Role and Mechanisms of Phytochemicals in Hair Growth and Health
Abstract
1. Introduction
2. Types of Alopecia
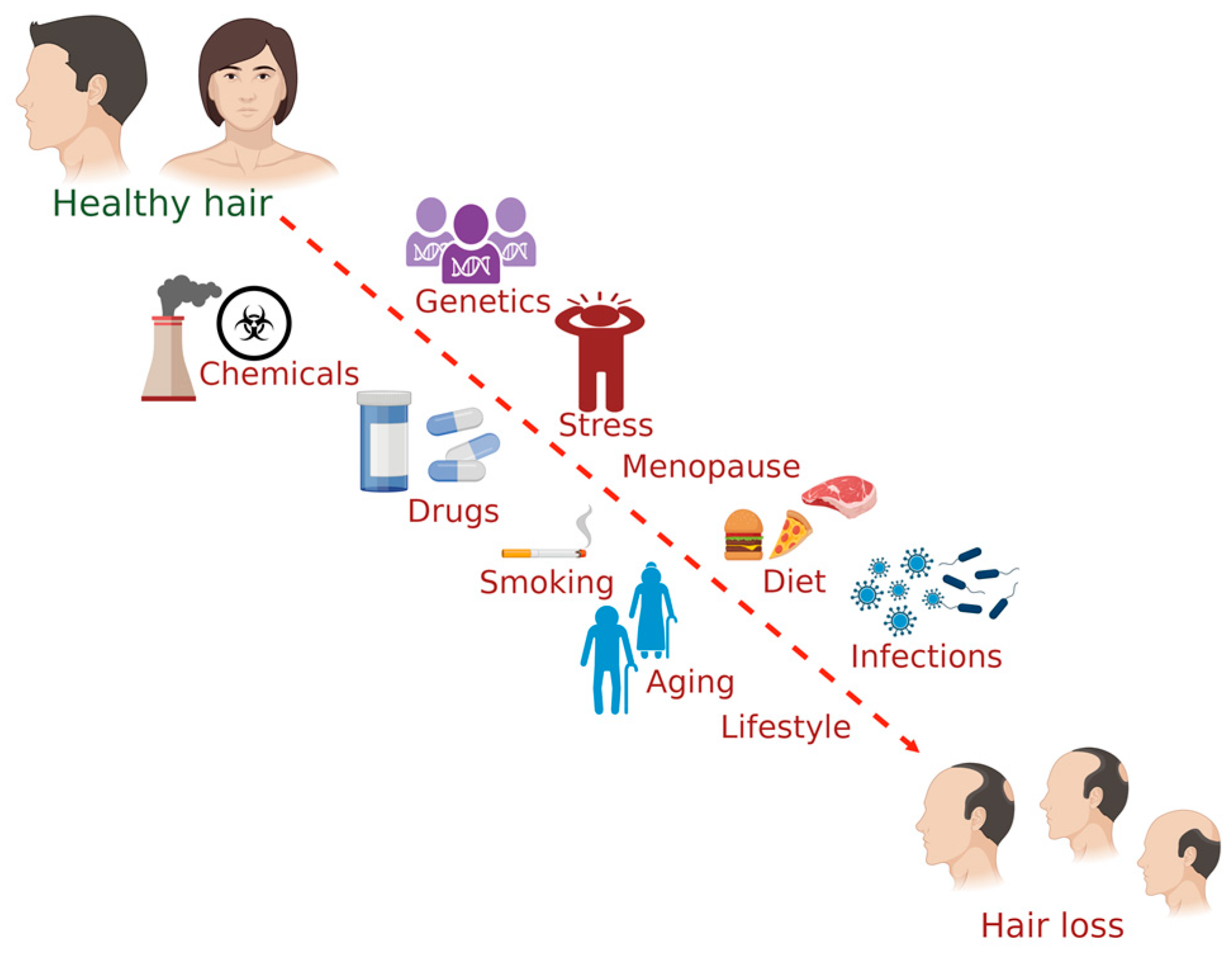
3. Factors Affecting Hair Health
3.1. Diet
3.2. Chemical Exposure
3.3. Drugs
3.4. Diseases or Disorders
3.5. Smoking
3.6. Genetics
3.7. Stress
3.8. Menopause
4. Treatments for Alopecia
5. Role of Phytochemicals in Managing Hair Loss
6. Role of Phytochemicals in Hair Growth
6.1. Evidence from In Vitro Studies
6.2. Evidence from In Vivo Studies
6.3. Evidence from Clinical Trials
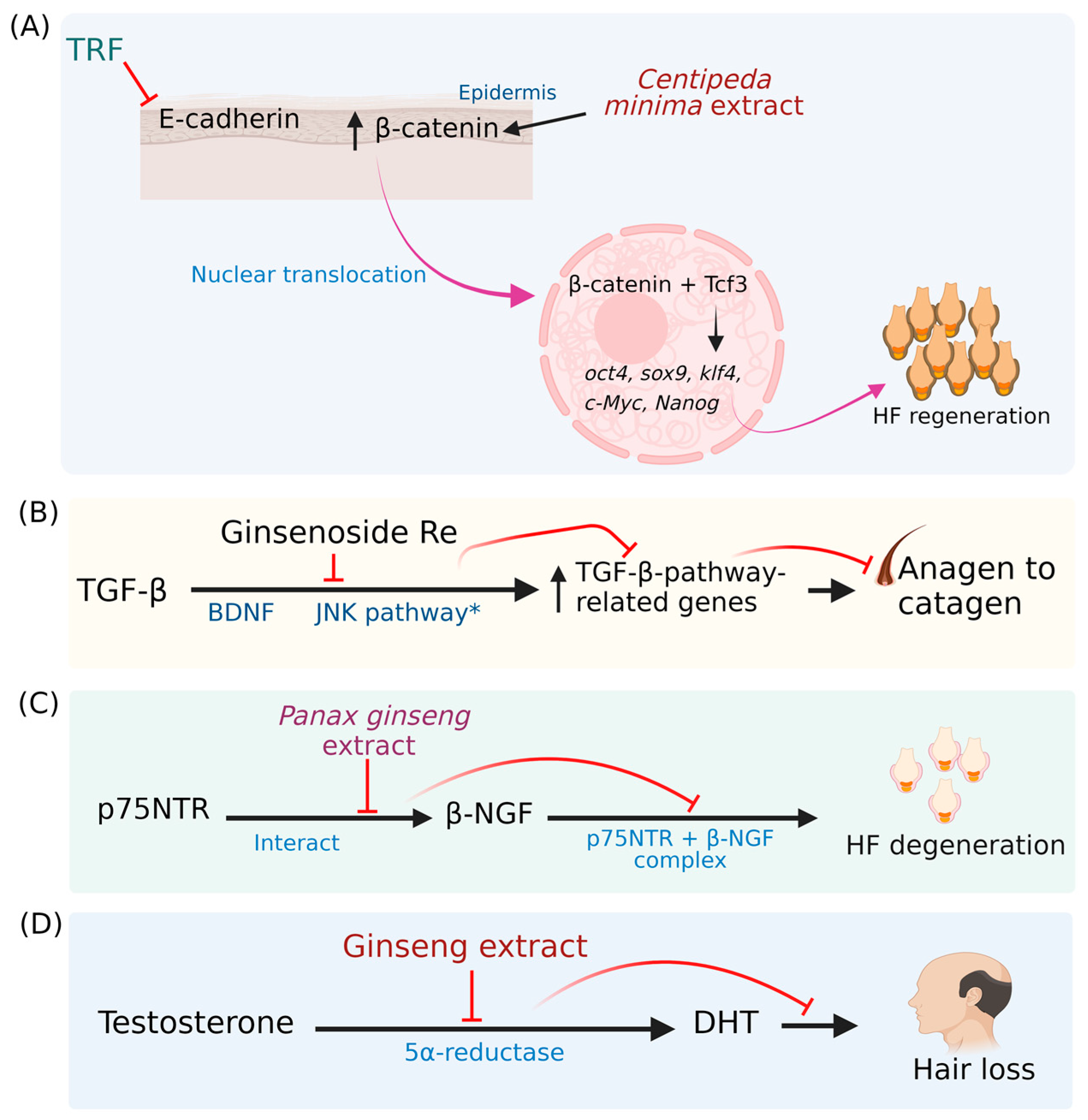
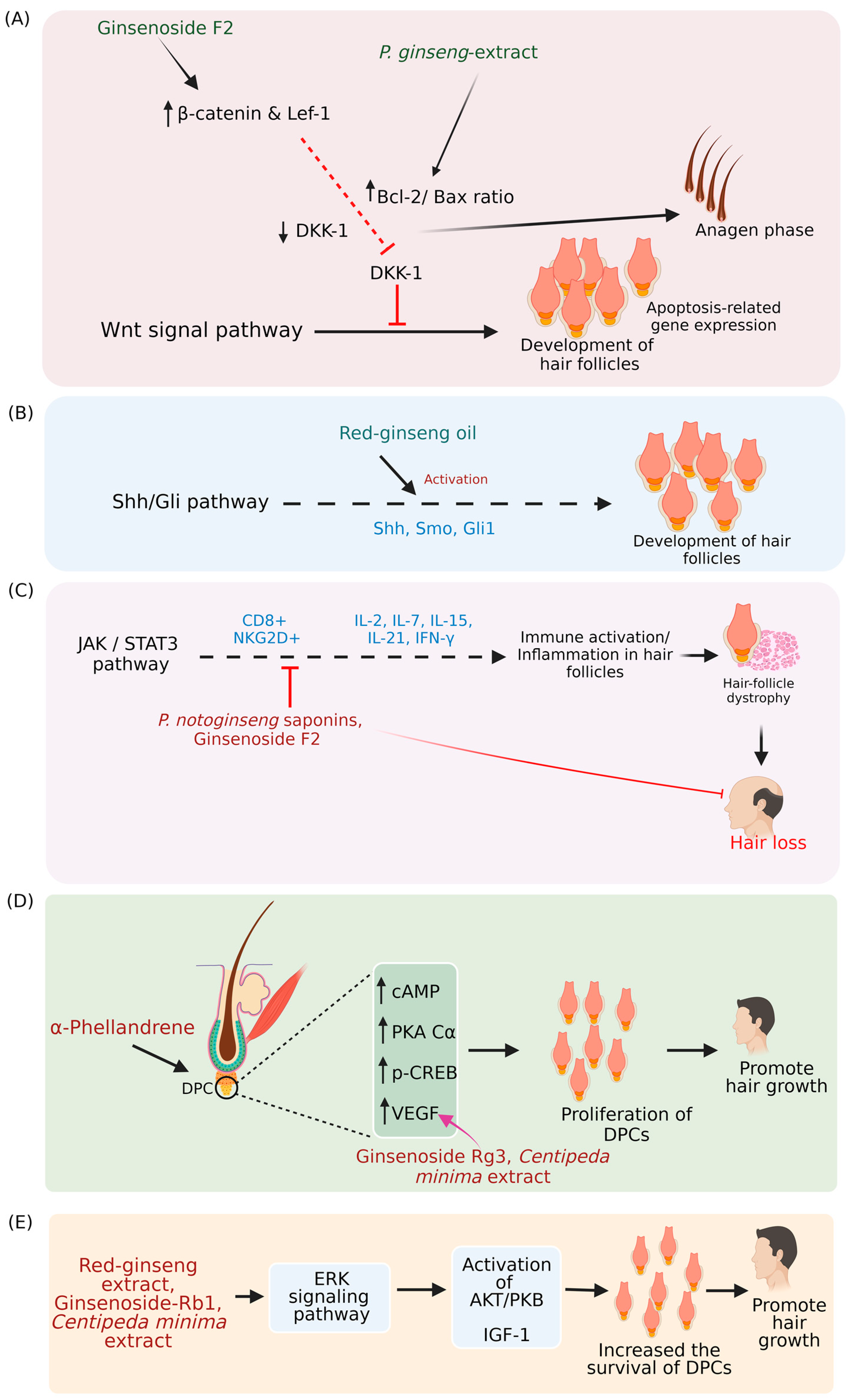
7. Mechanisms Associated with Hair Loss Prevention/Hair Growth-Stimulating Property of Phytochemicals
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Broadley, D.; McElwee, K.J. A “hair-raising” history of alopecia areata. Exp. Dermatol. 2020, 29, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Park, A.M.; Khan, S.; Rawnsley, J. Hair biology: Growth and pigmentation. Facial Plast. Surg. Clin. N. Am. 2018, 26, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Mulinari-Brenner, F.; Neto, F.J.; Rosas, F.M.B.; Torres, L.F.B. Morphometry of normal scalp hair follicles. An. Bras. Dermatol. 2006, 81, 46–52. [Google Scholar] [CrossRef]
- Wosicka, H.; Cal, K. Targeting to the hair follicles: Current status and potential. J. Dermatol. Sci. 2010, 57, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Aging of hair. J. Cosmet. Dermatol. 2005, 4, 60–72. [Google Scholar] [CrossRef]
- Trüeb, R.M.; Henry, J.P.; Davis, M.G.; Schwartz, J.R. Scalp condition impacts hair growth and retention via oxidative stress. Int. J. Trichol. 2018, 10, 262–270. [Google Scholar] [CrossRef]
- Yu, V.; Juhász, M.; Chiang, A.; Atanaskova Mesinkovska, N. Alopecia and associated toxic agents: A systematic review. Skin Appendage Disord. 2018, 4, 245–260. [Google Scholar] [CrossRef]
- Mercke, Y.; Sheng, H.; Khan, T.; Lippmann, S. Hair loss in psychopharmacology. Ann. Clin. Psychiatry 2000, 12, 35–42. [Google Scholar] [CrossRef]
- Chumlea, W.C.; Rhodes, T.; Girman, C.J.; Johnson-Levonas, A.; Lilly, F.R.; Wu, R.; Guo, S.S. Family history and risk of hair loss. Dermatology 2004, 209, 33–39. [Google Scholar] [CrossRef]
- Łukasik, A.; Kozicka, K.; Kłosowicz, A.; Jaworek, A.; Wojas-Pelc, A. The role of family history and its influence on the onset time in female pattern hair loss. Postepy Dermatol. Alergol. 2021, 38, 815–818. [Google Scholar] [CrossRef]
- Choi, S.; Zhang, B.; Ma, S.; Gonzalez-Celeiro, M.; Stein, D.; Jin, X.; Kim, S.T.; Kang, Y.L.; Besnard, A.; Rezza, A.; et al. Corticosterone inhibits GAS6 to govern hair follicle stem-cell quiescence. Nature 2021, 592, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Su, L.H.; Chen, T.H. Association of androgenetic alopecia with smoking and its prevalence among Asian men: A community-based survey. Arch. Dermatol. 2007, 143, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Chaikittisilpa, S.; Rattanasirisin, N.; Panchaprateep, R.; Orprayoon, N.; Phutrakul, P.; Suwan, A.; Jaisamrarn, U. Prevalence of female pattern hair loss in postmenopausal women: A cross-sectional study. Menopause 2022, 29, 415–420. [Google Scholar] [CrossRef]
- Guo, E.L.; Katta, R. Diet and hair loss: Effects of nutrient deficiency and supplement use. Dermatol. Pract. Concept. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Messenger, A.G.; Rundegren, J. Minoxidil: Mechanisms of action on hair growth. Br. J. Dermatol. 2004, 150, 186–194. [Google Scholar] [CrossRef]
- Rogers, N.E.; Avram, M.R. Medical treatments for male and female pattern hair loss. J. Am. Acad. Dermatol. 2008, 59, 547–566. [Google Scholar] [CrossRef] [PubMed]
- Arca, E.; Açikgöz, G.; Taştan, H.B.; Köse, O.; Kurumlu, Z. An open, randomized, comparative study of oral finasteride and 5% topical minoxidil in male androgenetic alopecia. Dermatology 2004, 209, 117–125. [Google Scholar] [CrossRef]
- Olsen, E.A.; Whiting, D.; Bergfeld, W.; Miller, J.; Hordinsky, M.; Wanser, R.; Zhang, P.; Kohut, B. A multicenter, randomized, placebo-controlled, double-blind clinical trial of a novel formulation of 5% minoxidil topical foam versus placebo in the treatment of androgenetic alopecia in men. J. Am. Acad. Dermatol. 2007, 57, 767–774. [Google Scholar] [CrossRef]
- Leavitt, M.; Perez-Meza, D.; Rao, N.A.; Barusco, M.; Kaufman, K.D.; Ziering, C. Effects of finasteride (1 mg) on hair transplant. Dermatol. Surg. 2005, 31, 1268–1276. [Google Scholar] [CrossRef]
- Sung, J.H. Effective and economical cell therapy for hair regeneration. Biomed. Pharmacother. 2023, 157, 13988. [Google Scholar] [CrossRef]
- Hosking, A.M.; Juhasz, M.; Atanaskova Mesinkovska, N. Complementary and alternative treatments for alopecia: A comprehensive review. Skin Appendage Disord. 2019, 5, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Daniels, G.; Akram, S.; Westgate, G.E.; Tamburic, S. Can plant-derived phytochemicals provide symptom relief for hair loss? A critical review. Int. J. Cosmet. Sci. 2019, 41, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Padule, K.; Shinde, S.; Chitlange, S.; Giram, P.; Nagore, D. The advancement of herbal-based nanomedicine for hair. Cosmetics 2022, 9, 118. [Google Scholar] [CrossRef]
- Saansoomchai, P.; Limmongkon, A.; Surangkul, D.; Chewonarin, T.; Srikummool, M. Enhanced VEGF expression in hair follicle dermal papilla cells by Centella asiatica Linn. Chiang Mai Univ. J. Nat. Sci. 2018, 17, 25–37. [Google Scholar] [CrossRef]
- Pandit, S.; Chauhan, N.S.; Dixit, V.K. Effect of Cuscuta reflexa Roxb on androgen-induced alopecia. J. Cosmet. Dermatol. 2008, 7, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Mustarichie, R.; Wicaksono, I.A. Hair growth stimulants activity from Sterculia urceolata JE Smith ethanol extract. Res. J. Pharm. Technol. 2019, 12, 4111–4116. [Google Scholar] [CrossRef]
- Yuen, G.K.; Ho, B.S.; Lin, L.S.; Dong, T.T.; Tsim, K.W. Tectoridin stimulates the activity of human dermal papilla cells and promotes hair shaft elongation in mouse vibrissae hair follicle culture. Molecules 2022, 27, 400. [Google Scholar] [CrossRef]
- Ahmed, N.S.; Ghatak, S.; El Masry, M.S.; Gnyawali, S.C.; Roy, S.; Amer, M.; Everts, H.; Sen, C.K.; Khanna, S. Epidermal E-cadherin dependent β-catenin pathway is phytochemical inducible and accelerates anagen hair cycling. Mol. Ther. 2017, 5, 2502–2512. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y. Hair-growth potential of ginseng and its major metabolites: A review on its molecular mechanisms. Int. J. Mol. Sci. 2018, 19, 2703. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.H.; Xiang, L.J.; Shi, H.X.; Zhang, J.; Jiang, L.P.; Cai, P.T.; Lin, Z.L.; Lin, B.B.; Huang, Y.; Zhang, H.L.; et al. Fibroblast growth factors stimulate hair growth through β-catenin and Shh expression in C57BL/6 mice. BioMed Res. Int. 2015, 2015, 730139. [Google Scholar]
- Coleman, E. Types and Treatment of Hair Loss in Men and Women. Plast. Surg. Nurs. 2020, 40, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Asghar, F.; Shamim, N.; Farooque, U.; Sheikh, H.; Aqeel, R. Telogen effluvium: A review of the literature. Cureus 2020, 12, e8320. [Google Scholar] [CrossRef]
- Harrison, S. Sinclair, R. Telogen effluvium. Clin. Exp. Dermatol. 2002, 27, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Nnoruka, E.N.; Obiagboso, I.; Maduechesi, C. Hair loss in children in South-East Nigeria: Common and uncommon cases. Int. J. Dermatol. 2007, 46, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Headington, J. Telogen effluvium. new concepts and review. Arch. Dermatol. 1993, 129, 356–363. [Google Scholar] [CrossRef]
- Messenger, A.G.; McKillop, J.; Farrant, P.; McDonagh, A.J.; Sladden, M. British Association of Dermatologists’ guidelines for the management of alopecia areata 2012. Br. J. Dermatol. 2012, 166, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Lundin, M.; Chawa, S.; Sachdev, A.; Bhanusali, D.; Seiffert-Sinha, K.; Sinha, A.A. Gender differences in alopecia areata. J. Drugs Dermatol. 2014, 13, 409–413. [Google Scholar] [PubMed]
- Filbrandt, R.; Rufaut, N.; Jones, L.; Sinclair, R. Primary cicatricial alopecia: Diagnosis and treatment. CMAJ 2013, 185, 1579–1585. [Google Scholar] [CrossRef]
- Villablanca, S.; Fischer, C.; García-García, S.C.; Mascaró-Galy, J.M.; Ferrando, J. Primary scarring alopecia: Clinical-pathological review of 72 cases and review of the literature. Skin Appendage Disord. 2017, 3, 132–143. [Google Scholar] [CrossRef]
- Kyei, A.; Bergfeld, W.F.; Piliang, M.; Summers, P. Medical and environmental risk factors for the development of central centrifugal cicatricial alopecia. Arch. Dermatol. 2011, 147, 909–914. [Google Scholar] [CrossRef]
- Finner, A.M. Nutrition and hair. Deficiencies and supplements. Dermatol. Clin. 2013, 31, 167–172. [Google Scholar] [CrossRef] [PubMed]
- MacFarquhar, J.K.; Broussard, D.L.; Melstrom, P.; Hutchinson, R.; Wolkin, A.; Martin, C.; Burk, R.F.; Dunn, J.R.; Green, A.L.; Hammond, R.; et al. Acute selenium toxicity associated with a dietary supplement. Arch. Intern. Med. 2010, 170, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Hammel, C.; Kyriakopoulos, A.; Behne, D.; Gawlik, D.; Brätter, P. Protein-bound selenium in the seeds of coco de mono (Lecythis ollaria). J. Trace. Elem. Med. Biol. 1996, 10, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.; Desel, H. Acute selenium poisoning by paradise nuts (Lecythis ollaria). Hum. Exp. Toxicol. 2010, 29, 431–434. [Google Scholar] [CrossRef]
- Morinaga, H.; Mohri, Y.; Grachtchouk, M.; Asakawa, K.; Matsumura, H.; Oshima, M.; Takayama, N.; Kato, T.; Nishimori, Y.; Sorimachi, Y.; et al. Obesity accelerates hair thinning by stem cell-centric converging mechanisms. Nature 2021, 595, 266–271. [Google Scholar] [CrossRef]
- James, M.J.; Gibson, R.A.; Cleland, L.G. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am. J. Clin. Nutr. 2000, 71, 343S–348S. [Google Scholar] [CrossRef]
- Goluch-Koniuszy, Z.S. Nutrition of women with hair loss problem during the period of menopause. Menopause Rev. 2016, 15, 56–61. [Google Scholar] [CrossRef]
- Lee, W.S.; Ro, B.I.; Hong, S.P.; Bak, H.; Sim, W.Y.; Kim, D.W.; Park, J.K.; Ihm, C.W.; Eun, H.C.; Kwon, O.S.; et al. A new classification of pattern hair loss that is universal for men and women: Basic and specific (BASP) classification. J. Am. Acad. Dermatol. 2007, 57, 37–46. [Google Scholar] [CrossRef]
- Flores, A.; Schell, J.; Krall, A.S.; Jelinek, D.; Miranda, M.; Grigorian, M.; Braas, D.; White, A.C.; Zhou, J.L.; Graham, N.A.; et al. Lactate dehydrogenase activity drives hair follicle stem cell activation. Nat. Cell Biol. 2017, 19, 1017–1026. [Google Scholar] [CrossRef]
- Figlak, K.; Paus, R.; Williams, G.; Philpott, M. 597 Outer root sheath is able to synthesise glycogen from lactate-investigating glycogen metabolism in human hair follicles. J. Investig. Dermatol. 2019, 139, S317. [Google Scholar] [CrossRef]
- Shi, X.; Tuan, H.; Na, X.; Yang, H.; Yang, Y.; Zhang, Y.; Xi, M.; Tan, Y.; Yang, C.; Zhang, J.; et al. The association between sugar-sweetened beverages and male pattern hair loss in young men. Nutrients 2023, 15, 214. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.L.; Deng, J.F.; Lin, K.P.; Tsai, W.J. Lead, mercury, and arsenic poisoning due to topical use of traditional Chinese medicines. Am. J. Med. 2013, 126, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Namba, Y.; Suzuki, R.; Sasaki, J.; Takayasu, M.; Watanabe, K.; Kenji, D.; Hayashi, M.; Kitamura, Y.; Kawamo, M.; Masaki, H.; et al. Thallium group poisoning incident in Japan 2011. Crit. Care 2013, 17 (Suppl. 2), P269. [Google Scholar] [CrossRef][Green Version]
- Sharquie, K.E.; Ibrahim, G.A.; Noaimi, A.A.; Hamudy, H.K. Outbreak of thallium poisoning among Iraqi patients. JSSDDS 2011, 15, 29–32. [Google Scholar] [CrossRef]
- Moeschlin, S. Thallium poisoning. Clin. Toxicol. 1980, 17, 133–146. [Google Scholar] [CrossRef]
- Fernando, R.; Fernando, D.N. Poisoning with plants and mushrooms in Sri Lanka: A retrospective hospital-based study. Vet. Hum. Toxicol. 1990, 32, 579–581. [Google Scholar]
- Nakamura, N.; Fujita, T.; Murakami, R.; Kumasaka, R.; Shimada, M.; Shimaya, Y.; Osawa, H.; Yamabe, H.; Okumura, K.; Yachie, A. A case of familial Mediterranean fever-associated systemic amyloidosis. CEN Case Rep. 2012, 1, 4–6. [Google Scholar] [CrossRef][Green Version]
- Alpsoy, E.; Leccese, P.; Emmi, G.; Ohno, S. Treatment of Behçet’s disease: An algorithmic multidisciplinary approach. Front. Med. 2021, 8, 624795. [Google Scholar] [CrossRef]
- Richette, P.; Bardin, T. Colchicine for the treatment of gout. Expert Opin. Pharmacother. 2010, 11, 2933–2938. [Google Scholar] [CrossRef]
- Finkelstein, Y.; Aks, S.E.; Hutson, J.R.; Juurlink, D.N.; Nguyen, P.; Dubnov-Raz, G.; Pollak, U.; Koren, G.; Bentur, Y. Colchicine poisoning: The dark side of an ancient drug. Clin. Toxicol. 2010, 48, 407–414. [Google Scholar] [CrossRef]
- Folpini, A.; Furfori, P. Colchicine toxicity-clinical features and treatment. Massive overdose case report. J. Toxicol. Clin. Toxicol. 1995, 33, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Levsky, M.E.; Miller, M.A.; Masneri, D.A.; Borys, D. Colchicine exposures: The Texas experience. South. Med. J. 2008, 101, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Premaratna, R.; Weerasinghe, M.S.; Premawardana, N.P.; de Silva, H.J. Gloriosa superba poisoning mimicking an acute infection- a case report. BMC Pharmacol. Toxicol. 2015, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Biçer, S.; Soysal, D.D.; Ctak, A.; Uçsel, R.; Karaböcüoğlu, M.; Uzel, N. Acute colchicine intoxication in a child: A case report. Pediatr. Emerg. Care 2007, 23, 314–317. [Google Scholar]
- Combalia, A.; Baliu-Piqué, C.; Fortea, A.; Ferrando, J. Anagen effluvium following acute colchicine poisoning. Int. J. Trichol. 2016, 8, 171–172. [Google Scholar] [CrossRef]
- Alaygut, D.; Kilic, S.C.; Kaya, A.; Oflaz, M.B.; Bolat, F.; Cevit, Ö.; Icagasioglu, F.D. Assessment of 17 pediatric cases with colchicine poisoning in a 2-year period. Pediatr. Emerg. Care 2016, 32, 168–172. [Google Scholar] [CrossRef]
- Kim, K.; Shin, M.K.; Kim, J.H.; Kim, M.H.; Haw, C.R.; Park, H.K. Effects of atopic dermatitis on the morphology and water content of scalp hair. Micros. Res. Tech. 2012, 75, 620–625. [Google Scholar] [CrossRef]
- Plozzer, C.; Coletti, C.; Kokelj, F.; Trevisan, G. Scanning electron microscopy study of hair shaft disorders in psoriasis. Acta Derm. Venereol. Suppl. (Stockh) 2000, 211, 9–11. [Google Scholar] [CrossRef]
- Kumar, B.; Soni, A.; Saraswat, A.; Kaur, I.; Dogra, S. Hair in psoriasis: A prospective, blinded scanning electron microscopic study. Clin. Exp. Dermatol. 2008, 33, 491–494. [Google Scholar] [CrossRef]
- Shin, M.K.; Kim, K.S.; Ahn, J.J.; Kim, N.I.; Park, H.K.; Haw, C.R. Investigation of the hair of patients with scalp psoriasis using atomic force microscopy. Clin. Exp. Dermatol. 2012, 37, 156–163. [Google Scholar] [CrossRef]
- Kim, K.S.; Shin, M.K.; Ahn, J.J.; Haw, C.R.; Park, H.K. A comparative study of hair shafts in scalp psoriasis and seborrheic dermatitis using atomic force microscopy. Skin Res. Technol. 2013, 19, e60–e64. [Google Scholar]
- Pitney, L.; Weedon, D.; Pitney, M. Is seborrhoeic dermatitis associated with a diffuse, low-grade folliculitis and progressive cicatricial alopecia? Australas J. Dermatol. 2016, 57, e105–e107. [Google Scholar] [CrossRef]
- Dhaille, F.; Dillies, A.S.; Dessirier, F.; Reygagne, P.; Diouf, M.; Baltazard, T.; Lombart, F.; Hébert, V.; Chopinaud, M.; Verneuil, L.; et al. A single typical trichoscopic feature is predictive of tinea capitis: A prospective multicentre study. Br. J. Dermatol. 2019, 181, 1046–1051. [Google Scholar] [CrossRef]
- Mosley, J.G.; Gibbs, A.C. Premature grey hair and hair loss among smokers: A new opportunity for health education? BMJ 1996, 313, 1616. [Google Scholar] [CrossRef]
- Chaudhry, M.; Ashraf, T.; Zeeshan, N.; Hanif, A.; Khan, M.; Ghazanfar, I. Association of smoking with baldness and graying of hair among male adults. Biomedica 2018, 34, 53–56. [Google Scholar]
- Babadjouni, A.; Pouldar Foulad, D.; Hedayati, B.; Evron, E.; Mesinkovska, N. The effects of smoking on hair health: A systematic review. Skin Appendage Disord. 2021, 7, 251–264. [Google Scholar] [CrossRef]
- Thom, E. Stress and the hair growth cycle: Cortisol-induced hair growth disruption. J. Drugs Dermatol. 2016, 15, 1001–1004. [Google Scholar]
- Tosti, A.; Duque-Estrada, B. Treatment strategies for alopecia. Expert Opin. Pharmacother. 2009, 10, 1017–1026. [Google Scholar] [CrossRef]
- Diani, A.R.; Mulholland, M.J.; Shull, K.L.; Kubicek, M.F.; Johnson, G.A.; Schostarez, H.J.; Brunden, M.N.; Buhl, A.E. Hair growth effects of oral administration of finasteride, a steroid 5-alpha reductase inhibitor, alone and in combination with topical minoxidil in the balding stump-tail macaque. J. Clin. Endocrinol. Metab. 1992, 74, 345–350. [Google Scholar]
- Olsen, E.A.; Hordinsky, M.; Whiting, D.; Stough, D.; Hobbs, S.; Ellis, M.L.; Wilson, T.; Rittmaster, R.S.; Dutasteride Alopecia Research Team. The importance of dual 5 alpha redutase inhibition in the treatment of male pattern hair loss: Results of a randomized placebo-controlled study of dutasteride versus finasteride. J. Am. Acad. Dermatol. 2006, 55, 1014–1023. [Google Scholar] [CrossRef]
- Vexiau, P.; Chaspoux, C.; Boudou, P. Effects of minoxidil 2% vs. cyproterone acetate treatment on female androgenetic alopecia: A controlled, 12-month randomized trial. Br. J. Dermatol. 2002, 146, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Hordinsky, M.; Roberts, J.L.; Whiting, D.A.; Dermatologic Consortium for Women’s Health. Female pattern hair loss. J. Am. Acad. Dermatol. 2002, 47, 795. [Google Scholar] [CrossRef]
- Iorizzo, M.; Vincenzi, C.; Voudouris, S.; Piraccini, B.M.; Tosti, A. Finasteride treatment of female pattern hair loss. Arch. Dermatol. 2006, 142, 298–302. [Google Scholar] [CrossRef]
- Kohler, C.; Tschumi, K.; Bodmer, C.; Schneiter, M.; Birkhaeuser, M. Effect of finasteride 5 mg (Proscar) on acne and alopecia in female patients with normal serum levels of free testosterone. Gynecol. Endocrinol. 2007, 23, 142–145. [Google Scholar] [CrossRef]
- Pekmezci, E.; Dundar, C.; Turkoglu, M. Proprietary herbal extract downregulates the gene expression of IL-1α in HaCaT Cells: Possible implications against nonscarring alopecia. Med. Arch. 2018, 72, 136–140. [Google Scholar] [CrossRef]
- Kang, W.; Park, S.; Choi, D.; Son, B.; Park, T. Activation of cAMP signaling in response to α-Phellandrene promotes vascular endothelial growth factor levels and proliferation in human dermal papilla cells. Int. J. Mol. Sci. 2022, 23, 8959. [Google Scholar] [CrossRef]
- Himaniarwati; Arba, M.; Susilawati, Y.; Mustarichie, R. Hair growth promoting activity of Langir bark (Albizia saponaria Lour.) ethanol extract: In vivo assay. Rasayan J. Chem. 2022, 15, 2065–2071. [Google Scholar] [CrossRef]
- León, F.; Hernandez-Zapata, V.; Bacab, M.C.; Maldonado, G.; Lezama, J.A.; Monteon, V. The wound healing action of a cream latex formulation of Jatropha gaumeri Greenm. in a pre-clinical model. Vet. World 2020, 13, 2508–2514. [Google Scholar] [CrossRef]
- Marcovici, G.; Bauman, A. An uncontrolled case series using a botanically derived, β-cyclodextrin inclusion complex in two androgenetic alopecia-affected male subjects. Cosmetics 2020, 7, 65. [Google Scholar] [CrossRef]
- Skowrońska, W.; Granica, S.; Dziedzic, M.; Kurkowiak, J.; Ziaja, M.; Bazylko, A. Arctium lappa and Arctium tomentosum, sources of Arctii radix: Comparison of anti-lipoxygenase and antioxidant activity as well as the chemical composition of extracts from aerial parts and from roots. Plants 2021, 10, 78. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, M.J.; Lee, W.Y.; Pyo, J.; Shin, M.S.; Hwang, G.S.; Shin, D.; Kim, C.E.; Park, E.S.; Kang, K.S. Hair growth stimulation effect of Centipeda minima extract: Identification of active compounds and anagen-activating signaling pathways. Biomolecules 2021, 11, 976. [Google Scholar] [CrossRef] [PubMed]
- Madhunithya, E.; Venkatesh, G.; Shyamala, G.; Manjari, V.; Ramesh, S.; Karuppaiah, A.; Sankar, V. Development of ethosome comprising combined herbal extracts and its effect on hair growth. Adv. Trad. Med. 2021, 21, 131–141. [Google Scholar] [CrossRef]
- Serruya, R.; Maor, Y. Hair growth-promotion effects at the cellular level and antioxidant activity of the plant-based extract Phyllotex™. Heliyon 2021, 7, e07888. [Google Scholar] [CrossRef] [PubMed]
- Ruksiriwanich, W.; Khantham, C.; Muangsanguan, A.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; Sringarm, K.; Ferrer, E.; et al. Phytochemical constitution, anti-inflammation, anti-androgen, and hair growth-promoting potential of shallot (Allium ascalonicum L.) extract. Plants 2022, 11, 1499. [Google Scholar] [CrossRef]
- Hyun, J.; Im, J.; Kim, S.-W.; Kim, H.Y.; Seo, I.; Bhang, S.H. Morus alba root extract induces the anagen phase in the human hair follicle dermal papilla cells. Pharmaceutics 2021, 13, 1155. [Google Scholar] [CrossRef]
- Im, J.; Hyun, J.; Kim, S.W.; Bhang, S.H. Enhancing the angiogenic and proliferative capacity of dermal fibroblasts with mulberry (Morus alba L) root extract. Tissue Eng. Regen. Med. 2022, 19, 49–57. [Google Scholar] [CrossRef]
- Galkin, A.Y.; Solovjova, V.F.; Dugan, A.M. Anti-inflammatory and immunomodulating properties of the herbal preparation indicated for prevention and treatment of alopecia. Botanics 2013, 3, 49–55. [Google Scholar] [CrossRef]
- Park, S.O.; Park, B.S.; Noh, G.Y. Action mechanism of natural plant extracts for hair loss prevention and hair growth promotion in C57BL/6 Mice. Int. J. Pharmacol. 2015, 11, 588–595. [Google Scholar] [CrossRef]
- Susanti, L.; Mustarichie, R.; Halimah, E.; Kurnia, D.; Setiawan, A.; Maladan, Y. Anti-alopecia activity of alkaloids group from noni fruit against dihydrotestosterone-induced male rabbits and its molecular mechanism: In vivo and in silico studies. Pharmaceuticals 2022, 15, 1557. [Google Scholar] [CrossRef]
- Joshi, P.S.; Patil, Y.B.; Nagarkar, B.; Paul, T.S.; Apte, K.G. In vivo phytotherapy in BALB/c athymic nude mice: Hair growth promotion using Ficus religiosa L. and Morus alba L. J. Nat. Remedies 2021, 21, 51–60. [Google Scholar] [CrossRef]
- Park, S.O.; Park, B.S.; Noh, G.Y. Effect of natural plant extract (Abelmo) on action mechanism and hair growth activities in C57BL/6 mice. J. Korean Oil chem. Soc. 2014, 31, 653–662. [Google Scholar] [CrossRef]
- Rao, G.V.; Mukhopadhyay, T.; Ranganathan, S.; Madhavi, M.S.L.; Annamalai, T.; Lavakumar, S. Chemical examination of three Indian medicinal plants and their hair growth evaluation studies. Arch. Appl. Sci. Res. 2013, 5, 126–130. [Google Scholar]
- Rusu, M.; Csedo, C.; Marcus, G.; Lupuliasa, D. Preclinical study on the hair growth and regeneration of external use lotions containing castor oil (Ricini oleum) in rabbits. Farmacia 2008, 56, 507–512. [Google Scholar]
- Kporou, E.; Sitapha, O.; Moussa, G.; Gouedji, Y.; Kra, A.; Djaman, J. Quality, safety and activity of an ointment formulated from Butyrospermum parkii and Ricinus communis oils on rabbits hair growth. Rev. RAMReS-Ser. Pharm. Med. Trad. Afr. 2021, 20, 38–46. [Google Scholar]
- Tiwari, R.; Tiwari, G.; Yadav, A.; Ramachandran, V. Development and evaluation of herbal hair serum: A traditional way to improve hair quality. Open Dermatol. J. 2021, 15, 52–58. [Google Scholar] [CrossRef]
- Begum, S.; Lee, M.R.; Gu, L.J.; Hossain, M.J.; Kim, H.K.; Sung, C.K. Comparative hair restorer efficacy of medicinal herb on nude (Foxn1nu) mice. BioMed Res. Int. 2014, 2014, 319795. [Google Scholar] [CrossRef]
- Kumar, N.; Chaiyasut, C. Hair growth promoting activity of Carthamus tinctorius florets extract-loaded nanostructured lipid carriers. Int. J. Pharm. Pharm. Sci. 2015, 7, 252–257. [Google Scholar]
- Imtiaz, F.; Islam, M.; Saeed, H.; Saleem, B.; Asghar, M.; Saleem, Z. Impact of Trigonella foenum-graecum leaves extract on mice hair growth. Pakistan J. Zool. 2017, 49, 1405–1412. [Google Scholar] [CrossRef]
- Dahmani, M.M.; Laoufi, R.; Selama, O.; Arab, K. Gas chromatography coupled to mass spectrometry characterization, anti-inflammatory effect, wound-healing potential, and hair growth-promoting activity of Algerian Carthamus caeruleus L. (Asteraceae). Indian J. Pharmacol. 2018, 50, 123–129. [Google Scholar] [CrossRef]
- Trivedi, R.V.; Bansod, P.G.; Taksande, J.B.; Mahore, J.G.; Tripurneni, S.R.; Rai, K.R.; Umekar, M.J. Investigation of hair growth promoting ability of herbal gel containing Zingiber officinale. Int. J. Res. Pharm. Sci. 2019, 10, 3498–3507. [Google Scholar] [CrossRef]
- Sakib, S.A.; Tareq, A.M.; Islam, A.; Rakib, A.; Islam, M.N.; Uddin, M.A.; Rahman, M.M.; Seidel, V.; Emran, T.B. Anti-inflammatory, thrombolytic and hair-growth promoting activity of the n-hexane fraction of the methanol extract of Leea indica leaves. Plants 2021, 10, 1081. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.C.; Nam, G.W.; Jeong, N.H.; Choi, B.Y. Hair growth promotion by extracts of Inula Helenium and Caesalpinia sappan bark in patients with androgenetic alopecia: A pre-clinical study using phototrichogram analysis. Cosmetics 2019, 6, 66. [Google Scholar] [CrossRef]
- Ablon, G. A 3-month, randomized, double-blind, placebo-controlled study evaluating the ability of an extra-strength marine protein supplement to promote hair growth and decrease shedding in women with self-perceived thinning hair. Dermatol. Res. Pract. 2015, 2015, 841570. [Google Scholar] [CrossRef]
- Ablon, G.; Kogan, S. A randomized, double-blind, placebo-controlled study of a nutraceutical supplement for promoting hair growth in perimenopausal, menopausal, and postmenopausal women with thinning hair. J. Drugs Dermatol. 2021, 20, 55–61. [Google Scholar] [CrossRef]
- Ablon, G.; Kogan, S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J. Drugs Dermatol. 2018, 17, 558–565. [Google Scholar] [PubMed]
- Kim, B.H.; Lee, W.-Y.; Trinh, T.A.; Pyo, J.S.; Lee, S.; Kim, C.-E.; Lee, D.H.; Park, E.-S.; Kang, K.S. Hair growth effect of emulsion extracted brevilin A, a JAK3 inhibitor, from Centipeda minima. Processes 2020, 8, 767. [Google Scholar] [CrossRef]
- Sim, J.B.; Park, S.O.; Park, B.S.; Noh, G.Y. Effect of natural plant extracts on hair loss prevent in people with alopecia. Asian J. Dermatol. 2016, 8, 8–13. [Google Scholar] [CrossRef]
- Shin, S.; Kim, K.; Lee, M.J.; Lee, J.; Choi, S.; Kim, K.S.; Ko, J.M.; Han, H.; Kim, S.Y.; Youn, H.J.; et al. Epigallocatechin gallate-mediated alteration of the microRNA expression profile in 5-dihydrotestosterone-treated human dermal papilla cells. Ann. Dermatol. 2016, 28, 327–334. [Google Scholar] [CrossRef]
- Fischer, T.W.; Herczeg-Lisztes, E.; Funk, W.; Zillikens, D.; Bıro, T.; Paus, R. Differential effects of caffeine on hair shaft elongation, matrix and outer root sheath keratinocyte proliferation, and transforming growth factor-beta2/insulin-like growth factor- 1-mediated regulation of the hair cycle in male and female human hair follicles in vitro. Br. J. Dermatol. 2014, 171, 1031–1043. [Google Scholar]
- Ehsani, A.H.; Toosi, S.; Seirafi, H.; Akhyani, M.; Hosseini, M.; Azadi, R.; Noormohamadpour, P.; Ghanadan, A. Capsaicin vs. clobetasol for the treatment of localized alopecia areata. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1451–1453. [Google Scholar] [CrossRef]
- Ablon, G. A 6-month, randomized, double-blind, placebo-controlled study evaluating the ability of a marine complex supplement to promote hair growth in men with thinning hair. J. Cosmet. Dermatol. 2016, 15, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Sharquie, K.E.; Al-Obaidi, H.K. Onion juice (Allium cepa L.), a new topical treatment for alopecia areata. J. Dermatol. 2002, 29, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kamimura, A.; Yokoo, Y.; Honda, S.; Watanabe, Y. The first clinical trial of topical application of procyanidin B-2 to investigate its potential as a hair growing agent. Phytother. Res. 2001, 15, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.H.; Lee, S.Y.; Jeong, D.W.; Choi, E.J.; Kim, Y.J.; Lee, J.G.; Yi, Y.H.; Cha, H.S. Effect of pumpkin seed oil on hair growth in men with androgenetic alopecia: A randomized, double-blind, placebo-controlled trial. Evid.-Based Complement. Altern. Med. 2014, 2014, 549721. [Google Scholar] [CrossRef]
- Park, G.H.; Park, K.Y.; Cho, H.I.; Lee, S.M.; Han, J.S.; Won, C.H.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Moon, K.C.; et al. Red ginseng extract promotes the hair growth in cultured human hair follicles. J. Med. Food. 2015, 18, 354–362. [Google Scholar] [CrossRef]
- Wessagowit, V.; Tangjaturonrusamee, C.; Kootiratrakarn, T.; Bunnag, T.; Pimonrat, T.; Muangdang, N.; Pichai, P. Treatment of male androgenetic alopecia with topical products containing Serenoa repens extract. Australas J. Dermatol. 2016, 57, e76–e82. [Google Scholar] [CrossRef]
- Fischer, T.W.; Hipler, U.C.; Elsner, P. Effect of caffeine and testosterone on the proliferation of human hair follicles in vitro. Int. J. Dermatol. 2007, 46, 27–35. [Google Scholar] [CrossRef]
- Dhurat, R.; Chitallia, J.; May, T.W.; Jayaraaman, A.M.; Madhukara, J.; Anandan, S.; Vaidya, P.; Klenk, A. An open-label randomized multicenter study assessing the noninferiority of a caffeine based topical liquid 0.2% versus minoxidil 5% solution in male androgenetic alopecia. Skin Pharmacol. Physiol. 2017, 30, 298–305. [Google Scholar] [CrossRef]
- Hamed, F.N.; McDonagh, A.J.G.; Almaghrabi, S.; Bakri, Y.; Messenger, A.G.; Tazi-Ahnini, R. Epigallocatechin-3 gallate inhibits STAT-1/JAK2/IRF-1/HLA-DR/HLAB and reduces CD8 MKG2D lymphocytes of alopecia areata patients. Int. J. Environ. Res. Public Health 2018, 15, 2882. [Google Scholar] [CrossRef]
- Shin, H.; Cho, A.R.; Kim, D.Y.; Munkhbayer, S.; Choi, S.J.; Jang, S.; Kim, S.H.; Shin, H.C.; Kwon, O. Enhancement of human hair growth using Ecklonia cava Polyphenols. Ann. Dermatol. 2016, 28, 15–21. [Google Scholar] [CrossRef]
- Rastegar, H.; Ashtiani, H.A.; Aghaei, M.; Barikbin, B.; Ehsani, A. Herbal extracts induce dermal papilla cell proliferation of human hair follicles. Ann. Dermatol. 2015, 27, 667–675. [Google Scholar] [CrossRef]
- Pekmezci, E.; Dundar, C.; Turkoglu, M. A proprietary herbal extract against hair loss in androgenetic alopecia and telogen effluvium: A placebo-controlled, single-blind, clinical-instrumental study. Acta Dermatovenerol. Alp. Pannonica Adriat. 2018, 27, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Mari, E.; Scarno, M.; Garelli, V.; Maxia, C.; Scali, E.; Iorio, A.; Carlesimo, M. Comparative effectiveness of finasteride vs Serenoa repens in male androgenetic alopecia: A two-year study. Int. J. Immunopathol. Pharmacol. 2012, 25, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Prager, N.; Bickett, K.; French, N.; Marcovici, G. A randomized, double-blind, placebo-controlled trial to determine the effectiveness of botanically derived inhibitors of 5-alpha-reductase in the treatment of androgenetic alopecia. J. Altern. Complement. Med. 2002, 8, 143–152. [Google Scholar] [CrossRef]
- Li, Z.; Ryu, S.W.; Lee, J.; Choi, K.; Kim, S.; Choi, C. Protopanaxatirol type ginsenoside Re promotes cyclic growth of hair follicles via inhibiting transforming growth factor β signaling cascades. Biochem. Biophys. Res. Commun. 2016, 470, 924–929. [Google Scholar] [CrossRef]
- Hibino, T.; Nishiyama, T. Role of TGF-beta2 in the human hair cycle. J. Dermatol. Sci. 2004, 35, 9–18. [Google Scholar] [CrossRef]
- Suzuki, A.; Matsuura, D.; Kanatani, H.; Yano, S.; Tsunakawa, M.; Matsuyama, S.; Shigemori, H. Inhibitory effects of polyacetylene compounds from Panax ginseng on neurotrophin receptor-mediated hair growth. Biol. Pharm. Bull. 2017, 40, 1784–1788. [Google Scholar] [CrossRef]
- Shin, D.H.; Cha, Y.J.; Yang, K.E.; Jang, I.S.; Son, C.G.; Kim, B.H.; Kim, J.M. Ginsenoside rg3 up-regulates the expression of vascular endothelial growth factor in human dermal papilla cells and mouse hair follicles. Phytother. Res. 2014, 28, 1088–1095. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.N.; Hong, Y.D.; Park, B.C.; Na, Y. Panax ginseng extract antagonizes the effect of DKK1-induced catagen-like changes of hair follicles. Int. J. Mol. Med. 2017, 40, 1194–1200. [Google Scholar] [CrossRef]
- Shin, H.S.; Park, S.Y.; Hwang, E.S.; Lee, D.G.; Song, H.G.; Mavlonov, G.T.; Yi, T.H. The inductive effect of ginsenoside F2 on hair growth by altering the Wnt signal pathway in telogen mouse skin. Eur. J. Pharmacol. 2014, 730, 82–89. [Google Scholar] [CrossRef]
- Truong, V.L.; Bak, M.J.; Lee, C.; Jun, M.; Jeong, W.S. Hair regenerative mechanisms of red ginseng oil and its major components in the testosterone-induced delay of anagen entry in C57BL/6 mice. Molecules 2017, 22, 1505. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Huyghues-Despointes, B.M.; Tsai, J.; Scholtz, J.M. NMR study and molecular dynamics simulations of optimized beta-hairpin fragments of protein G. Proteins 2007, 69, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Seo, W.; Eun, H.S.; Kim, S.Y.; Jo, E.; Kim, M.H.; Choi, W.M.; Lee, J.H.; Shim, Y.R.; Cui, C.H.; et al. Protective effects of ginsenoside F2 on 12-O-tetradecanoylphorbol-13-acetate-induced skin inflammation in mice. Biochem. Biophys. Res. Commun. 2016, 478, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Andl, T.; Reddy, S.T.; Gaddapara, T.; Millar, S.E. Wnt signals are required for the initiation of hair follicle development. Dev. Cell 2002, 2, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Ito, N.; Takigawa, M.; Ito, T. The hair follicle and immune privilege. J. Investig. Dermatol. Symp. Proc. 2003, 8, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Dai, Z.; Jabbari, A.; Cerise, J.E.; Higgins, C.A.; Gong, W.; de Jong, A.; Harel, S.; DeStefano, G.M.; Rothman, L.; et al. Alopecia areata is driven by cytotoxic t lymphocytes and is reversed by Jak inhibition. Nat. Med. 2014, 20, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ito, N.; Saathoff, M.; Bettermann, A.; Takigawa, M.; Paus, R. Interferon-gamma is a potent inducer of catagen-like changes in cultured human anagen hair follicles. Br. J. Dermatol. 2005, 152, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Bratty, M.A.; Alhazmi, H.A.; Thangavel, N. GC–MS profiling and in silico prediction of MAPK receptor activation by fatty acids of watercress oil for hair growth marketed in Saudi Arabia. J. Saudi Chem. Soc. 2021, 25, 101196. [Google Scholar] [CrossRef]
| Hair Loss Type | Treatments |
|---|---|
| Androgenic alopecia | Topical minoxidil (2 to 5%), oral finasteride/dutasteride, hair transplantation |
| Alopecia areata | Topical minoxidil (5%), corticosteroids (Topical or oral) |
| Alopecia totalis | Hair transplantation |
| Cicatricial alopecia | Topical or oral antibiotics, corticosteroids, scalp reduction surgery, hair transplantation |
| Senescent alopecia | Topical minoxidil |
| Traction alopecia | Anti-inflammatory agents, hair transplantation |
| Telogen effluvium | 5-α reductase inhibitors |
| Phytochemicals | Mechanism of Action | References |
|---|---|---|
| Caffeine | 5α-reductase inhibition. Stimulates the HFK and ORS proliferation. Reduces oxidative stress. Reduces apoptosis and necrosis. | [119,127,128] |
| Epigallocatechin gallate (Camellia sinensis (L.) Kuntze) | Extension of analgen phase. Reduces oxidative cell damage and oxidative stress. Reduces the senescence-related gene expression. Inhibits the IFN-γ signaling. | [118,129,130] |
| Procyanidin B2 | Suppresses inflammation. | [123] |
| Herbal mix * (Chamaemelum nobile (L.) All., Althaea officinalis L., Persea Americana Mill., Rosmarinus officinalis L., Aloe vera (L.) Burm. F., Urtica dioica, Thymus vulgaris L.) | 5α-reductase 2 inhibition. Inhibits apoptosis. | [131] |
| Herbal mix (Urtica uren L., Urtica dioica, Matricaria chamomilla, Achillea millefolium, Ceratonia siliqua, Equisetum arvense) contains vitamins B1, B2, B6 and C, and myricetin, quercetin, kaempferol, and trace elements (iron, zinc, and copper) | Induces the HF effects. | [132] |
| Vitamin C, zinc, horsetail stem extract, and flax seed extract * | Systemic effects. | [121] |
| Capsaicin (Capsicum annuum L.) | Stimulates the perifollicular nerves. Stimulates the HF immune system. | [120] |
| Crude onion juice (Allium cepa L.) * | Induction of immunological reaction and antigenic competition. | [122] |
| Red ginseng extract (Panax ginseng C.A. Mey.) | Increased cell proliferation. | [125] |
| Red ginseng extract + ginsenoside Rb1 and ginsenoside Rg3 | Induced upregulation of androgen receptor. | [125] |
| Pumpkin seed oil * | 5α-reductase inhibition. | [124] |
| Saw palmetto extract * | 5α-reductase inhibition. | [133,134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kesika, P.; Sivamaruthi, B.S.; Thangaleela, S.; Bharathi, M.; Chaiyasut, C. Role and Mechanisms of Phytochemicals in Hair Growth and Health. Pharmaceuticals 2023, 16, 206. https://doi.org/10.3390/ph16020206
Kesika P, Sivamaruthi BS, Thangaleela S, Bharathi M, Chaiyasut C. Role and Mechanisms of Phytochemicals in Hair Growth and Health. Pharmaceuticals. 2023; 16(2):206. https://doi.org/10.3390/ph16020206
Chicago/Turabian StyleKesika, Periyanaina, Bhagavathi Sundaram Sivamaruthi, Subramanian Thangaleela, Muruganantham Bharathi, and Chaiyavat Chaiyasut. 2023. "Role and Mechanisms of Phytochemicals in Hair Growth and Health" Pharmaceuticals 16, no. 2: 206. https://doi.org/10.3390/ph16020206
APA StyleKesika, P., Sivamaruthi, B. S., Thangaleela, S., Bharathi, M., & Chaiyasut, C. (2023). Role and Mechanisms of Phytochemicals in Hair Growth and Health. Pharmaceuticals, 16(2), 206. https://doi.org/10.3390/ph16020206










