Novel Cationic Meso-Arylporphyrins and Their Antiviral Activity against HSV-1
Abstract
1. Introduction
2. Results and Discussions
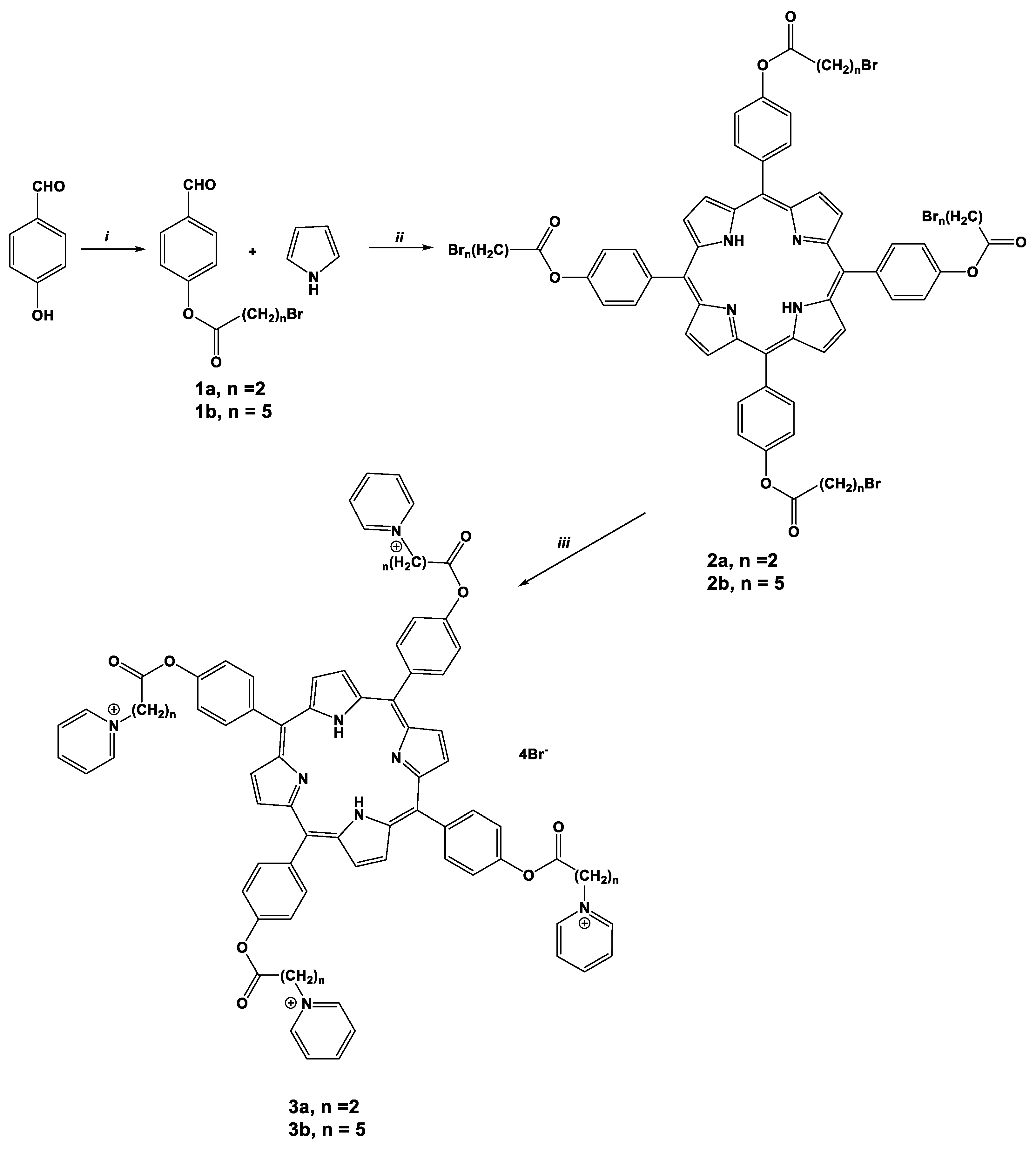
2.1. Synthesis of Porphyrins
2.2. Spectral Parameters and Photophysical Properties
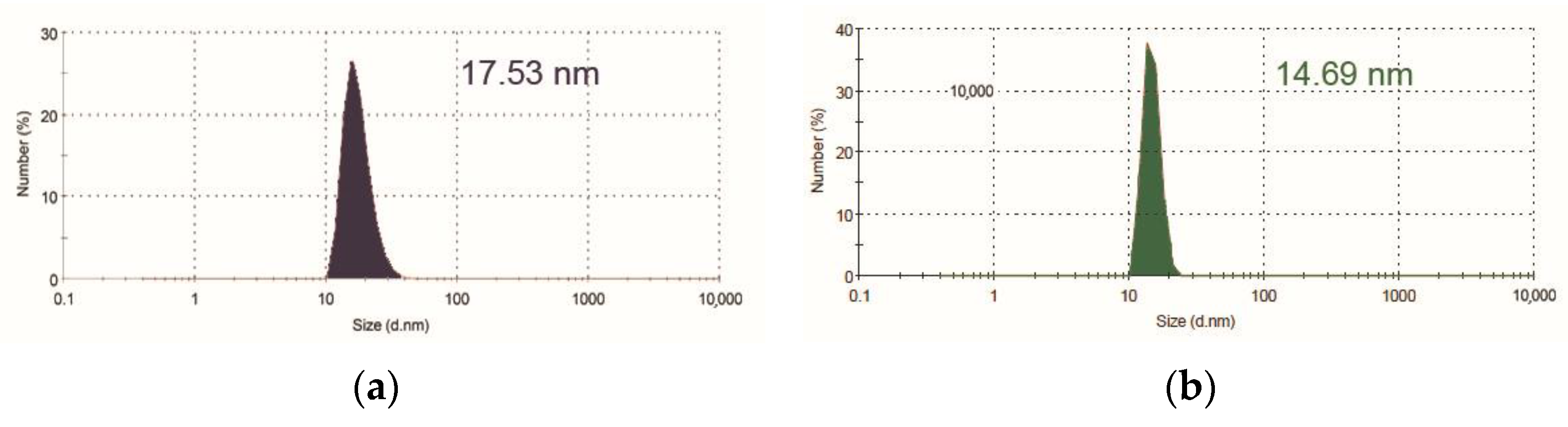
2.3. The Inclusion of Porphyrins in Pluronic F127 Micelles
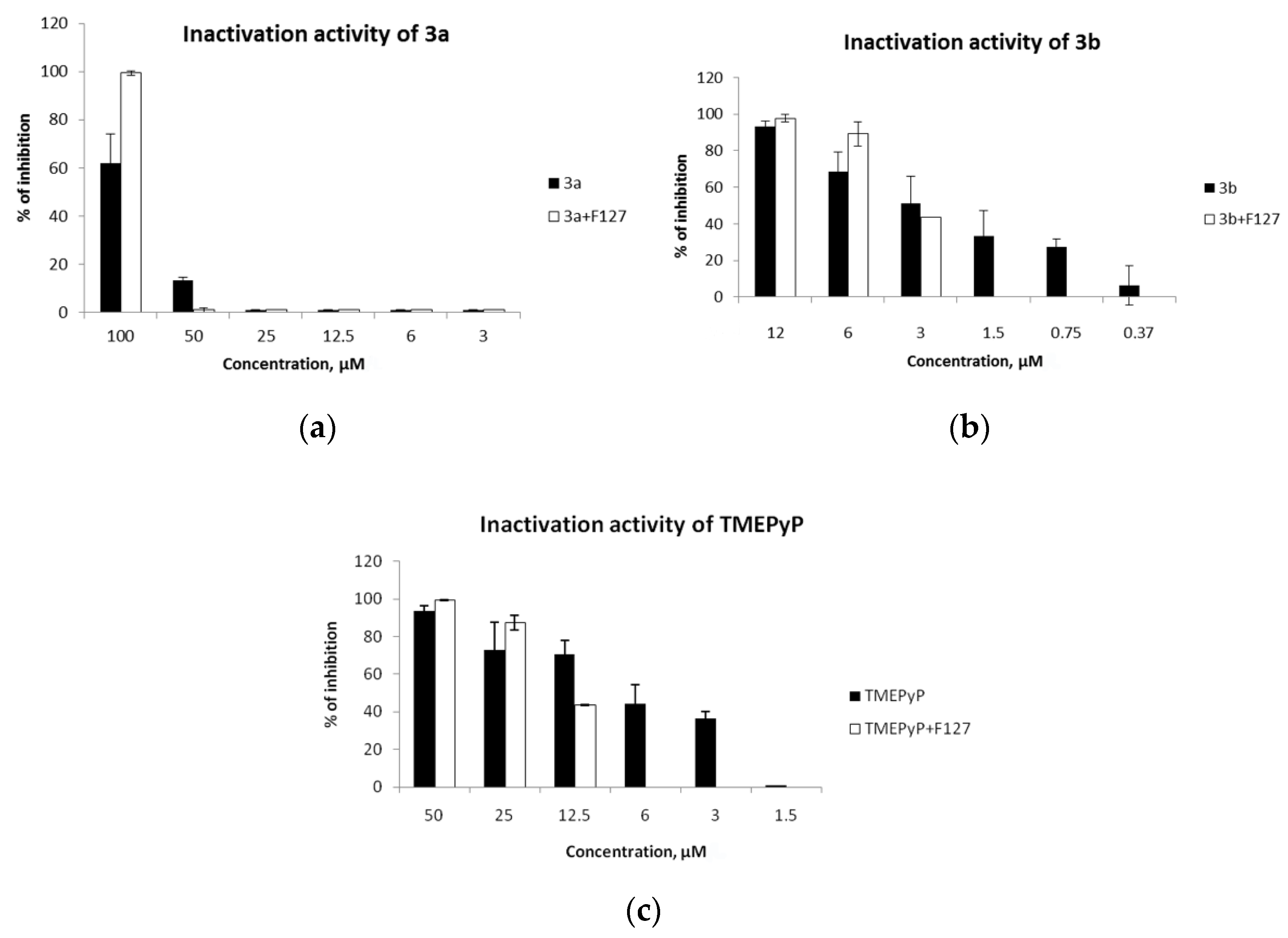
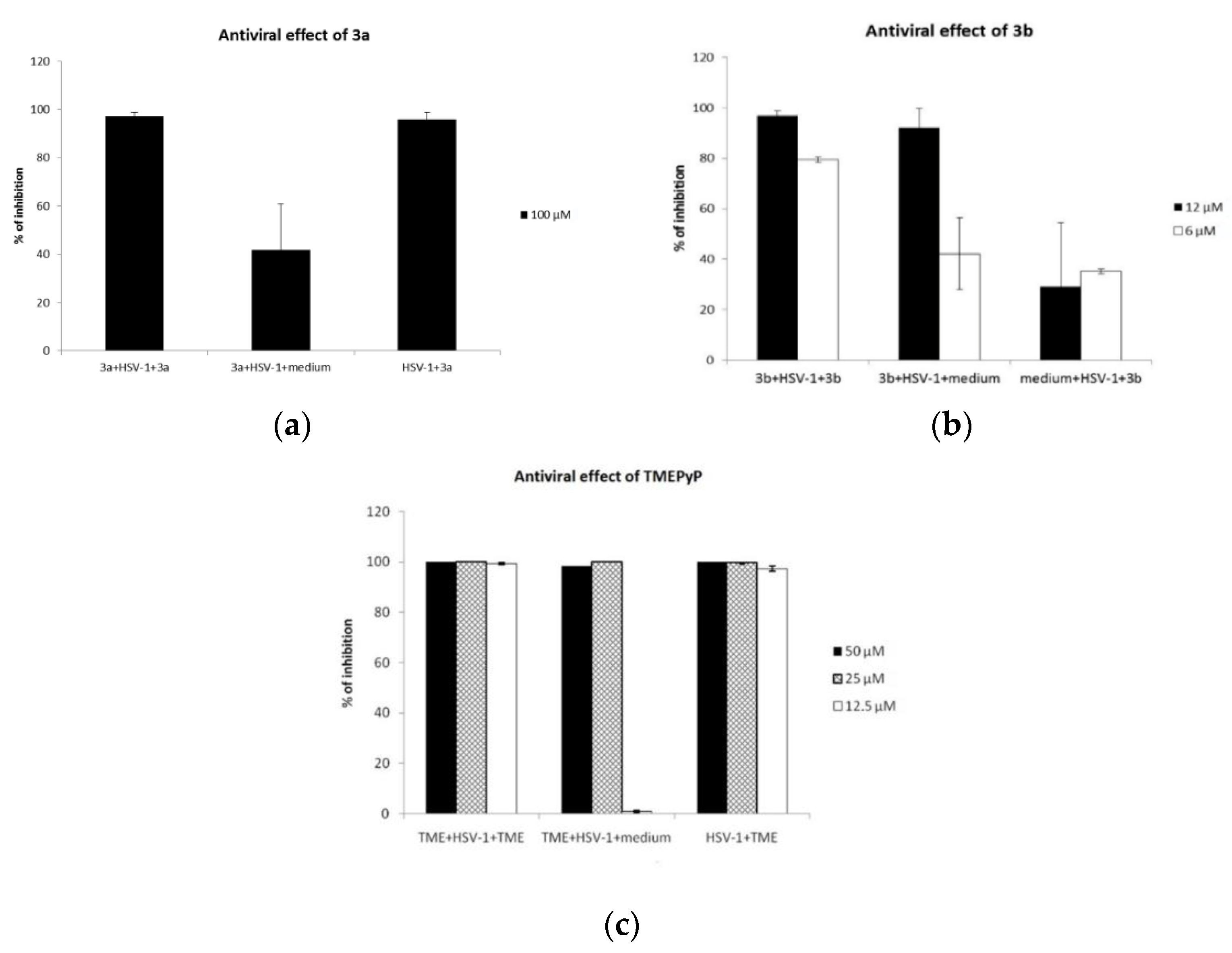
2.4. Virucidal Activity
2.5. Antiviral Activity
3. Materials and Methods
3.1. Cells
3.2. Virus
3.3. Cell Viability
3.4. Virucidal Effect
3.5. Influence of the Compounds on Virus Yield during Infection
3.6. Synthesis
3.6.1. Reagents
3.6.2. Measurements
3.6.3. General Procedure for the Substituted Benzaldehydes 1a,b Preparation
3.6.4. General Procedure for the Porphyrins 2a,b Preparation
3.6.5. General Procedure for the Preparation of Porphyrins 3a,b
3.6.6. n-Octanol/PBS Partition Coefficients
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chayavichitsilp, P.; Buckwalter, J.V.; Krakowski, A.C.; Friedlander, S.F. Herpes Simplex. Pediatr. Rev. 2009, 30, 119–130. [Google Scholar] [CrossRef]
- Cardone, G.; Heymann, J.B.; Cheng, N.; Trus, B.L.; Steven, A.C. Procapsid Assembly, Maturation, Nuclear Exit: Dynamic Steps in the Production of Infectious Herpesvirions. Adv. Exp. Med. Biol. 2011, 726, 423–439. [Google Scholar] [CrossRef]
- Imbronito, A.V.; Grande, S.R.; Freitas, N.M.; Okuda, O.; Lotufo, R.F.; Nunes, F.D. Detection of Epstein-Barr virus and human cytomegalovirus in blood and oral samples: Comparison of three sampling methods. J. Oral. Sci. 2008, 50, 25–31. [Google Scholar] [CrossRef]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.E.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS ONE 2015, 10, e0140765. [Google Scholar] [CrossRef] [PubMed]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.; Vickerman, P.; Newman, L.M.; Gottlieb, S.L. First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob. Health 2017, 5, e300–e309. [Google Scholar] [CrossRef]
- Nicoll, M.P.; Proença, J.T.; Efstathiou, S. The molecular basis of herpes simplex virus latency. FEMS Microbiol. Rev. 2012, 36, 684–705. [Google Scholar] [CrossRef]
- Mangold, C.A.; Szpara, M.L. Persistent Infection with Herpes Simplex Virus 1 and Alzheimer’s Disease—A Call to Study How Variability in Both Virus and Host may Impact Disease. Viruses 2019, 11, 966. [Google Scholar] [CrossRef]
- Khoury-Hanold, W.; Yordy, B.; Kong, P.; Kong, Y.; Ge, W.; Szigeti-Buck, K.; Ralevski, A.; Horvath, T.L.; Iwasaki, A. Viral Spread to Enteric Neurons Links Genital HSV-1 Infection to Toxic Megacolon and Lethality. Cell Host Microbe 2016, 19, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Bibert, S.; Piret, J.; Quinodoz, M.; Collinet, E.; Zoete, V.; Michielin, O.; Menasria, R.; Meylan, P.; Bihl, T.; Erard, V.; et al. Herpes simplex encephalitis in adult patients with MASP-2 deficiency. PLOS Pathog. 2019, 15, e1008168. [Google Scholar] [CrossRef]
- Grünewald, K.; Desai, P.; Winkler, D.C.; Heymann, J.B.; Belnap, D.M.; Baumeister, W.; Steven, A.C. Three-Dimensional Structure of Herpes Simplex Virus from Cryo-Electron Tomography. Science 2003, 302, 1396–1398. [Google Scholar] [CrossRef]
- Baumeister, J.; Fischer, R.; Eckenberg, P.; Henninger, K.; Ruebsamen-Waigmann, H.; Kleymann, G. Superior Efficacy of Helicase-Primase Inhibitor BAY 57–1293 for Herpes Infection and Latency in the Guinea Pig Model of Human Genital Herpes Disease. Antivir. Chem. Chemother. 2007, 18, 35–48. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E.; Field, H.J. Antiviral prodrugs—The development of successful prodrug strategies for antiviral chemotherapy. Br. J. Pharmacol. 2006, 147, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Elion, G.B.; Furman, P.A.; Fyfe, J.A.; De Miranda, P.; Beauchamp, L.; Schaeffer, H.J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl)guanine. Proc. Natl. Acad. Sci. USA 1977, 74, 5716–5720. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.M.; Erhard, K.; Hardwicke, M.A.; Lin, H.; McSurdy-Freed, J.; Plant, R.; Raha, K.; Rominger, C.M.; Schaber, M.D.; Spengler, M.D.; et al. Synthesis and structure–activity relationships of 1,2,4-triazolo [1,5-a]pyrimidin-7(3H)-ones as novel series of potent β isoform selective phosphatidylinositol 3-kinase inhibitors. Bioorganic Med. Chem. Lett. 2012, 22, 3198–3202. [Google Scholar] [CrossRef]
- Sergerie, Y.; Boivin, G. Hydroxyurea enhances the activity of acyclovir and cidofovir against herpes simplex virus type 1 resistant strains harboring mutations in the thymidine kinase and/or the DNA polymerase genes. Antivir. Res. 2008, 77, 77–80. [Google Scholar] [CrossRef]
- Urbani, M.; Grätzel, M.; Nazeeruddin, M.K.; Torres, T. Meso-Substituted Porphyrins for Dye-Sensitized Solar Cells. Chem. Rev. 2014, 114, 12330–12396. [Google Scholar] [CrossRef]
- Li, L.-L.; Diau, E.W.-G. Porphyrin-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 291–304. [Google Scholar] [CrossRef]
- Ezhov, A.V.; Vyalba, F.Y.; Zhdanova, K.A.; Mironov, A.F.; Zhizhin, K.Y.; Bragina, N.A. Synthesis and properties comparison of meso-arylporphyrins metal complexes as potential dyes for solar cells. Fine Chem. Technol. 2018, 13, 21–30. [Google Scholar] [CrossRef][Green Version]
- Ezhov, A.V.; Zhdanova, K.A.; Bragina, N.A.; Mironov, A.F. Approaches to Improve Efficiency of Dye-Sensitized Solar Cells. Macroheterocycles 2016, 9, 337–352. [Google Scholar] [CrossRef]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef]
- Castillero, P.; Roales, J.; Lopes-Costa, T.; Sánchez-Valencia, J.R.; Barranco, A.; González-Elipe, A.R.; Pedrosa, J.M. Optical Gas Sensing of Ammonia and Amines Based on Protonated Porphyrin/TiO2 Composite Thin Films. Sensors 2016, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, A.; Huang, Z.-H.; Wang, L.-N.; Kang, F. Porphyrin-Based Nanostructures for Photocatalytic Applications. Nanomaterials 2016, 6, 51. [Google Scholar] [CrossRef]
- Li, J.; Ren, Q.; Liu, L.; Sun, K.; Gu, X. Non-covalent conjugation of sulfonated porphyrins to polyethylenimine-grafted multiwalled carbon nanotubes as efficient recyclable heterogeneous catalysts for dihydroxynaphthalenes photooxidation. Mol. Catal. 2019, 470, 97–103. [Google Scholar] [CrossRef]
- Ezhov, A.V.; Vyal’Ba, F.Y.; Zhdanova, K.A.; Zhdanov, A.P.; Zhizhin, K.Y.; Kluykin, I.N.; Bragina, N.A.; Mironov, A.F. Synthesis of donor-π-acceptor porphyrins for DSSC: DFT-study, comparison of anchoring mode and effectiveness. J. Porphyr. Phthalocyanines 2020, 24, 538–547. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Buczek, K.; Trytek, M.; Deryło, K.; Borsuk, G.; Rybicka-Jasińska, K.; Gryko, D.; Cytryńska, M.; Tchórzewski, M. Bioactivity studies of porphyrinoids against microsporidia isolated from honeybees. Sci. Rep. 2020, 10, 11553. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.F.; Zhdanova, K.; Bragina, N. Nanosized vehicles for delivery of photosensitizers in photodynamic diagnosis and therapy of cancer. Russ. Chem. Rev. 2018, 87, 859–881. [Google Scholar] [CrossRef]
- Civantos, F.J.; Karakullukcu, B.; Biel, M.; Silver, C.E.; Rinaldo, A.; Saba, N.F.; Takes, R.P.; Poorten, V.V.; Ferlito, A. A Review of Photodynamic Therapy for Neoplasms of the Head and Neck. Adv. Ther. 2018, 35, 324–340. [Google Scholar] [CrossRef] [PubMed]
- Basso, G.; Cargnelutti, J.F.; Oliveira, A.L.; Acunha, T.V.; Weiblen, R.; Flores, E.F.; Iglesias, B.A. Photodynamic inactivation of selected bovine viruses by isomeric cationic tetra-platinated porphyrins. J. Porphyr. Phthalocyanines 2019, 23, 1041–1046. [Google Scholar] [CrossRef]
- Costa, L.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Almeida, A. Photodynamic Inactivation of Mammalian Viruses and Bacteriophages. Viruses 2012, 4, 1034–1074. [Google Scholar] [CrossRef]
- Tomé, J.P.; Silva, E.M.; Pereira, A.M.; Alonso, C.M.; Faustino, M.A.; Neves, M.G.; Tomé, A.C.; Cavaleiro, J.A.; Tavares, S.A.; Duarte, R.R.; et al. Synthesis of neutral and cationic tripyridylporphyrin-d-galactose conjugates and the photoinactivation of HSV-1. Bioorganic Med. Chem. 2007, 15, 4705–4713. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.M.; Giuntini, F.; Faustino, M.A.; Tomé, J.P.; Neves, M.G.; Tomé, A.C.; Silva, A.M.; Santana-Marques, M.G.; Ferrer-Correia, A.J.; Cavaleiro, J.A.; et al. Synthesis of cationic β-vinyl substituted meso-tetraphenylporphyrins and their in vitro activity against herpes simplex virus type. Bioorganic Med. Chem. Lett. 2005, 15, 3333–3337. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, D.; Timilsina, U.; Mazumder, Z.H.; Mukherjee, A.; Ghimire, D.; Markandey, M.; Upadhyaya, K.; Sharma, D.; Mishra, N.; Jha, T.; et al. Dual activity of amphiphilic Zn(II) nitroporphyrin derivatives as HIV-1 entry inhibitors and in cancer photodynamic therapy. Eur. J. Med. Chem. 2019, 174, 66–75. [Google Scholar] [CrossRef]
- Neris, R.L.S.; Figueiredo, C.M.; Higa, L.M.; Araujo, D.F.; Carvalho, C.A.M.; Verçoza, B.R.F.; Silva, M.O.L.; Carneiro, F.A.; Tanuri, A.; Gomes, A.M.O.; et al. Co-protoporphyrin IX and Sn-protoporphyrin IX inactivate Zika, Chikungunya and other arboviruses by targeting the viral envelope. Sci. Rep. 2018, 8, 9805. [Google Scholar] [CrossRef]
- Moura, N.M.; Esteves, M.; Vieira, C.; Rocha, G.M.; Faustino, M.A.F.; Almeida, A.; Cavaleiro, J.A.; Lodeiro, C.; Neves, M.G.P. Novel β-functionalized mono-charged porphyrinic derivatives: Synthesis and photoinactivation of Escherichia coli. Dyes Pigment. 2019, 160, 361–371. [Google Scholar] [CrossRef]
- Tsolekile, N.; Nelana, S.; Oluwafemi, O.S. Porphyrin as Diagnostic and Therapeutic Agent. Molecules 2019, 24, 2669. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Song, H.; Liao, W.; Zhuo, L.; Wang, G.; Wei, H.; Yang, Y.; Luo, S.; Zhou, Z. Visible light-induced biocidal activities and mechanistic study of neutral porphyrin derivatives against S. aureus and E. coli. J. Photochem. Photobiol. Biol. 2018, 185, 199–205. [Google Scholar] [CrossRef]
- Sampedro, A.G.; Tabero, A.; Mahamed, I.; Acedo, P. Multimodal use of the porphyrin TMPyP: From cancer therapy to antimicrobial applications. J. Porphyr. Phthalocyanines 2019, 23, 11–27. [Google Scholar] [CrossRef]
- Moghnie, S.; Tovmasyan, A.; Craik, J.; Batinic-Haberle, I.; Benov, L. Cationic amphiphilic Zn-porphyrin with high antifungal photodynamic potency. Photochem. Photobiol. Sci. 2017, 16, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Cormick, M.P.; Alvarez, M.G.; Rovera, M.; Durantini, E.N. Photodynamic inactivation of Candida albicans sensitized by tri- and tetra-cationic porphyrin derivatives. Eur. J. Med. Chem. 2009, 44, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Musetti, C.; Spagnul, C.; Mion, G.; Da Ros, S.; Gianferrara, T.; Sissi, C. DNA Targeting by Cationic Porphyrin-Ruthenium(II) Conjugates. ChemPlusChem 2014, 80, 158–168. [Google Scholar] [CrossRef]
- Artusi, S.; Nadai, M.; Perrone, R.; Biasolo, M.A.; Palù, G.; Flamand, L.; Calistri, A.; Richter, S.N. The Herpes Simplex Virus-1 genome contains multiple clusters of repeated G-quadruplex: Implications for the antiviral activity of a G-quadruplex ligand. Antivir. Res. 2015, 118, 123–131. [Google Scholar] [CrossRef]
- Sun, R.W.-Y.; Yu, W.-Y.; Sun, H.; Che, C.-M. In Vitro Inhibition of Human Immunodeficiency Virus Type-1 (HIV-1) Reverse Transcriptase by Gold(III) Porphyrins. ChemBioChem 2004, 5, 1293–1298. [Google Scholar] [CrossRef]
- Majiya, H.; Adeyemi, O.O.; Stonehouse, N.J.; Millner, P. Photodynamic inactivation of bacteriophage MS2: The A-protein is the target of virus inactivation. J. Photochem. Photobiol. Biol. 2018, 178, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Zupán, K.; Egyeki, M.; Tóth, K.; Fekete, A.; Herényi, L.; Módos, K.; Csik, G. Comparison of the efficiency and the specificity of DNA-bound and free cationic porphyrin in photodynamic virus inactivation. J. Photochem. Photobiol. Biol. 2008, 90, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Casteel, M.J.; Jayaraj, K.; Gold, A.; Ball, L.M.; Sobsey, M.D. Photoinactivation of Hepatitis A Virus by Synthetic Porphyrins. Photochem. Photobiol. 2004, 80, 294. [Google Scholar] [CrossRef]
- Tabish, T.A.; Hamblin, M.R. Multivalent nanomedicines to treat COVID-19: A slow train coming. Nano Today 2020, 35, 100962. [Google Scholar] [CrossRef] [PubMed]
- Alekseeva, A.; Kapkaeva, M.; Shcheglovitova, O.; Boldyrev, I.; Pazynina, G.; Bovin, N.; Vodovozova, E. Interactions of antitumour Sialyl Lewis X liposomes with vascular endothelial cells. Biochim. Biophys. Acta Biomembr. 2015, 1848, 1099–1110. [Google Scholar] [CrossRef]
- Lamch, Ł.; Pucek, A.; Kulbacka, J.; Chudy, M.; Jastrzębska, E.; Tokarska, K.; Bułka, M.; Brzózka, Z.; Wilk, K.A. Recent progress in the engineering of multifunctional colloidal nanoparticles for enhanced photodynamic therapy and bioimaging. Adv. Colloid Interface Sci. 2018, 261, 62–81. [Google Scholar] [CrossRef]
- Solov’Eva, A.B.; Savko, M.A.; Glagolev, N.N.; Aksenova, N.A.; Timashev, P.S.; Bragina, N.A.; Zhdanova, K.A.; Mironov, A.F. Photogeneration of Singlet Oxygen by Tetra(p-Hydroxyphenyl)porphyrins Modified with Oligo- and Polyalkylene Oxides. Russ. J. Phys. Chem. 2018, 92, 1621–1626. [Google Scholar] [CrossRef]
- Zhdanova, K.; Savelyeva, I.; Ignatova, A.; Gradova, M.; Gradov, O.; Lobanov, A.; Feofanov, A.; Mironov, A.; Bragina, N. Synthesis and photodynamic antimicrobial activity of amphiphilic meso-arylporphyrins with pyridyl moieties. Dyes Pigment. 2020, 181, 108561. [Google Scholar] [CrossRef]
- Lindsey, J.S.; Hsu, H.C.; Schreiman, I.C. Synthesis of tetraphenylporphyrins under very mild conditions. Tetrahedron Lett. 1986, 27, 4969–4970. [Google Scholar] [CrossRef]
- Fedulova, I.N.; Bragina, N.A.; Novikov, N.V.; Mironov, A.F.; Bykova, V.V.; Usol’Tseva, N.V.; Ananieva, G.A. Synthesis and mesomorphism of tetraphenylporphyrin derivatives. Mendeleev Commun. 2008, 18, 324–326. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Effects of substituents on the photophysical properties of symmetrical porphyrins. Dyes Pigment. 2013, 96, 440–448. [Google Scholar] [CrossRef]
- Kano, K.; Fukuda, K.; Wakami, H.; Nishiyabu, R.; Pasternack, R.F. Factors Influencing Self-Aggregation Tendencies of Cationic Porphyrins in Aqueous Solution. J. Am. Chem. Soc. 2000, 122, 7494–7502. [Google Scholar] [CrossRef]
- Tozoni, J.R.; Neto, N.B.; Ribeiro, C.; Pazin, W.; Ito, A.; Borissevitch, I.; Marletta, A. Relationship between porphyrin aggregation and formation of porphyrin ring structures in poly(n-alkyl methacrylate)/porphyrin blends. Polymers 2016, 102, 136–142. [Google Scholar] [CrossRef]
- Sigge, U.; Bindig, C.; Endisch, T.; Komatsu, E.; Tsuchida, J.; Voigt, J.H. Fuhrhop. Photophysical and photochemical prop-erties of porphyrin aggregates. Ber. Bunsenges. Phys. Chem. 1996, 100, 2070–2075. [Google Scholar] [CrossRef]
- Gradova, M.A.; Gradov, O.; Zhdanova, K.A.; Bragina, N.A.; Lobanov, A.V. Self-assembly of amphiphilic meso-aryl-substituted porphyrin derivatives in the presence of surfactants. J. Porphyr. Phthalocyanines 2020, 24, 505–514. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Mathias, J.P.; Seto, C.T. Molecular self-assembly and nanochemistry: A chemical strategy for the synthesis of nanostructures. Science 1991, 254, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Managa, M.; Ngoy, B.P.; Mafukidze, D.; Nyokong, T. Incorporation of metal free and Ga 5,10,15,20-tetrakis(4-bromophenyl) porphyrin into Pluronic F127-folic acid micelles. J. Lumin 2018, 194, 739–746. [Google Scholar] [CrossRef]
- Managa, M.; Achadu, O.J.; Nyokong, T. Photophysical studies of graphene quantum dots—Pyrene-derivatized porphyrins conjugates when encapsulated within Pluronic F127 micelles. Dyes Pigment. 2018, 148, 405–416. [Google Scholar] [CrossRef]
- Vilsinski, B.H.; Aparicio, J.L.; Pereira, P.C.D.S.; Fávaro, S.L.; Campanholi, K.S.S.; Gerola, A.P.; Tessaro, A.L.; Hioka, N.; Caetano, W. Physico-chemical properties of meso-tetrakis(p-methoxyphenyl)porphyrin (tmpp) incorporated into pluronic tm p-123 and f-127 polymeric micelles. Química Nova 2014, 37, 1650–1656. [Google Scholar] [CrossRef]
- Kadish, K.M.; Smith, K.M.; Guilard, R. The Porphyrin Handbook; Synthesis and Organic Chemistry; Academic Press: New York, NY, USA, 2000; Volume 1. [Google Scholar]


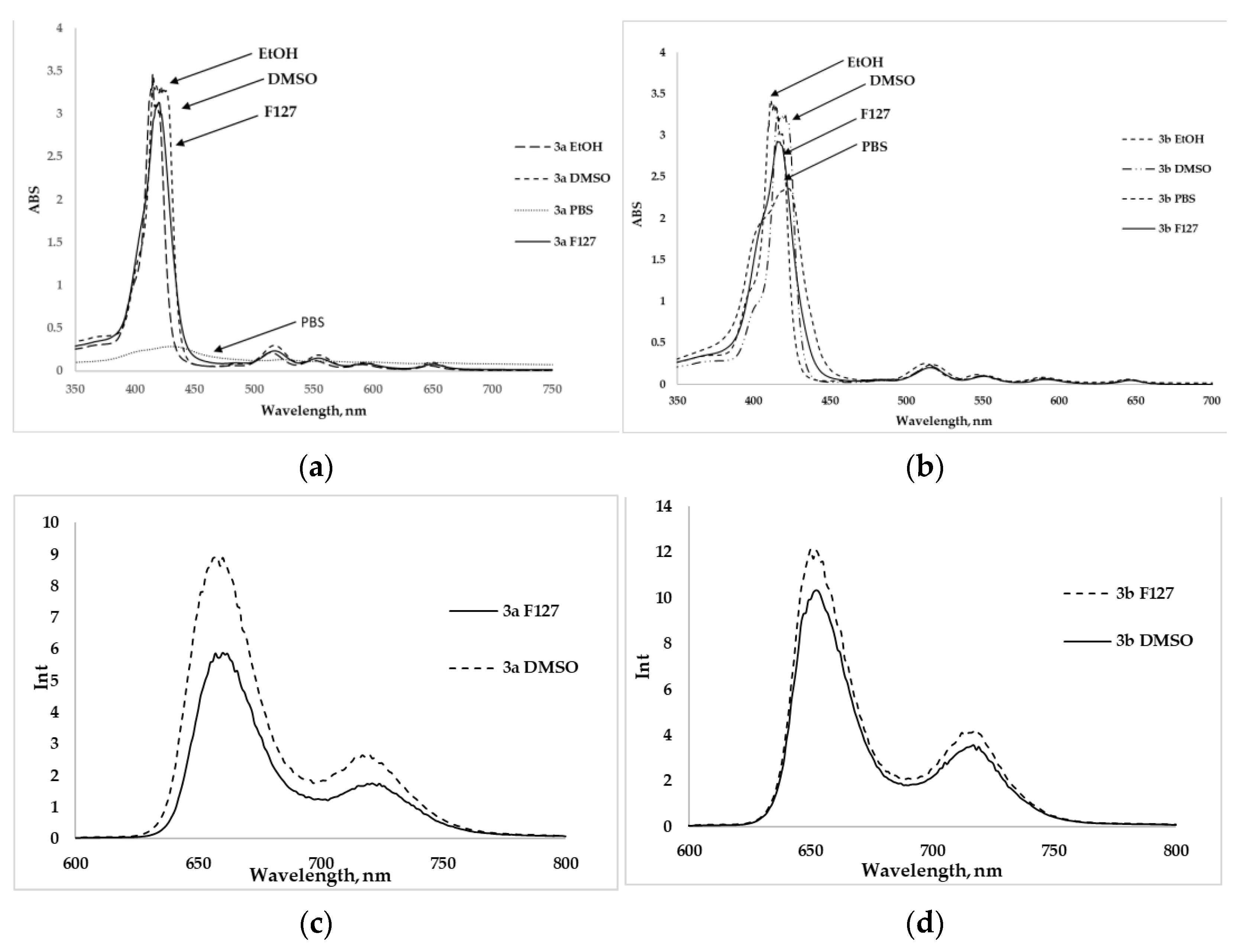

| No. | λabs (nm) (ε 10−3 L mol−1 cm−1) a | λem (nm) | ΦF b | Stokes Shift | LogP c | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Soret | QI | QII | QIII | QIV | Q (0,0) | Q (0,1) | ||||
| 3a | 415 (312) | 512 (14.5) | 546 (6.2) | 590 (3.7) | 645 (3.5) | 651 | 721 | 0.14 | 238 | −4.86 |
| 3b | 418 (328) | 513 (13.37) | 548 (6.07) | 592 (3.64) | 648 (3.56) | 652 | 723 | 0.13 | 236 | −4.6 |
| Compounds | CC50, µM | IC50, µM | ||
|---|---|---|---|---|
| PBS | F127 | PBS | F127 | |
| 3a | >100 | >100 | 53.6 | 99.8 |
| 3b | 43.0 | 27.0 | 3.4 | 3.6 |
| TMePyP | >100 | >100 | 2.3 | 13.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhdanova, K.A.; Savelyeva, I.O.; Ezhov, A.V.; Zhdanov, A.P.; Zhizhin, K.Y.; Mironov, A.F.; Bragina, N.A.; Babayants, A.A.; Frolova, I.S.; Filippova, N.I.; et al. Novel Cationic Meso-Arylporphyrins and Their Antiviral Activity against HSV-1. Pharmaceuticals 2021, 14, 242. https://doi.org/10.3390/ph14030242
Zhdanova KA, Savelyeva IO, Ezhov AV, Zhdanov AP, Zhizhin KY, Mironov AF, Bragina NA, Babayants AA, Frolova IS, Filippova NI, et al. Novel Cationic Meso-Arylporphyrins and Their Antiviral Activity against HSV-1. Pharmaceuticals. 2021; 14(3):242. https://doi.org/10.3390/ph14030242
Chicago/Turabian StyleZhdanova, Kseniya A., Inga O. Savelyeva, Artem V. Ezhov, Andrey P. Zhdanov, Konstantin Yu. Zhizhin, Andrey F. Mironov, Natal’ya A. Bragina, Alla A. Babayants, Irina S. Frolova, Nadezhda I. Filippova, and et al. 2021. "Novel Cationic Meso-Arylporphyrins and Their Antiviral Activity against HSV-1" Pharmaceuticals 14, no. 3: 242. https://doi.org/10.3390/ph14030242
APA StyleZhdanova, K. A., Savelyeva, I. O., Ezhov, A. V., Zhdanov, A. P., Zhizhin, K. Y., Mironov, A. F., Bragina, N. A., Babayants, A. A., Frolova, I. S., Filippova, N. I., Scliankina, N. N., & Scheglovitova, O. N. (2021). Novel Cationic Meso-Arylporphyrins and Their Antiviral Activity against HSV-1. Pharmaceuticals, 14(3), 242. https://doi.org/10.3390/ph14030242







