Identification of Anti-Inflammatory and Anti-Proliferative Neolignanamides from Warburgia ugandensis Employing Multi-Target Affinity Ultrafiltration and LC-MS
Abstract
1. Introduction
2. Results and Discussion
2.1. Anti-Inflammatory Activities of Different Extracts and Fractions of Stem Barks from W. ugandensis
2.2. AUF-HPLC-MS/MS Analysis of COX-2/5-LOX/Top I/Top II Ligands
2.2.1. Screening for the Potential COX-2/5-LOX/Top I/Top II Ligands in WE-P
2.2.2. Structural Identification of the Potential COX-2/5-LOX/Top I/Top II Ligands
2.3. Compounds Isolated and Identified from WE-P
2.4. Anti-Inflammatory Activities of Compounds Isolated and Identified from WE-P
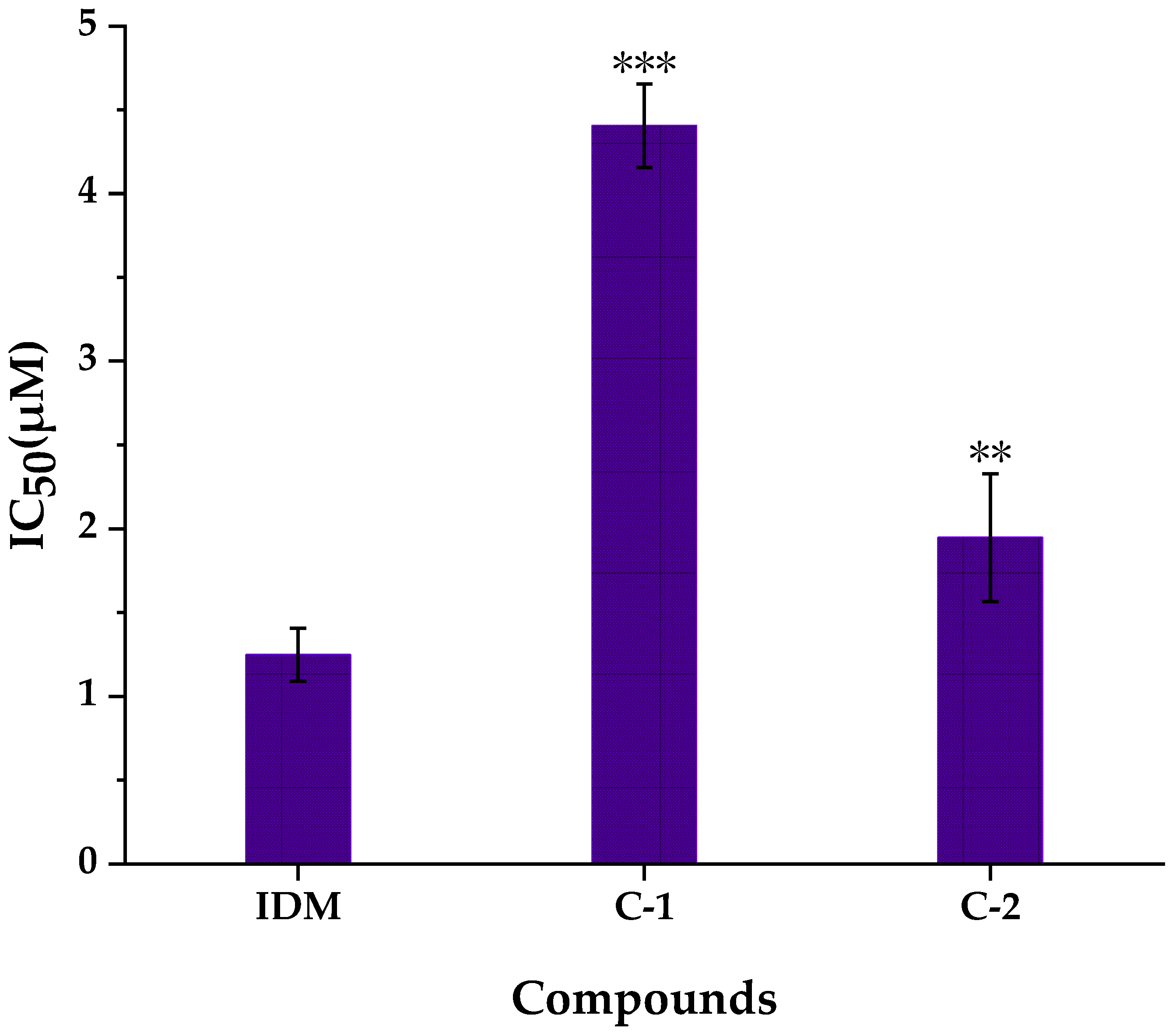
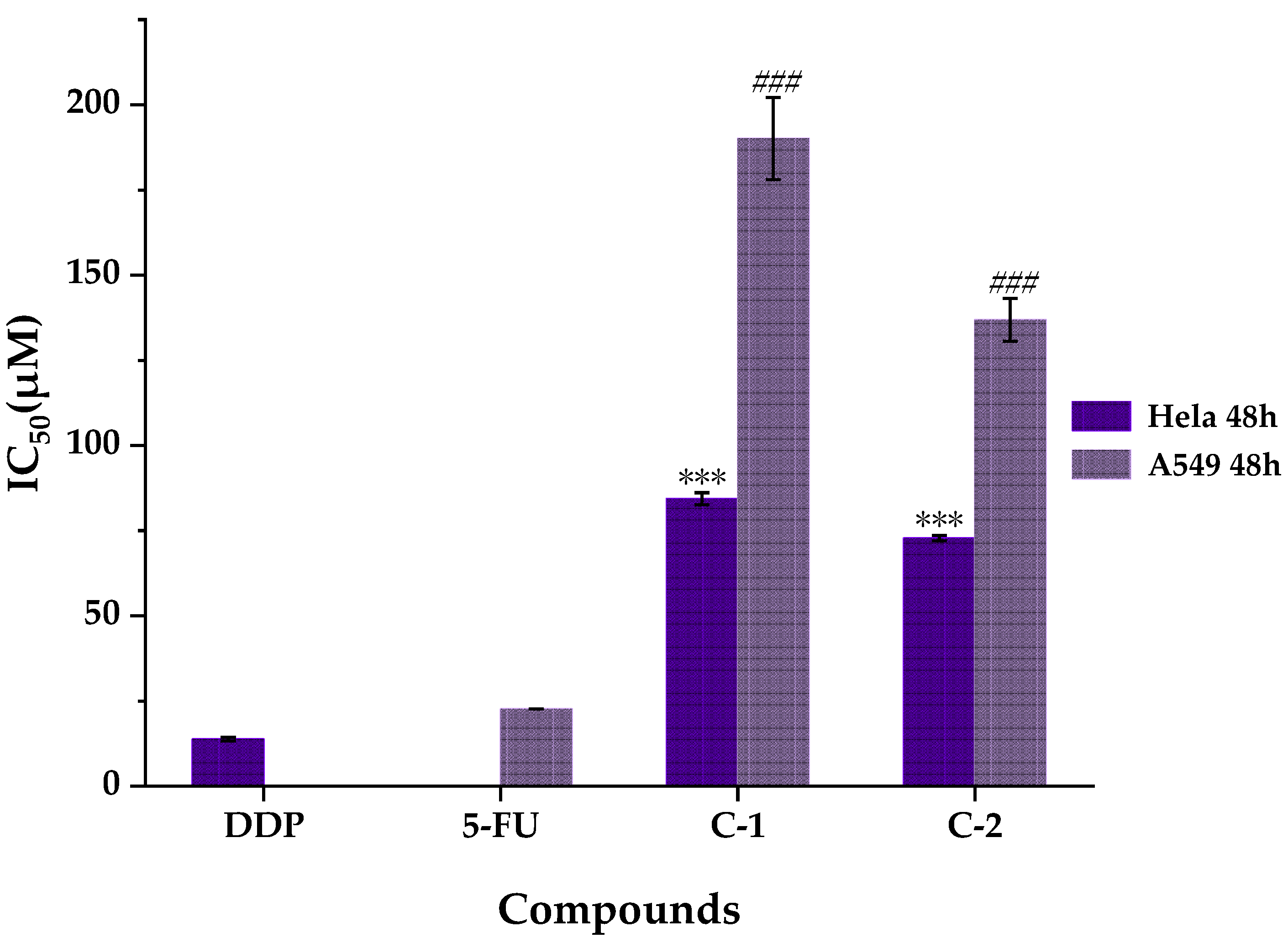
2.5. Anti-Proliferative Activities of Compounds Isolated and Identified from WE-P
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals and Reagents
3.3. Instruments and Materials
3.4. Preparation of Samples
3.4.1. Preparation of Extracts and Fractions
3.4.2. Isolation of Compounds from WE-P
3.5. Screening and Identification of the Potential Ligands with AUF-HPLC-MS/MS
3.5.1. Affinity Ultrafiltration Procedures
3.5.2. HPLC-UV/ESI-MS/MS Analysis
3.6. In Vitro COX-2 Inhibitory Assay
3.7. Cell Culture and Cell Proliferative Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leonard, C.M.; Viljoen, A.M. Warburgia: A comprehensive review of the botany, traditional uses and phytochemistry. J. Ethnopharmacol. 2015, 165, 260–285. [Google Scholar] [CrossRef] [PubMed]
- Wube, A.A.; Gibbons, S.; Asres, K.; Streit, B.; Adams, M.; Bauer, R.; Bucar, F. In vitro 12(S)-HETE and leukotriene metabolism inhibitory activity of sesquiterpenes of Warburgia ugandensis. Planta. Med. 2006, 72, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Litaudon, M.; Krief, S.; Martin, M.T.; Kasenene, J.; Kiremire, B.; Dumontet, V.; Guéritte, F. Ugandenial A, a new drimane-type sesquiterpenoid from Warburgia ugandensis. Molecules 2009, 14, 3844–3850. [Google Scholar] [CrossRef] [PubMed]
- Mbieda, J.N.; Lissouck, D.; Onguene, P.P.A.; Amana, B.A.; Ongagna, J.M.; Toze, F.A.; Mama, D.B. Insight into the antioxidant and antiradical properties of colorotane sesquiterpenes extracted from Warburgia ugandensis: Theoretical evaluation. Struct. Chem. 2021, 32, 667–677. [Google Scholar] [CrossRef]
- Mbwambo, Z.H.; Erasto, P.; Innocent, E.; Masimba, P.J. Antimicrobial and cytotoxic activities of fresh leaf extracts of Warburgia ugandensis. Tanzania. J. Health Res. 2009, 11, 75–78. [Google Scholar] [CrossRef][Green Version]
- Merawie, Y.; Sahile, S.; Moges, F.; Husen, A. Antimicrobial activity of crude and semi-purified fractions of Warburgia ugandensis against some pathogens. J. Coast. Life Med. 2013, 1, 184–191. [Google Scholar]
- Drage, S.; Mitter, B.; Engelmeier, D.; Chobot, V.; Gorfer, M.; Muchugi, A.; Jamnadass, R.H.; Sessitsch, A.; Hadacek, F. Antimicrobial drimane sesquiterpenes contribute to balanced antagonism but do not structure bacterial and fungal endophytes in the African pepper bark tree Warburgia ugandensis. Front. Ecol. Evol. 2017, 5, 138. [Google Scholar] [CrossRef]
- Drage, S.; Mitter, B.; Tröls, C.; Muchugi, A.; Jamnadass, R.H.; Sessitsch, A.; Hadacek, F. Antimicrobial drimane sesquiterpenes and their effect on endophyte communities in the medical tree Warburgia ugandensis. Front. Microbiol. 2014, 5, 13. [Google Scholar] [CrossRef]
- Kubo, I.; Fujita, K.; Lee, S.H.; Ha, T.J. Antibacterial activity of polygodial. Phytother. Res. 2005, 19, 1013–1017. [Google Scholar] [CrossRef]
- Ngure, P.K.; Tonui, W.K.; Ingonga, J.; Mutai, C.; Kigondu, E.; Ng’ang’a, Z.; Rukunga, G.; Kimutai, A. In vitro antileishmanial activity of extracts of Warburgia ugandensis (Canellaceae), a Kenyan medicinal plant. J. Med. Plants Res. 2009, 3, 61–66. [Google Scholar]
- Irungu, B.N.; Rukunga, G.M.; Mungai, G.M.; Muthaura, C.N. In vitro antiplasmodial and cytotoxicity activities of 14 medicinal plants from Kenya. S. Afr. J. Bot. 2007, 73, 204–207. [Google Scholar] [CrossRef]
- Lacroix, D.; Prado, S.; Kamoga, D.; Kasenene, J.; Namukobe, J.; Krief, S.; Dumontet, V.; Mouray, E.; Bodo, B.; Brunois, F. Antiplasmodial and cytotoxic activities of medicinal plants traditionally used in the village of Kiohima, Uganda. J. Ethnopharmacol. 2011, 133, 850–855. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet. Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer. J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Banik, K.; Ranaware, A.M.; Harsha, C.; Nitesh, T.; Girisa, S.; Deshpande, V.; Fan, L.; Nalawade, S.P.; Sethi, G.; Kunnumakkara, A.B. Piceatannol: A natural stilbene for the prevention and treatment of cancer. Pharmacol. Res. 2020, 153, 104635. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Hashemi, F.; Moghadam, E.R.; Owrang, M.; Hashemi, F.; Makvandi, P.; Goharrizi, M.A.S.B.; Najafi, M.; et al. Lung cancer cells and their sensitivity/resistance to cisplatin chemotherapy: Role of microRNAs and upstream mediators. Cell. Signal. 2021, 78, 109871. [Google Scholar] [CrossRef] [PubMed]
- Baglini, E.; Salerno, S.; Barresi, E.; Robello, M.; Da Settimo, F.; Taliani, S.; Marini, A.M. Multiple topoisomerase I (TopoI), topoisomerase II (TopoII) and tyrosyl-DNA phosphodiesterase (TDP) inhibitors in the development of anticancer drugs. Eur. J. Pharm. Sci. 2021, 156, 105594. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.H.H.; Abbas, S.H.; Hayallah, A.M.; Abuo-Rahma, G.E.D.A.; Mostafa, Y.A. Novel urea linked ciprofloxacin-chalcone hybrids having antiproliferative topoisomerases I/II inhibitory activities and caspases-mediated apoptosis. Bioorg. Chem. 2021, 106, 104422. [Google Scholar] [CrossRef]
- Chang, J.Y.; Guo, X.; Chen, H.X.; Jiang, Z.L.; Fu, Q.; Wang, H.K.; Bastow, K.F.; Zhu, X.K.; Guan, J.; Lee, K.H.; et al. Unique biochemical, cytotoxic, and antitumor activity of camptothecin and 4β-amino-4′-O-demethylepipodophyllotoxin conjugates. Biochem. Pharmacol. 2000, 59, 497–508. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef]
- Pommier, Y.; Leo, E.; Zhang, H.L.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Rolle, C.E.; Kanteti, R.; Surati, M.; Nandi, S.; Dhanasingh, I.; Yala, S.; Tretiakova, M.; Arif, Q.; Hembrough, T.; Brand, T.M.; et al. Combined MET inhibition and topoisomerase I inhibition block cell growth of small cell lung cancer. Mol. Cancer Ther. 2014, 13, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pandey, V.P.; Yadav, K.; Yadav, A.; Dwivedi, U.N. Natural products as anti-cancerous therapeutic molecules targeted towards topoisomerases. Curr. Protein Pept. Sci. 2020, 21, 1103–1142. [Google Scholar] [CrossRef]
- Xie, L.L.; Lee, D.Y.W.; Shang, Y.; Cao, X.T.; Wang, S.Q.; Liao, J.; Zhang, T.; Dai, R.H. Characterization of spirostanol glycosides and furostanol glycosides from Anemarrhenae rhizoma as dual targeted inhibitors of 5-lipoxygenase and cyclooxygenase-2 by employing a combination of affinity ultrafiltration and HPLC/MS. Phytomedicine 2020, 77, 153284. [Google Scholar] [CrossRef]
- Tiwari, M. The role of serratiopeptidase in the resolution of inflammation. Asian J. Pharm. Sci. 2017, 12, 209–215. [Google Scholar] [CrossRef]
- Ouellet, M.; Riendeau, D.; Percival, M.D. A high level of cyclooxygenase-2 inhibitor selectivity is associated with a reduced interference of platelet cyclooxygenase-1 inactivation by aspirin. Proc. Natl. Acad. Sci. USA 2001, 98, 14583–14588. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Yun, C.W.; Park, W.K.; Kong, J.Y.; Kim, K.S.; Park, Y.; Lee, S.; Kim, B.K. Modulation of the activity of pro-inflammatory enzymes, COX-2 and iNOS, by chrysin derivatives. Pharmacol. Res. 2004, 49, 37–43. [Google Scholar] [CrossRef]
- Rajaram, A.; Vanaja, G.R.; Vyakaranam, P.; Rachamallu, A.; Reddy, G.V.; Anilkumar, K.; Arunasree, K.M.; Dhyani, A.; Prasad, N.K.; Sharma, S.; et al. Anti-inflammatory profile of Aegle marmelos (L) Correa (Bilva) with special reference to young roots grown in different parts of India. J. Ayurveda Integr. Med. 2018, 9, 90–98. [Google Scholar] [CrossRef]
- Zhao, A.Q.; Li, L.; Li, B.; Zheng, M.Z.; Tsao, R. Ultrafiltration LC-ESI-MSn screening of 5-lipoxygenase inhibitors from selected Chinese medicinal herbs Saposhnikovia divaricata, Smilax glabra, Pueraria lobata and Carthamus tinctorius. J. Funct. Foods 2016, 24, 244–253. [Google Scholar] [CrossRef]
- Zhu, H.B.; Liu, S.; Li, X.; Song, F.R.; Liu, Z.Q.; Liu, S.Y. Bioactivity fingerprint analysis of cyclooxygenase-2 ligands from radix Aconiti by ultrafiltration-UPLC-MSn. Anal. Bioanal. Chem. 2013, 405, 7437–7445. [Google Scholar] [CrossRef]
- Petrovic, N.; Murray, M. Using N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD) to assay cyclooxygenase activity in vitro. Methods Mol. Biol. 2010, 594, 129–140. [Google Scholar]
- Zimmermann, K.C.; Sarbia, M.; Weber, A.A.; Borchard, F.; Gabbert, H.E.; Schrör, K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999, 59, 198–204. [Google Scholar] [PubMed]
- El-Dash, Y.; Khalil, N.A.; Ahmed, E.M.; Hassan, M.S.A. Synthesis and biological evaluation of new nicotinate derivatives as potential anti-inflammatory agents targeting COX-2 enzyme. Bioorg. Chem. 2021, 107, 104610. [Google Scholar] [CrossRef]
- Wube, A.A.; Bucar, F.; Gibbons, S.; Asres, K. Sesquiterpenes from Warburgia ugandensis and their antimycobacterial activity. Phytochemistry 2005, 66, 2309–2315. [Google Scholar] [CrossRef]
- Maroyi, A. The genus Warburgia: A review of its traditional uses and pharmacology. Pharm. Biol. 2014, 52, 378–391. [Google Scholar] [CrossRef]
- Munigunti, R.; Mulabagal, V.; Calderón, A.I. Screening of natural compounds for ligands to PfTrxR by ultrafiltration and LC-MS based binding assay. J. Pharm. Biomed. Anal. 2011, 55, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Cai, H.; Li, W.; Cai, B. Ultrafiltration coupled with high-performance liquid chromatography and quadrupole-time-of-flight mass spectrometry for screening lipase binders from different extracts of Dendrobium officinale. Anal. Bioanal. Chem. 2015, 407, 6081–6093. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.L.; Wu, J.L.; Li, N.; Guo, M.Q. Screening for anti-proliferative and anti-inflammatory components from Rhamnus davurica Pall. using bio-affinity ultrafiltration with multiple drug targets. Anal. Bioanal. Chem. 2018, 410, 3587–3595. [Google Scholar] [CrossRef] [PubMed]
- Fratoni, E.; Claudino, V.D.; Yunes, R.A.; Franchi, G.C., Jr.; Nowill, A.E.; Filho, V.C.; Monache, F.D.; Malheiros, A. Further drimane sesquiterpenes from Drimys brasiliensis stem barks with cytotoxic potential. Naunyn. Schmiedebergs. Arch. Pharmacol. 2016, 389, 791–797. [Google Scholar] [CrossRef]
- King, R.R.; Calhoun, L.A. Characterization of cross-linked hydroxycinnamic acid amides isolated from potato common scab lesions. Phytochemistry 2005, 66, 2468–2473. [Google Scholar] [CrossRef]
- Madikane, V.E.; Bhakta, S.; Russell, A.J.; Campbell, W.E.; Claridge, T.D.; Elisha, B.G.; Davies, S.G.; Smith, P.; Sim, E. Inhibition of mycobacterial arylamine N-acetyltransferase contributes to anti-mycobacterial activity of Warburgia salutaris. Bioorg. Med. Chem. 2007, 15, 3579–3586. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Hossain, R.; Adhikari, A.; Ali, Z.; Yousuf, S.; Choudhary, M.I.; Ali, M.Y.; Zaman, M.S. Drimane-type sesquiterpenes from Polygonum hydropiper. Planta Med. 2011, 77, 1848–1851. [Google Scholar] [CrossRef]
- Kioy, D.; Gray, A.I.; Waterman, P.G. A comparative study of the stem-bark drimane sesquiterpenes and leaf volatile oils of Warburgia ugandensis, and W. Stuhlmannii. Phytochemistry 1990, 29, 3535–3538. [Google Scholar] [CrossRef]
- Appel, H.H.; Bond, R.P.M.; Overton, K.H. Sesquiterpenoids-III the constitution and stereochemistry of valdiviolide, fuegin, winterin and futronolide. Tetrahedron 1963, 19, 635–641. [Google Scholar] [CrossRef]
- Fukuyama, Y.; Sato, T.; Miura, I.; Asakawa, Y. Drimane-type sesqui- and norsesquiterpenoids from Polygonum hydropiper. Phytochemistry 1985, 24, 1521–1524. [Google Scholar] [CrossRef]
- Bastos, J.K.; Kaplan, M.A.C.; Gottlieb, O.R. Drimane-type sesquiterpenoids as chemosystematic markers of Canellaceae. J. Braz. Chem. Soc. 1999, 10, 136–139. [Google Scholar] [CrossRef][Green Version]
- Rajab, M.S.; Ndegwa, J.M. 11α-Hydroxy muzigadiolide, a novel drimane sesquiterpene from the stem bark of warburgia ugandensis. Bull. Chem. Soc. Ethiop. 2000, 14, 45–49. [Google Scholar] [CrossRef]
- Mudyiwa, M.; Rajab, M.S.; Fronczek, F.R.; Watkins, S.F. (1R,4R,5aS,7S,9aS)-7,9a-Dimethyl-6-methylene-3-oxo-1,3,4,5,5a,6,7,8,9,9a-decahydronaphtho-[1,2-c]furan-1,4-diyldiacetate. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, 2612–2613. [Google Scholar] [CrossRef]
- Opiyo, S.A.; Manguro, L.O.A.; Okinda-Owuor, P.; Ateka, E.M.; Lemmen, P. 7α-Acetylugandensolide and antimicrobial properties of Warburgia ugandensis extracts and isolates against sweet potato pathogens. Phytochem. Lett. 2011, 4, 161–165. [Google Scholar] [CrossRef]
- Yang, B.Y.; Yin, X.; Liu, Y.; Ye, H.L.; Zhang, M.L.; Guan, W.; Kuang, H.X. Bioassay-guided isolation of lignanamides with potential anti-inflammatory effect from the roots of Solanum melongena L. Phytochem. Lett. 2019, 30, 160–164. [Google Scholar] [CrossRef]
- Nchiozem-Ngnitedem, V.A.; Omosa, L.K.; Bedane, K.G.; Derese, S.; Brieger, L.; Strohmann, C.; Spiteller, M. Anti-inflammatory steroidal sapogenins and a conjugated chalcone-stilbene from Dracaena usambarensis Engl. Fitoterapia 2020, 146, 104717. [Google Scholar] [CrossRef]
- Zhuang, X.C.; Chen, G.L.; Liu, Y.; Zhang, Y.L.; Guo, M.Q. New lignanamides with antioxidant and anti-inflammatory activities screened out and identified from Warburgia ugandensis combining affinity ultrafiltration LC-MS with SOD and XOD enzymes. Antioxidants 2021, 10, 370. [Google Scholar] [CrossRef]
- Gao, X.X.; Gao, Y.N.; Wang, D.D.; Hu, G.S.; Yan, T.; Jia, J.M.; Wang, A.H. Six novel lignanoids with complex structures from Sigesbeckia glabrescens Makino with their cytotoxic activities. Fitoterapia 2021, 148, 104799. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.H.; Cuong, N.H.; Dang, T.H.; Hanh, N.T.M.; Thi, V.L.; Hong, H.T.T.; Quang, T.H.; Nguyen, H.D.; Xuan, C.N.; Hoai, N.N.; et al. Cytotoxic constituents from Isotrema tadungense. J. Asian Nat. Prod. Res. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Y.; Liu, W.K.; Che, C.T. Lignanamides and nonalkaloids components of Hyoscyamus niger seeds. J. Nat. Prod. 2002, 65, 206–209. [Google Scholar] [CrossRef]
- Cardullo, N.; Pulvirenti, L.; Spatafora, C.; Musso, N.; Barresi, V.; Condorelli, D.F.; Tringali, C. Dihydrobenzofuran neolignanamides: Laccase-mediated biomimetic synthesis and antiproliferative activity. J. Nat. Prod. 2016, 79, 2122–2134. [Google Scholar] [CrossRef]

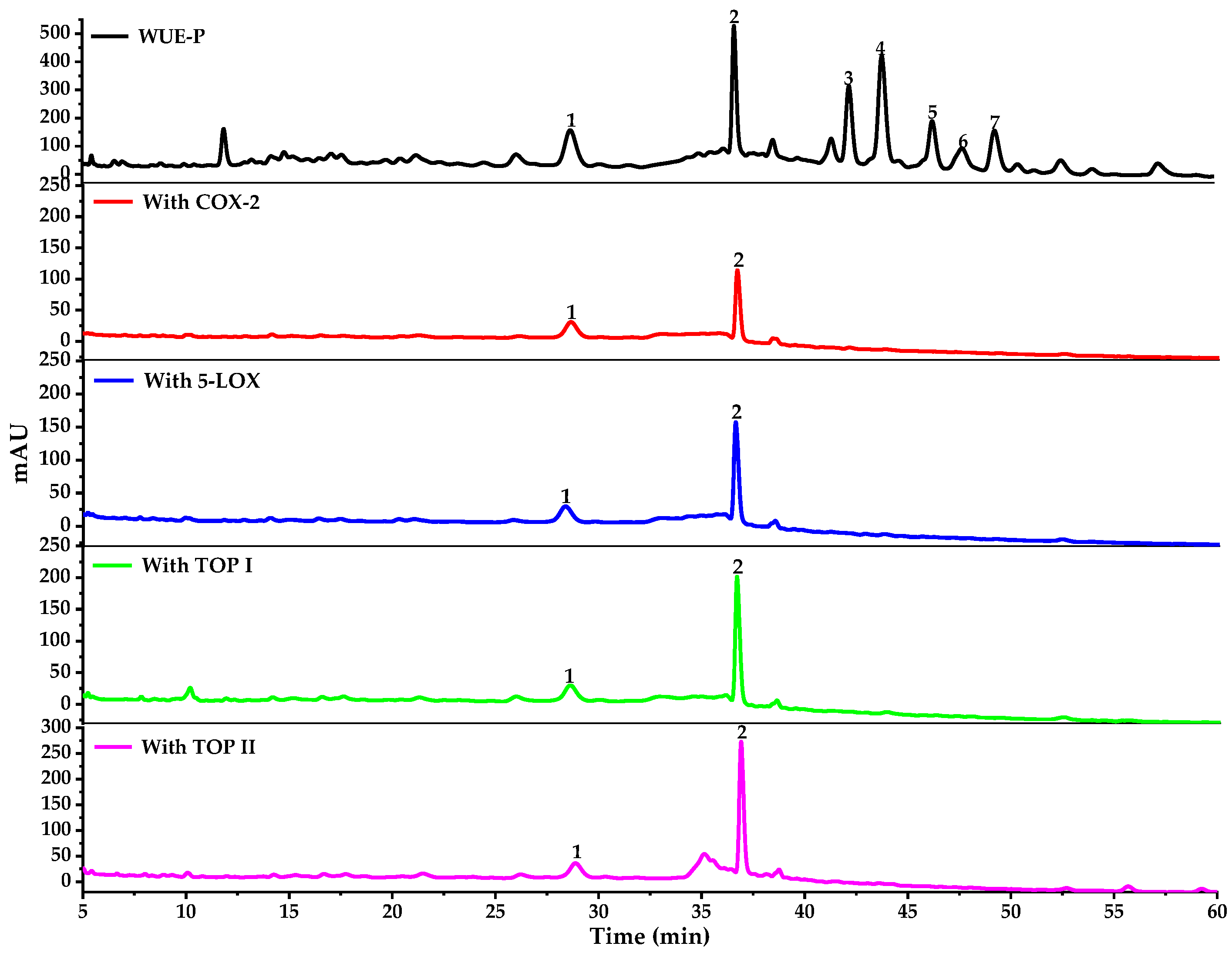
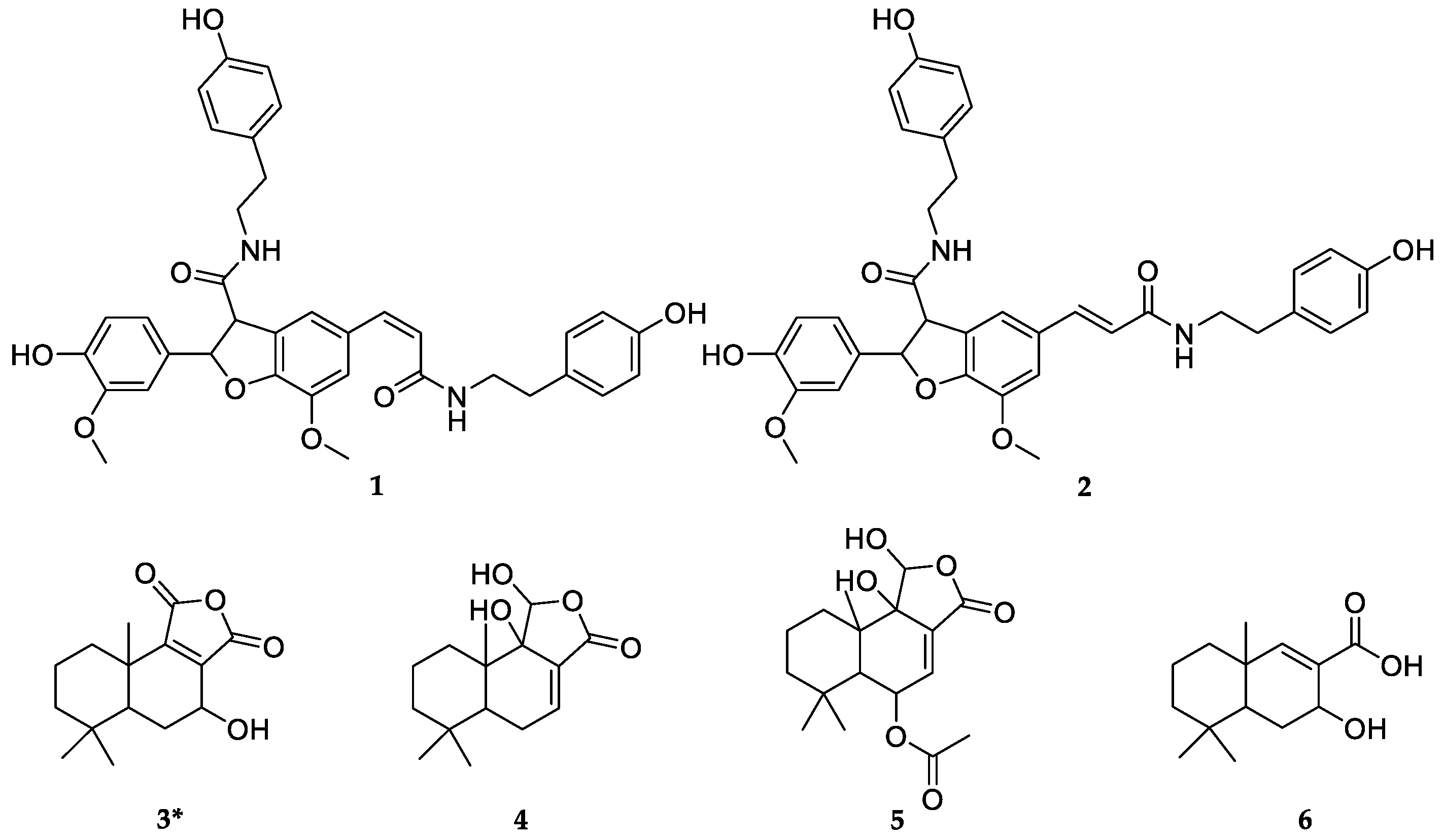
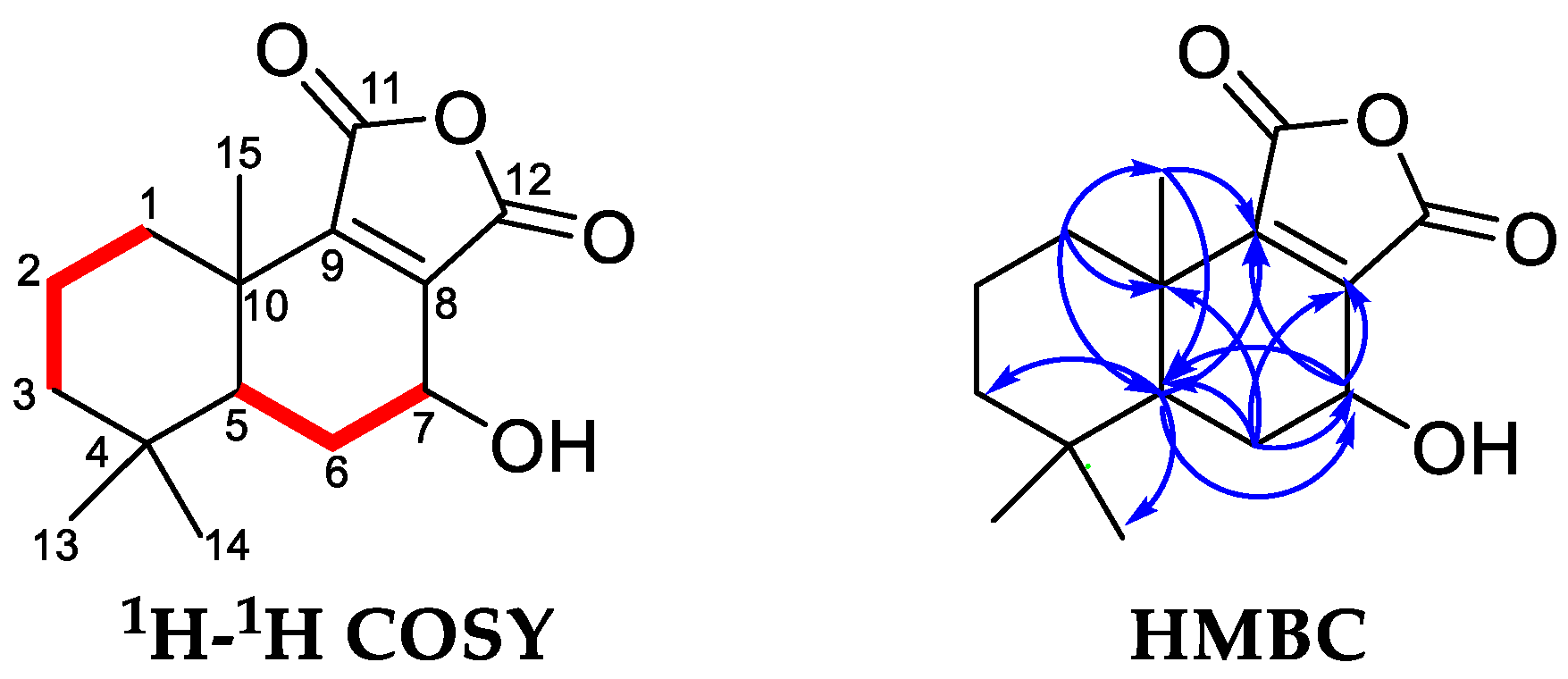
| No. | Rt (min) | UF-RBS (%) # | |||
|---|---|---|---|---|---|
| COX-2 | 5-LOX | Top I | Top II | ||
| 1 | 28.64 | 20.15 ± 0.12 | 16.74 ± 0.65 | 34.25 ± 2.81 | 24.53 ± 1.54 |
| 2 | 36.67 | 27.39 ± 1.58 | 42.00 ± 1.26 | 76.2 ± 5.20 | 58.57 ± 3.82 |
| No. | AUF-HPLC-MS/MS | UPLC-QTOF-MS/MS # | Identified Compounds | |||||
|---|---|---|---|---|---|---|---|---|
| Rt (min) | Observed Mass m/z * | MS/MS Fragments | Observed Mass m/z * | Calculated Mass m/z * (Δ ppm) | Molecular Formula | MS/MS Fragments | ||
| 1 | 28.64 | 623 | 485 (4.30), 461 (73.00), 431 (11.40), 298 (100), 282 (16.85), 267 (8.37) | 625.2546 ** | 625.2544 ** (0.32) | C36H36N2O8 | 488 (1.20), 462 (20.71), 351 (51.09), 325 (100), 307 (31.23), 293 (11.37), 201 (1.71), 138 (2.64), 121 (7.31) | N-cis-grossamide a |
| 2 | 36.67 | 623 | 622 (2.40), 471 (12.43), 460 (100), 445 (18.72), 432 (32.44), 416 (7.55), 401 (4.32), 351 (5.81), 307 (4.40), 297 (79.60), 282 (10.74) | 625.2548 ** | 625.2544 ** (0.64) | C36H36N2O8 | 625 (35.87), 488 (10.84), 462 (93.45), 351 (42.94), 325 (100), 307 (9.98), 293 (2.27), 201 (1.58), 121 (17.01) | N-trans-grossamide a |
| 3 | 42.22 | 263 | 263 (50.10), 235 (22.46), 219 (100), 201 (33.88), 183 (24.12), 173 (8.38), 146 (3.73), 97 (1.53), 71 (12.36) | 263.1298 | 263.1289 (3.42) | C15H20O4 | 263 (100), 235 (1.47), 219 (7.93), 191 (1.49) | 7-hydroxywinterin b |
| 4 | 43.81 | 265 | 265 (57.24), 237 (9.03), 221 (100), 203 (4.06), 185 (8.93), 175 (0.20), 149 (0.11), 97 (0.25), 71 (10.76) | 265.1445 | 265.1445 (0.00) | C15H22O4 | 265 (100), 237 (3.08), 221 (63.34), 203 (2.04), 185 (2.61), 71 (17.89) | ugandenial A a |
| 5 | 46.23 | 323 | 323 (11.88), 280 (4.56), 264 (100), 235 (15.16), 219 (14.69), 191 (7.94), 175 (1.85), 147 (2.63), 59 (4.67) | 323.1508 | 323.1500 (2.48) | C17H24O6 | 323 (94.57), 279 (7.18), 263 (100), 235 (27.05), 219 (15.90), 201 (4.96), 191 (2.15), 147 (3.86), 59 (31.03) | 11α-hydroxycinnamosmolide a |
| 6 | 47.65 | 237 | 237 (100), 219 (4.34), 193 (11.14), 163 (1.65), 145 (0.09), 106 (0.09) | 237.1491 | 237.1496 (−2.11) | C14H22O3 | 237 (25.19), 191 (100), 177 (8.02), 163 (29.86), 147 (8.31), 135 (1.69), 107 (3.23) | polygonal acid a |
| 7 | 49.30 | 391 | 391 (51.79), 347 (85.39), 328 (13.48), 275 (68.95), 257 (60.29), 229 (100), 185 (0.72), 115 (25.78) | - | - | - | - | unknown |
| Position | Compound 3 a | |
|---|---|---|
| δH | δC | |
| 1 | 1.32 (1H, overlap), 2.51 (1H, dt, J = 13.0, 3.6 Hz) | 35.8 (t) |
| 2 | 1.56 (1H, dq-like, J = 13.6, 3.6 Hz) 1.78 (1H, dt, J = 13.6, 3.6 Hz) | 19.4 (t) |
| 3 | 1.31 (1H, overlap), 1.52 (1H, m) | 42.7 (t) |
| 4 | - | 33.8 (s) |
| 5 | 1.65 (1H, d, J = 13.0 Hz) | 47.0 (d) |
| 6 | 1.72 (1H, dd-like, J = 13.0, 4.1 Hz), 1.92 (1H, d-like, J = 14.0 Hz) | 29.4 (t) |
| 7 | 4.53 (1H, d-like, J = 3.0 Hz) | 60.3 (d) |
| 8 | - | 139.8 (s) |
| 9 | - | 153.9 (s) |
| 10 | - | 38.1 (s) |
| 11 | - | 172.7 (s) |
| 12 | - | 172.3 (s) |
| 13 | 0.96 (3H, s) | 33.6 (q) |
| 14 | 0.94 (3H, s) | 21.9 (q) |
| 15 | 1.18 (3H, s) | 19.1 (q) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, X.-C.; Zhang, Y.-L.; Chen, G.-L.; Liu, Y.; Hu, X.-L.; Li, N.; Wu, J.-L.; Guo, M.-Q. Identification of Anti-Inflammatory and Anti-Proliferative Neolignanamides from Warburgia ugandensis Employing Multi-Target Affinity Ultrafiltration and LC-MS. Pharmaceuticals 2021, 14, 313. https://doi.org/10.3390/ph14040313
Zhuang X-C, Zhang Y-L, Chen G-L, Liu Y, Hu X-L, Li N, Wu J-L, Guo M-Q. Identification of Anti-Inflammatory and Anti-Proliferative Neolignanamides from Warburgia ugandensis Employing Multi-Target Affinity Ultrafiltration and LC-MS. Pharmaceuticals. 2021; 14(4):313. https://doi.org/10.3390/ph14040313
Chicago/Turabian StyleZhuang, Xiao-Cui, Yong-Li Zhang, Gui-Lin Chen, Ye Liu, Xiao-Lan Hu, Na Li, Jian-Lin Wu, and Ming-Quan Guo. 2021. "Identification of Anti-Inflammatory and Anti-Proliferative Neolignanamides from Warburgia ugandensis Employing Multi-Target Affinity Ultrafiltration and LC-MS" Pharmaceuticals 14, no. 4: 313. https://doi.org/10.3390/ph14040313
APA StyleZhuang, X.-C., Zhang, Y.-L., Chen, G.-L., Liu, Y., Hu, X.-L., Li, N., Wu, J.-L., & Guo, M.-Q. (2021). Identification of Anti-Inflammatory and Anti-Proliferative Neolignanamides from Warburgia ugandensis Employing Multi-Target Affinity Ultrafiltration and LC-MS. Pharmaceuticals, 14(4), 313. https://doi.org/10.3390/ph14040313






