Quantitation of the A2A Adenosine Receptor Density in the Striatum of Mice and Pigs with [18F]FLUDA by Positron Emission Tomography
Abstract
1. Introduction
2. Results
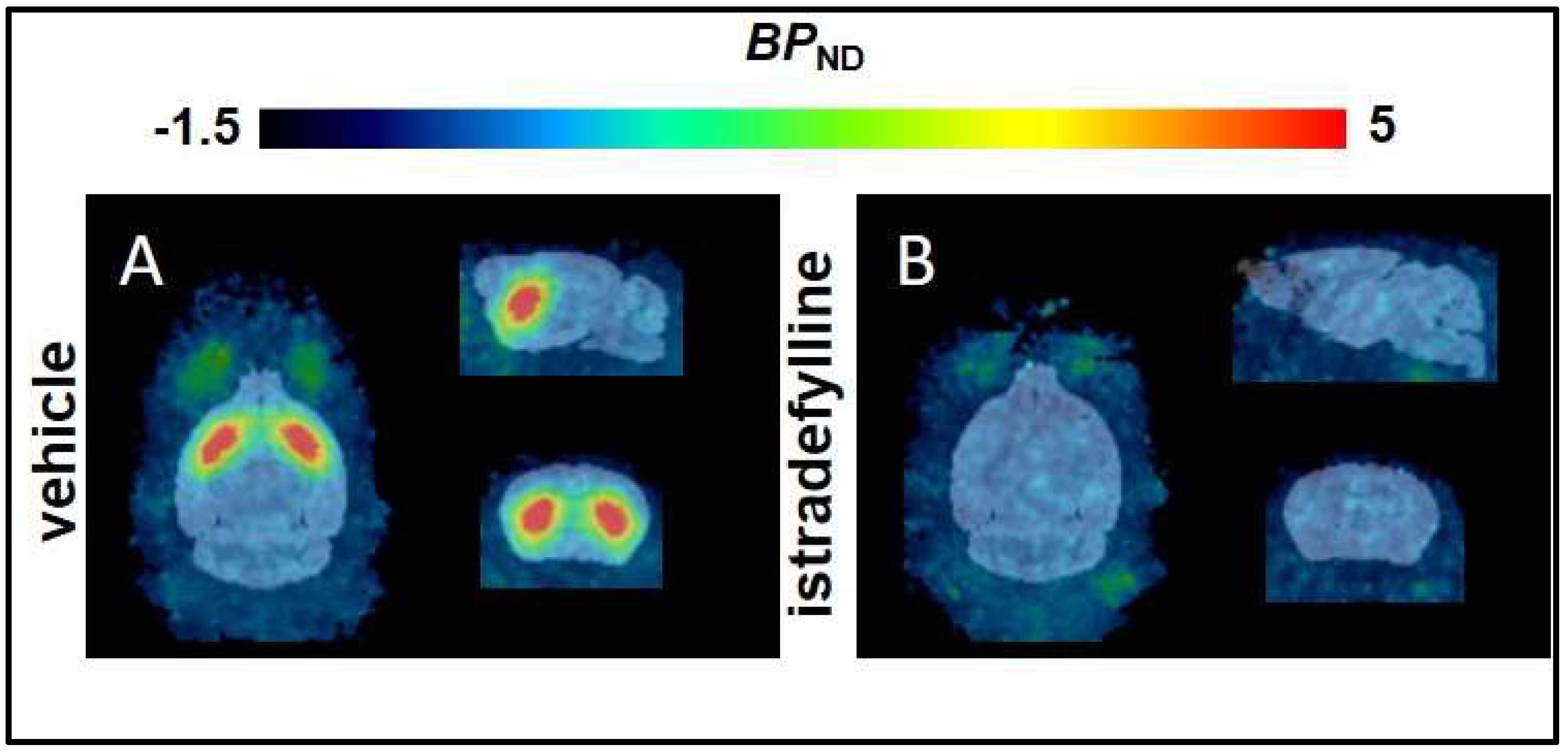
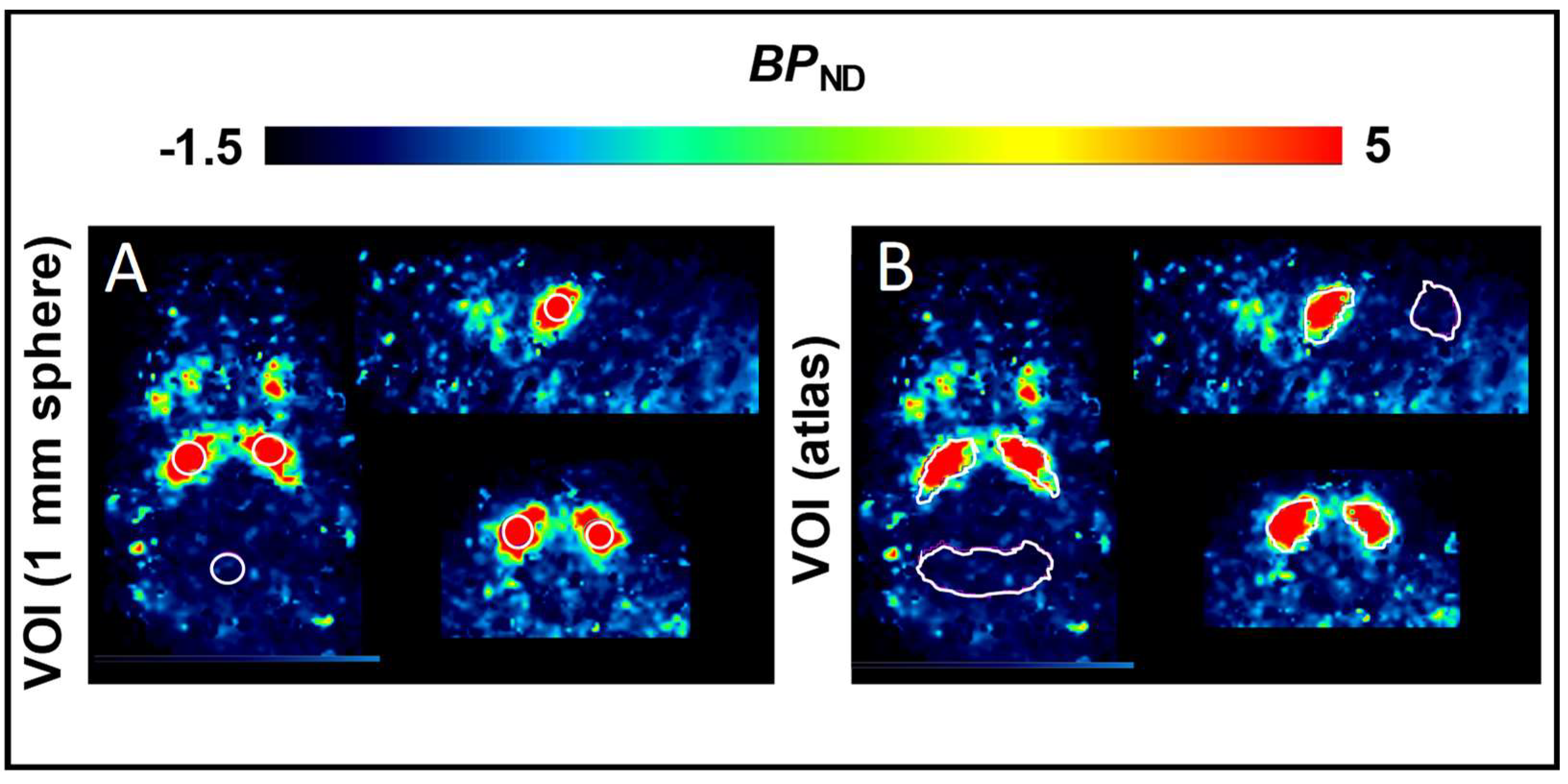
2.1. Non–Compartmental Analysis and Determination of [18F]FLUDA BPND in Healthy CD–1 Mice
| Brain region | BPND | R2 | AIC | |||||
|---|---|---|---|---|---|---|---|---|
| Veh | Istra | p–Value | Veh | Istra | Veh | Istra | ||
| Striatum | T2–Atlas VOI | 3.9 ± 1.2 | 0.0 ± 0.0 | <0.0001 | 0.8 ± 0.1 | 0.8 ± 0.1 | 100 ± 16 | 142 ± 22 |
| 1 mm sphere VOI | 5.9 ± 1.7 | 0.1 ± 0.2 | <0.0001 | 0.8 ± 0.1 | 0.7 ± 0.1 | 106 ± 16 | 120 ± 16 | |
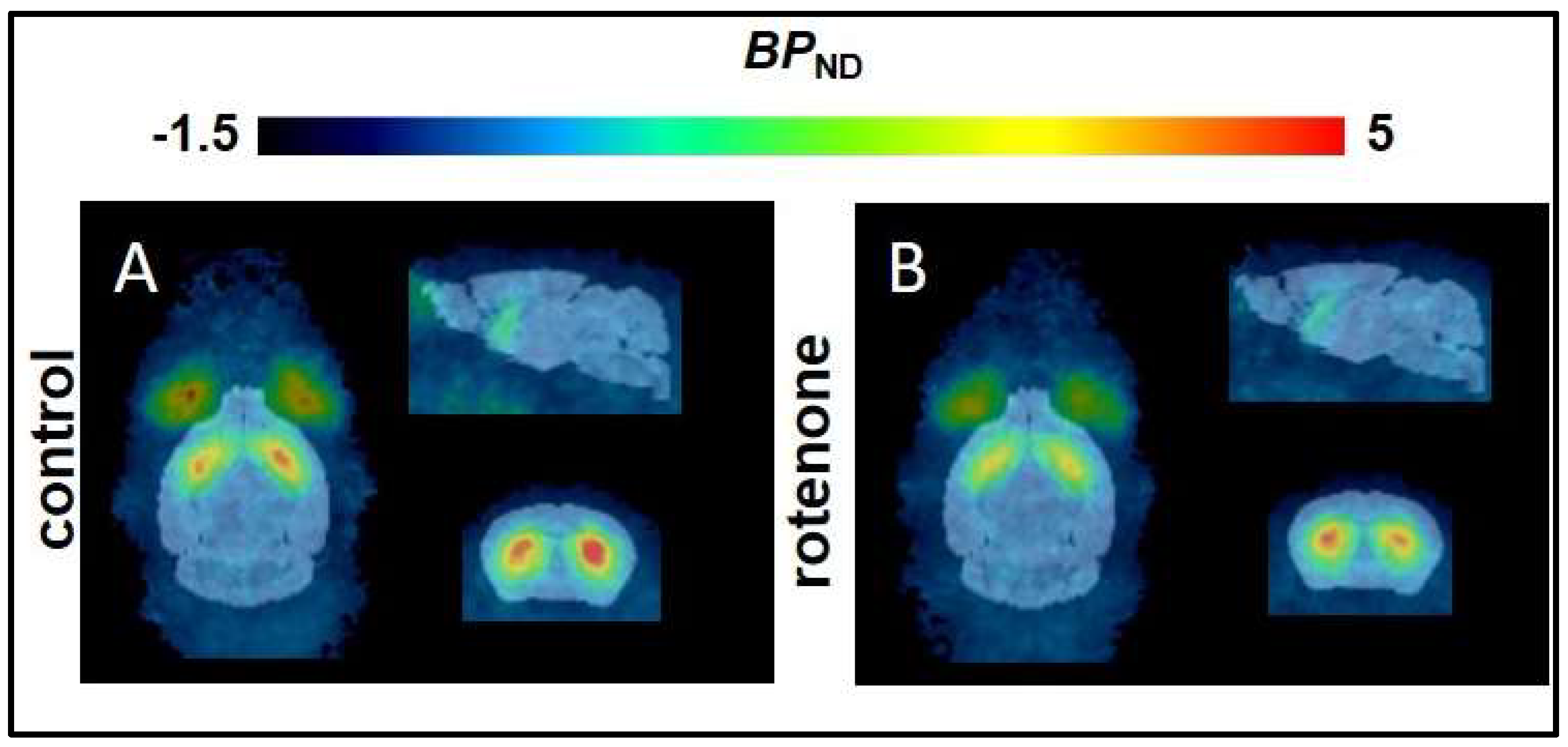
2.2. Non–Compartmental Analysis and Determination of the BPND in a C57BL/6JRj Murine Rotenone–Induced Parkinson Disease Model
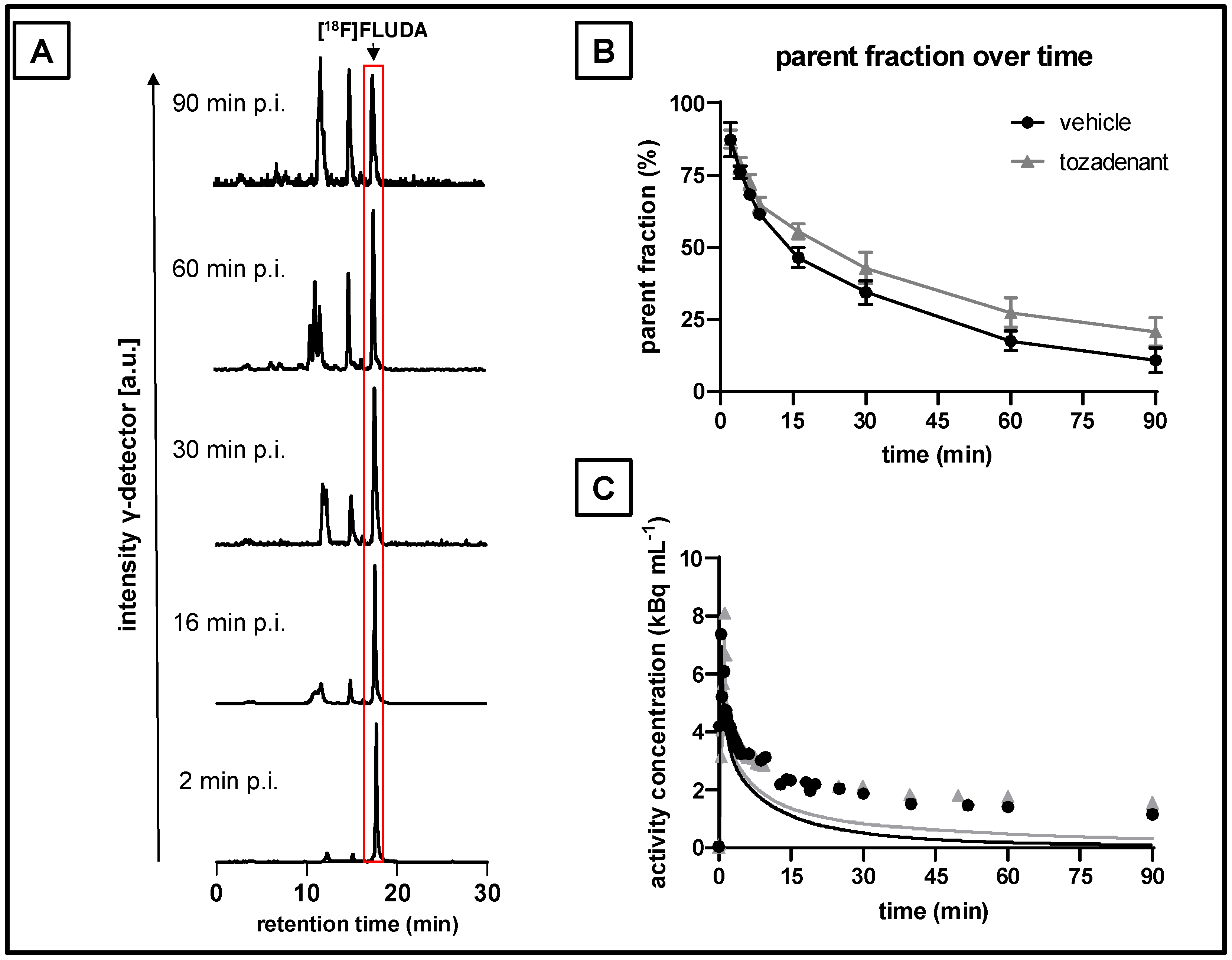
2.3. Plasma Metabolism of [18F]FLUDA in Pigs
2.4. Kinetic Analysis of [18F]FLUDA Uptake into Different Porcine Brain Regions
2.5. Determination of the VT and BPND of [18F]FLUDA in the Pig Brain
3. Materials and Methods
3.1. General Information
3.2. Radiosynthesis of [18F]FLUDA
3.3. Animals
3.4. Oral Rotenone Administration
3.5. Small Animal PET Imaging
3.6. PET Imaging of Pigs
3.7. Blood Sampling of Pigs
3.8. Analysis of Radiometabolites
3.9. Data Analysis and Model Description
4. Discussion
| Radiotracer | Ki (nM) of ligands | Ratio A1/A2A | BPND in Striatum and Striatal Substructures | References |
|---|---|---|---|---|
| [11C]TMSX ([11C]KF18446) | Rat (forebrain membranes) a: 5.9 | Rat: 270 | Human: 1.5 (DVR + 1) | [34,35] |
| [11C]KW-6002 | Human (CHO cells) a: 12/9.1 Rat (synaptosome preparations) a: 2.2/1.6 Mouse (synaptosome preparations) a: 18.9 | Human: >31.5 Rat: 32.4 Mouse: 56 | Human: Caudate 3.4; Putamen 2.9; Nucleus accumbens 2.4 | [5,36,37] |
| [11C]SCH442416 | Human (CHO cells) b: 0.05 Rat (striatal membranes) b: 0.5 | Human: 23145 Rat: 3630 | Macaca nemestrina: 0.74 Human: Caudate 0.53/0.40/0.96 *; Putamen 0.99/0.97/1.67 * | [14,38,39] |
| [11C]Preladenant (SCH 420814) | Human(HEK293 cells) b: 1.1 Rat: 2.5 | Human: 343 Rat: 1340 | Wistar rat: 5.0 to 6.1 | [40,41,42] |
| [18F]FPSCH | 53.6 | Wistar rat: 1.4–2.6 | [30] | |
| [18F]MRS5425/[18F]FESCH | Human (HEK293 cells/CHO-K1 cells): 12.4 a/0.6 c | Human: ~ 806/338 | Wistar rat: 1.6–3.4 CD-1 mouse: 2.7–3.8 | [26,30,43] Present study |
| [18F]MNI-444 | Human (HEK293 cells) a: 2.8 | Macaca mulatta: Caudate 5.5–6.8; Putamen 8.0–9.6; Nucleus accumbens 2.6–3.5 Human: Caudate 2.6–3.6; Putamen 4.1–5.5; Nucleus accumbens 1.3–2.5 | [21,44] | |
| [18F]FLUDA | Human (CHO-K1 cells): 0.7 | Human: >1400 | CD-1 mouse: 3.9–5.9 Mouse (C57BL/6JRj, rotenone treated): 2.5–3.5 Pigs: Caudate 1.1–2.1; Putamen 0.9–1.9; Nucleus accumbens: 0.8–1.5 | [25] Present study |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ishiwata, K.; Kimura, Y.; de Vries, E.J.; Elsinga, P. PET Tracers for Mapping Adenosine Receptors as Probes for Diagnosis of CNS Disorders. Cent. Nerv. Syst. Agents Med. Chem. 2007, 7, 57–77. [Google Scholar] [CrossRef][Green Version]
- Jacobson, K.A. Introduction to Adenosine Receptors as Therapeutic Targets. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; Volume 193, pp. 1–24. [Google Scholar]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef] [PubMed]
- Lanciego, J.L.; Luquin, N.; Obeso, J.A. Functional Neuroanatomy of the Basal Ganglia. Cold Spring Harb. Perspect. Med. 2012, 2, a009621. [Google Scholar] [CrossRef]
- Brooks, D.J.; Doder, M.; Osman, S.; Luthra, S.K.; Hirani, E.; Hume, S.; Kase, H.; Kilborn, J.; Martindill, S.; Mori, A. Positron Emission Tomography Analysis of [11C]KW–6002 Binding to Human and Rat Adenosine A2A Receptors in the Brain. Synapse 2008, 62, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Borroto–Escuela, D.O.; Fuxe, K. Adenosine Heteroreceptor Complexes in the Basal Ganglia Are Implicated in Parkinson’s Disease and Its Treatment. J. Neural Transm. 2019, 126, 455–471. [Google Scholar] [CrossRef]
- Froestl, W.; Muhs, A.; Pfeifer, A. Cognitive Enhancers (Nootropics). Part 1: Drugs Interacting with Receptors. J. Alzheimers Dis. 2012, 32, 793–887. [Google Scholar] [CrossRef]
- Müller, C.; Jacobson, K.A. Xanthines as Adenosine Receptor Antagonists. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 200, pp. 151–199. [Google Scholar] [CrossRef]
- Cappelletti, S.; Piacentino, D.; Sani, G.; Aromatario, M. Caffeine: Cognitive and Physical Performance Enhancer or Psychoactive Drug? Curr. Neuropharmacol. 2015, 13, 71–88. [Google Scholar] [CrossRef]
- Jenner, P. An Overview of Adenosine A2A Receptor Antagonists in Parkinson’s Disease. In International Review of Neurobiology; Academic Press Inc.: Cambridge, MA, USA, 2014; Volume 119, pp. 71–86. [Google Scholar]
- Xu, K.; Bastia, E.; Schwarzschild, M. Therapeutic Potential of Adenosine A2A Receptor Antagonists in Parkinson’s Disease. Pharmacol. Ther. 2005, 105, 267–310. [Google Scholar] [CrossRef]
- Varani, K.; Bachoud–Lévi, A.-C.; Mariotti, C.; Tarditi, A.; Abbracchio, M.P.; Gasperi, V.; Borea, P.A.; Dolbeau, G.; Gellera, C.; Solari, A.; et al. Biological Abnormalities of Peripheral A2A Receptors in a Large Representation of Polyglutamine Disorders and Huntington’s Disease Stages. Neurobiol. Dis. 2007, 27, 36–43. [Google Scholar] [CrossRef]
- Matos, M.; Augusto, E.; MacHado, N.J.; Dos Santos–Rodrigues, A.; Cunha, R.A.; Agostinho, P. Astrocytic Adenosine A2A Receptors Control the Amyloid–β Peptide–Induced Decrease of Glutamate Uptake. J. Alzheimers Dis. 2012, 31, 555–567. [Google Scholar] [CrossRef]
- Ramlackhansingh, A.F.; Bose, S.K.; Ahmed, I.; Turkheimer, F.E.; Pavese, N.; Brooks, D.J. Adenosine 2A Receptor Availability in Dyskinetic and Nondyskinetic Patients with Parkinson Disease. Neurology 2011, 76, 1811–1816. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Ceruti, S.; Abbracchio, M.P. Adenosine Receptors and Neurological Disease: Neuroprotection and Neurodegeneration. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; Volume 193, pp. 535–587. [Google Scholar]
- Sebastião, A.M.; Ribeiro, J.A. Triggering Neurotrophic Factor Actions through Adenosine A2A Receptor Activation: Implications for Neuroprotection. Br. J. Pharmacol. 2009, 158, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-F.; Pedata, F. Modulation of Ischemic Brain Injury and Neuroinflammation by Adenosine A2A Receptors. Curr. Pharm. Des. 2008, 14, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Weiner, W.J. Levodopa—Toxic or Neuroprotective? Nat. Clin. Pract. Neurol. 2006, 2, 518–519. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Mizuno, Y. A Long–Term Study of Istradefylline Safety and Efficacy in Patients with Parkinson Disease. Clin. Neuropharmacol. 2015, 38, 41–46. [Google Scholar] [CrossRef] [PubMed]
- LeWitt, P.A.; Aradi, S.D.; Hauser, R.A.; Rascol, O. The Challenge of Developing Adenosine A2A Antagonists for Parkinson Disease: Istradefylline, Preladenant, and Tozadenant. Parkinsonism Relat. Disord. 2020, 80, S54–S63. [Google Scholar] [CrossRef]
- Barret, O.; Hannestad, J.; Alagille, D.; Vala, C.; Tavares, A.; Papin, C.; Morley, T.; Fowles, K.; Lee, H.; Seibyl, J.; et al. Adenosine 2A Receptor Occupancy by Tozadenant and Preladenant in Rhesus Monkeys. J. Nucl. Med. 2014, 55, 1712–1718. [Google Scholar] [CrossRef]
- Hauser, R.A.; Olanow, C.W.; Kieburtz, K.D.; Pourcher, E.; Docu–Axelerad, A.; Lew, M.; Kozyolkin, O.; Neale, A.; Resburg, C.; Meya, U.; et al. Tozadenant (SYN115) in Patients with Parkinson’s Disease Who Have Motor Fluctuations on Levodopa: A Phase 2b, Double–Blind, Randomised Trial. Lancet Neurol. 2014, 13, 767–776. [Google Scholar] [CrossRef]
- Seibyl, J.; Russell, D.; Jennings, D.; Marek, K. Neuroimaging Over the Course of Parkinson’s Disease: From Early Detection of the At–Risk Patient to Improving Pharmacotherapy of Later–Stage Disease. Semin. Nucl. Med. 2012, 42, 406–414. [Google Scholar] [CrossRef]
- McCluskey, S.P.; Plisson, C.; Rabiner, E.A.; Howes, O. Advances in CNS PET: The State–of–the–Art for New Imaging Targets for Pathophysiology and Drug Development. Eur. J. Nucl. Med. Mol. Imaging 2019, 47, 451–489. [Google Scholar] [CrossRef]
- Lai, T.H.; Toussaint, M.; Teodoro, R.; Dukić–Stefanović, S.; Gündel, D.; Ludwig, F.-A.; Wenzel, B.; Schröder, S.; Sattler, B.; Moldovan, R.-P.; et al. Improved in Vivo PET Imaging of the Adenosine A2A Receptor in the Brain Using [18F]FLUDA, a Deuterated Radiotracer with High Metabolic Stability. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2727–2736. [Google Scholar] [CrossRef] [PubMed]
- Schröder, S.; Lai, T.H.; Toussaint, M.; Kranz, M.; Chovsepian, A.; Shang, Q.; Dukić–Stefanović, S.; Deuther–Conrad, W.; Teodoro, R.; Wenzel, B.; et al. PET Imaging of the Adenosine A2A Receptor in the Rotenone–Based Mouse Model of Parkinson’s Disease with [18F]FESCH Synthesized by a Simplified Two–Step One–Pot Radiolabeling Strategy. Molecules 2020, 25, 1633. [Google Scholar] [CrossRef] [PubMed]
- Pond, W.G.; Boleman, S.L.; Fiorotto, M.L.; Ho, H.; Knabe, D.A.; Mersmann, H.J.; Savell, J.W.; Su, D.R. Perinatal Ontogeny of Brain Growth in the Domestic Pig. Proc. Soc. Exp. Biol. Med. 2000, 223, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Saikali, S.; Meurice, P.; Sauleau, P.; Eliat, P.-A.; Bellaud, P.; Randuineau, G.; Vérin, M.; Malbert, C.-H. A Three–Dimensional Digital Segmented and Deformable Brain Atlas of the Domestic Pig. J. Neurosci. Methods 2010, 192, 102–109. [Google Scholar] [CrossRef]
- Lai, T.H.; Wenzel, B.; Moldovan, R.-P.; Brust, P.; Kopka, K.; Teodoro, R. Automated Radiosynthesis of the Adenosine A2A Receptor–Targeting Radiotracer [18F]FLUDA. J. Label. Compd. Radiopharm. 2022, 1–5. [Google Scholar] [CrossRef]
- Khanapur, S.; Van Waarde, A.; Dierckx, R.A.J.O.; Elsinga, P.H.; Koole, M.J.B. Preclinical Evaluation and Quantification of 18F–Fluoroethyl and 18F–Fluoropropyl Analogs of SCH442416 as Radioligands for PET Imaging of the Adenosine A2A Receptor in Rat Brain. J. Nucl. Med. 2017, 58, 466–472. [Google Scholar] [CrossRef]
- Li, J.; Hong, X.; Li, G.; Conti, P.S.; Zhang, X.; Chen, K. PET Imaging of Adenosine Receptors in Diseases. Curr. Top. Med. Chem. 2019, 19, 1445–1463. [Google Scholar] [CrossRef]
- Vuorimaa, A.; Rissanen, E.; Airas, L. In Vivo PET Imaging of Adenosine 2A Receptors in Neuroinflammatory and Neurodegenerative Disease. Contrast Media Mol. Imaging 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Ma, Y.; Hof, P.R.; Grant, S.C.; Blackband, S.J.; Bennett, R.; Slatest, L.; Mcguigan, M.D.; Benveniste, H. A Three–Dimensional Digital Atlas Database of the Adult C57BL/6J Mouse Brain by Magnetic Resonance Microscopy. Neuroscience 2005, 135, 1203–1215. [Google Scholar] [CrossRef]
- Ishiwata, K.; Noguchi, J.; Wakabayashi, S.; Shimada, J.; Ogi, N.; Nariai, T.; Tanaka, A.; Endo, K.; Suzuki, F.; Senda, M. 11C-Labeled KF18446: A Potential Central Nervous System Adenosine A2a Receptor Ligand. J. Nucl. Med. 2000, 41, 345–354. [Google Scholar]
- Mishina, M.; Ishiwata, K.; Naganawa, M.; Kimura, Y.; Kitamura, S.; Suzuki, M.; Hashimoto, M.; Ishibashi, K.; Oda, K.; Sakata, M.; et al. Adenosine A2A Receptors Measured with [11C]TMSX PET in the Striata of Parkinson’s Disease Patients. PLoS ONE 2011, 6, e17338. [Google Scholar] [CrossRef] [PubMed]
- Mihara, T.; Mihara, K.; Yarimizu, J.; Mitani, Y.; Matsuda, R.; Yamamoto, H.; Aoki, S.; Akahane, A.; Iwashita, A.; Matsuoka, N. Pharmacological Characterization of a Novel, Potent Adenosine A1 and A2A Receptor Dual Antagonist, 5-[5-Amino-3-(4-Fluorophenyl) Pyrazin-2-Yl]-1-Isopropylpyridine-2(1H)-One (ASP5854), in Models of Parkinson’s Disease and Cognition. J. Pharmacol. Exp. Ther. 2007, 323, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Shimada, J.; Koike, N.; Nonaka, H.; Shiozaki, S.; Yanagawa, K.; Kanda, T.; Kobayashi, H.; Ichimura, M.; Nakamura, J.; Kase, H.; et al. Adenosine A(2A) Antagonists with Potent Anti-Cataleptic Activity. Bioorganic Med. Chem. Lett. 1997, 7, 2349–2352. [Google Scholar] [CrossRef]
- Moresco, R.M.; Todde, S.; Belloli, S.; Simonelli, P.; Panzacchi, A.; Rigamonti, M.; Galli-Kienle, M.; Fazio, F. In Vivo Imaging of Adenosine A2A Receptors in Rat and Primate Brain Using [11C]SCH442416. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Todde, S.; Moresco, R.M.; Simonelli, P.; Baraldi, P.G.; Cacciari, B.; Spalluto, G.; Varani, K.; Monopoli, A.; Matarrese, M.; Carpinelli, A.; et al. Design, Radiosynthesis, and Biodistribution of a New Potent and Selective Ligand for in Vivo Imaging of the Adenosine A2A Receptor System Using Positron Emission Tomography [2]. J. Med. Chem. 2000, 43, 4359–4362. [Google Scholar] [CrossRef]
- Zhou, X.; Khanapur, S.; De Jong, J.R.; Willemsen, A.T.M.; Dierckx, R.A.J.O.; Elsinga, P.H.; De Vries, E.F.J. In Vivo Evaluation of [11C]Preladenant Positron Emission Tomography for Quantification of Adenosine A2A Receptors in the Rat Brain. J. Cereb. Blood Flow Metab. 2017, 37, 577–589. [Google Scholar] [CrossRef]
- Hodgson, R.A.; Bertorelli, R.; Varty, G.B.; Lachowicz, J.E.; Forlani, A.; Fredduzzi, S.; Cohen-Williams, M.E.; Higgins, G.A.; Impagnatiello, F.; Nicolussi, E.; et al. Characterization of the Potent and Highly Selective A2A Receptor Antagonists Preladenant and SCH 412348 [7-[2-[4-2,4-Difluorophenyl]-1-Piperazinyl]Ethyl]-2-(2-Furanyl)-7hpyrazolo[ 4,3-e][1,2,4]Triazolo[1,5-c] Pyrimidin-5-Amine in Rodent Models of Movemen. J. Pharmacol. Exp. Ther. 2009, 330, 294–303. [Google Scholar] [CrossRef]
- Neustadt, B.R.; Hao, J.; Lindo, N.; Greenlee, W.J.; Stamford, A.W.; Tulshian, D.; Ongini, E.; Hunter, J.; Monopoli, A.; Bertorelli, R.; et al. Potent, Selective, and Orally Active Adenosine A2A Receptor Antagonists: Arylpiperazine Derivatives of Pyrazolo[4,3-e]-1,2,4-Triazolo[1,5-c]Pyrimidines. Bioorganic Med. Chem. Lett. 2007, 17, 1376–1380. [Google Scholar] [CrossRef]
- Shinkre, B.A.; Kumar, T.S.; Gao, Z.G.; Deflorian, F.; Jacobson, K.A.; Trenkle, W.C. Synthesis and Evaluation of 1,2,4-Triazolo[1,5-c]Pyrimidine Derivatives as A2A Receptor-Selective Antagonists. Bioorganic Med. Chem. Lett. 2010, 20, 5690–5694. [Google Scholar] [CrossRef]
- Barret, O.; Hannestad, J.; Vala, C.; Alagille, D.; Tavares, A.; Laruelle, M.; Jennings, D.; Marek, K.; Russell, D.; Seibyl, J.; et al. Characterization in Humans of 18F-MNI-444, a PET Radiotracer for Brain Adenosine 2A Receptors. J. Nucl. Med. 2015, 56, 586–591. [Google Scholar] [CrossRef]
- Wan, W.; Sutherland, G.R.; Geiger, J.D. Binding of the Adenosine A2 Receptor Ligand [3H]CGS 21680 to Human and Rat Brain: Evidence for Multiple Affinity Sites. J. Neurochem. 1990, 55, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Sihver, W.; Schulze, A.; Wutz, W.; Stüsgen, S.; Olsson, R.A.; Bier, D.; Holschbach, M.H. Autoradiographic Comparison of in Vitro Binding Characteristics of Various Tritiated Adenosine A2A Receptor Ligands in Rat, Mouse and Pig Brain and First Ex Vivo Results. Eur. J. Pharmacol. 2009, 616, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Dionisotti, S.; Ongini, E.; Zocchi, C.; Kull, B.; Arslan, G.; Fredholm, B.B. Characterization of Human A(2A) Adenosine Receptors with the Antagonist Radioligand [3H]-SCH 58261. Br. J. Pharmacol. 1997, 121, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Uustare, A.; Vonk, A.; Terasmaa, A.; Fuxe, K.; Rinken, A. Kinetic and Functional Properties of [3H]ZM241385, a High Affinity Antagonist for Adenosine A2A Receptors. Life Sci. 2005, 76, 1513–1526. [Google Scholar] [CrossRef] [PubMed]
- Hirani, E.; Gillies, J.; Karasawa, A.; Shimada, J.; Kase, H.; Opacka-Juffry, J.; Osman, S.; Luthra, S.K.; Hume, S.P.; Brooks, D.J. Evaluation of [4-O-Methyl-11<C]KW-6002 as a Potential PET Ligand for Mapping Central Adenosine A2A Receptors in Rats. Synapse 2001, 42, 164–176. [Google Scholar] [CrossRef]
- Lammertsma, A.A.; Hume, S.P. Simplified Reference Tissue Model for PET Receptor Studies. Neuroimage 1996, 4, 153–158. [Google Scholar] [CrossRef]
- Black, K.J.; Koller, J.M.; Campbell, M.C.; Gusnard, D.A.; Bandak, S.I. Quantification of Indirect Pathway Inhibition by the Adenosine A2a Antagonist SYN115 in Parkinson Disease. J. Neurosci. 2010, 30, 16284–16292. [Google Scholar] [CrossRef]
- Rominger, A.; Wagner, E.; Mille, E.; Böning, G.; Esmaeilzadeh, M.; Wängler, B.; Gildehaus, F.-J.; Nowak, S.; Bruche, A.; Tatsch, K.; et al. Endogenous Competition against Binding of [18F]DMFP and [ 18 F]Fallypride to Dopamine D 2/3 Receptors in Brain of Living Mouse. Synapse 2010, 64, 313–322. [Google Scholar] [CrossRef]
- Mishina, M.; Kimura, Y.; Naganawa, M.; Ishii, K.; Oda, K.; Sakata, M.; Toyohara, J.; Kobayashi, S.; Katayama, Y.; Ishiwata, K. Differential Effects of Age on Human Striatal Adenosine A1 and A2A Receptors. Synapse 2012, 66, 832–839. [Google Scholar] [CrossRef]
- Cunha, R.A.; Constantino, M.D.; Sebastião, A.M.; Ribeiro, J.A. Modification of A1 and A2a Adenosine Receptor Binding in Aged Striatum, Hippocampus and Cortex of the Rat. NeuroReport 1995, 6, 1583–1588. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Vijayraja, D.; Mohankumar, T.; Manimaran, D.; Ganesan, P.; Choi, D.-K.; Elangovan, N. Isolongifolene Mitigates Rotenone-Induced Dopamine Depletion and Motor Deficits through Anti-Oxidative and Anti-Apoptotic Effects in a Rat Model of Parkinson’s Disease. J. Chem. Neuroanat. 2021, 112, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Doorduin, J.; Elsinga, P.H.; Dierckx, R.A.J.O.; de Vries, E.F.J.; Casteels, C. Altered Adenosine 2A and Dopamine D2 Receptor Availability in the 6-Hydroxydopamine-Treated Rats with and without Levodopa-Induced Dyskinesia. Neuroimage 2017, 157, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.; Nandhu, M.S.; Kuruvilla, K.P.; Paulose, C.S. Dopamine D1 and D2 Receptor Subtypes Functional Regulation in Corpus Striatum of Unilateral Rotenone Lesioned Parkinson’s Rat Model: Effect of Serotonin, Dopamine and Norepinephrine. Neurol. Res. 2010, 32, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Villar-Menéndez, I.; Porta, S.; Buira, S.P.; Pereira-Veiga, T.; Díaz-Sánchez, S.; Albasanz, J.L.; Ferrer, I.; Martín, M.; Barrachina, M. Increased Striatal Adenosine A2A Receptor Levels Is an Early Event in Parkinson’s Disease-Related Pathology and It Is Potentially Regulated by MiR-34b. Neurobiol. Dis. 2014, 69, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.K.; Lang, L.; Jacobson, O.; Shinkre, B.; Ma, Y.; Niu, G.; Trenkle, W.C.; Jacobson, K.A.; Chen, X.; Kiesewetter, D.O. Striatal Adenosine A2A Receptor-Mediated Positron Emission Tomographic Imaging in 6-Hydroxydopamine-Lesioned Rats Using [18F]-MRS5425. Nucl. Med. Biol. 2011, 38, 897–906. [Google Scholar] [CrossRef]
- Tomiyama, M.; Kimura, T.; Maeda, T.; Tanaka, H.; Kannari, K.; Baba, M. Upregulation of Striatal Adenosine A2A Receptor MRNA in 6-Hydroxydopamine-Lesioned Rats Intermittently Treated with L-DOPA. Synapse 2004, 52, 218–222. [Google Scholar] [CrossRef]
- Gebicke-Haerter, P.J.; Christoffel, F.; Timmer, J.; Northoff, H.; Berger, M.; Van Calker, D. Both Adenosine A1- and A2-Receptors Are Required to Stimulate Microglial Proliferation. Neurochem. Int. 1996, 29, 37–42. [Google Scholar] [CrossRef]
- Pickel, V.M.; Chan, J.; Linden, J.; Rosin, D.L. Subcellular Distributions of Adenosine A1 and A2A Receptors in the Rat Dorsomedial Nucleus of the Solitary Tract at the Level of the Area Postrema. Synapse 2006, 60, 496–509. [Google Scholar] [CrossRef]
- Martinez-Mir, M.I.; Probst, A.; Palacios, J.M. Adenosine A2 Receptors: Selective Localization in the Human Basal Ganglia and Alterations with Disease. Neuroscience 1991, 42, 697–706. [Google Scholar] [CrossRef]
- Li, W.; Silva, H.B.; Real, J.; Wang, Y.M.; Rial, D.; Li, P.; Payen, M.P.; Zhou, Y.; Muller, C.E.; Tomé, A.R.; et al. Inactivation of Adenosine A2A Receptors Reverses Working Memory Deficits at Early Stages of Huntington’s Disease Models. Neurobiol. Dis. 2015, 79, 70–80. [Google Scholar] [CrossRef]
- Chou, S.Y.; Lee, Y.C.; Chen, H.M.; Chiang, M.C.; Lai, H.L.; Chang, H.H.; Wu, Y.C.; Sun, C.N.; Chien, C.L.; Lin, Y.S.; et al. CGS21680 Attenuates Symptoms of Huntington’s Disease in a Transgenic Mouse Model. J. Neurochem. 2005, 93, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-Y.; Coelho, J.E.; Ohtsuka, N.; Canas, P.M.; Day, Y.-J.; Huang, Q.-Y.; Rebola, N.; Yu, L.; Boison, D.; Cunha, R.A.; et al. A Critical Role of the Adenosine A2A Receptor in Extrastriatal Neurons in Modulating Psychomotor Activity as Revealed by Opposite Phenotypes of Striatum and Forebrain A2A Receptor Knock-Outs. J. Neurosci. 2008, 28, 2970–2975. [Google Scholar] [CrossRef] [PubMed]

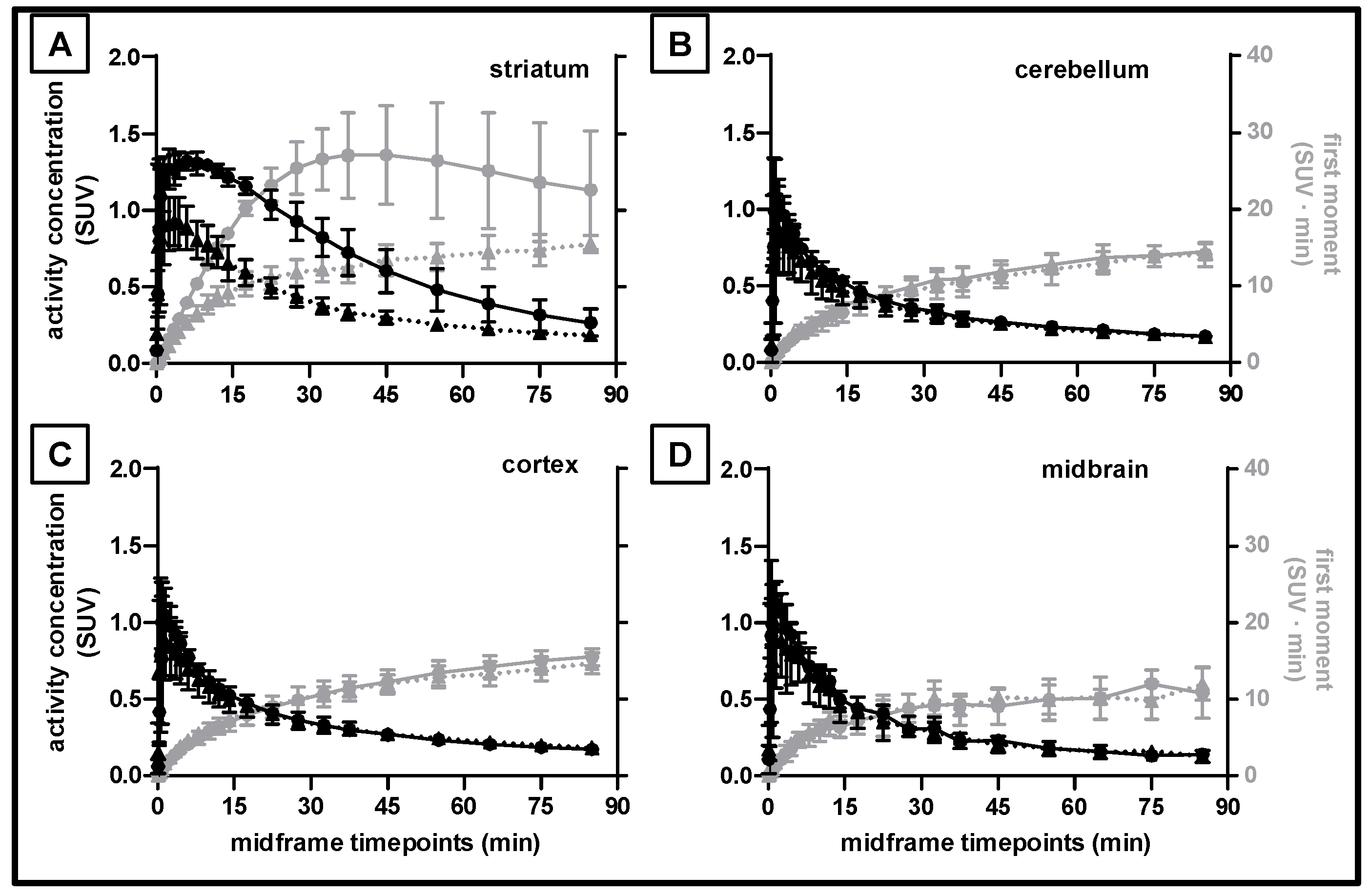

| Brain Region | Time–to–Peak (min) | TAC Peak Value (SUV) | AUC0–60 min a (SUV ∙ min) | MRT (min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Veh | Toz | p–Value | Veh | Toz | p–Value | Veh | Toz | p–Value | Veh | Toz | p–Value | |
| Striatum | 3.0 ± 0.8 | 3.6 ± 2.0 | 0.22 | 1.0 ± 0.2 | 0.8 ± 0.1 | 0.18 | 23 ± 8 | 18 ± 4 | 0.12 | 20 ± 2 | 18 ± 8 | 0.30 |
| Cerebellum | 0.9 ± 0.2 | 1.0 ± 0.3 | 0.22 | 0.7 ± 0.3 | 0.7 ± 0.1 | 0.49 | 6 ± 3 | 5 ± 1 | 0.44 | 16 ± 1 | 15 ± 1 | 0.35 |
| p–value (Striatum vs. Cerebellum) | <0.01 | 0.02 | – | 0.05 | 0.14 | – | <0.01 | <0.01 | – | <0.01 | 0.24 | – |
| Brain Region | Time–to–Peak (min) | TAC Peak Value (SUV) | AUC0–60 min (SUV ∙ min) | MRT (min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Veh | Istra | p–Value | Veh | Istra | p–Value | Veh | Istra | p–Value | Veh | Istra | p–Value | |
| Striatum | 3.0 ± 0.8 | 1.0 ± 0.3 | <0.01 | 1.0 ± 0.2 | 0.7 ± 0.2 | 0.04 | 23 ± 8 | 5 ± 2 | <0.01 | 20 ± 2 | 14 ± 0 | <0.01 |
| Cerebellum | 0.9 ± 0.2 | 0.9 ± 0.3 | 0.32 | 0.7 ± 0.3 | 0.8 ± 0.5 | 0.31 | 6 ± 3 | 6 ± 3 | 0.36 | 16 ± 1 | 17 ± 2 | 0.47 |
| p–value (Striatum vs. Cerebellum) | <0.01 | 0.50 | – | 0.05 | 0.28 | – | <0.01 | 0.21 | – | <0.01 | 0.02 | – |
| Brain Region | Time–to–Peak (min) | Peak TAC Value (SUV) | AUC0–60 min (SUV min) | MRT (min) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ctrl | Rot | Ctrl | Rot | p–Value | Ctrl | Rot | p–Value | Ctrl | Rot | p–Value | |
| Striatum | 2.3 | 2.3 | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.91 | 20 ± 3 | 19 ± 2 | 0.43 | 17 ± 1 | 17 ± 1 | 0.87 |
| Cerebellum | 0.8 | 0.8 | 0.7 ± 0.2 | 0.8 ± 0.1 | 0.45 | 6 ± 1 | 7 ± 1 | 0.19 | 17 ± 1 | 17 ± 1 | 0.59 |
| Brain Region | BPND | R2 | AIC | |||||
|---|---|---|---|---|---|---|---|---|
| Ctrl | Rot | p–Value | Ctrl | Rot | Ctrl | Rot | ||
| Striatum | T2–Atlas VOI | 2.5 ± 0.4 | 2.0 ± 0.4 | <0.001 | 0.9 ± 0.1 | 0.9 ± 0.0 | 91 ± 16 | 89 ± 11 |
| 1 mm sphere VOI | 3.5 ± 0.7 | 3.2 ± 0.5 | <0.001 | 0.8 ± 0.1 | 0.8 ± 0.1 | 121 ± 9 | 120 ± 13 | |
| Brain Region | Time–to–Peak Time (min) | TAC Peak Value (SUV) | AUC0–90 min (SUV min) | MRT (min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Veh | Toz | p–Value | Veh | Toz | p–Value | Veh | Toz | p–Value | Veh | Toz | p–Value | |
| Striatum | 5.5 ± 2.8 | 1.6 ± 1.7 | 0.05 | 1.3 ± 0.1 | 0.9 ± 0.2 | 0.03 | 61 ± 9 | 34 ± 5 | 0.01 | 31 ± 2 | 30 ± 2 | 0.38 |
| Cerebellum | 1.5 ± 0.0 | 1.0 ± 0.5 | 0.43 | 1.0 ± 0.2 | 0.9 ± 0.3 | 0.32 | 30 ± 2 | 27 ± 5 | 0.27 | 31 ± 1 | 32 ± 2 | 0.22 |
| Midbrain | 2.5 ± 1.0 | 1.3 ± 1.0 | 0.11 | 1.1 ± 0.1 | 1.0 ± 0.3 | 0.30 | 27 ± 2 | 26 ± 6 | 0.35 | 27 ± 1 | 29 ± 2 | 0.17 |
| Cortex | 1.5 ± 0.0 | 1.3 ± 1.0 | 0.40 | 1.0 ± 0.2 | 0.9 ± 0.3 | 0.29 | 30 ± 2 | 29 ± 5 | 0.42 | 30 ± 1 | 32 ± 1 | 0.07 |
| Brain Region | VT (mL · g−1) | BPND | |||
|---|---|---|---|---|---|
| Veh (n = 2) | Toz (n = 2) | Veh (n = 3) | Toz (n = 3) | p–Value | |
| Striatum | 14.6 | 8.5 | 1.32 ± 0.37 | 0.31 ± 0.17 | <0.001 |
| Cerebellum | 8.7 | 7.3 | reference | reference | – |
| Midbrain | 6.5 | 7.0 | 0.04 ± 0.08 | 0.05 ± 0.10 | 0.47 |
| Cortex | 7.84 | 7.90 | 0.08 ± 0.10 | 0.1 ± 0.04 | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gündel, D.; Toussaint, M.; Lai, T.H.; Deuther-Conrad, W.; Cumming, P.; Schröder, S.; Teodoro, R.; Moldovan, R.-P.; Pan-Montojo, F.; Sattler, B.; et al. Quantitation of the A2A Adenosine Receptor Density in the Striatum of Mice and Pigs with [18F]FLUDA by Positron Emission Tomography. Pharmaceuticals 2022, 15, 516. https://doi.org/10.3390/ph15050516
Gündel D, Toussaint M, Lai TH, Deuther-Conrad W, Cumming P, Schröder S, Teodoro R, Moldovan R-P, Pan-Montojo F, Sattler B, et al. Quantitation of the A2A Adenosine Receptor Density in the Striatum of Mice and Pigs with [18F]FLUDA by Positron Emission Tomography. Pharmaceuticals. 2022; 15(5):516. https://doi.org/10.3390/ph15050516
Chicago/Turabian StyleGündel, Daniel, Magali Toussaint, Thu Hang Lai, Winnie Deuther-Conrad, Paul Cumming, Susann Schröder, Rodrigo Teodoro, Rareş-Petru Moldovan, Francisco Pan-Montojo, Bernhard Sattler, and et al. 2022. "Quantitation of the A2A Adenosine Receptor Density in the Striatum of Mice and Pigs with [18F]FLUDA by Positron Emission Tomography" Pharmaceuticals 15, no. 5: 516. https://doi.org/10.3390/ph15050516
APA StyleGündel, D., Toussaint, M., Lai, T. H., Deuther-Conrad, W., Cumming, P., Schröder, S., Teodoro, R., Moldovan, R.-P., Pan-Montojo, F., Sattler, B., Kopka, K., Sabri, O., & Brust, P. (2022). Quantitation of the A2A Adenosine Receptor Density in the Striatum of Mice and Pigs with [18F]FLUDA by Positron Emission Tomography. Pharmaceuticals, 15(5), 516. https://doi.org/10.3390/ph15050516









