Immunomodulatory, Anticancer, and Antimicrobial Effects of Rice Bran Grown in Iraq: An In Vitro and In Vivo Study
Abstract
1. Introduction
2. Results
2.1. LC–MS Analysis of Rice Bran Extracts
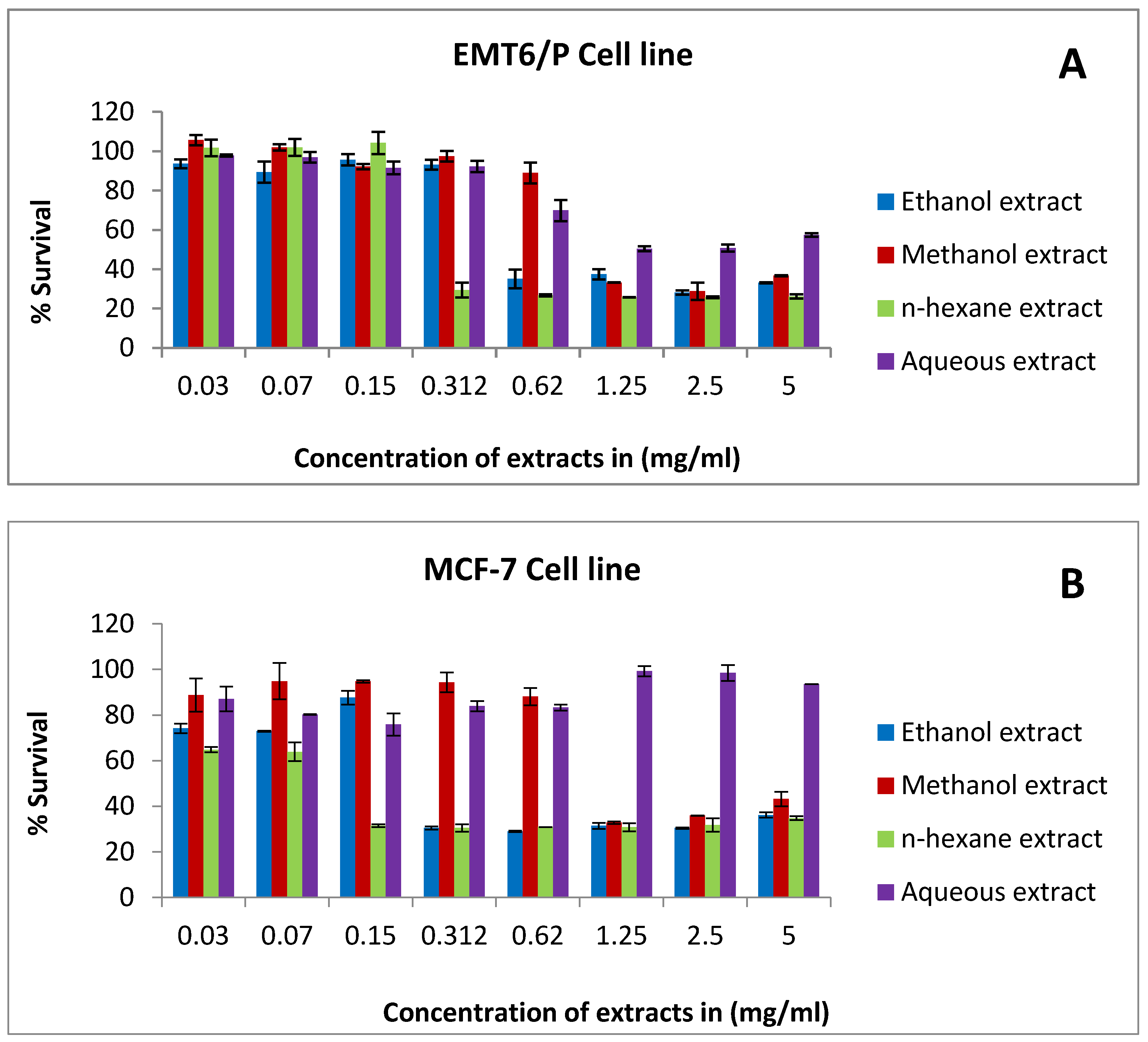
2.2. The Antiproliferative Activity of Rice Bran Extracts
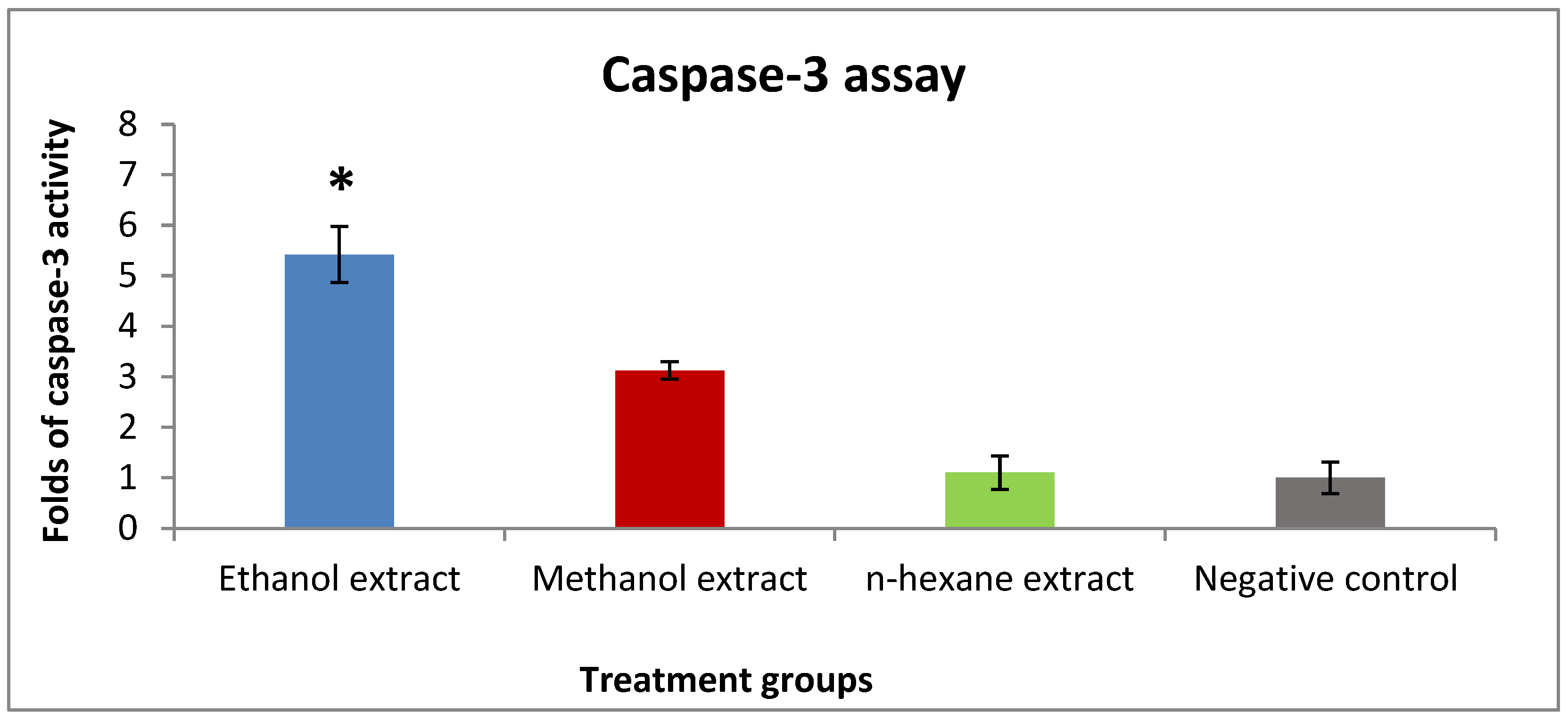
2.3. Apoptotic Activity of Rice Bran Extracts against the T47D Cell Line
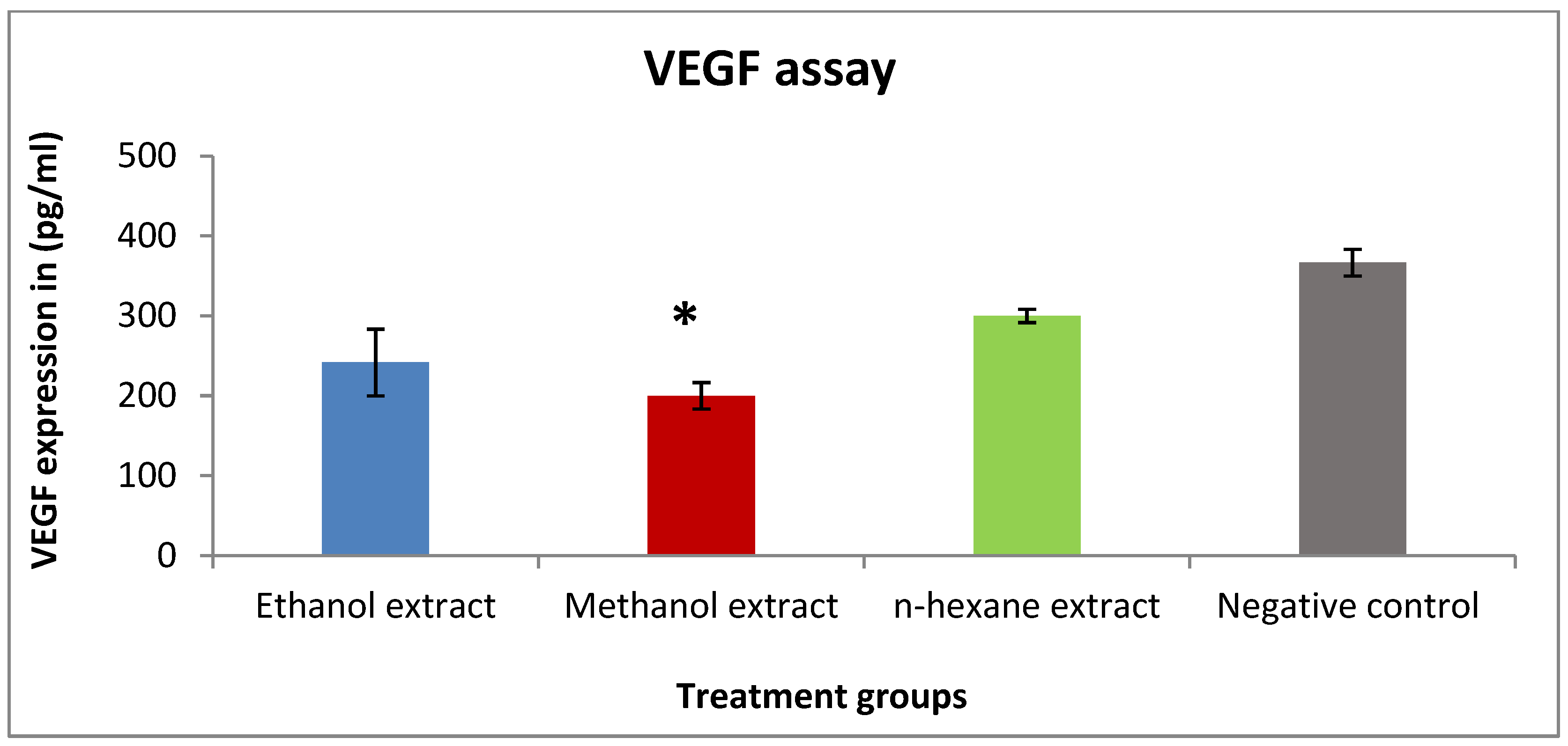
2.4. The Effect of Rice Bran Extracts on VEGF Expression in T47D Cells
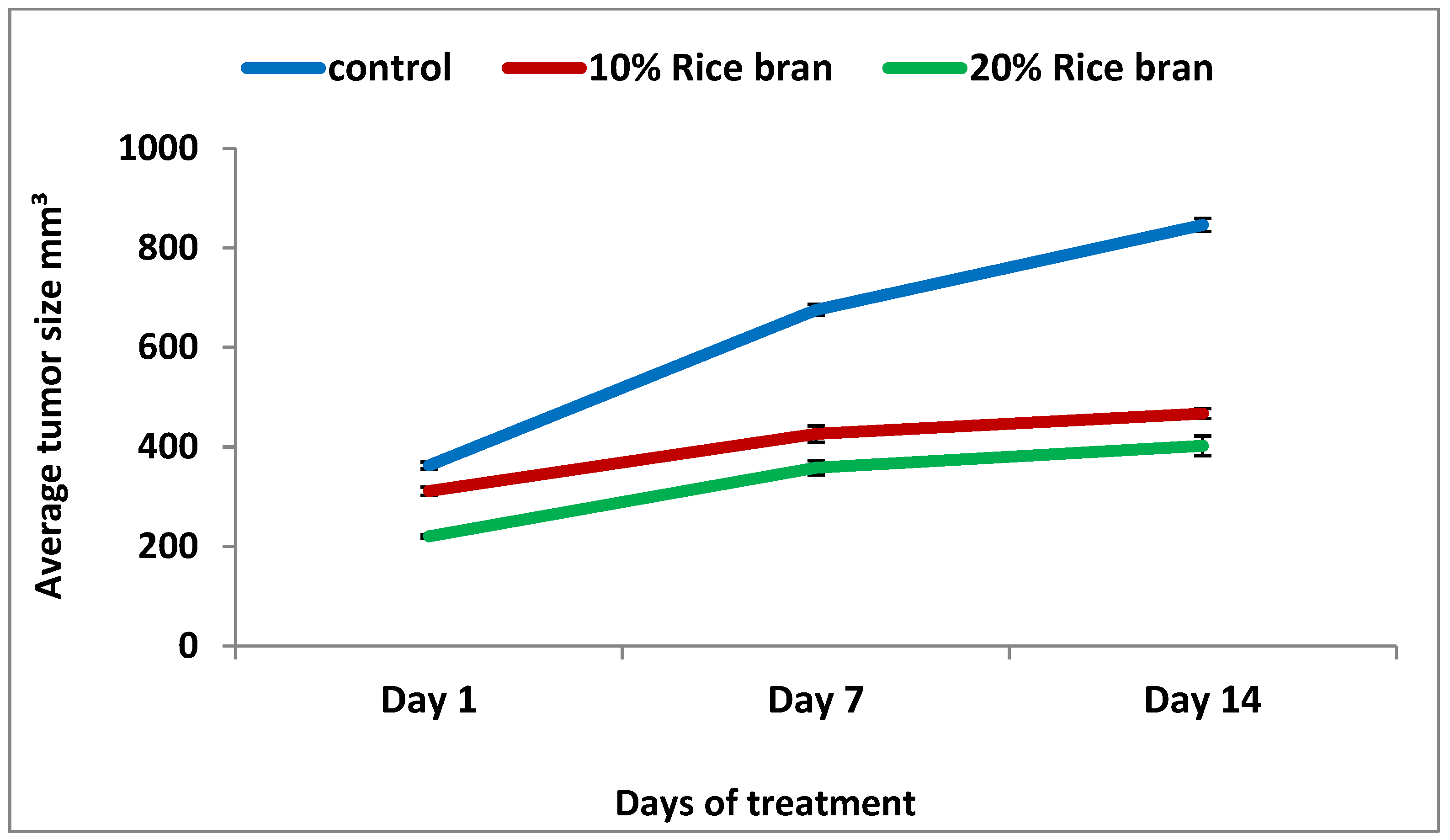
2.5. Effect of Rice Bran Consumption on Tumor Development and Growth
2.6. The Effect of Rice Bran Extracts on the Proliferation of Splenic Lymphocytes
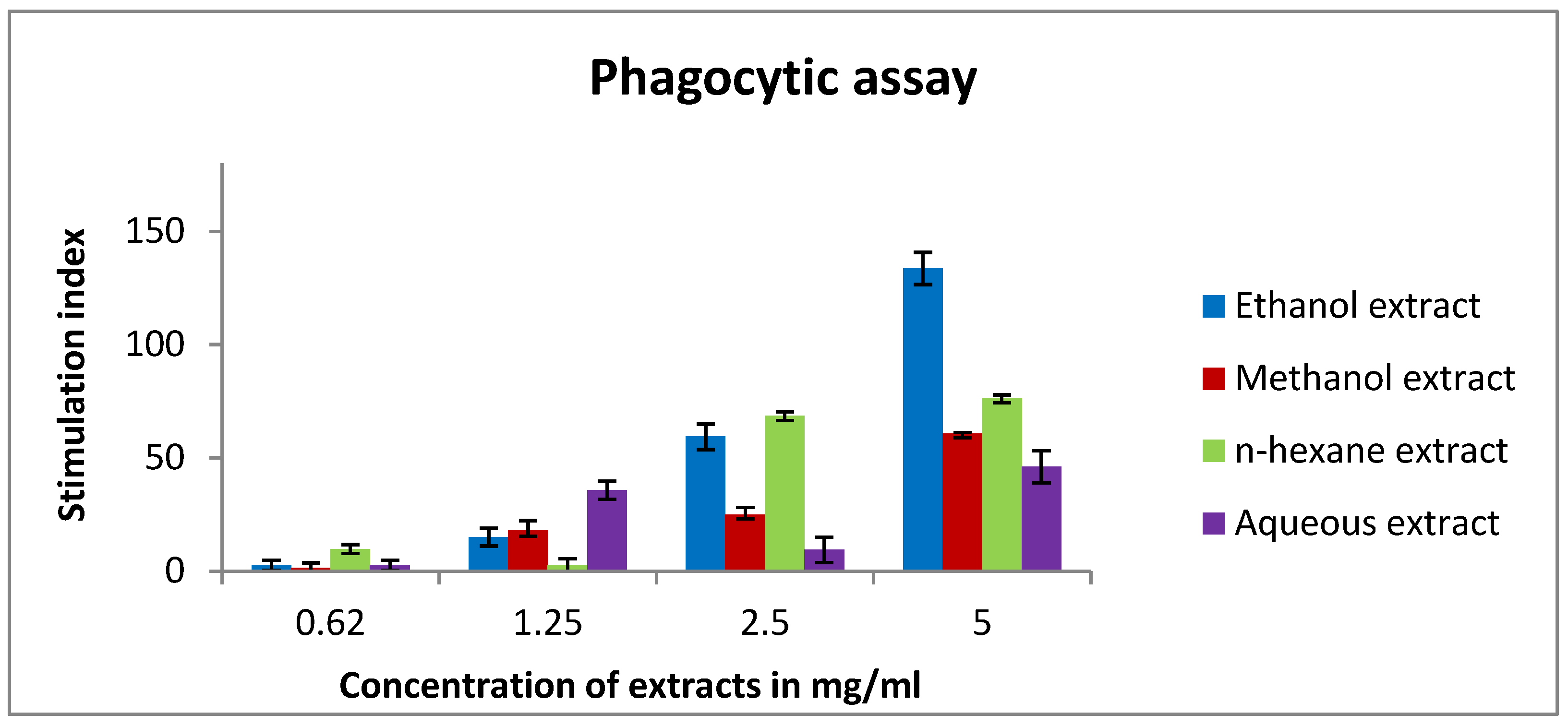
2.7. The Effect of Rice Bran Extracts on the Activity of Mouse Peritoneal Macrophages
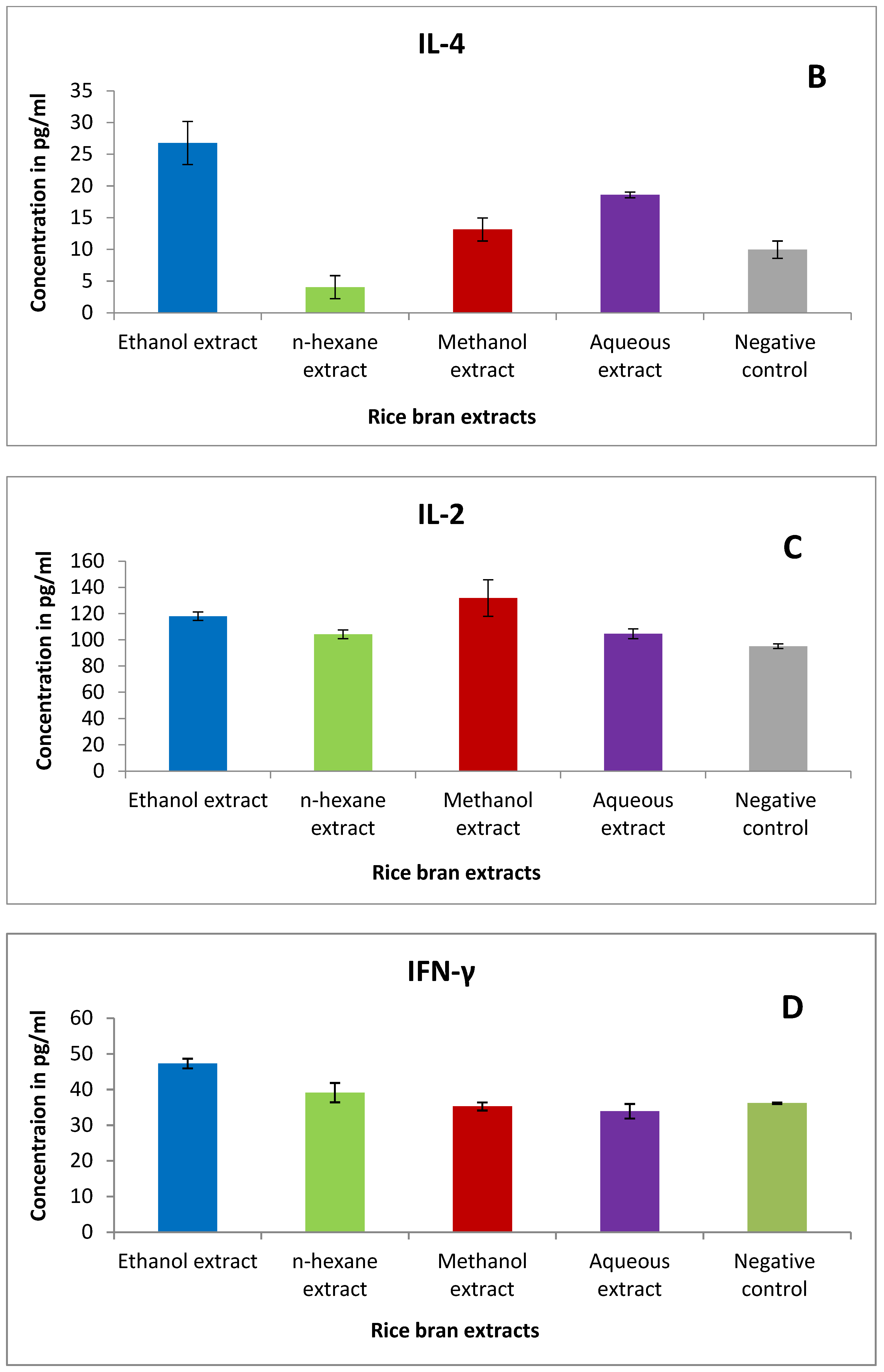
2.8. The Effect of Rice Bran Extracts on Cytokines of Murine Splenic Lymphocytes
2.9. Antibacterial Activity of Rice Bran Extracts
2.10. Antioxidant Activity of Rice Bran Extracts
3. Discussion
3.1. Anticancer Effect
3.2. Immunomodulatory Effect
3.3. Antibacterial and Antioxidant Activity
4. Materials and Methods
4.1. Rice Bran Supply and Extracts Preparation
4.2. Quantitative Analysis of Rice Bran Extracts by Liquid Chromatography–Mass Spectrometry (LC–MS)
4.3. Cell Lines and Cell Culturing Condition
4.4. Experimental Animals
4.5. MTT Cell Viability Assay
4.6. Apoptosis Assay
4.7. VEGF Assay
4.8. Preparation of the Experimental Diet for In Vivo Assay
4.9. Tumor Prophylaxis Treatment
4.10. Preparation of Murine Splenocytes
4.11. The Lymphocyte Proliferation Assay
4.12. The Lymphocyte Proliferation Assay
- In the presence of Con A or LPS
- In the absence of Con A or LPS
4.13. Isolation of Murine Peritoneal Macrophage
4.14. In Vitro Phagocytic Assay (Nitro Blue Tetrazolium (NBT) Reduction Test)
4.15. TH1/TH2 Assay
4.16. Microbial Strains
4.17. Antibacterial Assay
4.18. DPPH Radical Scavenging Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.J. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, A.M.; Rudzki, M.; Rudzki, S.; Lewandowski, T.; Laskowska, B. Environmental risk factors for cancer-review paper. Ann. Agric. Environ. Med. 2019, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Canfell, K.; Kim, J.J.; Brisson, M.; Keane, A.; Simms, K.T.; Caruana, M.; Burger, E.A.; Martin, D.; Nguyen, D.T.; Bénard, É.; et al. Mortality impact of achieving WHO cervical cancer elimination targets: A comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 2020, 395, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, J.K.; Duggan, C.; Anderson, B.O.; Etzioni, R. Early detection and treatment strategies for breast cancer in low-income and upper middle-income countries: A modelling study. Lancet Glob. Health 2018, 6, e885–e893. [Google Scholar] [CrossRef] [PubMed]
- Horton, S.; Gauvreau, C.L. Cancer in low-and middle-income countries: An economic overview. Cancer Dis. Control Priorities 2015, 3, 263–280. [Google Scholar]
- Abdel-Razeq, H.; Attiga, F.; Mansour, A. Cancer care in Jordan. Hematol. Oncol. Stem Cell Ther. 2015, 8, 64–70. [Google Scholar] [CrossRef]
- Abdel-Razeq, H.; Mansour, A.; Jaddan, D. Breast cancer care in Jordan. JCO Glob. Oncol. 2020, 6, 260–268. [Google Scholar] [CrossRef]
- Al Tamwneh, M.; Khatib, S.; Arqub, K. Cancer incidence in Jordan, 1996–2005. East Mediterr. Health J. 2010, 16, 837–845. [Google Scholar] [CrossRef]
- Mansour, A.; Al-Omari, A.; Sultan, I. Burden of cancer among Syrian refugees in Jordan. J. Glob. Oncol. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Xu, G.; McLeod, H.L. Strategies for enzyme/prodrug cancer therapy. Clin. Cancer Res. 2001, 7, 3314–3324. [Google Scholar]
- Rahman, M.S.; Suresh, S.; Waly, M.I. Risk factors for cancer: Genetic and environment. In Bioactive Components, Diet and Medical Treatment in Cancer Prevention; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–23. [Google Scholar]
- Mucci, L.A.; Hjelmborg, J.B.; Harris, J.R.; Czene, K.; Havelick, D.J.; Scheike, T.; Graff, R.E.; Holst, K.; Möller, S.; Unger, R.H.; et al. Familial risk and heritability of cancer among twins in Nordic countries. JAMA 2016, 315, 68–76. [Google Scholar] [CrossRef]
- Dai, J.; Lv, J.; Zhu, M.; Wang, Y.; Qin, N.; Ma, H.; He, Y.-Q.; Zhang, R.; Tan, W.; Fan, J.; et al. Identification of risk loci and a polygenic risk score for lung cancer: A large-scale prospective cohort study in Chinese populations. Lancet Respir. Med. 2019, 7, 881–891. [Google Scholar] [CrossRef]
- Boffetta, P.; Nyberg, F. Contribution of environmental factors to cancer risk. Br. Med. Bull. 2003, 68, 71–94. [Google Scholar] [CrossRef]
- Sung, B.; Prasad, S.; Yadav, V.R.; Lavasanifar, A.; Aggarwal, B.B. Cancer and diet: How are they related? Free Radic. Res. 2011, 45, 864–879. [Google Scholar] [CrossRef]
- English, D.; Armstrong, B.; Kricker, A.; Fleming, C. Sunlight and cancer. Cancer Causes Control 1997, 8, 271–283. [Google Scholar] [CrossRef]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health benefits of the Mediterranean diet: Metabolic and molecular mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef]
- Shin, H.-Y.; Kim, S.-M.; Lee, J.H.; Lim, S.-T. Solid-state fermentation of black rice bran with Aspergillus awamori and Aspergillus oryzae: Effects on phenolic acid composition and antioxidant activity of bran extracts. Food Chem. 2019, 272, 235–241. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Ausman, L.M.; Carrasco, W.; Gualtieri, L.J.; Jenner, J.L.; Ordovas, J.M.; Nicolosi, R.J.; Goldin, B.R.; Schaefer, E.J. Rice bran oil consumption and plasma lipid levels in moderately hypercholesterolemic humans. Arter. Thromb. 1994, 14, 549–556. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Sami, S.A.; Khan, F.A. Effects of stabilized rice bran, its soluble and fiber fractions on blood glucose levels and serum lipid parameters in humans with diabetes mellitus Types I and II. J. Nutr. Biochem. 2002, 1, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, J.; Wang, J.; Sun, B. The anti-cancer activity and potential clinical application of rice bran extracts and fermentation products. RSC Adv. 2019, 9, 18060–18069. [Google Scholar] [CrossRef]
- Kannan, A.; Hettiarachchy, N.S.; Lay, J.O.; Liyanage, R.J.P. Human cancer cell proliferation inhibition by a pentapeptide isolated and characterized from rice bran. Peptides 2010, 31, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, M. Enhancement of human natural killer cell activity by modified arabinoxylane from rice bran (MGN-3). Int. J. Immunother. 1998, 14, 89–99. [Google Scholar]
- Ryan, E.P. Bioactive food components and health properties of rice bran. J. Am. Vet. Med. Assoc. 2011, 238, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Zarei, I.; Brown, D.G.; Nealon, N.J.; Ryan, E.P. Rice bran metabolome contains amino acids, vitamins & cofactors, and phytochemicals with medicinal and nutritional properties. Rice 2017, 10, 24. [Google Scholar]
- Zarei, I.; Luna, E.; Leach, J.E.; McClung, A.; Vilchez, S.; Koita, O.; Ryan, E.P. Comparative rice bran metabolomics across diverse cultivars and functional rice gene–bran metabolite relationships. Metabolites 2018, 8, 63. [Google Scholar] [CrossRef]
- Al Azzawi, T.N.I.; Khan, M.; Hussain, A.; Shahid, M.; Imran, Q.M.; Mun, B.-G.; Lee, S.-U.; Yun, B.-W. Evaluation of Iraqi rice cultivars for their tolerance to drought stress. Agronomy 2020, 10, 1782. [Google Scholar] [CrossRef]
- Knecht, K.; Kinder, D.; Stockert, A. Biologically-based complementary and alternative medicine (CAM) use in cancer patients: The good, the bad, the misunderstood. Front. Nutr. 2020, 6, 196. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Adhami, V.M.; Saleem, M.; Mukhtar, H. Beneficial effects of tea and its polyphenols against prostate cancer. Mol. Nutr. Food Res. 2006, 50, 130–143. [Google Scholar] [CrossRef]
- Dy, G.K.; Bekele, L.; Hanson, L.J.; Furth, A.; Mandrekar, S.; Sloan, J.A.; Adjei, A.A. Complementary and alternative medicine use by patients enrolled onto phase I clinical trials. J. Clin. Oncol. 2004, 22, 4810–4815. [Google Scholar] [CrossRef]
- Kappauf, H.; Leykauf-Ammon, D.; Bruntsch, U.; Horneber, M.; Kaiser, G.; Büschel, G.; Gallmeier, W.M. Use of and attitudes held towards unconventional medicine by patients in a department of internal medicine/oncology and haematology. Support Care Cancer 2000, 8, 314–322. [Google Scholar] [CrossRef]
- Moko, E.M.; Purnomo, H.; Kusnadi, J.; Ijong, F.G. Phytochemical content and antioxidant properties of colored and non colored varieties of rice bran from Minahasa, North Sulawesi, Indonesia. Int. Food Res. J. 2014, 21, 1017. [Google Scholar]
- Oki, T.; Masuda, M.; Furuta, S.; Nishiba, Y.; Terahara, N.; Suda, I. Involvement of anthocyanins and other phenolic compounds in radical-scavenging activity of purple-fleshed sweet potato cultivars. Food Sci. 2002, 67, 1752–1756. [Google Scholar] [CrossRef]
- He, Z.; Li, B.; Rankin, G.O.; Rojanasakul, Y.; Chen, Y.C. Selecting bioactive phenolic compounds as potential agents to inhibit proliferation and VEGF expression in human ovarian cancer cells. Oncol. Lett. 2015, 9, 1444–1450. [Google Scholar] [CrossRef]
- Ahmed, S.I.; Hayat, M.Q.; Tahir, M.; Mansoor, Q.; Ismail, M.; Keck, K.; Bates, R.B. Pharmacologically active flavonoids from the anticancer, antioxidant and antimicrobial extracts of Cassia angustifolia Vahl. BMC Complement. Altern. Med. 2016, 16, 460. [Google Scholar] [CrossRef]
- Brusselmans, K.; Vrolix, R.; Verhoeven, G.; Swinnen, J.V. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J. Biol. Chem. 2005, 280, 5636–5645. [Google Scholar] [CrossRef]
- Brusselmans, K.; De Schrijver, E.; Heyns, W.; Verhoeven, G.; Swinnen, J.V. Epigallocatechin-3-gallate is a potent natural inhibitor of fatty acid synthase in intact cells and selectively induces apoptosis in prostate cancer cells. Int. J. Cancer 2003, 106, 856–862. [Google Scholar] [CrossRef]
- Mishra, A.; Sharma, A.K.; Kumar, S.; Saxena, A.K.; Pandey, A.K. Bauhinia variegata leaf extracts exhibit considerable antibacterial, antioxidant, and anticancer activities. BioMed Res. Int. 2013, 2013, 15436. [Google Scholar] [CrossRef]
- Lindenmeyer, F.; Li, H.; Menashi, S.; Soria, C.; Lu, H. Apigenin acts on the tumor cell invasion process and regulates protease production. Nutr. Cancer 2001, 39, 139–147. [Google Scholar] [CrossRef]
- Li, Y.-L.; Gan, G.-P.; Zhang, H.-Z.; Wu, H.-Z.; Li, C.-L.; Huang, Y.-P.; Liu, Y.-W.; Liu, J.-W. A flavonoid glycoside isolated from Smilax china L. rhizome in vitro anticancer effects on human cancer cell lines. J. Ethnopharmacol. 2007, 113, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.R.; Sharma, S.; Mandal, S.; Goswami, A.; Mukhopadhyay, S.; Majumder, H.K. Luteolin, an emerging anti-cancer flavonoid, poisons eukaryotic DNA topoisomerase I. Biochem. J. 2002, 366, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Jiang, B.-H.; King, S.M.; Chen, Y.C. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr. Cancer 2008, 60, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.P.; Guijas, C.; Astudillo, A.M.; Rubio, J.M.; Balboa, M.A.; Balsinde, J. Sequestration of 9-hydroxystearic acid in FAHFA (fatty acid esters of hydroxy fatty acids) as a protective mechanism for colon carcinoma cells to avoid apoptotic cell death. Cancers 2019, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Page, Y.L.; Percevault, F.; Ferriere, F.; Flouriot, G.; Pakdel, F. Apigenin, a partial antagonist of the estrogen receptor (ER), inhibits ER-positive breast cancer cell proliferation through Akt/FOXM1 signaling. Int. J. Mol. Sci. 2021, 22, 470. [Google Scholar] [CrossRef]
- Maggioni, D.; Garavello, W.; Rigolio, R.; Pignataro, L.; Gaini, R.; Nicolini, G. Apigenin impairs oral squamous cell carcinoma growth in vitro inducing cell cycle arrest and apoptosis. Int. J. Oncol. 2013, 43, 1675–1682. [Google Scholar] [CrossRef]
- Kamiyama, M.; Shibamoto, T. Flavonoids with potent antioxidant activity found in young green barley leaves. J. Agric. Food Chem. 2012, 60, 6260–6267. [Google Scholar] [CrossRef]
- Hoai, T.T.; Yen, P.T.; Dao, T.T.B.; Long, L.H.; Anh, D.X.; Minh, L.H.; Anh, B.Q.; Thuong, N.T. Evaluation of the cytotoxic effect of rutin prenanoemulsion in lung and colon cancer cell lines. J. Nanomater. 2020, 2020, 8867669. [Google Scholar] [CrossRef]
- Maeda, J.; Roybal, E.J.; Brents, C.A.; Uesaka, M.; Aizawa, Y.; Kato, T.A. Natural and glucosyl flavonoids inhibit poly (ADP-ribose) polymerase activity and induce synthetic lethality in BRCA mutant cells. Oncol. Rep. 2014, 31, 551–556. [Google Scholar] [CrossRef]
- Khan, F.; Pandey, P.; Upadhyay, T.K.; Jafri, A.; Jha, N.K.; Mishra, R.; Singh, V. Anti-cancerous effect of rutin against HPV-C33A cervical cancer cells via G0/G1 cell cycle arrest and apoptotic induction. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 409–418. [Google Scholar] [CrossRef]
- Guon, T.E.; Chung, H.S. Hyperoside and rutin of Nelumbo nucifera induce mitochondrial apoptosis through a caspase-dependent mechanism in HT-29 human colon cancer cells. Oncol. Lett. 2016, 11, 2463–2470. [Google Scholar] [CrossRef]
- Rana, P.; Vadhera, S.; Soni, G. In vivo antioxidant potential of rice bran oil (RBO) in albino rats. Indian J. Physiol. Pharmacol. 2004, 48, 428–436. [Google Scholar]
- Badr El-Din, N.K.; Noaman, E.; Ghoneum, M. In vivo tumor inhibitory effects of nutritional rice bran supplement MGN-3/Biobran on Ehrlich carcinoma-bearing mice. Nutr. Cancer 2008, 60, 235–244. [Google Scholar] [CrossRef]
- Choi, S.P.; Kim, S.P.; Nam, S.H.; Friedman, M. Antitumor effects of dietary black and brown rice brans in tumor-bearing mice: Relationship to composition. Mol. Nutr. Food Res. 2013, 57, 390–400. [Google Scholar] [CrossRef]
- Kaur, A.; Fang, C.-M. An overview of the human immune system and the role of interferon regulatory factors (IRFs). Prog. Microbes Mol. Biol. 2020, 3, a0000129. [Google Scholar] [CrossRef]
- Smith, N.C.; Rise, M.L.; Christian, S.L. A comparison of the innate and adaptive immune systems in cartilaginous fish, ray-finned fish, and lobe-finned fish. Front. Immunol. 2019, 10, 2292. [Google Scholar] [CrossRef]
- da Costa, T.A.; Lang, J.; Torres, R.M.; Pelanda, R. The development of human immune system mice and their use to study tolerance and autoimmunity. J. Transl. Autoimmun. 2019, 2, 100021. [Google Scholar] [CrossRef]
- Moslehi, M.; Moazamiyanfar, R.; Dakkali, M.S.; Rezaei, S.; Rastegar-Pouyani, N.; Jafarzadeh, E.; Mouludi, K.; Khodamoradi, E.; Taeb, S.; Najafi, M. Modulation of the immune system by melatonin; implications for cancer therapy. Int. Immunopharmacol. 2022, 108, 108890. [Google Scholar] [CrossRef]
- Xu, H.-S.; Wu, Y.-W.; Xu, S.-F.; Sun, H.-X.; Chen, F.-Y.; Yao, L. Antitumor and immunomodulatory activity of polysaccharides from the roots of Actinidia eriantha. J. Ethnopharmacol. 2009, 125, 310–317. [Google Scholar] [CrossRef]
- Mitchell, S.; Roy, K.; Zangle, T.A.; Hoffmann, A. Nongenetic origins of cell-to-cell variability in B lymphocyte proliferation. Proc. Natl. Acad. Sci. USA 2018, 115, E2888–E2897. [Google Scholar] [CrossRef]
- Thitilertdecha, P.; Tantithavorn, V.; Poungpairoj, P.; Onlamoon, N. Determination of suppressive effect on human T-cell activation by hispidulin, nepetin, and vanillic acid. Immunopharmacol. Immunotoxicol. 2019, 41, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Mudgal, J.; Mallik, S.B.; Nampoothiri, M.; Kinra, M.; Hall, S.; Grant, G.D.; Anoopkumar-Dukie, S.; Davey, A.K.; Rao, C.M.; Arora, D. Effect of coffee constituents, caffeine and caffeic acid on anxiety and lipopolysaccharide-induced sickness behavior in mice. J. Funct. Foods 2020, 64, 103638. [Google Scholar] [CrossRef]
- Cortés-Ferre, H.E.; Martínez-Avila, M.; Antunes-Ricardo, M.; Guerrero-Analco, J.A.; Monribot-Villanueva, J.L.; Gutierrez-Uribe, J.A. In vitro evaluation of anti-inflammatory activity of “Habanero” chili pepper (Capsicum chinense) seeds extracts pretreated with cellulase. Plant Foods Hum. Nutr. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Gao, Y.; Ji, M.; Yang, Y.; Wang, Z.; Wang, B.; Jin, J.; Li, L.; Wang, H.; Xu, X.; et al. Oral SMEDDS promotes lymphatic transport and mesenteric lymph nodes target of chlorogenic acid for effective T-cell antitumor immunity. J. Immunother. Cancer 2021, 9, 002753. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.-W.; Lin, B.-F. Bioactivities of major constituents isolated from Angelica sinensis (Danggui). Chin. Med. 2011, 6, 29. [Google Scholar] [CrossRef]
- Kilani-Jaziri, S.; Mokdad-Bzeouich, I.; Krifa, M.; Nasr, N.; Ghedira, K.; Chekir-Ghedira, L. Immunomodulatory and cellular anti-oxidant activities of caffeic, ferulic, and p-coumaric phenolic acids: A structure–activity relationship study. Drug Chem. Toxicology 2017, 40, 416–424. [Google Scholar] [CrossRef]
- Srinivasan, A.; Ekambaram, S.P.; Perumal, S.S.; Aruldhas, J.; Erusappan, T. Chemical characterization and immunostimulatory activity of phenolic acid bound arabinoxylans derived from foxtail and barnyard millets. J. Food Biochem. 2020, 44, e13116. [Google Scholar] [CrossRef]
- Rathor, R.; Meena, D.; Shyam, R.; Misra, K. Immunostimulatory Activity Investigation of Aqueous and Hydroethanolic Extract of Wheatgrass Using THP1 Cells. MOJ Immunol. 2017, 5, 00146. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, B.; Kaur, A.; Yadav, M.P.; Singh, N.; Ahlawat, A.K.; Singh, A.M. Effect of growing conditions on proximate, mineral, amino acid, phenolic composition and antioxidant properties of wheatgrass from different wheat (Triticum aestivum L.) varieties. Food Chem. 2021, 341, 128201. [Google Scholar] [CrossRef]
- Constant, S.L.; Bottomly, K. Induction of Th1 and Th2 CD4plus T cell responses: The alternative approaches. Annu. Rev. Immunol. 1997, 15, 297. [Google Scholar] [CrossRef]
- Berger, A. Th1 and Th2 responses: What are they? BMJ 2000, 321, 424. [Google Scholar] [CrossRef]
- Borish, L.; Rosenwasser, L. T H 1/T H 2 lymphocytes: Doubt some more. J. Allergy Clin. Immunol. 1997, 99, 161–164. [Google Scholar] [CrossRef]
- Kidd, P. Th1/Th2 balance: The hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 2003, 8, 223–246. [Google Scholar]
- Braun, C.M.; Huang, S.-K.; Bashian, G.G.; Kagey-Sobotka, A.; Lichtenstein, L.M.; Essayan, D.M. Corticosteroid modulation of human, antigen-specific Th1 and Th2 responses. J. Allergy Clin. Immunol. 1997, 100, 400–407. [Google Scholar] [CrossRef]
- Satyam, A.; Singh, P.; Badjatia, N.; Seth, A.; Sharma, A. A disproportion of TH1/TH2 cytokines with predominance of TH2, in urothelial carcinoma of bladder. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2011; pp. 58–65. [Google Scholar]
- Sato, M.; Goto, S.; Kaneko, R.; Ito, M.; Sato, S.; Takeuchi, S. Impaired production of Th1 cytokines and increased frequency of Th2 subsets in PBMC from advanced cancer patients. Anticancer. Res. 1998, 18, 3951–3955. [Google Scholar]
- Mohammadi, M.; Soltani, M.; Siahpoosh, A.; Shamsaie, M. Effects of dietary supplementation of date palm (Phoenix dactylifera) seed extract on body composition, lipid peroxidation and tissue quality of common carp (Cyprinus carpio) juveniles based on the total volatile nitrogen test. Iran. J. Fish. Sci. 2018, 17, 394–402. [Google Scholar]
- Al Harthi, S.; Mavazhe, A.; Al Mahroqi, H.; Khan, S.A. Quantification of phenolic compounds, evaluation of physicochemical properties and antioxidant activity of four date (Phoenix dactylifera L.) varieties of Oman. J. Taibah Univ. Med. Sci. 2015, 10, 346–352. [Google Scholar] [CrossRef]
- Dardjito, E.; Proverawati, A.; Sumeru, A.; Setiyani, R.; Upoyo, A.; Kamaludin, R. Date seeds (Phoenix dactylifera L.) consumption as anti-inflammatory and immunostimulant: A systematic review. IOP Conf. Ser. Earth Environ. Sci. 2019, 850, 012038. [Google Scholar]
- Yasuma, T.; Toda, M.; Abdel-Hamid, A.M.; D’Alessandro-Gabazza, C.; Kobayashi, T.; Nishihama, K.; D’alessandro, V.F.; Pereira, G.V.; Mackie, R.I.; Gabazza, E.C. Degradation Products of Complex Arabinoxylans by Bacteroides intestinalis Enhance the Host Immune Response. Microorganisms 2021, 9, 1126. [Google Scholar] [CrossRef]
- He, M.; Wu, T.; Pan, S.; Xu, X. Antimicrobial mechanism of flavonoids against Escherichia coli ATCC 25922 by model membrane study. Appl. Surf. Sci. 2014, 305, 515–521. [Google Scholar] [CrossRef]
- Lima, V.N.; Oliveira-Tintino, C.D.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.; Cruz, R.P.; Menezes, I.R. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Orhan, D.D.; Özçelik, B.; Özgen, S.; Ergun, F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010, 165, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Karbalaii, M.T.; Jaafar, H.Z.E.; Rahmat, A. Phytochemical constituents, antioxidant activity, and antiproliferative properties of black, red, and brown rice bran. Chem. Cent. J. 2018, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Chatha, S.A.S.; Anwar, F.; Manzoor, M. Evaluation of the antioxidant activity of rice bran extracts using different antioxidant assays. Grasas Y Aceites 2006, 57, 328–335. [Google Scholar]
- Arab, F.; Alemzadeh, I.; Maghsoudi, V. Determination of antioxidant component and activity of rice bran extract. Sci. Iran. 2011, 18, 1402–1406. [Google Scholar] [CrossRef]
- Moongngarm, A.; Daomukda, N.; Khumpika, S. Chemical Compositions, Phytochemicals, and Antioxidant Capacity of Rice Bran, Rice Bran Layer, and Rice Germ. APCBEE Procedia 2012, 2, 73–79. [Google Scholar] [CrossRef]
- Rainard, P. A colorimetric microassay for opsonins by reduction of NBT in phagocytosing bovine polymorphs. J. Immunol. Methods 1986, 90, 197–201. [Google Scholar] [CrossRef]
- Boothapandi, M.; Ramanibai, R. Immunomodulatory activity of Indigofera tinctoria leaf extract on in vitro macrophage responses and lymphocyte proliferation. Int. J. Pharm. Pharm. Sci. 2016, 8, 58–63. [Google Scholar]
- Talib, W.H.; Zarga, M.H.A.; Mahasneh, A.M. Antiproliferative, antimicrobial and apoptosis inducing effects of compounds isolated from Inula viscosa. Molecules 2012, 17, 3291–3303. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]






| NO | Compounds | Formula | RT (Retention Time) | Relative % (Aqueous Extract) | Relative % (Methanol Extract) | Relative % (n-Hexane Extract) | Relative % (Ethanol Extract) |
|---|---|---|---|---|---|---|---|
| 1 | Succinic acid | C4H6O4 | 0.98 | 70.254 | 2.805 | 0.007 | 0.966 |
| 2 | Gallic acid | C7H6O5 | 1.04 | 0 | 0.029 | 0 | 0.023 |
| 3 | Protocatechuic aldehyde | C7H6O3 | 2.09 | 0.029 | 0.386 | 0 | 0.208 |
| 4 | Vanillic acid | C8H8O4 | 2.62 | 1.318 | 1.414 | 0 | 0.733 |
| 5 | Chlorogenic acid | C16H18O9 | 2.88 | 0 | 0.008 | 0 | 0 |
| 6 | Vanillic acid | C8H8O4 | 3.2 | 0.163 | 0.784 | 0 | 0.367 |
| 7 | Caffeic acid | C9H8O4 | 3.27 | 0.150 | 2.873 | 0 | 1.533 |
| 8 | Anthranilic acid | C7H7NO2 | 3.88 | 0 | 0 | 0 | 0 |
| 9 | Anthranilic acid | C7H7NO2 | 4.07 | 0 | 1.120 | 0 | 3.155 |
| 10 | p-Coumaric acid | C9H8O3 | 4.44 | 0 | 5.307 | 0.001 | 1.841 |
| 11 | Ferulic acid (trans) | C10H10O4 | 4.55 | 0.183 | 0.266 | 0 | 0.075 |
| 12 | Anthranilic acid | C7H7NO2 | 4.56 | 0 | 3.866 | 0 | 0 |
| 13 | 3,5-Dimethoxy-4-hydroxyacetophenone | C10H12O4 | 4.87 | 5.934 | 6.771 | 0.017 | 2.524 |
| 14 | Apiin | C26H28O14 | 5.11 | 10.173 | 31.189 | 0 | 15.968 |
| 15 | Ferulic acid (trans) | C10H10O4 | 5.13 | 4.024 | 13.796 | 0.022 | 4.860 |
| 16 | Salicylic acid | C7H6O3 | 5.13 | 0.203 | 0.918 | 0.013 | 0.292 |
| 17 | 2,4-Dihydroxyacetophenone | C8H8O3 | 5.29 | 0 | 0.349 | 0 | 0.167 |
| 18 | Saponarin | C27H30O15 | 5.48 | 1.149 | 4.231 | 0.001 | 2.024 |
| 19 | Rutin | C27H30O16 | 5.58 | 0.074 | 3.852 | 0 | 2.193 |
| 20 | 3,5-Dimethoxy-4-hydroxyacetophenone | C10H12O4 | 5.63 | 0.066 | 0.017 | 0 | 0.036 |
| 21 | 3-Rha-7-Rha quercetin (NMR) | C27H30O15 | 5.77 | 0.016 | 0.445 | 0 | 0.120 |
| 22 | Salicylic acid | C7H6O3 | 5.78 | 1.832 | 1.762 | 0.001 | 0.676 |
| 23 | Spiraeoside | C21H20O12 | 5.78 | 0 | 1.011 | 0 | 0.563 |
| 24 | 3-O-Methyl quercetin | C16H12O7 | 8.8 | 0 | 0.263 | 0 | 0.201 |
| 25 | Apigenin | C15H10O5 | 9.92 | 0.462 | 0.803 | 0 | 0.545 |
| 26 | Kaempferol | C15H10O6 | 10.13 | 2.738 | 0.382 | 0 | 0.164 |
| 27 | 5,6,4′-Trihydroxy-7,3′-dimethoxyflavone | C17H14O7 | 10.34 | 0.764 | 8.002 | 0 | 5.173 |
| 28 | ISOHAMNETIN | C16H12O7 | 10.51 | 0.339 | 0.452 | 0 | 0.067 |
| 29 | FAHFA 26:2; FAHFA 18:2/8:0; [M-H]- | C26H46O4 | 29.53 | 0.075 | 2.943 | 49.380 | 29.837 |
| 30 | FAHFA 27:2; FAHFA 18:2/9:0; [M-H]- | C27H48O4 | 29.99 | 0.045 | 3.938 | 50.555 | 25.676 |
| Cell Line | T47D | MCF-7 | MDA-MB-231 | EMT6/P | Fibroblast |
|---|---|---|---|---|---|
| Rice bran extracts | IC50 mg/mL ±SEM | IC50 mg/mL ±SEM | IC50 mg/mL ±SEM | IC50 mg/mL ±SEM | IC50 mg/mL ±SEM |
| Ethanol extract | 0.37 ± 0.01 | 0.36 ± 0.01 | 0.64 ± 0.09 | 0.71 ± 0.15 | >5 mg |
| Methanol extract | 1.01 ± 0.29 | 1.29 ± 0.04 | 2.13 ± 0.14 | 1.19 ± 0.09 | >5 mg |
| n-hexane extract | 0.33 ± 0.01 | 0.12 ± 0.07 | 0.51 ± 0.03 | 0.44 ± 0.01 | >5 mg |
| Aqueous extract | >5 | >5 | >5 | >5 | >5 mg |
| Treatment Groups (n = 10) | Av. Initial Tumor Size (mm³) ± SEM | Av. Final Tumor Size (mm³) ± SEM | % Change in Tumor Size | % of Mice with No Detectable Tumor | Average Tumor Weight (g) |
|---|---|---|---|---|---|
| Control | 362.8 ± 1.2 | 846.2 ± 1.3 | 133.2 | 50% | 1.31 |
| 1:10% rice bran | 311.2 ± 2.6 | 467.2 ± 4.9 | 50.1 | 60% | 0.60 |
| 2:20% rice bran | 220.2 ± 0.9 | 402.2 ± 4.3 | 82.6 | 70% | 0.64 |
| Tested Microorganisms | MIC of the Rice Bran Extracts (mg/mL) | MIC of the Positive Control (mg/mL) | |||
|---|---|---|---|---|---|
| Ethanol Extract | Methanol Extract | n-Hexane Extract | Aqueous Extract | Gentamycin | |
| E. coli | 150 | 150 | >150 | >150 | 0.012 |
| P. auriginosa | >150 | >150 | >150 | >150 | 0.012 |
| B. subtilis | 150 | >150 | 150 | >150 | 0.003 |
| Rice Bran Extracts | IC50 (µg/mL) ± SEM |
|---|---|
| Ethanol extract | 168.7 ± 9 |
| Methanol extract | 114.6 ± 13 |
| n-hexane extract | >400 |
| Aqueous extract | 233.1 ± 22 |
| Ascorbic acid | 1.74 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talib, W.H.; Mahmod, A.I.; Awajan, D.; Hamed, R.A.; Al-Yasari, I.H. Immunomodulatory, Anticancer, and Antimicrobial Effects of Rice Bran Grown in Iraq: An In Vitro and In Vivo Study. Pharmaceuticals 2022, 15, 1502. https://doi.org/10.3390/ph15121502
Talib WH, Mahmod AI, Awajan D, Hamed RA, Al-Yasari IH. Immunomodulatory, Anticancer, and Antimicrobial Effects of Rice Bran Grown in Iraq: An In Vitro and In Vivo Study. Pharmaceuticals. 2022; 15(12):1502. https://doi.org/10.3390/ph15121502
Chicago/Turabian StyleTalib, Wamidh H., Asma Ismail Mahmod, Dima Awajan, Reem Ali Hamed, and Intisar Hadi Al-Yasari. 2022. "Immunomodulatory, Anticancer, and Antimicrobial Effects of Rice Bran Grown in Iraq: An In Vitro and In Vivo Study" Pharmaceuticals 15, no. 12: 1502. https://doi.org/10.3390/ph15121502
APA StyleTalib, W. H., Mahmod, A. I., Awajan, D., Hamed, R. A., & Al-Yasari, I. H. (2022). Immunomodulatory, Anticancer, and Antimicrobial Effects of Rice Bran Grown in Iraq: An In Vitro and In Vivo Study. Pharmaceuticals, 15(12), 1502. https://doi.org/10.3390/ph15121502







