Pembrolizumab-Associated Cardiotoxicity: A Retrospective Analysis of the FDA Adverse Events Reporting System
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Data Source and Extraction Criteria
4.2. Data Analysis—Descriptive Analysis
4.3. Data Analysis—Disproportionality Analysis
- ROR025 (lower limit of the 95% confidence interval of ROR) > 1 and adverse events > 3;
- IC025 (lower limit of the 95% credibility interval of IC) > 0.
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teixidó, C.; Vilariño, N.; Reyes, R.; Reguart, N. PD-L1 Expression Testing in Non-Small Cell Lung Cancer. Ther. Adv. Med. Oncol. 2018, 10, 1758835918763493. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Workman, C.J.; Vignali, D.A.A. LAG-3 as the Third Checkpoint Inhibitor. Nat. Immunol. 2023, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Laenens, D.; Yu, Y.; Santens, B.; Jacobs, J.; Beuselinck, B.; Bechter, O.; Wauters, E.; Staessen, J.; Janssens, S.; Van Aelst, L. Incidence of Cardiovascular Events in Patients Treated With Immune Checkpoint Inhibitors. J. Clin. Oncol. 2022, 40, 3430–3438. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wu, X. Cardiovascular Toxicities Associated with Immune Checkpoint Inhibitors: An Updated Comprehensive Disproportionality Analysis of the FDA Adverse Event Reporting System. J. Clin. Pharm. Ther. 2022, 47, 1576–1584. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.-E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Salem, J.-E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.-P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular Toxicities Associated with Immune Checkpoint Inhibitors: An Observational, Retrospective, Pharmacovigilance Study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Michel, L.; Rassaf, T.; Totzeck, M. Cardiotoxicity from Immune Checkpoint Inhibitors. Int. J. Cardiol. Heart Vasc. 2019, 25, 100420. [Google Scholar] [CrossRef] [PubMed]
- Lal, J.C.; Brown, S.-A.; Collier, P.; Cheng, F. A Retrospective Analysis of Cardiovascular Adverse Events Associated with Immune Checkpoint Inhibitors. Cardiooncology 2021, 7, 19. [Google Scholar] [CrossRef]
- Chen, C.; Chen, T.; Liang, J.; Guo, X.; Xu, J.; Zheng, Y.; Guo, Z.; Chi, L.; Wei, L.; Chen, X.; et al. Cardiotoxicity Induced by Immune Checkpoint Inhibitors: A Pharmacovigilance Study from 2014 to 2019 Based on FAERS. Front. Pharmacol. 2021, 12, 616505. [Google Scholar] [CrossRef]
- Dolladille, C.; Akroun, J.; Morice, P.-M.; Dompmartin, A.; Ezine, E.; Sassier, M.; Da-Silva, A.; Plane, A.-F.; Legallois, D.; L’Orphelin, J.-M.; et al. Cardiovascular Immunotoxicities Associated with Immune Checkpoint Inhibitors: A Safety Meta-Analysis. Eur. Heart J. 2021, 42, 4964–4977. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.A.; Sorbara, E.E.; Cicala, G.; Santoro, V.; Cutroneo, P.M.; Franchina, T.; Spina, E. Adverse Drug Reactions with HER2-Positive Breast Cancer Treatment: An Analysis from the Italian Pharmacovigilance Database. Drugs Real World Outcomes 2022, 9, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Wittayanukorn, S.; Qian, J.; Johnson, B.S.; Hansen, R.A. Cardiotoxicity in Targeted Therapy for Breast Cancer: A Study of the FDA Adverse Event Reporting System (FAERS). J. Oncol. Pharm. Pract. 2017, 23, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Long, P.; Li, S.; Pan, L.; Wang, Y.; Chen, W.; Wang, X. Cardiovascular Adverse Events Associated with Antibody-Drug Conjugates (ADCs): A Pharmacovigilance Study Based on the FAERS Database. Front. Pharmacol. 2024, 15, 1378010. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research. FDA Adverse Event Reporting System (FAERS); FDA: Silver Spring, MD, USA, 2021. [Google Scholar]
- Lee, L.; Gupta, M.; Sahasranaman, S. Immune Checkpoint Inhibitors: An Introduction to the next-Generation Cancer Immunotherapy. J. Clin. Pharmacol. 2016, 56, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune Checkpoint Inhibitors: Recent Progress and Potential Biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Abou Alaiwi, S.; Xie, W.; Nassar, A.H.; Dudani, S.; Martini, D.; Bakouny, Z.; Steinharter, J.A.; Nuzzo, P.V.; Flippot, R.; Martinez-Chanza, N.; et al. Safety and Efficacy of Restarting Immune Checkpoint Inhibitors after Clinically Significant Immune-Related Adverse Events in Metastatic Renal Cell Carcinoma. J. Immunother. Cancer 2020, 8, e000144. [Google Scholar] [CrossRef]
- Lee, D.J.; Lee, H.J.; Farmer, J.R.; Reynolds, K.L. Mechanisms Driving Immune-Related Adverse Events in Cancer Patients Treated with Immune Checkpoint Inhibitors. Curr. Cardiol. Rep. 2021, 23, 98. [Google Scholar] [CrossRef]
- De Velasco, G.; Je, Y.; Bossé, D.; Awad, M.M.; Ott, P.A.; Moreira, R.B.; Schutz, F.; Bellmunt, J.; Sonpavde, G.P.; Hodi, F.S.; et al. Comprehensive Meta-Analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol. Res. 2017, 5, 312–318. [Google Scholar] [CrossRef]
- Tachibana, M.; Imagawa, A. Type 1 Diabetes Related to Immune Checkpoint Inhibitors. Best. Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101657. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-Analysis. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Vandiver, J.W.; Singer, Z.; Harshberger, C. Severe Hyponatremia and Immune Nephritis Following an Initial Infusion of Nivolumab. Target. Oncol. 2016, 11, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, S.; Neilan, T.G. Immune Checkpoint Inhibitor-Associated Myocarditis. Oncologist 2018, 23, 879–886. [Google Scholar] [CrossRef]
- Totzeck, M.; Lutgens, E.; Neilan, T.G. Are We Underestimating the Potential for Cardiotoxicity Related to Immune Checkpoint Inhibitors? Eur. Heart J. 2021, 42, 1632–1635. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated with Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef]
- Tarrio, M.L.; Grabie, N.; Bu, D.; Sharpe, A.H.; Lichtman, A.H. PD-1 Protects against Inflammation and Myocyte Damage in T Cell-Mediated Myocarditis. J. Immunol. 2012, 188, 4876–4884. [Google Scholar] [CrossRef]
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune Dilated Cardiomyopathy in PD-1 Receptor-Deficient Mice. Science 2001, 291, 319–322. [Google Scholar] [CrossRef]
- Okazaki, T.; Tanaka, Y.; Nishio, R.; Mitsuiye, T.; Mizoguchi, A.; Wang, J.; Ishida, M.; Hiai, H.; Matsumori, A.; Minato, N.; et al. Autoantibodies against Cardiac Troponin I Are Responsible for Dilated Cardiomyopathy in PD-1-Deficient Mice. Nat. Med. 2003, 9, 1477–1483. [Google Scholar] [CrossRef]
- Axelrod, M.L.; Meijers, W.C.; Screever, E.M.; Qin, J.; Carroll, M.G.; Sun, X.; Tannous, E.; Zhang, Y.; Sugiura, A.; Taylor, B.C.; et al. T Cells Specific for α-Myosin Drive Immunotherapy-Related Myocarditis. Nature 2022, 611, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Tay, W.T.; Fang, Y.-H.; Beh, S.T.; Liu, Y.-W.; Hsu, L.-W.; Yen, C.-J.; Liu, P.-Y. Programmed Cell Death-1: Programmed Cell Death-Ligand 1 Interaction Protects Human Cardiomyocytes Against T-Cell Mediated Inflammation and Apoptosis Response In Vitro. Int. J. Mol. Sci. 2020, 21, 2399. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, F.; Poto, R.; Aran, L.; Cuomo, A.; Galdiero, M.R.; Spadaro, G.; Abete, P.; Bonaduce, D.; Marone, G.; Tocchetti, C.G.; et al. Cardiovascular Toxicity of Immune Checkpoint Inhibitors: Clinical Risk Factors. Curr. Oncol. Rep. 2021, 23, 13. [Google Scholar] [CrossRef]
- Rubio-Infante, N.; Ramírez-Flores, Y.A.; Castillo, E.C.; Lozano, O.; García-Rivas, G.; Torre-Amione, G. Cardiotoxicity Associated with Immune Checkpoint Inhibitor Therapy: A Meta-Analysis. Eur. J. Heart Fail. 2021, 23, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Drobni, Z.D.; Zafar, A.; Gongora, C.A.; Zlotoff, D.A.; Alvi, R.M.; Taron, J.; Rambarat, P.K.; Schoenfeld, S.; Mosarla, R.C.; et al. Pre-Existing Autoimmune Disease Increases the Risk of Cardiovascular and Noncardiovascular Events After Immunotherapy. JACC CardioOncol. 2022, 4, 660–669. [Google Scholar] [CrossRef]
- Xie, W.; Huang, H.; Xiao, S.; Fan, Y.; Deng, X.; Zhang, Z. Immune Checkpoint Inhibitors Therapies in Patients with Cancer and Preexisting Autoimmune Diseases: A Meta-Analysis of Observational Studies. Autoimmun. Rev. 2020, 19, 102687. [Google Scholar] [CrossRef]
- Grabie, N.; Lichtman, A.H.; Padera, R. T Cell Checkpoint Regulators in the Heart. Cardiovasc. Res. 2019, 115, 869–877. [Google Scholar] [CrossRef]
- Knudsen, B.; Prasad, V. COVID-19 Vaccine Induced Myocarditis in Young Males: A Systematic Review. Eur. J. Clin. Investig. 2023, 53, e13947. [Google Scholar] [CrossRef]
- Oster, M.E.; Shay, D.K.; Su, J.R.; Gee, J.; Creech, C.B.; Broder, K.R.; Edwards, K.; Soslow, J.H.; Dendy, J.M.; Schlaudecker, E.; et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US from December 2020 to August 2021. JAMA 2022, 327, 331–340. [Google Scholar] [CrossRef]
- Patel, R.P.; Parikh, R.; Gunturu, K.S.; Tariq, R.Z.; Dani, S.S.; Ganatra, S.; Nohria, A. Cardiotoxicity of Immune Checkpoint Inhibitors. Curr. Oncol. Rep. 2021, 23, 79. [Google Scholar] [CrossRef]
- Moey, M.Y.Y.; Tomdio, A.N.; McCallen, J.D.; Vaughan, L.M.; O’Brien, K.; Naqash, A.R.; Cherry, C.; Walker, P.R.; Carabello, B.A. Characterization of Immune Checkpoint Inhibitor-Related Cardiotoxicity in Lung Cancer Patients from a Rural Setting. JACC CardioOncol. 2020, 2, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Ndjana Lessomo, F.Y.; Wang, Z.; Mukuka, C. Comparative Cardiotoxicity Risk of Pembrolizumab versus Nivolumab in Cancer Patients Undergoing Immune Checkpoint Inhibitor Therapy: A Meta-Analysis. Front. Oncol. 2023, 13, 1080998. [Google Scholar] [CrossRef] [PubMed]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 61, e78–e140. [Google Scholar] [CrossRef] [PubMed]
- Triggianese, P.; Novelli, L.; Galdiero, M.R.; Chimenti, M.S.; Conigliaro, P.; Perricone, R.; Perricone, C.; Gerli, R. Immune Checkpoint Inhibitors-Induced Autoimmunity: The Impact of Gender. Autoimmun. Rev. 2020, 19, 102590. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.S.; Montagnoli, T.L.; Rocha, B.S.; Tacco, M.L.C.A.; Marinho, S.C.P.; Zapata-Sudo, G. Estrogen Receptors: Therapeutic Perspectives for the Treatment of Cardiac Dysfunction after Myocardial Infarction. Int. J. Mol. Sci. 2021, 22, 525. [Google Scholar] [CrossRef]
- Ortona, E.; Pierdominici, M.; Rider, V. Editorial: Sex Hormones and Gender Differences in Immune Responses. Front. Immunol. 2019, 10, 1076. [Google Scholar] [CrossRef]
- Wang, F.; Wei, Q.; Wu, X. Cardiac Arrhythmias Associated with Immune Checkpoint Inhibitors: A Comprehensive Disproportionality Analysis of the FDA Adverse Event Reporting System. Front. Pharmacol. 2022, 13, 986357. [Google Scholar] [CrossRef]
- Escudier, M.; Cautela, J.; Malissen, N.; Ancedy, Y.; Orabona, M.; Pinto, J.; Monestier, S.; Grob, J.-J.; Scemama, U.; Jacquier, A.; et al. Clinical Features, Management, and Outcomes of Immune Checkpoint Inhibitor–Related Cardiotoxicity. Circulation 2017, 136, 2085–2087. [Google Scholar] [CrossRef]
- Fradley, M.G.; Brown, A.C.; Shields, B.; Viganego, F.; Damrongwatanasuk, R.; Patel, A.A.; Hartlage, G.; Roper, N.; Jaunese, J.; Roy, L.; et al. Developing a Comprehensive Cardio-Oncology Program at a Cancer Institute: The Moffitt Cancer Center Experience. Oncol. Rev. 2017, 11, 340. [Google Scholar] [CrossRef]
- Lyon, A.R.; Yousaf, N.; Battisti, N.M.L.; Moslehi, J.; Larkin, J. Immune Checkpoint Inhibitors and Cardiovascular Toxicity. Lancet Oncol. 2018, 19, e447–e458. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O.; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing Toxicities Associated with Immune Checkpoint Inhibitors: Consensus Recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on Cardio-Oncology Developed in Collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Bhatti, A.W.; Patel, R.; Dani, S.S.; Khadke, S.; Makwana, B.; Lessey, C.; Shah, J.; Al-Husami, Z.; Yang, E.H.; Thavendiranathan, P.; et al. SGLT2i and Primary Prevention of Cancer Therapy–Related Cardiac Dysfunction in Patients with Diabetes. JACC CardioOncol. 2024, in press. [Google Scholar] [CrossRef]
- Ha, A.; Langroudi, A.P.; Eisenberg, M.L. What Is the Validity of the Federal Adverse Event Reporting System in Contemporary Clinical Research? J. Sex. Med. 2024, 21, 744–745. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research. Questions and Answers on FDA’s Adverse Event Reporting System (FAERS). Available online: https://www.fda.gov/drugs/surveillance/questions-and-answers-fdas-adverse-event-reporting-system-faers (accessed on 25 September 2024).
- Ye, X.; Hu, F.; Zhai, Y.; Qin, Y.; Xu, J.; Guo, X.; Zhuang, Y.; He, J. Hematological Toxicities in Immune Checkpoint Inhibitors: A Pharmacovigilance Study from 2014 to 2019. Hematol. Oncol. 2020, 38, 565–575. [Google Scholar] [CrossRef]
- van Puijenbroek, E.P.; Bate, A.; Leufkens, H.G.M.; Lindquist, M.; Orre, R.; Egberts, A.C.G. A Comparison of Measures of Disproportionality for Signal Detection in Spontaneous Reporting Systems for Adverse Drug Reactions. Pharmacoepidemiol. Drug Saf. 2002, 11, 3–10. [Google Scholar] [CrossRef]
- Evans, S.J.; Waller, P.C.; Davis, S. Use of Proportional Reporting Ratios (PRRs) for Signal Generation from Spontaneous Adverse Drug Reaction Reports. Pharmacoepidemiol. Drug Saf. 2001, 10, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Bate, A.; Lindquist, M.; Edwards, I.R.; Olsson, S.; Orre, R.; Lansner, A.; De Freitas, R.M. A Bayesian Neural Network Method for Adverse Drug Reaction Signal Generation. Eur. J. Clin. Pharmacol. 1998, 54, 315–321. [Google Scholar] [CrossRef]
- Szarfman, A.; Machado, S.G.; O’Neill, R.T. Use of Screening Algorithms and Computer Systems to Efficiently Signal Higher-than-Expected Combinations of Drugs and Events in the US FDA’s Spontaneous Reports Database. Drug Saf. 2002, 25, 381–392. [Google Scholar] [CrossRef]
- Sakaeda, T.; Tamon, A.; Kadoyama, K.; Okuno, Y. Data Mining of the Public Version of the FDA Adverse Event Reporting System. Int. J. Med. Sci. 2013, 10, 796–803. [Google Scholar] [CrossRef]

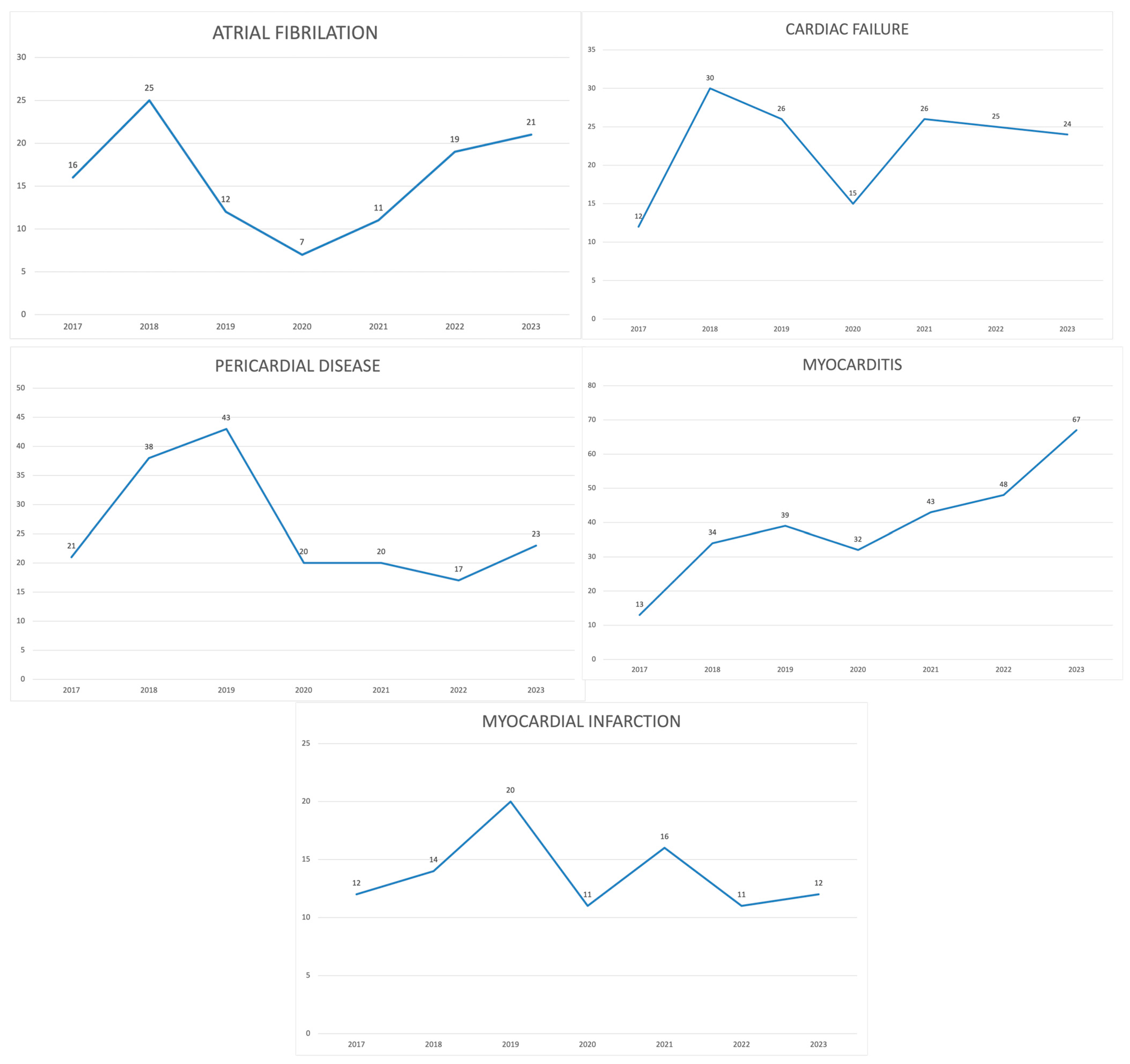
| n (%) | Cardiac Failure | Atrial Fibrillation | Myocardial Infarction | Pericardial Disease | Myocarditis | QT Prolongation | |
|---|---|---|---|---|---|---|---|
| Sex | Female | 52 (32.70) | 39 (35.14) | 23 (23.96) | 80 (43.48) | 107 (38.77) | 0 (0.00) |
| Male | 101 (63.52) | 68 (61.26) | 69 (71.88) | 104 (56.52) | 158 (57.25) | 0 (0.00) | |
| N/A | 5 (3.14) | 4 (3.60) | 4 (4.17) | 0 (0.00) | 11 (3.99) | 0 (0.00) | |
| Age | 71.62 ± 9.69 (N/A 37) | 71.58 ± 8.05 (N/A 24) | 70.00 ± 10.55 (N/A 45) | 63.80 ± 13.22 (N/A 43) | 69.59 ± 11.55 (N/A 41) | 0.00 ± 0.00 | |
| Reaction | Serious | 158 | 111 | 96 | 184 | 276 | 0 |
| Non-serious | 0 | 0 | 0 | 0 | 0 | 0 | |
| Outcome | Died | 67 (42.14) | 27 (24.32) | 45 (46.88) | 32 (17.39) | 112 (40.58) | 0 (0.00) |
| Life-threatening | 16 (10.06) | 15 (13.51) | 9 (9.38) | 20 (10.87) | 57 (20.65) | 0 (0.00) | |
| Hospitalized | 45 (28.30) | 35 (31.53) | 27 (28.13) | 76 (41.30) | 68 (24.64) | 0 (0.00) | |
| Disabled | 0 (0.00) | 0 (0.00) | 0 (0.0) | 0 (0.0) | 1(0.36) | 0 (0.00) | |
| Other | 30 (18.87) | 34 (30.63) | 15 (15.63) | 56 (30.43) | 38 (13.77) | 0 (0.00) |
| ROR (95% CI) | IC (97.5% CI) | |
|---|---|---|
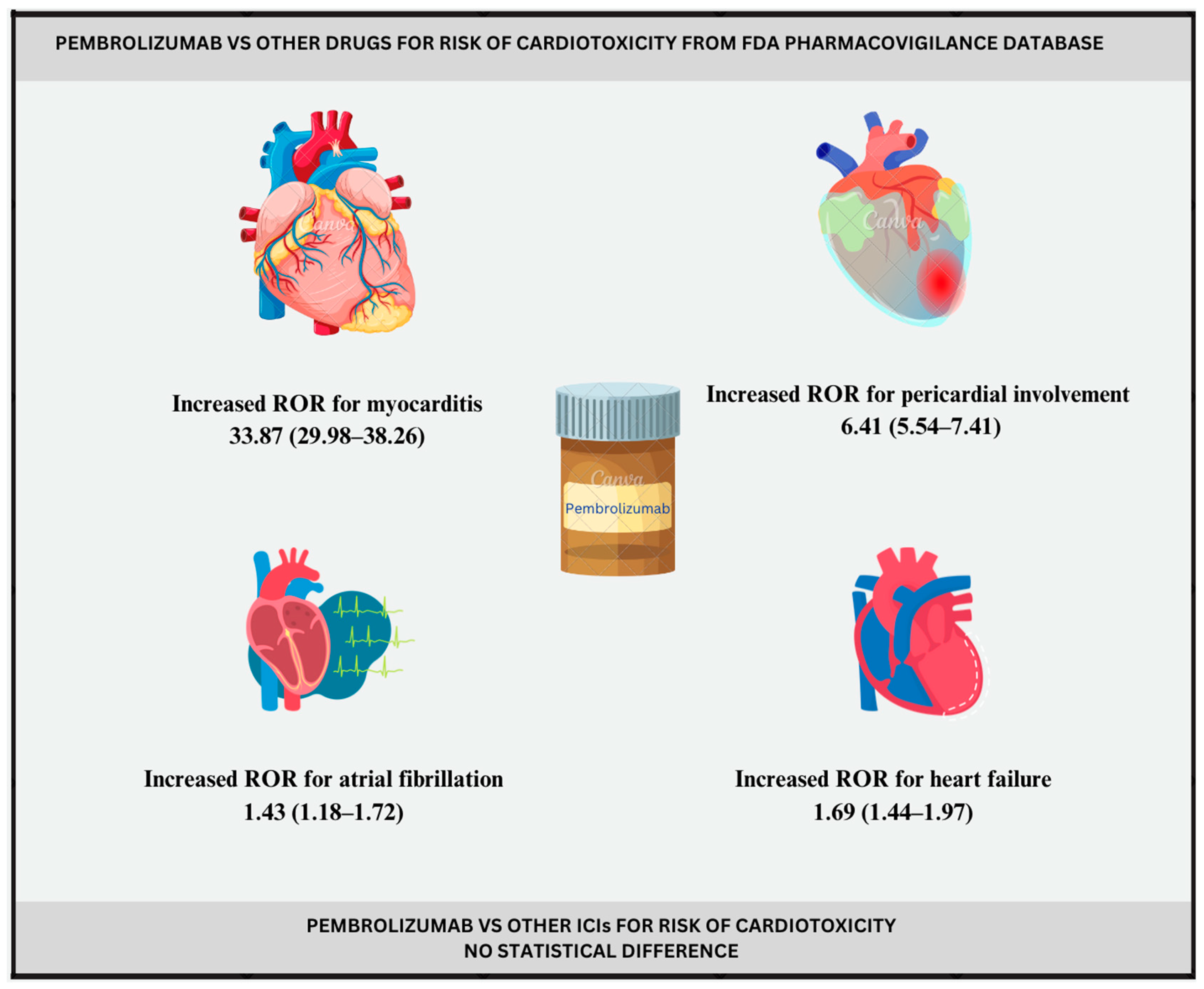
| Cardiac failure | 1.69 (1.44–1.97) | 0.75 (0.48–1.37) |
| Atrial fibrillation | 1.43 (1.18–1.72) | 0.51 (0.19–1.26) |
| Myocardial infarction | 1.22 (1.00–1.49) | 0.28 (−0.06–1.09) |
| Pericardial disease | 6.41 (5.54–7.41) | 2.64 (2.39–3.22) |
| Myocarditis | 33.87 (29.98–38.26) | 4.91 (4.71–5.39) |
| QT prolongation | / | / |
| Adverse Events | Pembrolizumab | Cemiplimab | Nivolumab | Atezolizumab | Avelumab | Durvalumab | Ipilimumab |
|---|---|---|---|---|---|---|---|
| Cardiac failure | 0.74 (0.63–0.87) | 2.05 (0.92–4.61) | 1.10 (0.97–1.26) | 0.65 (0.48–0.89) | 0.81 (0.42–1.56) | 0.74 (0.54–1.02) | 0.17 (0.07–0.40) |
| Atrial Fibrillation | 0.62 (0.51–0.75) | 0.44 (0.06–3.17) | 0.80 (0.68–0.95) | 0.86 (0.63–1.18) | 1.31 (0.72–2.38) | 0.52 (0.33–0.79) | 0.51 (0.28–0.92) |
| Myocardial infarction | 1.01 (0.81–1.26) | 1.52 (0.38–6.10) | 1.14 (0.94–1.38) | 1.18 (0.82–1.68) | 1.41 (0.67–2.97) | 1.40 (0.98–1.98) | 0.37 (0.15–0.89) |
| Pericardial disease | 1.13 (0.96–1.32) | 0.86 (0.21–3.44) | 1.14 (0.98–1.32) | 1.29 (1.00–1.66) | 0.45 (0.17–1.21) | 0.72 (0.50–1.04) | 0.17 (0.06–0.44) |
| Myocarditis | 1.01 (0.89–1.15) | 1.31 (0.54–3.16) | 0.85 (0.75–0.97) | 0.52 (0.38–0.70) | 0.97 (0.57–1.64) | 0.53 (0.38–0.74) | 0.53 (0.34–0.83) |
| QT prolongation | / | / | 0.31 (0.12–0.76) | / | / | / | 1.23 (0.3–5.02) |
| Adverse Events | Pembrolizumab | Cemiplimab | Nivolumab | Atezolizumab | Avelumab | Durvalumab | Ipilimumab |
|---|---|---|---|---|---|---|---|
| Cardiac failure | −0.37 (−0.63–0.26) | 0.91 (−0.50–3.99) | 0.12 (−0.08–0.59) | −0.59 (−1.11–0.66) | −0.29 (−1.43–2.26) | −0.41 (−0.94–0.83) | −2.45 (−4.01–0.89) |
| Atrial Fibrillation | −0.60 (−0.91–0.15) | −0.87 (−4.65–5.33) | −0.26 (−0.53–0.38) | −0.20 (−0.73–1.04) | 0.37 (−0.66–2.69) | −0.91 (−1.64–0.79) | −0.92 (−1.95–1.40) |
| Myocardial infarction | 0.02 (−0.32–0.82) | 0.46 (−2.14–5.34) | 0.15 (−0.14–0.84) | 0.22 (−0.37–1.60) | 0.45 (−0.85–3.32) | 0.45 (−0.13–1.82) | −1.33 (−2.89–2.01) |
| Pericardial disease | 0.14 (−0.10–0.72) | −0.18 (−2.77–4.70) | 0.15 (−0.07–0.67) | 0.34 (−0.08–1.34) | −1.05 (−2.81–2.64) | −0.45 (−1.06–0.98) | −2.42 (−4.18–1.26) |
| Myocarditis | 0.01 (−0.19–0.49) | 0.34 (−1.22–3.68) | −0.19 (−0.38–0.27) | −0.91 (−1.42–0.29) | −0.04 (−0.95–2.03) | −0.87 (−1.42–0.42) | −0.87 (−1.62–0.87) |
| QT prolongation | −4.77 (−15.10–5.01) | −0.46 (−10.79–9.32) | −1.42 (−2.99–1.92) | −3.15 (−13.47–6.64) | −1.27 (−11.60–8.51) | −2.96 (−13.28–6.83) | 0.23 (−2.36–5.12) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milutinovic, S.; Jancic, P.; Jokic, V.; Petrovic, M.; Dumic, I.; Rodriguez, A.M.; Tanasijevic, N.; Begosh-Mayne, D.; Stanojevic, D.; Escarcega, R.O.; et al. Pembrolizumab-Associated Cardiotoxicity: A Retrospective Analysis of the FDA Adverse Events Reporting System. Pharmaceuticals 2024, 17, 1372. https://doi.org/10.3390/ph17101372
Milutinovic S, Jancic P, Jokic V, Petrovic M, Dumic I, Rodriguez AM, Tanasijevic N, Begosh-Mayne D, Stanojevic D, Escarcega RO, et al. Pembrolizumab-Associated Cardiotoxicity: A Retrospective Analysis of the FDA Adverse Events Reporting System. Pharmaceuticals. 2024; 17(10):1372. https://doi.org/10.3390/ph17101372
Chicago/Turabian StyleMilutinovic, Stefan, Predrag Jancic, Vera Jokic, Marija Petrovic, Igor Dumic, Ambar Morales Rodriguez, Nikola Tanasijevic, Dustin Begosh-Mayne, Dragana Stanojevic, Ricardo O. Escarcega, and et al. 2024. "Pembrolizumab-Associated Cardiotoxicity: A Retrospective Analysis of the FDA Adverse Events Reporting System" Pharmaceuticals 17, no. 10: 1372. https://doi.org/10.3390/ph17101372
APA StyleMilutinovic, S., Jancic, P., Jokic, V., Petrovic, M., Dumic, I., Rodriguez, A. M., Tanasijevic, N., Begosh-Mayne, D., Stanojevic, D., Escarcega, R. O., Lopez-Mattei, J., & Cao, X. (2024). Pembrolizumab-Associated Cardiotoxicity: A Retrospective Analysis of the FDA Adverse Events Reporting System. Pharmaceuticals, 17(10), 1372. https://doi.org/10.3390/ph17101372








