Cytotoxic Labdane Diterpenes, Norlabdane Diterpenes and Bis-Labdanic Diterpenes from the Zingiberaceae: A Systematic Review
Abstract
1. Introduction
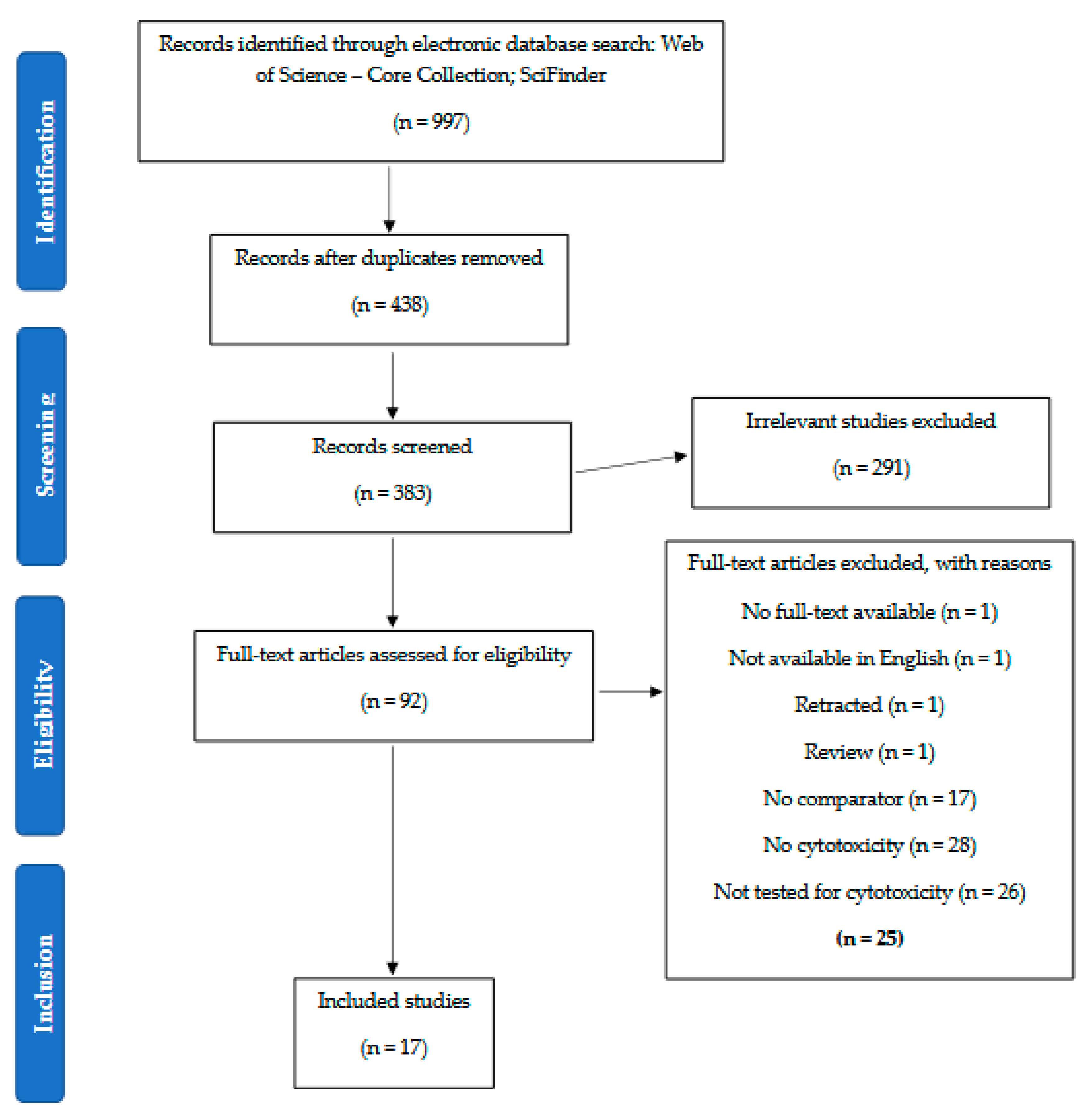
2. Methods
3. Results
4. Discussion
4.1. Phytochemical Investigation
4.1.1. Distribution of the Cytotoxic Labdane-Type Diterpenes within the Zingiberaceae
4.1.2. Extraction, Isolation, and Characterisation of the Cytotoxic Labdane-Type Diterpenes
4.1.3. Structures of the Cytotoxic Labdane-Type Diterpenes
Labdane Diterpenes
Norlabdane Diterpenes
Bis-Labdanic Diterpenes
4.2. Cytotoxic Activities
4.2.1. Breast Cancer Cell Lines
MCF-7 Cell Line
MDA-MB-231 and T-47D Cell Lines
4.2.2. Cervical Cancer Cell Lines
KB Cell Lines
SMMC-7721 and SGC-7901 Cell Lines
HeLa Cell Line
4.2.3. Liver Cancer Cell Lines
HepG2 Cell Line
HCC-S102 Cell Line
4.2.4. Colorectal Cancer Cell Lines
HCT-116 Cell Line
HT-29 Cell Line
COLO 205 Cell Line
DLD-1 Cell Line
4.2.5. Pancreatic Cancer Cell Lines
4.2.6. Lung Cancer Cell Lines
A-549 Cell Line
NCI-H187 Cell Line
4.2.7. Prostate Cancer Cell Lines
5. Strengths and Limitations
6. Future Research
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Acronyms
| A-549 | Lung adenocarcinoma |
| BxPC-3 | Pancreatic ductal adenocarcinoma |
| COLO 205 | Colon adenocarcinoma |
| DLD-1 | Colon adenocarcinoma |
| DU145 | Prostate carcinoma |
| GI50 | Half-maximal inhibition of cell proliferation |
| GLOBOCAN | Global Cancer, Incidence, Mortality and Prevalence |
| HCC-S102 | Hepatocellular carcinoma |
| HCT-116 | Colon carcinoma |
| HeLa | Human papillomavirus-related endocervical adenocarcinoma |
| HepG2 | Hepatoblastoma |
| HPLC | High performance liquid chromatography |
| HT-29 | Colon adenocarcinoma |
| IC50 | Half-maximal inhibitory concentration |
| IR | Infrared spectroscopy |
| KB | Human papillomavirus-related endocervical adenocarcinoma |
| MCF-7 | Michigan Cancer Foundation-7 (breast cancer) |
| MDA-MB-231 | MD Anderson-Metastatic Breast-231 (triple-negative breast cancer) |
| MS | Mass spectrometry |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide |
| NCI-H187 | Lung small-cell carcinoma |
| NMR | Nuclear magnetic resonance spectroscopy |
| PICO | Population, Intervention, Comparison and Outcome |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| REMA | Resazurin microtiter assay |
| SAR | Structure–activity relationship |
| SGC-7901 | Human papillomavirus-related endocervical adenocarcinoma |
| SMMC-7721 | Human papillomavirus-related endocervical adenocarcinoma |
| SRB | Sulforhodamine B |
| T-47D | Breast cancer |
| TLC | Thin-layer chromatography |
| UV-Vis | Ultraviolet–visible spectroscopy |
| WHO | World Health Organization |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yu, H. Global Burden of Cancer. Yale J. Biol. Med. 2006, 79, 85–94. [Google Scholar] [PubMed]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global Cancer Statistics, 2002. CA Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Shin, H.-R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of Worldwide Burden of Cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012: Globocan 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Elovainio, M.; Lumme, S.; Arffman, M.; Manderbacka, K.; Pukkala, E.; Hakulinen, C. Living Alone as a Risk Factor for Cancer Incidence, Case-Fatality and All-Cause Mortality: A Nationwide Registry Study. SSM—Popul. Health 2021, 15, 100826. [Google Scholar] [CrossRef]
- Dai, S.; Mo, Y.; Wang, Y.; Xiang, B.; Liao, Q.; Zhou, M.; Li, X.; Li, Y.; Xiong, W.; Li, G.; et al. Chronic Stress Promotes Cancer Development. Front. Oncol. 2020, 10, 1492. [Google Scholar] [CrossRef]
- Chinwong, D.; Mookmanee, N.; Chongpornchai, J.; Chinwong, S. A Comparison of Gender Differences in Smoking Behaviors, Intention to Quit, and Nicotine Dependence among Thai University Students. J. Addict. 2018, 2018, 8081670. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-Derived Anticancer Agents: A Green Anticancer Approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Pandya, P.H.; Murray, M.E.; Pollok, K.E.; Renbarger, J.L. The Immune System in Cancer Pathogenesis: Potential Therapeutic Approaches. J. Immunol. Res. 2016, 2016, 4273943. [Google Scholar] [CrossRef]
- Russo, J.; Russo, I.H. The Role of Estrogen in the Initiation of Breast Cancer. J. Steroid Biochem. Mol. Biol. 2006, 102, 89–96. [Google Scholar] [CrossRef]
- Danaei, G.; Vander Hoorn, S.; Lopez, A.D.; Murray, C.J.; Ezzati, M. Causes of Cancer in the World: Comparative Risk Assessment of Nine Behavioural and Environmental Risk Factors. Lancet 2005, 366, 1784–1793. [Google Scholar] [CrossRef]
- Bidram, E.; Esmaeili, Y.; Ranji-Burachaloo, H.; Al-Zaubai, N.; Zarrabi, A.; Stewart, A.; Dunstan, D.E. A Concise Review on Cancer Treatment Methods and Delivery Systems. J. Drug Deliv. Sci. Technol. 2019, 54, 101350. [Google Scholar] [CrossRef]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative Approaches for Cancer Treatment: Current Perspectives and New Challenges. eCancer 2019, 13, 961. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Merecz-Sadowska, A.; Rijo, P.; Mori, M.; Hatziantoniou, S.; Górski, K.; Szemraj, J.; Piekarski, J.; Śliwiński, T.; Bijak, M.; et al. Hidden in Plants—A Review of the Anticancer Potential of the Solanaceae Family in In Vitro and In Vivo Studies. Cancers 2022, 14, 1455. [Google Scholar] [CrossRef]
- Bhanot, A.; Sharma, R.; Noolvi, M.N. Natural Sources as Potential Anti-Cancer Agents: A Review. Int. J. Phytomed. 2011, 18, 9–26. [Google Scholar]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2016, 25, 41–59. [Google Scholar] [CrossRef]
- Fridlender, M.; Kapulnik, Y.; Koltai, H. Plant Derived Substances with Anti-Cancer Activity: From Folklore to Practice. Front. Plant Sci. 2015, 6, 799. [Google Scholar] [CrossRef]
- Tan, M.C.S.; Oyong, G.G.; Shen, C.-C.; Ragasa, C.Y. Cytotoxic Activities of the Dichloromethane Extracts from Andrographis paniculata (Burm. f.) Nees. J. Nat. Sci. Biol. Med. 2018, 9, 201–206. [Google Scholar] [CrossRef]
- Ivanović, M.; Makoter, K.; Islamčević Razboršek, M. Comparative Study of Chemical Composition and Antioxidant Activity of Essential Oils and Crude Extracts of Four Characteristic Zingiberaceae Herbs. Plants 2021, 10, 501. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-Q.; Liu, H.; He, M.-X.; Wang, R.; Zeng, Q.-Q.; Wang, Y.; Ye, W.-C.; Zhang, Q.-W. A Review of the Botany, Phytochemical, and Pharmacological Properties of Galangal. In Natural and Artificial Flavoring Agents and Food Dyes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 351–396. ISBN 978-0-12-811518-3. [Google Scholar]
- Tamokou, J.D.D.; Mbaveng, A.T.; Kuete, V. Antimicrobial Activities of African Medicinal Spices and Vegetables. In Medicinal Spices and Vegetables from Africa; Elsevier: Amsterdam, The Netherlands, 2017; pp. 207–237. ISBN 978-0-12-809286-6. [Google Scholar]
- Deng, M.; Yun, X.; Ren, S.; Qing, Z.; Luo, F. Plants of the Genus Zingiber: A Review of Their Ethnomedicine, Phytochemistry and Pharmacology. Molecules 2022, 27, 2826. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Yue, X.; Wang, Y.; Yang, Y.; Sun, D.; Li, H.; Chen, L. Chemistry and Bioactivity of Plants from the Genus Amomum. J. Ethnopharmacol. 2021, 281, 114563. [Google Scholar] [CrossRef] [PubMed]
- Van, H.T.; Pham, T.V.; Nguyen, H.T.D.; Nguyen, N.A.; Truong, D.H. A Review on Chemical Constituents of Essential Oils of Aframomum Genus. J. Phytol. 2021, 13, 161–170. [Google Scholar] [CrossRef]
- Sun, W.; Wang, S.; Zhao, W.; Wu, C.; Guo, S.; Gao, H.; Tao, H.; Lu, J.; Wang, Y.; Chen, X. Chemical Constituents and Biological Research on Plants in the Genus Curcuma. Crit. Rev. Food Sci. Nutr. 2017, 57, 1451–1523. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Luo, J.-G.; Kong, L.-Y. The Genus Alpinia: A Review of Its Phytochemistry and Pharmacology. World J. Tradit. Chin. Med. 2016, 2, 26. [Google Scholar] [CrossRef]
- Hartati, R.; Suganda, A.G.; Fidrianny, I. Botanical, Phytochemical and Pharmacological Properties of Hedychium (Zingiberaceae)—A Review. Procedia Chem. 2014, 13, 150–163. [Google Scholar] [CrossRef]
- Kong, L.-Y.; Qin, M.-J.; Niwa, M. New Cytotoxic Bis-Labdanic Diterpenoids from Alpinia Calcarata. Planta Med. 2002, 68, 813–817. [Google Scholar] [CrossRef]
- Chen, L.-G.; Su, P.-J.; Tsai, P.-W.; Yang, L.-L.; Wang, C.-C. Intermedin A, a New Labdane Diterpene Isolated from Alpinia Intermedia, Prolonged the Survival Time of P-388D1 Tumor-Bearing CDF1 Mice. Planta Med. 2017, 83, 151–157. [Google Scholar] [CrossRef]
- Luo, J.-G.; Yin, H.; Fan, B.-Y.; Kong, L.-Y. Labdane Diterpenoids from the Roots of Amomum Maximum and Their Cytotoxic Evaluation. Helv. Chim. Acta 2014, 97, 1140–1145. [Google Scholar] [CrossRef]
- Abas, F.; Lajis, N.H.; Shaari, K.; Israf, D.A.; Stanslas, J.; Yusuf, U.K.; Raof, S.M. A Labdane Diterpene Glucoside from the Rhizomes of Curcuma Mangga. J. Nat. Prod. 2005, 68, 1090–1093. [Google Scholar] [CrossRef]
- Soumya, T.; Lakshmipriya, T.; Klika, K.D.; Jayasree, P.R.; Manish Kumar, P.R. Anticancer Potential of Rhizome Extract and a Labdane Diterpenoid from Curcuma Mutabilis Plant Endemic to Western Ghats of India. Sci. Rep. 2021, 11, 552. [Google Scholar] [CrossRef]
- Zhan, Z.-J.; Wen, Y.-T.; Ren, F.-Y.; Rao, G.-W.; Shan, W.-G.; Li, C.-P. Diterpenoids and a Diarylheptanoid from Hedychium Coronarium with Significant Anti-Angiogenic and Cytotoxic Activities. Chem. Biodivers. 2012, 9, 2754–2760. [Google Scholar] [CrossRef]
- Suresh, G.; Reddy, P.P.; Babu, K.S.; Shaik, T.B.; Kalivendi, S.V. Two New Cytotoxic Labdane Diterpenes from the Rhizomes of Hedychium Coronarium. Bioorg. Med. Chem. Lett. 2010, 20, 7544–7548. [Google Scholar] [CrossRef]
- Chimnoi, N.; Sarasuk, C.; Khunnawutmanotham, N.; Intachote, P.; Seangsai, S.; Saimanee, B.; Pisutjaroenpong, S.; Mahidol, C.; Techasakul, S. Phytochemical Reinvestigation of Labdane-Type Diterpenes and Their Cytotoxicity from the Rhizomes of Hedychium Coronarium. Phytochem. Lett. 2009, 2, 184–187. [Google Scholar] [CrossRef]
- Chen, L.-C.; Wen, Z.-H.; Sung, P.-J.; Shu, C.-W.; Kuo, W.-L.; Chen, P.-Y.; Chen, J.-J. New Labdane-Type Diterpenoid and Cytotoxic Constituents of Hedychium Coronarium. Chem. Nat. Compd. 2017, 53, 72–76. [Google Scholar] [CrossRef]
- Songsri, S.; Nuntawong, N. Cytotoxic Labdane Diterpenes from Hedychium ellipticum Buch.-Ham. Ex Sm. Molecules 2016, 21, 749. [Google Scholar] [CrossRef]
- Zhao, Q.; Qing, C.; Hao, X.J.; Han, J.; Zuo, G.Y.; Zou, C.; Xu, G.L. Cytotoxicity of Labdane-Type Diterpenoids from Hedychium Forrestii. Chem. Pharm. Bull. 2008, 56, 210–212. [Google Scholar] [CrossRef]
- Kumrit, I.; Suksamrarn, A.; Meepawpan, P.; Songsri, S.; Nuntawong, N. Labdane-Type Diterpenes from Hedychium Gardnerianum with Potent Cytotoxicity against Human Small Cell Lung Cancer Cells. Phytother. Res. 2010, 24, 1009–1013. [Google Scholar] [CrossRef]
- Zhao, H.; Zeng, G.; Zhao, S.; Xu, J.; Kong, L.; Li, Y.; Tan, N.; Yang, S. Cytotoxic Labdane-Type Diterpenes from Hedychium Longipetalum Inhibiting Production of Nitric Oxide. Bioorg. Med. Chem. Lett. 2015, 25, 4572–4575. [Google Scholar] [CrossRef]
- Reddy, P.P.; Rao, R.R.; Shashidhar, J.; Sastry, B.S.; Rao, J.M.; Babu, K.S. Phytochemical Investigation of Labdane Diterpenes from the Rhizomes of Hedychium Spicatum and Their Cytotoxic Activity. Bioorg. Med. Chem. Lett. 2009, 19, 6078–6081. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.P.; Rao, R.R.; Rekha, K.; Babu, K.S.; Shashidhar, J.; Shashikiran, G.; Lakshmi, V.V.; Rao, J.M. Two New Cytotoxic Diterpenes from the Rhizomes of Hedychium Spicatum. Bioorg. Med. Chem. Lett. 2009, 19, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-P.; Zhao, S.-M.; Xu, J.-J.; Zeng, G.-Z.; Li, Y.; Tan, N.-H.; Yang, Y. New Labdane Diterpenes from Hedychium Yunnanense with Cytotoxicity and Inhibitory Effects on Nitric Oxide Production. Nat. Prod. Res. 2016, 30, 2669–2674. [Google Scholar] [CrossRef] [PubMed]
- Singamaneni, V.; Lone, B.; Singh, J.; Kumar, P.; Gairola, S.; Singh, S.; Gupta, P. Corrigendum: Coronarin K and L: Two Novel Labdane Diterpenes From Roscoea Purpurea: An Ayurvedic Crude Drug. Front. Chem. 2021, 9, 798461. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.T.N.; Wong, W.S.F.; Chai, C.L.L. Labdane Diterpenoids as Potential Anti-Inflammatory Agents. Pharmacol. Res. 2017, 124, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Comşa, Ş.; Cîmpean, A.M.; Raica, M. The Story of MCF-7 Breast Cancer Cell Line: 40 Years of Experience in Research. Anticancer Res. 2015, 8, 3147–3154. [Google Scholar]
- Hollestelle, A.; Nagel, J.H.A.; Smid, M.; Lam, S.; Elstrodt, F.; Wasielewski, M.; Ng, S.S.; French, P.J.; Peeters, J.K.; Rozendaal, M.J.; et al. Distinct Gene Mutation Profiles among Luminal-Type and Basal-Type Breast Cancer Cell Lines. Breast Cancer Res. Treat. 2010, 121, 53–64. [Google Scholar] [CrossRef]
- Vaughan, L.; Glänzel, W.; Korch, C.; Capes-Davis, A. Widespread Use of Misidentified Cell Line KB (HeLa): Incorrect Attribution and Its Impact Revealed through Mining the Scientific Literature. Cancer Res. 2017, 77, 2784–2788. [Google Scholar] [CrossRef]
- Ye, F.; Chen, C.; Qin, J.; Liu, J.; Zheng, C. Genetic Profiling Reveals an Alarming Rate of Cross-contamination among Human Cell Lines Used in China. FASEB J. 2015, 29, 4268–4272. [Google Scholar] [CrossRef]
- Rebouissou, S.; Zucman-Rossi, J.; Moreau, R.; Qiu, Z.; Hui, L. Note of Caution: Contaminations of Hepatocellular Cell Lines. J. Hepatol. 2017, 67, 896–897. [Google Scholar] [CrossRef]
- Bian, X.; Yang, Z.; Feng, H.; Sun, H.; Liu, Y. A Combination of Species Identification and STR Profiling Identifies Cross-Contaminated Cells from 482 Human Tumor Cell Lines. Sci. Rep. 2017, 7, 9774. [Google Scholar] [CrossRef]
- Abdurakhmonov, I.Y. Introduction to Microsatellites: Basics, Trends and Highlights. In Microsatellite Markers; Abdurakhmonov, I.Y., Ed.; InTech: Vienna, Austria, 2016; ISBN 978-953-51-2797-0. [Google Scholar]
- Vieira, M.L.C.; Santini, L.; Diniz, A.L.; Munhoz, C.d.F. Microsatellite Markers: What They Mean and Why They Are so Useful. Genet. Mol. Biol. 2016, 39, 312–328. [Google Scholar] [CrossRef]
- Laohathai, K.; Bhamarapravati, N. Culturing of Human Hepatocellular Carcinoma. A Simple and Reproducible Method. Am. J. Pathol. 1985, 118, 203–208. [Google Scholar] [PubMed]
- López-Terrada, D.; Cheung, S.W.; Finegold, M.J.; Knowles, B.B. Hep G2 Is a Hepatoblastoma-Derived Cell Line. Hum. Pathol. 2009, 40, 1512–1515. [Google Scholar] [CrossRef]
- Berg, K.C.G.; Eide, P.W.; Eilertsen, I.A.; Johannessen, B.; Bruun, J.; Danielsen, S.A.; Bjørnslett, M.; Meza-Zepeda, L.A.; Eknæs, M.; Lind, G.E.; et al. Multi-Omics of 34 Colorectal Cancer Cell Lines—A Resource for Biomedical Studies. Mol. Cancer 2017, 16, 116. [Google Scholar] [CrossRef]
- Liu, Y.; Bodmer, W.F. Analysis of P53 Mutations and Their Expression in 56 Colorectal Cancer Cell Lines. Proc. Natl. Acad. Sci. USA 2006, 103, 976–981. [Google Scholar] [CrossRef]
- Mashima, T.; Oh-hara, T.; Sato, S.; Mochizuki, M.; Sugimoto, Y.; Yamazaki, K.; Hamada, J.; Tada, M.; Moriuchi, T.; Ishikawa, Y.; et al. P53-Defective Tumors With a Functional Apoptosome-Mediated Pathway: A New Therapeutic Target. J. Natl. Cancer Inst. 2005, 97, 765–777. [Google Scholar] [CrossRef]
- Sabulal, N.; Baby, S. Chemistry of Amomum Essential Oils. J. Essent. Oil Res. 2021, 33, 427–441. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Blázquez, M.A. Curcuma longa L. Rhizome Essential Oil from Extraction to Its Agri-Food Applications. A Review. Plants 2020, 10, 44. [Google Scholar] [CrossRef]
- Dosoky, N.; Setzer, W. Chemical Composition and Biological Activities of Essential Oils of Curcuma Species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. A Review of the Composition of the Essential Oils and Biological Activities of Angelica Species. Sci. Pharm. 2017, 85, 33. [Google Scholar] [CrossRef] [PubMed]
| Keywords | |
|---|---|
| Bioactive compounds | “Labdane diterpene” OR “Labdane diterpenes” OR “Labdane diterpenoid” OR “Labdane diterpenoids” OR “Bis-labdanic diterpene” OR “Bis-labdanic diterpenes” OR “Bis-labdanic diterpenoid” OR “Bis-labdanic diterpenoids” OR “Dimeric labdane diterpene” OR “Dimeric labdane diterpenes” OR “Dimeric labdane diterpenoid” OR “Dimeric labdane diterpenoids” |
| Cytotoxic | “Cytotoxic” |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Population: in vitro studies | Studies with incomplete results/methodologies; in vivo and ex vivo studies |
| Intervention: naturally occurring labdane-type diterpenes from the Zingiberaceae | Synthetic labdane-type diterpenes |
| Comparators: standard (synthetic or natural) compounds | Articles that do not have comparators |
| Outcome: articles that report IC50 or GI50 values of the cytotoxic activities of the labdane-type diterpenes | Articles that do not report the IC50 or GI50 values of the cytotoxic activities of the labdane-type diterpenes |
| Articles in English | Articles not in English |
| Years: 2000–March 2022 | Human trials, clinical trials, randomized control trials, animal studies, literature reviews, systemic reviews, meta-analyses, conference proceedings, and patents |
| Original articles including short communications, notes and letters | Studies without accessible full text |
| Species | Name of Labdane-Type Diterpenes | Reference |
|---|---|---|
| Alpinia calcarata Rosc. | Calcaratarin D (1) Calcaratarin E (2) | [30] |
| Alpinia intermedia Gagnep | Intermedin A (3) | [31] |
| Amomum maximum Roxb | Amomax C (4) (12Z,14R)-labda-8(17),12- diene-14,15,16-triol (5) | [32] |
| Curcuma mangga Val.van Zip | Zerumin B (6) | [33] |
| Curcuma mutabilis Škorničk., M. Sabu & Prasanthk. | (E)-14,15-epoxylabda-8(17),12-dien-16-al (Cm epoxide) (7) | [34] |
| Hedychium coronarium J. Koenig | Hedycoronal A (8) Hedycoronal B (9) Coronarin D methyl ether (10) Coronarin D ethyl ether (11) Labda-8(17),11,13-trien-15(16)-olide (12) (12E)-Labda-8(17),12-dien-15(16)-olide (13) Coronarin A (14) 15-Hydroxy-11,15-epoxylabda-8(17),12-dien-16-al (15) 16-Hydroxylabda-8(17),11,13-trien-15,16-olide (16) Isocoronarin D (17) | [35] |
| Hedychium coronarium J. Koenig | Coronarin D methyl ether (10) 6-oxo-7,11,13-labdatrien-17-al-16,15-olide (18) 7,17-dihydroxy-6-oxo-7,11,13-labdatrien-16,15-olide (19) Coronarin D (20) Coronarin C (21) Hedychenone (22) 6-oxo-7,11,13-labdatriene-16,15-olide (23) Pacovatinin A (24) | [36] |
| Hedychium coronarium J. Koenig | Isocoronarin D (17) Coronarin D (20) Coronarin B (25) | [37] |
| Hedychium coronarium J. Koenig | Coronarin A (14) Calcaratarin A (26) | [38] |
| Hedychium ellipticum Buch-Ham. ex Sm. | Zerumin B (6) 16-Hydroxylabda-8(17),11,13-trien-15,16-olide (16) Coronarin D (20) Coronarin E (27) (E)-15,16-Bisnorlabda-8(17),11-dien-13-one (28) Villosin (29) 15-Methoxylabda-8(17),11,13-trien-15,16-olide (30) (E)-labda-8(17),12-dien-15,16-dial (31) | [39] |
| Hedychium forrestii Tong. | Yunnancoronarin A (32) Yunnancoronarin B (33) | [40] |
| Hedychium gardnerianum Sheppard ex Ker Gawl | Coronarin A (14) Coronarin E (27) Villosin (29) Yunnancoronarin A (32) Yunnancoronarin B (33) Hedyforrestin B (34) Hedyforrestin C (35) | [41] |
| Hedychium longipetalum X. Hu & N. Liu | Yunnancoronarin A (32) Hedyforrestin B (34) Hedyforrestin C (35) Hedylongnoid C (36) | [42] |
| Hedychium spicatum Buch.-Ham. ex Sm. | Coronarin E (27) 7-hydroxy,15,16-epoxy-17-al-7,11,13(16),14-labda-tetraene-6-one (7-hydroxy hedichinal) (37) 14,15,16-trinor-7,11-labdadien-13-oicacid (spicatanoic acid) (38) Yunnancoronarin D (39) | [43] |
| Hedychium spicatum Buch.-Ham. ex Sm. | Yunnancoronarin A (32) 7-hydroxy,6-oxo-7,11,13-labdatrien-16,15-olide (hedychilactone D) (40) 9-hydroxy,15,16-epoxy-7,11,13(16)14-labdatetraen-6-one (9-hydroxy hedychenone) (41) | [44] |
| Hedychium yunnanense Gagnep. | Hedychenone (22) Villosin (29) Hedychenoid B (42) | [45] |
| Roscoea purpurea Sm. | Coronarin A (14) Coronarin K (43) Coronarin L (44) | [46] |
| Compound | IC50/GI50 (µM) | Assay | Reference | |
|---|---|---|---|---|
| MCF-7 | Potency | |||
Amomax C (4)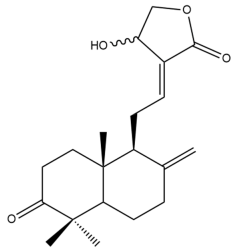 | IC50: 29.80 ± 2.41 | Moderate | MTT | [32] |
Zerumin B (6)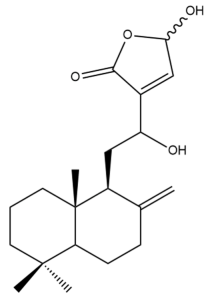 | IC50: 0.59 ± 1.8 | Potent | MTT | [33] |
| IC50: 39.71 | Weak | REMA | [39] | |
Coronarin D methyl ether (10) 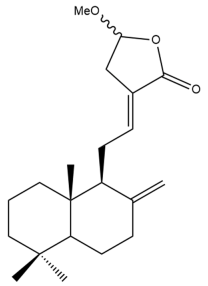 | GI50: 25.3 ± 2.8 | Moderate | SRB | [36] |
Coronarin A (14)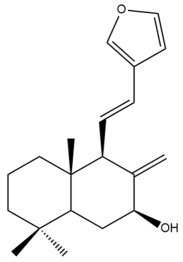 | IC50: >50 | Weak | MTT | [46] |
16-Hydroxylabda-8(17),11,13-trien-15,16-olide (16) | IC50: 8.75 | Strong | REMA | [39] |
6-oxo-7,11,13-labdatrien-17-al-16,15-olide (18) | GI50: 69.9 ± 8.5 | Weak | SRB | [36] |
7,17-dihydroxy-6-oxo-7,11,13-labdatrien-16,15-olide (19)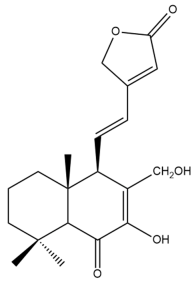 | GI50: 12.0 ± 1.4 | Moderate | SRB | [36] |
Coronarin D (20)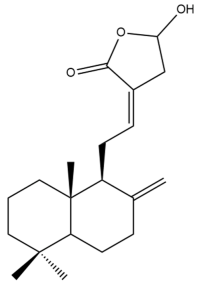 | GI50: 17.4 ± 2.1 | Moderate | SRB | [36] |
| IC50: 51.03 | Weak | REMA | [39] | |
Coronarin C (21)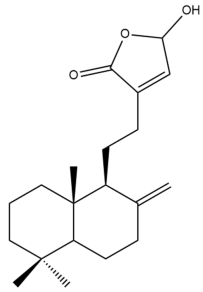 | GI50: 32.6 ± 3.6 | Weak | SRB | [36] |
Hedychenone (22) | GI50: 14.9 ± 2.3 | Moderate | SRB | [36] |
6-oxo-7,11,13-labdatriene-16,15-olide (23)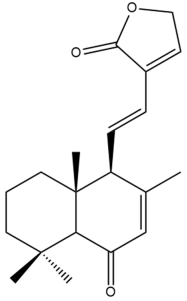 | GI50: 15.7 ± 2.4 | Moderate | SRB | [36] |
Pacovatinin A (24)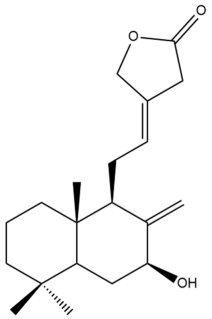 | GI50: 44.7 ± 5.3 | Weak | SRB | [36] |
Villosin (29)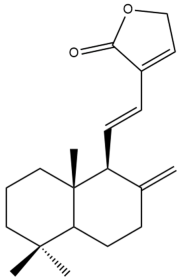 | IC50: 28.29 | Moderate | REMA | [39] |
(E)-labda-8(17),12-dien-15,16-dial (31) | IC50: 74.56 | Weak | REMA | [39] |
Yunnancoronarin A (32)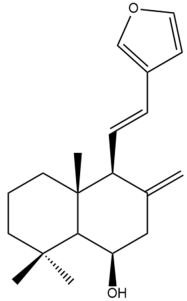 | IC50: 93.5 ± 0.27 | Weak | NA | [44] |
7-hydroxy,6-oxo-7,11,13-labdatrien-16,15-olide (hedychilactone D) (40)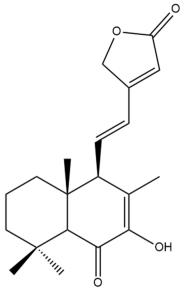 | IC50: 63.76 ± 0.12 | Weak | NA | [44] |
9-hydroxy,15,16-epoxy-7,11,13(16)14-labdatetraen-6-one (9-hydroxy hedychenone) (41) 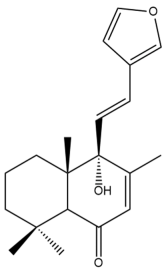 | IC50: 98.50 ± 0.19 | Weak | NA | [44] |
Coronarin K (43)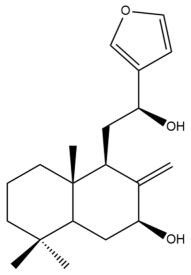 | IC50: 56.24 ± 0.83 | Weak | MTT | [46] |
Coronarin L (44) | IC50: 49.84 ± 2.61 | Weak | MTT | [46] |
| Compound | IC50/GI50 (µM) | Assay | Reference | |
|---|---|---|---|---|
| T-47D | Potency | |||
Isocoronarin D (17) 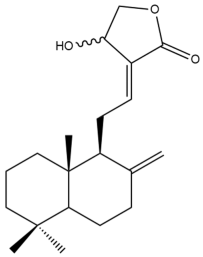 | IC50: 8.49 | Strong | MTT | [37] |
Coronarin D (20)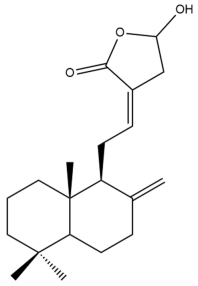 | IC50: 15.08 | Moderate | MTT | [37] |
| Compound | IC50/GI50 (µM) | Assay | Reference | |
|---|---|---|---|---|
| MDA-MB-231 | Potency | |||
Isocoronarin D (17)  | IC50: 7.23 | Strong | MTT | [37] |
Coronarin D (20)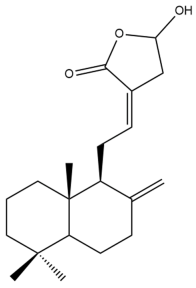 | IC50: 7.86 | Strong | MTT | [37] |
| Compound | IC50/GI50 (µM) | Assay | Reference | |
|---|---|---|---|---|
| KB | Potency | |||
Calcaratarin D (1)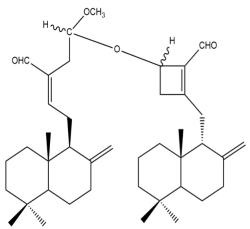 | IC50: 0.34 | Potent | MTT | [30] |
Calcaratarin E (2) | IC50: 0.24 | Potent | MTT | [30] |
Zerumin B (6)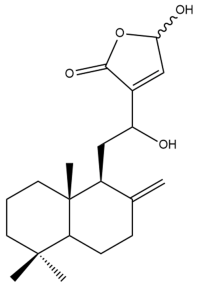 | IC50: 39.26 | Weak | REMA | [39] |
16-Hydroxylabda-8(17),11,13-trien-15,16-olide (16)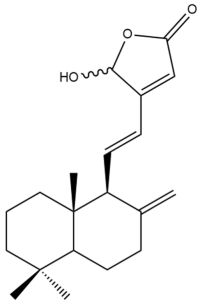 | IC50: 2.75 | Strong | REMA | [39] |
Isocoronarin D (17)  | IC50: 8.81 | Strong | MTT | [37] |
Coronarin D (20) 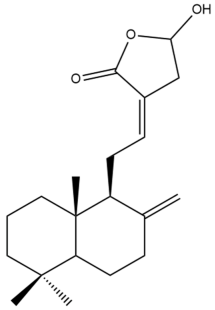 | IC50: 9.43 | Strong | MTT | [37] |
Coronarin E (27)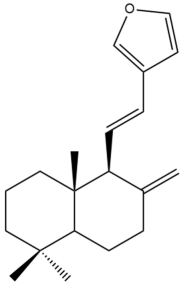 | IC50: 34.00 | Weak | REMA | [39] |
Villosin (29)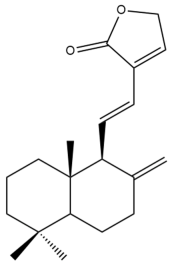 | IC50: 13.65 | Moderate | REMA | [39] |
15-Methoxylabda-8(17),11,13-trien-15,16-olide (30)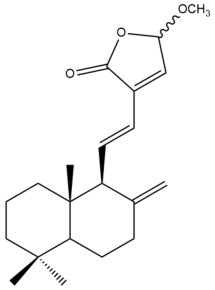 | IC50: 72.08 | Weak | REMA | [39] |
(E)-labda-8(17),12-dien-15,16-dial (31) 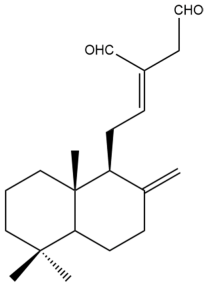 | IC50: 96.05 | Weak | REMA | [39] |
| Compound | IC50/GI50 (µM) | Assay | Reference | |
|---|---|---|---|---|
| SMMC-7721 | Potency | |||
Amomax C (4)  | IC50: 24.38 ± 1.81 | Moderate | MTT | [32] |
| Compound | IC50/GI50 (µM) | Assay | Reference | |
|---|---|---|---|---|
| SGC-7901 | Potency | |||
Hedychenone (22)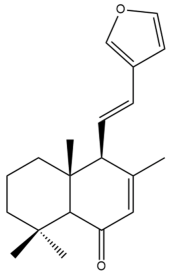 | IC50: 23.73 ± 0.7 | Moderate | SRB | [45] |
Villosin (29)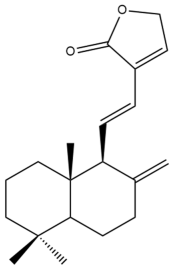 | IC50: 25.87 ± 0.7 | Moderate | SRB | [45] |
Yunnancoronarin A (32)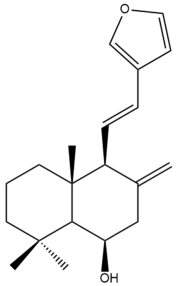 | IC50: 20.7 | Moderate | SRB | [42] |
Hedyforrestin B (34)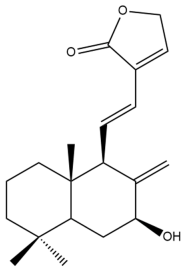 | IC50: 45.98 | Weak | SRB | [42] |
Hedyforrestin C (35)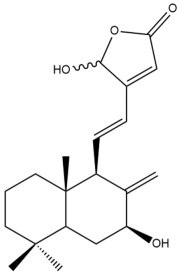 | IC50: 21.96 | Moderate | SRB | [42] |
Hedylongnoid C (36)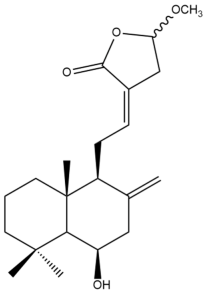 | IC50: 25.1 | Moderate | SRB | [42] |
Hedychenoid B (42) | IC50: 42.98 ± 1.5 | Weak | SRB | [45] |
| Compound | IC50/GI50 (µM) | Assay | Reference | |
|---|---|---|---|---|
| HeLa | Potency | |||
Hedycoronal A (8) | IC50: 37.0 ± 0.4 | Weak | SRB | [35] |
Coronarin D methyl ether (10) | IC50: 88.1 ± 0.6 | Weak | SRB | [35] |
(12E)-Labda-8(17),12-dien-15(16)-olide (13)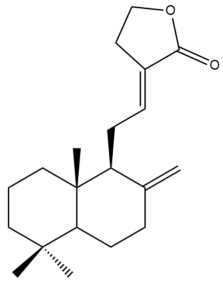 | IC50: 33.0 ± 0.5 | Weak | SRB | [35] |
Coronarin A (14) 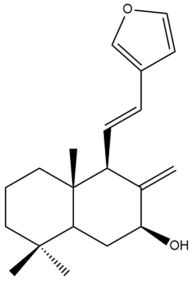 | IC50: 45.2 ± 0.3 | Weak | SRB | [35] |
15-Hydroxy-11,15-epoxylabda-8(17),12-dien-16-al (15)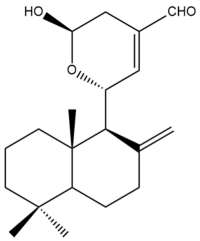 | IC50: 28.7 ± 0.4 | Moderate | SRB | [35] |
16-Hydroxylabda-8(17),11,13-trien-15,16-olide (16) | IC50: 23.0 ± 0.4 | Moderate | SRB | [35] |
Isocoronarin D (17) 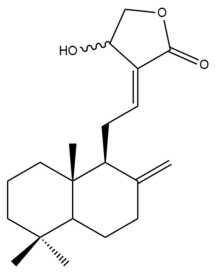 | IC50: 9.12 | Strong | MTT | [37] |
6-oxo-7,11,13-labdatrien-17-al-16,15-olide (18)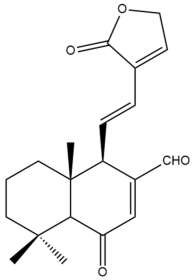 | GI50: 76.9 ± 6.2 | Weak | SRB | [36] |
7,17-dihydroxy-6-oxo-7,11,13-labdatrien-16,15-olide (19)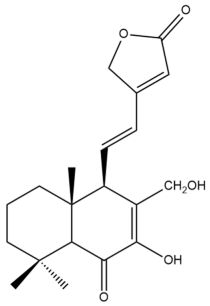 | GI50: 79.7 ± 5.8 | Weak | SRB | [36] |
Coronarin D (20)  | IC50: 12.57 | Moderate | MTT | [37] |
| GI50: 47.0 ± 5.6 | Weak | SRB | [36] | |
Coronarin C (21)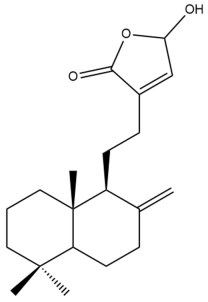 | GI50: 36.3 ± 4.3 | Weak | SRB | [36] |
Hedychenone (22) | GI50: 63.2 ± 5.4 | Weak | SRB | [36] |
| IC50: 32.71 ± 1.61 | Weak | SRB | [45] | |
6-oxo-7,11,13-labdatriene-16,15-olide (23) | GI50: 83.6 ± 6.7 | Weak | SRB | [36] |
Pacovatinin A (24)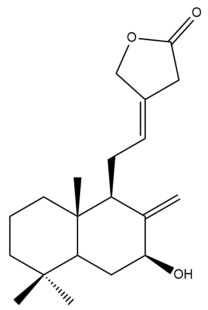 | GI50: 62.1 ± 4.7 | Weak | SRB | [36] |
Coronarin B (25) 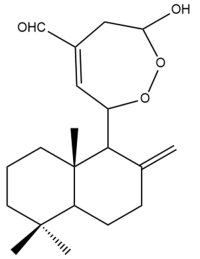 | IC50: 8.54 | Strong | MTT | [37] |
Villosin (29)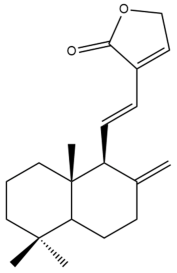 | IC50: 44.13 ± 2.1 | Weak | SRB | [45] |
Yunnancoronarin A (32)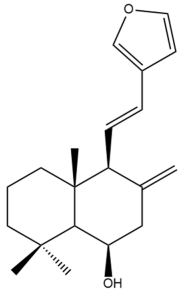 | IC50: 21.93 | Moderate | SRB | [42] |
Hedyforrestin B (34)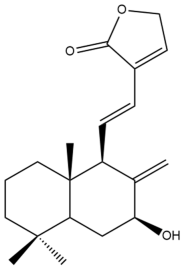 | IC50: 46.93 | Weak | SRB | [42] |
Hedyforrestin C (35) | IC50: 27.74 | Moderate | SRB | [42] |
Hedylongnoid C (36) | IC50: 32.48 | Weak | SRB | [42] |
| Compound | IC50 (µM) | Assay | Reference | |
|---|---|---|---|---|
| HepG2 | Potency | |||
Intermedin A (3)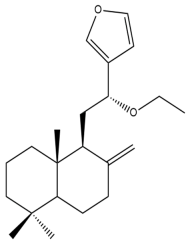 | IC50: 119.82 ± 2.37 | Weak | MTT | [31] |
(12Z,14R)-labda-8(17),12- diene-14,15,16-triol (5) | IC50: 30.1 ± 1.86 | Weak | MTT | [32] |
Zerumin B (6) | IC50: 25.33 ± 3.3 | Moderate | MTT | [33] |
Hedycoronal A (8)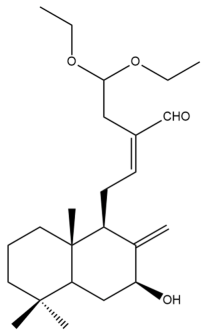 | IC50: 15.6 ± 0.6 | Moderate | SRB | [35] |
Hedycoronal B (9)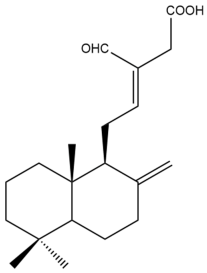 | IC50: 26.7 ± 0.4 | Moderate | SRB | [35] |
Coronarin D methyl ether (10)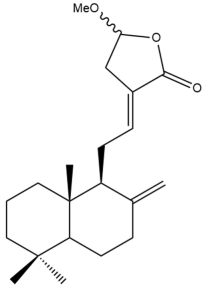 | IC50: 14.2 ± 0.3 | Moderate | SRB | [35] |
Coronarin D ethyl ether (11)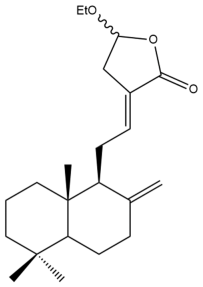 | IC50: 13.4 ± 0.2 | Moderate | SRB | [35] |
Labda-8(17),11,13-trien-15(16)-olide (12) | IC50: 12.6 ± 0.1 | Moderate | SRB | [35] |
(12E)-Labda-8(17),12-dien-15(16)-olide (13) | IC50: 10.6 ± 0.4 | Moderate | SRB | [35] |
Coronarin A (14) | IC50: 10.7 ± 0.4 | Moderate | SRB | [35] |
15-Hydroxy-11,15-epoxylabda-8(17),12-dien-16-al (15)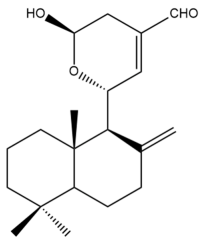 | IC50: 0.9 ± 0.2 | Potent | SRB | [35] |
16-Hydroxylabda-8(17),11,13-trien-15,16-olide (16)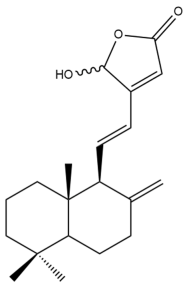 | IC50: 1.1 ± 0.2 | Strong | SRB | [35] |
Isocoronarin D (17)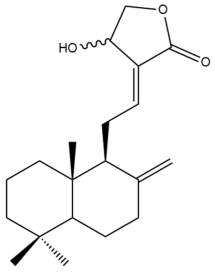 | IC50: 8.0 ± 0.5 | Strong | SRB | [35] |
| IC50: 16.67 | Moderate | MTT | [37] | |
Coronarin D (20)  | IC50: 56.56 | Weak | MTT | [37] |
Coronarin B (25) 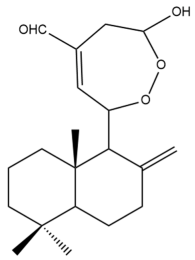 | IC50: 24.51 | Moderate | MTT | [37] |
| Compound | IC50 (µM) | Assay | Reference | |
|---|---|---|---|---|
| HCC-S102 | Potency | |||
Isocoronarin D (17) 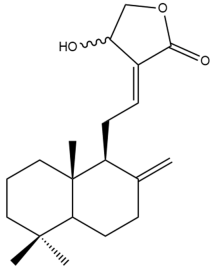 | IC50: 36.16 | Weak | MTT | [37] |
Coronarin D (20) 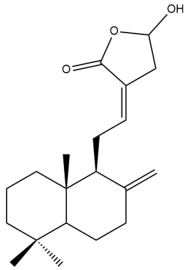 | IC50: 67.56 | Weak | MTT | [37] |
| Compound | IC50 (µM) | Assay | Reference | |
|---|---|---|---|---|
| HCT-116 | Potency | |||
(E)-14,15-epoxylabda-8(17),12-dien-16-al (Cm epoxide) (7) | IC50: 3.21 | Strong | MTT | [34] |
Coronarin A (14)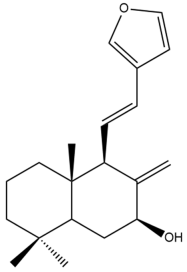 | IC50: >50 | Weak | MTT | [46] |
Coronarin K (43)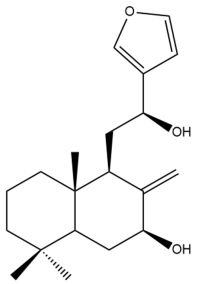 | IC50: 26.03 ± 1.46 | Moderate | MTT | [46] |
Coronarin L (44)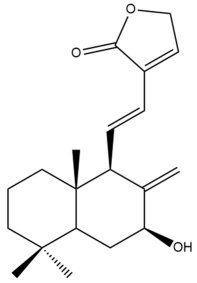 | IC50: >50 | Weak | MTT | [46] |
| Compound | IC50 (µM) | Assay | Reference | |
|---|---|---|---|---|
| HT-29 | Potency | |||
Hedycoronal A (8) | IC50: 18.7 ± 0.4 | Moderate | SRB | [35] |
Hedycoronal B (9)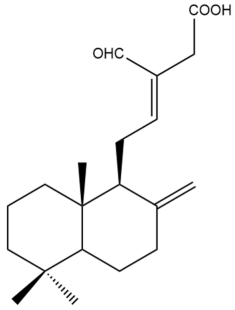 | IC50: 16.2 ± 0.2 | Moderate | SRB | [35] |
Coronarin D methyl ether (10) | IC50: 7.8 ± 0.2 | Strong | SRB | [35] |
Coronarin D ethyl ether (11) | IC50: 9.8 ± 0.4 | Strong | SRB | [35] |
Labda-8(17),11,13-trien-15(16)-olide (12) | IC50: 7.8 ± 0.3 | Strong | SRB | [35] |
(12E)-Labda-8(17),12-dien-15(16)-olide (13) | IC50: 13.9 ± 0.1 | Moderate | SRB | [35] |
Coronarin A (14)  | IC50: 14.7 ± 0.6 | Moderate | SRB | [35] |
15-Hydroxy-11,15-epoxylabda-8(17),12-dien-16-al (15) 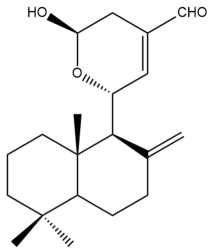 | IC50: 15.6 ± 0.6 | Moderate | SRB | [35] |
16-Hydroxylabda-8(17),11,13-trien-15,16-olide (16)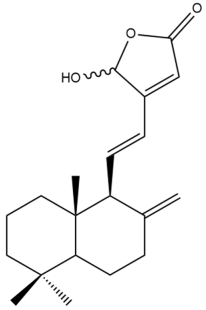 | IC50: 12.3 ± 0.3 | Moderate | SRB | [35] |
Isocoronarin D (17)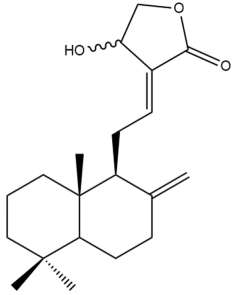 | IC50: 8.8 ± 0.1 | Strong | SRB | [35] |
| Compound | IC50 (µM) | Assay | Reference | |
|---|---|---|---|---|
| COLO 205 | Potency | |||
Yunnancoronarin A (32)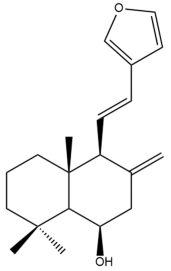 | IC50: 90.47 ± 0.1 | Weak | NA | [44] |
7-hydroxy,6-oxo-7,11,13-labdatrien-16,15-olide (hedychilactone D) (40)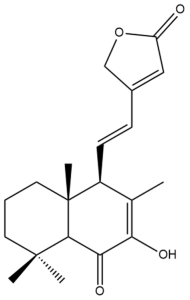 | IC50: 36.44 ± 0.09 | Weak | NA | [44] |
9-hydroxy,15,16-epoxy-7,11,13(16)14-labdatetraen-6-one (9-hydroxy hedychenone) (41) | IC50: 76.47 ± 0.03 | Weak | NA | [44] |
| Compound | IC50 (µM) | Assay | Reference | |
|---|---|---|---|---|
| DLD-1 | Potency | |||
Coronarin A (14) 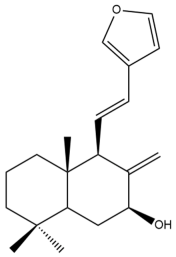 | IC50: 41.67 ± 6.33 | Weak | MTT | [38] |
Calcaratarin A (26) | IC50: 35.34 ± 6.61 | Weak | MTT | [38] |
| Compound | IC50 (µM) | Assay | Reference | |
|---|---|---|---|---|
| Bxpc-3 | Potency | |||
Coronarin A (14) 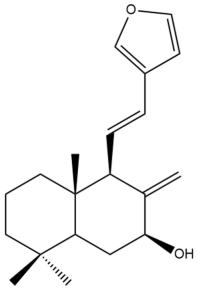 | IC50: 22.83 ± 1.47 | Moderate | MTT | [46] |
Coronarin K (43) 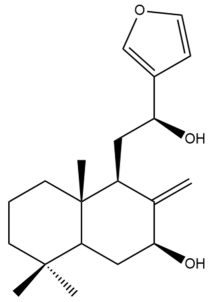 | IC50: 56.70 ± 2.17 | Weak | MTT | [46] |
Coronarin L (44) 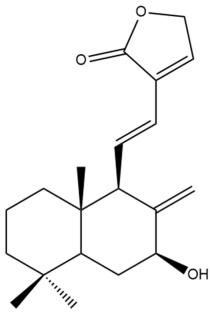 | IC50: 56.83 ± 1.92 | Weak | MTT | [46] |
| Compound | IC50/GI50 (µM) | Assay | Reference | |
|---|---|---|---|---|
| A-549 | Potency | |||
Coronarin D methyl ether (10) | GI50: 30 ± 3.9 | Moderate | SRB | [36] |
Coronarin A (14) | IC50: 61.8 ± 2.82 | Weak | MTT | [46] |
Isocoronarin D (17)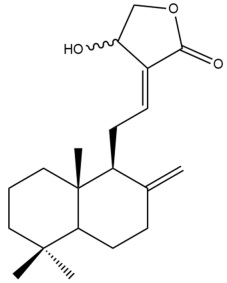 | IC50: 17.3 | Moderate | MTT | [37] |
6-oxo-7,11,13-labdatrien-17-al-16,15-olide (18) | GI50: 18.5 ± 0.95 | Moderate | SRB | [36] |
7,17-dihydroxy-6-oxo-7,11,13-labdatrien-16,15-olide (19)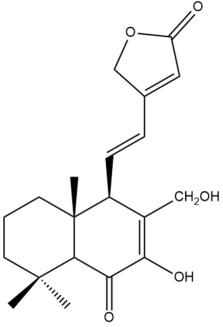 | GI50: 26 ± 1.7 | Moderate | SRB | [36] |
Coronarin D (20) | GI50: 8 ± 0.95 | Strong | SRB | [36] |
| IC50: 86.42 | Weak | MTT | [37] | |
Coronarin C (21) | GI50: 4.8 ± 0.19 | Strong | SRB | [36] |
Hedychenone (22)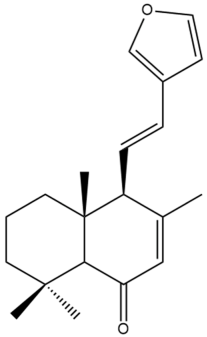 | GI50: 7.4 ± 0.65 | Strong | SRB | [36] |
6-oxo-7,11,13-labdatriene-16,15-olide (23) | GI50: 21.7 ± 0.44 | Moderate | SRB | [36] |
Pacovatinin A (24)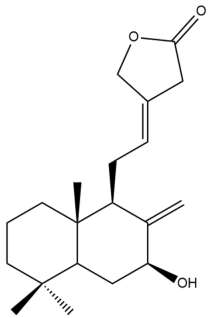 | GI50: 15.1 ± 0.5 | Moderate | SRB | [36] |
Coronarin B (25)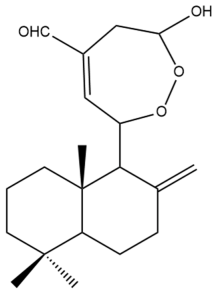 | IC50: 85.39 | Weak | MTT | [37] |
Coronarin E (27) | IC50: 53.26 ± 1.09 | Weak | MTT | [43] |
Yunnancoronarin A (32)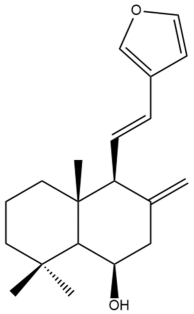 | IC50: 11.08 | Moderate | MTT | [40] |
Yunnancoronarin B (33) 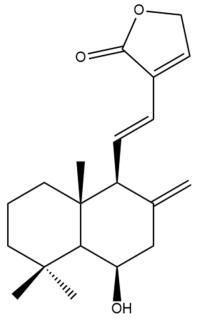 | IC50: 0.92 | Potent | MTT | [40] |
7-hydroxy,15,16-epoxy-17-al-7,11,13(16),14-labda-tetraene-6-one (7-hydroxy hedichinal) (37) | IC50: 37.85 ± 1.01 | Weak | MTT | [43] |
14,15,16-trinor-7,11-labdadien-13-oicacid (spicatanoic acid) (38)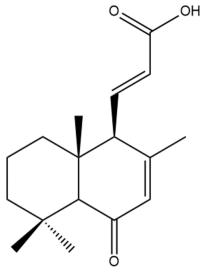 | IC50: 51.97 ± 1.09 | Weak | MTT | [43] |
Yunnancoronarin D (39)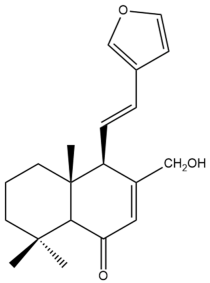 | IC50: 51.24 ± 1.21 | Weak | MTT | [43] |
Coronarin K (43)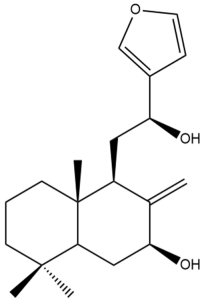 | IC50: 13.49 ± 0.62 | Moderate | MTT | [46] |
Coronarin L (44)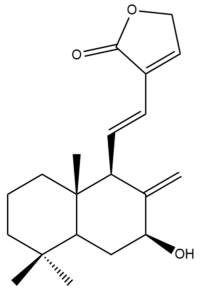 | IC50: 33.78 ± 1.37 | Weak | MTT | [46] |
| Compound | IC50/GI50 (µM) | Assay | Reference | |
|---|---|---|---|---|
| NCI-H187 | Potency | |||
Zerumin B (6)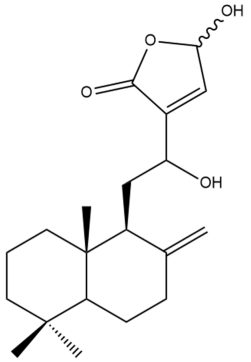 | IC50: 18.36 | Moderate | REMA | [39] |
Coronarin A (14)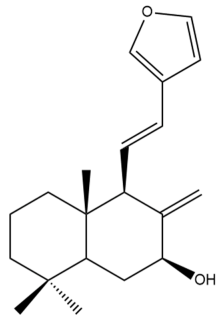 | IC50: 40.77 | Weak | REMA | [41] |
16-Hydroxylabda-8(17),11,13-trien-15,16-olide (16) | IC50: 2.18 | Strong | REMA | [39] |
Coronarin D (20)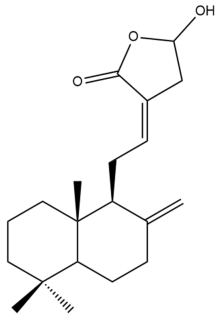 | IC50: 80.77 | Weak | REMA | [39] |
Coronarin E (27)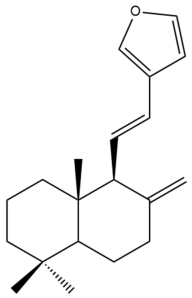 | IC50: 63.50 | Weak | REMA | [39] |
| IC50: 49.73 | Weak | REMA | [41] | |
(E)-15,16-Bisnorlabda-8(17),11-dien-13-one (28)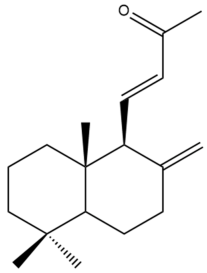 | IC50: 83.22 | Weak | REMA | [39] |
Villosin (29)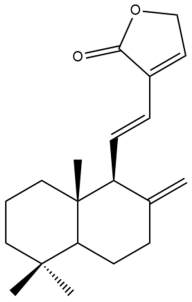 | IC50: 0.40 | Potent | REMA | [39] |
| IC50: 0.40 | Potent | REMA | [41] | |
15-Methoxylabda-8(17),11,13-trien-15,16-olide (30)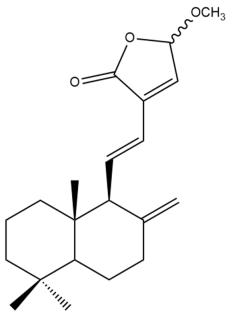 | IC50: 2.72 | Strong | REMA | [39] |
(E)-labda-8(17),12-dien-15,16-dial (31)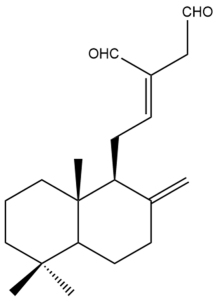 | IC50: 36.93 | Weak | REMA | [39] |
Yunnancoronarin A (32)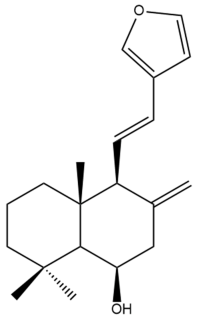 | IC50: 36.78 | Weak | REMA | [41] |
Yunnancoronarin B (33) 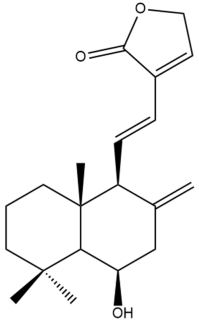 | IC50: 44.57 | Weak | REMA | [41] |
Hedyforrestin B (34)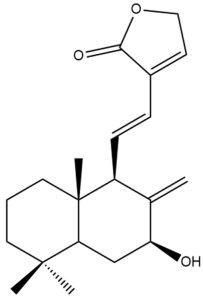 | IC50: 3.10 | Strong | REMA | [41] |
Hedyforrestin C (35)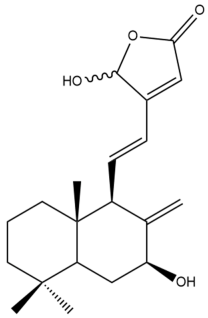 | IC50: 2.46 | Strong | REMA | [41] |
| Compound | IC50 (µM) | Assay | Reference | |
|---|---|---|---|---|
| DU145 | Potency | |||
Zerumin B (6) | IC50: 11.21 ± 3.1 µM | Moderate | MTT | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voon, K.J.; Sivasothy, Y.; Sundralingam, U.; Lalmahomed, A.; Goh, A.P.-T. Cytotoxic Labdane Diterpenes, Norlabdane Diterpenes and Bis-Labdanic Diterpenes from the Zingiberaceae: A Systematic Review. Pharmaceuticals 2022, 15, 1517. https://doi.org/10.3390/ph15121517
Voon KJ, Sivasothy Y, Sundralingam U, Lalmahomed A, Goh AP-T. Cytotoxic Labdane Diterpenes, Norlabdane Diterpenes and Bis-Labdanic Diterpenes from the Zingiberaceae: A Systematic Review. Pharmaceuticals. 2022; 15(12):1517. https://doi.org/10.3390/ph15121517
Chicago/Turabian StyleVoon, Kelvin Jianmin, Yasodha Sivasothy, Usha Sundralingam, Aicha Lalmahomed, and Asly Poh-Tze Goh. 2022. "Cytotoxic Labdane Diterpenes, Norlabdane Diterpenes and Bis-Labdanic Diterpenes from the Zingiberaceae: A Systematic Review" Pharmaceuticals 15, no. 12: 1517. https://doi.org/10.3390/ph15121517
APA StyleVoon, K. J., Sivasothy, Y., Sundralingam, U., Lalmahomed, A., & Goh, A. P.-T. (2022). Cytotoxic Labdane Diterpenes, Norlabdane Diterpenes and Bis-Labdanic Diterpenes from the Zingiberaceae: A Systematic Review. Pharmaceuticals, 15(12), 1517. https://doi.org/10.3390/ph15121517









