Transgene Delivery to Human Induced Pluripotent Stem Cells Using Nanoparticles
Abstract
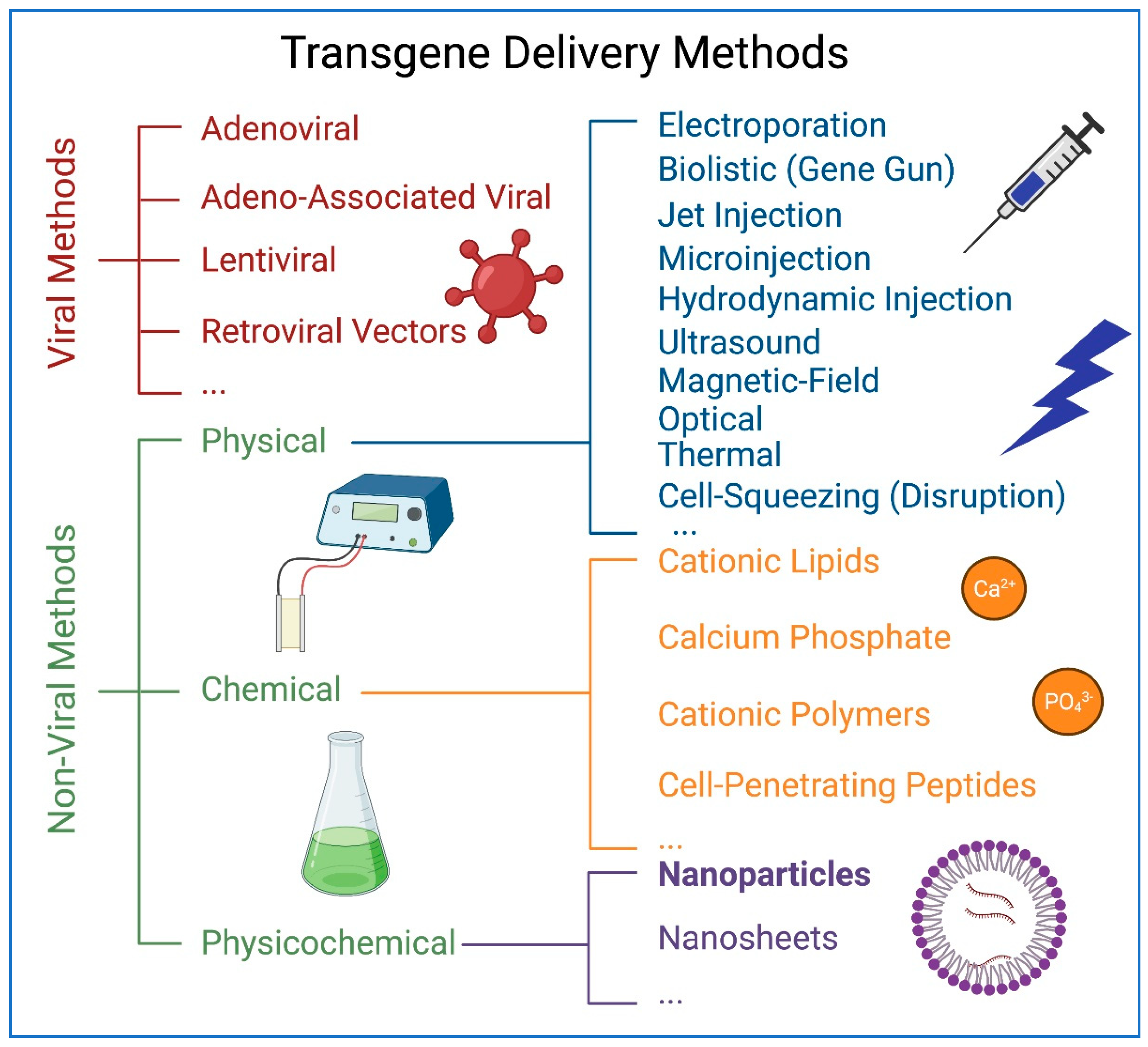
1. Introduction
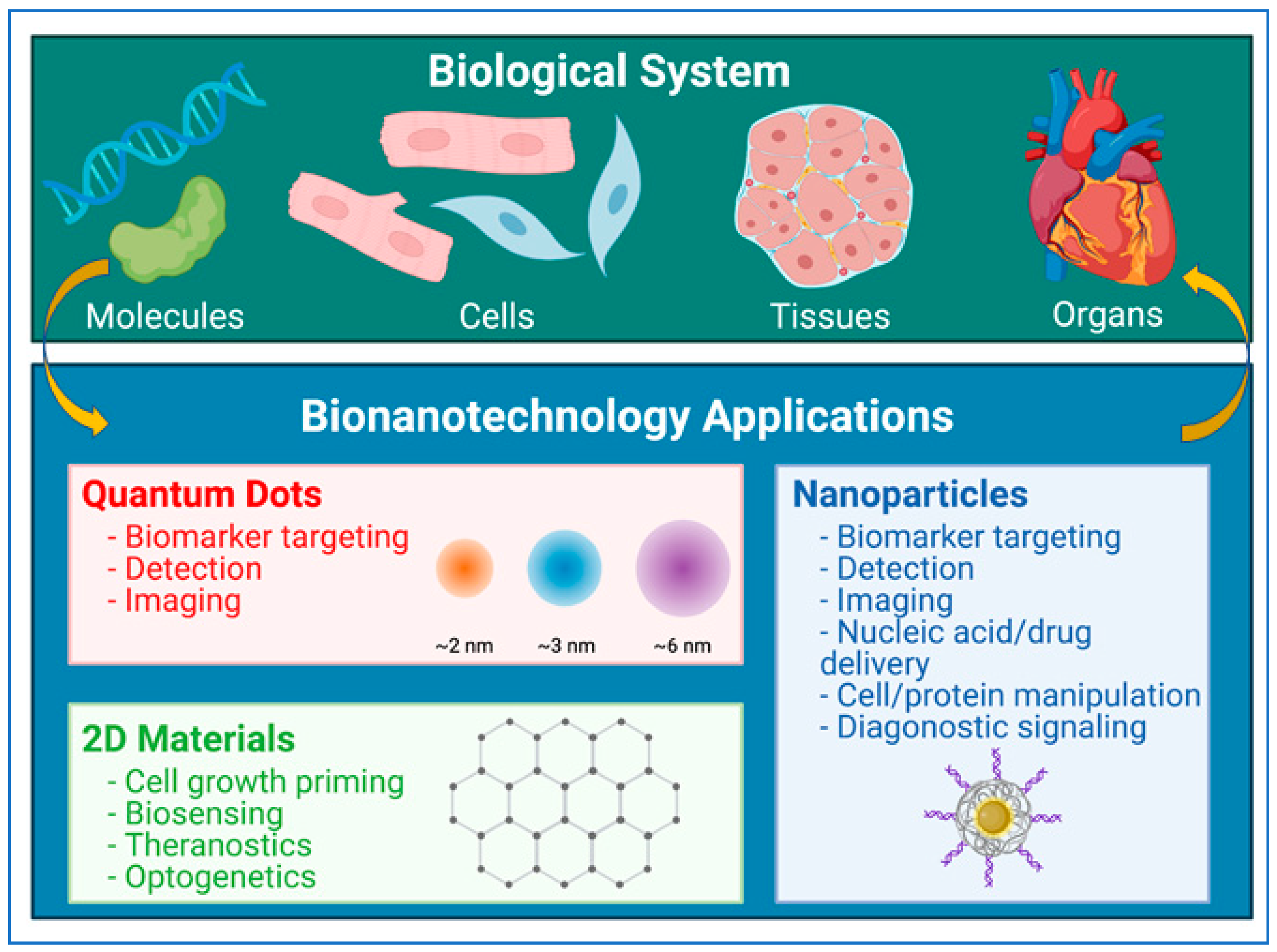
2. Physical, Chemical, and Biological Properties of Nanoparticles
3. Nanoparticles in Biomedicine
4. Nanoparticles in the Development of COVID-19 Vaccines
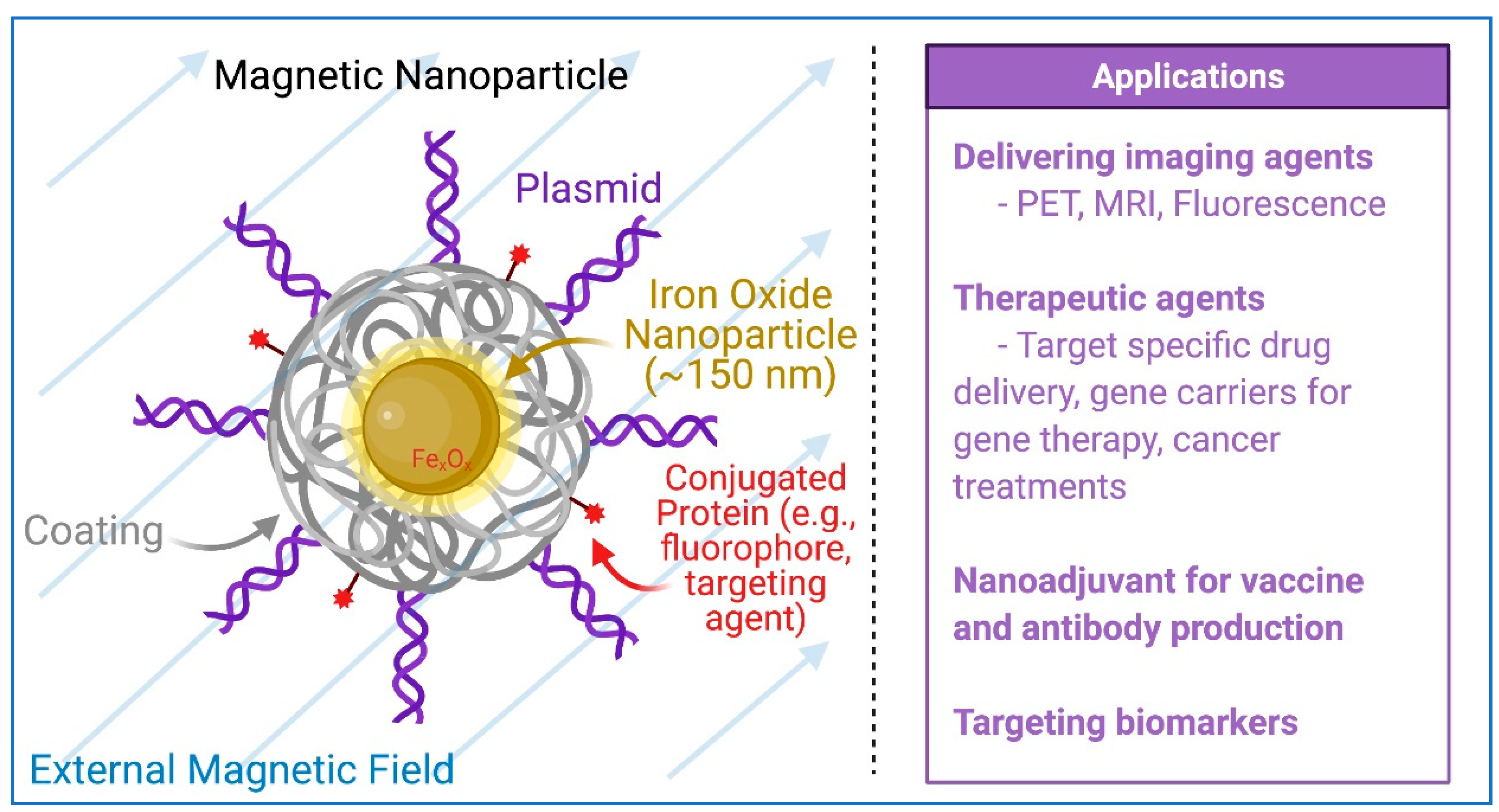
5. Magnetic Nanoparticles
6. HiPSCs and hiPSC-Derived Cardiomyocytes (hiPSC-CMs)
7. Nanoparticles in iPSC Generation and Precision Medicine
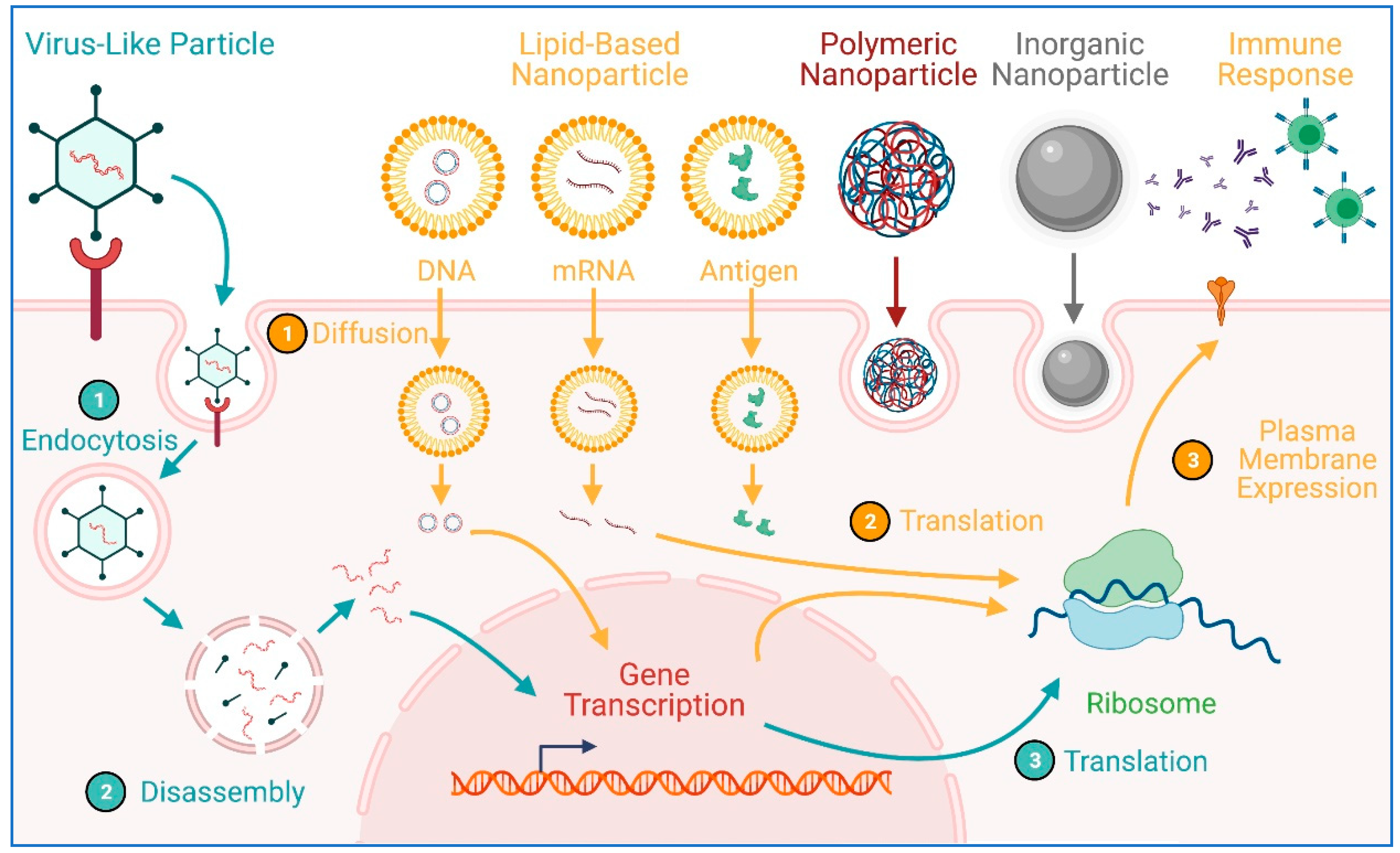
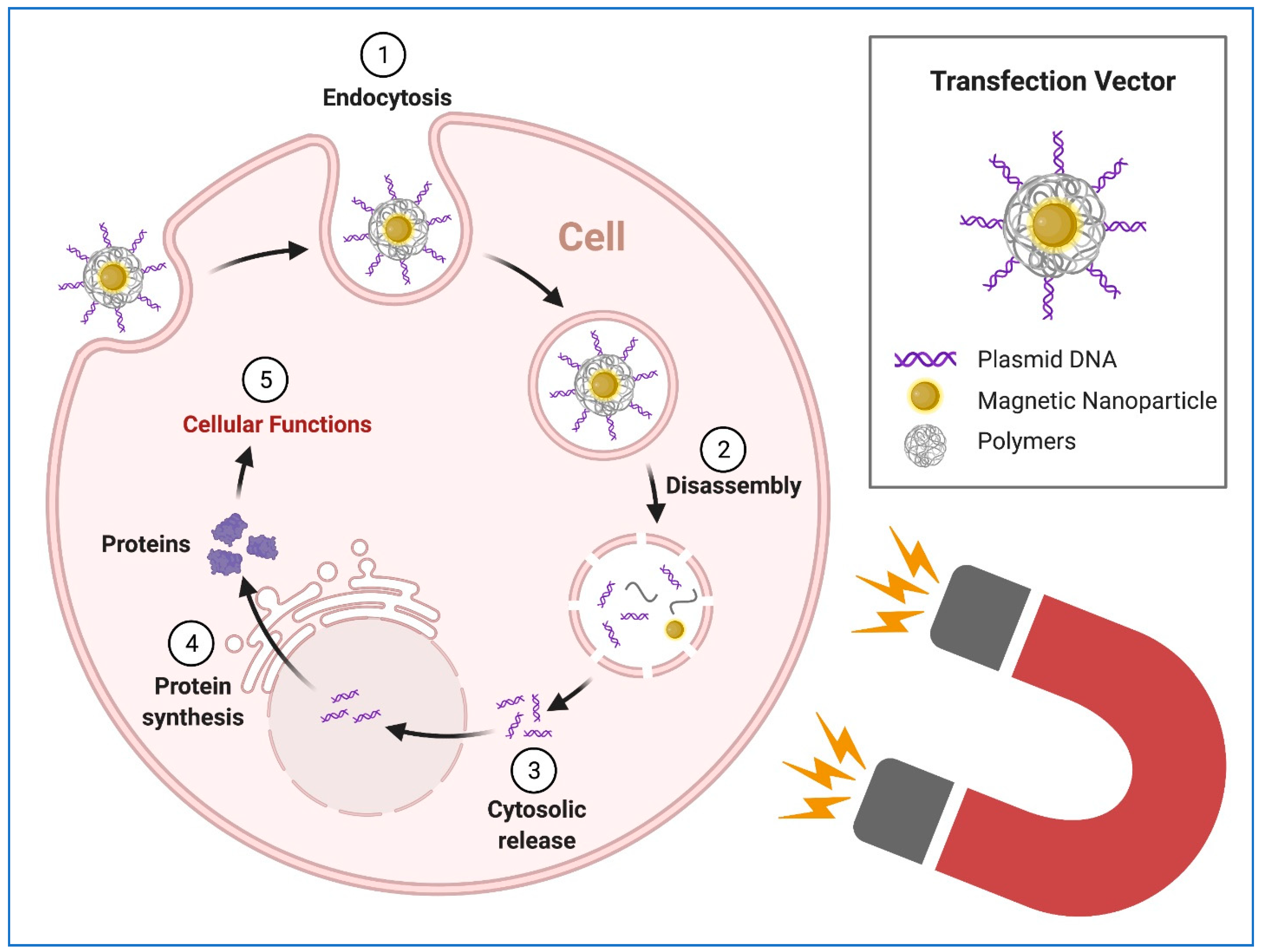
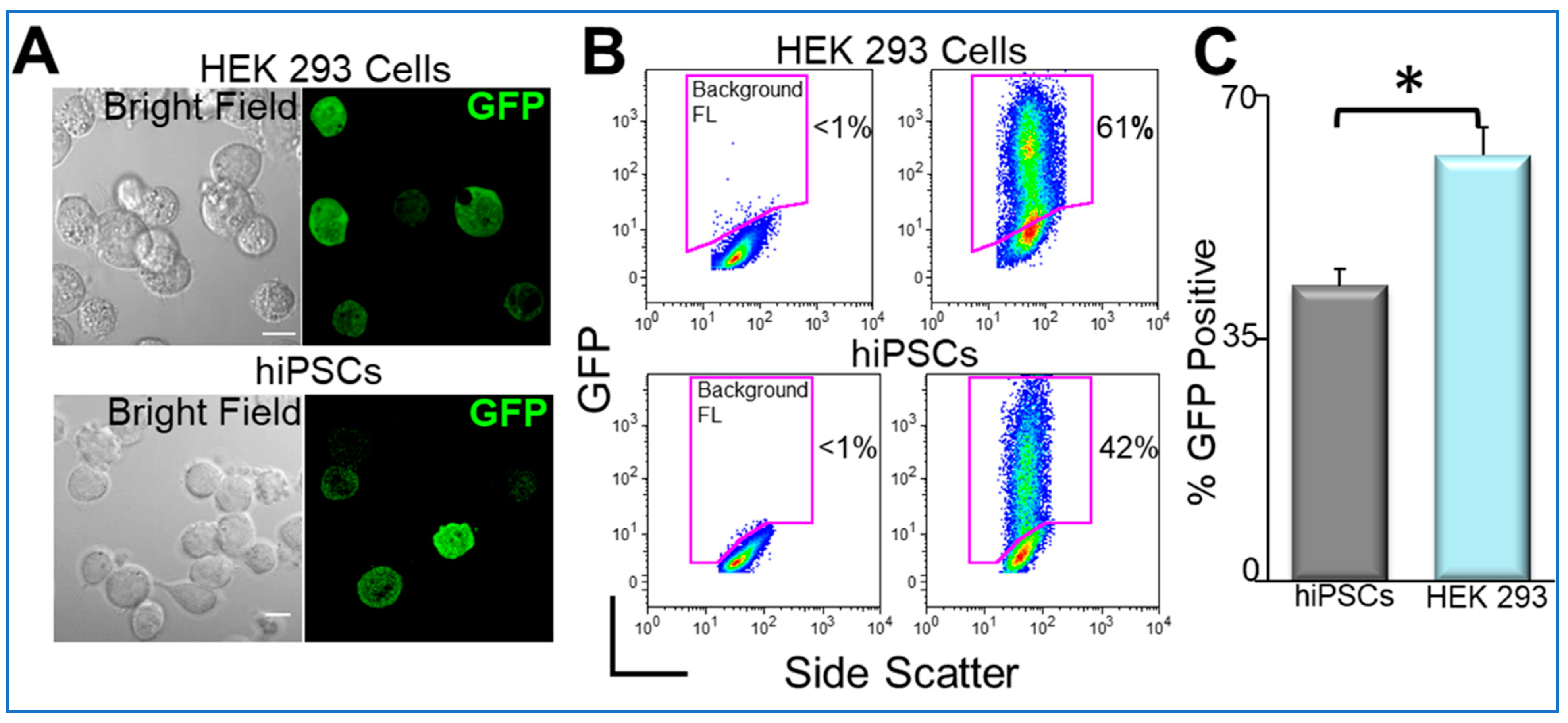
8. Transgene Delivery to hiPSCs Using Nanoparticles
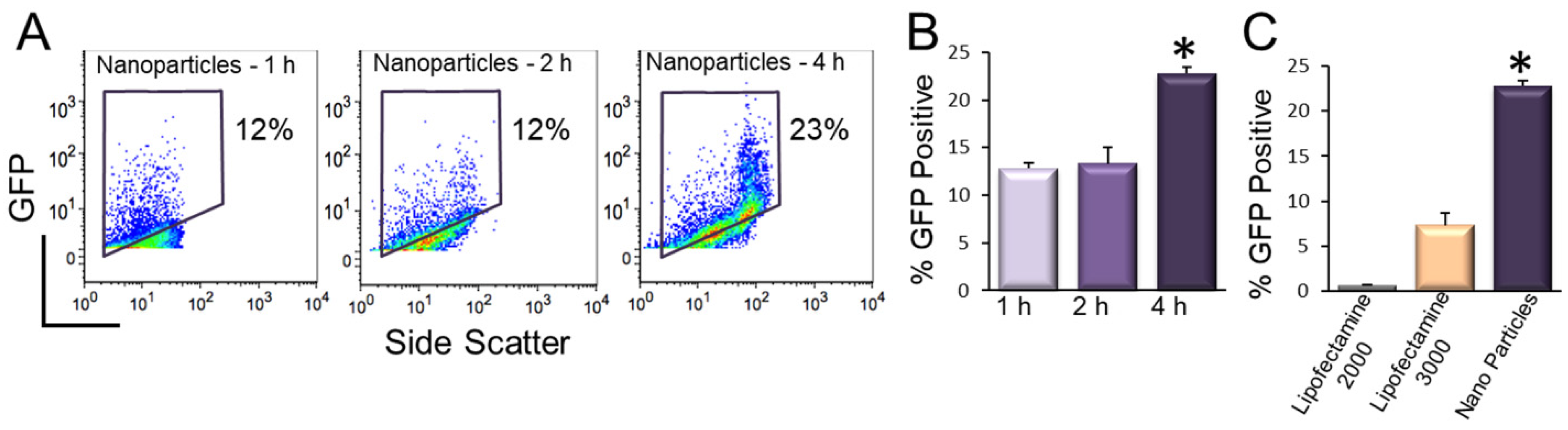
9. Transfection of hiPSC-CMs Using Nanoparticles
10. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 2017, 16, 115–130. [Google Scholar] [CrossRef]
- Karagiannis, P.; Takahashi, K.; Saito, M.; Yoshida, Y.; Okita, K.; Watanabe, A.; Inoue, H.; Yamashita, J.K.; Todani, M.; Nakagawa, M.; et al. Induced Pluripotent Stem Cells and Their Use in Human Models of Disease and Development. Physiol. Rev. 2019, 99, 79–114. [Google Scholar] [CrossRef]
- Liu, G.; David, B.T.; Trawczynski, M.; Fessler, R.G. Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications. Stem Cell Rev. Rep. 2020, 16, 3–32. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat. Rev. Mol. Cell Biol. 2016, 17, 183–193. [Google Scholar] [CrossRef]
- Sharma, A.; Sances, S.; Workman, M.J.; Svendsen, C.N. Multi-Lineage human iPSC-Derived platforms for disease modeling and drug discovery. Cell Stem Cell 2020, 26, 309–329. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Rui, M.; Yu, C.; Chu, T.; Li, C.; Zhan, Z.; Cao, H.; Li, H.; Liu, Z.; Shen, H. Nanotechnology in Generation and Biomedical Application of Induced Pluripotent Stem Cells. Nano LIFE 2018, 8. [Google Scholar] [CrossRef]
- Al Abbar, A.; Ngai, S.C.; Nograles, N.; Alhaji, S.Y.; Abdullah, S. Induced Pluripotent Stem Cells: Reprogramming Platforms and Applications in Cell Replacement Therapy. BioRes. Open Access 2020, 9, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Pickar-Oliver, A.; Gersbach, C.A. The next generation of CRISPR–Cas technologies and applications. Nat. Rev. Mol. Cell Biol. 2019, 20, 490–507. [Google Scholar] [CrossRef]
- Şişli, H.B.; Hayal, T.B.; Seçkin, S.; Şenkal, S.; Kıratlı, B.; Şahin, F.; Doğan, A. Gene Editing in Human Pluripotent Stem Cells: Recent Advances for Clinical Therapies. Adv. Exp. Med. Biol. 2019, 1237, 17–28. [Google Scholar] [CrossRef]
- Doss, M.X.; Sachinidis, A. Current Challenges of iPSC-Based Disease Modeling and Therapeutic Implications. Cells 2019, 8, 403. [Google Scholar] [CrossRef]
- Chatterjee, P.; Cheung, Y.; Liew, C. Transfecting and Nucleofecting Human Induced Pluripotent Stem Cells. J. Vis. Exp. 2011, 2011, e3110. [Google Scholar] [CrossRef]
- Fontes, A.; Lakshmipathy, U. Advances in genetic modification of pluripotent stem cells. Biotechnol. Adv. 2013, 31, 994–1001. [Google Scholar] [CrossRef]
- Rapti, K.; Stillitano, F.; Karakikes, I.; Nonnenmacher, M.; Weber, T.; Hulot, J.-S.; Hajjar, R.J. Effectiveness of gene delivery systems for pluripotent and differentiated cells. Mol. Ther. Methods Clin. Dev. 2015, 2, 14067. [Google Scholar] [CrossRef]
- Czerwińska, P.; Mazurek, S.; Kołodziejczak, I.; Wiznerowicz, M. Gene delivery methods and genome editing of human pluripotent stem cells. Rep. Pr. Oncol. Radiother. 2019, 24, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in Medicine: Therapeutic Applications and Developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Dayem, A.A.; Choi, H.Y.; Yang, G.-M.; Kim, K.; Saha, S.K.; Kim, J.-H.; Cho, S.-G. The potential of nanoparticles in stem cell differentiation and further therapeutic applications. Biotechnol. J. 2016, 11, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; De Serres, G. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2021, 384. [Google Scholar] [CrossRef]
- Nanomedicine and the COVID-19 vaccines. Nat. Nanotechnol. 2020, 15, 963. Available online: https://www.nature.com/articles/s41565-020-00820-0 (accessed on 5 April 2021). [CrossRef] [PubMed]
- Chauhan, G.; Madou, M.J.; Kalra, S.; Chopra, V.; Ghosh, D.; Martinez-Chapa, S.O. Nanotechnology for COVID-19: Therapeutics and Vaccine Research. ACS Nano 2020, 14, 7760–7782. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Beiss, V.; Fiering, S.N.; Steinmetz, N.F. COVID-19 Vaccine Frontrunners and Their Nanotechnology Design. ACS Nano 2020, 14, 12522–12537. [Google Scholar] [CrossRef]
- Powell, A.E.; Zhang, K.; Sanyal, M.; Tang, S.; Weidenbacher, P.A.; Li, S.; Pham, T.D.; Pak, J.E.; Chiu, W.; Kim, P.S. A single immunization with spike-functionalized ferritin vaccines elicits neutralizing antibody responses against SARS-CoV-2 in mice. bioRxiv 2020. [Google Scholar] [CrossRef]
- Shin, M.D.; Shukla, S.; Chung, Y.H.; Beiss, V.; Chan, S.K.; Ortega-Rivera, O.A.; Wirth, D.M.; Chen, A.; Sack, M.; Pokorski, J.K.; et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020, 15, 646–655. [Google Scholar] [CrossRef]
- Nanoscale Science, E.; Technology Subcommittee. A Progress Review of the NNI Nanotechnology Signature Initiatives; White House Office of Science and Technology Policy: Washington, DC, USA, 2015.
- Choi, H.; Mody, C.C.M. The Long History of Molecular Electronics: Microelectronics Origins of Nanotechnology. Soc. Stud. Sci. 2009, 39, 11–50. [Google Scholar] [CrossRef]
- Chaudhuri, R.G.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2011, 112, 2373–2433. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Caruthers, S.D.; A Wickline, S.; Lanza, G.M. Nanotechnological applications in medicine. Curr. Opin. Biotechnol. 2007, 18, 26–30. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.; Park, Y.I.; Lee, N.; Hyeon, T. Recent Development of Inorganic Nanoparticles for Biomedical Imaging. ACS Central Sci. 2018, 4, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Elizondo, G.; Wittenberg, J.; Lee, A.S.; Josephson, L.; Brady, T.J. Ultrasmall superparamagnetic iron oxide: An intravenous contrast agent for assessing lymph nodes with MR imaging. Radiology 1990, 175, 494–498. [Google Scholar] [CrossRef]
- Gao, X.; Chung, L.W.K.; Nie, S.; Marcel, B.; Charles, H.Z. Quantum Dots for In Vivo Molecular and Cellular Imaging. In Quantum Dots; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2007; Volume 374, pp. 135–146. [Google Scholar]
- Tan, A.; Yildirimer, L.; Rajadas, J.; De La Peña, H.; Pastorin, G.; Seifalian, A. Quantum dots and carbon nanotubes in oncology: A review on emerging theranostic applications in nanomedicine. Nanomedicine 2011, 6, 1101–1114. [Google Scholar] [CrossRef]
- Banerjee, R. Liposomes: Applications in Medicine. J. Biomater. Appl. 2001, 16, 3–21. [Google Scholar] [CrossRef]
- Hirsch, L.R.; Gobin, A.M.; Lowery, A.R.; Tam, F.; Drezek, R.A.; Halas, N.J.; West, J.L. Metal Nanoshells. Ann. Biomed. Eng. 2006, 34, 15–22. [Google Scholar] [CrossRef]
- Tóth, É.; Bolskar, R.D.; Borel, A.; González, G.; Helm, L.; Merbach, A.E.; Sitharaman, B.; Wilson, L.J. Water-Soluble Gadofullerenes: Toward High-Relaxivity, pH-Responsive MRI Contrast Agents. J. Am. Chem. Soc. 2005, 127, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; DeGiovanni, P.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef]
- Gao, W.; Chan, J.M.; Farokhzad, O.C. pH-Responsive nanoparticles for drug delivery. Mol. Pharm. 2010, 7, 1913–1920. [Google Scholar] [CrossRef]
- Jhaveri, A.; Deshpande, P.; Torchilin, V. Stimuli-sensitive nanopreparations for combination cancer therapy. J. Control. Release 2014, 190, 352–370. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, N.; Gao, Z.; Kennedy, A. Multifunctional Nanoparticles for Combining Ultrasonic Tumor Imaging and Targeted Chemotherapy. J. Natl. Cancer Inst. 2007, 99, 1095–1106. [Google Scholar] [CrossRef]
- Guduru, R.; Liang, P.; Runowicz, C.; Nair, M.P.N.; Atluri, V.S.R.; Khizroev, S. Magneto-Electric Nanoparticles to Enable Field-Controlled High-Specificity Drug Delivery to Eradicate Ovarian Cancer Cells. Sci. Rep. 2013, 3, srep02953. [Google Scholar] [CrossRef]
- Jain, K. Role of nanobiotechnology in the development of personalized medicine. Nanomedicine 2009, 4, 249–252. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Plank, C.; Zelphati, O.; Mykhaylyk, O. Magnetically enhanced nucleic acid delivery. Ten years of magnetofection—Progress and prospects. Adv. Drug Deliv. Rev. 2011, 63, 1300–1331. [Google Scholar] [CrossRef] [PubMed]
- Corchero, J.L.; Villaverde, A. Biomedical applications of distally controlled magnetic nanoparticles. Trends Biotechnol. 2009, 27, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Liang, L.; Veiseh, O. Recent Advancements of Magnetic Nanomaterials in Cancer Therapy. Pharmaceutics 2020, 12, 147. [Google Scholar] [CrossRef]
- Ferreira, M.; Sousa, J.; Pais, A.; Vitorino, C. The Role of Magnetic Nanoparticles in Cancer Nanotheranostics. Materials 2020, 13, 266. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Schoeman, D.; Fielding, B.C. Coronavirus envelope protein: Current knowledge. Virol. J. 2019, 16, 1–22. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef]
- Astuti, I. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab. Syndr. 2020, 14, 407–412. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.F.; Xu, W.; Liu, S.W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Gregory, A.E.; Titball, R.; Williamson, D. Vaccine delivery using nanoparticles. Front. Cell Infect. Microbiol. 2013, 3, 13. [Google Scholar] [CrossRef]
- Neun, B.W.; Dobrovolskaia, M.A.; McNeil, S.E. Analysis of Nanoparticle-Adjuvant Properties In Vivo. Adv. Struct. Saf. Stud. 2017, 1682, 189–195. [Google Scholar] [CrossRef]
- Ballester, M.; Nembrini, C.; Dhar, N.; de Titta, A.; de Piano, C.; Pasquier, M.; Simeoni, E.; van der Vlies, A.J.; McKinney, J.D.; Hubbell, J.A.; et al. Nanoparticle conjugation and pulmonary delivery enhance the protective efficacy of Ag85B and CpG against tuberculosis. Vaccine 2011, 29, 6959–6966. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Mitchell, A.R.; Johnson, S.L.; Wagner-Bartak, C.; Morcol, T.; Bell, S.J.D. Calcium Phosphate Nanoparticle Adjuvant. Clin. Diagn. Lab. Immunol. 2000, 7, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; el Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Marks, F.; Clemens, J.D. Looking beyond COVID-19 vaccine phase 3 trials. Nat. Med. 2021, 27, 205–211. [Google Scholar] [CrossRef]
- Diaz-Arévalo, D.; Zeng, M. Nanoparticle-based vaccines: Opportunities and limitations. In Nanopharmaceuticals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 135–150. [Google Scholar]
- Livingston, E.H.; Malani, P.N.; Creech, C.B. The Johnson & Johnson Vaccine for COVID-19. JAMA 2021. [Google Scholar] [CrossRef]
- Gilchrist, R.K.; Medal, R.; Shorey, W.D.; Hanselman, R.C.; Parrott, J.C.; Taylor, C.B. Selective Inductive Heating of Lymph Nodes. Ann. Surg. 1957, 146, 596–606. [Google Scholar] [CrossRef]
- Dunnill, P.; Lilly, M.D. Letter: Purification of enzymes using magnetic bio-affinity materials. Biotechnol. Bioeng. 1974, 16, 987–990. [Google Scholar] [CrossRef]
- Widder, K.J.; Senyel, A.E.; Scarpelli, G.D. Magnetic microspheres: A model system of site specific drug delivery in vivo. Proc. Soc. Exp. Biol. Med. 1978, 158, 141–146. [Google Scholar] [CrossRef]
- Widder, K.J.; Senyei, A.E.; Ovadia, H.; Paterson, P.Y. Magnetic protein A microspheres: A rapid method for cell separation. Clin. Immunol. Immunopathol. 1979, 14, 395–400. [Google Scholar] [CrossRef]
- Krishnan, K.M. Biomedical Nanomagnetics: A Spin Through Possibilities in Imaging, Diagnostics, and Therapy. IEEE Trans. Magn. 2010, 46, 2523–2558. [Google Scholar] [CrossRef]
- Kudr, J.; Haddad, Y.A.E.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic Nanoparticles: From Design and Synthesis to Real World Applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Du, J. Superparamagnetic nanoparticles for biomedical applications. J. Mater. Chem. B 2020, 8, 354–367. [Google Scholar] [CrossRef] [PubMed]
- A Yamoah, M.; Moshref, M.; Sharma, J.; Chen, W.C.; A Ledford, H.; Lee, J.H.; Chavez, K.S.; Wang, W.; E López, J.; Lieu, D.K.; et al. Highly efficient transfection of human induced pluripotent stem cells using magnetic nanoparticles. Int. J. Nanomed. 2018, 13, 6073–6078. [Google Scholar] [CrossRef]
- Wahajuddin, S.A. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef] [PubMed]
- Amstad, E.; Zurcher, S.; Mashaghi, A.; Wong, J.Y.; Textor, M.; Reimhult, E. Surface Functionalization of Single Superparamagnetic Iron Oxide Nanoparticles for Targeted Magnetic Resonance Imaging. Small 2009, 5, 1334–1342. [Google Scholar] [CrossRef]
- McBain, S.C.; Yiu, H.H.; Dobson, J. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomed. 2008, 3, 169–180. [Google Scholar]
- Dakhore, S.; Nayer, B.; Hasegawa, K. Human Pluripotent Stem Cell Culture: Current Status, Challenges, and Advancement. Stem Cells Int. 2018, 2018, 7396905. [Google Scholar] [CrossRef]
- Musunuru, K.; Sheikh, F.; Gupta, R.M.; Houser, S.R.; Maher, K.O.; Milan, D.J.; Terzic, A.; Wu, J.C. Induced Pluripotent Stem Cells for Cardiovascular Disease Modeling and Precision Medicine: A Scientific Statement from the American Heart Association. Circ. Genom. Precis. Med. 2018, 11, e000043. [Google Scholar] [CrossRef]
- Paik, D.T.; Chandy, M.; Wu, J.C. Patient and Disease–Specific Induced Pluripotent Stem Cells for Discovery of Personalized Cardiovascular Drugs and Therapeutics. Pharmacol. Rev. 2020, 72, 320–342. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.K.; Neofytou, E.; Rhee, J.W.; Wu, J.C. Potential strategies to address the major clinical barriers facing stem cell regenerative therapy for cardiovascular disease: A review. JAMA Cardiol. 2016, 1, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhao, M.; Mattapally, S.; Chen, S.; Zhang, J. CCND2 overexpression enhances the regenerative potency of human induced pluripotent stem cell–derived cardiomyocytes: Remuscularization of injured ventricle. Circ. Res. 2018, 122, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Shiba, Y.; Gomibuchi, T.; Seto, T.; Wada, Y.; Ichimura, H.; Tanaka, Y.; Ogasawara, T.; Okada, K.; Shiba, N.; Sakamoto, K.; et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016, 538, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Shen, J.; Wang, Z.; Ji, J.; Song, H.; Wang, K.; Liu, B.-L.; Li, J.; Cui, D. Efficient preparation and labeling of human induced pluripotent stem cells by nanotechnology. Int. J. Nanomed. 2011, 6, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, J.-H.; Lee, H.J.; Jeon, K.; Lim, H.; Choi, H.y.; Lee, E.-R.; Park, S.H.; Park, J.-Y.; Hong, S.; et al. The generation of iPS cells using non-viral magnetic nanoparticle based transfection. Biomaterials 2011, 32, 6683–6691. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Noh, E.H.; Chung, H.-M.; Kang, M.-J.; Kim, E.Y.; Park, S.P. Efficient Generation of Virus-Free iPS Cells Using Liposomal Magnetofection. PLoS ONE 2012, 7, e45812. [Google Scholar] [CrossRef]
- Montserrat, N.; Garreta, E.; González, F.; Gutiérrez, J.; Eguizábal, C.; Ramos, V.; Borrós, S.; Carlos, J. Izpisua Belmonte Simple Generation of Human Induced Pluripotent Stem Cells Using Poly-β-amino Esters as the Non-viral Gene Delivery System Simple generation of human induced pluripotent stem cells using poly-beta-amino esters as the non-viral gene delivery system. J. Biol. Chem. 2011, 286, 12417–12428. [Google Scholar]
- Green, J.J.; Bhise, N.S.; Wahlin, K.J.; Zack, N.J. Evaluating the potential of poly(beta-amino ester) nanoparticles for reprogramming human fibroblasts to become induced pluripotent stem cells. Int. J. Nanomed. 2013, 8, 4641–4658. [Google Scholar] [CrossRef][Green Version]
- Wang, C.; Zhu, K.; Li, J.; Lai, H.; Yang, C.; Guo, C. Reprogramming fibroblasts to pluripotency using arginine-terminated polyamidoamine nanoparticles based non-viral gene delivery system. Int. J. Nanomed. 2014, 9, 5837–5847. [Google Scholar] [CrossRef]
- Varli, H.S.; Alkan, F.; Demirbilek, M.; Türkoğlu, N. A virus-free vector for the transfection of somatic cells to obtain IPSC. J. Nanoparticle Res. 2019, 21, 237. [Google Scholar] [CrossRef]
- Seo, B.J.; Hong, Y.J.; Do, J.T. Cellular Reprogramming Using Protein and Cell-Penetrating Peptides. Int. J. Mol. Sci. 2017, 18, 552. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Li, Q.; Jiang, L.; Deng, C.; Liu, Z.; Fu, Y.; Zhang, M.; Tan, H.; Feng, Y.; Shan, Z.; et al. Generation of Functional Human Cardiac Progenitor Cells by High-Efficiency Protein Transduction. STEM CELLS Transl. Med. 2015, 4, 1415–1424. [Google Scholar] [CrossRef]
- Kim, D.; Kim, C.-H.; Moon, J.-I.; Chung, Y.-G.; Chang, M.-Y.; Han, B.-S.; Ko, S.; Yang, E.; Cha, K.Y.; Lanza, R.; et al. Generation of Human Induced Pluripotent Stem Cells by Direct Delivery of Reprogramming Proteins. Cell Stem Cell 2009, 4, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wu, S.; Joo, J.Y.; Zhu, S.; Han, D.W.; Lin, T.; Trauger, S.; Bien, G.; Yao, S.; Zhu, Y.; et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 2009, 4, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Farkhani, S.M.; Valizadeh, A.; Karami, H.; Mohammadi, S.; Sohrabi, N.; Badrzadeh, F. Cell penetrating peptides: Efficient vectors for delivery of nanoparticles, nanocarriers, therapeutic and diagnostic molecules. Peptides 2014, 57, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.R.; Feng, T.; Zhang, Q.; Chan, H.Y.E.; Chau, Y. Co-Encapsulation and Co-Delivery of Peptide Drugs via Polymeric Nanoparticles. Polymers 2019, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, X.; Jia, J.; Fei, H. A peptide-lipid nanoparticle assembly platform with integrated functions for targeted cell delivery. J. Mater. Chem. B 2016, 4, 1535–1543. [Google Scholar] [CrossRef]
- Varanko, A.; Saha, S.; Chilkoti, A. Recent trends in protein and peptide-based biomaterials for advanced drug delivery. Adv. Drug Deliv. Rev. 2020, 156, 133–187. [Google Scholar] [CrossRef]
- Rowe, R.G.; Daley, G.Q. Induced pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Genet. 2019, 20, 377–388. [Google Scholar] [CrossRef]
- Chen, W.; Tsai, P.-H.; Hung, Y.; Chiou, S.-H.; Mou, C.-Y. Nonviral Cell Labeling and Differentiation Agent for Induced Pluripotent Stem Cells Based on Mesoporous Silica Nanoparticles. ACS Nano 2013, 7, 8423–8440. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.-H.; Jang, S.-F.; Mou, C.-Y. Mesoporous silica nanoparticles: A potential platform for generation of induced pluripotent stem cells? Nanomedicine 2014, 9, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S. Unmet needs in developing nanoparticles for precision medicine. Nanomedicine 2017, 12, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Beers, J.; Gulbranson, D.R.; George, N.; Siniscalchi, L.I.; Jones, J.; Thomson, J.A.; Chen, G. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat. Protoc. 2012, 7, 2029–2040. [Google Scholar] [CrossRef] [PubMed]
- Ohnuki, M.; Takahashi, K. Present and future challenges of induced pluripotent stem cells. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140367. [Google Scholar] [CrossRef]
- Li, X.-L.; Li, G.-H.; Fu, J.; Fu, Y.-W.; Zhang, L.; Chen, W.; Arakaki, C.; Zhang, J.-P.; Wen, W.; Zhao, M.; et al. Highly efficient genome editing via CRISPR-Cas9 in human pluripotent stem cells is achieved by transient BCL-XL overexpression. Nucleic Acids Res. 2018, 46, 10195–10215. [Google Scholar] [CrossRef]
- Rubinsky, B. Irreversible electroporation in medicine. Technol. Cancer Res. Treat. 2007, 6, 255–260. [Google Scholar] [CrossRef]
- Plank, C.; Scherer, F.; Schillinger, U.; Bergemann, C.; Anton, M. Magnetofection: Enhancing and Targeting Gene Delivery with Superparamagnetic Nanoparticles and Magnetic Fields. J. Liposome Res. 2003, 13, 29–32. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Elbaz, N.M.; Sedki, M.; Elgammal, A.; Yacoub, M.H. Magnetic nanoparticles-based drug and gene delivery systems for the treatment of pulmonary diseases. Nanomedicine 2017, 12, 387–402. [Google Scholar] [CrossRef]
- Yoon, T.-J.; Kim, J.S.; Kim, B.G.; Yu, K.N.; Cho, M.-H.; Lee, J.-K. Multifunctional Nanoparticles Possessing A? Magnetic Motor Effect? for Drug or Gene Delivery. Angew. Chem. Int. Ed. 2005, 44, 1068–1071. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.-P. Magnetic nanoparticles in nanomedicine: A review of recent advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef] [PubMed]
- Pickard, M.R.; Barraud, P.; Chari, D.M. The transfection of multipotent neural precursor/stem cell transplant populations with magnetic nanoparticles. Biomaterials 2011, 32, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.F.; Pickard, M.R.; Chari, D.M. Magnetic nanoparticle mediated transfection of neural stem cell suspension cultures is enhanced by applied oscillating magnetic fields. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 737–741. [Google Scholar] [CrossRef]
- Park, W.; Na Yang, H.; Ling, D.; Yim, H.; Kim, K.S.; Hyeon, T.; Na, K.; Park, K.-H. Multi-modal transfection agent based on monodisperse magnetic nanoparticles for stem cell gene delivery and tracking. Biomaterials 2014, 35, 7239–7247. [Google Scholar] [CrossRef]
- Pickard, M.R.; Adams, C.F.; Barraud, P.; Chari, D.M. Using Magnetic Nanoparticles for Gene Transfer to Neural Stem Cells: Stem Cell Propagation Method Influences Outcomes. J. Funct. Biomater. 2015, 6, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Pickard, M.R.; Adams, C.F.; Chari, D.M. Magnetic Nanoparticle-Mediated Gene Delivery to Two- and Three-Dimensional Neural Stem Cell Cultures: Magnet-Assisted Transfection and Multifection Approaches to Enhance Outcomes. Curr. Protoc. Stem Cell Biol. 2017, 40, 2D.19.1–2D.19.16. [Google Scholar] [CrossRef]
- Fernandes, F.; Kotharkar, P.; Chakravorty, A.; Kowshik, M.; Talukdar, I. Nanocarrier Mediated siRNA Delivery Targeting Stem Cell Differentiation. Curr. Stem Cell Res. Ther. 2020, 15, 155–172. [Google Scholar] [CrossRef]
- Underhill, S.M.; Wheeler, D.S.; Li, M.; Watts, S.D.; Ingram, S.L.; Amara, S.G. Amphetamine Modulates Excitatory Neurotransmission through Endocytosis of the Glutamate Transporter EAAT3 in Dopamine Neurons. Neuron 2014, 83, 404–416. [Google Scholar] [CrossRef]
- Wang, R.; Palavicini, J.P.; Wang, H.; Maiti, P.; Bianchi, E.; Xu, S.; Lloyd, B.N.; Dawson-Scully, K.; Kang, D.E.; Lakshmana, M.K. RanBP9 overexpression accelerates loss of dendritic spines in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2014, 69, 169–179. [Google Scholar] [CrossRef]
- Lee, A.S.; Wu, J.C. Imaging of embryonic stem cell migration in vivo. Methods Mol. Biol. 2011, 750, 101–114. [Google Scholar]
- Zhang, J.; Wilson, G.F.; Soerens, A.G.; Koonce, C.H.; Yu, J.; Palecek, S.P.; Thomson, J.A.; Kamp, T.J. Functional Cardiomyocytes Derived from Human Induced Pluripotent Stem Cells. Circ. Res. 2009, 104, e30–e41. [Google Scholar] [CrossRef]
- Yoshida, Y.; Yamanaka, S. Induced Pluripotent Stem Cells 10 Years Later: For Cardiac Applications. Circ. Res. 2017, 120, 1958–1968. [Google Scholar] [CrossRef]
- Wu, J.C.; Garg, P.; Yoshida, Y.; Yamanaka, S.; Gepstein, L.; Hulot, J.-S.; Knollmann, B.C.; Schwartz, P.J. Towards Precision Medicine with Human iPSCs for Cardiac Channelopathies. Circ. Res. 2019, 125, 653–658. [Google Scholar] [CrossRef]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Cozzarelli Prize Winner: Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef]
- Lalit, P.A.; Hei, D.J.; Raval, A.N.; Kamp, T.J. Induced Pluripotent Stem Cells for Post–Myocardial Infarction Repair: Remarkable Opportunities and Challenges. Circ. Res. 2014, 114, 1328–1345. [Google Scholar] [CrossRef]
- Tan, S.; Tao, Z.; Loo, S.; Su, L.; Chen, X.; Ye, L. Non-viral vector based gene transfection with human induced pluripotent stem cells derived cardiomyocytes. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- He, Q.; Wu, Z.; Huang, C. Hollow Magnetic Nanoparticles: Synthesis and Applications in Biomedicine. J. Nanosci. Nanotechnol. 2012, 12, 2943–2954. [Google Scholar] [CrossRef]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic Nanoparticles: Surface Effects and Properties Related to Biomedicine Applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef]
- Bao, Y.; Wen, T.; Samia, A.C.S.; Khandhar, A.P.; Krishnan, K.M. Magnetic nanoparticles: Material engineering and emerging applications in lithography and biomedicine. J. Mater. Sci. 2016, 51, 513–553. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Z.; Sun, J.; Ma, T.; Hua, F.; Shen, Z. A brief review of cytotoxicity of nanoparticles on mesenchymal stem cells in regenerative medicine. Int. J. Nanomed. 2019, 14, 3875–3892. [Google Scholar] [CrossRef] [PubMed]
- Fayol, D.; Luciani, N.; Lartigue, L.; Gazeau, F.; Wilhelm, C. Managing magnetic nanoparticle aggregation and cellular uptake: A precondition for efficient stem-cell differentiation and MRI tracking. Adv. Healthc. Mater. 2013, 2, 313–325. [Google Scholar] [CrossRef]
- van de Walle, A.; Sangnier, A.P.; Abou-Hassan, A.; Curcio, A.; Hémadi, M.; Menguy, N.; Lalatonne, Y.; Luciani, N.; Wilhelm, C. Biosynthesis of magnetic nanoparticles from nano-degradation products revealed in human stem cells. Proc. Natl. Acad. Sci. USA 2019, 116, 4044–4053. [Google Scholar] [CrossRef]
- Sahakyan, N.; Haddad, A.; Richardson, S.; Forcha-Etieundem, V.; Christopher, L.; Alharbi, H.; Campbell, R. Personalized Nanoparticles for Cancer Therapy: A Call for Greater Precision. Anti Cancer Agents Med. Chem. 2017, 17, 1033–1039. [Google Scholar] [CrossRef]
- De Matteis, L.; Martín-Rapún, R.; De La Fuente, J.M. Nanotechnology in Personalized Medicine: A Promising Tool for Alzheimer’s Disease Treatment. Curr. Med. Chem. 2018, 25, 4602–4615. [Google Scholar] [CrossRef]
- Ryu, J.H.; Lee, S.; Son, S.; Kim, S.H.; Leary, J.F.; Choi, K.; Kwon, I.C. Theranostic nanoparticles for future personalized medicine. J. Control. Release 2014, 190, 477–484. [Google Scholar] [CrossRef]
- Yaari, Z.; Da Silva, D.; Zinger, A.; Goldman, E.; Kajal, A.; Tshuva, R.; Barak, E.; Dahan, N.; Hershkovitz, D.; Goldfeder, M.; et al. Theranostic barcoded nanoparticles for personalized cancer medicine. Nat. Commun. 2016, 7, 13325. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamoah, M.A.; Thai, P.N.; Zhang, X.-D. Transgene Delivery to Human Induced Pluripotent Stem Cells Using Nanoparticles. Pharmaceuticals 2021, 14, 334. https://doi.org/10.3390/ph14040334
Yamoah MA, Thai PN, Zhang X-D. Transgene Delivery to Human Induced Pluripotent Stem Cells Using Nanoparticles. Pharmaceuticals. 2021; 14(4):334. https://doi.org/10.3390/ph14040334
Chicago/Turabian StyleYamoah, Megan A., Phung N. Thai, and Xiao-Dong Zhang. 2021. "Transgene Delivery to Human Induced Pluripotent Stem Cells Using Nanoparticles" Pharmaceuticals 14, no. 4: 334. https://doi.org/10.3390/ph14040334
APA StyleYamoah, M. A., Thai, P. N., & Zhang, X.-D. (2021). Transgene Delivery to Human Induced Pluripotent Stem Cells Using Nanoparticles. Pharmaceuticals, 14(4), 334. https://doi.org/10.3390/ph14040334





