Ethnomedicinal Uses, Phytochemistry, and Therapeutic Potentials of Litsea glutinosa (Lour.) C. B. Robinson: A Literature-Based Review
Abstract
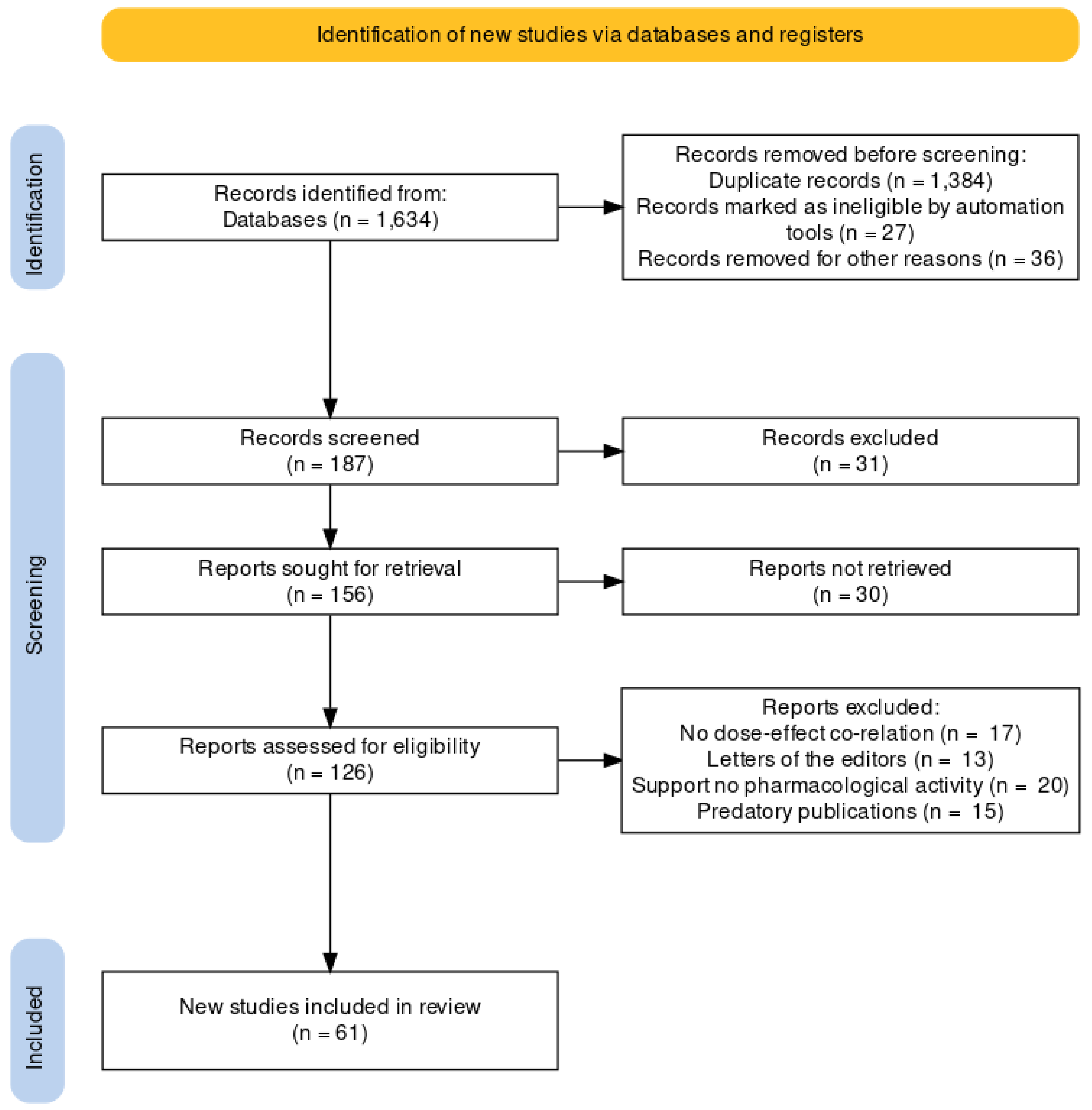
1. Introduction
2. Results
2.1. Traditional and Folk Values
2.2. Phytochemistry
2.2.1. Alkaloids
2.2.2. Alcohols
2.2.3. Carbohydrates
2.2.4. Glycosides
2.2.5. Esters
2.2.6. Terpenes
2.2.7. Flavonoids
2.2.8. Lactones
2.2.9. Steroids
2.2.10. Miscellaneous Constituents
2.3. Pharmacological Properties
2.3.1. Antioxidant Activity
2.3.2. Anti-Inflammatory Activity
2.3.3. Anti-Microbial Activity
2.3.4. Antipyretic Activity
2.3.5. Anti-Pyretic effect
2.3.6. Anti-Diabetic Effect
2.3.7. Analgesic Activity
2.3.8. Hepatoprotective Effect
2.3.9. Miscellaneous Effects
2.4. Toxicological Profile
3. Materials and Methods
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| BHT | Butylated hydroxytoluene |
| CCl4 | Carbon tetrachloride |
| DPPH | 1,1-diphenyl-2-picryl hydrazine |
| HL | Human leukemia |
| H2O2 | Hydrogen peroxide |
| HCl | Hydrochloric acid |
| HT29 | Human adenocarcinoma colorectal cell line |
| HepG2 | Hepatoma G2 |
| HPLC | High-performance liquid chromatography |
| LC/TOF-MS | Liquid chromatography/time-of-flight-mass spectrometry |
| MIC | Minimum inhibitory concentration |
| NMR | Nuclear magnetic resonance |
| SMMC-7721 | Surface Marker and Micro Cell-7721 |
| TLC | Thin-layer chromatography |
References
- Mondal, M.; Saha, S.; Sarkar, C.; Hossen, M.S.; Hossain, M.S.; Khalipha, A.B.R.; Islam, M.F.; Wahed, T.B.; Islam, M.T.; Rauf, A.; et al. Role of Citrus medica L. Fruits Extract in Combatting the Hematological and Hepatic Toxic Effects of Carbofuran. Chem. Res. Toxicol. 2021, 34, 1890–1902. [Google Scholar] [CrossRef]
- Sarkar, C.; Mondal, M.; Al-Khafaji, K.; El-Kersh, D.M.; Jamaddar, S.; Ray, P.; Roy, U.K.; Afroze, M.; Moniruzzaman, M.; Khan, M.; et al. GC–MS Analysis, and Evaluation of Protective Effect of Piper chaba Stem Bark against Paracetamol-Induced Liver Damage in Sprague-Dawley Rats: Possible Defensive Mechanism by Targeting CYP2E1 Enzyme through in Silico Study. Life Sci. 2022, 309, 121044. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Mondal, M.; Khanom, B.; Hossain, M.M.; Hossain, M.S.; Sureda, A.; Islam, M.T.; Martorell, M.; Kumar, M.; Sharifi-Rad, J.; et al. Heliotropium indicum L.: From Farm to a Source of Bioactive Compounds with Therapeutic Activity. Evid. Based Complement. Altern. Med. 2021, 2021, e9965481. [Google Scholar] [CrossRef] [PubMed]
- Hossain, R.; Quispe, C.; Herrera-Bravo, J.; Islam, M.S.; Sarkar, C.; Islam, M.T.; Martorell, M.; Cruz-Martins, N.; Al-Harrasi, A.; Al-Rawahi, A.; et al. Lasia spinosa Chemical Composition and Therapeutic Potential: A Literature-Based Review. Oxidative Med. Cell. Longev. 2021, 2021, e1602437. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Quispe, C.; Jamaddar, S.; Hossain, R.; Ray, P.; Mondal, M.; Abdulwanis Mohamed, Z.; Sani Jaafaru, M.; Salehi, B.; Islam, M.T.; et al. Therapeutic Promises of Ginkgolide A: A Literature-Based Review. Biomed. Pharmacother. 2020, 132, 110908. [Google Scholar] [CrossRef]
- Mondal, M.; Saha, S.; Hossain, M.; Al Foyjul, I.; Sarkar, C.; Hossain, S.; Khalipha, A.B.R.; Kundu, S.K. Phytochemical Profiling and Evaluation of Bioactivities of Methanolic and Ethyl Acetate Extracts of Marsdenia tenacissima Leaves. J. Herbs Spices Med. Plants 2020, 26, 405–422. [Google Scholar] [CrossRef]
- Bhowmick, R.; Sarwar, M.S.; RahmanDewan, S.M.; Das, A.; Das, B.; NasirUddin, M.M.; Islam, M.S.; Islam, M.S. In Vivo Analgesic, Antipyretic, and Anti-Inflammatory Potential in Swiss Albino Mice and in Vitro Thrombolytic Activity of Hydroalcoholic Extract from Litsea glutinosa Leaves. Biol. Res. 2014, 47, 56. [Google Scholar] [CrossRef]
- Lagudu, M.N.; Owk, A.K. Litsea glutinosa (Lauraceae): Evaluation of Its Foliar Phytochemical Constituents for Antimicrobial Activity. Not. Sci. Biol. 2018, 10, 21–25. [Google Scholar] [CrossRef][Green Version]
- Jacq, F.; Hladik, A.; Bellefontaine, R. Dynamique d’un Arbre Introduit à Mayotte, Litsea glutinosa (Lauraceae): Une Espèce Envahissante? Rev. Ecol. Terre Vie 2005, 60, 21–32. [Google Scholar] [CrossRef]
- Mohammad, N.; Dahayat, A.; Yadav, M.; Shirin, F.; Ansari, S.A. Genetic Diversity and Population Structure of Litsea glutinosa (Lour.) in Central India. Physiol. Mol. Biol. Plants 2018, 24, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Pareek, D.; Dobhal, S.; Sharma, M.C.; Joshi, Y.C.; Dobhal, M.P. Butanolides from Methanolic Extract of Litsea glutinosa. Chem. Biodivers. 2013, 10, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Franco, F.M.; Narasimhan, D. Plant Names and Uses as Indicators of Knowledge Patterns. Indian J. Tradit. Knowl. 2009, 8, 645–648. [Google Scholar]
- Kong, D.-G.; Zhao, Y.; Li, G.-H.; Chen, B.-J.; Wang, X.-N.; Zhou, H.-L.; Lou, H.-X.; Ren, D.-M.; Shen, T. The Genus Litsea in Traditional Chinese Medicine: An Ethnomedical, Phytochemical and Pharmacological Review. J. Ethnopharmacol. 2015, 164, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.; Schulp, C.J.E.; Kastner, T.; Verburg, P.H. Quantifying Spatial Variation in Ecosystem Services Demand: A Global Mapping Approach. Ecol. Econ. 2017, 136, 14–29. [Google Scholar] [CrossRef]
- Haque, T.; Uddin, M.Z.; Saha, M.L.; Mazid, M.A.; Hassan, M.A. Propagation, Antibacterial Activity and Phytochemical Profiles of Litsea glutinosa (Lour.) C. B. Robinson. Dhaka Univ. J. Biol. Sci. 2014, 23, 165–171. [Google Scholar] [CrossRef]
- Mishra, S.K.; Kumar, A.; Talukdar, A. Evaluation of Binding Property of Mucilage from Litsea glutinosa Wall. Pharmacogn. Res. 2010, 2, 289–292. [Google Scholar] [CrossRef]
- Chowdhury, J.U.; Bhuiyan, M.N.I.; Nandi, N.C. Aromatic Plants of Bangladesh: Essential Oils of Leaves and Fruits of Litsea glutinosa (Lour.) C.B. Robinson. Bangladesh J. Bot. 2008, 37, 81–83. [Google Scholar] [CrossRef]
- Uddin, M.Z.; Hassan, M.A. Determination of Informant Consensus Factor of Ethnomedicinal Plants Used in Kalenga Forest, Bangladesh. Bangladesh J. Plant Taxon. 2014, 21, 83–91. [Google Scholar] [CrossRef]
- Arora, D.S.; Kaur, G.J. Antibacterial Activity of Some Indian Medicinal Plants. J. Nat. Med. 2007, 61, 313–317. [Google Scholar] [CrossRef]
- Gerometta, E.; Grondin, I.; Smadja, J.; Frederich, M.; Gauvin-Bialecki, A. A Review of Traditional Uses, Phytochemistry and Pharmacology of the Genus Indigofera. J. Ethnopharmacol. 2020, 253, 112608. [Google Scholar] [CrossRef]
- Chinvorarat, J.; Pornpakakul, S. The Cleansing Performance of the Crude Extracts from the Fresh and Dried Litsea glutinosa Leaves. In Proceedings of the RSU International Research Conference (2022), Online Conference, 29 April 2022; p. 6. [Google Scholar]
- Prusti, A. Antibacterial Activity of Some Indian Medicinal Plants. Ethnobot. Leafl. 2008, 2008, 27. [Google Scholar]
- Victor, O.N.; Chidi, O.B.I. Phytochemical Constituents of Some Selected Medicinal Plants. Afr. J. Pure Appl. Chem. 2011, 10, 1948. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, Y.; Li, Y.; Zhang, C.; Sun, W.; Dongand, L.; Zhang, X. Litsine A: A New Aporphine Alkaloid from the Root Barks of Litsea glutinosa. Rec. Nat. Prod. 2018, 13, 167–171. [Google Scholar] [CrossRef]
- Yang, J.-H.; Li, L.; Wang, Y.-S.; Zhao, J.-F.; Zhang, H.-B.; Luo, S.-D. Two New Aporphine Alkaloids from Litsea glutinosa. Helv. Chim. Acta 2005, 88, 2523–2526. [Google Scholar] [CrossRef]
- Sun, W.; Jin, Y.; Zhang, L.; Tan, Y.; Zhang, C.; Dong, L.; Zhang, X. Two New Aminoethylstilbene Isoquinoline Alkaloids with Glucose Consumption Increasing Activity from the Root Barks of Litsea glutinosa. Phytochem. Lett. 2019, 34, 96–98. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, C.; Zhang, Y.; Zhang, C.; Cui, D.; Zhang, X. Glutinosine A: A New Morphinandienone Alkaloid from Litsea glutinosa. Rec. Nat. Prod. 2019, 13, 363–366. [Google Scholar] [CrossRef]
- Ndi, C.P.; Sykes, M.J.; Claudie, D.J.; McKinnon, R.A.; Semple, S.J.; Simpson, B.S.; Ndi, C.P.; Sykes, M.J.; Claudie, D.J.; McKinnon, R.A.; et al. Antiproliferative Aporphine Alkaloids from Litsea glutinosa and Ethnopharmacological Relevance to Kuuku I’yu Traditional Medicine. Aust. J. Chem. 2015, 69, 145–151. [Google Scholar] [CrossRef]
- Ninh, P.T.; Luu, N.T.; Anh, N.T.; Loc, T.V.; Mi, N.T.H.; Thao, T.T.P.; Sung, T.V. Chemical Constituents of the Barks of Litsea glutinosa Collected in Thai Nguyen Province, Vietnam. Vietnam J. Chem. 2015, 53, 652. [Google Scholar] [CrossRef]
- Lin, X.-W. Chemical constituents from root bark of Litsea glutinosa. Chin. Tradit. Herb. Drugs 2019, 24, 2817–2821. [Google Scholar]
- Das, D.; Maiti, S.; Maiti, T.K.; Islam, S.S. A New Arabinoxylan from Green Leaves of Litsea glutinosa (Lauraeae): Structural and Biological Studies. Carbohydr. Polym. 2013, 92, 1243–1248. [Google Scholar] [CrossRef]
- Wu, Y.; Jin, Y.; Dong, L.; Li, Y.; Zhang, C.; Gui, M.; Zhang, X. New Lignan Glycosides from the Root Barks of Litsea glutinosa. Phytochem. Lett. 2017, 20, 259–262. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Huang, R.; Lu, H.; Li, F.-Y.; Yang, J.-H. A New 2′-Oxygenated Flavone Glycoside from Litsea glutinosa (Lour.) C. B. Rob. Biosci. Biotechnol. Biochem. 2010, 74, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-M.; Duan, H.; Shi, J.-L.; Zuo, T.-T.; Hu, X.-C.; Lang, S.-Y.; Yan, M.; Liang, J.-Y.; Yang, Y.-G.; Kong, Q.-H.; et al. In Situ Fluorinated Solid Electrolyte Interphase towards Long-Life Lithium Metal Anodes. Nano Res. 2020, 13, 430–436. [Google Scholar] [CrossRef]
- Thakral, S.; Singh, V. 2,4-Dichloro-5-[(N-Aryl/Alkyl)Sulfamoyl]Benzoic Acid Derivatives: In Vitro Antidiabetic Activity, Molecular Modeling and In Silico ADMET Screening. Med. Chem. 2019, 15, 186–195. [Google Scholar] [CrossRef]
- Wu, H.; He, K.; Wang, Y.; Xue, D.; Ning, N.; Zou, Z.; Ye, X.; Li, X.; Wang, D.; Pang, J. The Antihypercholesterolemic Effect of Jatrorrhizine Isolated from Rhizoma coptidis. Phytomedicine 2014, 21, 1373–1381. [Google Scholar] [CrossRef]
- Dong, J.-W.; Cai, L.; Fang, Y.-S.; Xiao, H.; Li, Z.-J.; Ding, Z.-T. Proaporphine and Aporphine Alkaloids with Acetylcholinesterase Inhibitory Activity from Stephania epigaea. Fitoterapia 2015, 104, 102–107. [Google Scholar] [CrossRef]
- Pathan, H.; Williams, J. Basic Opioid Pharmacology: An Update. Br. J. Pain 2012, 6, 11–16. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Liao, Z.; Li, Y.; Huang, R.; Zhang, H.-B.; Yang, J.-H. A New Megastigmane Diglycoside from Litsea glutinosa (Lour.) C. B. Rob. J. Braz. Chem. Soc. 2011, 22, 2234–2238. [Google Scholar] [CrossRef]
- Pereira, D.M.; Faria, J.; Gaspar, L.; Valentão, P.; Andrade, P.B. Boerhaavia diffusa: Metabolite Profiling of a Medicinal Plant from Nyctaginaceae. Food Chem. Toxicol. 2009, 47, 2142–2149. [Google Scholar] [CrossRef]
- Patil, K.S.; Bhalsing, S.R. Ethnomedicinal Uses, Phytochemistry and Pharmacological Properties of the Genus Boerhavia. J. Ethnopharmacol. 2016, 182, 200–220. [Google Scholar] [CrossRef]
- YouWei, X.; HongLei, Z.; DongMei, R.; HongXiang, L.; Tao, S. Chemical constituents from the aerial parts of Litsea glutinosa (Lour.) C. B. Rob. J. Shandong Univ. 2016, 54, 45–49. [Google Scholar]
- Rumzhum, N.N.; Rahman, M.M.; Sharukh, A.A.; Chowdhury, S.A.; Pervin, M.N. In Vitro Antioxidant and Antinociceptive Potentialities of Methanolic Extract of Litsea glutinosa. Bangladesh J. Sci. Ind. Res. 2012, 47, 401–406. [Google Scholar] [CrossRef]
- Mandal, S.C.; Kumar, C.K.A.; Majumder, A.; Majumder, R.; Maity, B.C. Antibacterial Activity of Litsea glutinosa Bark. Fitoterapia 2000, 71, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Pradeepa, K.; Krishna, V.; Venkatesh; Kumar, K.G.; Thirumalesh, B.V.; Naveen Kumar, K.J. Antibacterial Screening of the Stem Bark and Leaf Extracts of Litsea glutinosa (Lour.) C.B. Rob—An Ethnomedicinally Important Tree of the Western Ghats. Pharmacogn. J. 2011, 3, 72–76. [Google Scholar] [CrossRef]
- Lohitha, P.; Sagar, S.V.; Ramanjaneyulu, K.; Verma, V.H.K. Phytochemical Screening and Evaluation of In vitro Anti bacterial Activity of Litsea glutinosa (L) bark Ethanolic Extract. Pharmacologyonline 2010, 6, 618–623. [Google Scholar]
- Ingkachotivanich, P.; Chanbang, Y.; Chuttong, B.; Page, P.; Sommano, S. Phytochemical Composition and Larvicidal Activity of Litsea glutinosa Crude Leaf Extract against the Virus-Transmitting Mosquito, Aedes aegypti (L.). Med. Plants Int. J. Phytomed. Relat. Ind. 2017, 9, 88. [Google Scholar] [CrossRef]
- Changani, H.; Parikh, P. Molecular Insights for an Anti-Osteoporotic Properties of Litsea glutinosa on Saos-2 Cells: An in-Vitro Approach. J. Ayurveda Integr. Med. 2022, 13, 100501. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, Y.; Wu, Y.; Zhang, C.; Jin, D.; Zheng, Q.; Li, Y. Anti-Hyperglycemic and Anti-Hyperlipidemia Effects of the Alkaloid-Rich Extract from Barks of Litsea glutinosa in Ob/Ob Mice. Sci. Rep. 2018, 8, 12646. [Google Scholar] [CrossRef]
- Tan, Y.-F.; Wang, R.-Q.; Wang, W.-T.; Wu, Y.; Ma, N.; Lu, W.-Y.; Zhang, Y.; Zhang, X.-P. Study on the Pharmacokinetics, Tissue Distribution and Excretion of Laurolitsine from Litsea glutinosa in Sprague-Dawley Rats. Pharm. Biol. 2021, 59, 882–890. [Google Scholar] [CrossRef]
- Ghosh, N.; Chaki, R.; Pal, M.; Mandal, S.C. Hepatoprotective Activity of Methanol Extract of Litsea glutinosa against Hepatotoxin Induced Toxicity. Orient. Pharm. Exp. Med. 2016, 16, 139–146. [Google Scholar] [CrossRef]
- Al-Rifai, A.; Aqel, A.; Al-Warhi, T.; Wabaidur, S.M.; Al-Othman, Z.A.; Badjah-Hadj-Ahmed, A.Y. Antibacterial, Antioxidant Activity of Ethanolic Plant Extracts of Some Convolvulus Species and Their DART-ToF-MS Profiling. Evid. Based Complement. Altern. Med. 2017, 2017, e5694305. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Fang, J.; Wang, Z.; Wang, P.; Wang, M. Extraction, Structure and Bioactivities of the Polysaccharides from Ginkgo biloba: A Review. Int. J. Biol. Macromol. 2020, 162, 1897–1905. [Google Scholar] [CrossRef]
- Mondal, M.; Hossain, M.M.; Das, N.; Rahman, M.A.; Uddin, N.; Hasan, M.R.; Alam, M.J.; Islam, M.N.; Wahed, T.B.; Kundu, S.K. Investigation of Bioactivities of Methanolic and Ethyl Acetate Extracts of Dioscorea pentaphylla Leaf along with Its Phenolic Composition. Food Meas. 2019, 13, 622–633. [Google Scholar] [CrossRef]
- Solowey, E.; Lichtenstein, M.; Sallon, S.; Paavilainen, H.; Solowey, E.; Lorberboum-Galski, H. Evaluating Medicinal Plants for Anticancer Activity. Sci. World J. 2014, 2014, e721402. [Google Scholar] [CrossRef]
- Thun, M.J.; DeLancey, J.O.; Center, M.M.; Jemal, A.; Ward, E.M. The Global Burden of Cancer: Priorities for Prevention. Carcinogenesis 2010, 31, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Jamaddar, S.; Islam, T.; Mondal, M.; Islam, M.T.; Mubarak, M.S. Therapeutic Perspectives of the Black Cumin Component Thymoquinone: A Review. Food Funct. 2021, 12, 6167–6213. [Google Scholar] [CrossRef]
- Grover, J.K.; Yadav, S.; Vats, V. Medicinal Plants of India with Anti-Diabetic Potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef]
- Ahmadiani, A.; Fereidoni, M.; Semnanian, S.; Kamalinejad, M.; Saremi, S. Antinociceptive and Anti-Inflammatory Effects of Sambucus ebulus Rhizome Extract in Rats. J. Ethnopharmacol. 1998, 61, 229–235. [Google Scholar] [CrossRef]
- Sumithregowda, A.; Venkatarangaiah, K.; Honnenahally, K.; Manjunath, V. Cytotoxicity and Oral Acute Toxicity Studies of Litsea glutinosa C. B (ROB) Stem Bark Ethanol Extract. Pharmacogn. J. 2017, 9, 880–886. [Google Scholar] [CrossRef]




| Traditional Uses | Part Used | Mode of Administration | References |
|---|---|---|---|
| Antispasmodic, emollient, poultice, diarrhea, dysentery as well as for wounds and bruises, fever, swelling, furunculosis | Leaves | Leaf powder | [17,18] |
| Cleaning the hair and scalp | Leaves | Clear mucilage solution | [21] |
| Rheumatism | Berries oil | Essential oil | [17,18] |
| Antiseptic | Leaves | Essential oil | [22] |
| Energy tonic | Bark | [15] | |
| Binding agent in tablet formulations, as plasters for fractured limbs, treating pain, aphrodisiac or to arouse sexual power, for bruises inflicted by blows, skin diseases, as a soothing effect on the body, for wounds on the neck of bullocks and bleeding | Bark | Bark-powder paste is used, mucilage in the gum from the bark | [16] |
| Skin boils | Seed | Seed powder | [16] |
| Phytochemicals | Part(s) | Reference(s) |
|---|---|---|
| Alkaloids | ||
| Litsine A (1) | Root bark | [24] |
| Litseglutine A (2) | Leaves and twigs | [25] |
| Litseglutine B (3) | Leaves and twigs | [25] |
| Litsine B (4) | Root bark | [26] |
| Litsine C (5) | Root bark | [26] |
| Boldine (6) | Root bark | [24] |
| Laurolitsine (7) | Root bark | [24] |
| Glutinosine A (8) | Root bark | [27] |
| Morphinane (9) | - | [13] |
| Aporphine (10) | [13] | |
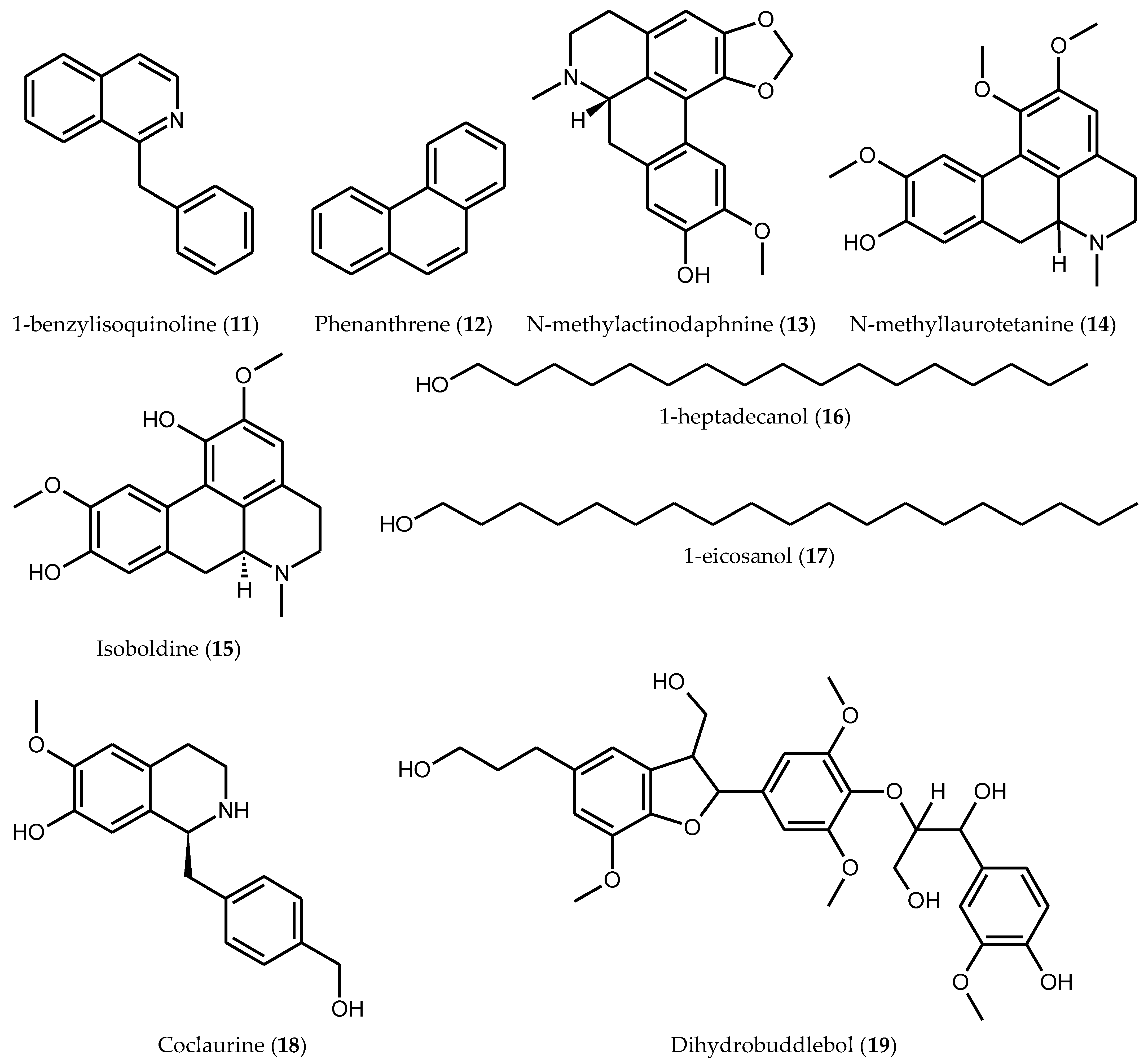
| 1-benzylisoquinoline (11) | [13] | |
| Phenanthrene (12) | [13] | |
| N-methylactinodaphnine (13) | Leaves | [28] |
| N-methyllaurotetanine (14) | Leaves | [28] |
| Isoboldine (15) | Leaves | [28] |
| Alcohols | ||
| 1-heptadecanol (16) | Bark | [29] |
| 1-eicosanol (17) | Bark | [29] |
| Coclaurine (18) | Root bark | [30] |
| Dihydrobuddlenol (19) | Root bark | [30] |
| Ssioriside (20) | Root bark | [30] |
| Carbohydrates | ||
| Xylose (21) | Leaves | [31] |
| Arabinose (22) | Leaves | [31] |
| Glycosides | ||
| Litseasins A (23) | Root bark | [32] |
| Litseasins B (24) | Root bark | [32] |
| Litseasins C (25) | Root bark | [32] |
| Glutin (26) | Leaves and twig | [33] |
| (7R,8S)-3,3′,5-trimethoxy-4′,7-epoxy-8,5′-neolignan4,9,9-triol 9-β-D-xylopyranoside (27) | Root bark | [32] |
| (6S, 7E, 9R)-6, 9-dihydroxy-4, 7-megastigmadien-3-one-9-O-[α-L-arabinofuranosyl-(l→6)]-β-D-glucopyranoside (28) | Leaves and twig | [33] |
| Roseoside (29) | Leaves and twig | [33] |
| (7′R, 8′R)-3, 5′-dimethoxy-9, 9′-dihydroxy-4, 7′-epoxylignan 4′-β-D-glucopyranoside (30) | Leaves and twig | [33] |
| (7′R, 8′S)-dihydrodehydrodiconifenyl alcohol 9′-O-β-D-xylopyranoside (31) | Leaves and twig | [33] |
| Pinoresinol 3-O-β-D-glucopyranoside (32) | Leaves and twig | [33] |
| 2′,5,7-trihydroxy-6-methoxyflavone 2′-O-β-D-glucopyranoside (33) | Leaves and twig | [33] |
| Schizandriside (34) | Root bark | [30] |
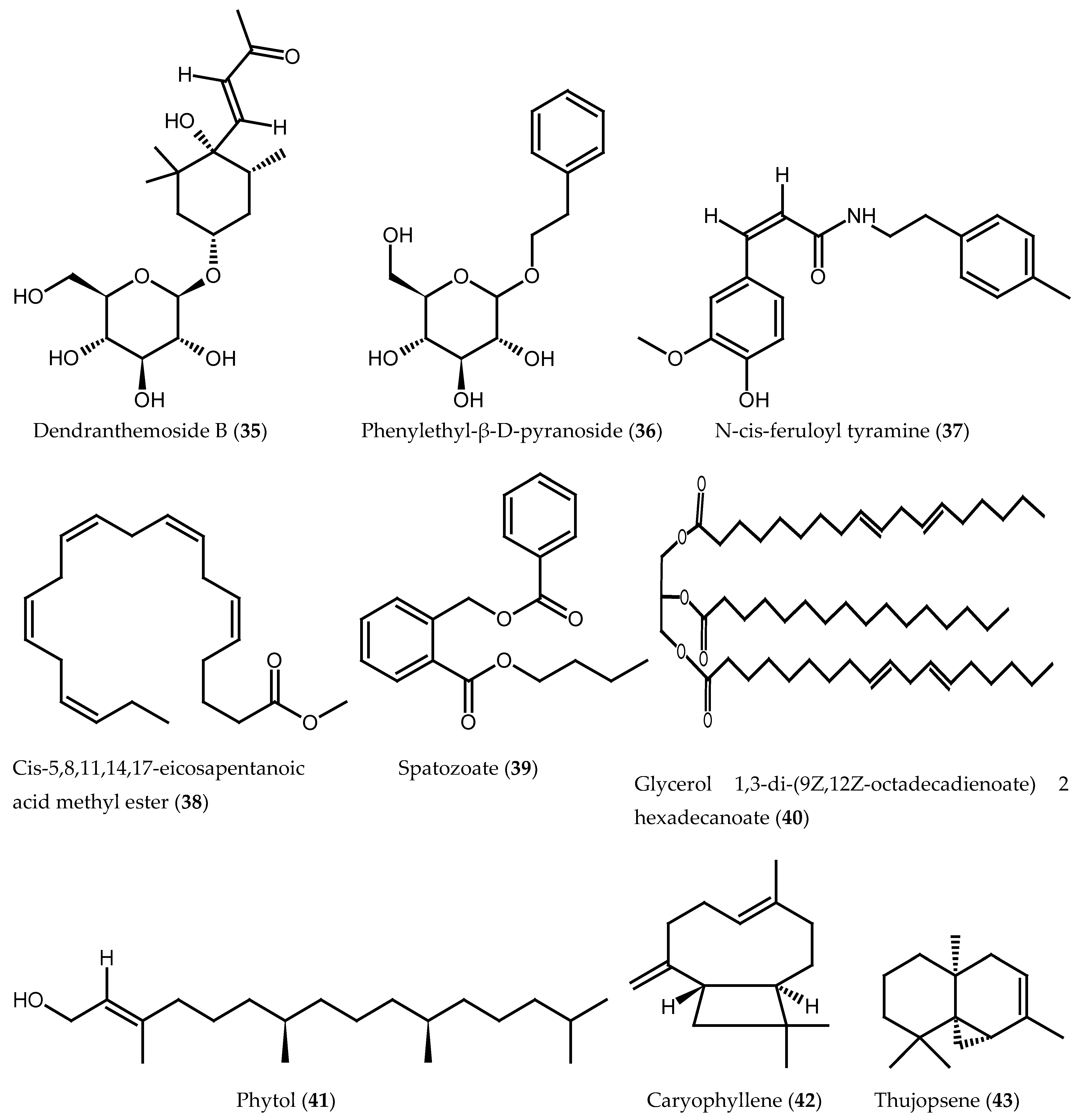
| Dendranthemoside B (35) | Root bark | [30] |
| Phenylethyl-β-D-glucopyranoside (36) | Root bark | [30] |
| N-cis-feruloyl tyramine (37) | Root bark | [30] |
| Esters | ||
| cis-5,8,11,14,17-eicosapentaenoic acid methyl ester (38) | Bark | [29] |
| Spatozoate (39) | Bark | [29] |
| Glycerol 1,3-di-(9Z,12Z-octadecadienoate) 2-hexadecanoate (40) | Bark | [29] |
| Terpenes | ||
| Phytol (41) | Leaf oil | [17] |
| Caryophyllene (42) | Leaf oil | [17] |
| Thujopsene (43) | Leaf oil | [17] |
| Myrcene (44) | Leaf oil | [17] |
| α-cubebene (45) | Fruit oil | [17] |
| β-ocimene (46) | Leaf oil | [17] |
| β-pinene (47) | Leaf oil | [17] |
| α-pinene (48) | Leaf oil | [17] |
| Caryophylleneoxide (49) | Leaf oil | [17] |
| Bicyclogermacrene (50) | Leaf oil | [17] |
| Ocimene (51) | Fruit oil | [17] |
| Flavonoids | ||
| Flavones (52) | - | [13] |
| Flavonols (53) | - | [13] |
| Flavan-3-ols (54) | - | [13] |
| Chalcones (55) | - | [13] |
| Flavanonols (56) | - | [13] |
| Anthocyanidins (57) | - | [13] |
| Lactones | ||
| Litsealactone C (58) | Bark | [11] |
| Litsealactone D (59) | Bark | [11] |
| Litsealactone G (60) | Bark | [11] |
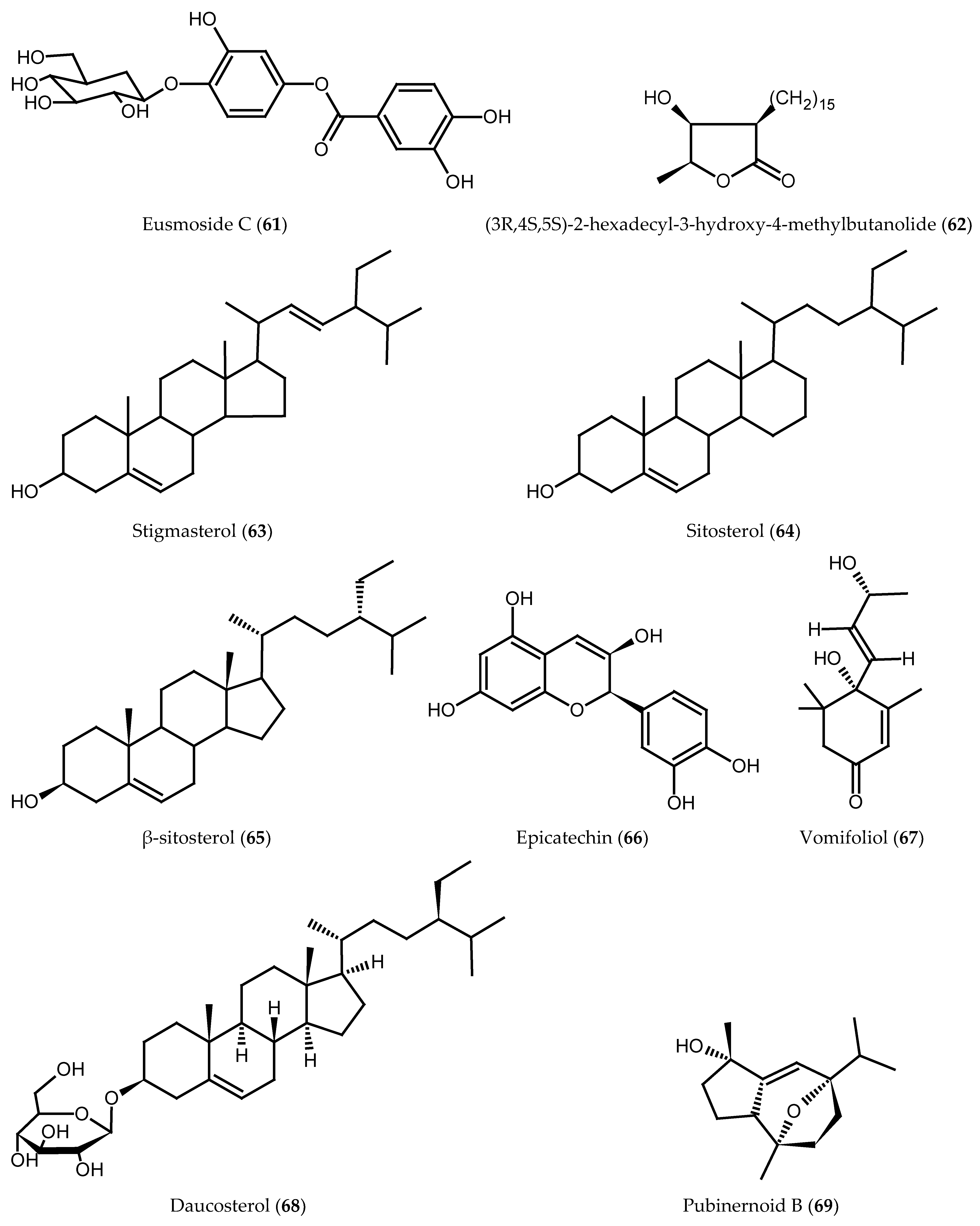
| Eusmoside C (61) | Bark | [11] |
| (3R,4S,5S)-2-hexadecyl-3-hydroxy-4-methylbutanolide (62) | Bark | [11] |
| Steroids | ||
| Stigmasterol (63) | Bark | [33] |
| Sitosterol (64) | Bark | [33] |
| β-sitosterol (65) | Bark | [29] |
| Epicatechin (66) | Bark | [15] |
| Vomifoliol (67) | Aerial parts | [34] |
| Daucosterol (68) | Aerial parts and bark | [29] |
| Pubinernoid B (69) | Aerial parts | [34] |
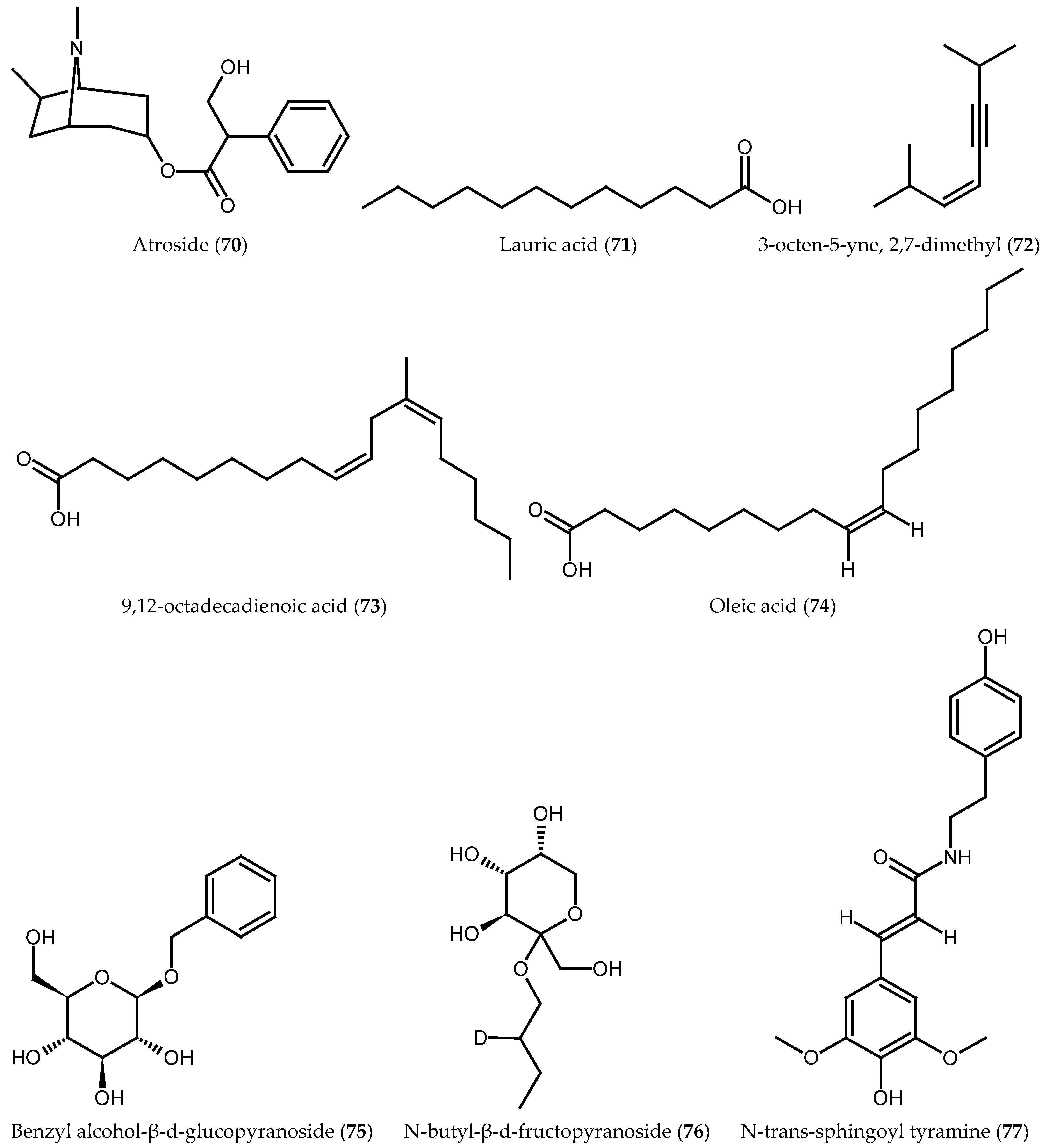
| Atroside (70) | Aerial parts | [34] |
| Miscellaneous constituents | ||
| Lauric acid (71) | Fruit oil | [17] |
| 3-octen-5-yne, 2,7-dimethyl (72) | Fruit oil | [17] |
| 9,12-octadecadienoic acid (73) | Bark oil | [34] |
| Oleic acid (74) | Fruit oil | [17] |
| Benzyl alcohol-β-d-glucopyranoside (75) | Root bark | [30] |
| N-butyl-β-d-fructopyranoside (76) | Root bark | [30] |
| N-trans-sphingoyl tyramine (77) | Root bark | [30] |
| Sources | Test Systems | Dose/Conc. | Results and Possible Mechanism | References |
|---|---|---|---|---|
| Antioxidant activity | ||||
| Methanolic extract of plant | Hydrogen peroxide scavenging activity, total antioxidant capacity, assay of nitric oxide scavenging activity and reducing-power test. | 50–250 mg/mL | Exhibited antioxidant effect in a concentration-dependent manner. | [43] |
| Leaf extract | DPPH-free-radical scavenging assay, reducing-power assay, total phenolic content. | 5–100 µg/mL | Antioxidant activity is dose-dependently increased. | [7] |
| Anti-inflammatory activity | ||||
| n-hexane, ethyl acetate, chloroform, and methanolic leaf extracts | Carrageenan-induced oedema test is carried out on Swiss albino mice. | 250 and 500 mg/kg | The crude methanolic extract showed significant potential against carrageenan-induced paw edema, by inhibiting either cyclooxygenase and/or lypooxygenase enzyme. | [7] |
| Anti-microbial activity | ||||
| Methanolic bark-extract | Staphylococcus aureus, Bacillus pumilus, Streptococcus pneumoniae, Escherichia coli, Bacillus subtilis, Lactobacillus arabinosus, Bacillus cereu, Sarcina lutea, Shigella dysenteriae, Shigella sonnei, Salmonella typhimurium, Vibrio cholera, Klebsiella pneumoniae, Escherichia coli. using an agar-diffusion method | 50–200 µg/mL | The bark extract is effectively used in diarrhea and dysentery by inhibiting both gram-positive and gram-negative bacteria. | [44] |
| Stem-bark and leaf-extracts | S. aureus, B. stubtilis, E.coli, Pseudomonas aeruginosa, K. pneumoniae, S. typhi, Salmonella paratyphi and Proteus sp. using agar-well diffusion method | 40, 20, 10, 5 and 2.5 mg/mL | Potent antibacterial agent. | [45] |
| Ethanolic and water-soluble leaf- and bark-extracts | E. coli, Enterobacter intermedium, Salmonella sp., S. aureus and Staphylococcus epidermis using Kirby–Bauer disc-diffusion method | Ethanol extract (1000 μg/disc), Distilled-water extract (10 μg/disc) | Ethanolic leaf-extract showed maximum antibacterial activity against E. coli with a zone of inhibition of 30 mm. | [15] |
| Aqueous leaf-extract | B. subtilis, Enterococcus faecalis, E. coli, K. pneumoniae, Micrococcus luteus, P. aeruginosa, Proteus vulgaris, S. aureus, S. pneumoniae, Aspergillus niger, Candida albicans and Saccharomyces cerevisiae. | 25, 50 and 100 mg/mL | Showed zone of inhibition of 50% more than the antibiotics investigated. | [8] |
| Ethanolic bark-extract | S. aureus, B. cereus, P. aeruginosa, E.Coli | 125–1000 mg/mL | Showed effective antimicrobial effect in dose-dependent manner against the test microbes. | [46] |
| Methanolic leaf-extract | Aedes aegypti larvae | 20, 40 and 60 g/L | At 60 g/L, the extract is considered to be most effective in larvicidal activity. | [47] |
| Anticancer activity | ||||
| New megastigmane diglycoside isolated from the plant. | Human cancer cell-lines myeloid leukemia HL-60, hepatocellular carcinoma SMMC-7721, lung cancer A-549, breast cancer MCF-7 and colon cancer SW480 cells. | (1 mg) in 1 mol L-1 HCl (2 mL) | Proved to be inactive (IC50 > 40 µM). | [39] |
| N-methylactinodaphnine, boldine, N-methyllaurotetanine, and isoboldine isolated from the ethanolic leaf-extract. | Cytotoxicity against HT29, SKMEL28, and primary human keratinocytes. | 100 μg/mL | Exerted cytotoxic effect through inhibiting DNA topo-II. | [28] |
| Methanolic bark-extract | In vitro studies on Saos-2 cell | 500 ng/mL, 10–400 μg/mL | Significantly downregulated the apoptotic and proliferative markers in Saos-2 osteocytes. | [48] |
| Antipyretic activity | ||||
| n-hexane, ethyl acetate, chloroform, and crude methanolic leaf-extracts | The subcutaneous injection of yeast suspension in Swiss albino mice. | 500 mg/kg | Exerted notable reduction in yeast-provoked elevation of body temperature (32.78 ± 0.46 °C) through inhibition of prostaglandin synthetase within the hypothalamus. | [7] |
| Anti-diabetic effect | ||||
| Ethanol bark-extract | Male ob/ob mice. | 50, 100 or 200 mg/kg | Ameliorated insulin resistance through alleviating obesity, hyperlipidemia and inflammation, and can be used as potent treatment of type 2 diabetes. | [49] |
| Glutinosine A isolated from the root bark. | HepG2 cells for glucose consumption assay. | 10 μM | Exerted no activity in stimulating glucose-consumption. | [27] |
| Laurolitsine isolated from the plant. | ob/ob mice | 2.0 mg/kg via the tail vein, 10.0 mg/kg by gavage | Demonstrated potent antihyperglycemic and antihyperlipidemic effect. | [50] |
| Litsine B and C isolated from the ethanolic root-bark extract. | Glucose-consumption assay on HepG2 cells. | 1–20 μM | Litsine C b significant increasing glucose-consumption. | [26] |
| Litsine A isolated from the root bark. | Glucose-uptake assay on C2C12 myoblasts. | 10 μM | Increased glucose uptake. | [24] |
| Analgesic activity | ||||
| n-hexane, ethyl acetate, chloroform, and crude-methanolic leaf extracts | Acetic-acid-induced writhing and hot-plate test in mice. | 250 and 500 mg/kg | Displayed significant analgesic-activity. | [7] |
| Leaf extract | Abdominal-writhing and tail-flick methods, using mice. | 100, 200 and 300 mg/kg | Provided significant analgesic activity by inhibiting prostaglandin synthetase, specifically endoperoxidase. | [45] |
| Methanolic extract of the plant | Acetic-acid-induced writhing model in Swiss albino mice. | 250 and 500 mg/kg | Provided a dose-dependent increase in analgesic effect. | [43] |
| Hepatoprotective effect | ||||
| Methanolic extract of the plant | CCl4- and paracetamol-induced hepatotoxicity in rats. | 100–200 mg/kg | Provided potent hepatoprotective-effect with controlled biological parameters. | [51] |
| Miscellaneous effects | ||||
| Bark extract | Immobilization stress-induced male Wistar albino rats. | 100, 300, and 500 mg/kg | Exhibited significant aphrodisiac and anti-infertility activity. | [46] |
| n-hexane, ethyl acetate, chloroform, and crude methanolic leaf extracts | Swiss albino mice. | 1 mg/mL | A significant clot-disruption was observed. | [7] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamaddar, S.; Raposo, A.; Sarkar, C.; Roy, U.K.; Araújo, I.M.; Coutinho, H.D.M.; Alkhoshaiban, A.S.; Alturki, H.A.; Saraiva, A.; Carrascosa, C.; et al. Ethnomedicinal Uses, Phytochemistry, and Therapeutic Potentials of Litsea glutinosa (Lour.) C. B. Robinson: A Literature-Based Review. Pharmaceuticals 2023, 16, 3. https://doi.org/10.3390/ph16010003
Jamaddar S, Raposo A, Sarkar C, Roy UK, Araújo IM, Coutinho HDM, Alkhoshaiban AS, Alturki HA, Saraiva A, Carrascosa C, et al. Ethnomedicinal Uses, Phytochemistry, and Therapeutic Potentials of Litsea glutinosa (Lour.) C. B. Robinson: A Literature-Based Review. Pharmaceuticals. 2023; 16(1):3. https://doi.org/10.3390/ph16010003
Chicago/Turabian StyleJamaddar, Sarmin, António Raposo, Chandan Sarkar, Uttam Kumar Roy, Isaac Moura Araújo, Henrique Douglas Melo Coutinho, Ali Saleh Alkhoshaiban, Hmidan A. Alturki, Ariana Saraiva, Conrado Carrascosa, and et al. 2023. "Ethnomedicinal Uses, Phytochemistry, and Therapeutic Potentials of Litsea glutinosa (Lour.) C. B. Robinson: A Literature-Based Review" Pharmaceuticals 16, no. 1: 3. https://doi.org/10.3390/ph16010003
APA StyleJamaddar, S., Raposo, A., Sarkar, C., Roy, U. K., Araújo, I. M., Coutinho, H. D. M., Alkhoshaiban, A. S., Alturki, H. A., Saraiva, A., Carrascosa, C., & Islam, M. T. (2023). Ethnomedicinal Uses, Phytochemistry, and Therapeutic Potentials of Litsea glutinosa (Lour.) C. B. Robinson: A Literature-Based Review. Pharmaceuticals, 16(1), 3. https://doi.org/10.3390/ph16010003











