Longitudinal Consumption of Ergothioneine Reduces Oxidative Stress and Amyloid Plaques and Restores Glucose Metabolism in the 5XFAD Mouse Model of Alzheimer’s Disease
Abstract
:1. Introduction
2. Results
2.1. ERGO Is a Metal Scavenger
2.2. ERGO Is a ROS Scavenger
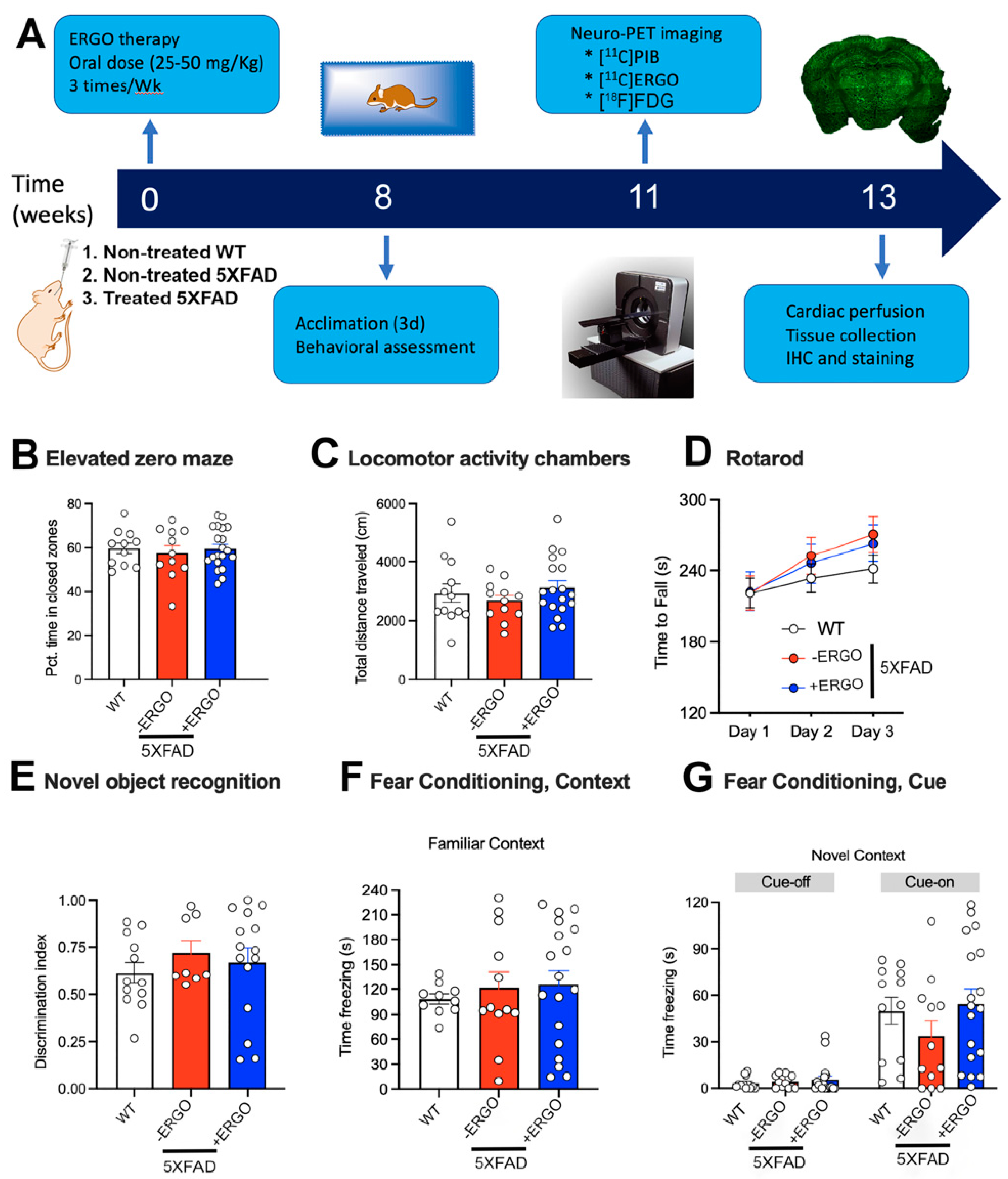
2.3. Timeline of the Therapy and Processing
2.4. ERGO Treatment Prevents Early Cognitive Deficits in 5XFAD Mice
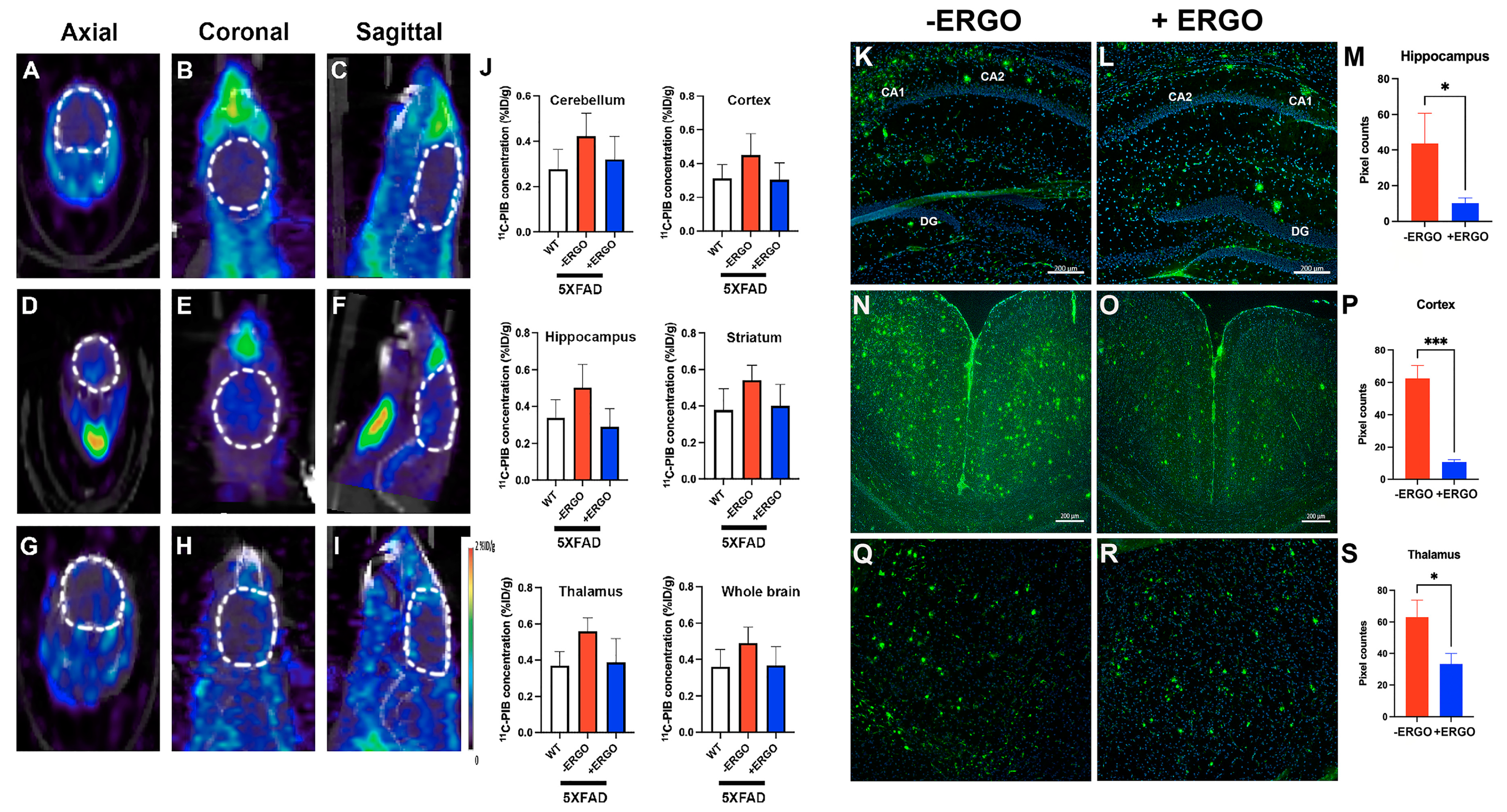
2.5. Longitudinal Consumption of ERGO Mitigates Aβ Aggregation in Young 5XFAD Mice
2.6. [11C]ERGO PET Radioligand Detects Oxidative Stress Reduction in ERGO-Treated 5XFAD Mice
2.7. ERGO Treatment Rescues Glucose Metabolism in 5XFAD Mice
3. Discussion
4. Materials and Methods
4.1. ERGO Formulation
4.2. Mass Spectrometry Analysis
4.3. ROS Measurement
4.4. Metal Scavenging Assays
4.4.1. Nickel Assay
4.4.2. Ferrous Ion Chelating (FIC) Assay
4.4.3. Cupric Ion Chelating (CIC) Assay
4.5. Animals
Gavage Treatment
4.6. Behavioral Experiments
4.6.1. Elevated Zero Maze (EZM)
4.6.2. Locomotor Activity
4.6.3. Rotarod
4.6.4. Novel Object Recognition
4.6.5. Fear Conditioning
4.7. Dynamic PET Imaging
4.8. Immunohistochemistry (IHC)
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramos-Rodriguez, J.J.; Pacheco-Herrero, M.; Thyssen, D.; Murillo-Carretero, M.I.; Berrocoso, E.; Spires-Jones, T.L.; Bacskai, B.J.; Garcia-Alloza, M. Rapid beta-amyloid deposition and cognitive impairment after cholinergic denervation in APP/PS1 mice. J. Neuropathol. Exp. Neurol. 2013, 72, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s disease. Cold Spring Harb. Perspect Biol. 2011, 3, a004457. [Google Scholar] [CrossRef]
- Gella, A.; Durany, N. Oxidative stress in Alzheimer disease. Cell Adhes. Migr. 2009, 3, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Knock, G.A.; Ward, J.P. Redox regulation of protein kinases as a modulator of vascular function. Antioxid. Redox Signal. 2011, 15, 1531–1547. [Google Scholar] [CrossRef]
- Son, Y.; Kim, S.; Chung, H.T.; Pae, H.O. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013, 528, 27–48. [Google Scholar]
- Verbon, E.H.; Post, J.A.; Boonstra, J. The influence of reactive oxygen species on cell cycle progression in mammalian cells. Gene 2012, 511, 1–6. [Google Scholar] [CrossRef]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar]
- Van Dyke, K. The possible role of peroxynitrite in Alzheimer’s disease: A simple hypothesis that could be tested more throughly. Med. Hypotheses 1997, 48, 375–380. [Google Scholar] [CrossRef]
- Xie, Z.; Wei, M.; Morgan, T.E. Peroxynitrite mediates neurotoxicity of amyloid beta-peptide-42- and lipopolysaccharide-activated microglia. J. Neurosci. 2002, 22, 3484–3492. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- Smith, M.A.; Harris, P.L.; Sayre, L.M.; Perry, G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl. Acad. Sci. USA 1997, 94, 9866–9868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohanka, M. Copper and copper nanoparticles toxicity and their impact on basic functions on the body. Bratsl. Med. 2019, 120, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Bolognin, S.; Messori, L.; Drago, D.; Gabbiani, C.; Cendron, L.; Zatta, P. Aluminum, copper, iron and zinc differentially alter amyloid-Abeta(1-42) aggregation and toxicity. Int. J. Biochem. Cell Biol. 2011, 43, 877–885. [Google Scholar] [CrossRef]
- Beelman, R.B.; Royse, D.J. Selenium enrichment of pleurotus cornucopiae rolland and grifola frondosa gray mushrooms. Int. J. Med. Mushrooms 2006, 8, 77–84. [Google Scholar] [CrossRef]
- Werner, A.R.; Beelman, R.B. Growing high-selenium edible and medicinal buttom mushrooms as ingredients for functional food or dietary supplements. Int. J. Med. Mushrooms 2002, 4, 167–171. [Google Scholar] [CrossRef]
- Kalaras, M.D.; Beelman, R.B.; Elias, R.J. Effects of postharvest pulsed UV light treatment of white button mushrooms on vitamin D2 content and quality attributes. J. Agric. Food Chem. 2012, 60, 220–225. [Google Scholar] [CrossRef]
- Kalaras, M.D.; Beelman, R.B.; Holick, M.F.; Elias, R.J. Generation of potentially bioactive ergosterol-derived products following pulsed ultraviolet light exposure of mushrooms (Agaricus bisporus). Food Chem. 2012, 135, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Kalaras, M.D.; Richie, J.P.; Calcagnotto, A.; Beelman, R.B. Mushrooms: A rich source of the antioxidants ergothioneine and glutathione. Food Chem. 2017, 233, 429–433. [Google Scholar] [CrossRef]
- Dubost, N.; Ou, B.; Beelman, R.B. Quantification of polyphenols and ergothioneine in cultivated mushrooms and correlation to total antioxidant capacity. Food Chem. 2007, 105, 727–735. [Google Scholar] [CrossRef]
- Dubost, N.J.; Beelman, R.B.; Royse, D.J. Influence of selected cultural factors and postharvest storage on ergothioneine content of common button mushroom Agaricus bisporus. Int. J. Med. Mushrooms 2007, 9, 163–176. [Google Scholar] [CrossRef]
- Borodina, I.; Kenny, L.C.; McCarthy, C.M.; Paramasivan, K.; Pretorius, E.; Roberts, T.J.; van der Hoek, S.A.; Kell, D.B. The biology of ergothioneine, an antioxidant nutraceutical. Nutr. Res. Rev. 2020, 33, 190–217. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Cheah, I.K.; Drum, C.L. Ergothioneine, an adaptive antioxidant for the protection of injured tissues? A hypothesis. Biochem. Biophys. Res. Commun. 2016, 470, 245–250. [Google Scholar] [CrossRef]
- Koh, S.S.; Ooi, S.C.; Lui, N.M.; Qiong, C.; Ho, L.T.; Cheah, I.K.; Halliwell, B.; Herr, D.R.; Ong, W.Y. Effect of Ergothioneine on 7-Ketocholesterol-Induced Endothelial Injury. Neuromol. Med. 2021, 23, 184–198. [Google Scholar] [CrossRef]
- Servillo, L.; D’Onofrio, N.; Balestrieri, M.L. Ergothioneine Antioxidant Function: From Chemistry to Cardiovascular Therapeutic Potential. J. Cardiovasc. Pharmacol. 2017, 69, 183–191. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Spencer, J.P.; Mahmood, N. Protection against oxidative damage and cell death by the natural antioxidant ergothioneine. Food Chem. Toxicol. 1999, 37, 1043–1053. [Google Scholar] [CrossRef]
- Colognato, R.; Laurenza, I.; Fontana, I.; Coppede, F.; Siciliano, G.; Coecke, S.; Aruoma, O.I.; Benzi, L.; Migliore, L. Modulation of hydrogen peroxide-induced DNA damage, MAPKs activation and cell death in PC12 by ergothioneine. Clin. Nutr. 2006, 25, 135–145. [Google Scholar] [CrossRef]
- Deiana, M.; Rosa, A.; Casu, V.; Piga, R.; Assunta Dessi, M.; Aruoma, O.I. L-ergothioneine modulates oxidative damage in the kidney and liver of rats in vivo: Studies upon the profile of polyunsaturated fatty acids. Clin. Nutr. 2004, 23, 183–193. [Google Scholar] [CrossRef]
- Markova, N.G.; Karaman-Jurukovska, N.; Dong, K.K.; Damaghi, N.; Smiles, K.A.; Yarosh, D.B. Skin cells and tissue are capable of using L-ergothioneine as an integral component of their antioxidant defense system. Free Radic. Biol. Med. 2009, 46, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Cheah, I.K.; Tang, R.M.Y. Ergothioneine—A diet-derived antioxidant with therapeutic potential. FEBS Lett. 2018, 592, 3357–3366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheah, I.K.; Feng, L.; Tang, R.M.Y.; Lim, K.H.C.; Halliwell, B. Ergothioneine levels in an elderly population decrease with age and incidence of cognitive decline; a risk factor for neurodegeneration? Biochem. Biophys. Res. Commun. 2016, 478, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Behof, W.J.; Whitmore, C.A.; Haynes, J.R.; Rosenberg, A.J.; Tantawy, M.N.; Peterson, T.E.; Harrison, F.E.; Beelman, R.B.; Pham, W. A novel antioxidant ergothioneine PET radioligand for in vivo imaging applications. Sci. Rep. 2021, 11, 18450. [Google Scholar] [CrossRef] [PubMed]
- Cheah, I.K.; Ng, L.T.; Ng, L.F.; Lam, V.Y.; Gruber, J.; Huang, C.Y.W.; Goh, F.Q.; Lim, K.H.C.; Halliwell, B. Inhibition of amyloid-induced toxicity by ergothioneine in a transgenic Caenorhabditis elegans model. FEBS Lett. 2019, 593, 2139–2150. [Google Scholar] [CrossRef]
- Song, T.Y.; Lin, H.C.; Chen, C.L.; Wu, J.H.; Liao, J.W.; Hu, M.L. Ergothioneine and melatonin attenuate oxidative stress and protect against learning and memory deficits in C57BL/6J mice treated with D-galactose. Free Radic. Res. 2014, 48, 1049–1060. [Google Scholar] [CrossRef]
- Tucker, L.B.; McCabe, J.T. Behavior of Male and Female C57BL/6J Mice Is More Consistent with Repeated Trials in the Elevated Zero Maze than in the Elevated Plus Maze. Front. Behav. Neurosci. 2017, 11, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klunk, W.E.; Engler, H.; Nordberg, A.; Wang, Y.; Blomqvist, G.; Holt, D.P.; Bergstrom, M.; Savitcheva, I.; Huang, G.F.; Estrada, S.; et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol. 2004, 55, 306–319. [Google Scholar] [CrossRef]
- Eimer, W.A.; Vassar, R. Neuron loss in the 5XFAD mouse model of Alzheimer’s disease correlates with intraneuronal Abeta42 accumulation and Caspase-3 activation. Mol. Neurodegener. 2013, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Behof, W.J.; Whitmore, C.A.; Haynes, J.R.; Rosenberg, A.J.; Tantawy, M.N.; Peterson, T.E.; Harrison, F.E.; Beelman, R.B.; Wijesinghe, P.; Matsubara, J.A.; et al. Improved synthesis of an ergothioneine PET radioligand for imaging oxidative stress in Alzheimer’s disease. FEBS Lett. 2022; Online ahead of print. [Google Scholar]
- Calsolaro, V.; Edison, P. Alterations in Glucose Metabolism in Alzheimer’s Disease. Recent Pat. Endocr. Metab. Immune Drug Discov. 2016, 10, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, A.; Perry, G.; Aliev, G.; Hirai, K.; Takeda, A.; Balraj, E.K.; Jones, P.K.; Ghanbari, H.; Wataya, T.; Shimohama, S.; et al. Oxidative damage is the earliest event in Alzheimer Disease. J. Neuopathol. Exp. Neurol. 2001, 60, 759–767. [Google Scholar] [CrossRef] [Green Version]
- Christen, Y. Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr. 2000, 71, 621S–629S. [Google Scholar] [CrossRef]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simao, A.N.; Vissoci Reiche, E.M. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef]
- Langkammer, C.; Ropele, S.; Pirpamer, L.; Fazekas, F.; Schmidt, R. MRI for iron mapping in Alzheimer’s disease. Neurodegener. Dis. 2014, 13, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Tisdall, M.D.; Ohm, D.T.; Lobrovich, R.; Das, S.R.; Mizsei, G.; Prabhakaran, K.; Ittyerah, R.; Lim, S.; McMillan, C.T.; Wolk, D.A.; et al. Ex vivo MRI and histopathology detect novel iron-rich cortical inflammation in frontotemporal lobar degeneration with tau versus TDP-43 pathology. Neuroimage Clin. 2021, 33, 102913. [Google Scholar] [CrossRef]
- Gong, N.J.; Dibb, R.; Bulk, M.; van der Weerd, L.; Liu, C. Imaging beta amyloid aggregation and iron accumulation in Alzheimer’s disease using quantitative susceptibility mapping MRI. Neuroimage 2019, 191, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Markesbery, W.R. The role of oxidative stress in Alzheimer disease. Arch. Neurol. 1999, 56, 1449–1452. [Google Scholar] [CrossRef] [Green Version]
- Filiz, G.; Price, K.A.; Caragounis, A.; Du, T.; Crouch, P.J.; White, A.R. The role of metals in modulating metalloprotease activity in the AD brain. Eur. Biophys. J. 2008, 37, 315–321. [Google Scholar] [CrossRef]
- Rao, K.S.J.; Rao, R.V.; Shanmugavelu, P.; Menon, R.B. Trace elements in Alzheimer’s disease brain: A new hypothesis. Alz Rep. 1999, 2, 241–246. [Google Scholar]
- Campbell, A.; Smith, M.A.; Sayre, L.M.; Bondy, S.C.; Perry, G. Mechanisms by which metals promote events connected to neurodegenerative diseases. Brain Res. Bull. 2001, 55, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Zatta, P.; Lucchini, R.; van Rensburg, S.J.; Taylor, A. The role of metals in neurodegenerative processes: Aluminum, manganese, and zinc. Brain Res. Bull. 2003, 62, 15–28. [Google Scholar] [CrossRef]
- Zecca, L.; Youdim, M.B.; Riederer, P.; Connor, J.R.; Crichton, R.R. Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 2004, 5, 863–873. [Google Scholar] [CrossRef]
- Hegde, M.L.; Bharathi, P.; Suram, A.; Venugopal, C.; Jagannathan, R.; Poddar, P.; Srinivas, P.; Sambamurti, K.; Rao, K.J.; Scancar, J.; et al. Challenges associated with metal chelation therapy in Alzheimer’s disease. J. Alzheimers Dis. 2009, 17, 457–468. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Atwood, C.S.; Moir, R.D.; Hartshorn, M.A.; Vonsattel, J.P.; Tanzi, R.E.; Bush, A.I. Zinc-induced Alzheimer’s Abeta1-40 aggregation is mediated by conformational factors. J. Biol. Chem. 1997, 272, 26464–26470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibach, B.; Haen, E.; Marienhagen, J.; Hajak, G. Clioquinol treatment in familiar early onset of Alzheimer’s disease: A case report. Pharmacopsychiatry 2005, 38, 178–179. [Google Scholar] [CrossRef]
- McLachlan, D.R.; Smith, W.L.; Kruck, T.P. Desferrioxamine and Alzheimer’s disease: Video home behavior assessment of clinical course and measures of brain aluminum. Ther. Drug Monit. 1993, 15, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Engelhart, M.J.; Geerlings, M.I.; Ruitenberg, A.; van Swieten, J.C.; Hofman, A.; Witteman, J.C.; Breteler, M.M. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA 2002, 287, 3223–3229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sano, M.; Ernesto, C.; Thomas, R.G.; Klauber, M.R.; Schafer, K.; Grundman, M.; Woodbury, P.; Growdon, J.; Cotman, C.W.; Pfeiffer, E.; et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N. Engl. J. Med. 1997, 336, 1216–1222. [Google Scholar] [CrossRef] [Green Version]
- Lannfelt, L.; Blennow, K.; Zetterberg, H.; Batsman, S.; Ames, D.; Harrison, J.; Masters, C.L.; Targum, S.; Bush, A.I.; Murdoch, R.; et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer’s disease: A phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008, 7, 779–786. [Google Scholar] [CrossRef]
- Lee, H.P.; Zhu, X.; Casadesus, G.; Castellani, R.J.; Nunomura, A.; Smith, M.A.; Lee, H.G.; Perry, G. Antioxidant approaches for the treatment of Alzheimer’s disease. Expert Rev. Neurother. 2010, 10, 1201–1208. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Curzon, P.; Rustay, N.R.; Browman, K.E. Chapter 2: Cued and contextual fear conditioning for rodents. In Methods of Behavior Analysis in Neuroscience, 2nd ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Boca Raton, FL, USA, 2009. [Google Scholar]
- Calvo, M.S.; Mehrotra, A.; Beelman, R.B.; Nadkarni, G.; Wang, L.; Cai, W.; Goh, B.C.; Kalaras, M.D.; Uribarri, J. A Retrospective Study in Adults with Metabolic Syndrome: Diabetic Risk Factor Response to Daily Consumption of Agaricus bisporus (White Button Mushrooms). Plant Foods Hum. Nutr. 2016, 71, 245–251. [Google Scholar] [CrossRef]
- McClure, R.; Ong, H.; Janve, V.; Barton, S.; Zhu, M.; Li, B.; Dawes, M.; Jerome, W.G.; Anderson, A.; Massion, P.; et al. Aerosol Delivery of Curcumin Reduced Amyloid-beta Deposition and Improved Cognitive Performance in a Transgenic Model of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 55, 797–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, C.P.; Boyd, K.L.; Wallace, J.M. Evaluation of Mice Undergoing Serial Oral Gavage While Awake or Anesthetized. J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 805–810. [Google Scholar] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whitmore, C.A.; Haynes, J.R.; Behof, W.J.; Rosenberg, A.J.; Tantawy, M.N.; Hachey, B.C.; Wadzinski, B.E.; Spiller, B.W.; Peterson, T.E.; Paffenroth, K.C.; et al. Longitudinal Consumption of Ergothioneine Reduces Oxidative Stress and Amyloid Plaques and Restores Glucose Metabolism in the 5XFAD Mouse Model of Alzheimer’s Disease. Pharmaceuticals 2022, 15, 742. https://doi.org/10.3390/ph15060742
Whitmore CA, Haynes JR, Behof WJ, Rosenberg AJ, Tantawy MN, Hachey BC, Wadzinski BE, Spiller BW, Peterson TE, Paffenroth KC, et al. Longitudinal Consumption of Ergothioneine Reduces Oxidative Stress and Amyloid Plaques and Restores Glucose Metabolism in the 5XFAD Mouse Model of Alzheimer’s Disease. Pharmaceuticals. 2022; 15(6):742. https://doi.org/10.3390/ph15060742
Chicago/Turabian StyleWhitmore, Clayton A., Justin R. Haynes, William J. Behof, Adam J. Rosenberg, Mohammed N. Tantawy, Brian C. Hachey, Brian E. Wadzinski, Benjamin W. Spiller, Todd E. Peterson, Krista C. Paffenroth, and et al. 2022. "Longitudinal Consumption of Ergothioneine Reduces Oxidative Stress and Amyloid Plaques and Restores Glucose Metabolism in the 5XFAD Mouse Model of Alzheimer’s Disease" Pharmaceuticals 15, no. 6: 742. https://doi.org/10.3390/ph15060742
APA StyleWhitmore, C. A., Haynes, J. R., Behof, W. J., Rosenberg, A. J., Tantawy, M. N., Hachey, B. C., Wadzinski, B. E., Spiller, B. W., Peterson, T. E., Paffenroth, K. C., Harrison, F. E., Beelman, R. B., Wijesinghe, P., Matsubara, J. A., & Pham, W. (2022). Longitudinal Consumption of Ergothioneine Reduces Oxidative Stress and Amyloid Plaques and Restores Glucose Metabolism in the 5XFAD Mouse Model of Alzheimer’s Disease. Pharmaceuticals, 15(6), 742. https://doi.org/10.3390/ph15060742







