Radiotracers for the Central Serotoninergic System
Abstract
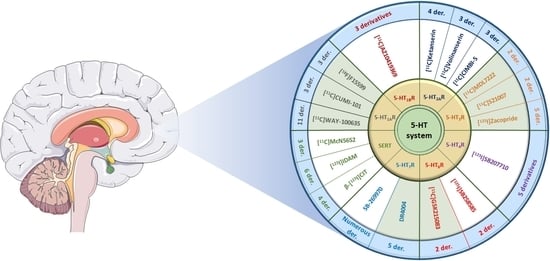
1. Introduction
2. 5-HT1 Receptors
2.1. 5-HT1A Receptors
2.2. N-Acetamide Pyridine Series: WAY-100635 Derivatives
2.3. 1,2,4-Triazine-3,5-Dione Series: CUMI-101 Derivatives
2.4. 2-Pyridinemethylamine Series: F15599 Derivatives
2.5. 5-HT1B Receptors
2.6. Chromen-4-One Series: AZ10419369 Derivatives
3. 5-HT2 Receptors
3.1. Quinazoline-2,4-Dione and Thiazolo [3,2-a]Pyrimidin-5-One Series: Ketanserin Derivatives
3.2. Piperidin-4-Ylmethanol Series: Volinanserin Derivatives
3.3. N-Benzylphenethylamine Series: CIMBI-5 Derivatives
3.4. Miscellaneous Derivatives as SPECT Imaging Tracers
4. 5-HT3 Receptors
4.1. Tropane Series: MDL7222 Derivatives
4.2. Pyrrolo[1,2-a]Pyrazine Series: S21007 Derivatives
4.3. (S)-Quinuclidin-3-Amine Series: Zacopride Derivatives
4.4. Miscellaneous Derivatives as 5-HT3R Radiotracers
5. 5-HT4 Receptors
5.1. Benzodioxane Series: SB207710 Derivatives
5.2. 5-HT5 Receptors
5.3. 5-HT6 Receptors
5.4. Benzene Sulfonamide Derivatives: SB258585 Derivatives
5.5. 5-(Piperazin-1-yl)Quinolone Derivatives: GSK215083 Derivatives
5.6. 5-HT7 Receptors
5.7. Oxindoles Series: DR4004 Derivatives
5.8. N-Sulfopyrrolidine Series: SB-269970 Derivatives
6. Serotonin Transporter
6.1. Isoquinoline Series: McN5652 Derivatives
6.2. Diarylthioether Series: [123I]IDAM Derivatives
6.3. Tropane Series: β-[123I]CIT Derivatives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Švob Štrac, D.; Pivac, N.; Mück-Šeler, D. The serotonergic system and cognitive function. Transl. Neurosci. 2016, 7, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E.; Nichols, C.D. Serotonin receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, D. Serotonin receptors nomenclature. In The Serotonin System; Elsevier: Amsterdam, The Netherlands, 2019; pp. 63–93. ISBN 9780128133231. [Google Scholar]
- Homberg, J.R.; Schubert, D.; Gaspar, P. New perspectives on the neurodevelopmental effects of SSRIs. Trends Pharmacol. Sci. 2010, 31, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, P.P.A.; Hartig, P.; Hoyer, D. A proposed new nomenclature for 5-HT receptors. Trends Pharmacol. Sci. 1993, 14, 233–236. [Google Scholar] [CrossRef]
- Kobilka, B.K.; Frielle, T.; Collins, S.; Yang-Feng, T.; Kobilka, T.S.; Francke, U.; Lefkowitz, R.J.; Caron, M.G. An intronless gene encoding a potential member of the family of receptors coupled to guanine nucleotide regulatory proteins. Nature 1987, 329, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Fargin, A.; Raymond, J.R.; Lohse, M.J.; Kobilka, B.K.; Caron, M.G.; Lefkowitz, R.J. The genomic clone G-21 which resembles a β-adrenergic receptor sequence encodes the 5-HT1A receptor. Nature 1988, 335, 358–360. [Google Scholar] [CrossRef]
- Zhou, F.C.; Patel, T.D.; Swartz, D.; Xu, Y.; Kelley, M.R. Production and characterization of an anti-serotonin 1A receptor antibody which detects functional 5-HT(1A) binding sites. Mol. Brain Res. 1999, 69, 186–201. [Google Scholar] [CrossRef]
- Pompeiano, M.; Palacios, J.M.; Mengod, G. Distribution and cellular localization of mRNA coding for 5-HT(1A) receptor in the rat brain: Correlation with receptor binding. J. Neurosci. 1992, 12, 440–453. [Google Scholar] [CrossRef]
- Richardson-Jones, J.W.; Craige, C.P.; Guiard, B.P.; Stephen, A.; Metzger, K.L.; Kung, H.F.; Gardier, A.M.; Dranovsky, A.; David, D.J.; Beck, S.G.; et al. 5-HT1A Autoreceptor Levels Determine Vulnerability to Stress and Response to Antidepressants. Neuron 2010, 65, 40–52. [Google Scholar] [CrossRef]
- Sprouse, J.S.; Aghajanian, G.K. Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse 1987, 1, 3–9. [Google Scholar] [CrossRef]
- Riad, M.; Garcia, S.; Watkins, K.C.; Jodoin, N.; Doucet, E.; Langlois, X.; El Mestikawy, S.; Hamon, M.; Descarries, L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J. Comp. Neurol. 2000, 417, 181–194. [Google Scholar] [CrossRef]
- Maier, S.F.; Grahn, R.E.; Watkins, L.R. 8-OH-DPAT microinjected in the region of the dorsal raphe nucleus blocks and reverses the enhancement of fear conditioning and interference with escape produced by exposure to inescapable shock. Behav. Neurosci. 1995, 109, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, S.D.; Quartermain, D.; Francisco, T.; Shemer, A. 5-HT1A receptor agonists induce anterograde amnesia in mice through a postsynaptic mechanism. Eur. J. Pharmacol. 1993, 236, 177–182. [Google Scholar] [CrossRef]
- Zhuang, X.; Gross, C.; Santarelli, L.; Compan, V.; Trillat, A.C.; Hen, R. Altered emotional states in knockout mice lacking 5-HT1A or 5-HT1B receptors. Neuropsychopharmacology 1999, 21, 52S–60S. [Google Scholar] [CrossRef]
- Billard, T.; Bars, D.; Zimmer, L. PET Radiotracers for Molecular Imaging of Serotonin 5-HT1A Receptors. Curr. Med. Chem. 2014, 21, 70–81. [Google Scholar] [CrossRef]
- Forster, E.A.; Cliffe, I.A.; Bill, D.J.; Dover, G.M.; Jones, D.; Reilly, Y.; Fletcher, A. A pharmacological profile of the selective silent 5-HT1A receptor antagonist, WAY-100635. Eur. J. Pharmacol. 1995, 281, 81–88. [Google Scholar] [CrossRef]
- Hall, H.; Lundkvist, C.; Halldin, C.; Farde, L.; Pike, V.W.; McCarron, J.A.; Fletcher, A.; Cliffe, I.A.; Barf, T.; Wikström, H.; et al. Autoradiographic localization of 5-HT(1A) receptors in the post-mortem human brain using [3H]WAY-100635. Brain Res. 1997, 745, 96–108. [Google Scholar] [CrossRef]
- Mathis, C.A.; Simpson, N.R.; Mahmood, K.; Kinahan, P.E.; Mintun, M.A. [11C]WAY 100635: A radioligand for imaging 5-HT1A receptors with positron emission tomography. Life Sci. 1994, 55, PL403–PL407. [Google Scholar] [CrossRef]
- Pike, V.W.; McCarron, J.A.; Lammerstma, A.A.; Hume, S.P.; Poole, K.; Grasby, P.M.; Malizia, A.; Cliffe, I.A.; Fletcher, A.; Bench, C.J. First delineation of 5-HT1A receptors in human brain with PET and [11C]WAY-100635. Eur. J. Pharmacol. 1995, 283, 3–5. [Google Scholar] [CrossRef]
- Hwang, D.R.; Simpson, N.R.; Montoya, J.; Mann, J.J.; Laruelle, M. An improved one-pot procedure for the preparation of [11C-carbonyl]-WAY100635. Nucl. Med. Biol. 1999, 26, 815–819. [Google Scholar] [CrossRef]
- Rabiner, E.A.; Messa, C.; Sargent, P.A.; Husted-Kjaer, K.; Montgomery, A.; Lawrence, A.D.; Bench, C.J.; Gunn, R.N.; Cowen, P.; Grasby, P.M. A database of [11C]WAY-100635 binding to 5-HT1A receptors in normal male volunteers: Normative data and relationship to methodological, demographic, physiological, and behavioral variables. Neuroimage 2002, 15, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.M.; Ogden, R.T.; Oquendo, M.A.; Kumar, J.S.D.; Simpson, N.; Huang, Y.-y.; Mann, J.J.; Parsey, R.V. Positron Emission Tomography Quantification of Serotonin-1A Receptor Binding in Medication-Free Bipolar Depression. Biol. Psychiatry 2009, 66, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Penttilä, J.; Hirvonen, J.; Tuominen, L.; Lumme, V.; Ilonen, T.; Någren, K.; Hietala, J. Verbal memory and 5-HT1A receptors in healthy volunteers—A PET study with [carbonyl-11C]WAY-100635. Eur. Neuropsychopharmacol. 2016, 26, 570–577. [Google Scholar] [CrossRef]
- Metts, A.V.; Rubin-Falcone, H.; Ogden, R.T.; Lin, X.; Wilner, D.E.; Burke, A.K.; Sublette, M.E.; Oquendo, M.A.; Miller, J.M.; Mann, J.J. Antidepressant medication exposure and 5-HT 1A autoreceptor binding in major depressive disorder. Synapse 2019, 73, e22089. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Kung, M.-P.; Kung, H.F. Synthesis And Evaluation of 4-(2′-Methoxyphenyl)-1-[2′-[N-(2″-pyridinyl)-p-iodobenzamido]ethyl]piperazine (p-MPPI): A New Iodinated 5-HT1A Ligand. J. Med. Chem. 1994, 37, 1406–1407. [Google Scholar] [CrossRef] [PubMed]
- Shiue, C.-Y.; Shiue, G.G.; Mozley, P.D.; Kung, M.-P.; Zhuang, Z.-P.; Kim, H.-J.; Kung, H.F. p-[18F]-MPPF: A potential radioligand for PET studies of 5-HT1A receptors in humans. Synapse 1997, 25, 147–154. [Google Scholar] [CrossRef]
- Aznavour, N.; Zimmer, L. [18F]MPPF as a tool for the in vivo imaging of 5-HT1A receptors in animal and human brain. Neuropharmacology 2007, 52, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Pauwelyn, G.; Vlerick, L.; Dockx, R.; Verhoeven, J.; Dobbeleir, A.; Peremans, K.; Goethals, I.; Bosmans, T.; Vanhove, C.; De Vos, F.; et al. PET quantification of [18F]MPPF in the canine brain using blood input and reference tissue modelling. PLoS ONE 2019, 14, e0218237. [Google Scholar] [CrossRef]
- Plenevaux, A.; Lemaire, C.; Aerts, J.; Lacan, G.; Rubins, D.; Melega, W.; Brihaye, C.; Degueldre, C.; Fuchs, S.; Salmon, E.; et al. [18F]p-MPPF: A Radiolabeled Antagonist for the Study of 5-HT1A Receptors with PET. Nucl. Med. Biol. 2000, 27, 467–471. [Google Scholar] [CrossRef]
- Costes, N.; Merlet, I.; Ostrowsky, K.; Faillenot, I.; Lavenne, F.; Zimmer, L.; Ryvlin, P.; Le Bars, D. A 18F-MPPF PET normative database of 5-HT1A receptor binding in men and women over aging. J. Nucl. Med. 2005, 46, 1980–1989. [Google Scholar]
- Kepe, V.; Barrio, J.R.; Huang, S.-C.; Ercoli, L.; Siddarth, P.; Shoghi-Jadid, K.; Cole, G.M.; Satyamurthy, N.; Cummings, J.L.; Small, G.W.; et al. Serotonin 1A receptors in the living brain of Alzheimer’s disease patients. Proc. Natl. Acad. Sci. USA 2006, 103, 702–707. [Google Scholar] [CrossRef]
- Merlet, I.; Ostrowsky, K.; Costes, N.; Ryvlin, P.; Isnard, J.; Faillenot, I.; Lavenne, F.; Dufournel, D.; Le Bars, D.; Mauguière, F. 5-HT1A receptor binding and intracerebral activity in temporal lobe epilepsy: An [18F]MPPF-PET study. Brain 2004, 127, 900–913. [Google Scholar] [CrossRef] [PubMed]
- Lothe, A.; Didelot, A.; Hammers, A.; Costes, N.; Saoud, M.; Gilliam, F.; Ryvlin, P. Comorbidity between temporal lobe epilepsy and depression: A [18 F]MPPF PET study. Brain 2008, 131, 2765–2782. [Google Scholar] [CrossRef] [PubMed]
- Lang, L.; Jagoda, E.; Schmall, B.; Vuong, B.; Adams, H.R.; Nelson, D.L.; Carson, R.E.; Eckelman, W.C. Development of Fluorine-18-Labeled 5-HT 1A Antagonists. J. Med. Chem. 1999, 42, 1576–1586. [Google Scholar] [CrossRef]
- Toczek, M.T.; Carson, R.E.; Lang, L.; Ma, Y.; Spanaki, M.V.; Der, M.G.; Fazilat, S.; Kopylev, L.; Herscovitch, P.; Eckelman, W.C.; et al. PET imaging of 5-HT1A receptor binding in patients with temporal lobe epilepsy. Neurology 2003, 60, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Neumeister, A.; Bain, E.; Nugent, A.C.; Carson, R.E.; Bonne, O.; Luckenbaugh, D.A.; Eckelman, W.; Herscovitch, P.; Charney, D.S.; Drevets, W.C. Reduced Serotonin Type 1A Receptor Binding in Panic Disorder. J. Neurosci. 2004, 24, 589–591. [Google Scholar] [CrossRef]
- Bonne, O.; Bain, E.; Neumeister, A.; Nugent, A.C.; Vythilingam, M.; Carson, R.E.; Luckenbaugh, D.A.; Eckelman, W.; Herscovitch, P.; Drevets, W.C.; et al. No Change in Serotonin Type 1A Receptor Binding in Patients with Posttraumatic Stress Disorder. Am. J. Psychiatry 2005, 162, 383–385. [Google Scholar] [CrossRef]
- Ryu, Y.H.; Liow, J.-S.; Zoghbi, S.; Fujita, M.; Collins, J.; Tipre, D.; Sangare, J.; Hong, J.; Pike, V.W.; Innis, R.B. Disulfiram Inhibits Defluorination of 18F-FCWAY, Reduces Bone Radioactivity, and Enhances Visualization of Radioligand Binding to Serotonin 5-HT1A Receptors in Human Brain. J. Nucl. Med. 2007, 48, 1154–1161. [Google Scholar] [CrossRef]
- Saigal, N.; Bajwa, A.K.; Faheem, S.S.; Coleman, R.A.; Pandey, S.K.; Constantinescu, C.C.; Fong, V.; Mukherjee, J. Evaluation of serotonin 5-HT 1A receptors in rodent models using [18F]mefway PET. Synapse 2013, 67, 596–608. [Google Scholar] [CrossRef][Green Version]
- Wooten, D.W.; Hillmer, A.T.; Moirano, J.M.; Ahlers, E.O.; Slesarev, M.; Barnhart, T.E.; Mukherjee, J.; Schneider, M.L.; Christian, B.T. Measurement of 5-HT 1A receptor density and in-vivo binding parameters of 18Fmefway in the nonhuman primate. J. Cereb. Blood Flow Metab. 2012, 32, 1546–1558. [Google Scholar] [CrossRef]
- Hillmer, A.T.; Wooten, D.W.; Bajwa, A.K.; Higgins, A.T.; Lao, P.J.; Betthauser, T.J.; Barnhart, T.E.; Rowley, H.A.; Stone, C.K.; Johnson, S.C.; et al. First-in-Human Evaluation of 18F-Mefway, a PET Radioligand Specific to Serotonin-1A Receptors. J. Nucl. Med. 2014, 55, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Lyoo, C.H.; Kim, J.S.; Kim, K.M.; Kang, J.H.; Choi, S.-H.; Kim, J.-J.; Ryu, Y.H. 18F-Mefway PET Imaging of Serotonin 1A Receptors in Humans: A Comparison with 18F-FCWAY. PLoS ONE 2015, 10, e0121342. [Google Scholar] [CrossRef]
- Defraiteur, C.; Lemaire, C.; Luxen, A.; Plenevaux, A. Radiochemical synthesis and tissue distribution of p-[18F]DMPPF, a new 5-HT1A ligand for PET, in rats. Nucl. Med. Biol. 2006, 33, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Andrée, B.; Halldin, C.; Pike, V.W.; Gunn, R.N.; Olsson, H.; Farde, L. The PET radioligand [carbonyl-(11)C]desmethyl-WAY-100635 binds to 5-HT(1A) receptors and provides a higher radioactive signal than [carbonyl-(11)C]WAY-100635 in the human brain. J. Nucl. Med. 2002, 43, 292–303. [Google Scholar]
- Houle, S.; Wilson, A.A.; Inaba, T.; Fisher, N.; Dasilva, J.N. Imaging 5-ht1a receptors with positron emission tomography initial human studies with [x11c]cpc-222. Nucl. Med. Commun. 1997, 18, 1130–1134. [Google Scholar] [CrossRef]
- Sandell, J.; Halldin, C.; Pike, V.W.; Chou, Y.H.; Varnäs, K.; Hall, H.; Marchais, S.; Nowick, B.; Wikström, H.V.; Swahn, C.G.; et al. New halogenated [11C]WAY analogues, [11C]6FPWAY and [11C]6BPWAY-Radiosynthesis and assessment as radioligands for the study of brain 5-HT1A receptors in living monkey. Nucl. Med. Biol. 2001, 28, 177–185. [Google Scholar] [CrossRef]
- Karramkam, M.; Hinnen, F.; Berrehouma, M.; Hlavacek, C.; Vaufrey, F.; Halldin, C.; McCarron, J.A.; Pike, V.W.; Dollé, F. Synthesis of a [6-Pyridinyl-18F]-labelled fluoro derivative of WAY-100635 as a candidate radioligand for brain 5-HT 1A receptor imaging with PET. Bioorg. Med. Chem. 2003, 11, 2769–2782. [Google Scholar] [CrossRef]
- McCarron, J. The Pyridinyl-6 Position of WAY-100635 as a site for radiofluorination—Effect on 5-HT1A receptor radioligand behavior in vivo. Mol. Imaging Biol. 2004, 6, 17–26. [Google Scholar] [CrossRef]
- Kumar, J.S.D.; Majo, V.J.; Hsiung, S.; Millak, M.S.; Liu, K.; Tamir, H.; Prabhakaran, J.; Simpson, N.R.; Van Heertum, R.L.; Mann, J.J.; et al. Synthesis and in Vivo Validation of [O-Methyl-11C]2-{4-[4-(7-methoxynaphthalen-1-yl)piperazin-1-yl]butyl}-4-methyl-2H-[1,2,4]triazine-3,5-dione: A Novel 5-HT 1A Receptor Agonist Positron Emission Tomography Ligand. J. Med. Chem. 2006, 49, 125–134. [Google Scholar] [CrossRef]
- Kumar, J.S.D.; Prabhakaran, J.; Majo, V.J.; Milak, M.S.; Hsiung, S.-C.; Tamir, H.; Simpson, N.R.; Van Heertum, R.L.; Mann, J.J.; Parsey, R.V. Synthesis and in vivo evaluation of a novel 5-HT1A receptor agonist radioligand [O-methyl-11C]2-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-4-methyl-1,2,4-triazine-3,5(2H,4H)dione in nonhuman primates. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1050–1060. [Google Scholar] [CrossRef]
- Milak, M.S.; DeLorenzo, C.; Zanderigo, F.; Prabhakaran, J.; Kumar, J.S.D.; Majo, V.J.; Mann, J.J.; Parsey, R.V. In Vivo Quantification of Human Serotonin 1A Receptor Using 11 C-CUMI-101, an Agonist PET Radiotracer. J. Nucl. Med. 2010, 51, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
- Hines, C.S.; Liow, J.-S.; Zanotti-Fregonara, P.; Hirvonen, J.; Morse, C.; Pike, V.W.; Innis, R.B. Human Biodistribution and Dosimetry of 11C-CUMI-101, an Agonist Radioligand for Serotonin-1A Receptors in Brain. PLoS ONE 2011, 6, e25309. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hendry, N.; Christie, I.; Rabiner, E.A.; Laruelle, M.; Watson, J. In vitro assessment of the agonist properties of the novel 5-HT1A receptor ligand, CUMI-101 (MMP), in rat brain tissue. Nucl. Med. Biol. 2011, 38, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Girgis, R.R.; Forbes, A.; Abi-Dargham, A.; Slifstein, M. A positron emission tomography occupancy study of brexpiprazole at dopamine D2 and D3 and serotonin 5-HT1A and 5-HT2A receptors, and serotonin reuptake transporters in subjects with schizophrenia. Neuropsychopharmacology 2020, 45, 786–792. [Google Scholar] [CrossRef]
- Majo, V.J.; Milak, M.S.; Prabhakaran, J.; Mali, P.; Savenkova, L.; Simpson, N.R.; Mann, J.J.; Parsey, R.V.; Kumar, J.S.D. Synthesis and in vivo evaluation of [18F]2-(4-(4-(2-(2-fluoroethoxy)phenyl)piperazin-1-yl)butyl)-4-methyl-1,2,4-triazine-3,5(2H,4H)-dione ([18F]FECUMI-101) as an imaging probe for 5-HT1A receptor agonist in nonhuman primates. Bioorg. Med. Chem. 2013, 21, 5598–5604. [Google Scholar] [CrossRef][Green Version]
- Kumar, J.S.D.; Underwood, M.D.; Simpson, N.R.; Kassir, S.A.; Prabhakaran, J.; Majo, V.J.; Bakalian, M.J.; Parsey, R.V.; Mann, J.J.; Arango, V. Autoradiographic Evaluation of [18F]FECUMI-101, a High Affinity 5-HT1AR Ligand in Human Brain. ACS Med. Chem. Lett. 2016, 7, 482–486. [Google Scholar] [CrossRef]
- Collier, T.L.; Liang, S.H.; Mann, J.J.; Vasdev, N.; Kumar, J.S.D. Microfluidic radiosynthesis of [ 18 F]FEMPT, a high affinity PET radiotracer for imaging serotonin receptors. Beilstein J. Org. Chem. 2017, 13, 2922–2927. [Google Scholar] [CrossRef]
- Vacher, B.; Bonnaud, B.; Funes, P.; Jubault, N.; Koek, W.; Assié, M.B.; Cosi, C.; Kleven, M. Novel derivatives of 2-pyridinemethylamine as selective, potent, and orally active agonists at 5-HT(1A) receptors. J. Med. Chem. 1999, 42, 1648–1660. [Google Scholar] [CrossRef]
- Koek, W.; Vacher, B.; Cosi, C.; Assié, M.B.; Patoiseau, J.F.; Pauwels, P.J.; Colpaert, F.C. 5-HT1A receptor activation and antidepressant-like effects: F 13714 has high efficacy and marked antidepressant potential. Eur. J. Pharmacol. 2001, 420, 103–112. [Google Scholar] [CrossRef]
- Lemoine, L.; Becker, G.; Vacher, B.; Billard, T.; Lancelot, S.; Newman-Tancredi, A.; Zimmer, L. Radiosynthesis and Preclinical Evaluation of 18 F-F13714 as a Fluorinated 5-HT 1A Receptor Agonist Radioligand for PET Neuroimaging. J. Nucl. Med. 2012, 53, 969–976. [Google Scholar] [CrossRef]
- Yokoyama, C.; Mawatari, A.; Kawasaki, A.; Takeda, C.; Onoe, K.; Doi, H.; Newman-Tancredi, A.; Zimmer, L.; Onoe, H. Marmoset Serotonin 5-HT 1A Receptor Mapping with a Biased Agonist PET Probe 18 F-F13714: Comparison with an Antagonist Tracer 18 F-MPPF in Awake and Anesthetized States. Int. J. Neuropsychopharmacol. 2016, 19, pyw079. [Google Scholar] [CrossRef] [PubMed]
- Heusler, P.; Palmier, C.; Tardif, S.; Bernois, S.; Colpaert, F.C.; Cussac, D. [3H]-F13640, a novel, selective and high-efficacy serotonin 5-HT1A receptor agonist radioligand. Naunyn Schmiedebergs Arch. Pharmacol. 2010, 382, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-J.; Colpaert, F.; Wiesenfeld-Hallin, Z. Opioid hyperalgesia and tolerance versus 5-HT1A receptor-mediated inverse tolerance. Trends Pharmacol. Sci. 2003, 24, 634–639. [Google Scholar] [CrossRef]
- Vidal, B.; Fieux, S.; Colom, M.; Billard, T.; Bouillot, C.; Barret, O.; Constantinescu, C.; Tamagnan, G.; Newman-Tancredi, A.; Zimmer, L. 18F-F13640 preclinical evaluation in rodent, cat and primate as a 5-HT1A receptor agonist for PET neuroimaging. Brain Struct. Funct. 2018, 223, 2973–2988. [Google Scholar] [CrossRef] [PubMed]
- Vidal, B.; Sebti, J.; Verdurand, M.; Fieux, S.; Billard, T.; Streichenberger, N.; Troakes, C.; Newman-Tancredi, A.; Zimmer, L. Agonist and antagonist bind differently to 5-HT1A receptors during Alzheimer’s disease: A post-mortem study with PET radiopharmaceuticals. Neuropharmacology 2016, 109, 88–95. [Google Scholar] [CrossRef]
- Colom, M.; Costes, N.; Redouté, J.; Dailler, F.; Gobert, F.; Le Bars, D.; Billard, T.; Newman-Tancredi, A.; Zimmer, L. 18F-F13640 PET imaging of functional receptors in humans. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 220–221. [Google Scholar] [CrossRef]
- Colom, M.; Vidal, B.; Fieux, S.; Redoute, J.; Costes, N.; Lavenne, F.; Mérida, I.; Irace, Z.; Iecker, T.; Bouillot, C.; et al. [18F]F13640, a 5-HT1A Receptor Radiopharmaceutical Sensitive to Brain Serotonin Fluctuations. Front. Neurosci. 2021, 15, 622423. [Google Scholar] [CrossRef]
- Sari, Y. Serotonin receptors: From protein to physiological function and behavior. Neurosci. Biobehav. Rev. 2004, 28, 565–582. [Google Scholar] [CrossRef]
- Rutz, S.; Riegert, C.; Rothmaier, A.K.; Buhot, M.C.; Cassel, J.C.; Jackisch, R. Presynaptic serotonergic modulation of 5-HT and acetylcholine release in the hippocampus and the cortex of 5-HT1B-receptor knockout mice. Brain Res. Bull. 2006, 70, 81–93. [Google Scholar] [CrossRef]
- Olivier, B.; van Oorschot, R. 5-HT1B receptors and aggression: A review. Eur. J. Pharmacol. 2005, 526, 207–217. [Google Scholar] [CrossRef]
- Pierson, M.E.; Andersson, J.; Nyberg, S.; McCarthy, D.J.; Finnema, S.J.; Varnäs, K.; Takano, A.; Karlsson, P.; Gulyás, B.; Medd, A.M.; et al. [11C]AZ10419369: A selective 5-HT1B receptor radioligand suitable for positron emission tomography (PET). Characterization in the primate brain. Neuroimage 2008, 41, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Maier, D.L.; Sobotka-Briner, C.; Ding, M.; Powell, M.E.; Jiang, Q.; Hill, G.; Heys, J.R.; Elmore, C.S.; Pierson, M.E.; Mrzljak, L. [N-methyl-3H3]AZ10419369 binding to the 5-HT 1B receptor: In vitro characterization and in vivo receptor occupancy. J. Pharmacol. Exp. Ther. 2009, 330, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Varnäs, K.; Nyberg, S.; Halldin, C.; Varrone, A.; Takano, A.; Karlsson, P.; Andersson, J.; McCarthy, D.; Smith, M.; Pierson, M.E.; et al. Quantitative Analysis of [ 11 C]AZ10419369 Binding to 5-HT 1B Receptors in Human Brain. J. Cereb. Blood Flow Metab. 2011, 31, 113–123. [Google Scholar] [CrossRef]
- Nord, M.; Finnema, S.J.; Schain, M.; Halldin, C.; Farde, L. Test–retest reliability of [11C]AZ10419369 binding to 5-HT1B receptors in human brain. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Tiger, M.; Farde, L.; Rück, C.; Varrone, A.; Forsberg, A.; Lindefors, N.; Halldin, C.; Lundberg, J. Low serotonin1B receptor binding potential in the anterior cingulate cortex in drug-free patients with recurrent major depressive disorder. Psychiatry Res. Neuroimaging 2016, 253, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Varrone, A.; Svenningsson, P.; Forsberg, A.; Varnäs, K.; Tiger, M.; Nakao, R.; Halldin, C.; Nilsson, L.-G.; Farde, L. Positron emission tomography imaging of 5-hydroxytryptamine1B receptors in Parkinson’s disease. Neurobiol. Aging 2014, 35, 867–875. [Google Scholar] [CrossRef]
- Lindberg, A.; Nag, S.; Schou, M.; Takano, A.; Matsumoto, J.; Amini, N.; Elmore, C.S.; Farde, L.; Pike, V.W.; Halldin, C. [11C]AZ10419096—A full antagonist PET radioligand for imaging brain 5-HT1B receptors. Nucl. Med. Biol. 2017, 54, 34–40. [Google Scholar] [CrossRef]
- Lindberg, A.; Arakawa, R.; Nogami, T.; Nag, S.; Schou, M.; Elmore, C.S.; Farde, L.; Pike, V.W.; Halldin, C. Potential for imaging the high-affinity state of the 5-HT1B receptor: A comparison of three PET radioligands with differing intrinsic activity. EJNMMI Res. 2019, 9, 100. [Google Scholar] [CrossRef]
- Lindberg, A.; Nag, S.; Schou, M.; Arakawa, R.; Nogami, T.; Moein, M.M.; Elmore, C.S.; Pike, V.W.; Halldin, C. Development of a 18F-labeled PET radioligand for imaging 5-HT1B receptors: [18F]AZ10419096. Nucl. Med. Biol. 2019, 78–79, 11–16. [Google Scholar] [CrossRef]
- Ridler, K.; Plisson, C.; Rabiner, E.A.; Gunn, R.N.; Easwaramoorthy, B.; Abi-Dargham, A.; Laruelle, M.; Slifstein, M. Characterization of in vivo pharmacological properties and sensitivity to endogenous serotonin of [11C] P943: A positron emission tomography study in Papio anubis. Synapse 2011, 65, 1119–1127. [Google Scholar] [CrossRef]
- Murrough, J.W.; Henry, S.; Hu, J.; Gallezot, J.-D.; Planeta-Wilson, B.; Neumaier, J.F.; Neumeister, A. Reduced ventral striatal/ventral pallidal serotonin1B receptor binding potential in major depressive disorder. Psychopharmacology 2011, 213, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Matuskey, D.; Bhagwagar, Z.; Planeta, B.; Pittman, B.; Gallezot, J.-D.; Chen, J.; Wanyiri, J.; Najafzadeh, S.; Ropchan, J.; Geha, P.; et al. Reductions in Brain 5-HT1B Receptor Availability in Primarily Cocaine-Dependent Humans. Biol. Psychiatry 2014, 76, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Henry, S.; Gallezot, J.D.; Ropchan, J.; Neumaier, J.F.; Potenza, M.N.; Sinha, R.; Krystal, J.H.; Huang, Y.; Ding, Y.S.; et al. Serotonin 1B Receptor Imaging in Alcohol Dependence. Biol. Psychiatry 2010, 67, 800–803. [Google Scholar] [CrossRef]
- Leysen, J.E. 5-HT2 receptors. Curr. Drug Targets CNS Neurol. Disord. 2004, 3, 11–26. [Google Scholar] [CrossRef]
- Conn, P.J.; Sanders-Bush, E. Selective 5ht-2 antagonists inhibit serotonin stimulated phosphatidylinositol metabolism in cerebral cortex. Neuropharmacology 1984, 23, 993–996. [Google Scholar] [CrossRef]
- Kim, J.; Moon, B.S.; Lee, B.C.; Lee, H.Y.; Kim, H.J.; Choo, H.; Pae, A.N.; Cho, Y.S.; Min, S.J. A Potential PET Radiotracer for the 5-HT2C Receptor: Synthesis and in Vivo Evaluation of 4-(3-[18F]fluorophenethoxy)pyrimidine. ACS Chem. Neurosci. 2017, 8, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Nye, J.A.; Voll, R.J.; Howell, L.; Goodman, M.M. Synthesis and Evaluation of Pyridyloxypyridyl Indole Carboxamides as Potential PET Imaging Agents for 5-HT2C Receptors. ACS Med. Chem. Lett. 2018, 9, 188–192. [Google Scholar] [CrossRef]
- Branchek, T.; Adham, N.; Macchi, M.; Kao, H.T.; Hartig, P.R. [3H]-DOB(4-bromo-2,5-dimethoxyphenylisopropylamine) and [3H] ketanserin label two affinity states of the cloned human 5-hydroxytryptamine2 receptor. Mol. Pharmacol. 1990, 38, 604–609. [Google Scholar]
- Nichols, D.E. Hallucinogens. Pharmacol. Ther. 2004, 101, 131–181. [Google Scholar] [CrossRef]
- Hall, H.; Farde, L.; Halldin, C.; Lundkvist, C.; Sedvall, G. Autoradiographic localization of 5-HT(2A) receptors in the human brain using [3H]M100907 and [11C]M100907. Synapse 2000, 38, 421–431. [Google Scholar] [CrossRef]
- Hasuo, H.; Matsuoka, T.; Akasu, T. Activation of presynaptic 5-hydroxytryptamine 2A receptors facilitates excitatory synaptic transmission via protein kinase C in the dorsolateral septal nucleus. J. Neurosci. 2002, 22, 7509–7517. [Google Scholar] [CrossRef] [PubMed]
- Gijsman, H.J.; Verkes, R.J.; Schouten-Verhagen, J.C.M.; Schoemaker, R.C.; Van Gerven, J.M.A.; De Rijk, R.H.; Van Kempen, G.M.J. A review of the role of serotonin receptors in psychiatric disorders. Hum. Psychopharmacol. 2000, 15, 397–415. [Google Scholar] [CrossRef]
- Berridge, M.; Comar, D.; Crouzel, C.; Baron, J.-C. 11C-Labeled ketanserin: A selective serotonin S2 antagonist. J. Label. Compd. Radiopharm. 1983, 20, 73–78. [Google Scholar] [CrossRef]
- Blin, J.; Pappata, S.; Kiyosawa, M.; Crouzel, C.; Baron, J.C. [18F]Setoperone: A new high-affinity ligand for positron emission tomography study of the serotonin-2 receptors in baboon brain in vivo. Eur. J. Pharmacol. 1988, 147, 73–82. [Google Scholar] [CrossRef]
- Blin, J.; Sette, G.; Fiorelli, M.; Bletry, O.; Elghozi, J.L.; Crouzel, C.; Baron, J.C. A Method for the In Vivo Investigation of the Serotonergic 5-HT2 Receptors in the Human Cerebral Cortex Using Positron Emission Tomography and 18F-Labeled Setoperone. J. Neurochem. 1990, 54, 1744–1754. [Google Scholar] [CrossRef]
- Meyer, J.H.; Kapur, S.; Houle, S.; DaSilva, J.; Owczarek, B.; Brown, G.M.; Wilson, A.A.; Kennedy, S.H. Prefrontal cortex 5-HT2 receptors in depression: An [18F]setoperone PET imaging study. Am. J. Psychiatry 1999, 156, 1029–1034. [Google Scholar] [CrossRef]
- Massou, J.M.; Trichard, C.; Attar-Levy, D.; Feline, A.; Corruble, E.; Beaufils, B.; Martinot, J.L. Frontal 5-HT(2A) receptors studied in depressive patients during chronic treatment by selective serotonin reuptake inhibitors. Psychopharmacology 1997, 133, 99–101. [Google Scholar] [CrossRef]
- Chabriat, H.; Vera, P.; Samson, Y.; Pappata, S.; Boullais, N.; Tehindrazanarivelo, A.; Bousser, M. 5HT2 receptors in cerebral cortex of migraineurs studied using PET and 18F-fluorosetoperone. Cephalalgia 1995, 15, 104–108. [Google Scholar] [CrossRef]
- Blin, J.; Baron, J.C.; Dubois, B.; Crouzel, C.; Fiorelli, M.; Attar-Lévy, D.; Pillon, B.; Fournier, D.; Vidailhet, M.; Agid, Y. Loss of brain 5-HT 2 receptors in Alzheimer’s disease. Brain 1993, 116, 497–510. [Google Scholar] [CrossRef]
- Lemaire, C.; Cantineau, R.; Guillaume, M.; Plenevaux, A.; Christiaens, L. Fluorine-18-altanserin: A radioligand for the study of serotonin receptors with PET: Radiolabeling and in vivo biologic behavior in rats. J. Nucl. Med. 1991, 32, 2266–2272. [Google Scholar]
- Biver, F.; Goldman, S.; Luxen, A.; Monclus, M.; Forestini, M.; Mendlewicz, J.; Lotstra, F. Multicompartmental study of fluorine-18 altanserin binding to brain 5HT2 receptors in humans using positron emission tomography. Eur. J. Nucl. Med. 1994, 21, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Price, J.C.; Lopresti, B.J.; Mason, N.S.; Holt, D.P.; Huang, Y.; Mathis, C.A. Analyses of [18F]altanserin bolus injection PET data. I: Consideration of radiolabeled metabolites in baboons. Synapse 2001, 41, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.Z.; Baldwin, R.M.; Van Dyck, C.H.; Al-Tikriti, M.; Roth, B.; Khan, N.; Charney, D.S.; Innis, R.B. Characterization of radioactive metabolites of 5-HT(2A) receptor PET ligand [18F]altanserin in human and rodent. Nucl. Med. Biol. 1999, 26, 601–608. [Google Scholar] [CrossRef]
- Adams, K.H.; Pinborg, L.H.; Svarer, C.; Hasselbalch, S.G.; Holm, S.; Haugbøl, S.; Madsen, K.; Frøkjær, V.; Martiny, L.; Paulson, O.B.; et al. A database of [18F]-altanserin binding to 5-HT2A receptors in normal volunteers: Normative data and relationship to physiological and demographic variables. Neuroimage 2004, 21, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.K.; Kaye, W.H.; Meltzer, C.C.; Price, J.C.; Greer, P.; McConaha, C.; Skovira, K. Reduced 5-HT2A receptor binding after recovery from anorexia nervosa. Biol. Psychiatry 2002, 52, 896–906. [Google Scholar] [CrossRef]
- Rasmussen, H.; Frokjaer, V.G.; Hilker, R.W.; Madsen, J.; Anhøj, S.; Oranje, B.; Pinborg, L.H.; Glenthøj, B.; Knudsen, G.M. Low frontal serotonin 2A receptor binding is a state marker for schizophrenia? Eur. Neuropsychopharmacol. 2016, 26, 1248–1250. [Google Scholar] [CrossRef]
- Mintun, M.A.; Sheline, Y.I.; Moerlein, S.M.; Vlassenko, A.G.; Huang, Y.; Snyder, A.Z. Decreased hippocampal 5-HT2A receptor binding in major depressive disorder: In vivo measurement with [18F]altanserin positron emission tomography. Biol. Psychiatry 2004, 55, 217–224. [Google Scholar] [CrossRef]
- Marner, L.; Frokjaer, V.G.; Kalbitzer, J.; Lehel, S.; Madsen, K.; Baaré, W.F.C.; Knudsen, G.M.; Hasselbalch, S.G. Loss of serotonin 2A receptors exceeds loss of serotonergic projections in early Alzheimer’s disease: A combined [11C]DASB and [18F]altanserin-PET study. Neurobiol. Aging 2012, 33, 479–487. [Google Scholar] [CrossRef]
- Staley, J.K.; Van Dyck, C.H.; Tan, P.Z.; Al Tikriti, M.; Ramsby, Q.; Klump, H.; Ng, C.; Garg, P.; Soufer, R.; Baldwin, R.M.; et al. Comparison of [18F]altanserin and [18F]deuteroaltanserin for PET imaging of serotonin2A receptors in baboon brain: Pharmacological studies. Nucl. Med. Biol. 2001, 28, 271–279. [Google Scholar] [CrossRef]
- Van Dyck, C.H.; Soares, J.C.; Tan, P.Z.; Staley, J.K.; Baldwin, R.M.; Amici, L.A.; Fu, X.; Garg, P.K.; Seibyl, J.P.; Charney, D.S.; et al. Equilibrium modeling of 5-HT2A receptors with [18F]deuteroaltanserin and PET: Feasibility of a constant infusion paradigm. Nucl. Med. Biol. 2000, 27, 715–722. [Google Scholar] [CrossRef]
- Soares, J.C.; Van Dyck, C.H.; Tan, P.Z.; Zoghbi, S.S.; Garg, P.; Soufer, R.; Baldwin, R.M.; Fujita, M.; Staley, J.K.; Fu, X.; et al. Reproducibility of in vivo brain measures of 5-HT2A receptors with PET and [18F]deuteroaltanserin. Psychiatry Res. Neuroimaging 2001, 106, 81–93. [Google Scholar] [CrossRef]
- Santhosh, L.; Estok, K.M.; Vogel, R.S.; Tamagnan, G.D.; Baldwin, R.M.; Mitsis, E.M.; MacAvoy, M.G.; Staley, J.K.; van Dyck, C.H. Regional distribution and behavioral correlates of 5-HT2A receptors in Alzheimer’s disease with [18F]deuteroaltanserin and PET. Psychiatry Res. Neuroimaging 2009, 173, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Nyberg, S.; Halldin, C.; Lundkvist, C.; Farde, L. PET imaging of central 5-HT(2A) receptors with carbon-11-MDL 100,907. J. Nucl. Med. 1998, 39, 208–214. [Google Scholar] [PubMed]
- Lopez-Gimenez, J.F.; Vilaro, M.T.; Palacios, M.; Mengod, G. [3H] MDL 100, 907 labels 5-HT 2A serotonin receptors selectively in primate brain. Neuropharmacology 1998, 37, 1147–1158. [Google Scholar] [CrossRef]
- López-Giménez, J.F.; Mengod, G.; Palacios, J.M.; Vilaró, M.T. Selective visualization of rat brain 5-HT(2A) receptors by autoradiography with [3H]MDL 100,907. Naunyn Schmiedebergs Arch. Pharmacol. 1997, 356, 446–454. [Google Scholar] [CrossRef]
- Lundkvist, C.; Halldin, C.; Ginovart, N.; Nyberg, S.; Swahn, C.G.; Carr, A.A.; Brunner, F.; Farde, L. [11C]MDL 100907, a radioligand for selective imaging of 5-HT2A receptors with positron emission tomography. Life Sci. 1996, 58, 187–192. [Google Scholar] [CrossRef]
- Talbot, P.S.; Slifstein, M.; Hwang, D.R.; Huang, Y.; Scher, E.; Abi-Dargham, A.; Laruelle, M. Extended characterisation of the serotonin 2A (5-HT 2A) receptor-selective PET radiotracer 11C-MDL100907 in humans: Quantitative analysis, test-retest reproducibility, and vulnerability to endogenous 5-HT tone. Neuroimage 2012, 59, 271–285. [Google Scholar] [CrossRef]
- Bhagwagar, Z.; Hinz, R.; Taylor, M.; Fancy, S.; Cowen, P.; Grasby, P. Increased 5-HT2A receptor binding in euthymic, medication-free patients recovered from depression: A positron emission study with [11C]MDL 100,907. Am. J. Psychiatry 2006, 163, 1580–1587. [Google Scholar] [CrossRef]
- Girgis, R.R.; Slifstein, M.; Xu, X.; Frankle, W.G.; Anagnostou, E.; Wasserman, S.; Pepa, L.; Kolevzon, A.; Abi-Dargham, A.; Laruelle, M.; et al. The 5-HT 2A receptor and serotonin transporter in Asperger’s Disorder: A PET study with [11C]MDL 100907 and [11C]DASB. Psychiatry Res. Neuroimaging 2011, 194, 230–234. [Google Scholar] [CrossRef]
- Simpson, H.B.; Slifstein, M.; Bender, J.; Xu, X.; Hackett, E.; Maher, M.J.; Abi-Dargham, A. Serotonin 2A receptors in obsessive-compulsive disorder: A positron emission tomography study with [11C]MDL 100907. Biol. Psychiatry 2011, 70, 897–904. [Google Scholar] [CrossRef]
- Scott, D.O.; Heath, T.G. Investigation of the CNS penetration of a potent 5-HT(2a) receptor antagonist (MDL 100,907) and an active metabolite (MDL 105,725) using in vivo microdialysis sampling in the rat. J. Pharm. Biomed. Anal. 1998, 17, 17–25. [Google Scholar] [CrossRef]
- Ren, H.; Wey, H.Y.; Strebl, M.; Neelamegam, R.; Ritter, T.; Hooker, J.M. Synthesis and imaging validation of [18F]MDL100907 enabled by Ni-mediated fluorination. ACS Chem. Neurosci. 2014, 5, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Herth, M.M.; Piel, M.; Debus, F.; Schmitt, U.; Lüddens, H.; Rösch, F. Preliminary in vivo and ex vivo evaluation of the 5-HT2A imaging probe [18F]MH.MZ. Nucl. Med. Biol. 2009, 36, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Debus, F.; Herth, M.M.; Piel, M.; Buchholz, H.G.; Bausbacher, N.; Kramer, V.; Lüddens, H.; Rösch, F. 18F-Labeling and evaluation of novel MDL 100907 derivatives as potential 5-HT2A antagonists for molecular imaging. Nucl. Med. Biol. 2010, 37, 487–495. [Google Scholar] [CrossRef]
- Hansen, H.D.; Ettrup, A.; Herth, M.M.; Dyssegaard, A.; Ratner, C.; Gillings, N.; Knudsen, G.M. Direct comparison of [18F]MH.MZ and [18F]altanserin for 5-HT2A receptor imaging with PET. Synapse 2013, 67, 328–337. [Google Scholar] [CrossRef]
- Ettrup, A.; Palner, M.; Gillings, N.; Santini, M.A.; Hansen, M.; Kornum, B.R.; Rasmussen, L.K.; Någren, K.; Madsen, J.; Begtrup, M.; et al. Radiosynthesis and evaluation of11C-CIMBI-5 as a 5-HT 2A receptor agonist radioligand for PET. J. Nucl. Med. 2010, 51, 1763–1770. [Google Scholar] [CrossRef]
- Prabhakaran, J.; DeLorenzo, C.; Zanderigo, F.; Knudsen, G.M.; Gilling, N.; Pratap, M.; Jorgensen, M.J.; Daunais, J.; Kaplan, J.R.; Parsey, R.V.; et al. In vivo PET Imaging of [11C]CIMBI-5, a 5-HT2AR Agonist Radiotracer in Nonhuman Primates. J. Pharm. Pharm. Sci. 2019, 22, 352–364. [Google Scholar] [CrossRef]
- Ettrup, A.; Hansen, M.; Santini, M.A.; Paine, J.; Gillings, N.; Palner, M.; Lehel, S.; Herth, M.M.; Madsen, J.; Kristensen, J.; et al. Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT2A agonist PET tracers. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 681–693. [Google Scholar] [CrossRef]
- Johansen, A.; Hansen, H.D.; Svarer, C.; Lehel, S.; Leth-Petersen, S.; Kristensen, J.L.; Gillings, N.; Knudsen, G.M. The importance of small polar radiometabolites in molecular neuroimaging: A PET study with [11C]Cimbi-36 labeled in two positions. J. Cereb. Blood Flow Metab. 2018, 38, 659–668. [Google Scholar] [CrossRef]
- Johansen, A.; Holm, S.; Dall, B.; Keller, S.; Kristensen, J.L.; Knudsen, G.M.; Hansen, H.D. Human biodistribution and radiation dosimetry of the 5-HT2A receptor agonist Cimbi-36 labeled with carbon-11 in two positions. EJNMMI Res. 2019, 9, 71. [Google Scholar] [CrossRef]
- Jørgensen, L.M.; Weikop, P.; Villadsen, J.; Visnapuu, T.; Ettrup, A.; Hansen, H.D.; Baandrup, A.O.; Andersen, F.L.; Bjarkam, C.R.; Thomsen, C.; et al. Cerebral 5-HT release correlates with [11C]Cimbi36 PET measures of 5-HT2A receptor occupancy in the pig brain. J. Cereb. Blood Flow Metab. 2017, 37, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha-Bang, S.; Ettrup, A.; Mc Mahon, B.; Skibsted, A.P.; Schain, M.; Lehel, S.; Dyssegaard, A.; Jørgensen, L.M.; Møller, K.; Gillings, N.; et al. Measuring endogenous changes in serotonergic neurotransmission with [11C]Cimbi-36 positron emission tomography in humans. Transl. Psychiatry 2019, 9, 134. [Google Scholar] [CrossRef]
- Yang, K.C.; Stepanov, V.; Martinsson, S.; Ettrup, A.; Takano, A.; Knudsen, G.M.; Halldin, C.; Farde, L.; Finnema, S.J. Fenfluramine Reduces [11C]Cimbi-36 Binding to the 5-HT2A Receptor in the Nonhuman Primate Brain. Int. J. Neuropsychopharmacol. 2017, 20, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Erritzoe, D.; Ashok, A.H.; Searle, G.E.; Colasanti, A.; Turton, S.; Lewis, Y.; Huiban, M.; Moz, S.; Passchier, J.; Saleem, A.; et al. Serotonin release measured in the human brain: A PET study with [11C]CIMBI-36 and d-amphetamine challenge. Neuropsychopharmacology 2020, 45, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Ettrup, A.; Svarer, C.; McMahon, B.; da Cunha-Bang, S.; Lehel, S.; Møller, K.; Dyssegaard, A.; Ganz, M.; Beliveau, V.; Jørgensen, L.M.; et al. Serotonin 2A receptor agonist binding in the human brain with [11C]Cimbi-36: Test-retest reproducibility and head-to-head comparison with the antagonist [18F]altanserin. Neuroimage 2016, 130, 167–174. [Google Scholar] [CrossRef]
- Prabhakaran, J.; Underwood, M.D.; Kumar, J.S.D.; Simpson, N.R.; Kassir, S.A.; Bakalian, M.J.; Mann, J.J.; Arango, V. Synthesis and in vitro evaluation of [18F]FECIMBI-36: A potential agonist PET ligand for 5-HT2A/2C receptors. Bioorg. Med. Chem. Lett. 2015, 25, 3933–3936. [Google Scholar] [CrossRef]
- Prabhakaran, J.; Solingapuram Sai, K.K.; Zanderigo, F.; Rubin-Falcone, H.; Jorgensen, M.J.; Kaplan, J.R.; Tooke, K.I.; Mintz, A.; Mann, J.J.; Kumar, J.S.D. In vivo evaluation of [18F]FECIMBI-36, an agonist 5-HT2A/2Creceptor PET radioligand in nonhuman primate. Bioorg. Med. Chem. Lett. 2017, 27, 21–23. [Google Scholar] [CrossRef]
- Sargent, T.; Budinger, T.F.; Braun, G.; Shulgin, A.T.; Braun, U. An iodinated catecholamine congener for brain imaging and metabolic studies. J. Nucl. Med. 1978, 19, 71–76. [Google Scholar]
- Glennon, R.A.; Seggel, M.R.; Soine, W.H.; Herrick-Davis, K.; Lyon, R.A.; Titeler, M. Iodine-125 labeled 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane: An iodinated radioligand that specifically labels the agonist high-affinity state of 5-HT2 serotonin receptors. J. Med. Chem. 1988, 31, 5–7. [Google Scholar] [CrossRef]
- Appel, M.; Mitchell, M.; Garlick, R.K.; Glennon, R.A.; Teitler, M.; Souza, B.D.E. Autoradiographic Characterization ([1251]DOI) Binding to 5-HT2 and 5-HT1 Receptors in Rat Brain. J. Pharmacol. Exp. Ther. 1990, 233, 843–857. [Google Scholar]
- Zea-Ponce, Y.; Kegeles, L.S.; Guo, N.; Raskin, L.; Bakthavachalam, V.; Laruelle, M. Pharmacokinetics and brain distribution in non human primate of R(-)[123I]DOI, A 5HT2A/2C serotonin agonist. Nucl. Med. Biol. 2002, 29, 575–583. [Google Scholar] [CrossRef]
- Terriere, D.; Janssen, P.M.; Gommeren, W.; Gysemans, M.; Mertens, J.J.; Leysen, J.E. Evaluation of radioiodo-4-amino-N-[1-[3-(4-fluorophenoxy)-propyl]-4- methyl-4-piperidinyl]-5-iodo-2-methoxybenzamide as a potential 5HT2 receptor tracer for SPE(C)T. Nucl. Med. Biol. 1995, 22, 1005–1010. [Google Scholar] [CrossRef]
- Abi-Dargham, A.; Zea-Ponce, Y.; Terriere, D.; Al-Tikriti, M.; Baldwin, R.M.; Hoffer, P.; Charney, D.; Leysen, J.E.; Laruelle, M.; Mertens, J.; et al. Preclinical evaluation of [123I]R93274 as a SPECT radiotracer for imaging 5-HT(2A) receptors. Eur. J. Pharmacol. 1997, 321, 285–293. [Google Scholar] [CrossRef]
- Busatto, G.F.; Pilowsky, L.S.; Costa, D.C.; Mertens, J.; Terriere, D.; Ell, P.J.; Mulligan, R.; Travis, M.J.; Leysen, J.E.; Lui, D.; et al. Initial evaluation of 123I-5-I-R91150, a selective 5-HT2A ligand for single-photon emission tomography, in healthy human subjects. Eur. J. Nucl. Med. 1997, 24, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Catafau, A.M.; Danus, M.; Bullich, S.; Llop, J.; Perich, J.; Cunningham, V.J.; Plaza, P.; Penengo, M.M.; Eersels, J.L.H.; Squassante, L.; et al. Characterization of the SPECT 5-HT2A receptor ligand 123I-R91150 in healthy volunteers: Part 1—Pseudoequilibrium interval and quantification methods. J. Nucl. Med. 2006, 47, 919–928. [Google Scholar]
- Baeken, C.; D’haenen, H.; Flamen, P.; Mertens, J.; Terriere, D.; Chavatte, K.; Boumon, R.; Bossuyt, A. 123I-5-I-R91150, a new single-photon emission tomography ligand for 5-HT(2A) receptors: Influence of age and gender in healthy subjects. Eur. J. Nucl. Med. 1998, 25, 1617–1622. [Google Scholar] [CrossRef]
- Travis, M.J.; Busatto, G.F.; Pilowsky, L.S.; Mulligan, R.; Acton, P.D.; Gacinovic, S.; Mertens, J.; Terrière, D.; Costa, D.C.; Ell, P.J.; et al. 5-HT 2A receptor blockade in patients with schizophrenia treated with risperidone or clozapine. Br. J. Psychiatry 1998, 173, 236–241. [Google Scholar] [CrossRef]
- Melse, M.; Tan, S.K.H.; Temel, Y.; van Kroonenburgh, M.J.P.G.; Leentjens, A.F.G. Changes in 5-HT2A Receptor Expression in Untreated, de novo Patients with Parkinson’s Disease. J. Parkinsons Dis. 2014, 4, 283–287. [Google Scholar] [CrossRef]
- Versijpt, J.; Van Laere, K.J.; Dumont, F.; Decoo, D.; Vandecapelle, M.; Santens, P.; Goethals, I.; Audenaert, K.; Slegers, G.; Dierckx, R.A.; et al. Imaging of the 5-HT2A system: Age-, gender-, and Alzheimer’s disease-related findings. Neurobiol. Aging 2003, 24, 553–561. [Google Scholar] [CrossRef]
- Baeken, C.; De Raedt, R.; Bossuyt, A.; Van Hove, C.; Mertens, J.; Dobbeleir, A.; Blanckaert, P.; Goethals, I. The impact of HF-rTMS treatment on serotonin2A receptors in unipolar melancholic depression. Brain Stimul. 2011, 4, 104–111. [Google Scholar] [CrossRef]
- Vermeire, S.T.; Audenaert, K.R.; Dobbeleir, A.A.; De Meester, R.H.; De Vos, F.J.; Peremans, K.Y. Evaluation of the brain 5-HT2A receptor binding index in dogs with anxiety disorders, measured with 123I-5I-R91150 and SPECT. J. Nucl. Med. 2009, 50, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Peremans, K.; De Spiegeleer, B.; Buntinx, E.; Dobbeleir, A.; Vermeire, S.; Vandermeulen, E.; De Vos, F.; Megens, A.; Eersels, J.; Audenaert, K. Evaluation of serotonin-2A receptor occupancy with 123I-5-I-R91150 and single-photon emission tomography before and after low-dose pipamperone administration in the canine brain. Nucl. Med. Commun. 2008, 29, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Goethals, I.; Vervaet, M.; Audenaert, K.; Jacobs, F.; Ham, H.; Van de Wiele, C.; Vandecapelle, M.; Slegers, G.; Dierckx, R.; van Heeringen, C. Differences of cortical 5-HT2A receptor binding index with SPECT in subtypes of anorexia nervosa: Relationship with personality traits? J. Psychiatr. Res. 2007, 41, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Audenaert, K.; Van Laere, K.; Dumont, F.; Vervaet, M.; Goethals, I.; Slegers, G.; Mertens, J.; van Heeringen, C.; Dierckx, R.A. Decreased 5-HT2a receptor binding in patients with anorexia nervosa. J. Nucl. Med. 2003, 44, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Van Heeringen, C.; Audenaert, K.; Van Laere, K.; Dumont, F.; Slegers, G.; Mertens, J.; Dierckx, R.A. Prefrontal 5-HT2a receptor binding index, hopelessness and personality characteristics in attempted suicide. J. Affect. Disord. 2003, 74, 149–158. [Google Scholar] [CrossRef]
- Dumas, N.; Moulin-Sallanon, M.; Ginovart, N.; Tournier, B.B.; Suzanne, P.; Cailly, T.; Fabis, F.; Rault, S.; Charnay, Y.; Millet, P. Small-animal single-photon emission computed tomographic imaging of the brain serotoninergic systems in wild-type and Mdr1a knockout rats. Mol. Imaging 2014, 13, 1–12. [Google Scholar] [CrossRef]
- Mühlhausen, U.; Ermert, J.; Coenen, H.H. Synthesis, labelling and first evaluation of [18F]R91150 as a serotonin 5-HT2A receptor antagonist for PET. J. Label. Compd. Radiopharm. 2009, 52, 13–22. [Google Scholar] [CrossRef]
- Samnick, S.; Remy, N.; Ametamey, S.; Bader, J.B.; Brandau, W.; Kirsch, C.-M. 123I-MSP and F[1 1C]MSP: New selective 5-HT2A receptor radiopharmaceuticals for in vivo studies of neuronal 5-HT2 serotonin receptors. Synthesis, in vitro binding study with unlabelled analogues and preliminary in vivo evaluation in mice. Life Sci. 1998, 63, 2001–2013. [Google Scholar] [CrossRef]
- Blanckaert, P.B.M.; Burvenich, I.; Wyffels, L.; De Bruyne, S.; Moerman, L.; De Vos, F. In vivo evaluation in rodents of [123I]-3-I-CO as a potential SPECT tracer for the serotonin 5-HT2A receptor. Nucl. Med. Biol. 2008, 35, 861–867. [Google Scholar] [CrossRef]
- Lummis, S.C.R. 5-HT 3 Receptors. J. Biol. Chem. 2012, 287, 40239–40245. [Google Scholar] [CrossRef]
- Niesler, B.; Kapeller, J.; Hammer, C.; Rappold, G. Serotonin type 3 receptor genes: HTR3A, B, C, D, E. Pharmacogenomics 2008, 9, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, G.J.; Jones, B.J.; Tyers, M.B. Identification and distribution of 5-HT3 receptors in rat brain using radioligand binding. Nature 1987, 330, 746–748. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.M.C.; Barnes, J.M.; Ge, J.; Barber, P.C.; Barnes, N.M. Autoradiographic distribution of [3H]-(S)-zacopride-labelled 5-HT3 receptors in human brain. J. Neurol. Sci. 1996, 144, 119–127. [Google Scholar] [CrossRef]
- Thompson, A.J.; Lummis, S.C.R. The 5-HT3 receptor as a therapeutic target. Expert Opin. Ther. Targets 2007, 11, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Walstab, J.; Rappold, G.; Niesler, B. 5-HT3 receptors: Role in disease and target of drugs. Pharmacol. Ther. 2010, 128, 146–169. [Google Scholar] [CrossRef] [PubMed]
- Fakhfouri, G.; Rahimian, R.; Dyhrfjeld-Johnsen, J.; Zirak, M.R.; Beaulieu, J.M. 5-HT3 receptor antagonists in neurologic and neuropsychiatric disorders: The iceberg still lies beneath the surface. Pharmacol. Rev. 2019, 71, 383–412. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J. Recent developments in 5-HT3 receptor pharmacology. Trends Pharmacol. Sci. 2013, 34, 100–109. [Google Scholar] [CrossRef]
- Paterson, L.M.; Kornum, B.R.; Nutt, D.J.; Pike, V.W.; Knudsen, G.M. 5-HT radioligands for human brain imaging with PET and SPECT. Med. Res. Rev. 2013, 33, 54–111. [Google Scholar] [CrossRef]
- Camsonne, R.; Barre, L.; Petit-Taboué, M.C.; Travére, J.M.; Jones, R.; Debruyne, D.; Moulin, M.A.; MacKenzie, E.T.; Baron, J.C. Positron emission tomographic studies of [11C]MDL 72222, a potential 5-HT3 receptor radioligand: Distribution, kinetics and binding in the brain of the baboon. Neuropharmacology 1993, 32, 65–71. [Google Scholar] [CrossRef]
- Ishiwata, K.; Saito, N.; Yanagawa, K.; Furuta, R.; Ishii, S.I.; Kiyosawa, M.; Homma, Y.; Ishii, K.; Suzuki, F.; Senda, M. Synthesis and evaluation of 5-HT3 receptor antagonist [11C]KF17643. Nucl. Med. Biol. 1996, 23, 285–290. [Google Scholar] [CrossRef]
- Besret, L.; Dauphin, F.; Guillouet, G.; Dhilly, M.; Gourand, F.; Blaizot, X.; Young, A.R.; Petit-taboué, M.C.; Mickala, P.; Barbelivien, A.; et al. [11C] S21OO7, a Putative Partial Agonist for 5-HT3 Receptors PET Studies. Rat and Primate in vio Biological Evaluation. Life Sci. 1998, 62, 115–129. [Google Scholar] [CrossRef]
- Katounina, T.; Besret, L.; Dhilly, M.; Petit-Taboué, M.C.; Barbelivien, A.; Baron, J.C.; Dauphin, F.; Barré, L. Synthesis and biological investigations of [18F]MR18445, a 5-HT3 receptor partial agonist. Bioorg. Med. Chem. 1998, 6, 789–795. [Google Scholar] [CrossRef]
- Smith, W.W.; Sancilio, L.F.; Owera-Atepo, J.B.; Naylor, R.J.; Lambert, L. Zacopride, a potent 5-HT3 antagonist. J. Pharm. Pharmacol. 1988, 301–302. [Google Scholar] [CrossRef] [PubMed]
- Hewlett, W.A.; Fridman, S.; Trivedi, B.L.; Schmidt, D.E.; De Paulis, T.; Ebert, M.H. Characterization of desamino-5-[125I]iodo-3-methoxy-zacopride ([125I]MIZAC) binding to 5-HT3 receptors in the rat brain. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1998, 22, 397–410. [Google Scholar] [CrossRef]
- Hewlett, W.A.; Trivedi, B.L.; Zhang, Z.; Lovinger, D.M.; Ansari, M.S.I.B.; Ebert, M.H.; Schmidt, D.E. Characterization of (S)-Des-4-amino-3-[125I]iodozacopride ([125I]DAIZAC), a Selective High-Affinity Radioligand for 5-hydroxytryptamine3 receptors. J. Pharmacol. Exp. Ther. 1999, 288, 221–231. [Google Scholar]
- Pithia, N.K.; Liang, C.; Pan, X.Z.; Pan, M.L.; Mukherjee, J. Synthesis and evaluation of (S)-[18F]fesetron in the rat brain as a potential PET imaging agent for serotonin 5-HT3 receptors. Bioorg. Med. Chem. Lett. 2016, 26, 1919–1924. [Google Scholar] [CrossRef]
- Mu, L.; Müller Herde, A.; Rüefli, P.M.; Sladojevich, F.; Milicevic Sephton, S.; Krämer, S.D.; Thompson, A.J.; Schibli, R.; Ametamey, S.M.; Lochner, M. Synthesis and Pharmacological Evaluation of [11C]Granisetron and [18F]Fluoropalonosetron as PET Probes for 5-HT3 Receptor Imaging. ACS Chem. Neurosci. 2016, 7, 1552–1564. [Google Scholar] [CrossRef]
- Ishiwata, K.; Ishii, K.; Ishii, S.I.; Senda, M. Synthesis of 5-HT3 receptor antagonists, [11C]Y-25130 and [11C]YM060. Appl. Radiat. Isot. 1995, 46, 907–910. [Google Scholar] [CrossRef]
- Thorell, J.O.; Stone-Elander, S.; Eriksson, L.; Ingvar, M. N-methylquipazine: Carbon-11 labelling of the 5-HT3 agonist and in vivo evaluation of its biodistribution using PET. Nucl. Med. Biol. 1997, 24, 405–412. [Google Scholar] [CrossRef]
- Gao, M.; Wang, M.; Hutchins, G.D.; Zheng, Q.H. Synthesis of new carbon-11 labeled benzoxazole derivatives for PET imaging of 5-HT3 receptor. Eur. J. Med. Chem. 2008, 43, 1570–1574. [Google Scholar] [CrossRef]
- Dumuis, A.; Bouhelal, R.; Sebben, M.; Cory, R.; Bockaert, J. A nonclassical 5-hydroxytryptamine receptor positively coupled with adenylate cyclase in the central nervous system. Mol. Pharmacol. 1988, 34, 880–887. [Google Scholar] [PubMed]
- Bockaert, J.; Claeysen, S.; Compan, V.; Dumuis, A. 5-HT4 receptors: History, molecular pharmacology and brain functions. Neuropharmacology 2008, 55, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.S.; Eglen, R.M. Peripheral 5-HT 4 receptors. FASEB J. 1996, 10, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Bockaert, J.; Claeysen, S.; Compan, V.; Dumuis, A. 5-HT4 receptors, a place in the sun: Act two. Curr. Opin. Pharmacol. 2011, 11, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Hagena, H.; Manahan-Vaughan, D. The serotonergic 5-HT4 receptor: A unique modulator of hippocampal synaptic information processing and cognition. Neurobiol. Learn. Mem. 2017, 138, 145–153. [Google Scholar] [CrossRef]
- Claeysen, S.; Bockaert, J.; Giannoni, P. Serotonin: A New Hope in Alzheimer’s Disease? ACS Chem. Neurosci. 2015, 6, 940–943. [Google Scholar] [CrossRef]
- Lezoualc’h, F. 5-HT4 receptor and Alzheimer’s disease: The amyloid connection. Exp. Neurol. 2007, 205, 325–329. [Google Scholar] [CrossRef]
- Jean, A.; Conductier, G.; Manrique, C.; Bouras, C.; Berta, P.; Hen, R.; Charnay, Y.; Bockaert, J.; Compan, V. Anorexia induced by activation of serotonin 5-HT4 receptors is mediated by increases in CART in the nucleus accumbens. Proc. Natl. Acad. Sci. USA 2007, 104, 16335–16340. [Google Scholar] [CrossRef]
- Lucas, G.; Rymar, V.V.; Du, J.; Mnie-Filali, O.; Bisgaard, C.; Manta, S.; Lambas-Senas, L.; Wiborg, O.; Haddjeri, N.; Piñeyro, G.; et al. Serotonin4 (5-HT4) Receptor Agonists Are Putative Antidepressants with a Rapid Onset of Action. Neuron 2007, 55, 712–725. [Google Scholar] [CrossRef]
- Kaumann, A.J.; Gaster, L.M.; King, F.D.; Brown, A.M. Blockade of human atrial 5-HT4 receptors by SB 207710, a selective and high affinity 5-HT4 receptor antagonist. Naunyn Schmiedebergs Arch. Pharmacol. 1994, 349, 546–548. [Google Scholar] [CrossRef]
- Varnäs, K.; Halldin, C.; Pike, V.W.; Hall, H. Distribution of 5-HT4 receptors in the postmortem human brain—An autoradiographic study using [125I]SB 207710. Eur. Neuropsychopharmacol. 2003, 13, 228–234. [Google Scholar] [CrossRef]
- Patel, S.; Roberts, J.; Moorman, J.; Reavill, C. Localization of serotonin-4 receptors in the striatonigral pathway in rat brain. Neuroscience 1995, 69, 1159–1167. [Google Scholar] [CrossRef]
- Pike, V.W.; Halldin, C.; Nobuhara, K.; Hiltunen, J.; Mulligan, R.S.; Swahn, C.G.; Karlsson, P.; Olsson, H.; Hume, S.P.; Hirani, E.; et al. Radioiodinated SB 207710 as a radioligand in vivo: Imaging of brain 5-HT4 receptors with SPET. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Gee, A.D.; Martarello, L.; Passchier, J.; Wishart, M.; Parker, C.; Matthews, J.; Comley, R.; Hopper, R.; Gunn, R. Synthesis and evaluation of [11C]SB207145 as the first in vivo serotonin 5-HT4 receptor radioligand for PET imaging in man. Curr. Radiopharm. 2008, 1, 110–114. [Google Scholar] [CrossRef]
- Kornum, B.R.; Lind, N.M.; Gillings, N.; Marner, L.; Andersen, F.; Knudsen, G.M. Evaluation of the novel 5-HT4 receptor PET ligand [11C]SB207145 in the Göttingen minipig. J. Cereb. Blood Flow Metab. 2009, 29, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Marner, L.; Gillings, N.; Comley, R.A.; Baaré, W.F.C.; Rabiner, E.A.; Wilson, A.A.; Houle, S.; Hasselbalch, S.G.; Svarer, C.; Gunn, R.N.; et al. Kinetic modeling of11C-SB207145 binding to 5-HT4 receptors in the human brain in vivo. J. Nucl. Med. 2009, 50, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Marner, L.; Gillings, N.; Madsen, K.; Erritzoe, D.; Baaré, W.F.C.; Svarer, C.; Hasselbalch, S.G.; Knudsen, G.M. Brain imaging of serotonin 4 receptors in humans with [11C]SB207145-PET. Neuroimage 2010, 50, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.; Marner, L.; Haahr, M.; Gillings, N.; Knudsen, G.M. Mass dose effects and in vivo affinity in brain PET receptor studies—A study of cerebral 5-HT 4 receptor binding with [ 11C]SB207145. Nucl. Med. Biol. 2011, 38, 1085–1091. [Google Scholar] [CrossRef]
- Haahr, M.E.; Fisher, P.M.; Jensen, C.G.; Frokjaer, V.G.; McMahon, B.; Madsen, K.; Baaré, W.F.C.; Lehel, S.; Norremolle, A.; Rabiner, E.A.; et al. Central 5-HT4 receptor binding as biomarker of serotonergic tonus in humans: A [11C]SB207145 PET study. Mol. Psychiatry 2014, 19, 427–432. [Google Scholar] [CrossRef]
- Perfalk, E.; da Cunha-Bang, S.; Holst, K.K.; Keller, S.; Svarer, C.; Knudsen, G.M.; Frokjaer, V.G. Testosterone levels in healthy men correlate negatively with serotonin 4 receptor binding. Psychoneuroendocrinology 2017, 81, 22–28. [Google Scholar] [CrossRef]
- Jakobsen, G.R.; Fisher, P.M.; Dyssegaard, A.; McMahon, B.; Holst, K.K.; Lehel, S.; Svarer, C.; Jensen, P.S.; Knudsen, G.M.; Frokjaer, V.G. Brain serotonin 4 receptor binding is associated with the cortisol awakening response. Psychoneuroendocrinology 2016, 67, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Deen, M.; Hansen, H.D.; Hougaard, A.; Nørgaard, M.; Eiberg, H.; Lehel, S.; Ashina, M.; Knudsen, G.M. High brain serotonin levels in migraine between attacks: A 5-HT4 receptor binding PET study. NeuroImage Clin. 2018, 18, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Hong, J.; Morse, C.L.; Pike, V.W. Synthesis, structure-affinity relationships, and radiolabeling of selective high-affinity 5-HT4 receptor ligands as prospective imaging probes for positron emission tomography. J. Med. Chem. 2010, 53, 7035–7047. [Google Scholar] [CrossRef] [PubMed]
- Terry, G.E.; Hirvonen, J.; Liow, J.S.; Zoghbi, S.S.; Gladding, R.; Tauscher, J.T.; Schaus, J.M.; Phebus, L.; Felder, C.C.; Morse, C.L.; et al. Imaging and quantitation of cannabinoid CB1 receptors in human and monkey brains using 18F-labeled inverse agonist radioligands. J. Nucl. Med. 2010, 51, 112–120. [Google Scholar] [CrossRef]
- Lohith, T.G.; Xu, R.; Tsujikawa, T.; Morse, C.L.; Anderson, K.B.; Gladding, R.L.; Zoghbi, S.S.; Fujita, M.; Innis, R.B.; Pike, V.W. Evaluation in monkey of two candidate PET radioligands, [11C]RX-1 and [18F]RX-2, for imaging brain 5-HT4 receptors. Synapse 2014, 68, 613–623. [Google Scholar] [CrossRef]
- Caillé, F.; Morley, T.J.; Tavares, A.A.S.; Papin, C.; Twardy, N.M.; Alagille, D.; Lee, H.S.; Baldwin, R.M.; Seibyl, J.P.; Barret, O.; et al. Synthesis and biological evaluation of positron emission tomography radiotracers targeting serotonin 4 receptors in brain: [18F]MNI-698 and [18F]MNI-699. Bioorg. Med. Chem. Lett. 2013, 23, 6243–6247. [Google Scholar] [CrossRef]
- Tavares, A.A.S.; Caillé, F.; Barret, O.; Papin, C.; Lee, H.; Morley, T.J.; Fowles, K.; Holden, D.; Seibyl, J.P.; Alagille, D.; et al. Whole-body biodistribution and dosimetry estimates of a novel radiotracer for imaging of serotonin 4 receptors in brain: [18F]MNI-698. Nucl. Med. Biol. 2014, 41, 432–439. [Google Scholar] [CrossRef]
- Tavares, A.A.S.; Caillé, F.; Barret, O.; Papin, C.; Lee, H.; Morley, T.J.; Fowles, K.; Holden, D.; Seibyl, J.P.; Alagille, D.; et al. In vivo evaluation of 18F-MNI698: An 18F-labeled radiotracer for imaging of serotonin 4 receptors in brain. J. Nucl. Med. 2014, 55, 858–864. [Google Scholar] [CrossRef]
- Fresneau, N.; Dumas, N.; Tournier, B.B.; Fossey, C.; Ballandonne, C.; Lesnard, A.; Millet, P.; Charnay, Y.; Cailly, T.; Bouillon, J.P.; et al. Design of a serotonin 4 receptor radiotracer with decreased lipophilicity for single photon emission computed tomography. Eur. J. Med. Chem. 2015, 94, 386–396. [Google Scholar] [CrossRef]
- Monsma, F.J.; Shen, Y.; Ward, R.P.; Hamblin, M.W.; Sibley, D.R. Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol. Pharmacol. 1993, 43, 320–327. [Google Scholar]
- Ruat, M.; Traiffort, E.; Leurs, R.; Tardivel-Lacombe, J.; Diaz, J.; Arrang, J.M.; Schwartz, J.C. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc. Natl. Acad. Sci. USA 1993, 90, 8547–8551. [Google Scholar] [CrossRef]
- Kohen, R.; Metcalf, M.A.; Khan, N.; Druck, T.; Huebner, K.; Lachowicz, J.E.; Meltzer, H.Y.; Sibley, D.R.; Roth, B.L.; Hamblin, M.W. Cloning, characterization, and chromosomal localization of a human 5-HT6 serotonin receptor. J. Neurochem. 1996, 66, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, M.; Matsumoto, M.; Togashi, H.; Mori, K.; Saito, H. Central distribution and function of 5-HT6 receptor subtype in the rat brain. Life Sci. 1998, 62, 1473–1477. [Google Scholar] [CrossRef]
- Ward, R.P.; Hamblin, M.W.; Lachowicz, J.E.; Hoffman, B.J.; Sibley, D.R.; Dorsa, D.M. Localization of serotonin subtype 6 receptor messenger RNA in the rat brain by in situ hybridization histochemistry. Neuroscience 1995, 64, 1105–1111. [Google Scholar] [CrossRef]
- Gérard, C.; Martres, M.P.; Lefèvre, K.; Miquel, M.C.; Vergé, D.; Lanfumey, L.; Doucet, E.; Hamon, M.; El Mestikawy, S. Immune-localization of serotonin 5-HT6 receptor-like material in the rat central nervous system. Brain Res. 1997, 746, 207–219. [Google Scholar] [CrossRef]
- Roberts, J.C.; Reavill, C.; East, S.Z.; Harrison, P.J.; Patel, S.; Routledge, C.; Leslie, R.A. The distribution of 5-HT6 receptors in rat brain: An autoradiographic binding study using the radiolabelled 5-HT6 receptor antagonist [125I]SB-258585. Brain Res. 2002, 934, 49–57. [Google Scholar] [CrossRef]
- East, S.Z.; Burnet, P.W.J.; Leslie, R.A.; Roberts, J.C.; Harrison, P.J. 5-HT6 receptor binding sites in schizophrenia and following antipsychotic drug administration: Autoradiographic studies with [125I]SB-258585. Synapse 2002, 45, 191–199. [Google Scholar] [CrossRef]
- Barnes, N.M.; Ahern, G.P.; Becamel, C.; Bockaert, J.; Camilleri, M.; Chaumont-Dubel, S.; Claeysen, S.; Cunningham, K.A.; Fone, K.C.; Gershon, M.; et al. International union of basic and clinical pharmacology. Cx. Classification of receptors for 5-hydroxytryptamine; pharmacology and function. Pharmacol. Rev. 2021, 73, 310–520. [Google Scholar] [CrossRef]
- Meffre, J.; Chaumont-Dubel, S.; Mannoury la Cour, C.; Loiseau, F.; Watson, D.J.G.; Dekeyne, A.; Séveno, M.; Rivet, J.M.; Gaven, F.; Déléris, P.; et al. 5-HT6 receptor recruitment of mTOR as a mechanism for perturbed cognition in schizophrenia. EMBO Mol. Med. 2012, 4, 1043–1056. [Google Scholar] [CrossRef]
- Karila, D.; Freret, T.; Bouet, V.; Boulouard, M.; Dallemagne, P.; Rochais, C. Therapeutic Potential of 5-HT6 Receptor Agonists. J. Med. Chem. 2015, 58, 7901–7912. [Google Scholar] [CrossRef]
- Voigt, J.P.; Fink, H. Serotonin controlling feeding and satiety. Behav. Brain Res. 2015, 277, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.; Tousi, B.; Sabbagh, M.N. 5HT6 Antagonists in the Treatment of Alzheimer’s Dementia: Current Progress. Neurol. Ther. 2018, 7, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Khoury, R.; Grysman, N.; Gold, J.; Patel, K.; Grossberg, G.T. The role of 5-HT6-receptor antagonists in Alzheimer’s disease: An update. Expert Opin. Investig. Drugs 2018, 27, 523–533. [Google Scholar] [CrossRef]
- Ferrero, H.; Solas, M.; Francis, P.T.; Ramirez, M.J. Serotonin 5-HT6 Receptor Antagonsits in Alzheimer’s Disease: Therapeutic rationale and Current Development Status. CNS Drugs 2017, 31, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Hirst, W.D.; Minton, J.A.L.; Bromidge, S.M.; Moss, S.F.; Latter, A.J.; Riley, G.; Routledge, C.; Middlemiss, D.N.; Price, G.W. Characterization of [125I]-SB-258585 binding to human recombinant and native 5-HT6 receptors in rat, pig and human brain tissue. Br. J. Pharmacol. 2000, 130, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Verdurand, M.; Joseph, B.; Lemoine, L.; Daoust, A.; Billard, T.; Fournet, G.; Le Bars, D.; Zimmer, L. Synthesis and biological evaluation in rat and cat of [18F]12ST05 as a potential 5-HT6 PET radioligand. Nucl. Med. Biol. 2007, 34, 995–1002. [Google Scholar] [CrossRef]
- Zhou, P.; Yan, Y.; Bernotas, R.; Harrison, B.L.; Huryn, D.; Robichaud, A.J.; Zhang, G.M.; Smith, D.L.; Schechter, L.E. 4-(2-Aminoethoxy)-N-(phenylsulfonyl)indoles as novel 5-HT6 receptor ligands. Bioorg. Med. Chem. Lett. 2005, 15, 1393–1396. [Google Scholar] [CrossRef]
- Parker, C.A.; Gunn, R.N.; Rabiner, E.A.; Slifstein, M.; Comley, R.; Salinas, C.; Johnson, C.N.; Jakobsen, S.; Houle, S.; Laruelle, M.; et al. Radiosynthesis and characterization of 11C-GSK215083 as a PET radioligand for the 5-HT6 receptor. J. Nucl. Med. 2012, 53, 295–303. [Google Scholar] [CrossRef]
- Parker, C.A.; Rabiner, E.A.; Gunn, R.N.; Searle, G.; Martarello, L.; Comley, R.A.; Davy, M.; Wilson, A.A.; Houle, S.; Mizrahi, R.; et al. Human kinetic modeling of the 5HT6 PET radioligand 11C-GSK215083 and its utility for determining occupancy at both 5HT6 and 5HT2A receptors by SB742457 as a potential therapeutic mechanism of action in Alzheimer disease. J. Nucl. Med. 2015, 56, 1901–1909. [Google Scholar] [CrossRef][Green Version]
- Rosse, G. Quinoline Derivatives as 5-HT 6 Receptor PET Ligands. ACS Med. Chem. Lett. 2014, 5, 275–276. [Google Scholar] [CrossRef][Green Version]
- Colomb, J.; Becker, G.; Fieux, S.; Zimmer, L.; Billard, T. Syntheses, radiolabelings, and in vitro evaluations of fluorinated pet radioligands of 5-HT6 serotoninergic receptors. J. Med. Chem. 2014, 57, 3884–3890. [Google Scholar] [CrossRef] [PubMed]
- Emery, S.; Fieux, S.; Vidal, B.; Courault, P.; Bouvard, S.; Tourvieille, C.; Iecker, T.; Billard, T.; Zimmer, L.; Lancelot, S. Preclinical validation of [18F]2FNQ1P as a specific PET radiotracer of 5-HT6 receptors in rat, pig, non-human primate and human brain tissue. Nucl. Med. Biol. 2020, 82–83, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Bard, J.A.; Zgombick, J.; Adham, N.; Vaysse, P.; Branchek, T.A.; Weinshank, R.L. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J. Biol. Chem. 1993, 268, 23422–23426. [Google Scholar] [CrossRef]
- Lovenberg, T.W.; Baron, B.M.; de Lecea, L.; Miller, J.D.; Prosser, R.A.; Rea, M.A.; Foye, P.E.; Racke, M.; Slone, A.L.; Siegel, B.W. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 1993, 11, 449–458. [Google Scholar] [CrossRef]
- Varnäs, K.; Thomas, D.R.; Tupala, E.; Tiihonen, J.; Hall, H. Distribution of 5-HT7 receptors in the human brain: A preliminary autoradiographic study using [3H]SB-269970. Neurosci. Lett. 2004, 367, 313–316. [Google Scholar] [CrossRef]
- Cifariello, A.; Pompili, A.; Gasbarri, A. 5-HT7 receptors in the modulation of cognitive processes. Behav. Brain Res. 2008, 195, 171–179. [Google Scholar] [CrossRef]
- L’Estrade, E.T.; Erlandsson, M.; Edgar, F.G.; Ohlsson, T.; Knudsen, G.M.; Herth, M.M. Towards selective CNS PET imaging of the 5-HT7 receptor system: Past, present and future. Neuropharmacology 2020, 172, 107830–107848. [Google Scholar] [CrossRef]
- Kikuchi, C.; Nagaso, H.; Hiranuma, T.; Koyama, M. Tetrahydrobenzindoles: Selective Antagonists of the 5-HT 7 Receptor. J. Med. Chem. 1999, 42, 533–535. [Google Scholar] [CrossRef]
- Kikuchi, C.; Hiranuma, T.; Koyama, M. Tetrahydrothienopyridylbutyl-tetrahydrobenzindoles: New selective ligands of the 5-HT7 receptor. Bioorg. Med. Chem. Lett. 2002, 12, 2549–2552. [Google Scholar] [CrossRef]
- Zhang, M.R.; Haradahira, T.; Maeda, J.; Okauchi, T.; Kida, T.; Obayashi, S.; Suzuki, K.; Suhara, T. Synthesis and preliminary PET study of the 5-HT7 receptor antagonist [11C]DR4446. J. Label. Compd. Radiopharm. 2002, 45, 857–866. [Google Scholar] [CrossRef]
- Herth, M.M.; Volk, B.; Pallagi, K.; Kofoed Bech, L.; Antoni, F.A.; Knudsen, G.M.; Kristensen, J.L. Synthesis and in vitro evaluation of oxindole derivatives as potential radioligands for 5-HT7 receptor imaging with PET. ACS Chem. Neurosci. 2012, 3, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, L.; Andries, J.; Le Bars, D.; Billard, T.; Zimmer, L. Comparison of 4 radiolabeled antagonists for serotonin 5-HT(7) receptor neuroimaging: Toward the first PET radiotracer. J. Nucl. Med. 2011, 52, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Tampio L’Estrade, E.; Xiong, M.; Shalgunov, V.; Edgar, F.G.; Volk, B.; Baerentzen, S.L.; Palner, M.; Erlandsson, M.; Ohlsson, T.; Knudsen, G.M.; et al. Development and Evaluation of Two Potential 5-HT7 Receptor PET Tracers: [18F]ENL09 and [18F]ENL10. ACS Chem. Neurosci. 2019, 10, 3961–3968. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.D.; Constantinescu, C.C.; Barret, O.; Herth, M.M.; Magnussen, J.H.; Lehel, S.; Dyssegaard, A.; Colomb, J.; Billard, T.; Zimmer, L.; et al. Evaluation of [18F]2FP3 in pigs and non-human primates. J. Label. Compd. Radiopharm. 2019, 62, 34–42. [Google Scholar] [CrossRef]
- Colomb, J.; Becker, G.; Forcellini, E.; Meyer, S.; Buisson, L.; Zimmer, L.; Billard, T. Synthesis and pharmacological evaluation of a new series of radiolabeled ligands for 5-HT7 receptor PET neuroimaging. Nucl. Med. Biol. 2014, 41, 330–337. [Google Scholar] [CrossRef]
- L’Estrade, E.T.; Edgar, F.G.; Xiong, M.; Shalgunov, V.; Baerentzen, S.L.; Erlandsson, M.; Ohlsson, T.G.; Palner, M.; Knudsen, G.M.; Herth, M.M. Synthesis, Radiolabeling, and in Vitro and in Vivo Evaluation of [18F]ENL30: A Potential PET Radiotracer for the 5-HT 7 Receptor. ACS Omega 2019, 4, 7344–7353. [Google Scholar] [CrossRef]
- L’Estrade, E.T.; Shalgunov, V.; Edgar, F.G.; Strebl-Bantillo, M.G.; Xiong, M.; Crestey, F.; Neelamegam, R.; Dyssegaard, A.; Lehel, S.; Erlandsson, M.; et al. Radiosynthesis and preclinical evaluation of [11C]Cimbi-701—Towards the imaging of cerebral 5-HT7 receptors. J. Label. Compd. Radiopharm. 2020, 63, 46–55. [Google Scholar] [CrossRef]
- Rudnick, G. Serotonin transporters—Structure and function. J. Membr. Biol. 2006, 213, 101–110. [Google Scholar] [CrossRef]
- Suehiro, M.; Scheffel, U.; Ravert, H.T.; Dannals, R.F.; Wagner, H.N. [11C](+)McN5652 as a radiotracer for imaging serotonin uptake sites with pet. Life Sci. 1993, 53, 883–892. [Google Scholar] [CrossRef]
- Szabo, Z.; Scheffel, U.; Suehiro, M.; Dannais, R.F.; Kim, S.E.; Ravert, H.T.; Ricaurte, G.A.; Wagner, H.N. Positron emission tomography of 5-HT transporter sites in the baboon brain with [11C]McN5652. J. Cereb. Blood Flow Metab. 1995, 15, 798–805. [Google Scholar] [CrossRef]
- Parsey, R.V.; Kegeles, L.S.; Hwang, D.R.; Simpson, N.; Abi-Dargham, A.; Mawlawi, O.; Slifstein, M.; Van Heertum, R.L.; Mann, J.J.; Laruelle, M. In vivo quantification of brain serotonin transporters in humans using [11C]McN 5652. J. Nucl. Med. 2000, 41, 1465–1477. [Google Scholar] [PubMed]
- Ichimiya, T.; Suhara, T.; Sudo, Y.; Okubo, Y.; Nakayama, K.; Nankai, M.; Inoue, M.; Yasuno, F.; Takano, A.; Maeda, J.; et al. Serotonin transporter binding in patients with mood disorders: A PET study with [11C](+)McN5652. Biol. Psychiatry 2002, 51, 715–722. [Google Scholar] [CrossRef]
- Simpson, H.B.; Lombardo, I.; Slifstein, M.; Huang, H.Y.; Hwang, D.R.; Abi-Dargham, A.; Liebowitz, M.R.; Laruelle, M. Serotonin transporters in obsessive-compulsive disorder: A positron emission tomography study with [11C]McN 5652. Biol. Psychiatry 2003, 54, 1414–1421. [Google Scholar] [CrossRef]
- Reivich, M.; Amsterdam, J.D.; Brunswick, D.J.; Yann Shiue, C. PET brain imaging with [11C](+)McN5652 shows increased serotonin transporter availability in major depression. J. Affect. Disord. 2004, 82, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Marjamäki, P.; Zessin, J.; Eskola, O.; Grönroos, T.; Haaparanta, M.; Bergman, J.; Lehikoinen, P.; Forsback, S.; Brust, P.; Steinbach, J.; et al. S-[18F]fluoromethyl-(+)-McN5652, a PET tracer for the serotonin transporter: Evaluation in rats. Synapse 2003, 47, 45–53. [Google Scholar] [CrossRef]
- Brust, P.; Zessin, J.; Kuwabara, H.; Pawelke, B.; Kretzschmar, M.; Hinz, R.; Bergman, J.; Eskola, O.; Solin, O.; Steinbach, J.; et al. Positron emission tomography imaging of the serotonin transporter in the pig brain using [11C](+)-McN5652 and S-([18F]fluoromethyl)-(+)-McN5652. Synapse 2003, 47, 143–151. [Google Scholar] [CrossRef]
- Brust, P.; Hinz, R.; Kuwabara, H.; Hesse, S.; Zessin, J.; Pawelke, B.; Stephan, H.; Bergmann, R.; Steinbach, J.; Sabri, O. In vivo Measurement of the Serotonin Transporter with (S)-([18F]fluoromethyl)-(+)-McN5652. Neuropsychopharmacology 2003, 28, 2010–2019. [Google Scholar] [CrossRef]
- Oya, S.; Kung, M.P.; Acton, P.D.; Mu, M.; Hou, C.; Kung, H.F. A new single-photon emission computed tomography imaging agent for serotonin transporters: [123I]IDAM, 5-Iodo-2-((2- ((dimethylamino)methyl)phenyl)thio)benzyl alcohol. J. Med. Chem. 1999, 42, 333–335. [Google Scholar] [CrossRef]
- Wilson, A.A.; Ginovart, N.; Schmidt, M.; Meyer, J.H.; Threlkeld, P.G.; Houle, S. Novel radiotracers for imaging the serotonin transporter by positron emission tomography: Synthesis, radiosynthesis, and in vitro and ex vivo evaluation of 11C-labeled 2-(phenylthio)araalkylamines. J. Med. Chem. 2000, 43, 3103–3110. [Google Scholar] [CrossRef]
- Houle, S.; Ginovart, N.; Hussey, D.; Meyer, J.H.; Wilson, A.A. Imaging the serotonin transporter with positron emission tomography: Initial human studies with [11C]DAPP and [11C]DASB. Eur. J. Nucl. Med. 2000, 27, 1719–1722. [Google Scholar] [CrossRef]
- Szabo, Z.; McCann, U.D.; Wilson, A.A.; Scheffel, U.; Owonikoko, T.; Mathews, W.B.; Ravert, H.T.; Hilton, J.; Dannals, R.F.; Ricaurte, G.A. Comparison of (+)-11C-McN5652 and 11C-DASB as serotonin transporter radioligands under various experimental conditions. J. Nucl. Med. 2002, 43, 678–692. [Google Scholar] [PubMed]
- Frankle, W.G.; Huang, Y.; Hwang, D.R.; Talbot, P.S.; Slifstein, M.; Van Heertum, R.; Abi-Dargham, A.; Laruelle, M. Comparative evaluation of serotonin transporter radioligands 11C-DASB and 11C-McN 5652 in healthy humans. J. Nucl. Med. 2004, 45, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, M.J.; Simpson, N.R.; Wang, T.; Van Heertum, R.L.; Mann, J.J.; Parsey, R.V. Biodistribution and radiation dosimetry of [11C]DASB in baboons. Nucl. Med. Biol. 2004, 31, 1097–1102. [Google Scholar] [CrossRef]
- Wilson, A.A.; Ginovart, N.; Hussey, D.; Meyer, J.; Houle, S. In vitro and in vivo characterisation of [11C]-DASB: A probe for in vivo measurements of the serotonin transporter by positron emission tomography. Nucl. Med. Biol. 2004, 31, 1097–1102. [Google Scholar] [CrossRef]
- Ginovart, N.; Wilson, A.A.; Meyer, J.H.; Hussey, D.; Houle, S. [11C]-DASB, a tool for in vivo measurement of SSRI-induced occupancy of the serotonin transporter: PET characterization and evaluation in cats. Synapse 2003, 47, 123–133. [Google Scholar] [CrossRef]
- Meyer, J.H.; Wilson, A.A.; Ginovart, N.; Goulding, V.; Hussey, D.; Hood, K.; Houle, S. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: A [11C]DASB PET imaging study. Am. J. Psychiatry 2001, 158, 1843–1849. [Google Scholar] [CrossRef]
- Cannon, D.M.; Ichise, M.; Rollis, D.; Klaver, J.M.; Gandhi, S.K.; Charney, D.S.; Manji, H.K.; Drevets, W.C. Elevated Serotonin Transporter Binding in Major Depressive Disorder Assessed Using Positron Emission Tomography and [11C]DASB; Comparison with Bipolar Disorder. Biol. Psychiatry 2007, 62, 870–877. [Google Scholar] [CrossRef]
- Brown, A.K.; George, D.T.; Fujita, M.; Liow, J.S.; Ichise, M.; Hibbeln, J.; Ghose, S.; Sangare, J.; Hommer, D.; Innis, R.B. PET [11C]DASB imaging of serotonin transporters in patients with alcoholism. Alcohol. Clin. Exp. Res. 2007, 31, 28–32. [Google Scholar] [CrossRef]
- Matsumoto, R.; Ichise, M.; Ito, H.; Ando, T.; Takahashi, H.; Ikoma, Y.; Kosaka, J.; Arakawa, R.; Fujimura, Y.; Ota, M.; et al. Reduced serotonin transporter binding in the insular cortex in patients with obsessive-compulsive disorder: A [11C]DASB PET study. Neuroimage 2010, 49, 121–126. [Google Scholar] [CrossRef]
- Politis, M.; Wu, K.; Loane, C.; Kiferle, L.; Molloy, S.; Brooks, D.J.; Piccini, P. Staging of serotonergic dysfunction in Parkinson’s Disease: An in vivo 11C-DASB PET study. Neurobiol. Dis. 2010, 40, 216–221. [Google Scholar] [CrossRef]
- Kim, J.H.; Son, Y.D.; Kim, J.H.; Choi, E.J.; Lee, S.Y.; Lee, J.E.; Cho, Z.H.; Kim, Y.B. Serotonin transporter availability in thalamic subregions in schizophrenia: A study using 7.0-T MRI with [11C]DASB high-resolution PET. Psychiatry Res. Neuroimaging 2015, 231, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, B.H.; Kim, E.; Howes, O.D.; Cho, K.I.K.; Yoon, Y.B.; Kwon, J.S. Higher serotonin transporter availability in early-onset obsessive–compulsive disorder patients undergoing escitalopram treatment: A [11C]DASB PET study. Hum. Psychopharmacol. 2018, 33, 2–7. [Google Scholar] [CrossRef]
- Christensen, J.B.; Bechgaard, K.; Paquignon, G. Carbon-11 labelling of MADAM in two different positions: A highly selective PET radioligand for the serotonin transporter. J. Label. Compd. Radiopharm. 2001, 44, 1013–1023. [Google Scholar] [CrossRef]
- Halldin, C.; Lundberg, J.; Sóvágó, J.; Gulyás, B.; Guilloteau, D.; Vercouillie, J.; Emond, P.; Chalon, S.; Tarkiainen, J.; Hiltunen, J.; et al. [11C]MADAM, a new serotonin transporter radioligand characterized in the monkey brain by PET. Synapse 2005, 58, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.; Odano, I.; Olsson, H.; Halldin, C.; Farde, L. Quantification of 11C-MADAM binding to the serotonin transporter in the human brain. J. Nucl. Med. 2005, 46, 1505–1515. [Google Scholar] [PubMed]
- Lundberg, J.; Halldin, C.; Farde, L. Measurement of serotonin transporter binding with PET and [ 11C]MADAM: A test-retest reproducibility study. Synapse 2006, 60, 256–263. [Google Scholar] [CrossRef]
- Majuri, J.; Joutsa, J.; Johansson, J.; Voon, V.; Parkkola, R.; Alho, H.; Arponen, E.; Kaasinen, V. Serotonin transporter density in binge eating disorder and pathological gambling: A PET study with [11C]MADAM. Eur. Neuropsychopharmacol. 2017, 27, 1281–1288. [Google Scholar] [CrossRef]
- Oya, S.; Choi, S.R.; Hou, C.; Mu, M.; Kung, M.P.; Acton, P.D.; Siciliano, M.; Kung, H.F. 2-((2-((Dimethylamino)methyl)phenyl)thio)-5-iodophenylamine (ADAM): An improved serotonin transporter ligand. Nucl. Med. Biol. 2000, 27, 249–254. [Google Scholar] [CrossRef]
- Lin, K.J.; Ye, X.X.; Yen, T.C.; Wey, S.P.; Tzen, K.Y.; Ting, G.; Hwang, J.J. Biodistribution study of [123I] ADAM in mice: Correlation with whole body autoradiography. Nucl. Med. Biol. 2002, 29, 643–650. [Google Scholar] [CrossRef]
- Itti, E.; Klein, G.; Rosso, J.; Evangelista, E.; Monin, J.L.; Gueret, P.; Meignan, M.; Thirion, J.P. Assessment of myocardial reperfusion after myocardial infarction using automatic 3-dimensional quantification and template matching. J. Nucl. Med. 2004, 45, 1981–1988. [Google Scholar]
- Lin, K.J.; Yen, T.C.; Wey, S.P.; Hwang, J.J.; Ye, X.X.; Tzen, K.Y.; Fu, Y.K.; Chen, J.C. Characterization of the binding sites for 123I-ADAM and the relationship to the serotonin transporter in rat and mouse brains using quantitative autoradiography. J. Nucl. Med. 2004, 45, 673–681. [Google Scholar] [PubMed]
- Ye, X.X.; Hwang, J.J.; Hsieh, J.F.; Chen, J.C.; Chou, Y.T.; Tu, K.Y.; Wey, S.P.; Ting, G. In vivo quantification by SPECT of [ 123I] ADAM bound to serotonin transporters in the brains of rabbits. Nucl. Med. Biol. 2004, 31, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, T.A.; Bergström, K.A.; Heikman, P.; Hiltunen, J.; Ahonen, A.K. Biodistribution and radiation dosimetry of [123I]ADAM in healthy human subjects: Preliminary results. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Newberg, A.B.; Plössl, K.; Mozley, P.D.; Stubbs, J.B.; Wintering, N.; Udeshi, M.; Alavi, A.; Kauppinen, T.; Kung, H.F. Biodistribution and imaging with 123I-ADAM: A serotonin transporter imaging agent. J. Nucl. Med. 2004, 45, 834–841. [Google Scholar]
- Catafau, A.M.; Pérez, V.; Penengo, M.M.; Bullich, S.; Danús, M.; Puigdemont, D.; Pascual, J.C.; Corripio, I.; Llop, J.; Perich, J.; et al. SPECT of serotonin transporters using123I-ADAM: Optimal imaging time after bolus injection and long-term test-retest in healthy volunteers. J. Nucl. Med. 2005, 46, 1301–1309. [Google Scholar] [PubMed]
- Frokjaer, V.G.; Pinborg, L.H.; Madsen, J.; De Nijs, R.; Svarer, C.; Wagner, A.; Knudsen, G.M. Evaluation of the serotonin transporter ligand 123I-ADAM for SPECT studies on humans. J. Nucl. Med. 2008, 49, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.H.; Huang, W.S.; Huang, S.Y.; Cheng, C.Y.; Chen, C.Y.; Shen, L.H.; Liu, J.C.; Fu, Y.K. Imaging serotonin transporters using [123I]ADAM SPECT in a parkinsonian primate model. Appl. Radiat. Isot. 2008, 66, 1799–1803. [Google Scholar] [CrossRef]
- Van De Giessen, E.; Booij, J. The SPECT tracer [123I]ADAM binds selectively to serotonin transporters: A double-blind, placebo-controlled study in healthy young men. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1507–1511. [Google Scholar] [CrossRef][Green Version]
- Catafau, A.M.; Perez, V.; Plaza, P.; Pascual, J.C.; Bullich, S.; Suarez, M.; Penengo, M.M.; Corripio, I.; Puigdemont, D.; Danus, M.; et al. Serotonin transporter occupancy induced by paroxetine in patients with major depression disorder: A 123I-ADAM SPECT study. Psychopharmacology 2006, 189, 145–153. [Google Scholar] [CrossRef]
- Herold, N.; Uebelhack, K.; Franke, L.; Amthauer, H.; Luedemann, L.; Bruhn, H.; Felix, R.; Uebelhack, R.; Plotkin, M. Imaging of serotonin transporters and its blockade by citalopram in patients with major depression using a novel SPECT ligand [123I]-ADAM. J. Neural Transm. 2006, 113, 659–670. [Google Scholar] [CrossRef]
- Lundgren, J.D.; Newberg, A.B.; Allison, K.C.; Wintering, N.A.; Ploessl, K.; Stunkard, A.J. 123I-ADAM SPECT imaging of serotonin transporter binding in patients with Night Eating Syndrome: A preliminary report. Psychiatry Res. Neuroimaging 2008, 162, 214–220. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Amsterdam, J.D.; Newberg, A.B.; Newman, C.F.; Shults, J.; Wintering, N.; Soeller, I. Change over time in brain serotonin transporter binding in major depression: Effects of therapy measured with [123I]-ADAM SPECT. J. Neuroimaging 2013, 23, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Chang, C.S.; Yang, Y.K.; Shen, L.H.; Yao, W.J. Comparison of brain serotonin transporter using [I-123]-ADAM between obese and nonobese young adults without an eating disorder. PLoS ONE 2017, 12, e0170886. [Google Scholar] [CrossRef]
- Shiue, G.G.; Fang, P.; Shiue, C.Y. Synthesis of N,N-dimethyl-2-(2-amino-4-[18F]fluorophenylthio)benzylamine as a serotonin transporter imaging agent. Appl. Radiat. Isot. 2003, 58, 183–191. [Google Scholar] [CrossRef]
- Shiue, G.G.; Choi, S.R.; Fang, P.; Hou, C.; Acton, P.D.; Cardi, C.; Saffer, J.R.; Greenberg, J.H.; Karp, J.S.; Kung, H.F.; et al. N,N-dimethyl-2-(2-amino-4-18F-fluorophenylthio)-benzylamine (4-18F-ADAM): An improved PET radioligand for serotonin transporters. J. Nucl. Med. 2003, 44, 1890–1897. [Google Scholar] [PubMed]
- Chen, Y.A.; Huang, W.S.; Lin, Y.S.; Cheng, C.Y.; Liu, R.S.; Wang, S.J.; Li, I.H.; Huang, S.Y.; Shiue, C.Y.; Chen, C.Y.; et al. Characterization of 4-[18F]-ADAM as an imaging agent for SERT in non-human primate brain using PET: A dynamic study. Nucl. Med. Biol. 2012, 39, 279–285. [Google Scholar] [CrossRef]
- Yeh, Y.W.; Ho, P.S.; Chen, C.Y.; Kuo, S.C.; Liang, C.S.; Ma, K.H.; Shiue, C.Y.; Huang, W.S.; Cheng, C.Y.; Wang, T.Y.; et al. Incongruent reduction of serotonin transporter associated with suicide attempts in patients with major depressive disorder: A positron emission tomography study with 4-[18F]-ADAM. Int. J. Neuropsychopharmacol. 2015, 18, pyu065. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.W.; Ho, P.S.; Kuo, S.C.; Chen, C.Y.; Liang, C.S.; Yen, C.H.; Huang, C.C.; Ma, K.H.; Shiue, C.Y.; Huang, W.S.; et al. Disproportionate reduction of serotonin transporter may predict the response and adherence to antidepressants in patients with major depressive disorder: A positron emission tomography study with 4-[18F]-ADAM. Int. J. Neuropsychopharmacol. 2015, 18, pyu120. [Google Scholar] [CrossRef][Green Version]
- Wang, J.L.; Parhi, A.K.; Oya, S.; Lieberman, B.; Kung, H.F. In vivo characterization of a series of 18F-diaryl sulfides (18F-2-(2′-((dimethylamino)methyl)-4′-(fluoroalkoxy) phenylthio)benzenamine) for PET imaging of the serotonin transporter. J. Nucl. Med. 2009, 50, 1509–1517. [Google Scholar] [CrossRef]
- Qiao, H.; Zhang, Y.; Wu, Z.; Zhu, L.; Choi, S.R.; Ploessl, K.; Kung, H.F. One-step preparation of [18F]FPBM for PET imaging of serotonin transporter (SERT) in the brain. Nucl. Med. Biol. 2016, 43, 470–477. [Google Scholar] [CrossRef]
- Innis, R.; Baldwin, R.; Sybirska, E.; Zea, Y.; Laruelle, M.; Al-Tikriti, M.; Charney, D.; Zoghbi, S.; Smith, E.; Wisniewski, G.; et al. Single photon emission computed tomography imaging of monoamine reuptake sites in primate brain with [123I]CIT. Eur. J. Pharmacol. 1991, 200, 369–370. [Google Scholar] [CrossRef]
- Neumeyer, J.L.; Wang, S.; Milius, R.A.; Baldwin, R.M.; Zea-Ponce, Y.; Hoffer, P.B.; Sybirska, E.; Al-Tikriti, M.; Charney, D.S. [123I]-2.beta.-carbomethoxy-3.beta.-(4-iodophenyl)tropane: High-affinity SPECT (single photon emission computed tomography) radiotracer of monoamine reuptake sites in brain. J. Med. Chem. 1991, 34, 3144–3146. [Google Scholar] [CrossRef] [PubMed]
- Kuikka, J.T.; Åkerman, K.; Bergström, K.A.; Karhu, J.; Hiltunen, J.; Haukka, J.; Heikkinen, J.; Tiihonen, J.; Wang, S.; Neumeyer, J.L. Iodine-123 labelledN-(2-fluoroethyl)-2β-carbomethoxy-3β-(4-iodophenyl)nortropane for dopamine transporter imaging in the living human brain. Eur. J. Nucl. Med. 1995, 22, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Hiltunen, J.; Åkerman, K.K.; Kuikka, J.T.; Bergström, K.A.; Halldin, C.; Nikula, T.; Räsänen, P.; Tiihonen, J.; Vauhkonen, M.; Karhu, J.; et al. Iodine-123 labeled nor-β-CIT as a potential tracer for serotonin transporter imaging in the human brain with single-photon emission tomography. Eur. J. Nucl. Med. 1998, 25, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Zitterl, W.; Aigner, M.; Stompe, T.; Zitterl-Eglseer, K.; Gutierrez-Lobos, K.; Wenzel, T.; Zettinig, G.; Hornik, K.; Pirker, W.; Thau, K. Changes in thalamus-hypothalamus serotonin transporter availability during clomipramine administration in patients with obsessive-compulsive disorder. Neuropsychopharmacology 2008, 33, 3126–3134. [Google Scholar] [CrossRef] [PubMed]
- Makkonen, I.; Riikonen, R.; Kokki, H.; Airaksinen, M.M.; Kuikka, J.T. Serotonin and dopamine transporter binding in children with autism determined by SPECT. Dev. Med. Child Neurol. 2008, 50, 593–597. [Google Scholar] [CrossRef]
- Lehto, S.M.; Tolmunen, T.; Kuikka, J.; Valkonen-Korhonen, M.; Joensuu, M.; Saarinen, P.I.; Vanninen, R.; Ahola, P.; Tiihonen, J.; Lehtonen, J. Midbrain serotonin and striatum dopamine transporter binding in double depression: A one-year follow-up study. Neurosci. Lett. 2008, 441, 291–295. [Google Scholar] [CrossRef]
- Dam, J.H.; Bender, D.; Peters, D.; Någren, K. [11C]NS9531, [11C]NS9762 and [11C]NS6417, specific SERT tracers: Pre-clinical evaluation in pigs and optimization of synthesis conditions using [11C]methyl triflate. Nucl. Med. Biol. 2016, 43, 42–51. [Google Scholar] [CrossRef]
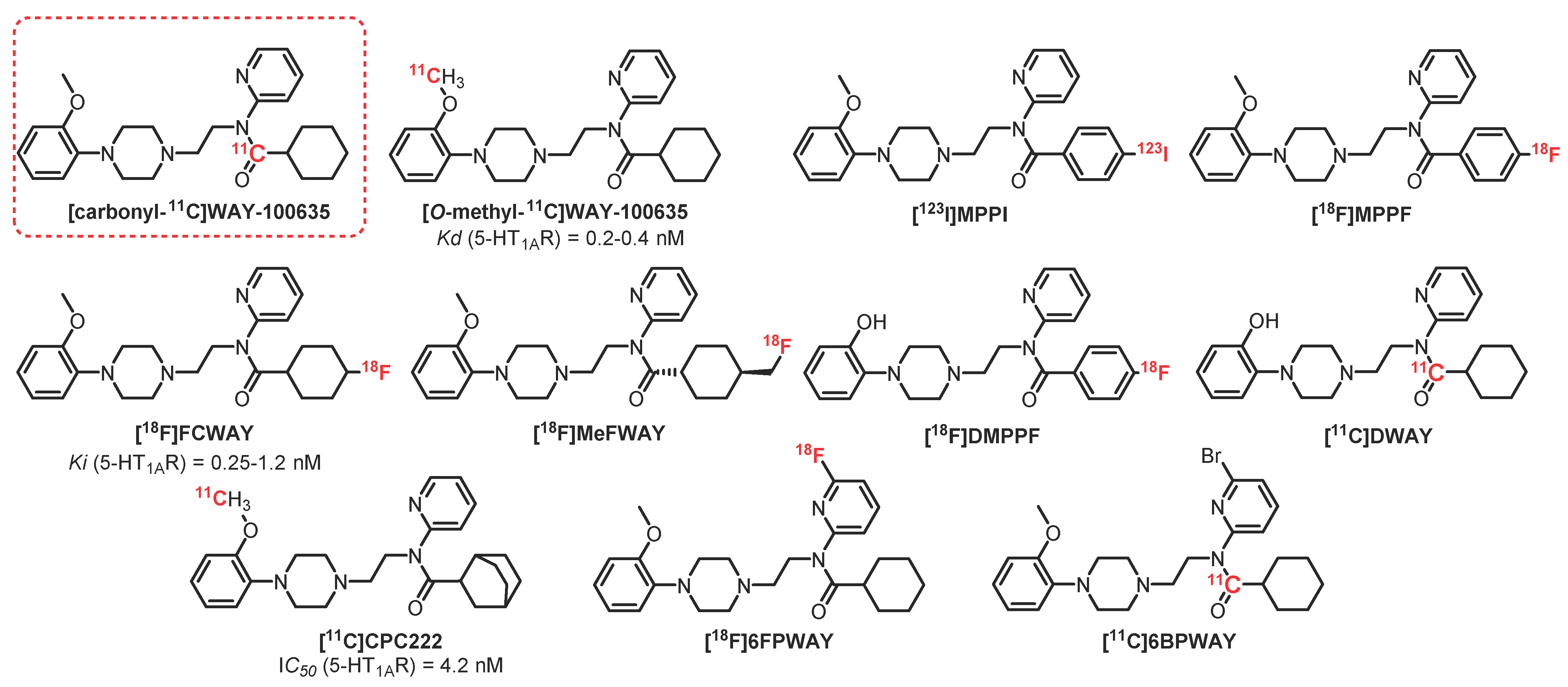
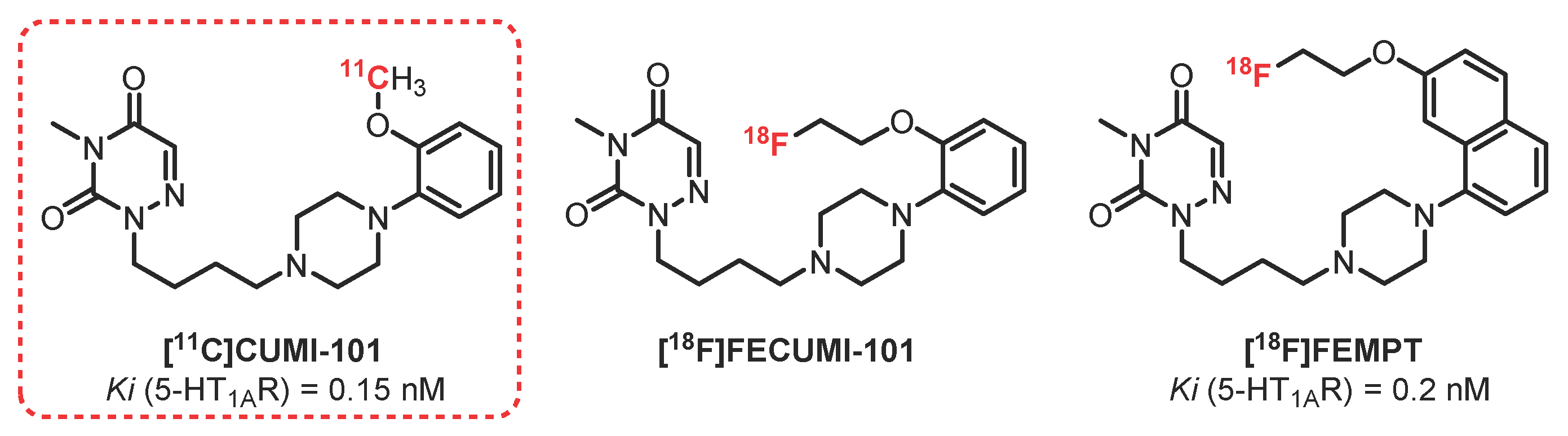
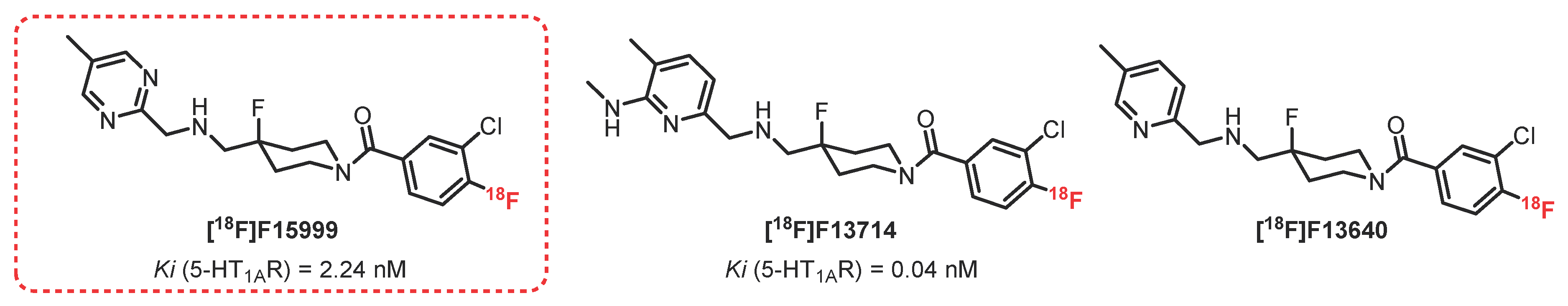
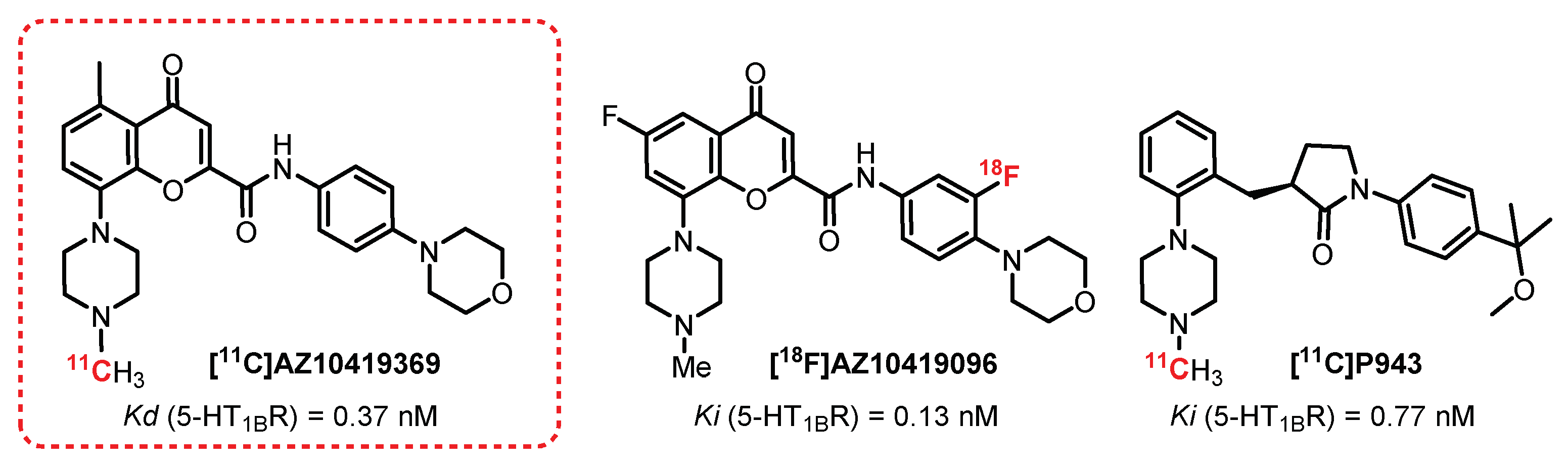

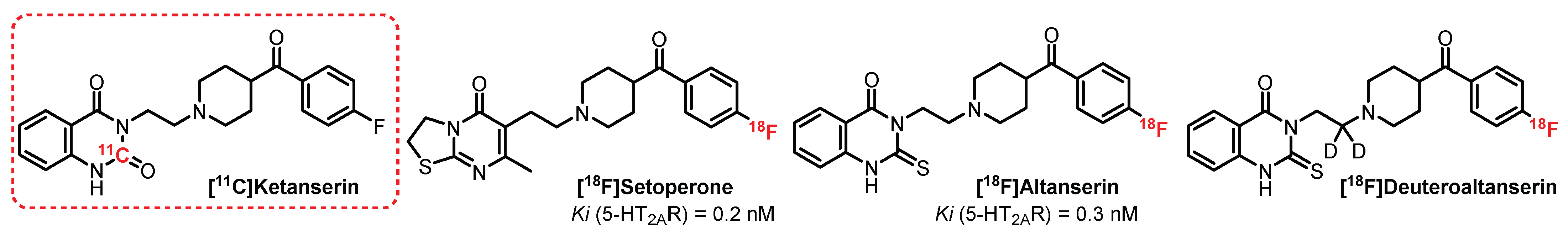
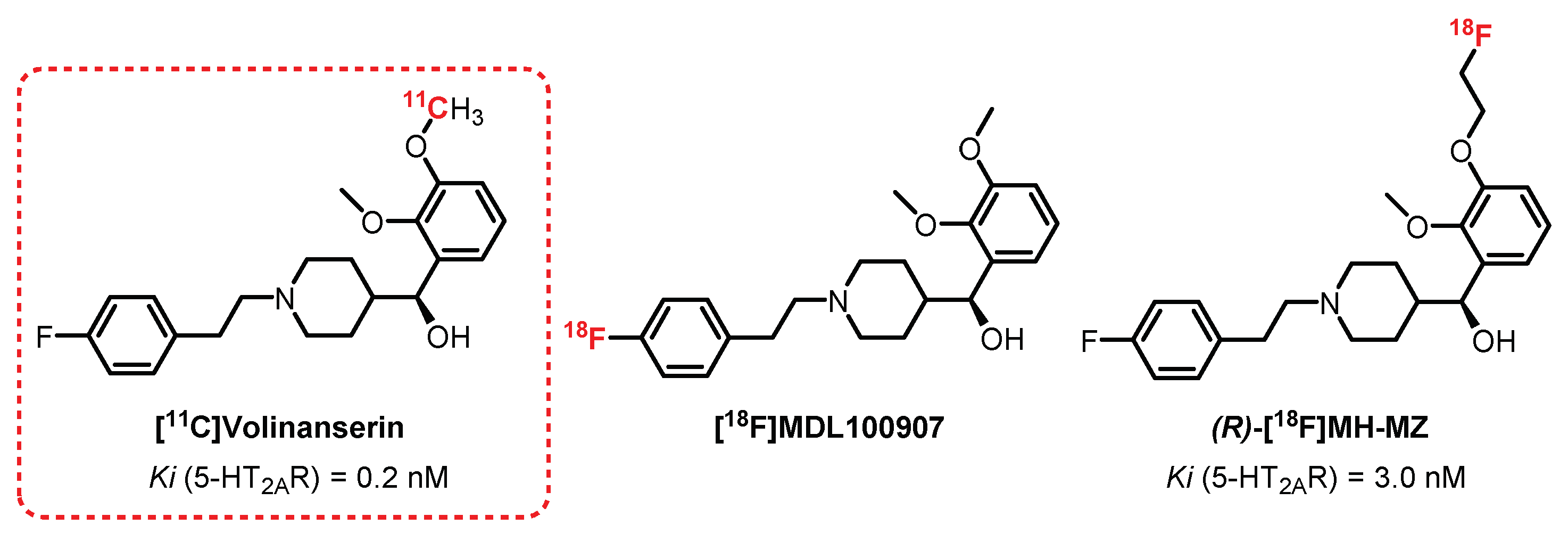
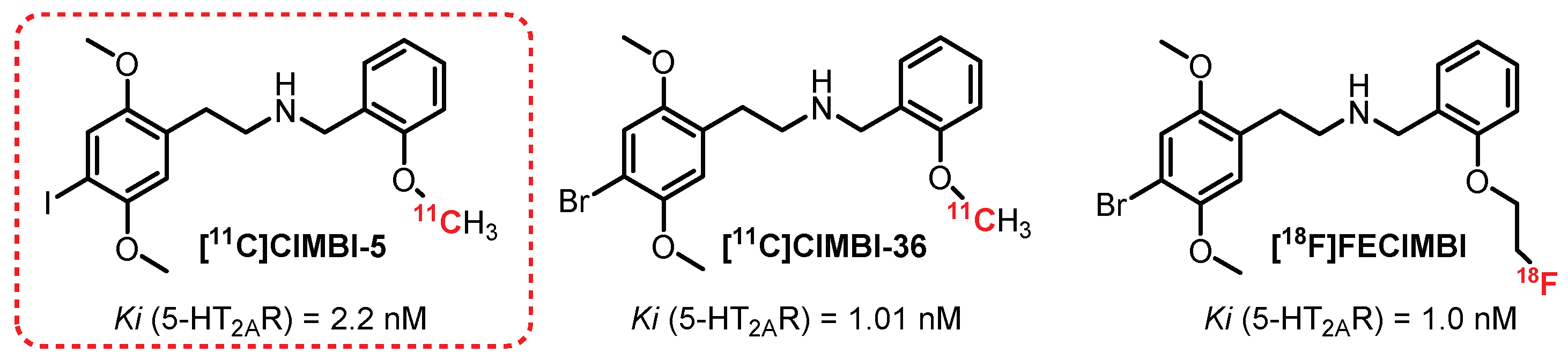
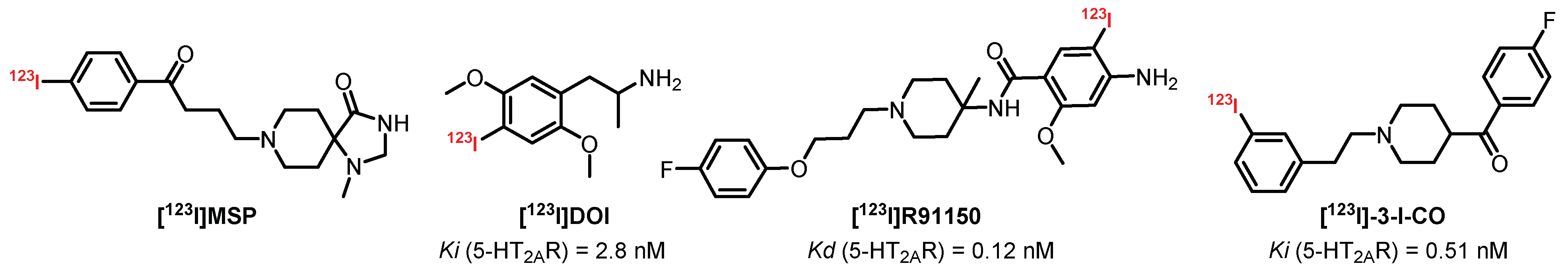
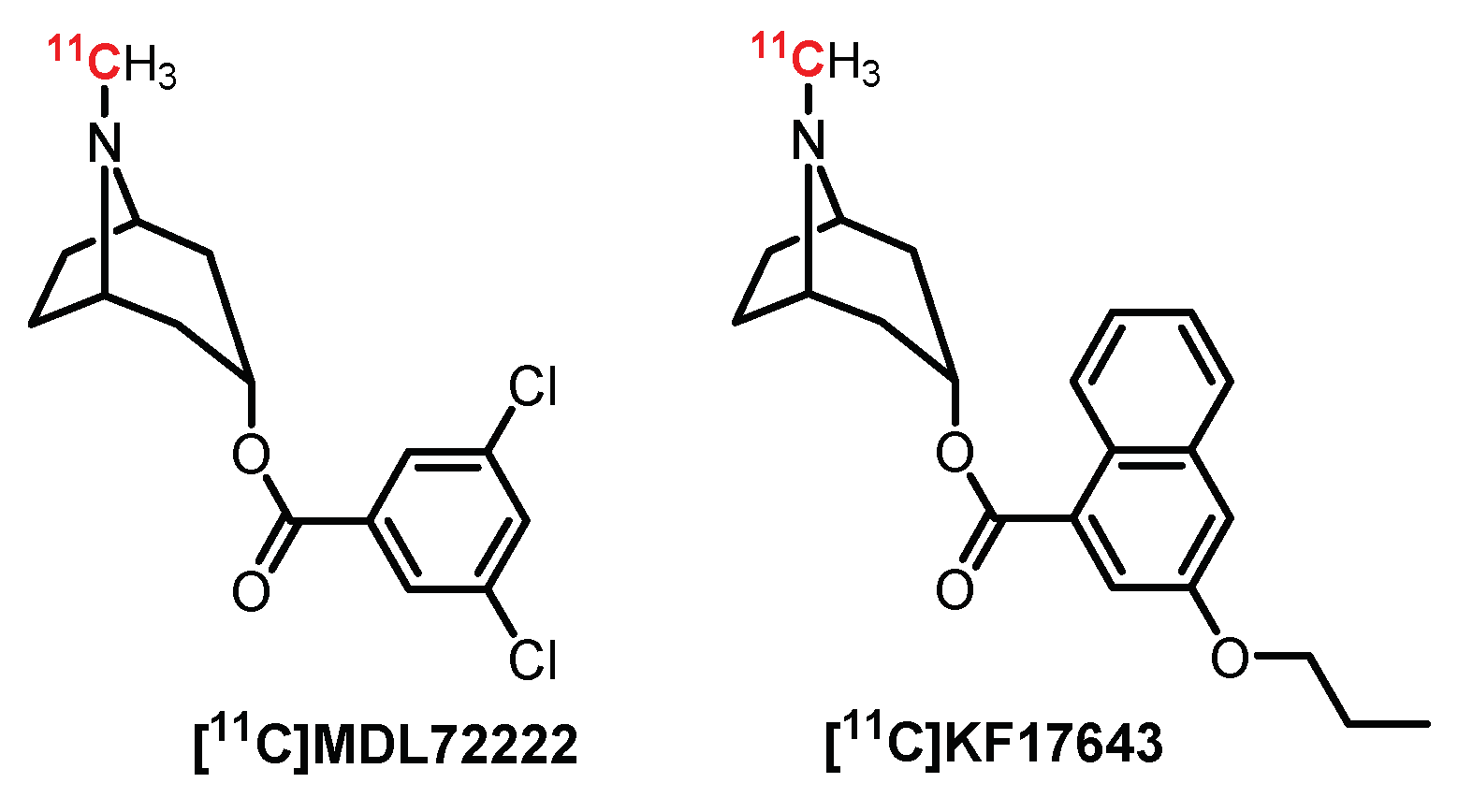
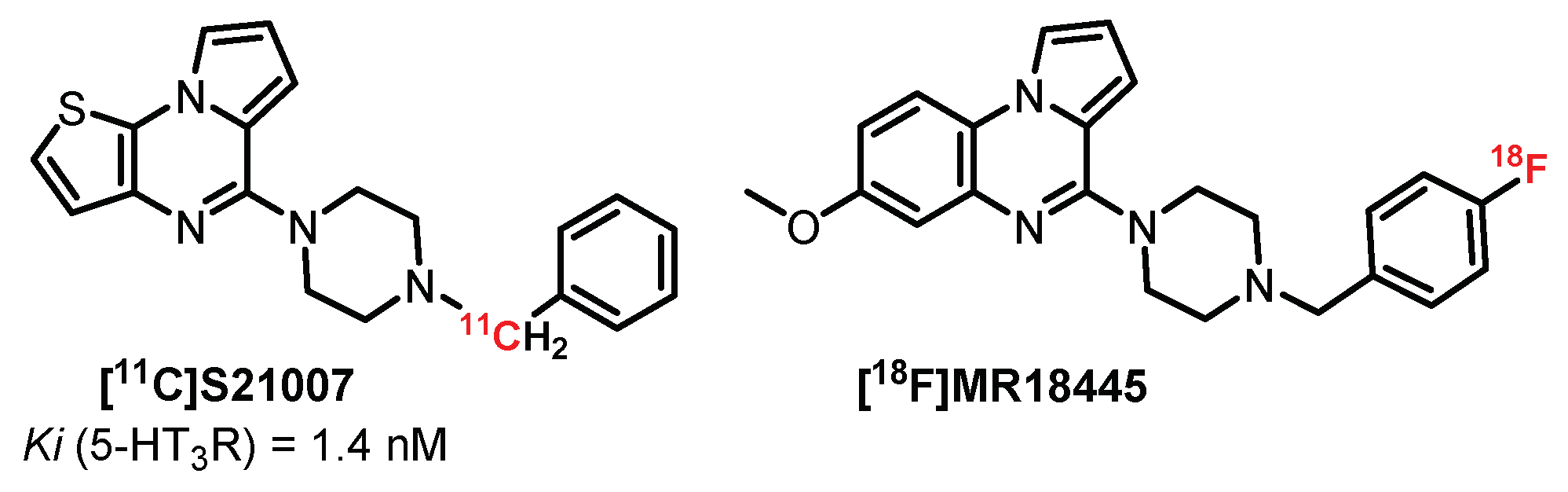
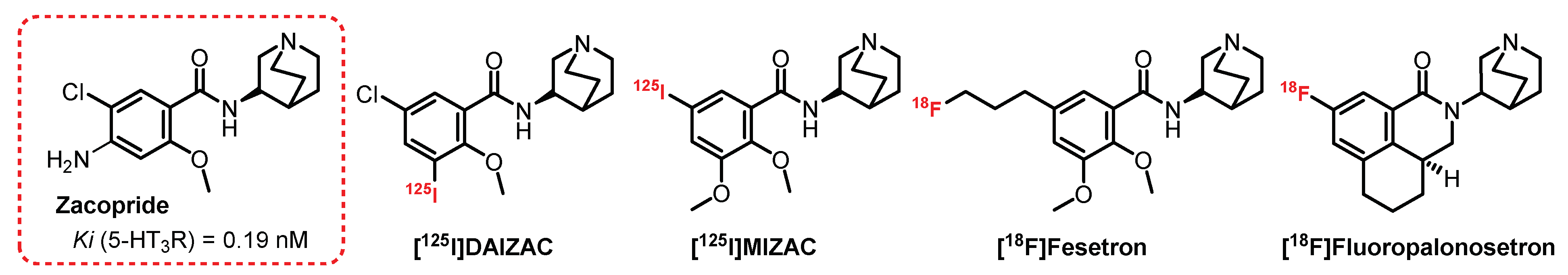
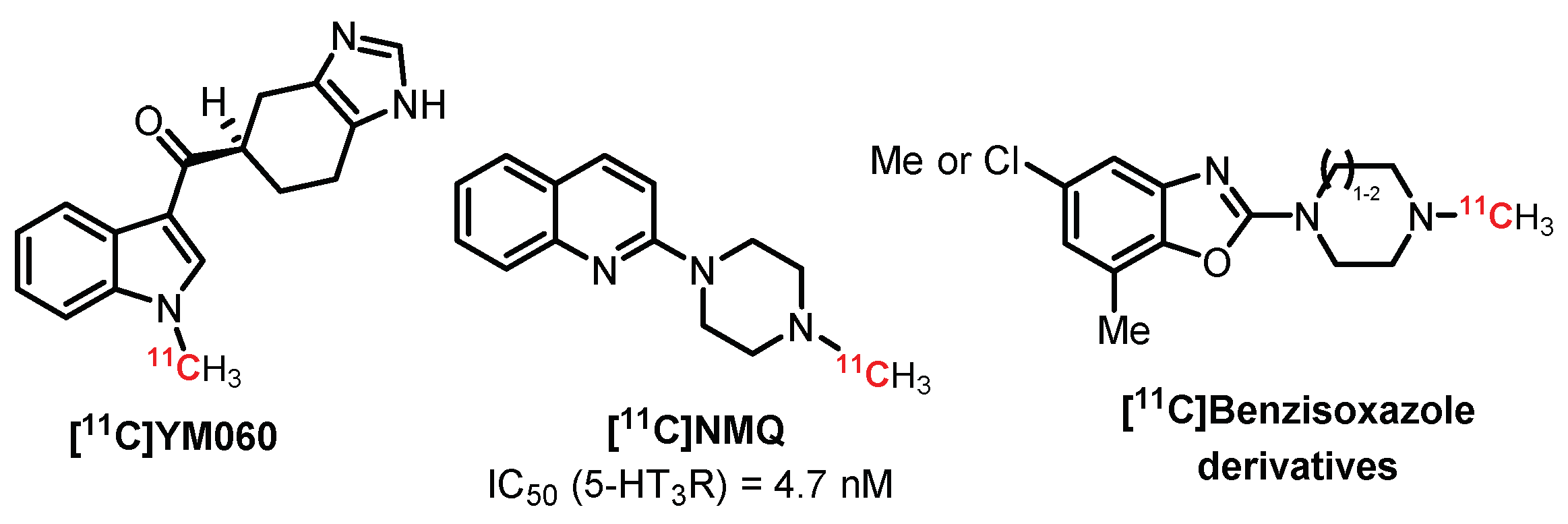
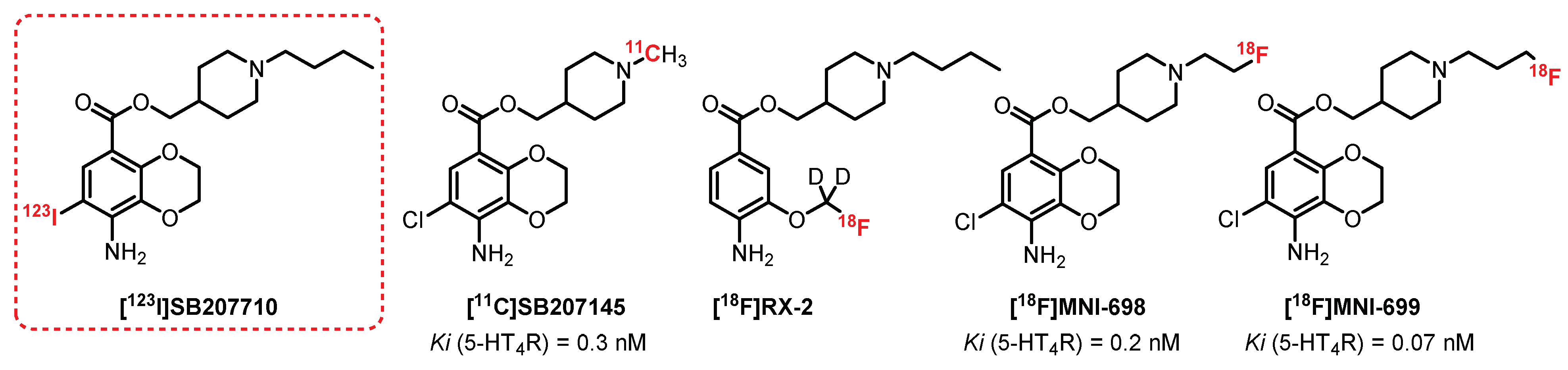
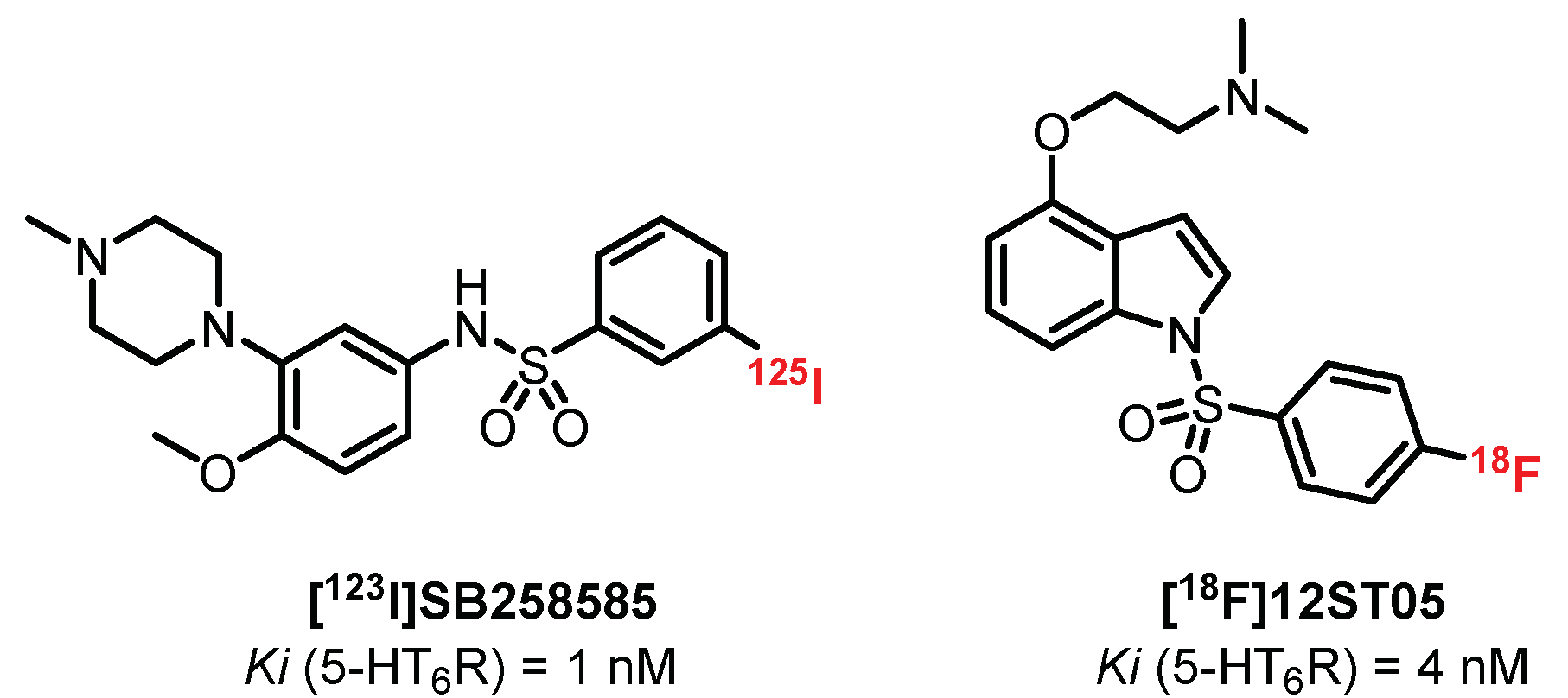
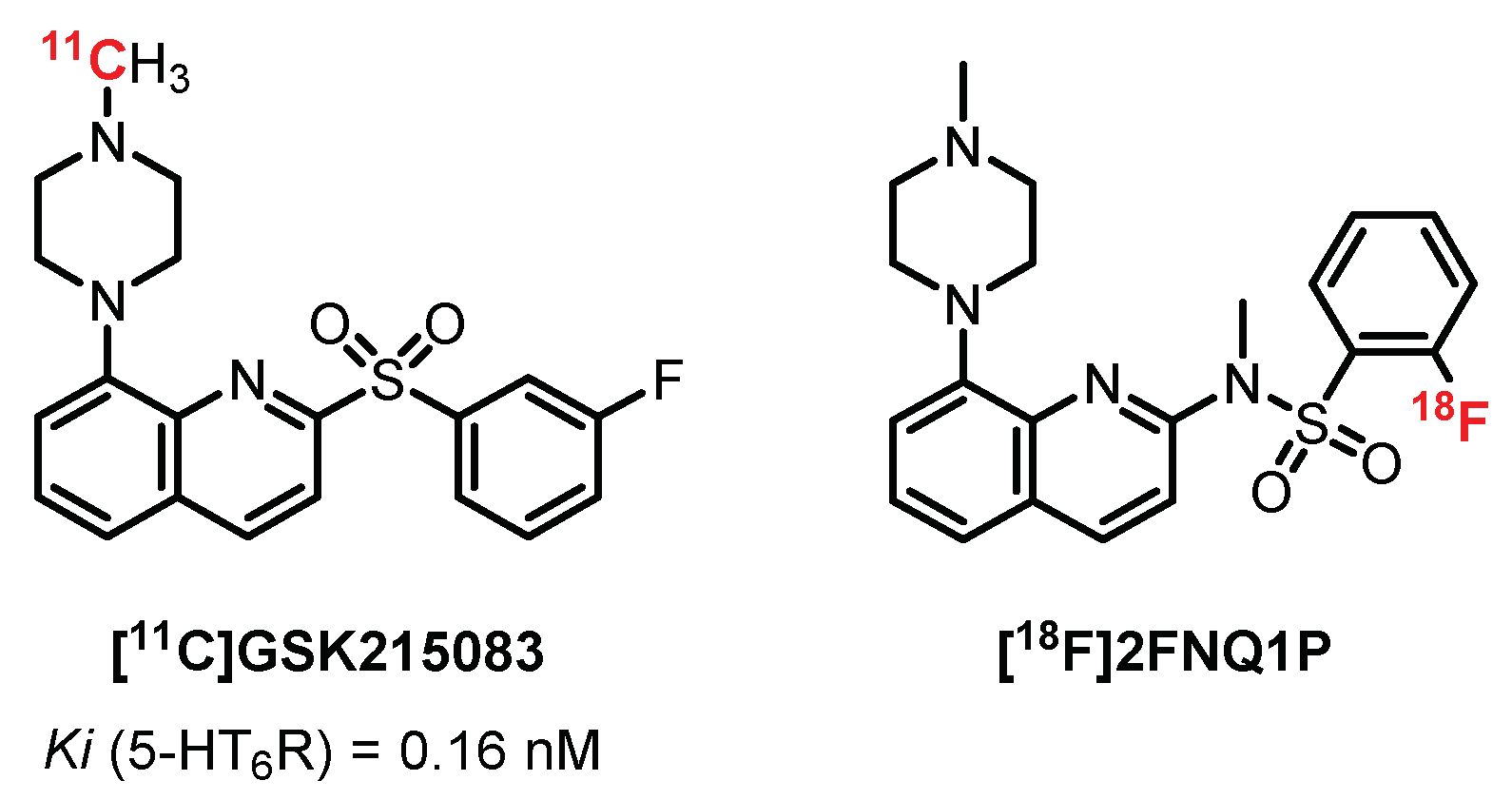
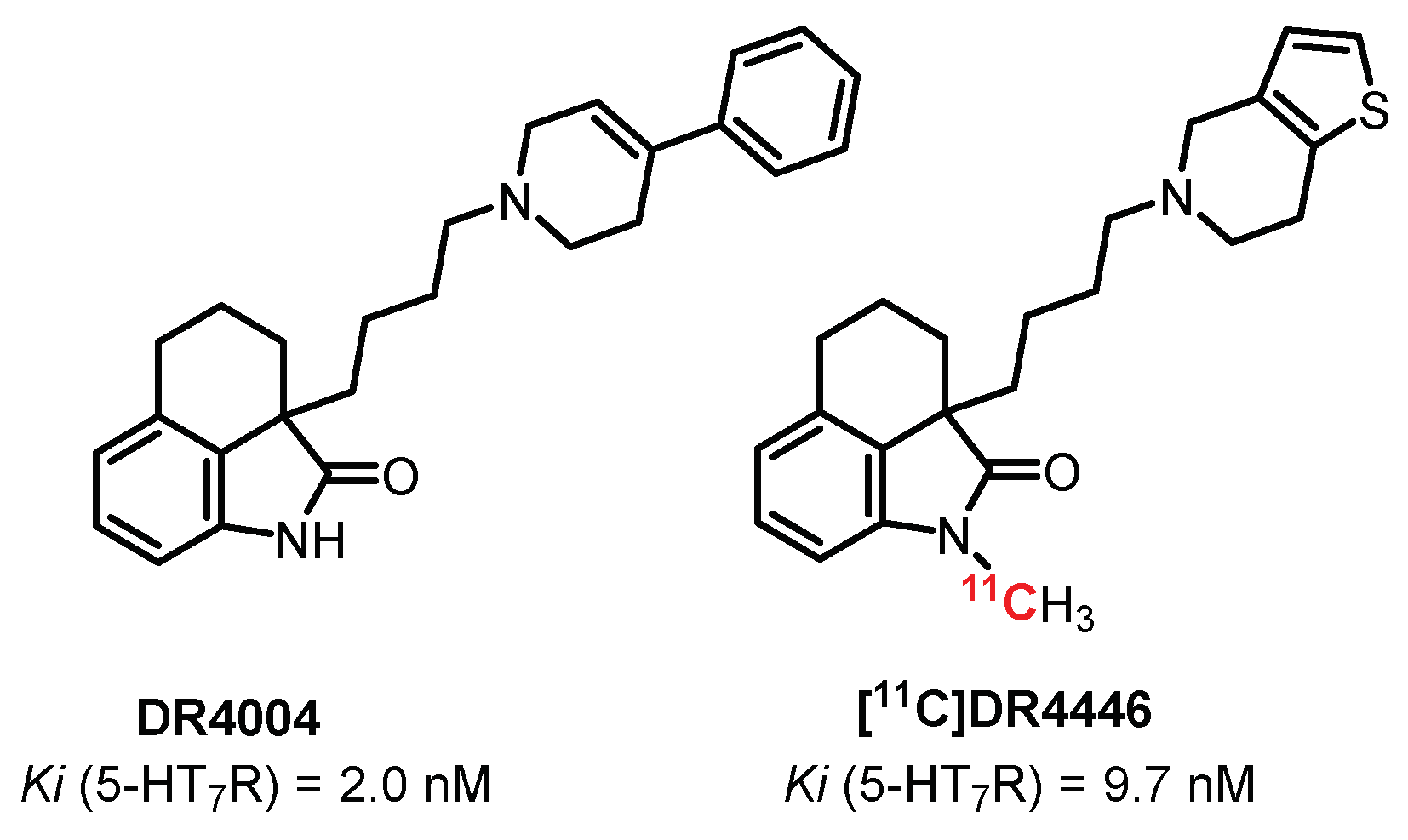
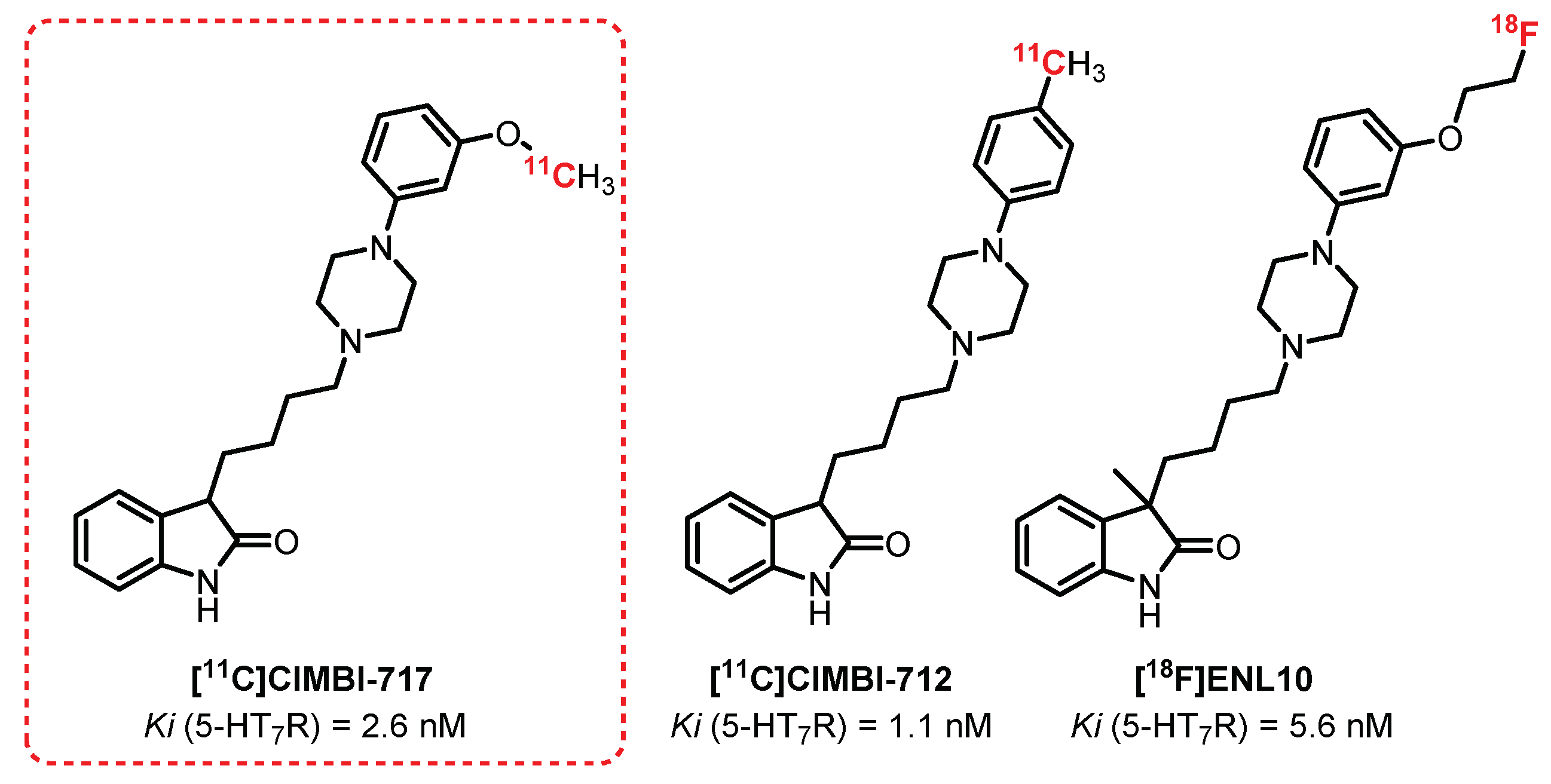
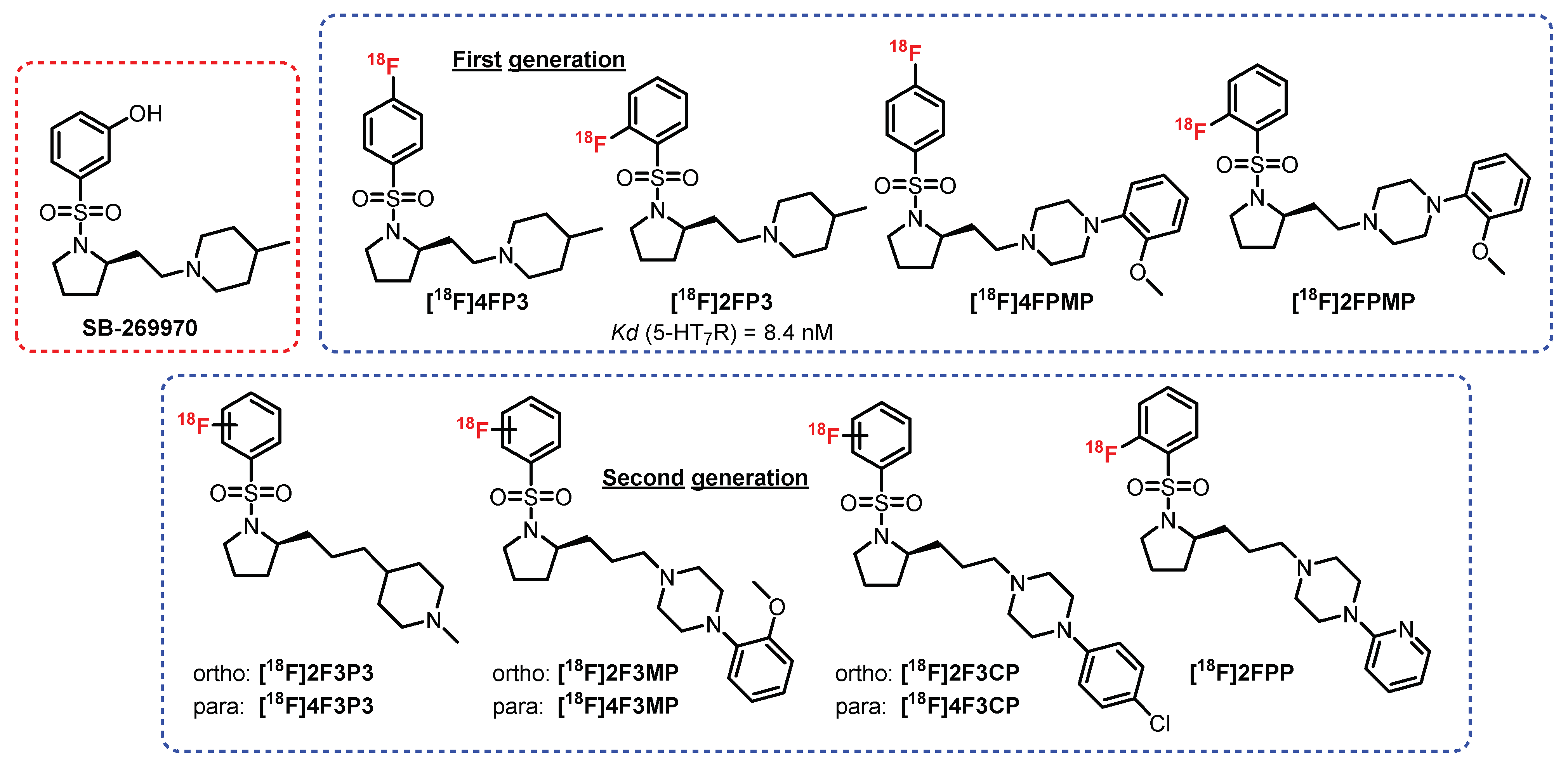
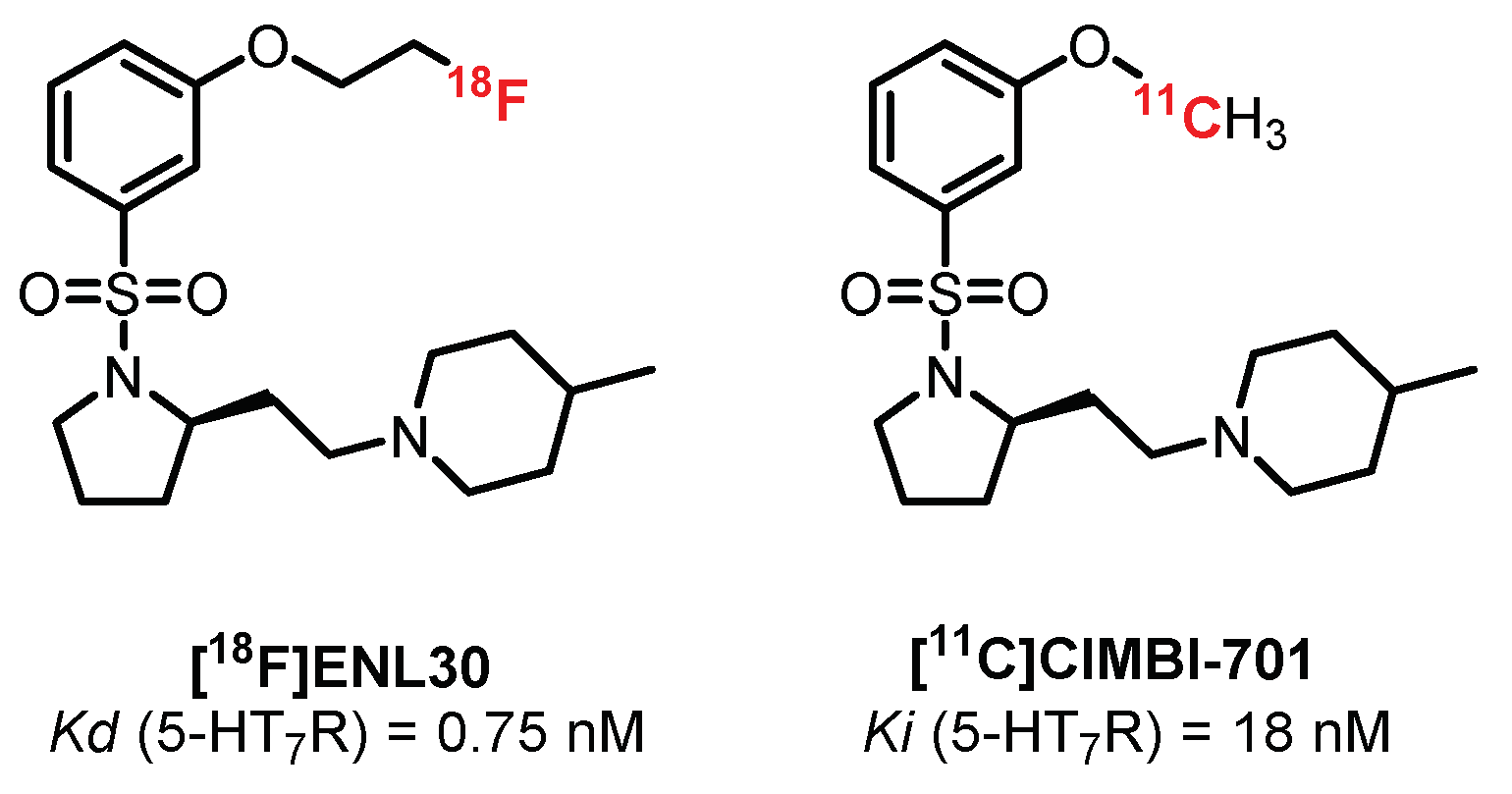
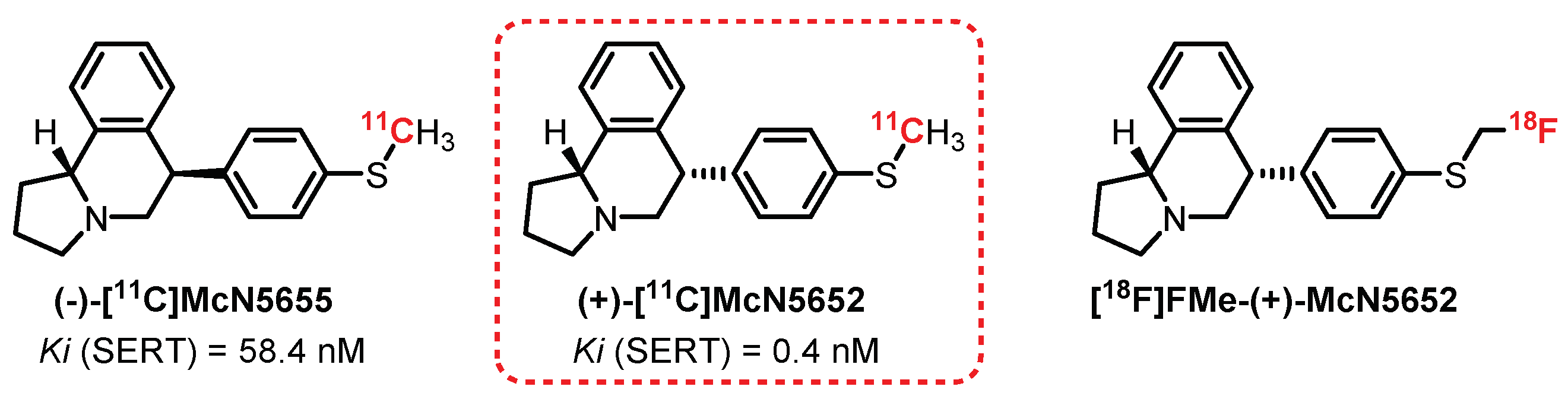
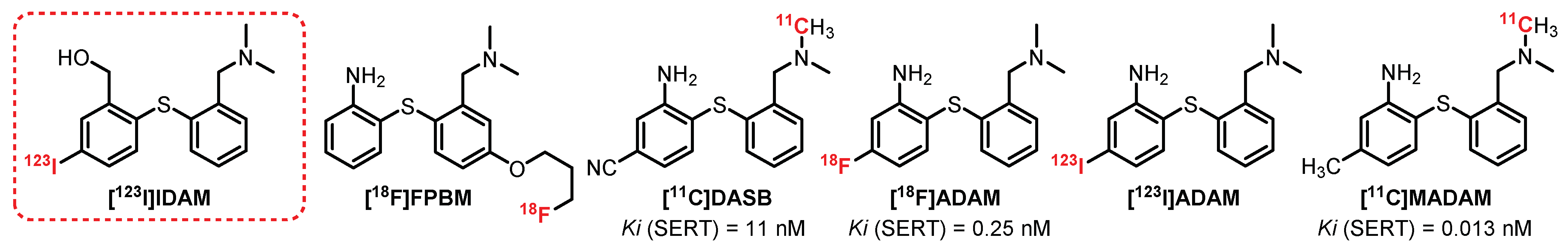
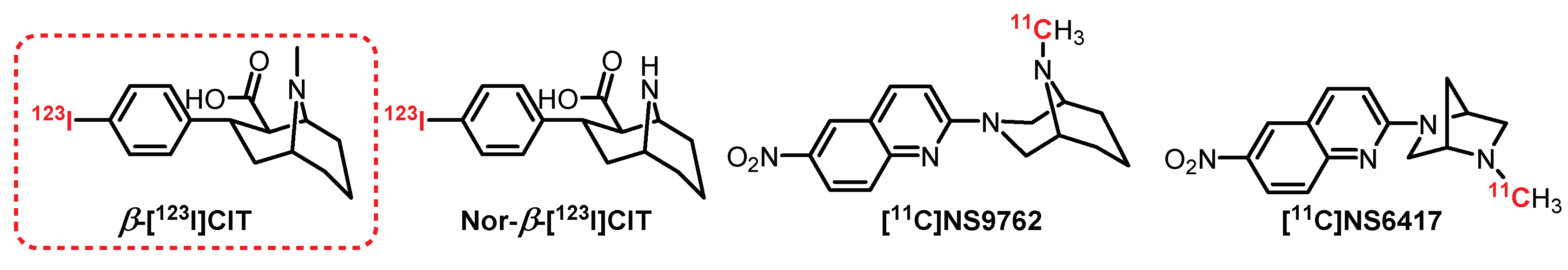
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangeant, R.; Dubost, E.; Cailly, T.; Collot, V. Radiotracers for the Central Serotoninergic System. Pharmaceuticals 2022, 15, 571. https://doi.org/10.3390/ph15050571
Mangeant R, Dubost E, Cailly T, Collot V. Radiotracers for the Central Serotoninergic System. Pharmaceuticals. 2022; 15(5):571. https://doi.org/10.3390/ph15050571
Chicago/Turabian StyleMangeant, Reynald, Emmanuelle Dubost, Thomas Cailly, and Valérie Collot. 2022. "Radiotracers for the Central Serotoninergic System" Pharmaceuticals 15, no. 5: 571. https://doi.org/10.3390/ph15050571
APA StyleMangeant, R., Dubost, E., Cailly, T., & Collot, V. (2022). Radiotracers for the Central Serotoninergic System. Pharmaceuticals, 15(5), 571. https://doi.org/10.3390/ph15050571