting from Multiple Epigenetic and Genetic Changes. Pharmacol. Rev. 2012, 64, 706–721. [Google Scholar] [CrossRef]
- Aboseada, H.A.; Hassanien, M.M.; El-Sayed, I.H.; Saad, E.A. Schiff Base 4-Ethyl-1-(Pyridin-2-Yl) Thiosemicarbazide up-Regulates the Antioxidant Status and Inhibits the Progression of Ehrlich Solid Tumor in Mice. Biochem. Biophys. Res. Commun. 2021, 573, 42–47. [Google Scholar] [CrossRef]
- Mohamed, H.R.H.; Amer, M.; Faky, A.S.A.E. Growth Retardation and Apoptotic Death of Tumor Cells by Artemisia Herba-Alba Oral Administration in Ehrlich Solid Carcinoma Bearing Mice. Rev. Bras. Farmacogn. 2020, 29, 763–772. [Google Scholar] [CrossRef]
- Saad, E.A.; Elsayed, S.A.; Hassanien, M.M.; AL-Adl, M.S. The New Iron(III) 3-Oxo-N-(Pyridin-2-Yl)Butanamide Complex Promotes Ehrlich Solid Tumor Regression in Mice via Induction of Apoptosis. Appl. Organomet. Chem. 2020, 34, e5282. [Google Scholar] [CrossRef]
- Saad, E.A.; Hassanien, M.M.; El-lban, F.W. Nickel(II) Diacetyl Monoxime-2-Pyridyl Hydrazone Complex Can Inhibit Ehrlich Solid Tumor Growth in Mice: A Potential New Antitumor Drug. Biochem. Biophys. Res. Commun. 2017, 484, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Oiso, S.; Ikeda, R.; Nakamura, K.; Takeda, Y.; Akiyama, S.-I.; Kariyazono, H. Involvement of NF-ΚB Activation in the Cisplatin Resistance of Human Epidermoid Carcinoma KCP-4 Cells. Oncol. Rep. 2012, 28, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Ehab, T.; Ahmed, S.N.E.; Mona, E.M.; Haneen, H.M. Antineoplastic Activities of Grape Seed Proanthocyanidin Extract against Ehrlich Solid Tumor Bearing Mice Induced Alterations in AFP, CEA, TNF-α and DNA Damage. Asian Oncol. Res. J. 2019, 1–12. [Google Scholar]
- Feitosa, I.B.; Mori, B.; Teles, C.B.G.; da Costa, A.G. What Are the Immune Responses during the Growth of Ehrlich’s Tumor in Ascitic and Solid Form? Life Sci. 2021, 264, 118578. [Google Scholar] [CrossRef] [PubMed]
- Sharawi, Z.W. Therapeutic Effect of Arthrocnemum Machrostachyum Methanolic Extract on Ehrlich Solid Tumor in Mice. BMC Complement. Med. Ther. 2020, 20, 153. [Google Scholar] [CrossRef]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-Related Inflammation, the Seventh Hallmark of Cancer: Links to Genetic Instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef]
- Hagemann, T.; Lawrence, T.; McNeish, I.; Charles, K.A.; Kulbe, H.; Thompson, R.G.; Robinson, S.C.; Balkwill, F.R. “Re-Educating” Tumor-Associated Macrophages by Targeting NF-KappaB. J. Exp. Med. 2008, 205, 1261–1268. [Google Scholar] [CrossRef]
- Porta, C.; Rimoldi, M.; Raes, G.; Brys, L.; Ghezzi, P.; Di Liberto, D.; Dieli, F.; Ghisletti, S.; Natoli, G.; De Baetselier, P.; et al. Tolerance and M2 (Alternative) Macrophage Polarization Are Related Processes Orchestrated by P50 Nuclear Factor B. Proc. Natl. Acad. Sci. USA 2009, 106, 14978–14983. [Google Scholar] [CrossRef]
- Xia, Y.; Shen, S.; Verma, I.M. NF-ΚB, an Active Player in Human Cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Quetin-Leclercq, J.; Elias, R.; Balansard, G.; Bassleer, R.; Angenot, L. Cytotoxic Activity of Some Triterpenoid Saponins. Planta Med. 1992, 58, 279–281. [Google Scholar] [CrossRef]
- Danloy, S.; Quetin-Leclercq, J.; Coucke, P.; Pauw-Gillet, M.-C.D.; Elias, R.; Balansard, G.; Angenot, L.; Bassleer, R. Effects of α-Hederin, a Saponin Extracted from Hedera Helix, on Cells Cultured in Vitro. Planta Med. 1994, 60, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Rooney, S.; Ryan, M.F. Effects of Alpha-Hederin and Thymoquinone, Constituents of Nigella Sativa, on Human Cancer Cell Lines. Anticancer Res. 2005, 25, 2199–2204. [Google Scholar] [PubMed]
- Tian, Z.; Liu, Y.-M.; Chen, S.-B.; Yang, J.-S.; Xiao, P.-G.; Wang, L.; Wu, E. Cytotoxicity of Two Triterpenoids from Nigella Glandulifera. Molecules 2006, 11, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-H.; Xu, L.-Z.; Lin, J.; Yang, S.-L.; Feng, Y.-L. Triterpenoid Saponins from the Stems of Clematis Parviloba. J. Asian Nat. Prod. Res. 2009, 11, 332–338. [Google Scholar] [CrossRef]
- Kumara, S.S.; Huat, B.T. Extraction, Isolation and Characterisation of Antitumor Principle, Alpha-Hederin, from the Seeds of Nigella Sativa. Planta Med. 2001, 67, 29–32. [Google Scholar] [CrossRef]
- Feller, G.; Kugel, A.; Moonshine, D.; Chalifa-Caspi, V.; Scholz, M.; Prüfer, D.; Rabinski, T.; Müller, K.J.; Ofir, R. African Descents Are More Sensitive than European Descents to the Antitumor Compounds α-Hederin and Kalopanaxsaponin I. Planta Med. 2010, 76, 1847–1851. [Google Scholar] [CrossRef]
- Pasi, S.; Aligiannis, N.; Pratsinis, H.; Skaltsounis, A.-L.; Chinou, I.B. Biologically Active Triterpenoids from Cephalaria Ambrosioides. Planta Med. 2009, 75, 163–167. [Google Scholar] [CrossRef]
- Swamy, S.M.K.; Huat, B.T.K. Intracellular Glutathione Depletion and Reactive Oxygen Species Generation Are Important in Alpha-Hederin-Induced Apoptosis of P388 Cells. Mol. Cell Biochem. 2003, 245, 127–139. [Google Scholar] [CrossRef]
- Rooney, S.; Ryan, M.F. Modes of Action of Alpha-Hederin and Thymoquinone, Active Constituents of Nigella Sativa, against HEp-2 Cancer Cells. Anticancer Res. 2005, 25, 4255–4259. [Google Scholar]
- Ding, Q.; Yang, L.-X.; Yang, H.-W.; Jiang, C.; Wang, Y.-F.; Wang, S. Cytotoxic and Antibacterial Triterpenoids Derivatives from Clematis Ganpiniana. J. Ethnopharmacol. 2009, 126, 382–385. [Google Scholar] [CrossRef]
- Abd-Alhaseeb, M.M.; Zaitone, S.A.; Abou-El-Ela, S.H.; Moustafa, Y.M. Olmesartan Potentiates the Anti-Angiogenic Effect of Sorafenib in Mice Bearing Ehrlich’s Ascites Carcinoma: Role of Angiotensin (1–7). PLoS ONE 2014, 9, e85891. [Google Scholar] [CrossRef] [PubMed]
- Elmeligie, S.; Khalil, N.A.; Ahmed, E.M.; Emam, S.H.; Zaitone, S.A.-B. Synthesis of New N1-Substituted-5-Aryl-3-(3,4,5-Trimethoxyphenyl)-2-Pyrazoline Derivatives as Antitumor Agents Targeting the Colchicine Site on Tubulin. Biol. Pharm. Bull. 2016, 39, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, M.; El-Sayed, R.M.; Helal, M.A.; Ibrahiem, A.T.; Elmahdi, H.S.; Eladl, M.A.; Bilay, S.E.; Alshahrani, A.M.; Tawfik, M.K.; Hamed, Z.E.; et al. Nifuroxazide Mitigates Angiogenesis in Ehlrich’s Solid Carcinoma: Molecular Docking, Bioinformatic and Experimental Studies on Inhibition of Il-6/Jak2/Stat3 Signaling. Molecules 2021, 26, 6858. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.-G.; Zeng, W.; Wong, V.K.-W.; Zhu, Y.-Z.; Lo, A.C.Y.; Liu, L.; Law, B.Y.-K. Hederagenin and α-Hederin Promote Degradation of Proteins in Neurodegenerative Diseases and Improve Motor Deficits in MPTP-Mice. Pharmacol. Res. 2017, 115, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.M.; Zaitone, S.A.; Shouman, S.A.; Moustafa, Y.M. Dorzolamide Synergizes the Antitumor Activity of Mitomycin C against Ehrlich’s Carcinoma Grown in Mice: Role of Thioredoxin-Interacting Protein. Naunyn-Schmiedeberg’s Arch. Pharm. 2015, 388, 1271–1282. [Google Scholar] [CrossRef]
- Ali, S.A.; Zaitone, S.A.; Moustafa, Y.M. Boswellic Acids Synergize Antitumor Activity and Protect against the Cardiotoxicity of Doxorubicin in Mice Bearing Ehrlich’s Carcinoma. Can. J. Physiol. Pharmacol. 2015, 93, 695–708. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Vasudevan, P.; Zhong, L.; Kim, G.; Samant, S.; Parekh, V.; Pillai, V.B.; Ravindra, P.V.; Gupta, M.; Jeevanandam, V.; et al. The Sirtuin SIRT6 Blocks IGF-Akt Signaling and Development of Cardiac Hypertrophy by Targeting c-Jun. Nat. Med. 2012, 18, 1643–1650. [Google Scholar] [CrossRef]
- Mishra, S.; Tamta, A.K.; Sarikhani, M.; Desingu, P.A.; Kizkekra, S.M.; Pandit, A.S.; Kumar, S.; Khan, D.; Raghavan, S.C.; Sundaresan, N.R. Subcutaneous Ehrlich Ascites Carcinoma Mice Model for Studying Cancer-Induced Cardiomyopathy. Sci. Rep. 2018, 8, 5599. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| Gene | Forward | Reverse |
|---|---|---|
| TNF-α | AGAACTCCAGGCGGTGTCTGT | CCTTGTCCCTTGAAGAGAACC |
| NFκB | AATTGCCCCGGCAT | ATGCGCCAATGCCCT |
| Caspase 3 | ATGTCAGCTCGCAATGG | AAGAAATTATGGAATTG |
| GAPDH | TGGCACAGTCAAGGCTGAGA | CTTCTGAGTGGCAGTGATGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elaidy, S.M.; El-Kherbetawy, M.K.; Abed, S.Y.; Alattar, A.; Alshaman, R.; Eladl, M.A.; Alamri, E.S.; Al balawi, A.N.; Zaid, A.; Elkazzaz, A.Y.; et al. α-Hederin Saponin Augments the Chemopreventive Effect of Cisplatin against Ehrlich Tumors and Bioinformatic Approach Identifying the Role of SDF1/CXCR4/p-AKT-1/NFκB Signaling. Pharmaceuticals 2023, 16, 405. https://doi.org/10.3390/ph16030405
Elaidy SM, El-Kherbetawy MK, Abed SY, Alattar A, Alshaman R, Eladl MA, Alamri ES, Al balawi AN, Zaid A, Elkazzaz AY, et al. α-Hederin Saponin Augments the Chemopreventive Effect of Cisplatin against Ehrlich Tumors and Bioinformatic Approach Identifying the Role of SDF1/CXCR4/p-AKT-1/NFκB Signaling. Pharmaceuticals. 2023; 16(3):405. https://doi.org/10.3390/ph16030405
Chicago/Turabian StyleElaidy, Samah M., Mohamed K. El-Kherbetawy, Sally Y. Abed, Abdullah Alattar, Reem Alshaman, Mohamed Ahmed Eladl, Eman Saad Alamri, Aisha Nawaf Al balawi, AbdelNaser Zaid, Amany Y. Elkazzaz, and et al. 2023. "α-Hederin Saponin Augments the Chemopreventive Effect of Cisplatin against Ehrlich Tumors and Bioinformatic Approach Identifying the Role of SDF1/CXCR4/p-AKT-1/NFκB Signaling" Pharmaceuticals 16, no. 3: 405. https://doi.org/10.3390/ph16030405
APA StyleElaidy, S. M., El-Kherbetawy, M. K., Abed, S. Y., Alattar, A., Alshaman, R., Eladl, M. A., Alamri, E. S., Al balawi, A. N., Zaid, A., Elkazzaz, A. Y., Abdelkhalig, S. M., Hamed, Z. E., & Zaitone, S. A. (2023). α-Hederin Saponin Augments the Chemopreventive Effect of Cisplatin against Ehrlich Tumors and Bioinformatic Approach Identifying the Role of SDF1/CXCR4/p-AKT-1/NFκB Signaling. Pharmaceuticals, 16(3), 405. https://doi.org/10.3390/ph16030405









