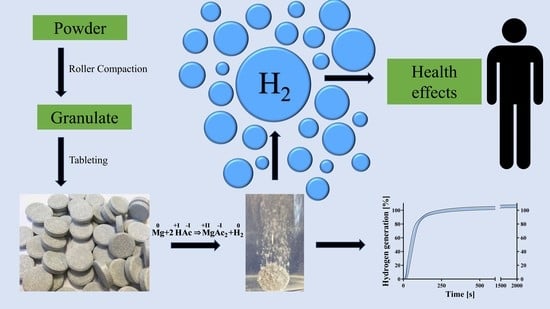
Formulation and Characterization of an Effervescent Hydrogen-Generating Tablet
Abstract
:1. Introduction
2. Results
2.1. Selection of Tableting Excipients
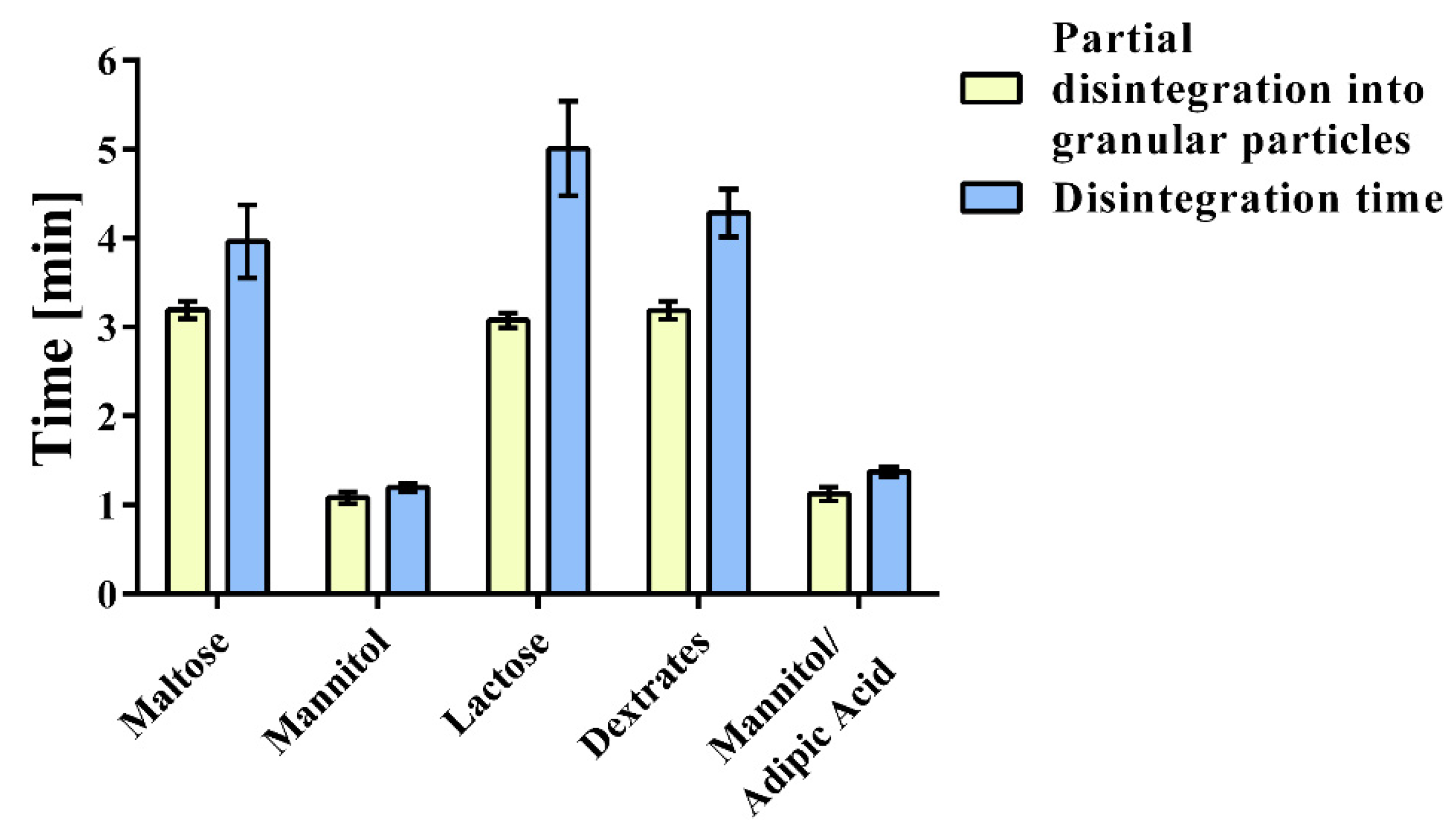
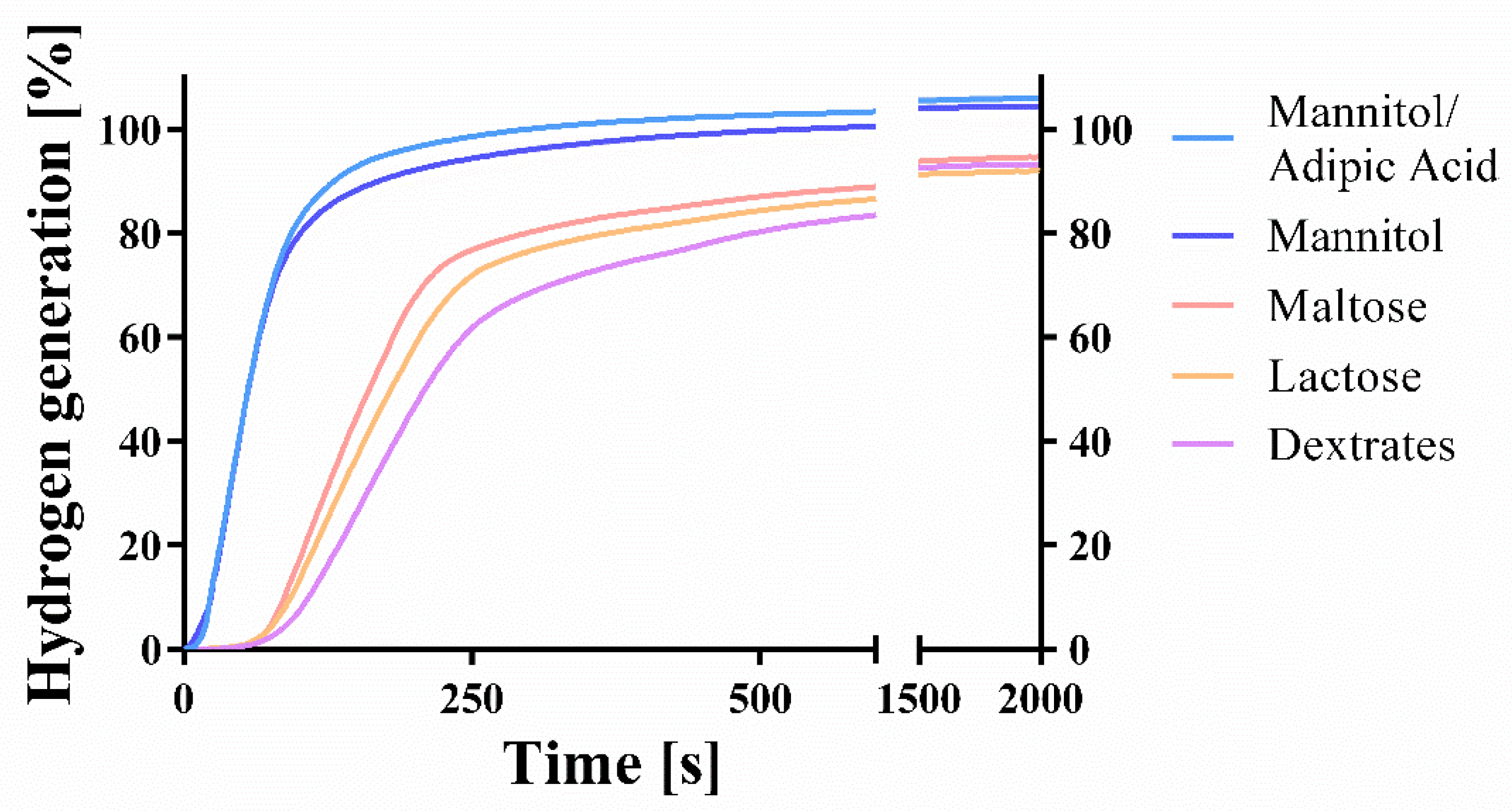
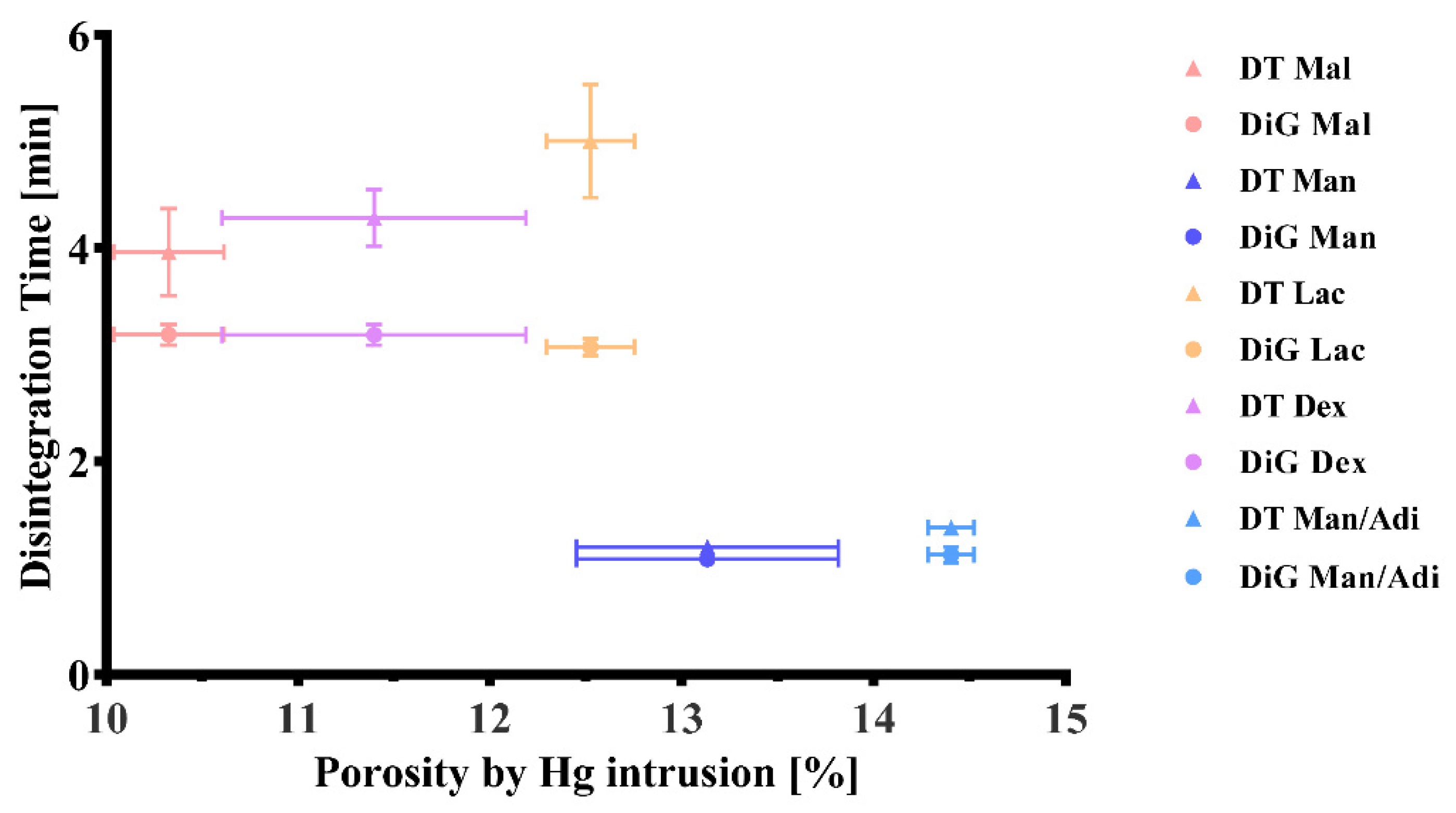
2.2. Disintegration, Porosity, Kinetic Hydrogen Generation, and Magnesium Content
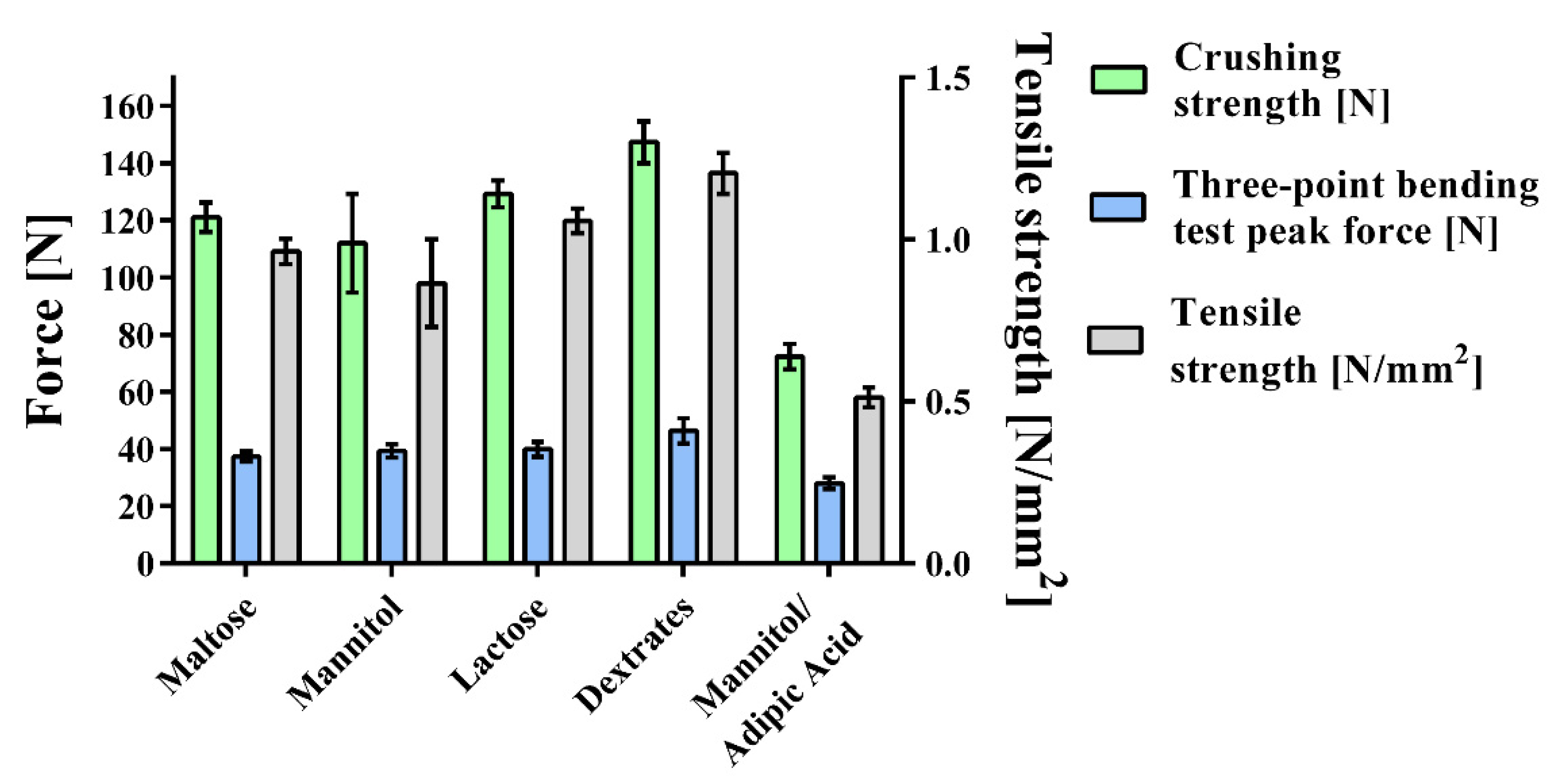
2.3. Tablet Hardness
2.3.1. Three-Point Bending Test
2.3.2. Friability of Uncoated Tablets
2.3.3. Resistance to Crushing
2.3.4. Tensile Strength
2.4. Stability
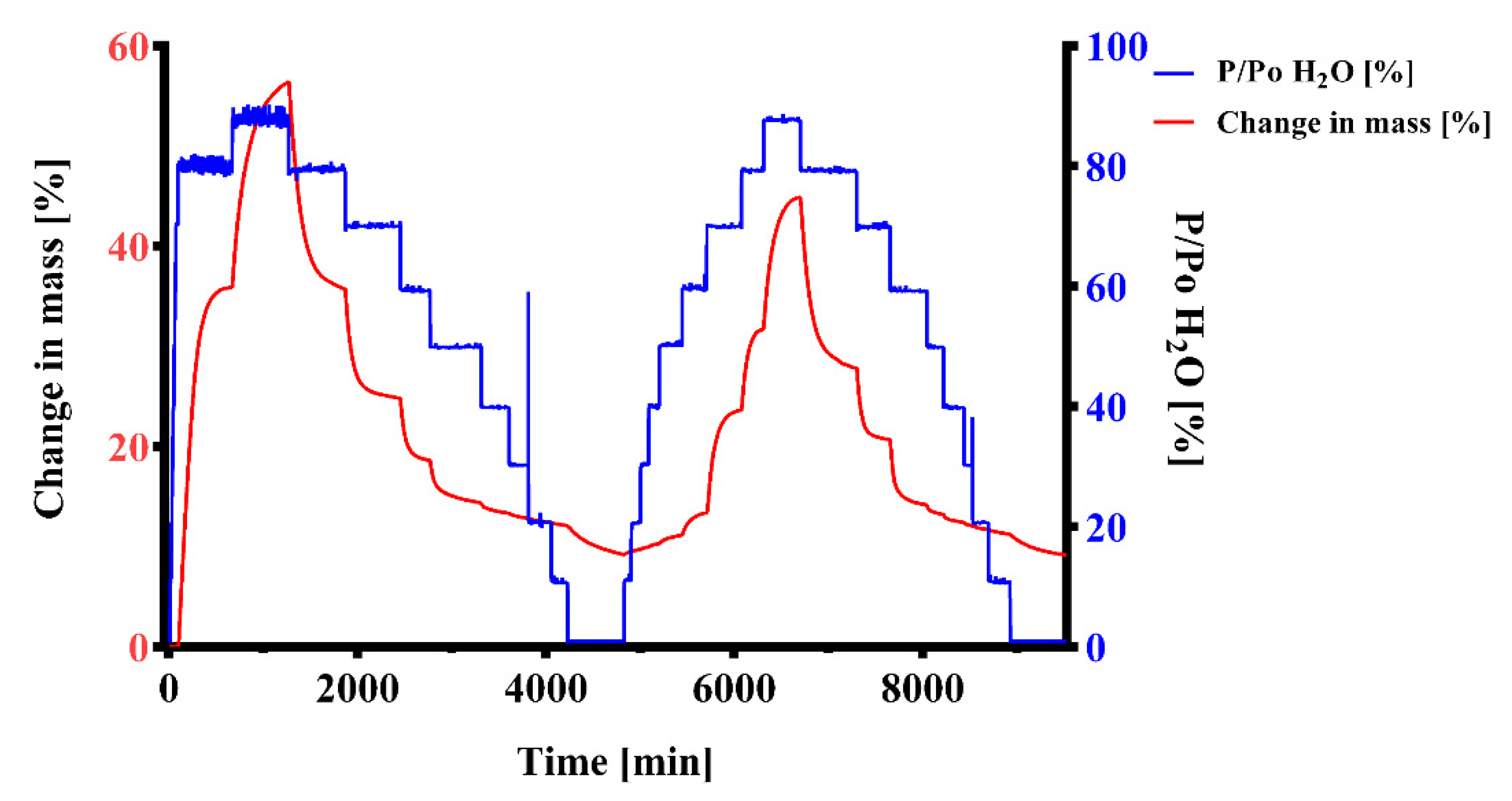
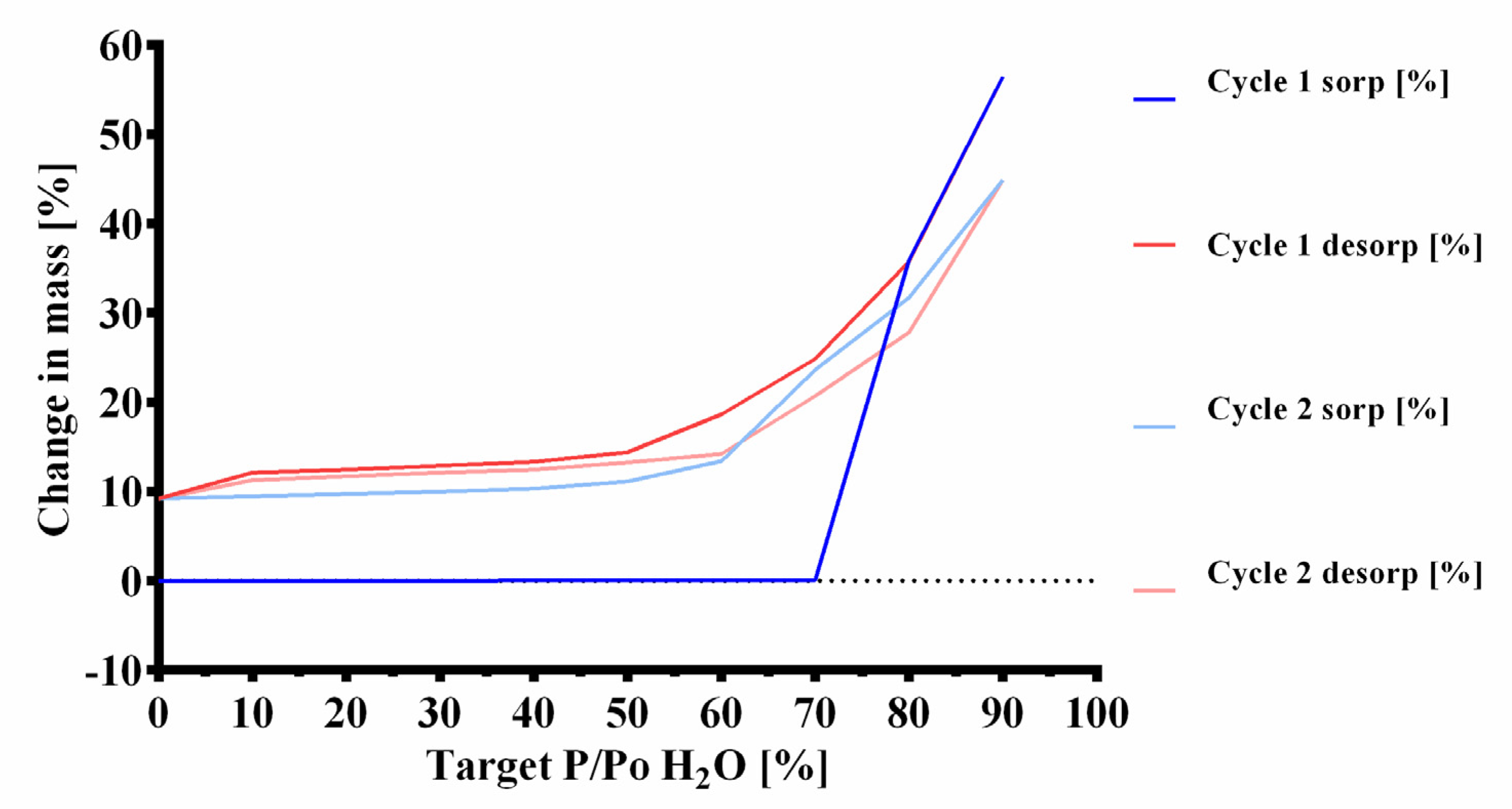
2.4.1. Dynamic Vapor Sorption (DVS)
2.4.2. Bulk Stability Testing
2.5. Granular Flow Properties
3. Discussion
3.1. Content, Kinetic Hydrogen Generation, and Disintegration
3.2. Tablet Hardness
3.3. Dynamic Vapor Sorption (DVS)
3.4. Bulk Stability Testing
3.5. Granular Flow Properties
4. Materials and Methods
4.1. Materials
4.2. Sieving of Powders
4.3. Milling of Adipic Acid
4.4. Blending of Powders
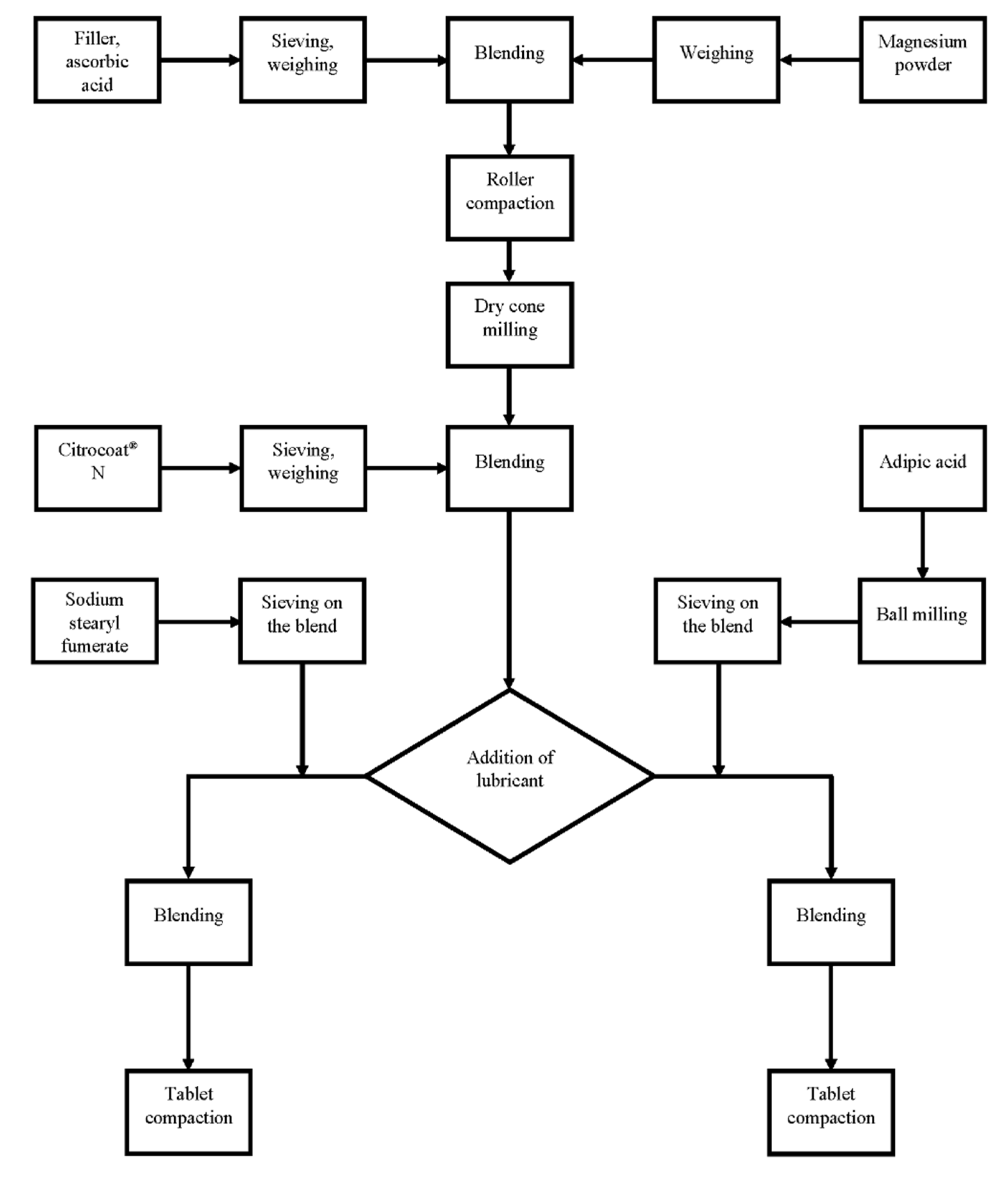
4.5. Roller Compaction/Dry Granulation
4.6. Dry Cone Milling
4.7. Blending of Dry Granules with Citrocoat® N
4.8. Addition of Lubricant
4.9. Compaction of Tablets
4.10. Kinetic Hydrogen Generation Measurement
4.11. Magnesium Content (Complexometric Titration)
4.12. Disintegration
4.13. Three-Point Bending Test
4.14. Friability of Uncoated Tablets
4.15. Resistance to Crushing
4.16. Tensile Strength
4.17. Porosity and Pore-Size Distribution of Solids by Mercury Porosity
4.18. Helium Pycnometry
4.19. Particle Size Analysis by Laser Light Diffraction
4.20. Bulk Density and Tapped Density of Powders
4.21. Angle of Repose
4.22. Flowability
4.23. Loss on Drying Analysis of the Granules
4.24. Dynamic Vapor Sorption (DVS)
4.25. Bulk Stability Testing
4.26. Scanning Electron Microscopy
4.27. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeBaron, T.W.; Kura, B.; Kalocayova, B.; Tribulova, N.; Slezak, J. A New Approach for the Prevention and Treatment of Cardiovascular Disorders. Molecular Hydrogen Significantly Reduces the Effects of Oxidative Stress. Molecules 2019, 24, 2076. [Google Scholar] [CrossRef] [Green Version]
- Ge, L.; Yang, M.; Yang, N.N.; Yin, X.X.; Song, W.G. Molecular hydrogen: A preventive and therapeutic medical gas for various diseases. Oncotarget 2017, 8, 102653–102673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichihara, M.; Sobue, S.; Ito, M.; Ito, M.; Hirayama, M.; Ohno, K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen-Comprehensive review of 321 original articles. Med. Gas Res. 2015, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Ohno, K.; Ito, M.; Ichihara, M.; Ito, M. Molecular hydrogen as an emerging therapeutic medical gas for neurodegenerative and other diseases. Oxidative Med. Cell. Longev. 2012, 2012, 353152. [Google Scholar] [CrossRef] [Green Version]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.-I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.T.; Qin, S.C. Neuroprotective Effects of Molecular Hydrogen: A Critical Review. Neurosci. Bull. 2020, 37, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Ito, M.; Fujita, Y.; Ito, M.; Ichihara, M.; Masuda, A.; Suzuki, Y.; Maesawa, S.; Kajita, Y.; Hirayama, M.; et al. Molecular hydrogen is protective against 6-hydroxydopamine-induced nigrostriatal degeneration in a rat model of Parkinson’s disease. Neurosci. Lett. 2009, 453, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, Y.; Inoue, T.; Uemura, Y.; Iwasaki, Y.; Yada, T.; Nakabeppu, Y.; Noda, M. Complexity of Stomach–Brain Interaction Induced by Molecular Hydrogen in Parkinson’s Disease Model Mice. Neurochem. Res. 2017, 42, 2658–2665. [Google Scholar] [CrossRef]
- Nagatani, K.; Nawashiro, H.; Takeuchi, S.; Tomura, S.; Otani, N.; Osada, H.; Wada, K.; Katoh, H.; Tsuzuki, N.; Mori, K. Safety of intravenous administration of hydrogen-enriched fluid in patients with acute cerebral ischemia: Initial clinical studies. Med. Gas Res. 2013, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Luo, Y.; Yang, P.; Liu, J. Hydrogen as a complementary therapy against ischemic stroke: A review of the evidence. J. Neurol. Sci. 2019, 396, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Nakano, H.; Hamada, H.; Itami, N.; Nakazawa, R.; Ito, S. A novel bioactive haemodialysis system using dissolved dihydrogen (H2) produced by water electrolysis: A clinical trial. Nephrol. Dial. Transplant. 2010, 25, 3026–3033. [Google Scholar] [CrossRef] [Green Version]
- Kajiyama, S.; Hasegawa, G.; Asano, M.; Hosoda, H.; Fukui, M.; Nakamura, N.; Kitawaki, J.; Imai, S.; Nakano, K.; Ohta, M.; et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res. 2008, 28, 137–143. [Google Scholar] [CrossRef]
- Nakao, A.; Toyoda, Y.; Sharma, P.; Evans, M.; Guthrie, N. Effectiveness of Hydrogen Rich Water on Antioxidant Status of Subjects with Potential Metabolic Syndrome—An Open Label Pilot Study. J. Clin. Biochem. Nutr. 2010, 46, 140–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeBaron, T.W.; Laher, I.; Kura, B.; Slezak, J. Hydrogen gas: From clinical medicine to an emerging ergogenic molecule for sports athletes. Can. J. Physiol. Pharmacol. 2019, 97, 797–807. [Google Scholar] [CrossRef]
- Mikami, T.; Tano, K.; Lee, H.; Lee, H.; Park, J.; Ohta, F.; LeBaron, T.W.; Ohta, S. Drinking hydrogen water enhances endurance and relieves psychometric fatigue: A randomized, double-blind, placebo-controlled study. Can. J. Physiol. Pharmacol. 2019, 97, 857–862. [Google Scholar] [CrossRef]
- Aoki, K.; Nakao, A.; Adachi, T.; Matsui, Y.; Miyakawa, S. Pilot study: Effects of drinking hydrogen-rich water on muscle fatigue caused by acute exercise in elite athletes. Med. Gas Res. 2012, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- Lucas, K.; Rosch, M.; Langguth, P. Molecular hydrogen (H2) as a potential treatment for acute and chronic fatigue. Arch. Pharm. 2021, 354, e2000378. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [Green Version]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Lucas, K.; Maes, M. Role of the Toll Like Receptor (TLR) Radical Cycle in Chronic Inflammation: Possible Treatments Targeting the TLR4 Pathway; Humana Press Inc.: Totowa, NJ, USA, 2013; Volume 48, pp. 190–204. [Google Scholar] [CrossRef]
- Ishihara, G.; Kawamoto, K.; Komori, N.; Ishibashi, T. Molecular hydrogen suppresses superoxide generation in the mitochondrial complex I and reduced mitochondrial membrane potential. Biochem. Biophys. Res. Commun. 2020, 522, 965–970. [Google Scholar] [CrossRef]
- Ishibashi, T.; Sato, B.; Rikitake, M.; Seo, T.; Kurokawa, R.; Hara, Y.; Naritomi, Y.; Hara, H.; Nagao, T. Consumption of water containing a high concentration of molecular hydrogen reduces oxidative stress and disease activity in patients with rheumatoid arthritis: An open-label pilot study. Med. Gas Res. 2012, 2, 27. [Google Scholar] [CrossRef] [Green Version]
- Song, G.; Tian, H.; Liu, J.; Zhang, H.; Sun, X.; Qin, S. H2 inhibits TNF-α-induced lectin-like oxidized LDL receptor-1 expression by inhibiting nuclear factor κB activation in endothelial cells. Biotechnol. Lett. 2011, 33, 1715–1722. [Google Scholar] [CrossRef]
- Ohta, S. Recent Progress Toward Hydrogen Medicine: Potential of Molecular Hydrogen for Preventive and Therapeutic Applications. Curr. Pharm. Des. 2011, 17, 2241–2252. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.G. Siddaiah M. Formulation and evaluation of effervescent tablets: A review. J. Drug Deliv. Ther. 2018, 8, 296–303. [Google Scholar] [CrossRef]
- Aslani, A.; Jahangiri, H. Formulation, characterization and physicochemical evaluation of ranitidine effervescent tablets. Adv. Pharm. Bull. 2013, 3, 315–322. [Google Scholar] [CrossRef]
- Aslani, A.; Sharifian, T. Formulation, characterization and physicochemical evaluation of amoxicillin effervescent tablets. Adv. Biomed. Res. 2014, 3, 209. [Google Scholar] [CrossRef]
- Aslani, A.; Fattahi, F. Formulation, characterization and physicochemical evaluation of potassium citrate effervescent tablets. Adv. Pharm. Bull. 2013, 3, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Mohrle, R.; Lieberman, L.; Lachmnann, L.; Schwartz, J.B. Pharmaceutical Dosage Forms: Tablets, 2nd ed.; CRC Press: New York, NY, USA, 1989; Volume 1, pp. 285–292. [Google Scholar]
- Coimbra, F.C.T.; Rocha, M.M.; Oliveira, V.C.; Macedo, A.P.; Pagnano, V.O.; Silva-Lovato, C.H.; Paranhos, H.D.F.O. Antimicrobial activity of effervescent denture tablets on multispecies biofilms. Gerodontology 2021, 38, 87–94. [Google Scholar] [CrossRef]
- Aiache, J.M. Les Comprimes Effervescents. Pharm. Acta Helv. 1974, 491, 169–178. [Google Scholar]
- Sheskey, P.J.; Cabelka, T.D. Use of roller compaction in the preparation of hydrophilic sustained-release matrix tablets. Pharm. Technol. 1994, 18, 132–150. [Google Scholar]
- He, X.; Han, X.; Ladyzhynsky, N.; Deanne, R. Assessing powder segregation potential by near infrared (NIR) spectroscopy and correlating segregation tendency to tabletting performance. Powder Technol. 2013, 236, 85–99. [Google Scholar] [CrossRef]
- Sendall, F.E.J.; Staniforth, J.N. A study of powder adhesion to metal surfaces during compression of effervescent pharmaceutical tablets. J. Pharm. Pharmacol. 1986, 38, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wu, F.; Hong, Y.; Shen, L.; Lin, X.; Feng, Y. Improvements in sticking, hygroscopicity, and compactibility of effervescent systems by fluid-bed coating. RSC Adv. 2019, 9, 31594–31608. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, P.C.; Christin, I. Effervescent tablets—A nearly forgotten drug form. Die Pharm. 1990, 45, 89–101. [Google Scholar]
- Yu, S.H.; Uan, J.Y.; Hsu, T.L. Effects of concentrations of NaCl and organic acid on generation of hydrogen from magnesium metal scrap. Int. J. Hydrogen Energy 2012, 37, 3033–3040. [Google Scholar] [CrossRef]
- Uan, J.Y.; Yu, S.H.; Lin, M.C.; Chen, L.F.; Lin, H.I. Evolution of hydrogen from magnesium alloy scraps in citric acid-added seawater without catalyst. Int. J. Hydrogen Energy 2009, 34, 6137–6142. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Schott, J. Experimental study of brucite dissolution and precipitation in aqueous solutions: Surface speciation and chemical affinity control. Geochim. Cosmochim. Acta 2004, 68, 31–45. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Schott, J.; Castillo, A. Kinetics of brucite dissolution at 25°C in the presence of organic and inorganic ligands and divalent metals. Geochim. Cosmochim. Acta 2005, 69, 905–918. [Google Scholar] [CrossRef]
- Wan, W.-L.; Lin, Y.-J.; Shih, P.-C.; Bow, Y.-R.; Cui, Q.; Chang, Y.; Chia, W.-T.; Sung, H.-W. An In Situ Depot for Continuous Evolution of Gaseous H 2 Mediated by a Magnesium Passivation/Activation Cycle for Treating Osteoarthritis. Angew. Chem. 2018, 130, 10023–10027. [Google Scholar] [CrossRef]
- Mou, F.; Chen, C.; Ma, H.; Yin, Y.; Wu, Q.; Guan, J. Self-Propelled Micromotors Driven by the Magnesium—Water Reaction and Their Hemolytic Properties. Angew. Chem. Int. Ed. 2013, 52, 7208–7212. [Google Scholar] [CrossRef]
- Taub, I.A.; Roberts, W.; LaGambina, S.; Kustin, K. Mechanism of Dihydrogen Formation in the Magnesium—Water Reaction. J. Phys. Chem. A 2002, 106, 8070–8078. [Google Scholar] [CrossRef]
- Ariyasu, A.; Hattori, Y.; Otsuka, M. Delay effect of magnesium stearate on tablet dissolution in acidic medium. Int. J. Pharm. 2016, 511, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Sendall, F.E.J.; Staniforth, J.N.; Rees, J.E.; Leatham, M.J. Effervescent tablets. Pharm. J. 1983, 230, 289–294. [Google Scholar]
- Carr, R.L. Evaluating flow properties of solids. Chem. Eng. 1965, 72, 163–168. [Google Scholar]
- Park, J.S.; Shim, J.Y.; Park, J.S.; Choi, Y.W.; Jeong, S.H. A novel three-layered tablet for extended release with various layer formulations and in vitro release profiles. Drug Dev. Ind. Pharm. 2011, 37, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, A.; Hofland, G.W.; Witkamp, G.-J. Properties of Sugar, Polyol, and Polysaccharide Water-Ethanol Solutions. J. Chem. Eng. Data 2007, 52, 1838–1842. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press and American Pharmacists Association: London, UK; Washington, DC, USA, 2009. [Google Scholar]
- Bi, Y.; Sunada, H.; Yonezawa, Y.; Danjo, K.; Otsuka, A.; Iida, K. Preparation and Evaluation of a Compressed Tablet Rapidly Disintegrating in the Oral Cavity. Chem. Pharm. Bull. 1996, 44, 2121–2127. [Google Scholar] [CrossRef] [Green Version]
- Wagner, C.M.; Pein, M.; Breitkreutz, J. Roll compaction of mannitol: Compactability study of crystalline and spray-dried grades. Int. J. Pharm. 2013, 453, 416–422. [Google Scholar] [CrossRef]
- Malkowska, S.; Khan, K.A. Effect of re-conpression on the properties of tablets prepared by dry granulation. Drug Dev. Ind. Pharm. 1983, 9, 331–347. [Google Scholar] [CrossRef]
- Sheskey, P.J.; Hendren, J. The effects of roll compaction equipment variables, granulation technique, and HPMC polymer level on a controlled-release matrix model drug formulation. Pharm. Technol. 1999, 23, 90–106. [Google Scholar]
- Sheskey, P.J.; Cabelka, T.D. Reworkability of Sustained-Release Tablet Formulations Containing HPMC Polymers. Pharm. Technol. 1992, 16, 60–74. [Google Scholar]
- Bultmann, J.M. Multiple compaction of microcrystalline cellulose in a roller compactor. Eur. J. Pharm. Biopharm. 2002, 54, 59–64. [Google Scholar] [CrossRef]
- Sun, C. Himmelspach MW. Reduced tabletability of roller compacted granules as a result of granule size enlargement. J. Pharm. Sci. 2006, 95, 200–206. [Google Scholar] [CrossRef]
- Herting, M.G.; Kleinebudde, P. Studies on the reduction of tensile strength of tablets after roll compaction/dry granulation. Eur. J. Pharm. Biopharm. 2008, 70, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Dahiya, S.; Calvin Sun, C.; Bansal, A.K. Understanding size enlargement and hardening of granules on tabletability of unlubricated granules prepared by dry granulation. J. Pharm. Sci. 2011, 100, 758–766. [Google Scholar] [CrossRef]
- Mosig, J.; Kleinebudde, P. Evaluation of lubrication methods: How to generate a comparable lubrication for dry granules and powder material for tableting processes. Powder Technol. 2014, 266, 156–166. [Google Scholar] [CrossRef]
- Heckel, R.W. Density-Pressure Relationships in Powder Compaction. Trans. Metall. Soc. AIME 1961, 221, 671–675. [Google Scholar]
- Hersey, J.A.; Rees, J.E. Deformation of Particles during Briquetting. Nat. Phys. Sci. 1971, 230, 96. [Google Scholar] [CrossRef]
- York, P. Crystal engineering and particle design for the powder compaction process. Drug Dev. Ind. Pharm. 1992, 18, 677–721. [Google Scholar] [CrossRef]
- Nordström, J.; Klevan, I.; Alderborn, G. A protocol for the classification of powder compression characteristics. Eur. J. Pharm. Biopharm. 2012, 80, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Humbert Droz, P.; Gurny, R.; Mordier, D.; Doelker, E. Densification behaviour of drugs presenting availability problems. Int. J. Pharm. Technol. Prod. Manuf. 1983, 4, 29–35. [Google Scholar]
- Humbert-Droz, P.; Mordier, D.; Doelker, E. Méthode rapide de détermination du comportement à la compression pour des études de préformulation. Pharm. Acta. Helv. 1982, 57, 136–143. [Google Scholar]
- Bowe, K.E. Recent advances in sugar-based excipients. Pharm. Sci. Technol. Today 1998, 1, 166–173. [Google Scholar] [CrossRef]
- Ohrem, H.L.; Schornick, E.; Kalivoda, A.; Ognibene, R. Why is mannitol becoming more and more popular as a pharmaceutical excipient in solid dosage forms? Pharm. Dev. Technol. 2014, 19, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Tarlier, N.; Soulairol, I.; Bataille, B.; Baylac, G.; Ravel, P.; Nofrerias, I.; Lefèvre, P.; Sharkawi, T. Compaction behavior and deformation mechanism of directly compressible textured mannitol in a rotary tablet press simulator. Int. J. Pharm. 2015, 495, 410–419. [Google Scholar] [CrossRef]
- Shukla, A.J.; Price, J.C. Effect of moisture content on compression properties of directly compressible high beta-content anhydrous lactose. Drug Dev. Ind. Pharm. 1991, 17, 2067–2081. [Google Scholar] [CrossRef]
- Vromans, H.; De Boer, A.H.; Bolhuis, G.K.; Lerk, C.F.; Kussendrager, K.D.; Bosch, H. Studies on tableting properties of lactose—Part 2. Consolidation and compaction of different types of crystalline lactose. Pharm. Weekbl. Sci. Ed. 1985, 7, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Lerk, C.F. Consolidation and compaction of lactose. Drug Dev. Ind. Pharm. 1993, 19, 2359–2398. [Google Scholar] [CrossRef]
- Olmo, I.G.; Ghaly, E.S. Compressional characterization of two dextrose-based directly compressible excipients using an instrumented tablet press. Pharm. Dev. Technol. 1999, 4, 221–231. [Google Scholar] [CrossRef]
- Olmo, I.G.; Ghaly, E.S. Evaluation of two dextrose-based directly compressible excipients. Drug Dev. Ind. Pharm. 1998, 24, 771–778. [Google Scholar] [CrossRef]
- Malamataris, S.; Goidas, P.; Dimitriou, A. Moisture sorption and tensile strength of some tableted direct compression excipients. Int. J. Pharm. 1991, 68, 51–60. [Google Scholar] [CrossRef]
- Shukla, A.J.; Price, J.C. Effect of Moisture Content on Compression Properties of Two Dextrose-Based Directly Compressible Diluents. Pharm. Res. 1991, 8, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Lennard-Jones, J.E. Processes of adsorption and diffusion on solid surfaces. Trans. Faraday Soc. 1932, 28, 333–359. [Google Scholar] [CrossRef]
- Huber, F.; Berwanger, J.; Polesya, S.; Mankovsky, S.; Ebert, H.; Giessibl, F.J. Chemical bond formation showing a transition from physisorption to chemisorption. Science 2019, 366, 235–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, M.; Schepky, G. The effect of hygroscopic formulation ingredients on the sorption characteristics of tablets. Drug Dev. Ind. Pharm. 1995, 21, 279–300. [Google Scholar] [CrossRef]
- Brack, P.; Dann, S.; Upul Wijayantha, K.G.; Adcock, P.; Foster, S. A simple, low-cost, and robust system to measure the volume of hydrogen evolved by chemical reactions with aqueous solutions. J. Vis. Exp. 2016, 2016, 54383. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.W.; Flanner, H.H. Mathematical comparison of dissolution profiles. Pharm. Technol. 1996, 20, 64–74. [Google Scholar]
- Fell, J.T.; Newton, J.M. The tensile strength of lactose tablets. J. Pharm. Pharmacol. 1968, 20, 657–659. [Google Scholar] [CrossRef]
- Price, R.; Young, P.M. Visualization of the Crystallization of Lactose from the Amorphous State. J. Pharm. Sci. 2004, 93, 155–164. [Google Scholar] [CrossRef]
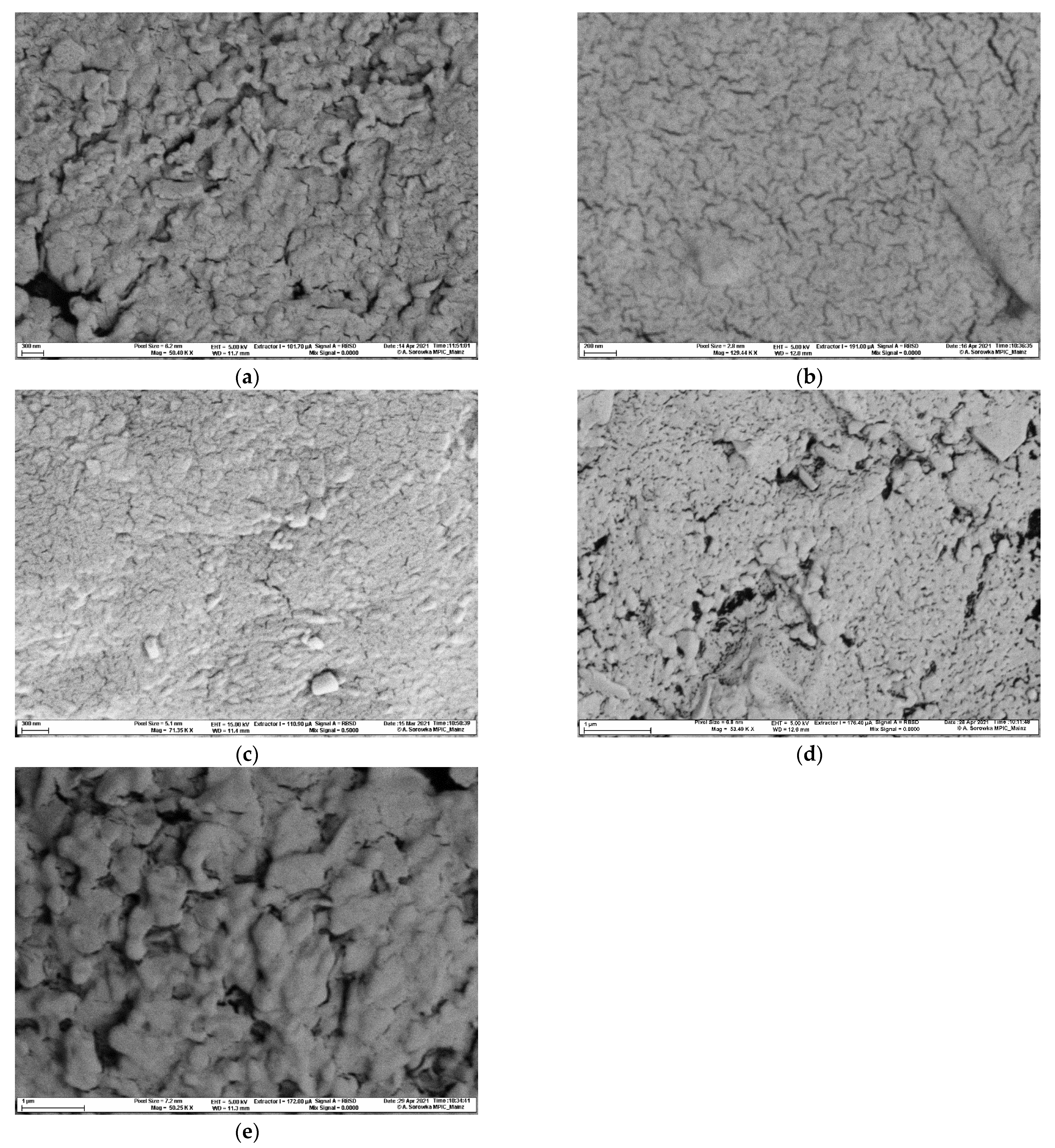
| Parameter | Maltose | Mannitol | Mannitol/ Adipic Acid | Lactose | Dextrates |
|---|---|---|---|---|---|
| H2 generation (mg) | 5.887 (0.027) | 6.488 (0.163) | 6.589 (0.129) | 5.719 (0.099) | 5.794 (0.110) |
| H2 generation (%) | 94.64 (0.43) | 104.30 (2.63) | 105.91 (2.08) | 91.93 (1.59) | 93.13 (1.76) |
| H2 generation (%) excluding weight incorrectness | 92.28 (0.28) | 102.80 (2.93) | 103.89 (1.77) | 92.23 (1.61) | 92.61 (1.18) |
| Mg; complexometric titration (mg) | 76.13 (0.36) | 78.02 (1.92) | 78.70 (1.13) | 75.40 (1.28) | 80.16 (1.46) |
| Mg; complexometric titration (%) | 101.51 (0.48) | 104.03 (2.56) | 104.94 (1.51) | 100.54 (1.70) | 106.88 (1.95) |
| Partial disintegration into granular particles (mm:ss) | 03:11(00:06) | 01:05 (00:04) | 01:08 (00:05) | 03:04 (00:05) | 03:11 (00:06) |
| Disintegration time (mm:ss) | 03:58 (00:25) | 01:12 (00:03) | 01:23 (00:03) | 05:00 (00:32) | 04:17 (00:15) |
| Porosity by Hg intrusion (%) | 10.33 (0.29) | 13.13 (0.68) | 14.40 (0.12) | 12.52 (0.23) | 11.4 (0.79) |
| Median pore radius (μm) | 0.1912 (0.0124) | 0.0502 (0.0073) | 0.0745 (0.0294) | 0.1330 (0.0143) | 0.1545 (0.0151) |
| Excipient | Maltose | Mannitol | Mannitol/ Adipic Acid | Lactose | Dextrates |
|---|---|---|---|---|---|
| Maltose | 16.08 | 16.18 | 64.24 | 44.18 | |
| Mannitol | 16.08 | 85.95 | 15.21 | 13.60 | |
| Mannitol/Adipic Acid | 16.18 | 85.95 | 15.62 | 14.59 | |
| Lactose | 64.24 | 15.21 | 15.62 | 54.65 | |
| Dextrates | 44.18 | 13.60 | 14.59 | 54.65 |
| Parameter | Maltose | Mannitol | Mannitol/ Adipic Acid | Lactose | Dextrates |
|---|---|---|---|---|---|
| Crushing strength (N) | 121.1 (5.1) | 112.0 (17.3) | 72.3 (4.4) | 129.3 (4.7) | 147.4 (7.3) |
| Tensile strength (N/mm2) | 0.963 (0.039) | 0.865 (0.135) | 0.513 (0.031) | 1.057 (0.038) | 1.204 (0.063) |
| Three-point bending test peak force (N) | 37.4 (1.7) | 39.3 (2.3) | 28.0 (2.1) | 39.8 (2.6) | 46.3 (4.5) |
| Friability (%) | broken tablets | broken tablets | not tested | broken tablets | 0.72 |
| True density (g/mL) | 1.624 (0.13) | 1.581 (0.007) | 1.569 (0.003) | 1.603 (0.025) | 1.633 (0.006) |
| Weight (g) | 1.545 (0.011) | 1.537 (0.019) | 1.631 (0.007) | 1.495 (0.005) | 1.515 (0.011) |
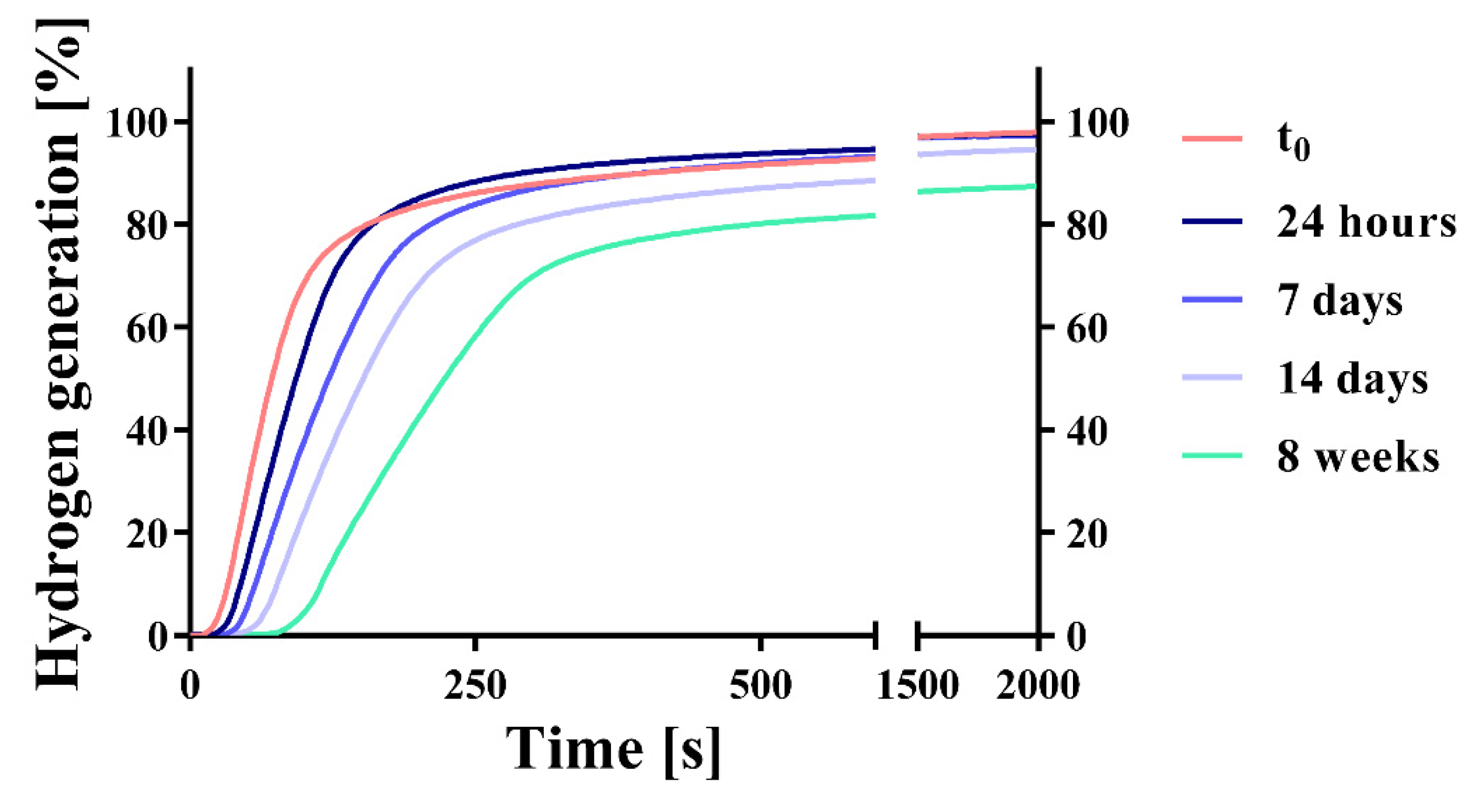
| Storage Time | H2 Generation (%) | Weight Gain (%) |
|---|---|---|
| No storage | 97.86 (2.98) | - |
| 24 h | 97.55 (3.04) | 0.504 (0.032) |
| 7 days | 97.31 (1.24) | 1.029 (0.005) |
| 14 days | 94.52 (1.84) | 1.289 (0.092) |
| 8 weeks | 87.33 (1.47) | 2.039 (0.161) |
| Storage Time | t0 | 24 h | 7 Days | 14 Days | 8 Weeks |
|---|---|---|---|---|---|
| t0 | 51.41 | 36.07 | 26.77 | 17.00 | |
| 24 h | 51.41 | 45.65 | 31.54 | 19.49 | |
| 7 days | 36.07 | 45.65 | 48.63 | 26.76 | |
| 14 days | 26.77 | 31.54 | 48.63 | 38.95 | |
| 8 weeks | 17.00 | 19.49 | 26.76 | 38.95 |
| Parameter | Maltose | Mannitol | Mannitol/ Adipic Acid | Lactose | Dextrates |
|---|---|---|---|---|---|
| Angle of repose (°) | 32.47 (0.3) | 39.3 (0.8) | - | 35.7 (0.6) | 33.4 (1.0) |
| Flow through an orifice (s/100 g) | 7.9 (0.1) | - | - | 11.5 (0.5) | 9.6 (0.0) |
| Bulk density (g/mL) | 0.872 (0.000) | 0.795 (0.005) | 0.803 (0.005) | 0.869 (0.012) | 0.855 (0.006) |
| Tapped density (g/mL) | 0.974 (0.000) | 0.949 (0.000) | 0.991 (0.008) | 1.005 (0.008) | 0.970 (0.007) |
| Hausner ratio | 1.12 (0.00) | 1.19 (0.01) | 1.23 (0.01) | 1.16 (0.03) | 1.13 (0.01) |
| Compressibility index (%) | 10.5 (0.0) | 16.3 (0.5) | 18.9 (0.5) | 13.5 (1.6) | 11.8 (0.6) |
| Particle size d10 (µm) | 130.9 (11.9) | 28.1 (3.5) | 14.0 (2.6) | 56.4 (3.0) | 90.5 (12.6) |
| Particle size d50 (µm) | 463 (22.6) | 461.9 (18.4) | 457.28 (15.2) | 429.9 (12.3) | 458.3 (23.7) |
| Particle size d90 (µm) | 1411.4 (117.4) | 1428.6 (40.3) | 1430.6 (41.6) | 1507.8 (40.0) | 1500.2 (116.1) |
| Loss on drying (%) | 1.80 (0.06) | 1.03 (0.11) | 1.02 (0.18) | 1.29 (0.11) | 4.32 (0.20) |
| Excipient/Tablet (mg) | Maltose | Mannitol | Mannitol/ Adipic Acid | Lactose | Dextrates |
|---|---|---|---|---|---|
| Magnesium powder | 75 | 75 | 75 | 75 | 75 |
| Ascorbic acid | 18 | 18 | 18 | 18 | 18 |
| Citrocoat® N | 721 | 721 | 721 | 721 | 721 |
| Maltose | 686 | ||||
| Mannitol | 686 | 686 | |||
| Lactose | 686 | ||||
| Dextrates | 686 | ||||
| Sodium stearyl fumarate | 7.5 | 7.5 | 7.5 | 7.5 | |
| Adipic acid | 150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosch, M.; Lucas, K.; Al-Gousous, J.; Pöschl, U.; Langguth, P. Formulation and Characterization of an Effervescent Hydrogen-Generating Tablet. Pharmaceuticals 2021, 14, 1327. https://doi.org/10.3390/ph14121327
Rosch M, Lucas K, Al-Gousous J, Pöschl U, Langguth P. Formulation and Characterization of an Effervescent Hydrogen-Generating Tablet. Pharmaceuticals. 2021; 14(12):1327. https://doi.org/10.3390/ph14121327
Chicago/Turabian StyleRosch, Moritz, Kurt Lucas, Jozef Al-Gousous, Ulrich Pöschl, and Peter Langguth. 2021. "Formulation and Characterization of an Effervescent Hydrogen-Generating Tablet" Pharmaceuticals 14, no. 12: 1327. https://doi.org/10.3390/ph14121327
APA StyleRosch, M., Lucas, K., Al-Gousous, J., Pöschl, U., & Langguth, P. (2021). Formulation and Characterization of an Effervescent Hydrogen-Generating Tablet. Pharmaceuticals, 14(12), 1327. https://doi.org/10.3390/ph14121327