A Biflavonoid-Rich Extract from Selaginella doederleinii Hieron. against Throat Carcinoma via Akt/Bad and IKKβ/NF-κB/COX-2 Pathways
Abstract
1. Introduction
2. Results
2.1. UPLC-Q-TOF-MS Analysis of SD-BFRE
2.2. SD-BFRE Inhibits Cell Viability and Alters Cell Morphology in TC Cells
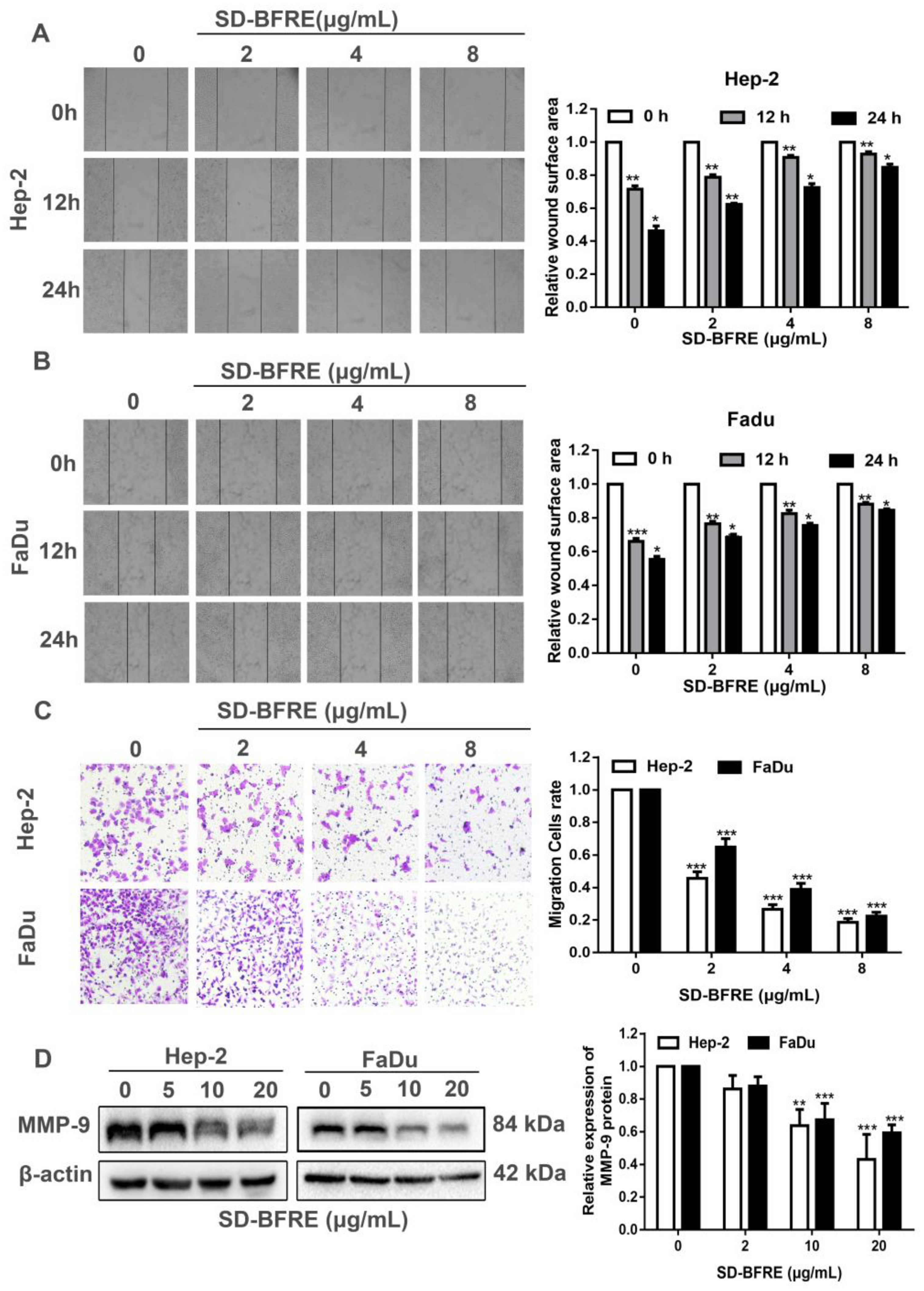
2.3. Effects of SD-BFRE on Cell Migration
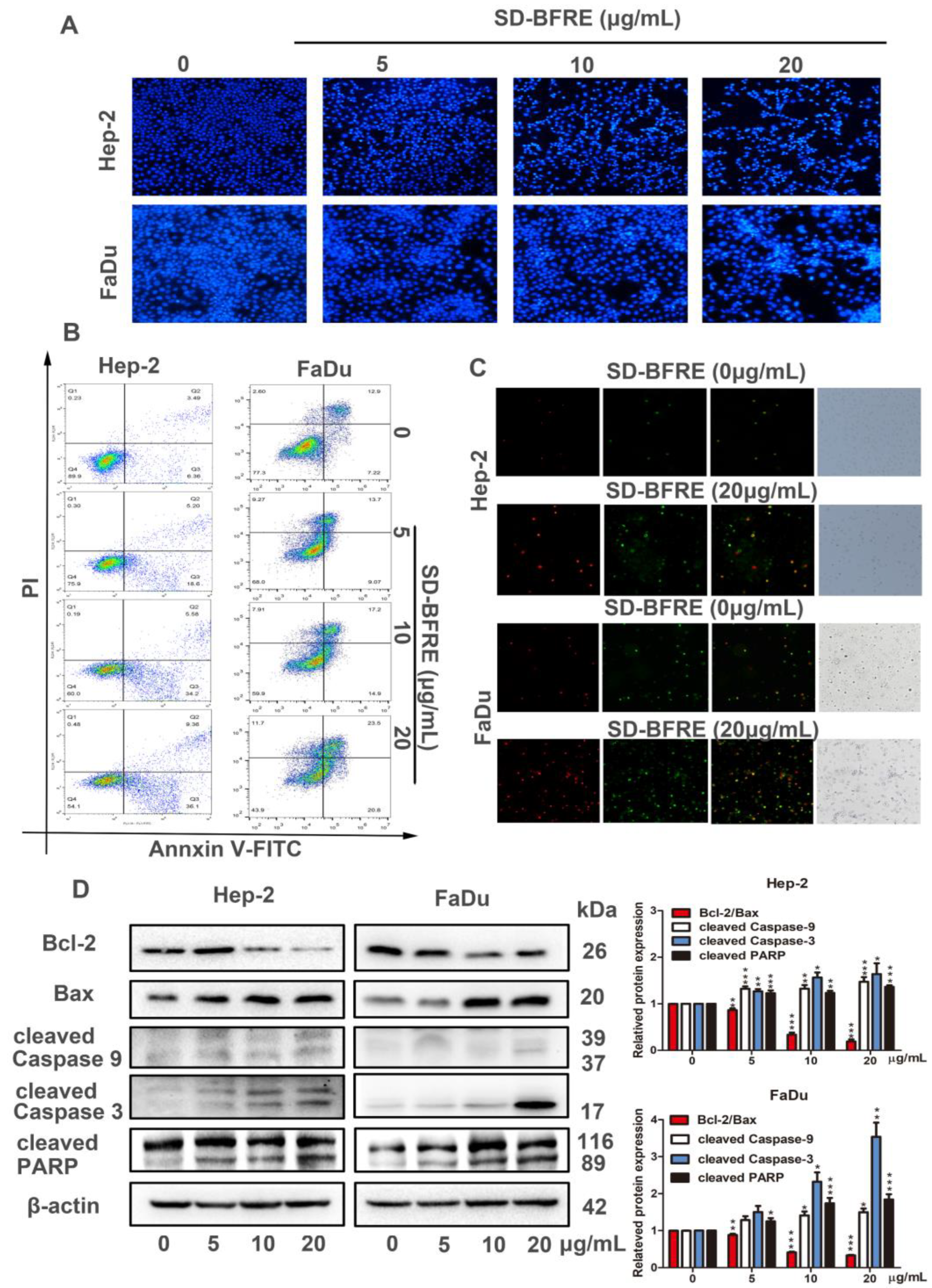
2.4. SD-BFRE Induces Apoptosis in TC Cells
2.5. SD-BFRE Inhibited Hep-2 Xenograft Growth and Induced Apoptosis In Vivo
2.6. SD-BFRE Inhibits NF-κB p65 and COX-2 Signaling In Vitro and In Vivo
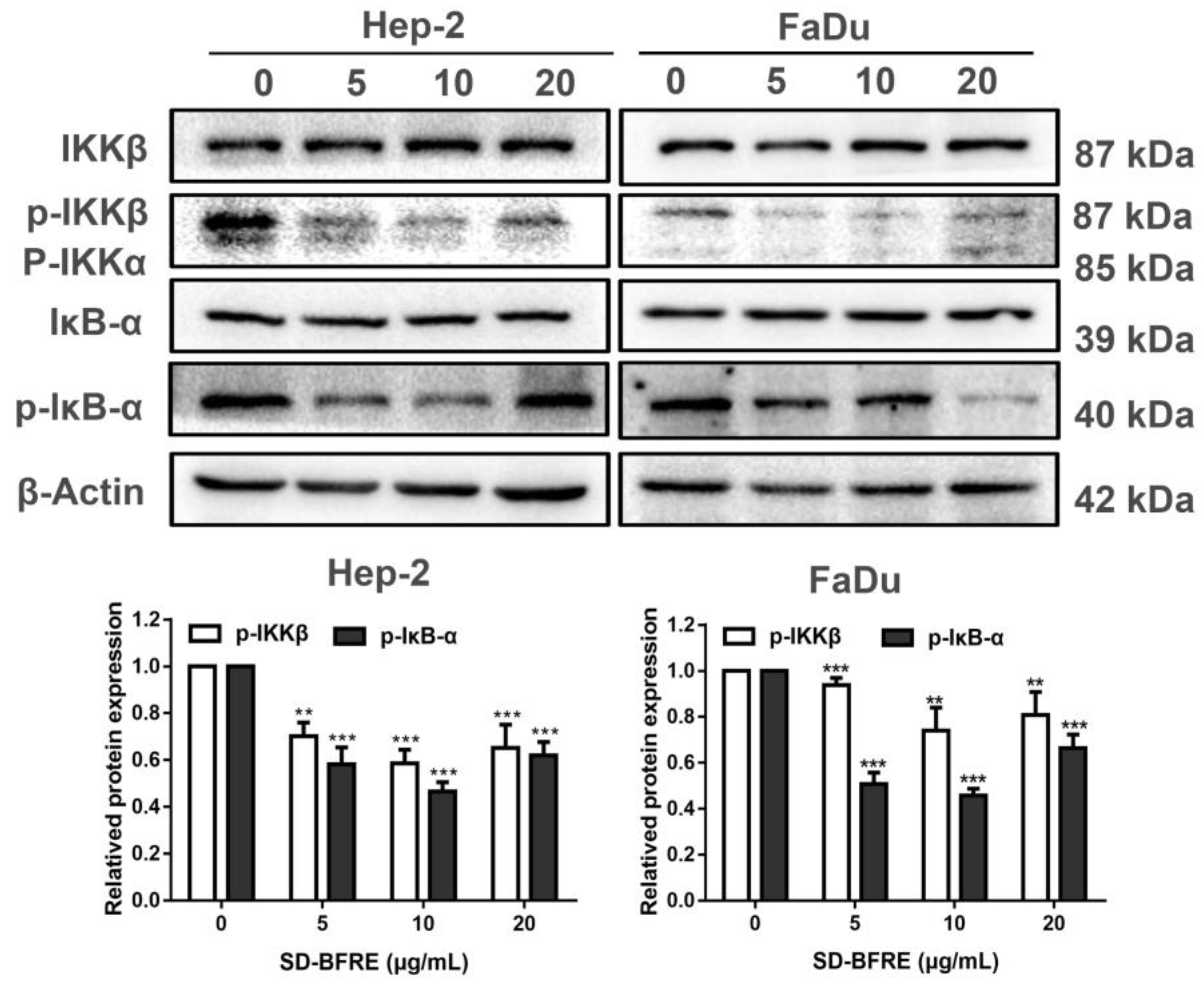
2.7. SD-BFRE Suppresses the NF-κB p65 Transcription by Regulating IKKβ Kinase In Vitro
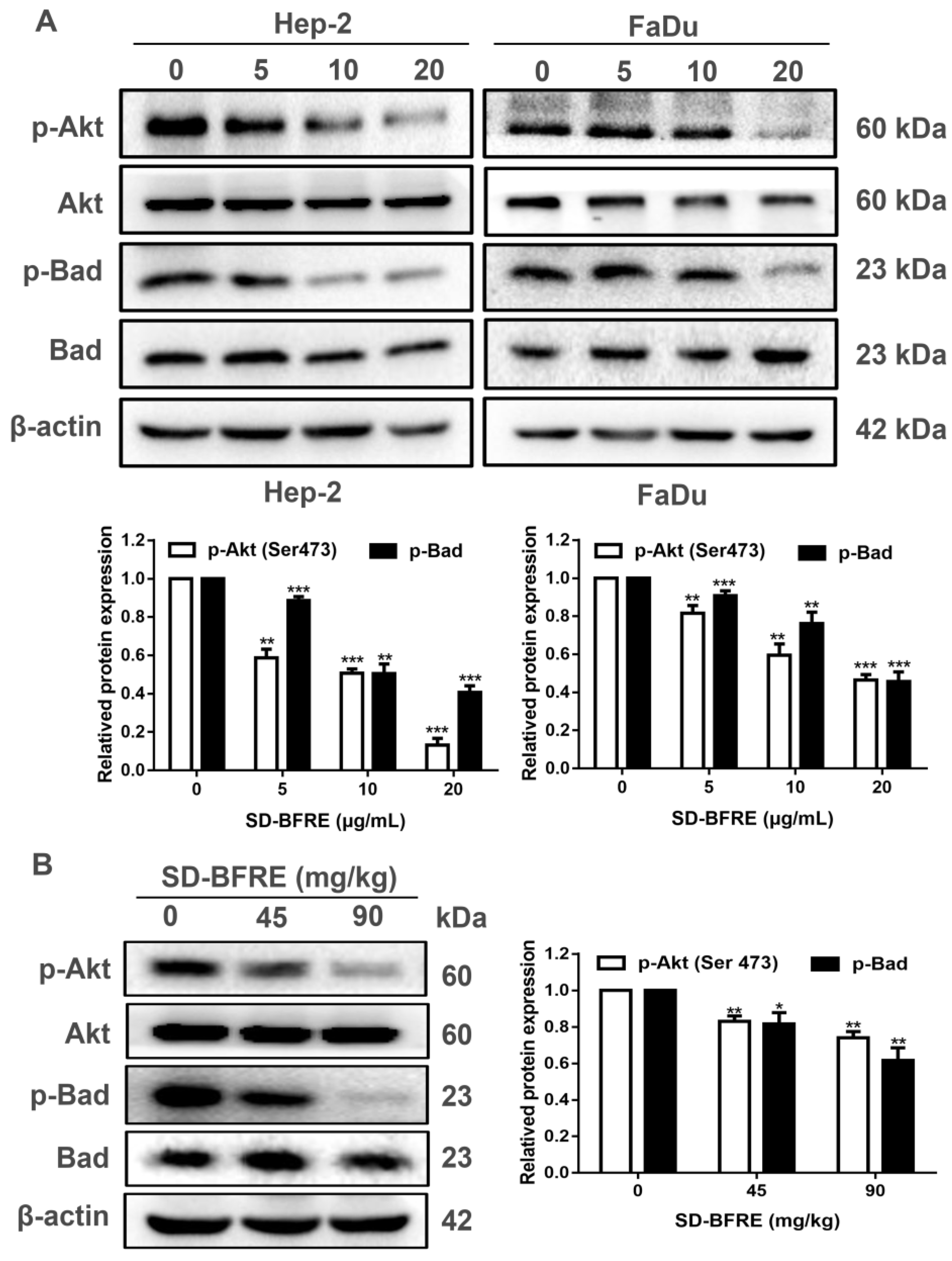
2.8. SD-BFRE Induces Apoptosis by Modulating the Akt/Bad Pathway In Vivo and In Vitro
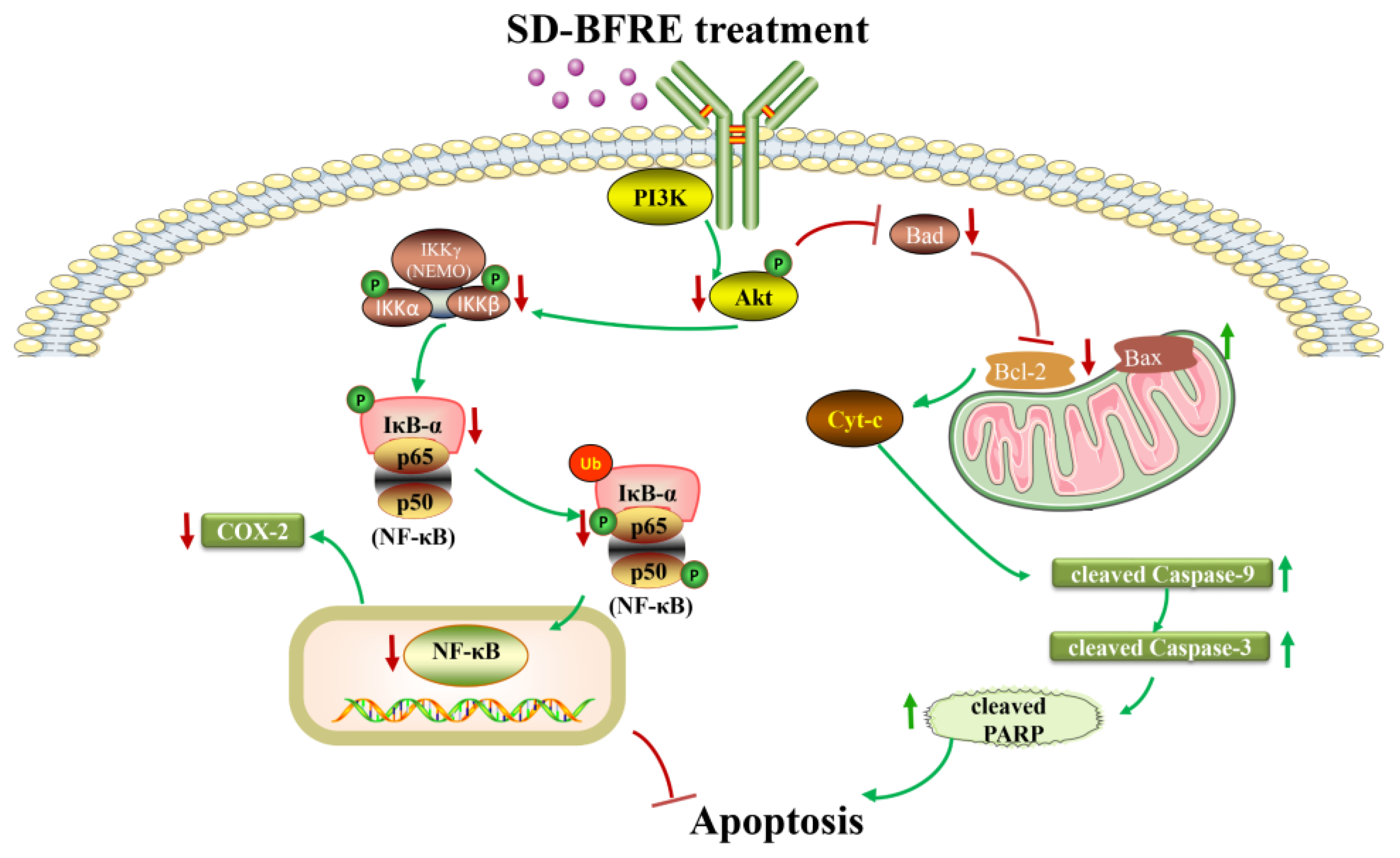
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Preparation and Chemical Elucidation of SD-BFRE
4.4. Cell Culture
4.5. Cytotoxicity by MTT Assay
4.6. Cell Migration Assays
4.7. Morphology Observation and Hoechst 33258 Staining
4.8. Annexin V-FITC/PI Staining Assay
4.9. Proteins Extracts and Western Blot Analysis
4.10. Tumor-Bearing Nude Mice Models and In Vivo Treatment
4.11. TUNEL Staining
4.12. Immunohistochemical Examinations of Transplanted Tumor Tissues (IHC)
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Katada, C.; Yokoyama, T.; Yano, T.; Kaneko, K.; Oda, I.; Shimizu, Y.; Doyama, H.; Koike, T.; Takizawa, K.; Hirao, M.; et al. Alcohol Consumption and Multiple Dysplastic Lesions Increase Risk of Squamous Cell Carcinoma in the Esophagus, Head, and Neck. Gastroenterology 2016, 151, 860–869 e867. [Google Scholar] [CrossRef] [PubMed]
- Calkovsky, V.; Wallenfels, P.; Calkovska, A.; Hajtman, A. Laryngeal Cancer: 12-Year Experience of a Single Center. Adv. Exp. Med. Biol. 2016, 911, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Bravi, F.; Lee, Y.A.; Hashibe, M.; Boffetta, P.; Conway, D.I.; Ferraroni, M.; La Vecchia, C.; Edefonti, V.; Investigators, I.C. Lessons learned from the INHANCE consortium: An overview of recent results on head and neck cancer. Oral Dis. 2021, 27, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, M.R.; Blakaj, A.; Blakaj, D. Organ preservation for advanced larynx cancer: A review of chemotherapy and radiation combination strategies. Oral Oncol. 2018, 86, 301–306. [Google Scholar] [CrossRef]
- Shibata, H.; Zhou, L.; Xu, N.; Egloff, A.M.; Uppaluri, R. Personalized cancer vaccination in head and neck cancer. Cancer Sci. 2021, 112, 978–988. [Google Scholar] [CrossRef]
- Wei, D.; Xu, J.; Liu, X.-Y.; Chen, Z.-N.; Bian, H. Fighting Cancer with Viruses: Oncolytic Virus Therapy in China. Hum. Gene Ther. 2018, 29, 151–159. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, H.; Li, C.; Cai, P.; Qi, F. The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. Biosci. Trends 2021, 15, 283–298. [Google Scholar] [CrossRef]
- McCulloch, M.; See, C.; Shu, X.J.; Broffman, M.; Kramer, A.; Fan, W.Y.; Gao, J.; Lieb, W.; Shieh, K.; Colford, J.M., Jr. Astragalus-based Chinese herbs and platinum-based chemotherapy for advanced non-small-cell lung cancer: Meta-analysis of randomized trials. J. Clin. Oncol. 2006, 24, 419–430. [Google Scholar] [CrossRef]
- So, T.H.; Chan, S.K.; Lee, V.H.; Chen, B.Z.; Kong, F.M.; Lao, L.X. Chinese Medicine in Cancer Treatment—How is it Practised in the East and the West? Clin. Oncol. 2019, 31, 578–588. [Google Scholar] [CrossRef]
- Zhang, Y.; Lou, Y.; Wang, J.; Yu, C.; Shen, W. Research Status and Molecular Mechanism of the Traditional Chinese Medicine and Antitumor Therapy Combined Strategy Based on Tumor Microenvironment. Front. Immunol. 2020, 11, 609705. [Google Scholar] [CrossRef]
- Lu, A.P.; Jia, H.W.; Xiao, C.; Lu, Q.P. Theory of traditional Chinese medicine and therapeutic method of diseases. World J. Gastroenterol. 2004, 10, 1854–1856. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Zhao, L.; Zhou, A.; Zhang, B.; Li, A.; Wang, Z.; Han, J. The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. Biosci. Trends 2015, 9, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Chen, B.; Zhang, Y.; Ou, H.; Li, Y.; Li, S.; Shi, P.; Lin, X. Analysis of the Total Biflavonoids Extract from Selaginella doederleinii by HPLC-QTOF-MS and Its In Vitro and In Vivo Anticancer Effects. Molecules 2017, 22, 325. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.P.; Dai, S.; Chen, R.S. Dictionary of Traditional Chinese Medicine, 1st ed.; Shanghai Science and Technology Press: Shanghai, China, 2006; pp. 831–832. [Google Scholar]
- Li, D.; Qian, Y.; Tian, Y.J.; Yuan, S.M.; Wei, W.; Wang, G. Optimization of Ionic Liquid-Assisted Extraction of Biflavonoids from Selaginella doederleinii and Evaluation of Its Antioxidant and Antitumor Activity. Molecules 2017, 22, 586. [Google Scholar] [CrossRef]
- Liu, H.; Peng, H.; Ji, Z.; Zhao, S.; Zhang, Y.; Wu, J.; Fan, J.; Liao, J. Reactive oxygen species-mediated mitochondrial dysfunction is involved in apoptosis in human nasopharyngeal carcinoma CNE cells induced by Selaginella doederleinii extract. J. Ethnopharmacol. 2011, 138, 184–191. [Google Scholar] [CrossRef]
- Sui, Y.; Li, S.; Shi, P.; Wu, Y.; Li, Y.; Chen, W.; Huang, L.; Yao, H.; Lin, X. Ethyl acetate extract from Selaginella doederleinii Hieron inhibits the growth of human lung cancer cells A549 via caspase-dependent apoptosis pathway. J. Ethnopharmacol. 2016, 190, 261–271. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Wang, G.; Shi, P.; Lin, S.; Xu, D.; Chen, B.; Liu, A.; Huang, L.; Lin, X.; et al. Ethyl Acetate Extract of Selaginella doederleinii Hieron Induces Cell Autophagic Death and Apoptosis in Colorectal Cancer via PI3K-Akt-mTOR and AMPKalpha-Signaling Pathways. Front. Pharmacol. 2020, 11, 565090. [Google Scholar] [CrossRef]
- Wang, J.Z.; Li, J.; Zhao, P.; Ma, W.T.; Feng, X.H.; Chen, K.L. Antitumor Activities of Ethyl Acetate Extracts from Selaginella doederleinii Hieron In Vitro and In Vivo and Its Possible Mechanism. Evid. Based Complement. Altern. Med. 2015, 2015, 865714. [Google Scholar] [CrossRef]
- Lin, L.C.; Kuo, Y.C.; Chou, C.J. Cytotoxic biflavonoids from Selaginella delicatula. J. Nat. Prod. 2000, 63, 627–630. [Google Scholar] [CrossRef]
- Chen, M.Y.; Wang, S.S.; Cheng, H.T.; Wan, D.R.; Lu, R.M.; Yang, X.Z. Seladoeflavones G-I, Three New flavonoids fromSelaginella doederleiniiHieron. ChemistrySelect 2022, 7, e202202242. [Google Scholar] [CrossRef]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, Z.; Wang, C.; Cheng, W.; Tian, X.; Huo, X.; Wang, Y.; Sun, C.; Feng, L.; Xing, J.; et al. Alantolactone, a natural sesquiterpene lactone, has potent antitumor activity against glioblastoma by targeting IKKbeta kinase activity and interrupting NF-kappaB/COX-2-mediated signaling cascades. J. Exp. Clin. Cancer Res. 2017, 36, 93. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, B.; Schmid, J.A. The complexity of NF-kappaB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef]
- Whiteside, S.T.; Epinat, J.C.; Rice, N.R.; Israel, A. I kappa B epsilon, a novel member of the I kappa B family, controls RelA and cRel NF-kappa B activity. EMBO J. 1997, 16, 1413–1426. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, Q.; Chen, H.; Guo, Q.; Zhang, L.; Zhang, Z.; Li, Y. Astragaloside IV enhanced carboplatin sensitivity in prostate cancer by suppressing AKT/NF-kappaB signaling pathway. Biochem. Cell Biol. 2021, 99, 214–222. [Google Scholar] [CrossRef]
- Kramer, N.; Walzl, A.; Unger, C.; Rosner, M.; Krupitza, G.; Hengstschlager, M.; Dolznig, H. In vitro cell migration and invasion assays. Mutat. Res. 2013, 752, 10–24. [Google Scholar] [CrossRef]
- Webb, A.H.; Gao, B.T.; Goldsmith, Z.K.; Irvine, A.S.; Saleh, N.; Lee, R.P.; Lendermon, J.B.; Bheemreddy, R.; Zhang, Q.; Brennan, R.C.; et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer 2017, 17, 434. [Google Scholar] [CrossRef]
- Goldar, S.; Khaniani, M.S.; Derakhshan, S.M.; Baradaran, B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac. J. Cancer Prev. 2015, 16, 2129–2144. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Kaczanowski, S. Apoptosis: Its origin, history, maintenance and the medical implications for cancer and aging. Phys. Biol. 2016, 13, 031001. [Google Scholar] [CrossRef] [PubMed]
- Villanova, L.; Careccia, S.; De Maria, R.; Fiori, M.E. Micro-Economics of Apoptosis in Cancer: NcRNAs Modulation of BCL-2 Family Members. Int. J. Mol. Sci. 2018, 19, 958. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, A.V.; Shilov, E.S.; Mufazalov, I.A.; Gogvadze, V.; Nedospasov, S.A.; Zhivotovsky, B. Cytochrome c: The Achilles’ heel in apoptosis. Cell. Mol. Life Sci. 2012, 69, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wei, M.; Xu, J.; Hua, J.; Liang, C.; Meng, Q.; Zhang, Y.; Liu, J.; Zhang, B.; Yu, X.; et al. PARP inhibitors in pancreatic cancer: Molecular mechanisms and clinical applications. Mol. Cancer 2020, 19, 49. [Google Scholar] [CrossRef]
- Adjei, A.A.; Hidalgo, M. Intracellular signal transduction pathway proteins as targets for cancer therapy. J. Clin. Oncol. 2005, 23, 5386–5403. [Google Scholar] [CrossRef]
- Ashkenazi, A. Targeting the extrinsic apoptotic pathway in cancer: Lessons learned and future directions. J. Clin. Investig. 2015, 125, 487–489. [Google Scholar] [CrossRef]
- Jung, Y.J.; Lee, E.H.; Lee, C.G.; Rhee, K.J.; Jung, W.S.; Choi, Y.; Pan, C.H.; Kang, K. AKR1B10-inhibitory Selaginella tamariscina extract and amentoflavone decrease the growth of A549 human lung cancer cells in vitro and in vivo. J. Ethnopharmacol. 2017, 202, 78–84. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, J.; Chen, X.; Fortenbery, N.; Eksioglu, E.; Kodumudi, K.N.; Pk, E.B.; Dong, J.; Djeu, J.Y.; Wei, S. Icariin and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int. Immunopharmacol. 2011, 11, 890–898. [Google Scholar] [CrossRef]
- Todoric, J.; Antonucci, L.; Karin, M. Targeting Inflammation in Cancer Prevention and Therapy. Cancer Prev. Res. 2016, 9, 895–905. [Google Scholar] [CrossRef]
- de Castro Barbosa, M.L.; da Conceicao, R.A.; Fraga, A.G.M.; Camarinha, B.D.; de Carvalho Silva, G.C.; Lima, A.G.F.; Cardoso, E.A.; de Oliveira Freitas Lione, V. NF-kappaB Signaling Pathway Inhibitors as Anticancer Drug Candidates. Anticancer Agents Med. Chem. 2017, 17, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lei, Y.; Chen, L.; Zhou, H.; Liu, H.; Jiang, J.; Yang, Y.; Wu, B. Phosphorylation of NF-kappaBp65 drives inflammation-mediated hepatocellular carcinogenesis and is a novel therapeutic target. J. Exp. Clin. Cancer Res. 2021, 40, 253. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Guo, F.; Tao, S.; Huang, R.; Ma, L.; Fu, P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-kappaB p65 and MAPK signaling pathways in septic AKI mice. Biomed. Pharmacother. 2020, 122, 109772. [Google Scholar] [CrossRef] [PubMed]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-kB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Viatour, P.; Merville, M.P.; Bours, V.; Chariot, A. Phosphorylation of NF-kappaB and IkappaB proteins: Implications in cancer and inflammation. Trends Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef]
- Greten, F.R.; Eckmann, L.; Greten, T.F.; Park, J.M.; Li, Z.W.; Egan, L.J.; Kagnoff, M.F.; Karin, M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004, 118, 285–296. [Google Scholar] [CrossRef]
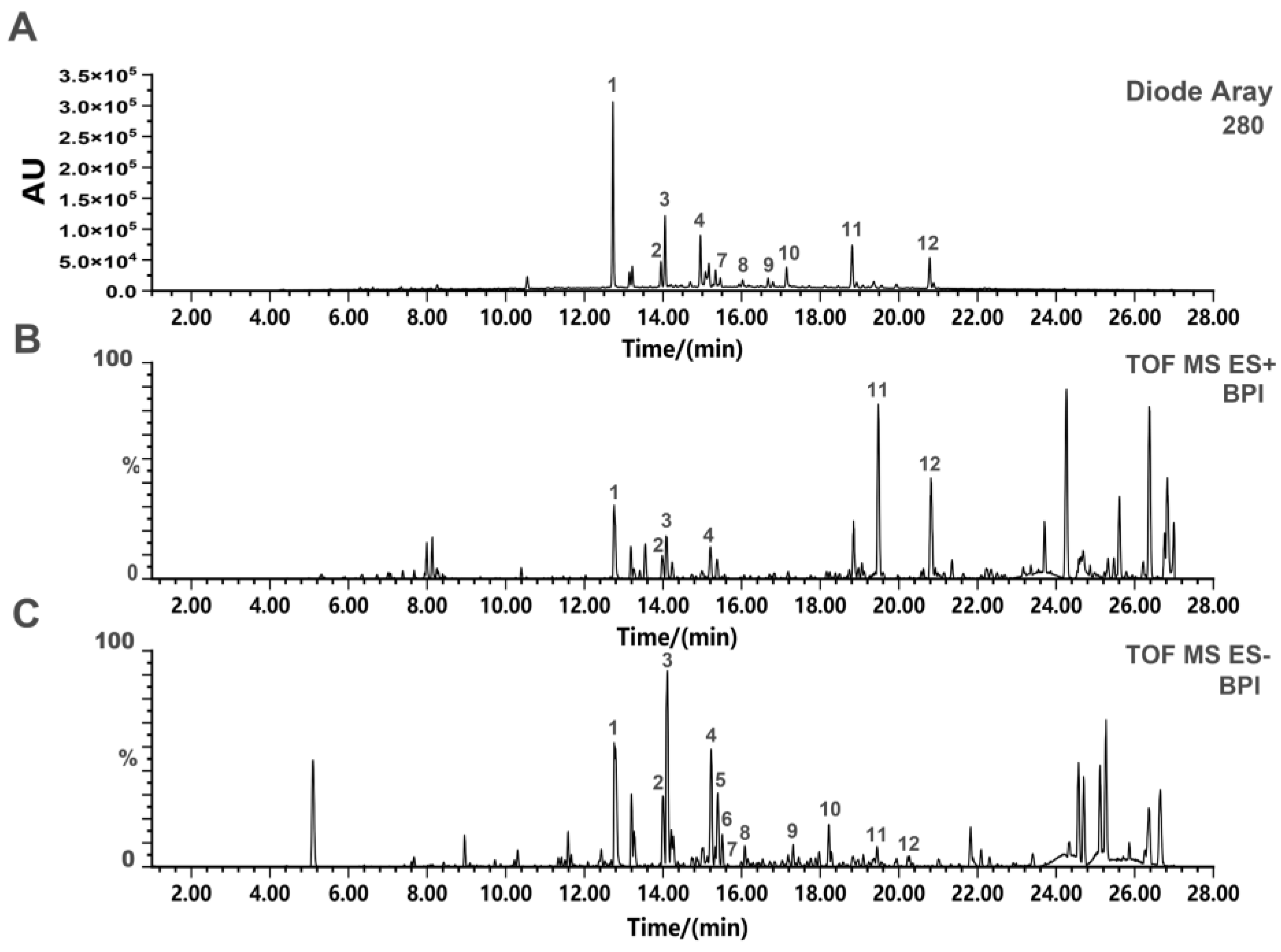



| No. | tR | Molecular | Ion Type | Molecular | Exact Mass | Compound | Reference |
|---|---|---|---|---|---|---|---|
| (min) | Iron (m/z) | Formula | |||||
| 1 | 12.81 | 537.0807 | [M-H]− | C30H18O10 | 538.0900 | Amentoflavone | [14] |
| 2 | 13.99 | 538.0969 | [M-H]− | C30H19O10 | 539.1056 | 2,3-Dihydro-3′,3′’’’-biapigenin | [14] |
| 3 | 14.06 | 541.1167 | [M-H]− | C30H22O10 | 542.1213 | 2,3,2′’,3′’-Tetrahydroochnaflavone | [22] |
| 4 | 15.00 | 539.1029 | [M-H]− | C30H20O10 | 540.1055 | 2,3-dihydroochnaflavone | [22] |
| 5 | 15.21 | 537.1248 | [M-H]− | C30H18O10 | 538.1369 | Delicaflavone | [22] |
| 6 | 15.22 | 537.1096 | [M-H]− | C30H18O10 | 538.1269 | Ochnaflavone | [22] |
| 7 | 15.37 | 537.0983 | [M-H]− | C30H18O10 | 538.1086 | Hinokifllavone | [14] |
| 8 | 16.06 | 551.0997 | [M-H]− | C31H20O10 | 552.1213 | Bilobetin | [14] |
| 9 | 16.84 | 565.1285 | [M-H]− | C32H22O10 | 566.1365 | Ginkgetin | [21] |
| 10 | 17.18 | 551.0983 | [M-H]− | C31H20O10 | 552.1666 | Podocarpusflavone A | [21] |
| 11 | 18.83 | 579.1281 | [M-H]− | C33H24O10 | 580.1395 | Heveaflavone | [14] |
| 12 | 20.82 | 593.1569 | [M-H]− | C34H26O10 | 594.1526 | 7,4′,7′’,4′’’-Tetra | [14] |
| O-methyl-amentoflavone |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wan, D.; Liu, W.; Kang, X.; Zhou, X.; Sefidkon, F.; Hosseini, M.M.Z.; Zhang, T.; Pan, X.; Yang, X. A Biflavonoid-Rich Extract from Selaginella doederleinii Hieron. against Throat Carcinoma via Akt/Bad and IKKβ/NF-κB/COX-2 Pathways. Pharmaceuticals 2022, 15, 1505. https://doi.org/10.3390/ph15121505
Wang S, Wan D, Liu W, Kang X, Zhou X, Sefidkon F, Hosseini MMZ, Zhang T, Pan X, Yang X. A Biflavonoid-Rich Extract from Selaginella doederleinii Hieron. against Throat Carcinoma via Akt/Bad and IKKβ/NF-κB/COX-2 Pathways. Pharmaceuticals. 2022; 15(12):1505. https://doi.org/10.3390/ph15121505
Chicago/Turabian StyleWang, Sisi, Dingrong Wan, Wenqi Liu, Xinyi Kang, Xiuteng Zhou, Fatemeh Sefidkon, Mohaddesehossadat Mahmoud Zadeh Hosseini, Ting Zhang, Xin Pan, and Xinzhou Yang. 2022. "A Biflavonoid-Rich Extract from Selaginella doederleinii Hieron. against Throat Carcinoma via Akt/Bad and IKKβ/NF-κB/COX-2 Pathways" Pharmaceuticals 15, no. 12: 1505. https://doi.org/10.3390/ph15121505
APA StyleWang, S., Wan, D., Liu, W., Kang, X., Zhou, X., Sefidkon, F., Hosseini, M. M. Z., Zhang, T., Pan, X., & Yang, X. (2022). A Biflavonoid-Rich Extract from Selaginella doederleinii Hieron. against Throat Carcinoma via Akt/Bad and IKKβ/NF-κB/COX-2 Pathways. Pharmaceuticals, 15(12), 1505. https://doi.org/10.3390/ph15121505




