Abstract
The biologically active components of the methanol extracts of R. mucronata were identified using GC/MS. The anticancer effects of each methanol extract from the leaves and stem were evaluated against cancer and non-cancer cell lines. The MTT assay was used in order to evaluate cell viability, and the IC50 and the selectivity indices were calculated in relation to a positive control (doxorubicin). The results showed that 11 and 8 different chemical compounds were found in the methanol extracts from the leaves and stems of R. mucronata, respectively. The active constituents of R. mucronata leaves and stems had anticancer effects against colon cancer (CaCo-2), with IC50 levels of 127 ± 4 μg/mL and 107 ± 6 μg/mL, respectively, and on breast cancer (MCF-7), with IC50 levels of 158 ± 10 μg/mL and 138 ± 4 μg/mL, respectively. These were both greater than their effects on prostate cancer (PC-3), for which they showed IC50 levels of 480 ± 14 μg/mL and 294 ± 3 μg/mL, respectively. However, the anticancer effect of the stems on lung cancer (A549) (IC50 = 155 ± 10 μg/mL) was greater than that of the leaves (IC50 = 376 ± 9 μg/mL) in comparison with doxorubicin. Neither the stems nor the leaves of R. mucronata showed any cytotoxicity against normal cells (WI-38), with the IC50 being 932 ± 30 μg/mL for the leaves and 629 ± 3 μg/mL for the stems.
Keywords:
alkaloids; cancers; cytotoxicity; flavonoids; phytochemistry; Rhizophora mucronata; steroids 1. Introduction
Chemotherapy remains the main treatment approach for cancer patients, particularly for those in the late stages of disease. Nevertheless, the development of drug resistance and severe adverse effects constrain the use of chemotherapy for cancer therapy [1]. The emergence of these chemotherapy problems is mainly due to drug inactivation and metabolic biotransformation by several enzymes, such as cytochromes P450 [2,3]. Identifying the molecular mechanisms that drive these problems remains a significant field of research that can help to identify novel pharmacological drug targets and to discover new drug leads, particularly from natural products, in order to improve patients’ therapeutic outcomes [4,5,6,7]. Recent scientific and technological developments have opened up new opportunities to better address the potential of natural-product-based drug discovery for cancer treatment. Nowadays, there is increasing interest in natural products as pharmacological drug leads, particularly to tackle cancer resistance [4,8,9,10]. Therefore, joint efforts must be made in order to identify novel natural-product-based cancer therapies.
Rhizophora L. (Linnaeus, Carl) is the only genus of the Rhizophoraceae family in Egypt. It is represented by only one species: Rhizophora mucronata Lam. (Lamarck, De) [11]. Along with Avicennia marina, it forms a certain type of vegetation in tidal saline wetlands and grows in scattered patches along the southern extension of the Red Sea coast of Egypt [11]. The traditional use of R. mucronata has recently been reviewed by Patra and Thatoi [12]. The most common traditional uses of R. mucronate are as an antipyretic and in the treatment of elephantiasis, hematoma, hepatitis, and ulcers [13,14]. It also possesses antiviral [15,16], antibacterial [17], antioxidant [18], and anti-inflammatory properties [19]. Additionally, the fruit flour of ripe R. mucronata may be used for diabetic patients [20], and its leaves may be used as an antidiarrheal medication [21]. R. mucronata contains several biologically active compounds, such as phenolics, alkaloids, steroids, saponins, flavonoids, tannins, triterpene, and diterpenoids [22]. R. mucronata has been studied in vitro and has proven to be able to inhibit the growth of cancer cells, including cervical [23], breast [24], and colon cancer cells [25]. The objective of this work is to identify and evaluate the possible anticancer activities of the biological substances present in the methanol extracts obtained from the leaves and stems of R. mucronata.
2. Results
2.1. Phytochemistry
The gas chromatography/mass spectrometry analysis of the methanolic extracts from the leaves and stems of R. mucronata were found to contain different bioactive compounds (Table 1, Figure 1 and Figure 2). The major components of the methanol extracts from both the leaves and stems were inositol (relative abundance (RA) = 33.67% and 27.59%, respectively) and 3-O-methyl-d-glucose (RA = 6.45% and 2.63%, respectively). The spectrum showed three m/z peaks in the methanol extracts from the leaves and stems, including a quinoline alkaloid derivative, benzo[H]quinoline-4-carboxylic acid (RA = 0.67% and 0.9%, respectively); a heterocyclic aromatic compound, 1H-purin-6-amine, N- [(3-fluorophenyl)methyl] (RA = 6.10% and 7.53%, respectively); and a glucosinolate, desulphosinigrin (RA = 3.97% and 1.4%, respectively). Alkaloid, 4-Chloro-3-methoxy-2-methylpyridine (RA = 1.94%); a fatty acid derivative, cyclopropane tetradecanoic acid, 2-octyl-, methyl ester (RA = 0.61%); methyl 14 methylpentadecanoate (RA = 4.34%); a doubly unsaturated fatty acid, 9,12-octadecadienoic acid (RA = 1.14%); a one cholestane steroid, (5α)-cholest-1-en-19-ol (RA = 2.30%); and an organo-silicone compound, tetradecamethyl-cycloheptasiloxane (RA = 2.37%) were identified in the leaves. However, the constituents found in the stems were α-tetraloxime, 5,6-dimethoxy (RA = 1.14%); a hexasiloxane, tetradecamethyl (RA = 3.82%); and a pyridine alkaloid derivative, thieno[3,4-c]pyridine, 1,3,4,7-tetraphenyl (RA = 0.95%).
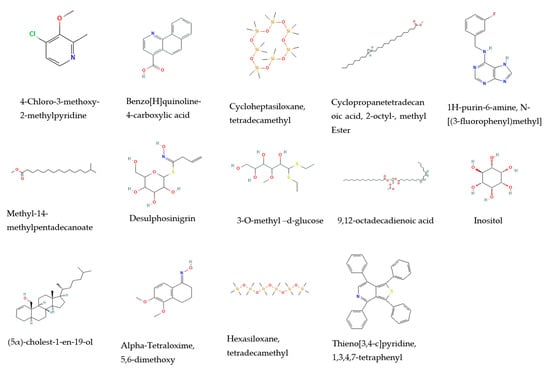
Figure 1.
The chemical structures of the chemical compounds identified from methanol extracts from the leaves and stems of Rhizophora mucronata via GC/MS.
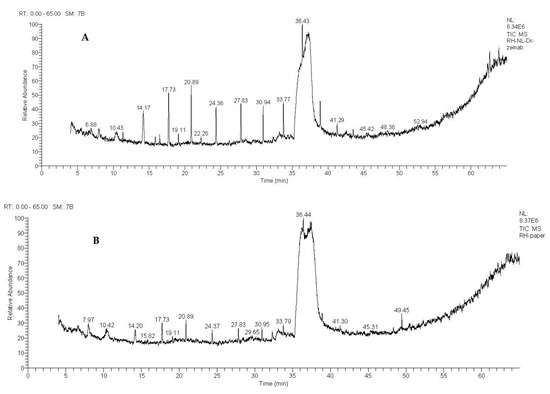
Figure 2.
Gas chromatography/mass spectrometry spectra of Rhizophora mucronate. (A) Stem extract. (B) Leaf extract.

Table 1.
Active principal identification of extracts from the leaves and stems of Rhizophora mucronata by GC/MS.
Table 1.
Active principal identification of extracts from the leaves and stems of Rhizophora mucronata by GC/MS.
| No. | Leaves | Stems | Biological Activity | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compounds | MW | M.F. | Category | Rt | RA% | Rt | RA% | |||
| 1 | 4-Chloro-3-methoxy-2-methylpyridine | 157 | C7H8ClNO | Alkaloid | 7.98 | 1.94 | No data available | |||
| 2 | Benzo[H]quinoline-4-carboxylic acid | 223 | C14H9NO2 | Quinoline alkaloid | 10.42 | 0.67 | 10.42 | 0.9 | Anticancer | [26] |
| 3 | Cycloheptasiloxane, tetradecamethyl | 518 | C14H42O7Si7 | Organo-silicone compound | 14.19 | 2.37 | Anticancer and antimicrobial | [27,28] | ||
| 4 | Cyclopropanetetradecanoic acid, 2-octyl-, methyl Ester | 394 | C26H50O2 | Fatty acid | 19.11 | 0.61 | Antimicrobial | [29,30] | ||
| 5 | 1H-purin-6-amine, N- [(3-fluorophenyl)methyl] | 243 | C12H10FN5 | Fluorinated aromatic compound | 20.89 | 6.10 | 27.8 | 7.53 | Antioxidant | [31] |
| 6 | Methyl-14-methylpentadecanoate | 270 | C17H34O2 | Fatty acid | 32.31 | 4.34 | Antimicrobial | [29,30] | ||
| 7 | Desulphosinigrin | 279 | C10H17NO6S | Glucosinolate | 33.10 | 3.97 | 33.2 | 1.49 | Anticancer and Antimicrobial | [32,33,34] |
| 8 | 3-O-methyl-d-glucose | 194 | C7H14O6 | D-aldohexose | 35.40 | 6.45 | 35.79 | 2.63 | Anti-inflammatory and antioxidant | [35] |
| 9 | 9,12-octadecadienoic acid | 280 | C18H32O2 | Fatty acid | 33.78 | 1.14 | Anticancer and antibacterial | [36,37] | ||
| 10 | Inositol | 180 | C6H12O6 | A cyclic carbohydrate | 35.99 | 33.67 | 37.36 | 27.59 | Anticancer | [38] |
| 11 | (5α)-cholest-1-en-19-ol | 386 | C27H46O | Cholestane steroids | 49.45 | 2.30 | No data available | |||
| 12 | Alpha-Tetraloxime, 5,6-dimethoxy | 221 | C12H15NO3 | Aromatic-organic compound | 22.26 | 1.14 | No data available | |||
| 13 | Hexasiloxane, tetradecamethyl | 458 | C14H42O5Si6 | Linear siloxanes | 24.36 | 3.82 | Antimicrobial | [39] | ||
| 14 | Thieno[3,4-c]pyridine, 1,3,4,7-tetraphenyl | 439 | C31H21NS | Pyridine alkaloid | 45.42 | 0.95 | Anticancer | [40] | ||
Rt: retention time; RA: relative abundance.
2.2. Cytotoxicity
The concentrations of the leaf and stem extracts were plotted on the X-axis in order to determine the half-maximal inhibitory concentration. The percentage of cytotoxicity is expressed as (cell viability % − 100) on the Y-axis (IC50). For instance, the IC50 of the leaf extract against colon cancer (CaCo-2) was 127 ± 4 µg/mL, whereas the IC50 of the stem extract against colon cancer was 107 ± 6 µg/mL (Figure 3).
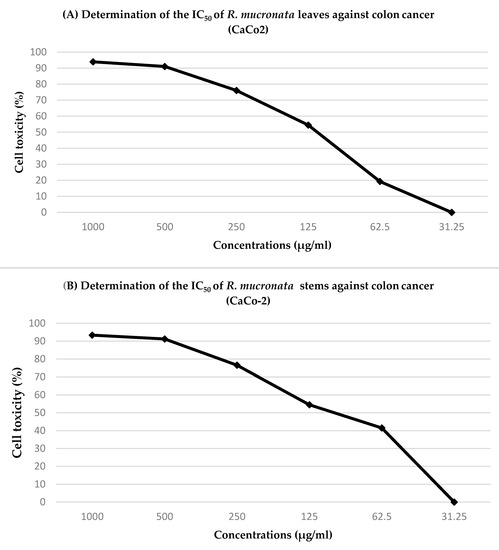
Figure 3.
Determination of the half-maximal inhibitory concentration (IC50) of (A) R. mucronata leaf extract and (B) R. mucronata stem extract against colon cancer (CaCo-2).
Using the MTT test, the anticancer properties of Rhizophora mucronata leaves and stems were studied with respect to the studied cancer and non-cancer cell lines (Table 2). The Dunnett’s test was used to compare all the IC50 values of the leaf and stem extracts to the IC50 values of doxorubicin, a positive control. Based on the NCI criteria, the methanol extracts from the leaves and stems exhibited various antitumor effects on the studied cancer cell lines, as shown in Table 2. The antitumor effects of R. mucronata leaves and stems on prostate cancer (PC-3), with IC50 values of 480 ± 14 μg/mL and 294 ± 3 μg/mL, respectively, were weak compared to doxorubicin. However, the anticancer effects of the leaves and stems on colon cancer (CaCo-2), with IC50 values of 107 ± 6 μg/mL and 127 ± 4 μg/mL, respectively, and breast cancer, with IC50 values of 158 ± 10 μg/mL and 138 ± 3 μg/mL, respectively, were moderate in compared to the positive control. In contrast, the anticancer effect of the stems (IC50 = 155 ± 10 μg/mL) on lung cancer (A549) was higher than that of the leaves (IC50 = 376 ± 9 μg/mL). Importantly, the extracts from the leaves and stems did not exhibit any cytotoxicity against non-cancer cells (WI-38), with IC50 values of 932 ± 30 μg/mL and 629 ± 3 μg/mL, respectively, relative to the positive control. The methanol extract from R. mucronata stems produced selective cytotoxicity in the CaCo-2, MCF-7, and A549 cell lines. However, no cytotoxic selectivity was shown for the leaf extract in the PC-3 or A549 cell lines (values < three) (Table 3).

Table 2.
Cytotoxic effects of methanol extract from R. mucronata against cancer cell lines.

Table 3.
Selectivity index values of R. mucronata methanol extracts from leaves and stems for CaCo-2, PC-3, MCF-7, and A549 cancer cells.
The various cell lines that were treated for 72 h with 1000 µg/mL of the leaf and stem extracts of R. mucronata were examined under a microscope. Compared to the untreated control cells, CaCo-2 cell lines exhibited significant shrinkage after treatment with the methanol extract from the leaves and stems of R. mucronata. These cells also became rounded and disconnected. However, the prostate cancer, breast cancer, lung cancer, and normal cell lines exhibited only small alterations in their morphology compared to the control cells, as shown in Figure 4, Figure 5 and Figure 6.
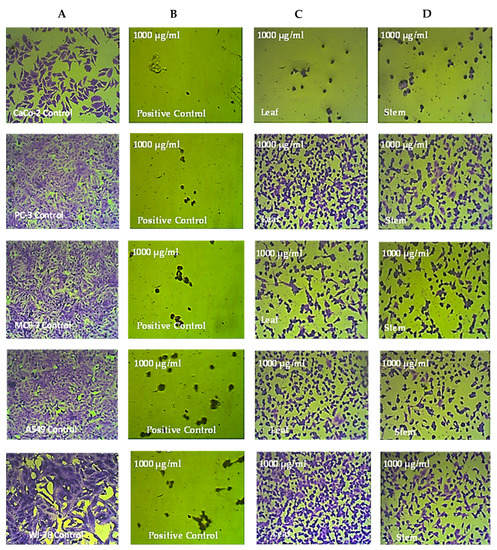
Figure 4.
The anticancer effects of R. micronata stem and leaf extracts in methanol on cancer cell lines. (A) Complete monolayer sheets are seen in all cancer cell lines that have not been treated. (B) Doxorubicin treatment results in rounded and shrunken cells in all cancer cell lines. (C) Tiny, shrunken, and rounded cells are visible in CaCo-2, MCF-7, and A549 cell lines, as well as in PC-3 and WI-38 cell lines. (D) The methanol extract of R. micronata stem used to treat cancer cell lines revealed significantly rounded and shrunken cells in colon cancer (CaCo-2), breast cancer (MCF-7), and lung cancer (A549), as well as smaller, rounded, and shrunken cells in PC-3 and WI-38.
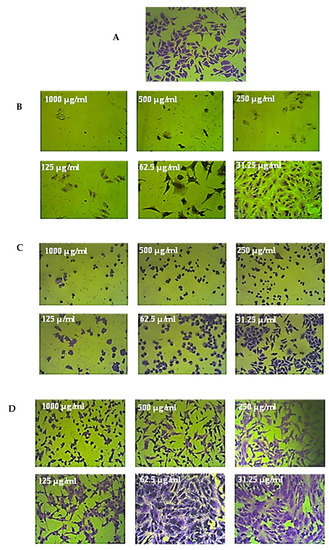
Figure 5.
An example of the moderate anticancer effects of the stem extract of R. micronata against colon cancer cell lines (CaCo-2). (A) Complete monolayer sheets of colon cancer cell lines (CaCo-2) that have not been treated. (B) The effect of doxorubicin treatment at different concentrations. (C) The effect of the stem extract of R. micronata against CaCo-2 cell lines at different concentrations. (D) The effect of the stem extract of R. micronata against normal human fetal lung fibroblasts (WI-38) at different concentrations.
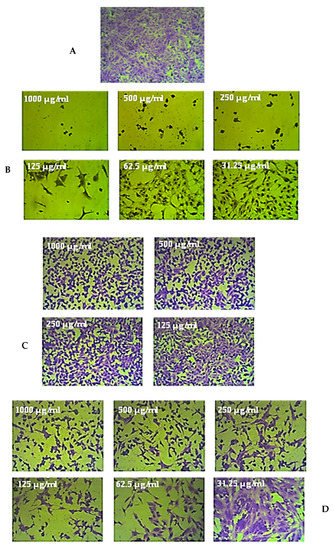
Figure 6.
An example of the weak anticancer effects of the leaf extract of R. micronata against prostate cancer cell lines (PC-3). (A) Complete monolayer sheets of prostate cancer cell lines (PC-3) that have not been treated. (B) The effect of doxorubicin treatment at different concentrations. (C) The effect of the leaf extract of R. micronata against PC-3 cell lines at different concentrations. (D) The effect of the leaf extract of R. micronata against normal human fetal lung fibroblasts (WI-38) at different concentrations.
3. Materials and Methods
3.1. Active Constituent Identification
3.1.1. Material of the Plant
R. mucronata was collected from the naturally growing stands of mangrove vegetation in the southern extension of the Red Sea, about 60 km south of Shalatin city along the coast (23°05′21.1″ N 35°33′08.0″ E), in July 2021. The leaves and stems were separated and cleaned with tap water and were allowed to dry for ten days in a shaded, ventilated location at a temperature of 25 °C. Every 24 h, any reduction in the weight of the leaves and stems was recorded until a fixed weight of 360 g was reached for the dry leaves and a fixed weight of 440 g was reached for the dry stems. Then, they were crushed into a fine powder [41].
3.1.2. Preparation of Plant Extract
A total of 200 g of R. mucronata leaves and stems that had been air-dried were extracted separately using the cold percolation technique three times in 500 mL of 70% methanol for 3 days [41]. A Buchner funnel was used to filter the two methanolic extracts [41]. The filtrates were then dried in a dissector after being evaporated in a rotary evaporator at a temperature lower than 40 °C. The residues were 25 g for the leaves and 18 g for the stems. The crude methanol extracts were subjected to GC/MS analysis in order to determine their bioactive components [41].
3.1.3. Phytochemical Screening
Different plant extract samples were analyzed using a gas chromatography/mass spectrometry (Thermo Scientific TRACE 1310) device (J & W Scientific). A continuous flow of 1 mL/min of helium was used as a carrier gas for the sample analysis. The oven’s temperature was ramped up from 40 °C to 280 °C at a rate of 5 °C per minute. The injection quantities were 1 µL, and the withholding period was 7.5 min. The ion source was adjusted to a temperature of 280 °C. The sample was ionized in the electron impact mode using a mass range of m/z 50–650 and an ionization voltage of 70 eV. The data interpretation was conducted utilizing databases from the Wiley and Nist libraries.
3.2. Cytotoxic Evaluation
The tissue culture laboratory at Vacsera, Dokkey, Giza, Egypt provided prostate cancer cells (PC-3), colon cancer cells (CaCo-2), breast cancer cells (MCF-7), lung cancer cells (A549), and normal human fetal lung fibroblasts (WI-38).
3.2.1. Culturing
The sterility of the process was maintained using a laminar airflow cabinet. The Roswell Park Memorial Institute medium (RPMI 1640) was used to sustain the cell culture. A mixture of 1% antibiotic and antimycotic (10,000 μg/mL streptomycin sulphate, 25 μg/mL amphotericin B, and 10,000 U/mL potassium penicillin) and 1% L-glutamine were added to the medium, and 10% heat-inactivated fetal bovine serum was used as a supplement [41].
3.2.2. MTT Assay
The MTT assay was employed in order to measure cytotoxicity. A purple formazan is created from the yellow MTT via mitochondrial reduction [41]. For inoculation, a 96-well microplate was filled with 1 × 105 cells per well in 100 µL of Roswell Park Memorial Institute medium (RPMI 1640). The microplates were incubated at 5% CO2 and 37 °C for 24 h in order to develop a completely formed monolayer sheet. After the cells formed a confluent layer, the growth medium was decanted from the 96-well microplates. Dimethyl sulfoxide (0.1%) was used to dissolve the methanol extracts from the leaves and stems. The dissolved extract was serially diluted using growth medium in order to achieve the final concentrations of 156.25, 312.5, 625, 1250, 5000, and 10,000 µg/mL. The confluent cell monolayers were injected with 0.1 mL of the extract at each concentration using a multichannel pipette, and then they were dispersed throughout the 96 wells. The cells that were treated with the extracts were incubated at 37 °C and 5% CO2 for 24 h. Three wells were used for each extract concentration. The control cells were incubated without leaf or stem extracts. Phosphate-buffered saline (Bio Basic Canada Inc.) was used to dissolve the MTT powder in order to provide a solution with a 5 mg/mL concentration. Each well received 20 µL of the MTT solution following completion of the incubation period. A shaker (MPS-1, Biosan, London, UK) was used for mixing, which was set to 150 rpm for 5 min. The 96-well microplates were then kept for 4 h at 37 °C and 5% CO2. A metabolic byproduct of the MTT, called formazan, was resuspended in 200 µL of DMSO and was aggressively shaken for five minutes at 150 rpm. The optical density at 560 nm was determined using a microplate reader. A background reference wavelength of 620 nm was used to adjust the results [41]. All experiments were performed in triplicate.
3.2.3. Determination of IC50 Values
Using GraphPad Prism version 7 software (San Diego, CA, USA), the IC50 values of the various concentrations of the methanol extracts of R. mucronata and doxorubicin (as a positive control) against CaCo-2, PC-3, MCF-7, A549, and WI-38 cell lines were computed. Equation (1) was used to determine the percentage of growth inhibition [41]:
Growth Inhibition (%) = 100 − (mean OD of individual test group/mean OD of control group) × 100
3.2.4. Criteria for Anticancer Effect Levels
The level of cytotoxicity of the methanol extract of R. mucronata was categorized using the Geran protocol and the protocol described by the National Cancer Institute (NCI) of the United States as a highly cytotoxic substance (IC50 < 20 µg/mL), a moderately cytotoxic substance (IC50 of 21–200 µg/mL), a weakly cytotoxic substance (IC50 of 201–500 µg/mL), or a non-cytotoxic substance (IC50 > 501 µg/mL) [42,43].
3.2.5. Selectivity Index
The ratio of a plant extract’s IC50 value in a non-cancer cell line (WI-38) to its IC50 value in each cancer cell line is known as the selectivity index. SI values below three indicate that the extract is not selective in relation to non-cancer cells [42,43]. The selectivity indices of the methanol extract were determined using Equation (2):
(Selectivity Index = IC50 of the non-cancer cell line (WI-38)/(IC50 of the cancer cell line)
3.2.6. Microscope
The morphological structures of the cell lines were examined using a Nikon 11,881 inverted microscope at 8× at various methanol Rhizophora mucronata extract concentrations.
4. Discussion
In this study, alkaloid derivatives, such as benzo[H]quinoline-4-carboxylic acid and thieno[3,4-c]pyridine, 1,3,4,7-tetraphenyl, were identified via GC/MS for the first time in Rhizophora mucronata. The anticancer effect of the stems of R. mucronata was evaluated for the first time against lung cancer cell lines and was compared to that of the plant’s leaves. It was found that the anticancer effect of the methanol extract from the stems of R. mucronata against lung cancer was greater than that of the leaf extract. Additionally, the stems did not show any cytotoxicity to the normal cell lines; therefore, the stem of R. mucronata may have potential selectivity for lung cancer without adversely affecting normal cells. The anticancer effects of the leaf and stem extracts of R. mucronata were similar against breast and colon cancer cell lines.
The GC/MS data for the leaf and stem extracts of Rhizophora mucronata revealed different chemical compounds, including hydrocarbons, fatty acids, alkaloids, carbohydrates, and sterols. The major component of the methanol extracts was inositol, which has a similar structure to glucose. The plant increases its osmotic pressure in the cell sap by increasing the concentration of the inositol compound to 33% in the leaves and 27% in the stems. This is an internal characteristic that is considered an adaptive response to resist the high salinity tolerance of the environment [44]. On the other hand, most of the identified compounds found in the methanol extracts of the leaves and stems have been reported to have anticancer effects. For example, Benzo[H]quinoline-4-carboxylic acid is a quinoline alkaloid with a reported potential anticancer effect [26]. The anticancer and antimicrobial effects of tetradecamethyl-cycloheptasiloxane have been reported [27,28]. A fatty acid, 9,12-octadecadienoic acid, has been previously reported to have anticancer and antimicrobial effects [36,37]. Although the anticancer effects of the other two identified fatty acids, cyclopropanetetradecanoic acid, 2-octyl-, methyl ester and methyl 14 methylpentadecanoate, have not been evaluated, their antimicrobial effects have been previously reported [29,30]. Desulphosinigrin is a glucosinolate that has also been reported to have anticancer and antimicrobial effects [32,33,34]. The major identified compound in the leaf and stem extracts was inositol, which has been reported to have anticancer effects [38]. Thieno[3,4-c]pyridine, 1,3,4,7-tetraphenyl, a pyridine alkaloid, has been reported to have anticancer effects [40]. Another identified compound, 1H-purin-6-amine, N- [(3-fluorophenyl)methyl], a heterocyclic aromatic, has not been evaluated for its anticancer effects, but it has been reported to have antioxidant activity [31]. Hexasiloxane, tetradecamethyl has been reported to have antimicrobial effects [39]. The carbohydrate 3-O-Methyl-d-glucose has been reported to possess anti-inflammatory and antioxidant effects [35]. The biological activities of the alkaloid, 4-Chloro-3-methoxy-2-methylpyridine, the sterol, (5α)-cholest-1-en-19-ol, and the aromatic organic compound, alpha-tetraloxime, 5,6-dimethoxy, have not been evaluated until now (Table 1).
This study revealed that the greatest anticancer effects of both of the parts of R. mucronata was on the colon (CaCo-2) and breast (MCF-7) cancer cell lines. This observation agrees with the results reported by Yunos et al. [45], who found that Epi-catechin, 4-O-caffeoyl quinic acid, 5-Ocaffeoyl quinic acid, and procyanidin B2 isolated from R. mucronata showed strong to moderate anticancer effects on colorectal (HT29) and breast (T47D) cancer cell lines. These results also agree with existing studies on the polyisoprenoids from R. mucronata leaves that induce apoptosis in WiDr colon cancer cells by reducing the expression of Bcl-2 and cyclin D1, consequently causing cell cycle arrest [25]. Furthermore, our results show that the leaves possess anticancer effects on breast (MCF-7) cancer cell lines, with an IC50 value of 158 ± 10 μg/mL. These results are consistent with those in the literature, where quinizarin isolated from the methanol extract of R. mucronata leaves demonstrated anticancer effects against breast (MDA-MB231) cancer cell lines [24]. Other anticancer effects of R. mucronata leaves have been reported in the literature [23,46] on cervical (Hela) and myeloid leukaemia (HL-60) cancer cell lines. Interestingly, the anticancer effects of R. mucronata was different between the leaf and stem extracts on lung (A549) cancer cell lines, which showed IC50 values of 376 ± 9 μg/mL and 155 ± 10 μg/mL, respectively. However, there was no difference between the anticancer effects of the leaves and stems of R. mucronata on prostate (PC-3) cancer cell lines, which were both ineffective with IC50 values of 480 ± 14 μg/mL and 294 ± 3 μg/mL, respectively. Our results also show that neither the leaves nor the stems of R. mucronata exhibited cytotoxicity against normal human fetal lung fibroblasts (WI-38), which showed IC50 values of 932 ± 30 μg/mL and 629 ± 3 μg/mL, respectively (Table 2). Moreover, the leaf and stem extracts had selective cytotoxicity towards the studied cancer cells, with the exception of prostate cancer cells (PC-3), and the stem extract’s selectivity for lung cancer (A549) was greater than that of the leaf extract when compared to normal cells (Table 3). Further investigations are necessary in order to explore the phenolic and flavonoid compounds present in R. mucronata and to estimate their anticancer effects on several cancer cell lines.
5. Conclusions
The leaves and stems of R. mucronata were analyzed via a phytochemical analysis, and both contained similar constituents, including benzo[H]quinoline-4-carboxylic acid, 1H-purin-6-amine, n- [(3-fluorophenyl)methyl], inositol, 3-O-methyl –d-glucose, and desulphosinigrin. However, the leaves contained active compounds, including 6-chloro-3-methoxy-2-phenylindole, cycloheptasiloxane, tetradecamethyl, cyclopropanetetradecanoic acid, 2-octyl-, methyl Ester, methyl 14 methylpentadecanoate, 9,12-octadecadienoic acid, and (5α)-cholest-1-en-19-ol. Furthermore, the stems contained active compounds, including alpha-Tetraloxime, 5,6-dimethoxy, hexasiloxane, tetradecamethyl, and thieno[3,4-c]pyridine, 1,3,4,7-tetraphenyl. In this study, the anticancer effects of both the leaves and stems of R. mucronata plants were evaluated against different cancer cell lines. Our results show that the leaf and stem extracts possess greater anticancer effects against colon and breast cancer compared to prostate cancer, and that the stems of R. mucronata have a greater anticancer effect against lung cancer than the leaves. The presence of the diverse variety of constituents identified via the phytochemical analysis may explain the anticancer effects of the leaf and stem extracts against different cancer cell lines. In the future, these constituents may be employed as monotherapy or in conjunction with other agents as treatments for a variety of medical conditions, particularly since normal cells are not adversely affected by the cytotoxic activities of either parts of the R. mucronata plant.
Author Contributions
Conceptualization, A.M.M.Y.; formal analysis, Y.M.A.-S.; investigation, D.A.M.M.; methodology, A.M.M.Y. and D.A.M.M.; supervision, Y.M.A.-S.; writing—original draft, A.M.M.Y.; writing—review and editing, A.M.M.Y. and Y.M.A.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We wish to thank Iman Al-Gohary, Professor of Plant Taxonomy, for identifying and collecting the R. mucronata plants.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Al-Saraireh, Y.M.; Alboaisa, N.S.; Alrawashdeh, H.M.; Hamdan, O.; Al-Sarayreh, S.; Al-Shuneigat, J.M.; Nofal, M.N. Screening of cytochrome 4Z1 expression in human non-neoplastic, pre-neoplastic and neoplastic tissues. Ecancermedicalscience 2020, 14, 1114. [Google Scholar] [CrossRef] [PubMed]
- Al-Saraireh, Y.M.; Alshammari, F.; Youssef, A.M.M.; Al-Sarayreh, S.; Almuhaisen, G.H.; Alnawaiseh, N.; Al Shuneigat, J.M.; Alrawashdeh, H.M. Profiling of CYP4Z1 and CYP1B1 expression in bladder cancers. Sci. Rep. 2021, 11, 5581. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Mai, Z.; Liu, C.; Yin, S.; Cai, Y.; Xia, C. Natural Products in Preventing Tumor Drug Resistance and Related Signaling Pathways. Molecules 2022, 27, 3513. [Google Scholar] [CrossRef]
- Al-Saraireh, Y.M.; Alshammari, F.; Youssef, A.M.M.; Al-Sarayra, Y.M.; Al-Saraireh, R.A.; Al-Muhaisen, G.H.; Al-Mahdy, Y.S.; Al-Kharabsheh, A.M.; Abufraijeh, S.M.; Alrawashdeh, H.M. Cytochrome 4Z1 Expression Is Correlated with Poor Prognosis in Patients with Cervical Cancer. Curr. Oncol. 2021, 28, 3573–3584. [Google Scholar] [CrossRef]
- Al-Saraireh, Y.M.; Alshammari, F.; Youssef, A.M.M.; Al-Sarayreh, S.; Almuhaisen, G.H.; Alnawaiseh, N.; Al-Shuneigat, J.M.; Alrawashdeh, H.M. Cytochrome 4Z1 Expression is Associated with Poor Prognosis in Colon Cancer Patients. OncoTargets Ther. 2021, 14, 5249–5260. [Google Scholar] [CrossRef]
- Al-Saraireh, Y.; Alrawashdeh, F.; Al-Shuneigat, J.; Alsbou, M.; Alnawaiseh, N.; Al-Shagahin, H. Screening of Glypican-3 Expression in Human Normal versus Benign and Malignant Tissues: A Comparative Study Glypican-3 expression in cancers. Biosci. Biotechnol. Res. Asia 2016, 13, 687–692. [Google Scholar] [CrossRef]
- Youssef, A.; El-Swaify, Z.; Al-saraireh, Y.; Dalain, S. Cytotoxic activity of methanol extract of Cynanchumacutum L. seeds on human cancer cell lines. Latin Am. J. Pharm. 2018, 37, 1997–2003. [Google Scholar]
- Youssef, A.M.M.; El-Swaify, Z.A.S. Anti-Tumour Effect of two Persicaria species seeds on colon and prostate cancers. Biomed. Pharmacol. J. 2018, 11, 635–644. [Google Scholar] [CrossRef]
- Youssef, A.M.M.; El-Swaify, Z.A.S.; Al-Saraireh, Y.M.; Al-Dalain, S.M. Anticancer effect of different extracts of Cynanchum acutum L. seeds on cancer cell lines. Pharmacogn. Mag. 2019, 15, 261. [Google Scholar] [CrossRef]
- Boulos, L. Flora of Egypt; Al Hadara Publishing: Cairo, Egypt, 1999; Volume 1. [Google Scholar]
- Patra, J.K.; Thatoi, H.N. Metabolic diversity and bioactivity screening of mangrove plants: A review. Acta Physiol. Plant. 2011, 33, 1051–1061. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetl. Ecol. Manag. 2002, 10, 421–452. [Google Scholar] [CrossRef]
- Ravikumar, S.; Nazar, S.; Nuralshiefa, A.; Abideen, S. Antibacterial activity of traditional therapeutic coastal medicinal plants against some pathogens. J. Environ. Biol. 2005, 26, 383–386. [Google Scholar] [PubMed]
- Padmakumar, K.; Ayyakkannu, K. Antiviral activity of marine plants. Indian J. Virol. 1997, 13, 33–36. [Google Scholar]
- Premanathan, M.; Kathiresan, K.; Yamamoto, N.; Nakashima, H. In vitro anti-human immunodeficiency virus activity of polysaccharide from Rhizophora mucronata Poir. Biosci. Biotechnol. Biochem. 1999, 63, 1187–1191. [Google Scholar] [CrossRef]
- Abeysinghe, P.D. Antibacterial activity of aqueous and ethanol extracts of mangrove species collected from Southern Sri Lanka. Asian J. Pharm. Biol Res. 2012, 2, 79–83. [Google Scholar]
- Sadeer, N.B.; Rocchetti, G.; Senizza, B.; Montesano, D.; Zengin, G.; Uysal, A.; Jeewon, R.; Lucini, L.; Mahomoodally, M.F. Untargeted metabolomic profiling, multivariate analysis and biological evaluation of the true mangrove (Rhizophora mucronata Lam.). Antioxidants 2019, 8, 489. [Google Scholar] [CrossRef]
- Manigandan, V.; Gurudeeban, S.; Satyavani, K.; Ramanathan, T. Molecular docking studies of Rhizophora mucronata alkaloids against neuroinflammatory marker cyclooxygenase 2. Int. J. Biol. Chem. 2014, 8, 91–99. [Google Scholar]
- Hardoko, E.S.; Puspitasari, Y.; Amalia, R. Study of ripe Rhizophora mucronata fruit flour as functional food for antidiabetic. Int. Food Res. J. 2015, 22, 953–959. [Google Scholar]
- Puspitasar, Y.E.; Hartiati, A.; Suprayitno, E. The potency of Rhizophora mucronata leaf extract as antidiarrhea. J. Appl. Sci. Res 2012, 8, 1180–1185. [Google Scholar]
- Faoziyah, A.R.; Kurniawan, W. Pemanfaatan ekstrak daun mangrove (Rhizophora mucronata sp.) dengan variasi pelarut sebagai bahan aktif sediaan farmasi terapi anti kanker. J. Health 2017, 4, 68–74. [Google Scholar] [CrossRef]
- Alsy, R. Effect Of Mangrove Leaf Extract Dosage Rhizophora Mucronata Lmk. On The Viability Of Hela Cells. J. Stem Cell Res. Tissue Eng. 2021, 5, 23–29. [Google Scholar] [CrossRef]
- Sachithanandam, V.; Lalitha, P.; Parthiban, A.; Muthukumaran, J.; Jain, M.; Misra, R.; Mageswaran, T.; Sridhar, R.; Purvaja, R.; Ramesh, R. A comprehensive in silico and in vitro studies on quinizarin: A promising phytochemical derived from Rhizophora mucronata Lam. J. Biomol. Struct. Dyn. 2021, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sari, D.P.; Basyuni, M.; Hasibuan, P.A.Z.; Wati, R. Cytotoxic effect of polyisoprenoids from Rhizophora mucronata and Ceriops tagal leaves against WiDr colon cancer cell lines. Sains Malays. 2018, 47, 1953–1959. [Google Scholar] [CrossRef]
- Iqbal, J.; Ejaz, S.A.; Khan, I.; Ausekle, E.; Miliutina, M.; Langer, P. Exploration of quinolone and quinoline derivatives as potential anticancer agents. DARU J. Pharm. Sci. 2019, 27, 613–626. [Google Scholar] [CrossRef]
- El-Fayoumy, E.A.; Shanab, S.M.; Hassan, O.; Shalaby, E.A. Enhancement of active ingredients and biological activities of Nostoc linckia biomass cultivated under modified BG-110 medium composition. Biomass Convers. Biorefin. 2021, 2021, 1–18. [Google Scholar] [CrossRef]
- Phuong, T.; Lam, P.; Diep, C. Bioactive compounds from marine streptomyces Sp. by gas chromatography-mass spectrometry. Pharm. Chem. J. 2018, 5, 196–203. [Google Scholar]
- Srivastava, R.; Mukerjee, A.; Verma, A. GC-MS analysis of Phytocomponents in, pet ether fraction of wrightia tinctoria seed. Pharmacogn. J. 2015, 7, 249–253. [Google Scholar] [CrossRef]
- Egbung, G.E.; Anosike, C.; Utu-Baku, A.B.; Ogar, I.; Nna, V.U. Phytochemical evaluation and GC-MS analysis of Hyptis verticillata cultivated in Calabar Cross River State, Nigeria. Int. J. Biol. Chem. Sci. 2017, 11, 2548–2559. [Google Scholar] [CrossRef][Green Version]
- Budayatin, B.; Waluyo, J.; Wahyuni, D.; Dafik, D. Antibacterial effects of Pheretima javanica extract and bioactive chemical analysis using Gas Chromatography Mass Spectrum. J. Phys. Conf. Ser. 2021, 1751, 012055. [Google Scholar] [CrossRef]
- Krishnaveni, M. Docking, simulation studies of desulphosinigrin—Cyclin dependent kinase 2, an anticancer drug target. Int. J. Pharm. Sci. Rev. Res. 2015, 30, 115–118. [Google Scholar]
- Kamal, S.A.; Hamza, L.F.; Hameed, I.H. Antibacterial activity of secondary metabolites isolated from Alternaria alternata. Afr. J. Biotechnol. 2015, 14, 2972–2994. [Google Scholar]
- Azhar, A.S.; Suhaila, H.B.; Imad, H.H. Analysis of bioactive chemical compounds of Euphorbia lathyrus using gas chromatography-mass spectrometry and fourier-transform infrared spectroscopy. J. Pharmacogn. Phytother. 2016, 8, 109–126. [Google Scholar] [CrossRef]
- Guerrero, R.V.; Vargas, R.A.; Petricevich, V.L. Chemical compounds and biological activity of an extract from bougainvillea x buttiana (var. rose) holttum and standl. Int. J. Pharm. Pharm. Sci. 2017, 9, 42–46. [Google Scholar] [CrossRef]
- Jayaraman, L.; Shivaji, S.; Anandakumar, S. Phytochemical screening, cytotoxic activity and molecular docking studies of Eclipta alba leaves extract against oral cancer. Rasayan J. Chem. 2022, 15, 676–685. [Google Scholar] [CrossRef]
- Rossellia, S.; Maggio, A.; Formisano, C.; Napolitano, F.; Senatore, F.; Spadaro, V.; Bruno, M. Chemical composition and antibacterial activity of extracts of Helleborus bocconei Ten. subsp. intermedius. Nat. Prod. Commun. 2007, 2, 1934578X0700200611. [Google Scholar] [CrossRef]
- Vucenik, I. Anticancer properties of inositol hexaphosphate and inositol: An overview. J. Nutr. Sci. Vitaminol. 2019, 65, S18–S22. [Google Scholar] [CrossRef]
- Bele, A.A.; Khale, A. Comparison of Constituents in Aloe Vera Gel Collected in Different Seasons by Chromatography and Spectroscopy Techniques. World J. Pharm. Res. 2016, 5, 1028–1040. [Google Scholar]
- El-Naggar, M.; Almahli, H.; Ibrahim, H.S.; Eldehna, W.M.; Abdel-Aziz, H.A. Pyridine-Ureas as Potential Anticancer Agents: Synthesis and In Vitro Biological Evaluation. Molecules 2018, 23, 1459. [Google Scholar] [CrossRef]
- Youssef, A.; El-Swaify, Z.; Maaty, D.; Youssef, M. Comparative study of two Lotus species: Phytochemistry, cytotoxicity and antioxidant capacity. J. Pharm. Pharmacogn. Res. 2020, 8, 537–548. [Google Scholar]
- Al-saraireh, Y.M.; Youssef, A.M.; Alshammari, F.O.; Al-Sarayreh, S.A.; Al-Shuneigat, J.M.; Alrawashdeh, H.M.; Mahgoub, S.S. Phytochemical characterization and anti-cancer properties of extract of Ephedra foeminea (Ephedraceae) aerial parts. Trop. J. Pharm. Res. 2021, 20, 1675–1681. [Google Scholar] [CrossRef]
- Al-Saraireh, Y.M.; Youssef, A.M.M.; Alsarayreh, A.Z.; Hujran, T.A.A.; Al-Sarayreh, S.; Al-Shuneigat, J.M.; Alrawashdeh, H.M. Phytochemical and anti-cancer properties of Euphorbia hierosolymitana Boiss. crude extracts. J. Pharm. Pharmacogn. Res. 2021, 9, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Watzka, M.; Medina, E. Mangroves in Contrasting Osmotic Environments: Photosynthetic Costs of High Salinity Tolerance. In Photosynthesis-From Its Evolution to Future Improvements in Photosynthetic Efficiency Using Nanomaterials; IntechOpen: London, UK, 2018. [Google Scholar]
- Yunos, N.M.; Ling, S.K.; Osman, A.; Abdullah, Z.; Sallehudin, N.J. Phytochemicals from Rhizophora mucronata Propagules, Its In Vitro Anti-Cancer and In Silico Drug-Likeness Potential. Chemistry 2021, 3, 979–990. [Google Scholar] [CrossRef]
- Taniguchi, K.; Funasaki, M.; Kishida, A.; Sadhu, S.K.; Ahmed, F.; Ishibashi, M.; Ohsaki, A. Two new coumarins and a new xanthone from the leaves of Rhizophora mucronata. Bioorg. Med. Chem. Lett. 2018, 28, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).