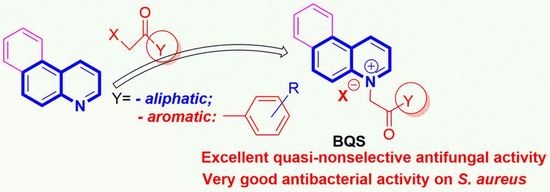
Benzoquinoline Derivatives: A Straightforward and Efficient Route to Antibacterial and Antifungal Agents
Abstract
1. Introduction
2. Results and Discussion
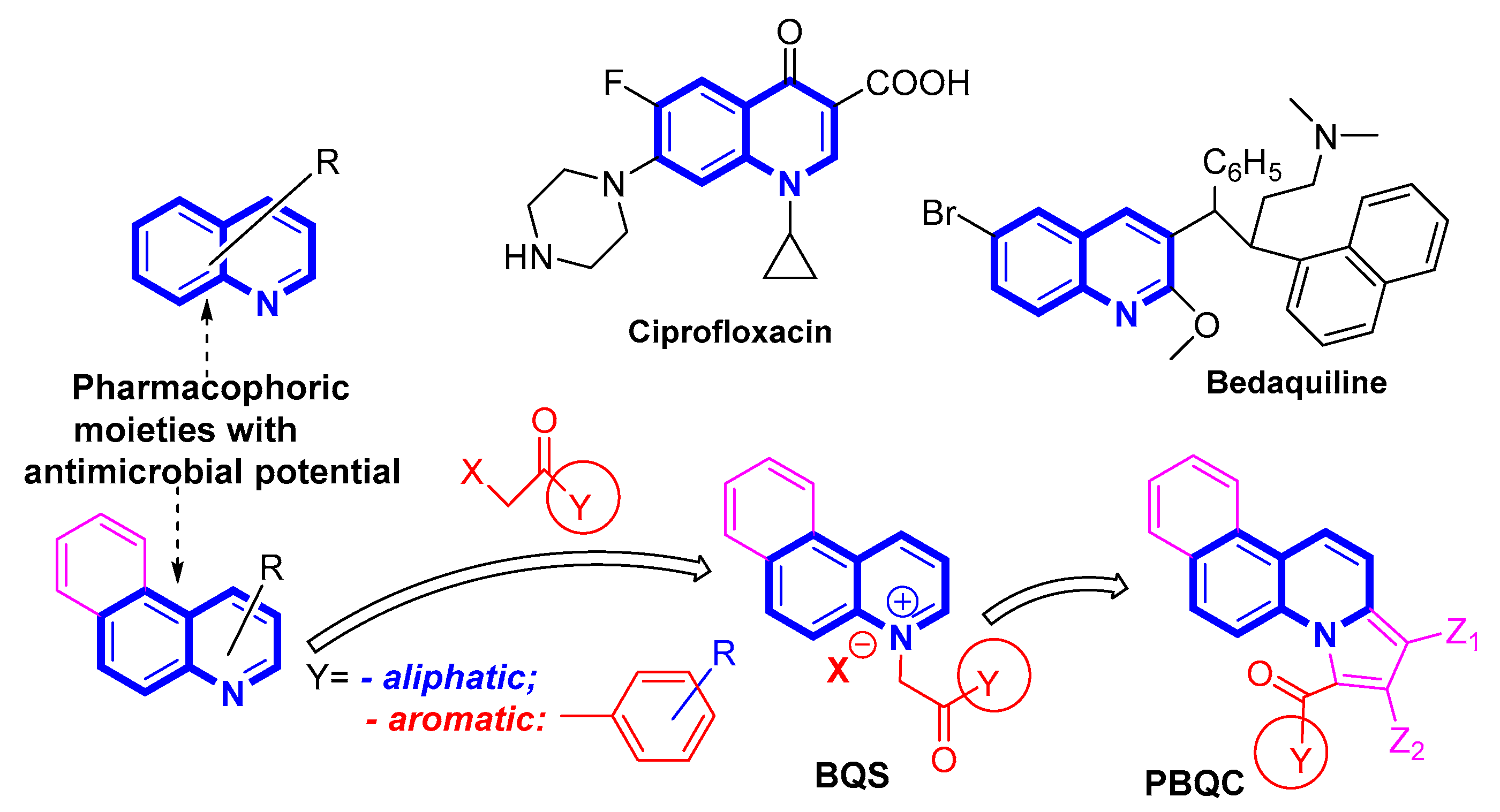
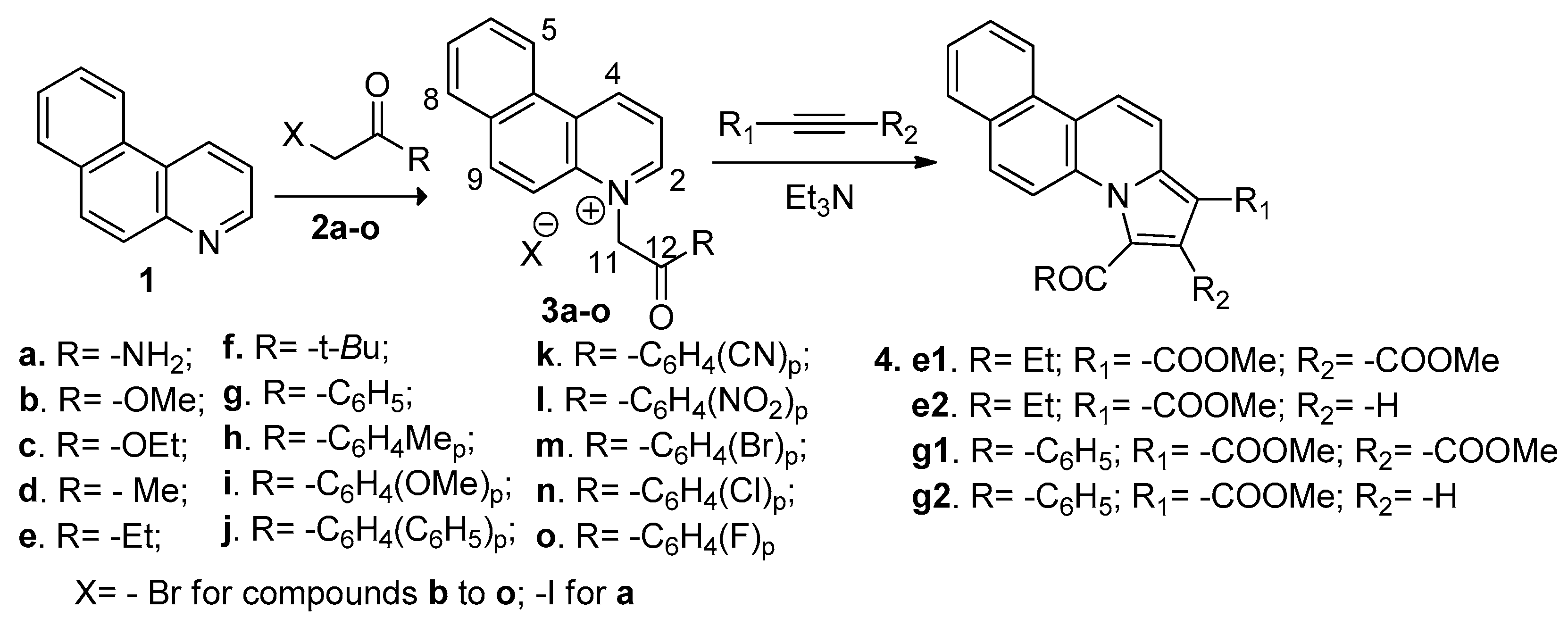
2.1. Desingn and Chemistry
2.2. Antimicrobial Assay
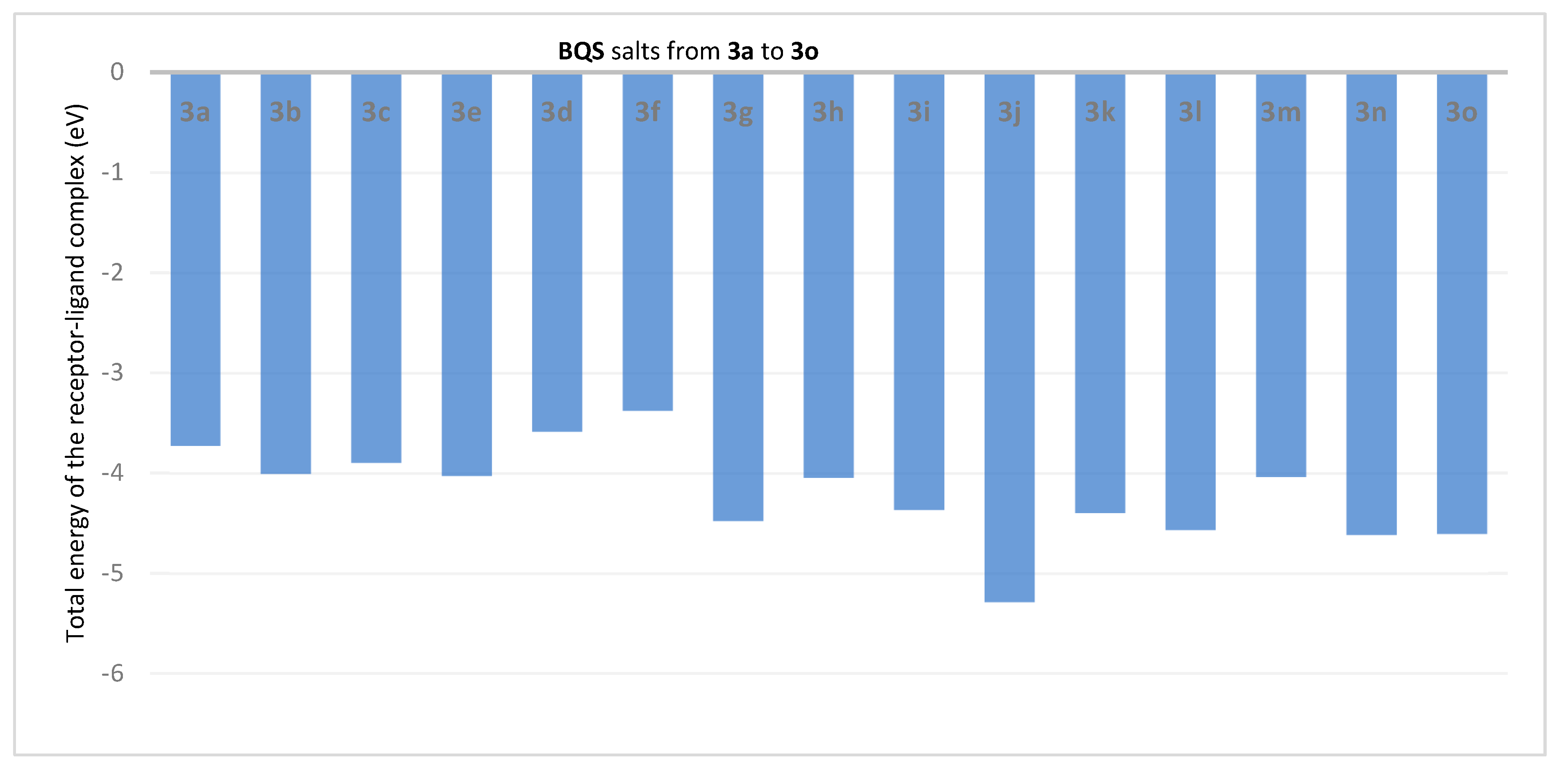
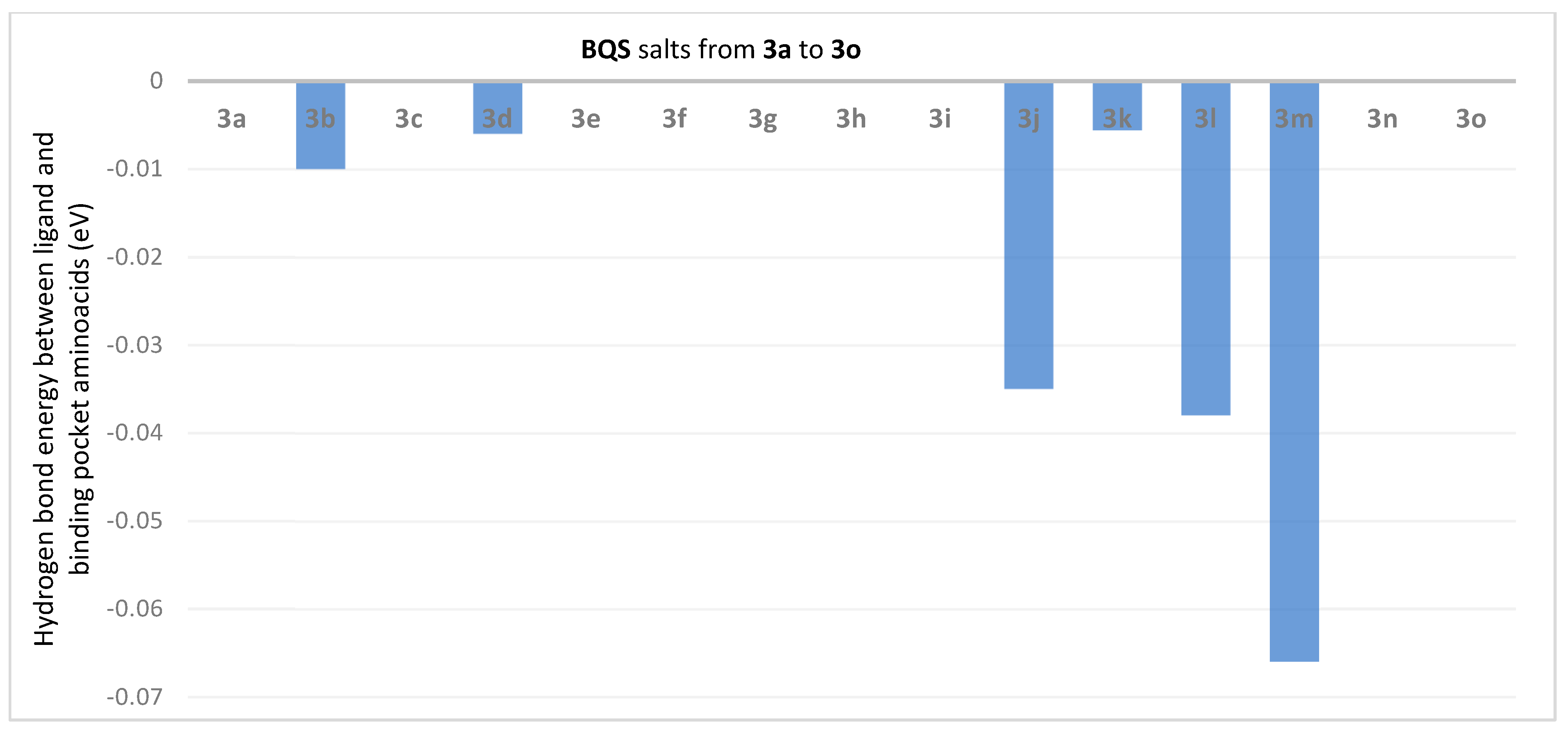
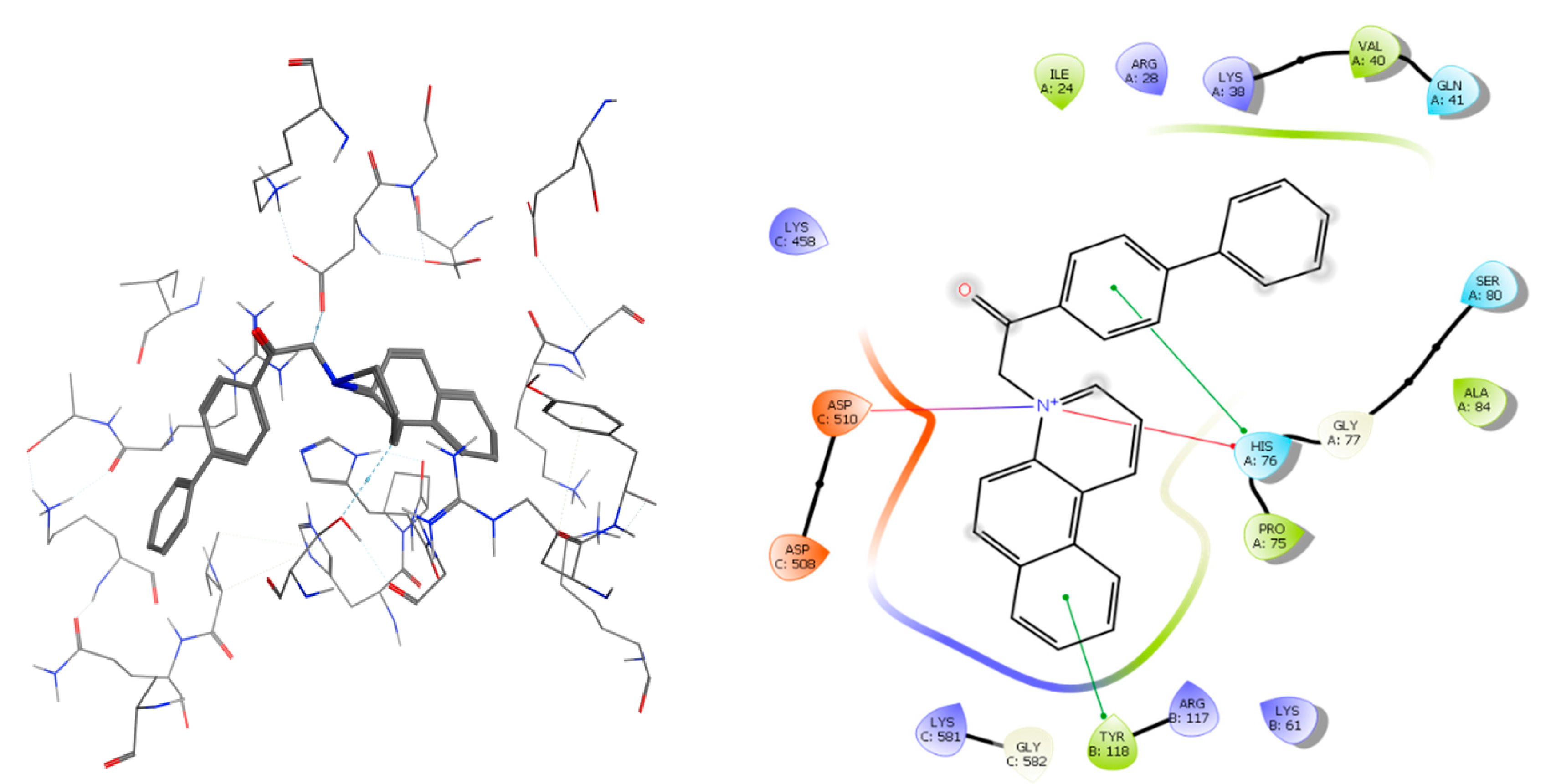
2.3. Molecular Docking
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of Benzo[f]quinoline Salts 3a–o
3.2.1. 1-(2-Amino-2-oxoethyl)benzo[f]quinolin-1-ium iodide (3a)
3.2.2. 1-(2-Methoxy-2-oxoethyl)benzo[f]quinolin-1-ium bromide (3b)
3.2.3. 1-(2-Ethoxy-2-oxoethyl)benzo[f]quinolin-1-ium bromide (3c)
3.2.4. 1-(2-Oxopropyl)benzo[f]quinolin-1-ium bromide (3d)
3.2.5. 1-(2-Oxobutyl)benzo[f]quinolin-1-ium bromide (3e)
3.2.6. 1-(3,3-Dimethyl-2-oxobutyl)benzo[f]quinolin-1-ium bromide (3f)
3.2.7. 1-(Phenacyl)benzo[f]quinolin-1-ium bromide (3g)
3.2.8. 1-(4-Methylphenacyl)benzo[f]quinolin-1-ium bromide (3h)
3.2.9. 1-(4-Methoxyphenacyl)benzo[f]quinolin-1-ium bromide (3i)
3.2.10. 1-(4-Phenylphenacyl)benzo[f]quinolin-1-ium bromide (3j)
3.2.11. 1-(4-Cyanophenacyl)benzo[f]quinolin-1-ium bromide (3k)
3.2.12. 1-(4-Nitrophenacyl)benzo[f]quinolin-1-ium bromide (3l)
3.2.13. 1-(4-Bromophenacyl)benzo[f]quinolin-1-ium bromide (3m)
3.2.14. 1-(4-Chlorophenacyl)benzo[f]quinolin-1-ium bromide (3n)
3.2.15. 1-(4-Fluorophenacyl)benzo[f]quinolin-1-ium bromide (3o)
3.3. Antimicrobial Assay
3.3.1. Disk-Diffusion Method
3.3.2. Broth Microdilution Method for Determining the Minimum Inhibitory Concentration (MIC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Global Strategy for Containment of Antimicrobial Resistance. Available online: https://www.who.int/drugresistance/WHO_Global_Strategy_English.pdf (accessed on 24 February 2021).
- Silverman, R.B.; Holladay, M.W. The Organic Chemistry of DRUG design and Drug Action, 3rd ed.; Academic Press: London, UK, 2014; ISBN 9780123820303. [Google Scholar]
- Brunton, L.; Knollmann, B.; Hilal-Dandan, R. Goodman & Gilman’s the Pharmacological Basis of Therapeutics, 13th ed.; McGraw-Hill: New York, NY, USA, 2013; ISBN 9781259584732. [Google Scholar]
- Eicher, T.; Hauptmann, S.; Speicher, A. The Chemistry of Heterocycles: Structures, Reactions, Synthesis, and Applications, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2013; ISBN 978-3-527-32747-8. [Google Scholar]
- Kumari, L.S.; Mazumder, A.; Kumar, V.; Gupta, S. Synthesis and biological potentials of quinoline analogues: A review of literature. Mini-Rev. Org. Chem. 2019, 16, 653–688. [Google Scholar] [CrossRef]
- Song, Y.; Xu, H.; Chen, W.; Zhan, P.; Liu, X. 8-Hydroxyquinoline: A privileged structure with a broad-ranging pharmacological potential. Med. Chem. Commun. 2015, 6, 61–74. [Google Scholar] [CrossRef]
- Kalaria, P.N.; Karad, S.C.; Raval, D.K. A review on diverse heterocyclic compounds as the privileged scaffolds in antimalarial drug discovery. Eur. J. Med. Chem. 2018, 158, 917–936. [Google Scholar] [CrossRef]
- Hu, Y.Q.; Gao, C.; Zhang, S.; Xu, L.; Xu, Z.; Feng, L.S.; Wu, X.; Zhao, F. Quinoline hybrids and their antiplasmodial and antimalarial activities. Eur. J. Med. Chem. 2017, 139, 22–47. [Google Scholar] [CrossRef]
- Mantu, D.; Antoci, V.; Moldoveanu, C.; Zbancioc, G.; Mangalagiu, I.I. Hybrid imidazole (benzimidazole)/pyridine(quinoline) derivatives and evaluation of their anticancer and antimycobacterial activity. J. Enz. Inhib. Med. Chem. 2016, 31, 96–103. [Google Scholar] [CrossRef]
- Afzal, O.; Kumar, S.; Haider, M.R.; Haider, R.; Ali, R.; Kumar, R.; Jaggi, M.; Bawa, S. A review on anticancer potential of bioactive heterocycle quinoline. Eur. J. Med. Chem. 2015, 97, 871–910. [Google Scholar] [CrossRef] [PubMed]
- Pinz, M.P.; Reis, A.S.; Oliveira, R.L.; Voss, G.T.; Vogt, A.G.; Sacramento, M.D.; Roehrs, J.A.; Alves, D.; Luchese, C.; Wilhelm, E.A. 7-Chloro-4-phenylsulfonylquinoline, a new antinociceptive and anti-inflammatory molecule: Structural improvement of a quinoline derivate with pharmacological activity. Regul. Toxicol. Pharmacol. 2017, 90, 72–77. [Google Scholar] [CrossRef]
- Zhong, F.; Geng, G.; Chen, B.; Pan, T.; Li, Q.; Zhang, H.; Bai, C. Identification of benzenesulfonamide quinoline derivatives as potent HIV-1 replication inhibitors targeting Rev protein. Org. Biomol. Chem. 2015, 13, 1792–1799. [Google Scholar] [CrossRef]
- Diaconu, D.; Mangalagiu, V.; Amariucai-Mantu, D.; Antoci, V.; Giuroiu, C.L.; Mangalagiu, I.I. Hybrid quinoline-sulfonamide complexes (M2+) derivatives with antimicrobial activity. Molecules 2020, 25, 2946. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Ba, Y.; Xu, Z. 1,2,4-Triazole-quinoline/quinolone hybrids as potential anti-bacterial agents. Eur. J. Med. Chem. 2019, 174, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Al Matarneh, C.; Sardaru, M.; Apostu, M.; Rosca, I.; Ciobanu, C.; Mangalagiu, I.I.; Danac, R. Synthesis and antibacterial evaluation of new pyrrolo[3′,4′:3,4]pyrrolo[1,2-a]quinoline and pyrrolo[3′,4′:3,4] pyrrolo[1,2-a]isoquinoline derivatives. Studia UBB Chemia 2019, LXIV, 67–80. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, S.; Pan, B.; Liu, X.; Feng, L.S. 4-Quinolone derivatives and their activities against Gram positive pathogens. Eur. J. Med. Chem. 2018, 143, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Ajani, O.O.; Iyaye, K.T.; Audu, O.Y.; Kuye, A.O.; Olanrewaju, I.O. Microwave assisted synthesis and antimicrobial potential of quinoline based 4-hydrazide-hydrazone derivatives. J. Heterocycl. Chem. 2018, 55, 302–312. [Google Scholar] [CrossRef]
- Nainwal, L.M.; Tasneem, S.; Akhtar, W.; Verma, G.; Khan, M.F.; Parvez, S.; Shaquiquzzaman, M.; Akhter, M.; Alam, M.M. Green recipes to quinoline: A review. Eur. J. Med. Chem. 2019, 164, 121–170. [Google Scholar] [CrossRef]
- Gattu, R.; Bagdi, P.R.; Sidick Basha, R.; Khan, A.T. Camphorsulfonic acid catalyzed one-pot three-component reaction for the synthesis of fused quinoline and benzoquinoline derivatives. J. Org. Chem. 2017, 82, 12416–12429. [Google Scholar] [CrossRef]
- Lungu, L.; Ciocarlan, A.; Simigon, C.; Ozer, I.; Shova, S.; Gutu, I.; Vornicu, N.; Mangalagiu, I.I.; D’Ambrosio, M.; Aricu, A. Synthesis and evaluation of biological activity of homodrimane sesquiterpenoids bearing 1,3,4-oxadiazole or 1,3,4-thiadiazole units. Chem. Heterocycl. Compd. 2020, 56, 578–585. [Google Scholar] [CrossRef]
- Aricu, A.; Ciocarlan, A.; Lungu, L.; Barba, A.; Shova, S.; Zbancioc, G.; Mangalagiu, I.I.; D’Ambrosio, M.; Vornicu, N. Synthesis of new antibacterial and antifungal drimane sesquiterpenoids with azaheterocyclic units. Med. Chem. Res. 2016, 25, 2316–2323. [Google Scholar] [CrossRef]
- Balan, A.M.; Miron, A.; Tuchilus, C.; Rotinberg, P.; Mihai, C.T.; Mangalagiu, I.I.; Zbancioc, G. Synthesis and in vitro analysis of novel dihydroxyacetophenone derivatives with antimicrobial and antitumor activities. Med. Chem. 2014, 10, 476–483. [Google Scholar]
- Kuchkova, K.; Aricu, A.; Barba, A.; Vlad, P.; Shova, S.; Secara, E.; Ungur, N.; Tuchilus, C.; Zbancioc, G.; Mangalagiu, I.I. Design, syntheses and antimicrobial activity of some novel homodrimanese squiterpenoids with diazine skeleton. Med. Chem. Res. 2014, 23, 1559–1568. [Google Scholar] [CrossRef]
- Tucaliuc, R.; Cotea, V.; Niculaua, M.; Tuchilus, C.; Mantu, D.; Mangalagiu, I.I. New pyridazine–fluorine derivatives: Synthesis, chemistry and biological activity. Part II. Eur. J. Med. Chem. 2013, 67, 367–372. [Google Scholar] [CrossRef]
- Balan, A.M.; Florea, O.; Moldoveanu, C.; Zbancioc, G.; Iurea, D.; Mangalagiu, I.I. Diazinium salts with dihydroxyacetophenone skeleton: Syntheses and antimicrobial activity. Eur. J. Med. Chem. 2009, 44, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- Larsen, A.K.; Grondard, L.; Couprie, J.; Desoize, B.; Comoe, L.; Jardillier, J.C.; Riou, J.F. The antileukemic alkaloid fagaronine is an inhibitor of DNA topoisomerases I and II. Biochem. Pharmacol. 1993, 46, 1403–1412. [Google Scholar] [CrossRef]
- Van, D.N.; Rucinschi, E.; Druta, I.; Zugravescu, I. Reaction de benzo[f]quinoline avec sels. Bul. Inst. Politeh. Iasi Sect. 2 Chim. 1977, 23, 51–57. [Google Scholar]
- Mangalagiu, I.I.; Mangalagiu, G.; Roman, M.; Olariu, I.; Petrovanu, M. Synthesis and spectral characterization of some new diazine salts. An. Stiint. Univ. Al. I. Cuza Iasi 1999, 7, 137–142. [Google Scholar]
- Georgescu, E.; Draghici, C.; Iuhas, P.C.; Georgescu, F. A new approach for the synthesis of benzo[f]pyrrolo [1,2-a]-quinolines. Arkivoc 2005, X, 95–104. [Google Scholar] [CrossRef]
- Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 11th ed.; Approved Standard, CLSI Document M07-A11; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; Available online: https://clsi.org/media/1928/m07ed11_sample.pdf (accessed on 24 February 2021).
- Konaté, K.; Mavoungou, J.F.; Lepengué, A.N.; Aworet-Samseny, R.R.R.; Hilou, A.; Souza, A.; Dicko, M.H.; M’Batchi, B. Antibacterial activity against β- lactamase producing Methicillin and Ampicillin-resistants Staphylococcus aureus: Fractional Inhibitory Concentration Index (FICI) determination. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 1–12. [Google Scholar] [CrossRef]
- Kavanagh, A.; Ramu, S.; Gong, Y.; Copper, M.A.; Blaskovich, M.A.T. Effects of microplate type and broth additives on microdilution MIC susceptibility assays. Antimicrob. Agents Chemother. 2019, 63, 1–17. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, K.S. Methods for in vitro evaluating antimicrobial activity. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Thakur, D.; Sahani, K. In vitro antimicrobial activity and MIC of the extracellular ethyl acetate crude extract of endophytic fungi Fusarium sp. isolated from Tephrosia purpurea root. Int. J. Pharm. Pharm. Sci. 2019, 11, 48–53. [Google Scholar] [CrossRef]
- Osaka, I.; Hefty, P.S. Simple resazurin-based microplate assay for measuring chlamydia infections. Antimicrob. Agents Chemother. 2013, 57, 2838–2840. [Google Scholar] [CrossRef]
- Hunter Lindsey, R.; Bromberg, K.D.; Felix, C.A.; Osheroff, N. 1,4-Benzoquinone is a topoisomerase II poison. Biochemistry 2004, 43, 7563–7574. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, M.; Xavier, A.S. Bedaquiline—The first ATP synthase inhibitor against multi drug resistant tuberculosis. J. Young Pharm. 2013, 5, 112–115. [Google Scholar] [CrossRef]
- SwissTargetPrediction. Available online: http://www.swisstargetprediction.ch/ (accessed on 4 December 2020).
- Wang, L.; Wu, D.; Robinson, C.V.; Wu, H.; Fu, T.M. Structures of a complete human V-ATPase reveal mechanisms of its assembly. Mol. Cell. 2020, 80, 501–5011. [Google Scholar] [CrossRef]
- Laponogov, I.; Pan, X.S.; Veselkov, D.A.; McAuley, K.E.; Fisher, L.M.; Sanderson, M.R. Structural basis of gate-DNA breakage and resealing by Type II Topoisomerases. PLoS ONE 2010, 5, e11338. [Google Scholar] [CrossRef]
- Dos Santos Maia, M.; Soares Rodrigues, G.C.; de Sousa, N.F.; Scotti, M.T.; Scotti, L.; Mendonça-Junior, F.J.B. Identification of new targets and the virtual screening of lignans against Alzheimer’s disease. Oxid. Med. Cell. Longev. 2020, 2020, 3098673. [Google Scholar] [CrossRef] [PubMed]
- Levy, Y.; Wolynes, P.G.; Onuchic, J.N. Protein topology determines binding mechanism. Proc. Natl. Acad. Sci. USA 2004, 101, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Vilar, S.; Cozza, G.; Moro, S. Medicinal chemistry and the molecular operating environment (MOE): Application of QSAR and molecular docking to drug discovery. Curr. Top Med. Chem. 2008, 8, 1555–1572. [Google Scholar] [CrossRef]
- Rollinger, J.M.; Stuppner, H.; Langer, T. Virtual screening for the discovery of bioactive natural products. In Natural Compounds as Drugs Volume I; Progress in Drug Research; Springer: Berlin/Heidelberg, Germany, 2008; Volume 65, pp. 211–249. [Google Scholar]
- Bohacek, R.S.; McMartin, C.; Guida, W.C. The art and practice of structure-based drug design: A molecular modeling perspective. Med. Res. Rev. 1999, 16, 3–50. [Google Scholar] [CrossRef]







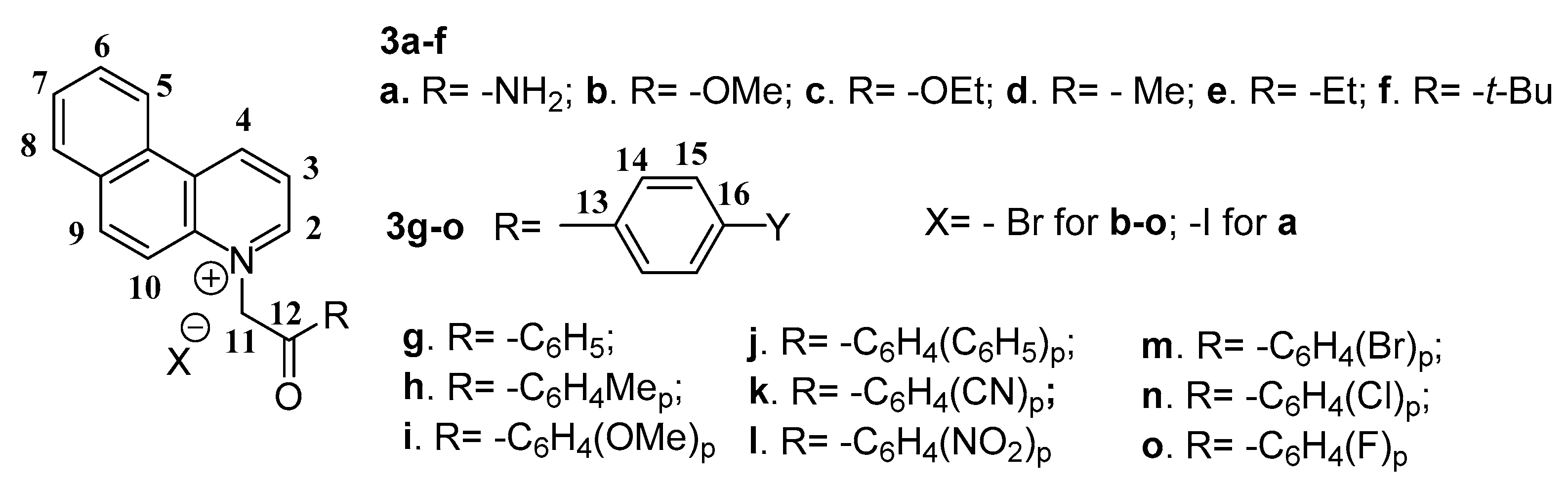
| Salt | IR, cm−1 | H-2 | H-4 | H-11 (CH2) | 13C-NMR |
|---|---|---|---|---|---|
| 3a | 1626 (νCO) | 9.47 | 10.18 | 5.91 | 166.0 (C-12: CO), 148.5 (C-2), 142.1 (C-4), 59.3 (C-11: CH2) |
| 3b | 1743 (νCO) | 9.62 | 10.23 | 6.33 | 166.6 (C-12: CO), 148.3 (C-2), 142.9 (C-4), 57.9 (C-11: CH2) |
| 3c | 1741 (νCO) | 9.60 | 10.25 | 6.30 | 166.1 (C-12: CO), 148.4 (C-2), 142.8 (C-4), 57.9 (C-11: CH2) |
| 3d | 1736 (νCO) | 9.39 | 10.20 | 6.43 | 199.7 (C-12: CO), 147.8 (C-2), 144.0 (C-4), 66.0 (C-11: CH2) |
| 3e | 1723 (νCO) | 9.40 | 10.21 | 6.42 | 202.3 (C-12: CO), 147.8 (C-2), 142.2 (C-4), 65.3 (C-11: CH2) |
| 3f | 1708 (νCO) | 9.46 | 10.24 | 6.68 | 207.0 (C-12: CO), 148.2 (C-2), 142.3 (C-4), 63.0 (C-11: CH2) |
| 3g | 1682 (νCO) | 9.45 | 10.20 | 7.05 | 190.6 (C-12: CO), 148.2 (C-2), 142.5 (C-4), 63.9 (C-11: CH2) |
| 3h | 1677 (νCO) | 9.55 | 10.25 | 7.12 | 190.2 (C-12: CO), 148.2 (C-2), 142.4 (C-4), 63.8 (C-11: CH2) |
| 3i | 1673 (νCO) | 9.52 | 10.25 | 7.08 | 188.9 (C-12: CO), 148.2 (C-2), 142.4 (C-4), 63.5 (C-11: CH2) |
| 3j | 1689 (νCO) | 9.56 | 10.28 | 7.18 | 190.3 (C-12: CO), 148.2 (C-2), 142.5 (C-4), 63.9 (C-11: CH2) |
| 3k | 1706 (νCO) | 9.51 | 10.28 | 7.15 | 190.3 (C-12: CO), 148.2 (C-2), 142.6 (C-4), 64.1 (C-11: CH2) |
| 3l | 1717 (νCO) | 9.52 | 10.29 | 7.18 | 190.1 (C-12: CO), 148.2 (C-2), 142.7 (C-4), 64.1 (C-11: CH2) |
| 3m | 1696 (νCO) | 9.52 | 10.27 | 7.11 | 190.1 (C-12: CO), 148.2 (C-2), 142.5 (C-4), 63.8 (C-11: CH2) |
| 3n | 1698 (νCO) | 9.47 | 10.28 | 7.08 | 189.9 (C-12: CO), 148.2 (C-2), 142.6 (C-4), 63.8 (C-11: CH2) |
| 3o | 1694 (νCO) | 9.51 | 10.27 | 7.11 | 189.5 (C-12: CO), 148.2 (C-2), 142.5 (C-4), 63.8 (C-11: CH2) |
| Cpd/R | a Diameter of Inhibition Zone (mm) | ||
|---|---|---|---|
| S. aureus ATCC 25923 | E. coli ATCC 25922 | C. albicans ATCC 10231 | |
| 3a/-NH2 | 0 | 0 | 10.5 ± 0.5 |
| 3b/-OMe | 19.5 ± 1.5 | 17 ± 1.73 | 20 ± 1.5 |
| 3c/-OEt | 20 ± 1 | 18.5 ± 1.5 | 22 ± 1.25 |
| 3d/-Me | 16 ± 1 | 11 ± 1 | 20 ± 1.80 |
| 3e/-Et | 17 ± 1.73 | 12.5 ± 1.5 | 30 ± 2 |
| 3f/-t-Bu | 20 ± 1 | 15.5 ± 2 | 27.5 ± 1.5 |
| 3g/-C6H5 | 18 ± 1.5 | 13 ± 1.5 | 20 ± 1.8 |
| 3h/-C6H4(Me)p | 20.5 ± 1.5 | 17 ± 2 | 21.5 ± 1.8 |
| 3i/-C6H4(OMe)p | 21.5 ± 1.73 | 18 ± 1.73 | 22 ± 1 |
| 3j/-C6H4(C6H5)p | 15 ± 1.5 | 13 ± 1.73 | 17 ± 1 |
| 3k/-C6H4(CN)p | 15 ± 2.5 | 12.5 ± 1.25 | 15 ± 1.73 |
| 3l/-C6H4(NO2)p | 15 ± 2 | 14 ± 2.6 | 17 ± 1 |
| 3m/-C6H4(Br)p | 16 ± 1.5 | 12 ± 1.5 | 19 ± 1.5 |
| 3n/-C6H4(Cl)p | 21 ± 1.5 | 18 ± 1.32 | 22.5 ± 1 |
| 3o/-C6H4(F)p | 14 ± 1.5 | 10.5 ± 1.5 | 14 ± 0.5 |
| 4e1 | 0 | 0 | 0 |
| 4e2 | 0 | 0 | 8 ± 1 |
| 4g1 | 0 | 0 | 0 |
| 4g2 C+ | 0 44 ± 1 | 0 46 ± 1.33 | 0 21 ± 1 |
| Strain | MIC (µg/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C+ | 3b | 3c | 3d | 3e | 3g | 3h | 3i | 3j | 3k | 3n | |
| S. aureus ATCC 25923 | 0.5 | 1.56 | 0.39 | 1.56 | 0.78 | 0.195 | 0.195 | 0.00304 | 0.39 | 1.19 | 0.0975 |
| E. coli ATCC 25922 | 0.25 | 1.56 | 0.78 | 1.56 | 0.78 | 0.195 | 0.195 | 0.00152 | 0.78 | 1.39 | 0.195 |
| C. albicans ATCC 10231 | 1.56 | 3.12 | 0.78 | 3.12 | 3.12 | 0.139 | 0.195 | 0.0575 | 0.78 | 0.81 | 0.195 |
| # | Structures | QP Logs | ClQP Logs | QP Log HERG | QP PCaco | QP Logbb | QP PMDCK | QP Logkp | Metab. | QP Log Khsa | %Ab-Sorbtion | Rule of 5 | Rule of 3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3a | −1.616 | −2.741 | −3.322 | −3.322 | −3.322 | 512.729 | 512.729 | 512.729 | 512.729 | 512.729 | 0 | 0 |
| 2 | 3b | −4.078 | −3.835 | −5.248 | −5.248 | −5.248 | 2017.43 | 2017.43 | 2017.43 | 2017.43 | 2017.43 | 0 | 0 |
| 3 | 3c | −4.668 | −4.133 | −5.585 | −5.585 | −5.585 | 2333.6 | 2333.6 | 2333.6 | 2333.6 | 2333.6 | 0 | 0 |
| 4 | 3d | −3.555 | −3.495 | −4.966 | −4.966 | −4.966 | 2001.37 | 2001.37 | 2001.37 | 2001.37 | 2001.37 | 0 | 0 |
| 5 | 3e | −3.805 | −3.793 | −5.012 | −5.012 | −5.012 | 2500.67 | 2500.67 | 2500.67 | 2500.67 | 2500.67 | 0 | 0 |
| 6 | 3f | −4.066 | −4.091 | −4.992 | −4.992 | −4.992 | 2271.19 | 2271.19 | 2271.19 | 2271.19 | 2271.19 | 0 | 0 |
| 7 | 3g | −4.291 | −4.766 | −5.876 | −5.876 | −5.876 | 1877 | 1877 | 1877 | 1877 | 1877 | 0 | 0 |
| 8 | 3h | −2.913 | −3.555 | −5.857 | −5.857 | −5.857 | 176.027 | 176.027 | 176.027 | 176.027 | 176.027 | 0 | 0 |
| 9 | 3i | −4.495 | −5.463 | −5.52 | −5.52 | −5.52 | 2244.4 | 2244.4 | 2244.4 | 2244.4 | 2244.4 | 0 | 0 |
| 10 | 3j | −6.680 | −6.742 | −7.42 | −7.42 | −7.42 | 2096.68 | 2096.68 | 2096.68 | 2096.68 | 2096.68 | 1 | 1 |
| 11 | 3k | −5.748 | −6.008 | −6.132 | −6.132 | −6.132 | 394.919 | 394.919 | 394.919 | 394.919 | 394.919 | 0 | 1 |
| 12 | 3l | −4.902 | −5.471 | −5.39 | −5.39 | −5.39 | 1898.89 | 1898.89 | 1898.89 | 1898.89 | 1898.89 | 0 | 0 |
| 13 | 3m | −5.181 | −6.442 | −5.408 | −5.408 | −5.408 | 5868.17 | 5868.17 | 5868.17 | 5868.17 | 5868.17 | 1 | 1 |
| 14 | 3n | −5.612 | −5.905 | −5.947 | −5.947 | −5.947 | 5702.91 | 5702.91 | 5702.91 | 5702.91 | 5702.91 | 1 | 1 |
| 15 | 3o | −5.223 | −5.555 | −5.898 | −5.898 | −5.898 | 4182.77 | 4182.77 | 4182.77 | 4182.77 | 4182.77 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoci, V.; Oniciuc, L.; Amariucai-Mantu, D.; Moldoveanu, C.; Mangalagiu, V.; Amarandei, A.M.; Lungu, C.N.; Dunca, S.; Mangalagiu, I.I.; Zbancioc, G. Benzoquinoline Derivatives: A Straightforward and Efficient Route to Antibacterial and Antifungal Agents. Pharmaceuticals 2021, 14, 335. https://doi.org/10.3390/ph14040335
Antoci V, Oniciuc L, Amariucai-Mantu D, Moldoveanu C, Mangalagiu V, Amarandei AM, Lungu CN, Dunca S, Mangalagiu II, Zbancioc G. Benzoquinoline Derivatives: A Straightforward and Efficient Route to Antibacterial and Antifungal Agents. Pharmaceuticals. 2021; 14(4):335. https://doi.org/10.3390/ph14040335
Chicago/Turabian StyleAntoci, Vasilichia, Liliana Oniciuc, Dorina Amariucai-Mantu, Costel Moldoveanu, Violeta Mangalagiu, Andreea Madalina Amarandei, Claudiu N. Lungu, Simona Dunca, Ionel I. Mangalagiu, and Gheorghita Zbancioc. 2021. "Benzoquinoline Derivatives: A Straightforward and Efficient Route to Antibacterial and Antifungal Agents" Pharmaceuticals 14, no. 4: 335. https://doi.org/10.3390/ph14040335
APA StyleAntoci, V., Oniciuc, L., Amariucai-Mantu, D., Moldoveanu, C., Mangalagiu, V., Amarandei, A. M., Lungu, C. N., Dunca, S., Mangalagiu, I. I., & Zbancioc, G. (2021). Benzoquinoline Derivatives: A Straightforward and Efficient Route to Antibacterial and Antifungal Agents. Pharmaceuticals, 14(4), 335. https://doi.org/10.3390/ph14040335