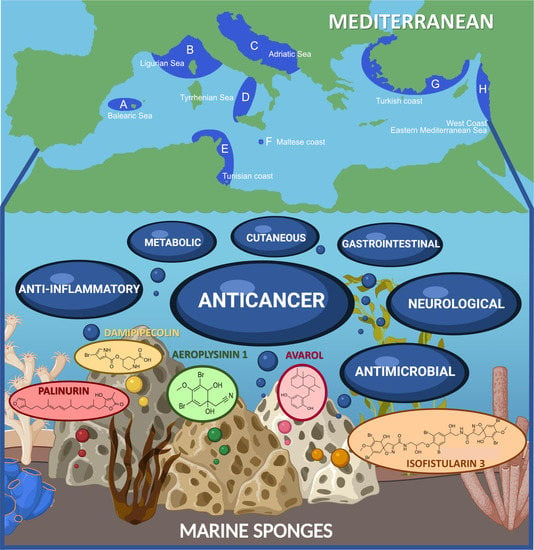
Pharmacological Activities of Extracts and Compounds Isolated from Mediterranean Sponge Sources
Abstract
1. Introduction
2. Methodology
3. Mediterranean Sea Sponges
4. Pharmacological Actions and Potential Therapeutic Applications
4.1. Anticancer Effects
4.2. Antimicrobial Effects
4.3. Anti-Inflammatory Effects
4.4. Neurological Effects
4.5. Other Effects: Cutaneous, Metabolic, and Gastrointestinal
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anjum, K.; Abbas, S.Q.; Shah, S.A.A.; Akhter, N.; Batool, S.; Hassan, S.S.U. Marine Sponges as a Drug Treasure. Biomol. Ther. 2016, 24, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Guerrero, A.J.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine Pharmacology in 2016–2017: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar. Drugs 2021, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Guerrero, A.J.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine Pharmacology in 2014–2015: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, Antiviral, and Anthelmintic Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar. Drugs 2020, 18, 5. [Google Scholar] [CrossRef]
- Amina, M.; Musayeib, N. Biological and Medicinal Importance of Sponge. In Biological Resources of Watet; Intech Open Limited: London, UK, 2018. [Google Scholar]
- Varijakzhan, D.; Loh, J.-Y.; Yap, W.-S.; Yusoff, K.; Seboussi, R.; Lim, S.-H.E.; Lai, K.-S.; Chong, C.-M. Bioactive Compounds from Marine Sponges: Fundamentals and Applications. Mar. Drugs 2021, 19, 246. [Google Scholar] [CrossRef]
- Bart, M.C.; Hudspith, M.; Rapp, H.T.; Verdonschot, P.F.M.; de Goeij, J.M. A Deep-Sea Sponge Loop? Sponges Transfer Dissolved and Particulate Organic Carbon and Nitrogen to Associated Fauna. Front. Mar. Sci. 2021, 8, 229. [Google Scholar] [CrossRef]
- Thomas, T.R.A.; Kavlekar, D.P.; LokaBharathi, P.A. Marine drugs from sponge-microbe association—A review. Mar. Drugs 2010, 8, 1417–1468. [Google Scholar] [CrossRef] [PubMed]
- Moitinho-Silva, L.; Díez-Vives, C.; Batani, G.; Esteves, A.I.S.; Jahn, M.T.; Thomas, T. Integrated metabolism in sponge–microbe symbiosis revealed by genome-centered metatranscriptomics. ISME J. 2017, 11, 1651–1666. [Google Scholar] [CrossRef] [PubMed]
- Perdikaris, S.; Vlachogianni, T.; Valavanidis, A. Bioactive Natural Substances from Marine Sponges: New Developments and Prospects for Future Pharmaceuticals. Nat. Prod. Chem. Res. 2013, 1, 3–8. [Google Scholar] [CrossRef]
- Sipkema, D.; Franssen, M.C.R.; Osinga, R.; Tramper, J.; Wijffels, R.H. Marine sponges as pharmacy. Mar. Biotechnol. 2005, 7, 142–162. [Google Scholar] [CrossRef] [PubMed]
- Gazave, E.; Lapébie, P.; Ereskovsky, A.V.; Vacelet, J.; Renard, E.; Cárdenas, P.; Borchiellini, C. No longer Demospongiae: Homoscleromorpha formal nomination as a fourth class of Porifera. Hydrobiologia 2012, 687, 3–10. [Google Scholar] [CrossRef]
- World Porifera Database. Available online: http://www.marinespecies.org/porifera (accessed on 16 September 2021).
- Renard, E.; Gazave, E.; Fierro-Constain, L.; Schenkelaars, Q.; Ereskovsky, A.; Vacelet, J.; Borchiellini, C. Porifera (Sponges): Recent Knowledge and New Perspectives. eLS 2013. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The Biodiversity of the Mediterranean Sea: Estimates, Patterns, and Threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef] [PubMed]
- Pronzato, R.; Ledda, F.; Manconi, R. Mediterranean horny sponges: How to drive a neverending story of exploitation toward a sustainable management and conservation. In Endangered Species: Habitat, Protection and Ecological Significance; Nova Science Publishers: Hauppauge, NY, USA, 2012; pp. 77–108. [Google Scholar]
- Gerovasileiou, V.; Voultsiadou, E. Marine Caves of the Mediterranean Sea: A Sponge Biodiversity Reservoir within a Biodiversity Hotspot. PLoS ONE 2012, 7, e39873. [Google Scholar] [CrossRef] [PubMed]
- Vacelet, J.; Duport, E. Prey capture and digestion in the carnivorous sponge Asbestopluma hypogea(Porifera: Demospongiae). Zoomorphology 2004, 123, 179–190. [Google Scholar] [CrossRef]
- Boury-Esnault, N.; Vacelet, J.; Dubois, M.; Goujard, A.; Fourt, M.; Pérez, T.; Chevaldonné, P. New hexactinellid sponges from deep Mediterranean canyons. Zootaxa 2017, 4236, 118–134. [Google Scholar] [CrossRef]
- Pansini, M.; Longo, C. A review of the Mediterranean Sea sponge biogeography with, in appendix, a list of the demosponges hitherto recorded from this sea. Biogeogr. J. Integr. Biogeogr. 2003, 24. [Google Scholar] [CrossRef]
- Pronzato, R. Mediterranean Sponge Fauna: A biological, historical and cultural heritage. Biogeogr. J. Integr. Biogeogr. 2003, 24. [Google Scholar] [CrossRef]
- Manconi, R.; Cadeddu, B.; Ledda, F.; Pronzato, R. An overview of the Mediterranean cave-dwelling horny sponges (Porifera, Demospongiae). ZooKeys 2013, 281, 1–68. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, R.W.M.; Boury-Esnault, N.; Vacelet, J.; Dohrmann, M.; Erpenbeck, D.; De Voogd, N.J.; Santodomingo, N.; Vanhoorne, B.; Kelly, M.; Hooper, J.N.A. Global Diversity of Sponges (Porifera). PLoS ONE 2012, 7, e35105. [Google Scholar] [CrossRef]
- Pronzato, R.; Manconi, R. Mediterranean commercial sponges: Over 5000 years of natural history and cultural heritage. Mar. Ecol. 2008, 29, 146–166. [Google Scholar] [CrossRef]
- Ferretti, C.; Marengo, B.; Ciucis, C.D.; Nitti, M.; Pronzato, M.; Marinari, U.; Pronzato, R.; Manconi, R.; Domenicotti, C. Effects of Agelas oroides and Petrosia ficiformis crude extracts on human neuroblastoma cell survival. Int. J. Oncol. 2007, 30, 161–169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- König, G.M.; Wright, A.D.; Linden, A. Antiplasmodial and cytotoxic metabolites from the Maltese sponge Agelas oroides. Planta Med. 1998, 64, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Ines, T.; Amina, B.; Khaled, S.; Kamel, G. Screening of antimicrobial activity of marine sponge extracts collected from Tunisian coast. Proc. West. Pharmacol. Soc. 2007, 50, 152–155. [Google Scholar] [PubMed]
- Bouaicha, N.; Amade, P.; Puel, D.; Roussakis, C. Zarzissine, a new cytotoxic guanidine alkaloid from the Mediterranean sponge Anchinoe paupertas. J. Nat. Prod. 1994, 57, 1455–1457. [Google Scholar] [CrossRef]
- Binnewerg, B.; Schubert, M.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Djurović, M.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; et al. Marine biomaterials: Biomimetic and pharmacological potential of cultivated Aplysina aerophoba marine demosponge. Mater. Sci. Eng. C 2020, 109, 110566. [Google Scholar] [CrossRef] [PubMed]
- Florean, C.; Schnekenburger, M.; Lee, J.Y.; Kim, K.R.; Mazumder, A.; Song, S.; Kim, J.M.; Grandjenette, C.; Kim, J.G.; Yoon, A.Y.; et al. Discovery and characterization of Isofistularin-3, a marine brominated alkaloid, as a new DNA demethylating agent inducing cell cycle arrest and sensitization to TRAIL in cancer cells. Oncotarget 2016, 7, 24027–24049. [Google Scholar] [CrossRef] [PubMed]
- García-Vilas, J.A.; Martínez-Poveda, B.; Quesada, A.R.; Medina, M.Á. Aeroplysinin-1, a Sponge-Derived Multi-Targeted Bioactive Marine Drug. Mar. Drugs 2015, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Fattorusso, E.; Giordano, A.; Menna, M.; Müller, W.E.G.; Perović-Ottstadt, S.; Schröder, H.C. Damipipecolin and damituricin, novel bioactive bromopyrrole alkaloids from the Mediterranean sponge Axinella damicornis. Bioorganic Med. Chem. 2007, 15, 5877–5887. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; D’Esposito, M.; Fattorusso, E.; Menna, M.; Müller, W.E.G.; Perović-Ottstadt, S.; Schröder, H.C. Novel bioactive bromopyrrole alkaloids from the Mediterranean sponge Axinella verrucosa. Bioorganic Med. Chem. 2006, 14, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.; Ammar, I.; Shabbar, A. Screening a Mediterranean Sponge Axinella verrucosa For Antibacterial Activity in Comparison to Some Antibiotics. J. Pharmacogn. Phytochem. 2013, 1, 66–75. [Google Scholar]
- Pozzolini, M.; Millo, E.; Oliveri, C.; Mirata, S.; Salis, A.; Damonte, G.; Arkel, M.; Scarfi, S. Elicited ROS Scavenging Activity, Photoprotective, and Wound-Healing Properties of Collagen-Derived Peptides from the Marine Sponge Chondrosia reniformis. Mar. Drugs 2018, 16, 465. [Google Scholar] [CrossRef]
- Scarfì, S.; Pozzolini, M.; Oliveri, C.; Mirata, S.; Salis, A.; Damonte, G.; Fenoglio, D.; Altosole, T.; Ilan, M.; Bertolino, M.; et al. Identification, Purification and Molecular Characterization of Chondrosin, a New Protein with Anti-tumoral Activity from the Marine Sponge Chondrosia Reniformis Nardo 1847. Mar. Drugs 2020, 18, 409. [Google Scholar] [CrossRef]
- Roué, M.; Domart-Coulon, I.; Ereskovsky, A.; Djediat, C.; Perez, T.; Bourguet-Kondracki, M.L. Cellular localization of clathridimine, an antimicrobial 2-aminoimidazole alkaloid produced by the Mediterranean calcareous sponge Clathrina clathrus. J. Nat. Prod. 2010, 73, 1277–1282. [Google Scholar] [CrossRef]
- Berlinck, R.G.; Braekman, J.C.; Daloze, D.; Bruno, I.; Riccio, R.; Ferri, S.; Spampinato, S.; Speroni, E. Polycyclic guanidine alkaloids from the marine sponge Crambe crambe and Ca++ channel blocker activity of crambescidin 816. J. Nat. Prod. 1993, 56, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Jares-Erijman, E.A.; Sakai, R.; Rinehart, K.L. Crambescidins: New antiviral and cytotoxic compounds from the sponge Crambe crambe. J. Org. Chem. 1991, 56, 5712–5715. [Google Scholar] [CrossRef]
- Roel, M.; Rubiolo, J.A.; Guerra-Varela, J.; Silva, S.B.; Thomas, O.P.; Cabezas-Sainz, P.; Sánchez, L.; López, R.; Botana, L.M. Marine guanidine alkaloids crambescidins inhibit tumor growth and activate intrinsic apoptotic signaling inducing tumor regression in a colorectal carcinoma zebrafish xenograft model. Oncotarget 2016, 7, 83071–83087. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-N.-D.; Feizbakhsh, O.; Sfecci, E.; Baratte, B.; Delehouzé, C.; Garcia, A.; Moulin, C.; Colas, P.; Ruchaud, S.; Mehiri, M.; et al. Kinase-Based Screening of Marine Natural Extracts Leads to the Identification of a Cytotoxic High Molecular Weight Metabolite from the Mediterranean Sponge Crambe tailliezi. Mar. Drugs 2019, 17, 569. [Google Scholar] [CrossRef]
- Ferrándiz, M.L.; Sanz, M.J.; Bustos, G.; Payá, M.; Alcaraz, M.J.; De Rosa, S. Avarol and avarone, two new anti-inflammatory agents of marine origin. Eur. J. Pharmacol. 1994, 253, 75–82. [Google Scholar] [CrossRef]
- Ciftci, H.I.; Can, M.; Ellakwa, D.E.; Suner, S.C.; Ibrahim, M.A.; Oral, A.; Sekeroglu, N.; Özalp, B.; Otsuka, M.; Fujita, M.; et al. Anticancer activity of Turkish marine extracts: A purple sponge extract induces apoptosis with multitarget kinase inhibition activity. Invest. New Drugs 2020, 38, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Pejin, B.; Iodice, C.; Kojic, V.; Jakimov, D.; Lazovic, M.; Tommonaro, G. In vitro evaluation of cytotoxic and mutagenic activity of avarol. Nat. Prod. Res. 2016, 30, 1293–1296. [Google Scholar] [CrossRef]
- Müller, W.E.; Maidhof, A.; Zahn, R.K.; Schröder, H.C.; Gasić, M.J.; Heidemann, D.; Bernd, A.; Kurelec, B.; Eich, E.; Seibert, G. Potent antileukemic activity of the novel cytostatic agent avarone and its analogues in vitro and in vivo. Cancer Res. 1985, 45, 4822–4826. [Google Scholar] [PubMed]
- De Giulio, A.; De Rosa, S.; Strazzullo, G.; Diliberto, L.; Obino, P.; Marongiu, M.E.; Pani, A.; La Colla, P. Synthesis and Evaluation of Cytostatic and Antiviral Activities of 3′ and 4′-Avarone Derivatives. Antivir. Chem. Chemother. 1991, 2, 223–227. [Google Scholar] [CrossRef]
- Hamed, A.N.E.-S.; Wätjen, W.; Schmitz, R.; Chovolou, Y.; Edrada-Ebel, R.; Youssef, D.T.A.; Kamel, M.S.; Proksch, P. A New Bioactive Sesquiterpenoid Quinone from the Mediterranean Sea Marine Sponge Dysidea avara. Nat. Prod. Commun. 2013, 8. [Google Scholar] [CrossRef]
- Pejin, B.; Ciric, A.; Marković, D.; Tommonaro, G.; Soković, M. In vitro avarol does affect the growth of Candida sp. Nat. Prod. Res. 2015, 30, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Posadas, I.; Terencio, M.C.; De Rosa, S.; Payá, M. Cavernolide: A new inhibitor of human sPLA2 sharing unusual chemical features. Life Sci. 2000, 67, 3007–3014. [Google Scholar] [CrossRef]
- Costantini, S.; Guerriero, E.; Teta, R.; Capone, F.; Caso, A.; Sorice, A.; Romano, G.; Ianora, A.; Ruocco, N.; Budillon, A.; et al. Evaluating the Effects of an Organic Extract from the Mediterranean Sponge Geodia cydonium on Human Breast Cancer Cell Lines. Int J. Mol. Sci. 2017, 18, 2112. [Google Scholar] [CrossRef]
- Nuzzo, G.; Ciavatta, M.; Villani, G.; Manzo, E.; Zanfardino, A.; Varcamonti, M.; Gavagnin, M. Fulvynes, antimicrobial polyoxygenated acetylenes from the Mediterranean sponge Haliclona fulva. Tetrahedron 2012, 68, 754–760. [Google Scholar] [CrossRef]
- Fiorini, L.; Tribalat, M.-A.; Sauvard, L.; Cazareth, J.; Lalli, E.; Broutin, I.; Thomas, O.P.; Mus-Veteau, I. Natural paniceins from mediterranean sponge inhibit the multidrug resistance activity of patched and increase chemotherapy efficiency on melanoma cells. Oncotarget 2015, 6, 22282. [Google Scholar] [CrossRef] [PubMed]
- Bidon-Chanal, A.; Fuertes, A.; Alonso, D.; Pérez, D.I.; Martínez, A.; Luque, F.J.; Medina, M. Evidence for a new binding mode to GSK-3: Allosteric regulation by the marine compound palinurin. Eur. J. Med. Chem. 2013, 60, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Wätjen, W.; Putz, A.; Chovolou, Y.; Kampkötter, A.; Totzke, F.; Kubbutat, M.H.; Proksch, P.; Konuklugil, B. Hexa-, hepta- and nonaprenylhydroquinones isolated from marine sponges Sarcotragus muscarum and Ircinia fasciculata inhibit NF-kappaB signalling in H4IIE cells. J. Pharm. Pharmacol. 2009, 61, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Erdogan-Orhan, I.; Sener, B.; de Rosa, S.; Perez-Baz, J.; Lozach, O.; Leost, M.; Rakhilin, S.; Meijer, L. Polyprenyl-hydroquinones and-furans from three marine sponges inhibit the cell cycle regulating phosphatase CDC25A. Nat. Prod. Res. 2004, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gil, B.; Sanz, M.J.; Terencio, M.C.; De Giulio, A.; De Rosa, S.; Alcaraz, M.J.; Payá, M. Effects of marine 2-polyprenyl-1,4-hydroquinones on phospholipase A2 activity and some inflammatory responses. Eur. J. Pharmacol. 1995, 285, 281–288. [Google Scholar] [CrossRef]
- Casapullo, A.; Bifulco, G.; Bruno, I.; Riccio, R. New Bisindole Alkaloids of the Topsentin and Hamacanthin Classes from the Mediterranean Marine Sponge Rhaphisia lacazei. J. Nat. Prod. 2000, 63, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Casapullo, A.; Minale, L.; Zollo, F. Paniceins and Related Sesquiterpenoids from the Mediterranean Sponge Reniera fulva. J. Nat. Prod. 1993, 56, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Sepčić, K.; Batista, U.; Vacelet, J.; Maček, P.; Turk, T. Biological Activities of Aqueous Extracts from Marine Sponges and Cytotoxic Effects of 3-Alkylpyridinium Polymers from Reniera sarai. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1997, 117, 47–53. [Google Scholar] [CrossRef]
- Žužek, M.C.; Grandič, M.; Benoit, E.; Frangež, R. Natural polymeric 3-alkylpyridinium salt affects vertebrate skeletal muscle contractility by preferentially blocking neuromuscular transmission. Toxicol. Lett. 2017, 281, 95–101. [Google Scholar] [CrossRef]
- BenRedjem Romdhane, Y.; Elbour, M.; Carbone, M.; Ciavatta, M.L.; Gavagnin, M.; Mathieu, V.; Lefranc, F.; Ktari, L.; Ben Mustapha, K.; Boudabous, A.; et al. In Vitro Growth Inhibitory Activities of Natural Products from Irciniid Sponges against Cancer Cells: A Comparative Study. BioMed Res. Int. 2016, 2016, 5318176-5318176. [Google Scholar] [CrossRef]
- Abed, C.; Legrave, N.; Dufies, M.; Robert, G.; Guérineau, V.; Vacelet, J.; Auberger, P.; Amade, P.; Mehiri, M. A new hydroxylated nonaprenylhydroquinone from the Mediterranean marine sponge Sarcotragus spinosulus. Mar. Drugs 2011, 9, 1210–1219. [Google Scholar] [CrossRef]
- Dellai, A.; Deghrigue, M.; Laroche-Clary, A.; Masour, H.B.; Chouchane, N.; Robert, J.; Bouraoui, A. Evaluation of antiproliferative and anti-inflammatory activities of methanol extract and its fractions from the Mediterranean sponge. Cancer Cell Int. 2012, 12, 18. [Google Scholar] [CrossRef]
- Garrido, L.; Zubía, E.; Ortega, M.J.; Salvá, J. New Furanoterpenoids from the Sponge Spongia officinalis. J. Nat. Prod. 1997, 60, 794–797. [Google Scholar] [CrossRef]
- Dellai, A.; Mansour, H.B.; Clary-Laroche, A.; Deghrigue, M.; Bouraoui, A. Anticonvulsant and analgesic activities of crude extract and its fractions of the defensive secretion from the Mediterranean sponge, Spongia officinalis. Cancer Cell International 2012, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Ercolano, G.; De Cicco, P.; Ianaro, A. New Drugs from the Sea: Pro-Apoptotic Activity of Sponges and Algae Derived Compounds. Mar. Drugs 2019, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Z.; Wang, H. Cytotoxic Natural Products from Marine Sponge-Derived Microorganisms. Mar. Drugs 2017, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, P.C.; Wilke, D.V.; Branco, P.C.; Bauermeister, A.; Rezende-Teixeira, P.; Gaudêncio, S.P.; Costa-Lotufo, L.V. Enriching cancer pharmacology with drugs of marine origin. Br. J. Pharmacol. 2020, 177, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Minale, L.; Riccio, R.; Sodano, G. Avarol a novel sesquiterpenoid hydroquinone with a rearranged drimane skeleton from the sponge disidea avara. Tetrahedron Lett. 1974, 15, 3401–3404. [Google Scholar] [CrossRef]
- Namba, T.; Kodama, R. Avarol induces apoptosis in pancreatic ductal adenocarcinoma cells by activating PERK-eIF2α-CHOP signaling. Mar. Drugs 2015, 13, 2376–2389. [Google Scholar] [CrossRef]
- Cimino, G.; De Stefano, S.; Minale, L. Paniceins, unusual aromatic sesquiterpenoids linked to a quinol or quinone system from the marine sponge Halichondria panicea. Tetrahedron 1973, 29, 2565–2570. [Google Scholar] [CrossRef]
- Lavecchia, A.; Coluccia, A.; Di Giovanni, C.; Novellino, E. Cdc25B phosphatase inhibitors in cancer therapy: Latest developments, trends and medicinal chemistry perspective. Anticancer Agents Med. Chem. 2008, 8, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Jares-Erijman, E.A.; Ingrum, A.A.; Sun, F.; Rinehart, K.L. On the structures of crambescins B and C1. J. Nat. Prod. 1993, 56, 2186–2188. [Google Scholar] [CrossRef] [PubMed]
- Dyson, L.; Wright, A.D.; Young, K.A.; Sakoff, J.A.; McCluskey, A. Synthesis and anticancer activity of focused compound libraries from the natural product lead, oroidin. Bioorganic Med. Chem. 2014, 22, 1690–1699. [Google Scholar] [CrossRef]
- Piña, I.C.; Gautschi, J.T.; Wang, G.Y.; Sanders, M.L.; Schmitz, F.J.; France, D.; Cornell-Kennon, S.; Sambucetti, L.C.; Remiszewski, S.W.; Perez, L.B.; et al. Psammaplins from the sponge Pseudoceratina purpurea: Inhibition of both histone deacetylase and DNA methyltransferase. J. Org. Chem. 2003, 68, 3866–3873. [Google Scholar] [CrossRef] [PubMed]
- Baud, M.G.; Leiser, T.; Haus, P.; Samlal, S.; Wong, A.C.; Wood, R.J.; Petrucci, V.; Gunaratnam, M.; Hughes, S.M.; Buluwela, L.; et al. Defining the mechanism of action and enzymatic selectivity of psammaplin A against its epigenetic targets. J. Med. Chem. 2012, 55, 1731–1750. [Google Scholar] [CrossRef]
- Pereira, R.; Benedetti, R.; Pérez-Rodríguez, S.; Nebbioso, A.; García-Rodríguez, J.; Carafa, V.; Stuhldreier, M.; Conte, M.; Rodríguez-Barrios, F.; Stunnenberg, H.G.; et al. Indole-Derived Psammaplin A Analogues as Epigenetic Modulators with Multiple Inhibitory Activities. J. Med. Chem. 2012, 55, 9467–9491. [Google Scholar] [CrossRef] [PubMed]
- Seidel, C.; Schnekenburger, M.; Dicato, M.; Diederich, M. Antiproliferative and proapoptotic activities of 4-hydroxybenzoic acid-based inhibitors of histone deacetylases. Cancer Lett. 2014, 343, 134–146. [Google Scholar] [CrossRef] [PubMed]
- 2019 AR Threats Report. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 2 February 2021).
- Rohde, S.; Schupp, P.J. Allocation of chemical and structural defenses in the sponge Melophlus sarasinorum. J. Exp. Mar. Biol. Ecol. 2011, 399, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Konuklugil, B.; Gözcelioğlu, K. Antımıcrobıal Actıvıty of Marıne Samples Collected From The Dıfferent Coasts of Turkey. Turk. J. Pharm. Sci. 2015, 12, 116–125. [Google Scholar] [CrossRef]
- Mancini, I.; Defant, A.; Guella, G. Recent Synthesis of Marine Natural Products with Antibacterial Activities. Anti-Infect. Agents Med. Chem. 2007, 600, 17–48. [Google Scholar] [CrossRef]
- Yalçin, F.N. Biological Activities of the Marine Sponge Axinella. Hacet. Univ. J. Fac. Pharm. 2007, 1, 47–60. [Google Scholar]
- Menna, M.; Aiello, A.; Aniello, F.; Fattorusso, E.; Imperatore, C.; Luciano, P.; Vitalone, R. Further Investigation of the Mediterranean Sponge Axinella polypoides: Isolation of a New Cyclonucleoside and a New Betaine. Mar. Drugs 2012, 10, 2509–2518. [Google Scholar] [CrossRef]
- Richards, J.J.; Ballard, T.E.; Huigens, R.W., III; Melander, C. Synthesis and Screening of an Oroidin Library against Pseudomonas aeruginosa Biofilms. ChemBioChem 2008, 9, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Sauleau, P.; Moriou, C.; Al Mourabit, A. Metabolomics approach to chemical diversity of the Mediterranean marine sponge Agelas oroides. Nat. Prod. Res. 2017, 31, 1625–1632. [Google Scholar] [CrossRef]
- Lira, N.; Montes, R.; Tavares, J.; Silva, M.; Cunha, E.; Athayde-Filho, P.; Da Silva Dias, C.; Barbosa Filho, J. Brominated Compounds from Marine Sponges of the Genus Aplysina and a Compilation of Their 13C NMR Spectral Data. Mar. Drugs 2011, 9, 2316–2368. [Google Scholar] [CrossRef] [PubMed]
- Tommonaro, G.; Iodice, C.; AbdEl-Hady, F.; Guerriero, G.; Pejin, B. The Mediterranean Sponge Dysidea avara as a 40 Year Inspiration of Marine Natural Product Chemists. J. Biodivers. Endanger. Species 2015. [Google Scholar] [CrossRef]
- Loaëc, N.; Attanasio, E.; Villiers, B.; Durieu, E.; Tahtouh, T.; Cam, M.; Davis, R.A.; Alencar, A.; Roué, M.; Bourguet-Kondracki, M.-L.; et al. Marine-Derived 2-Aminoimidazolone Alkaloids. Leucettamine B-Related Polyandrocarpamines Inhibit Mammalian and Protozoan DYRK & CLK Kinases. Mar. Drugs 2017, 15, 316. [Google Scholar] [CrossRef]
- Altuğ, G.; Çiftçi Türetken, P.S.; Kalkan, S.; Topaloğlu, B. The Distribution and Antibacterial Activity of Marine Sponge-Associated Bacteria in the Aegean Sea and the Sea of Marmara, Turkey. Curr. Microbiol. 2021, 78, 2275–2290. [Google Scholar] [CrossRef] [PubMed]
- Heydari, H.; Gozcelioglu, B.; Konulugil, B. Biodiversity and Secondary Metabolites of Marine Sponges from Turkey. Rec. Nat. Prod. 2019, 13, 367–378. [Google Scholar] [CrossRef]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 structure/function, mechanism, and signaling1. J. Lipid Res. 2009, 50, S237–S242. [Google Scholar] [CrossRef] [PubMed]
- Geerts, H.; Wikswo, J.; van der Graaf, P.H.; Bai, J.P.F.; Gaiteri, C.; Bennett, D.; Swalley, S.E.; Schuck, E.; Kaddurah-Daouk, R.; Tsaioun, K.; et al. Quantitative Systems Pharmacology for Neuroscience Drug Discovery and Development: Current Status, Opportunities, and Challenges. Pharmacomet. Syst. Pharmacol. 2020, 9, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Lunder, M.; Drevenšek, G.; Hawlina, S.; Sepcic, K.; Ziberna, L. Cardiovascular effects induced by polymeric 3-alkylpyridinium salts from the marine sponge Reniera sarai. Toxicon 2012, 60, 1041–1048. [Google Scholar] [CrossRef]
- Hill, R.T.; Fenical, W. Pharmaceuticals from marine natural products: Surge or ebb? Curr. Opin. Biotechnol. 2010, 21, 777–779. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Sheridan, C.; Osinga, R.; Dionísio, G.; Rocha, R.J.M.; Silva, B.; Rosa, R.; Calado, R. Marine Microorganism-Invertebrate Assemblages: Perspectives to Solve the “Supply Problem” in the Initial Steps of Drug Discovery. Mar. Drugs 2014, 12, 3929–3952. [Google Scholar] [CrossRef]
- Ryabinin, V.; Barbière, J.; Haugan, P.; Kullenberg, G.; Smith, N.; McLean, C.; Troisi, A.; Fischer, A.; Aricò, S.; Aarup, T.; et al. The UN Decade of Ocean Science for Sustainable Development. Front. Mar. Sci. 2019, 6, 470. [Google Scholar] [CrossRef]
- Brümmer, F.; Nickel, M. Sustainable use of marine resources: Cultivation of sponges. In Sponges(Porifera); Springer: Berlin, Germany, 2003; pp. 143–163. [Google Scholar]
- Schippers, K.; Sipkema, D.; Osinga, R.; Smidt, H.; Pomponi, S.; Martens, D.; Wijffels, R. Cultivation of Sponges, Sponge Cells and Symbionts. Adv. Mar. Biol. 2012, 62, 273–337. [Google Scholar] [CrossRef] [PubMed]
- Grasela, J.J.; Pomponi, S.A.; Rinkevich, B.; Grima, J. Efforts to develop a cultured sponge cell line: Revisiting an intractable problem. In Vitro Cell. Dev. Biol. Anim. 2012, 48, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gandelman, J.F.; Giambiagi-deMarval, M.; Oelemann, W.M.; Laport, M.S. Biotechnological potential of sponge-associated bacteria. Curr. Pharm. Biotechnol. 2014, 15, 143–155. [Google Scholar] [CrossRef]
- Wilson, M.C.; Mori, T.; Rückert, C.; Uria, A.R.; Helf, M.J.; Takada, K.; Gernert, C.; Steffens, U.A.E.; Heycke, N.; Schmitt, S.; et al. An environmental bacterial taxon with a large and distinct metabolic repertoire. Nature 2014, 506, 58–62. [Google Scholar] [CrossRef]
- Bertin, M.J.; Schwartz, S.L.; Lee, J.; Korobeynikov, A.; Dorrestein, P.C.; Gerwick, L.; Gerwick, W.H. Spongosine Production by a Vibrio harveyi Strain Associated with the Sponge Tectitethya crypta. J. Nat. Prod. 2015, 78, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Piel, J.; Hui, D.; Wen, G.; Butzke, D.; Platzer, M.; Fusetani, N.; Matsunaga, S. Antitumor polyketide biosynthesis by an uncultivated bacterial symbiont of the marine sponge Theonella swinhoei. Proc. Natl. Acad. Sci. USA 2004, 101, 16222–16227. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.; Helfrich, E.J.N.; Freeman, M.F.; Nanudorn, P.; Field, C.M.; Rückert, C.; Kündig, T.; Page, M.J.; Webb, V.L.; Kalinowski, J.; et al. A multiproducer microbiome generates chemical diversity in the marine sponge Mycale hentscheli. Proc. Natl. Acad. Sci. USA 2020, 117, 9508–9518. [Google Scholar] [CrossRef] [PubMed]
- Jaspars, M.; Challis, G. Microbiology: A talented genus. Nature 2014, 506, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Dat, T.T.H.; Steinert, G.; Cuc, N.T.K.; Smidt, H.; Sipkema, D. Bacteria Cultivated From Sponges and Bacteria Not Yet Cultivated From Sponges—A Review. Front. Microbiol. 2021, 12, 3427. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, M.A.; Tammam, M.A.; El-Demerdash, A.; Atanasov, A.G. Insights about clinically approved and Preclinically investigated marine natural products. Curr. Res. Biotechnol. 2020, 2, 88–102. [Google Scholar] [CrossRef]
- McCauley, E.P.; Piña, I.C.; Thompson, A.D.; Bashir, K.; Weinberg, M.; Kurz, S.L.; Crews, P. Highlights of marine natural products having parallel scaffolds found from marine-derived bacteria, sponges, and tunicates. J. Antibiot. 2020, 73, 504–525. [Google Scholar] [CrossRef]
- Schorn, M.A.; Jordan, P.A.; Podell, S.; Blanton, J.M.; Agarwal, V.; Biggs, J.S.; Allen, E.E.; Moore, B.S.; McFall-Ngai, M.J.; Donia, M.; et al. Comparative Genomics of Cyanobacterial Symbionts Reveals Distinct, Specialized Metabolism in Tropical Dysideidae Sponges. MBio 2019, 10, e00821-19. [Google Scholar] [CrossRef] [PubMed]
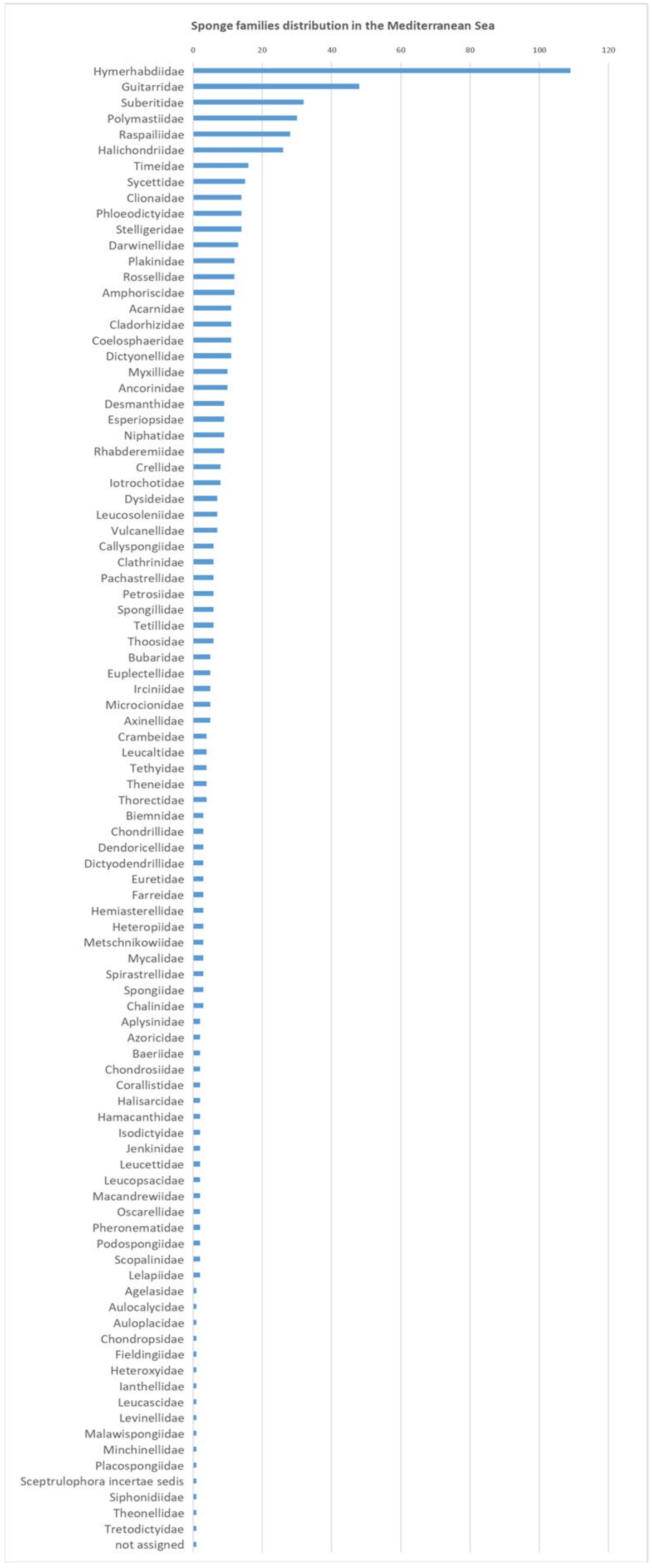
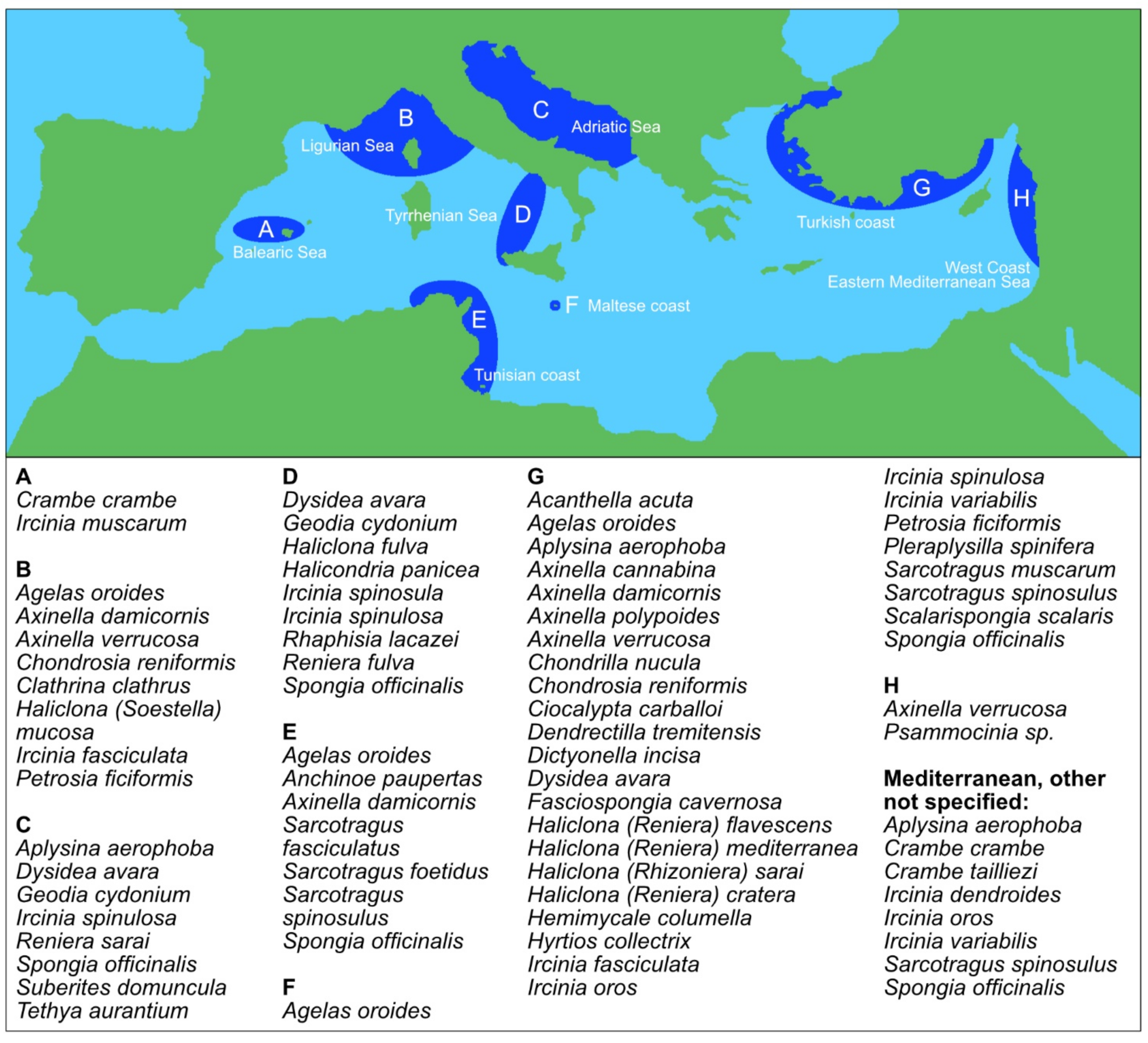


| Name | Acc. Species | Acc. Species Marine | Acc. Species Fresh |
|---|---|---|---|
| Phylum: Porifera | 9436 | 9174 | 264 |
| Class: Calcarea | 801 | 801 | 0 |
| Class: Demospongiae | 7817 | 7555 | 264 |
| Class: Hexactinellida | 687 | 687 | 0 |
| Class: Homoscleromorpha | 130 | 130 | 0 |
| Sponge | Extract/ Compounds (Collection Zone) | In Vitro/ Ex Vivo/ In Vivo | Effect | Active Dose/ Concentration | Reference |
|---|---|---|---|---|---|
| Agelas oroides | Methanol extract (Portofino’s promontory, Italy) | In vitro: LAN5 and SK-N-BE(2)-C cells | Anticancer | 10–20 ppm significantly increased cell death | [24] |
| Oroidin, 2-cyano-4,5-dibromopyrrole (Maltese Sea, at Rdum il Bies) | In vitro: KB, Lu1, KB-V, LNCaP, ZR-75-1 cells | Anticancer | Only 2-cyano-4,5-dibromopyrrole: IC50 = 1.7–10.8 µg/mL | [25] | |
| Ethyl acetate extract, Mixture of brominated pyrrole alkaloids (Monastir, Tunisia) | In vitro: S. epidermidis, S. aureus, M. luteus, E. feacalis, E. coli, P. aeruginosa, S. thyphymerium, L. monocytogenes. C. albicans, C. krusei, C. parapsilosis, C. glabrata and C. dubliniensis | Antimicrobial | 7–18 mm inhibition zone (5 mg/disk for bacteria and 10 mg/disk for yeasts) | [26] | |
| Anchinoe paupertas | Zarzissine (Zarzis, Tunisia) | In vitro: P-388, KB, NSCLC-N6 cells | Anticancer | IC50 = 5–12 µg/mL | [27] |
| Aplysina aerophoba | Aeroplysinin-1; Isofistularin-3 (Adriatic Sea, Kotor Bay, Montenegro) | In vitro: SH-SY5Y, MCF-7 cells | Anticancer | IC50 about 5 µM for aeroplysinin-1 on SH-SY5Ycells; IC50 > 25 µM for Isofistularin-3 on MCF-7 cells | [28] |
| Isofistularin-3 (Mediterranean Sea, unspecified area) | In vitro: RAJI, U-937, JURKAT, K-562, MEG-01, HL-60, SH-SY5Y, PC-3, MDA-MB-231 cells | Anticancer | IC50 = 8.1–50 μM | [29] | |
| Methanol extract, Aeroplysinin-1 (Rovinj, Croatia) | In vitro: B. cereus, B. subtilis, S. aureus, S. albus, V. anguillarum, Flexibacter sp., Moraxella sp. | Antimicrobial | 8–30 mm inhibition zone (100 μg/disc) | [30] | |
| Axinella damicornis | Damipipecolin and damituricin (Corsica island, Italy) | In vitro: rat neurons | Reversion of the increase of [Ca2+] induced by serotonin | IC50 = 0.1 µg/mL | [31] |
| Ethyl acetate extract, Mixture of brominated pyrrole alkaloids (Monastir, Tunisia) | In vitro, S. epidermidis, S. aureus, M. luteus, E. feacalis, E. coli, P. aeruginosa, S. thyphymerium, L. monocytogenes, C. tropicalis | Antimicrobial | 7–26 mm inhibition zone (5 mg/disk) | [26] | |
| Axinella verrucosa | Alkaloids (Corsica island, Italy) | In vitro: rat neurons | Reversion of the increase of [Ca2+] induced by serotonin, glutamic and quisqualic acid | IC50 = 10 µg/mL | [32] |
| Methanol extract, Hymenialdisine, 10- E-Hymenialdisine, Spongiacidine B (Latakia coast, Syria) | In vitro, S. aureus, A. Septicus, P. vulgaris, P. aeruginosa | Antimicrobial | >20 mm of inhibition zone (concentration not determined) | [33] | |
| Chondrosia reniformis | Marine collagen hydrolysates (Peptides rich in hydroxyproline)(Portofino, Italy) | In vitro: L929 and HaCaT cells | Enhanced proliferation of fibroblasts and keratinocytes; Enhanced collagen 1A expression and release;Protected from UV damages (cell death, keratin 1 and 10 expression);Promoted wound healing | IC50 = 10, 50, or 100 µg/mL | [34] |
| Proteic P4 fraction (from a crude extract) containing Chondrosin (Portofino, Italy) | In vitro: L929, RAW 264.7, MDA-MB-468 and HeLa cells | Anticancer | 1–100 µg/mL | [35] | |
| Clathrina clathrus | CH2Cl2/MeOH extract, Clathridimine, clathridine, clathridine zinc complex, preclathridine (Marseille, France) | In vitro, S. aureus, E. coli, C. albicans | Antimicrobial | 11–38 mm inhibition zone (concentration not determined) | [36] |
| Crambe crambe | Crambescidin 816 (Favignana island, Italy) | In vitro/ex vivo, (1) HCT-16 cells; (2) NG 108-15 cells; (3) Guinea pig ileum | (1) Cytotoxicity against colon cells; (2) potent Ca2+ antagonist activity; (3) inhibition of acetylcholine-induced contraction of ileum | (1) IC50 = 0.24 ug/mL; (2) EC50 = 1.5 × 10−4 µM; (3) −30% at 6 pM | [37] |
| Crambescidin 816, 844 and 800 (Isla de Formentor, Cueva, Spain) | In vitro: L-1210 cells | Anticancer | 98% cytotoxicity at 0.1 µg/mL | [38] | |
| Crambescidin 816, 830 and 800 (Mediterranean Sea, unspecified area) | In vitro: HepG2 In vivo: zebrafish xenografted colon cancer | Anticancer | IC50 = 0.18–2.66 μM Significant tumor growth decrease at 1 and 2 mM of embryos treatment | [39] | |
| Crambe tailliezi | P3 compound (Mediterranean Sea, unspecified area) | In vitro: U-2 OS cells | Anticancer | IC50 = 6.6 μM | [40] |
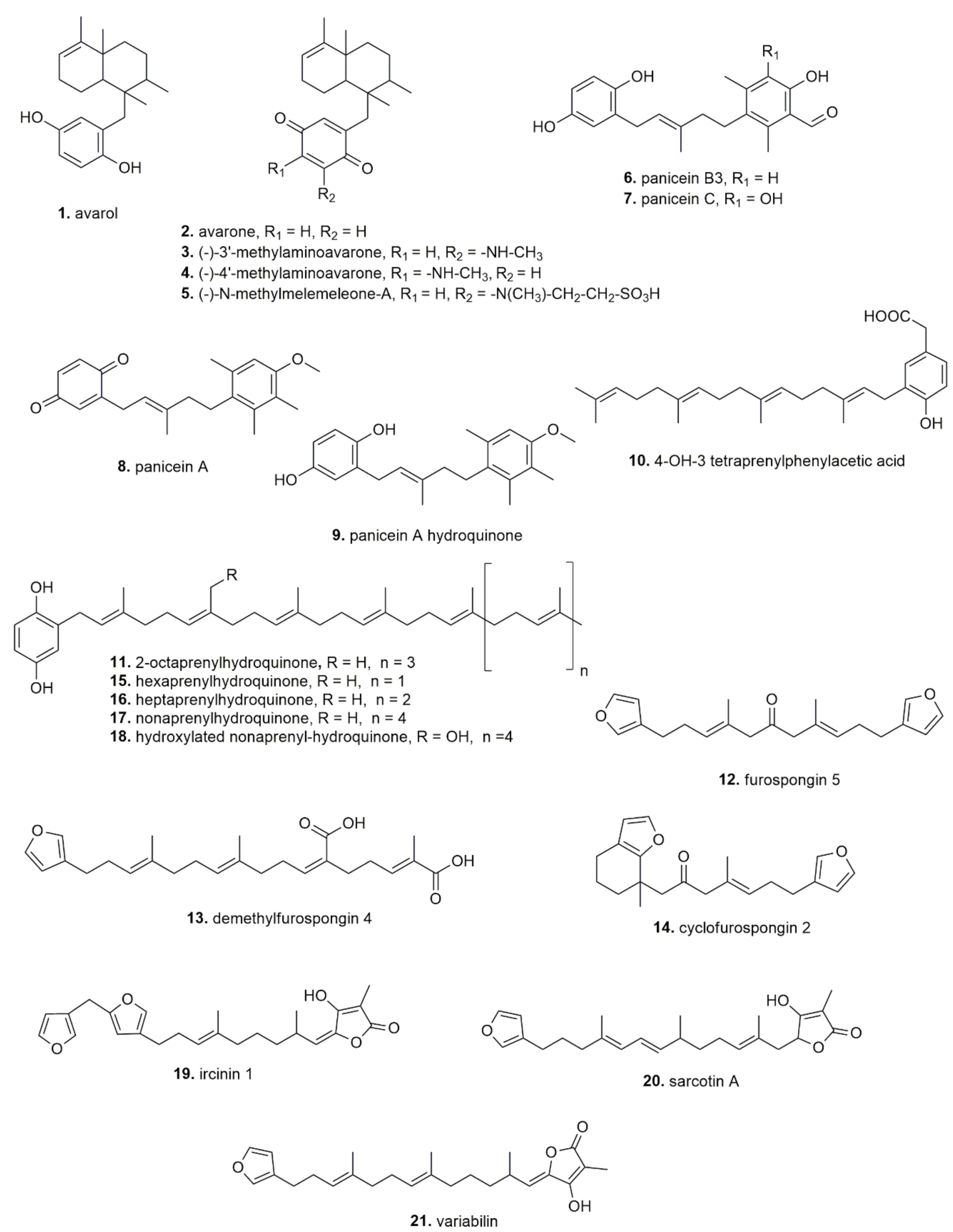
| Dysidea avara | Avarol (Mediterranean Sea, unspecified area) | In vivo, mouse | Reduction of paw edema | ED50: 9.2 mg/kg (orally) and 97 μg/ear (topically) | [41] |
| Avarol (Mediterranean Sea, unspecified area) | In vitro, human recombinant enzyme | Inhibition of human recombinant synovial PLA2 activity | IC50: 158 μM | ||
| Avarone (Mediterranean Sea, unspecified area) | In vivo, mouse | Reduction of ear edema | ED50: 4.6 mg/kg (orally) and 397 µg/ear (topically) | ||
| Avarol and avarone (Mediterranean Sea, unspecified area) | In vitro, human leukocytes | Inhibition of LTB4 and TXB2 release | IC50: 0.6 and 0.8 μM (LTB4); IC50 1.4 and 3.3 μM TXB2) | ||
| Etanolic extract (Dardanelles, Turkey) | In vitro: K562, KMS-12PE, A549, A375, H929, MCF7, HeLa, HCT116 cells | Anticancer Inhibition tyrosine kinase | IC50 = 2.91–25.15 µg/mL; Inhibition PDGFRβ (2.57 μg/mL) | [42] | |
| Avarol (Bay of Naples, Italy) | HT-29 cells | Anticancer | IC50 = <7 μM | [43] | |
| Avarol; Avarone (Bay of Kotor, Montenegro) | In vitro: L5178y cells In vivo: mouse | Anticancer | IC50 = 0.93 μM (avarol) and 0.62 μM (avarone) 10 mg/kg | [44] | |
| Avarol; Avarone (Bay of Naples, Italy) | In vitro: L1210, Raji C8166 cells | Anticancer | IC50 = 9.2–18.1 μM | [45] | |
| Avarol; Avarone; (−)-3′-methylaminoavarone; (−)-4′-methylaminoavarone; N-methylmelemeleone-A; (Fethiye, Turkey) | In vitro: HCT116 H4IIE cells | Anticancer | Avarone most potent: IC50 = 5.3–5.5 μM | [46] | |
| Avarol (Bay of Naples, Italy) | In vitro: C. albicans MH2, C. albicans 4/07, C. albicans 4/16, C. albicans 2/14, C. glabrata, C. krusei, C. albicans ATCC 10231, C. tropicalis ATCC 750 | Antimicrobial | MIC and MFC: 0.8–12 µg/mL | [47] | |
| Fasciospongia cavernosa | Carvenolide (Aegean Sea) | In vitro, human recombinant enzyme | Inhibition of human synovial PLA2 | IC50: 8.8 μM | [48] |
| In vitro, human macrophages | Reduction of TNF-α, nitrite and PGE2 production | 4.9 μM (TNFα); 7.7 μM (nitrite); IC50: 9.3 μM (PGE2) | |||
| Geodia cydonium | MeOH fraction (Bay of Naples, Italy) | In vitro: MCF-7, MDA-MB231, and MDA-MB468 | Anticancer | IC50 = 44–80 µg/mL | [49] |
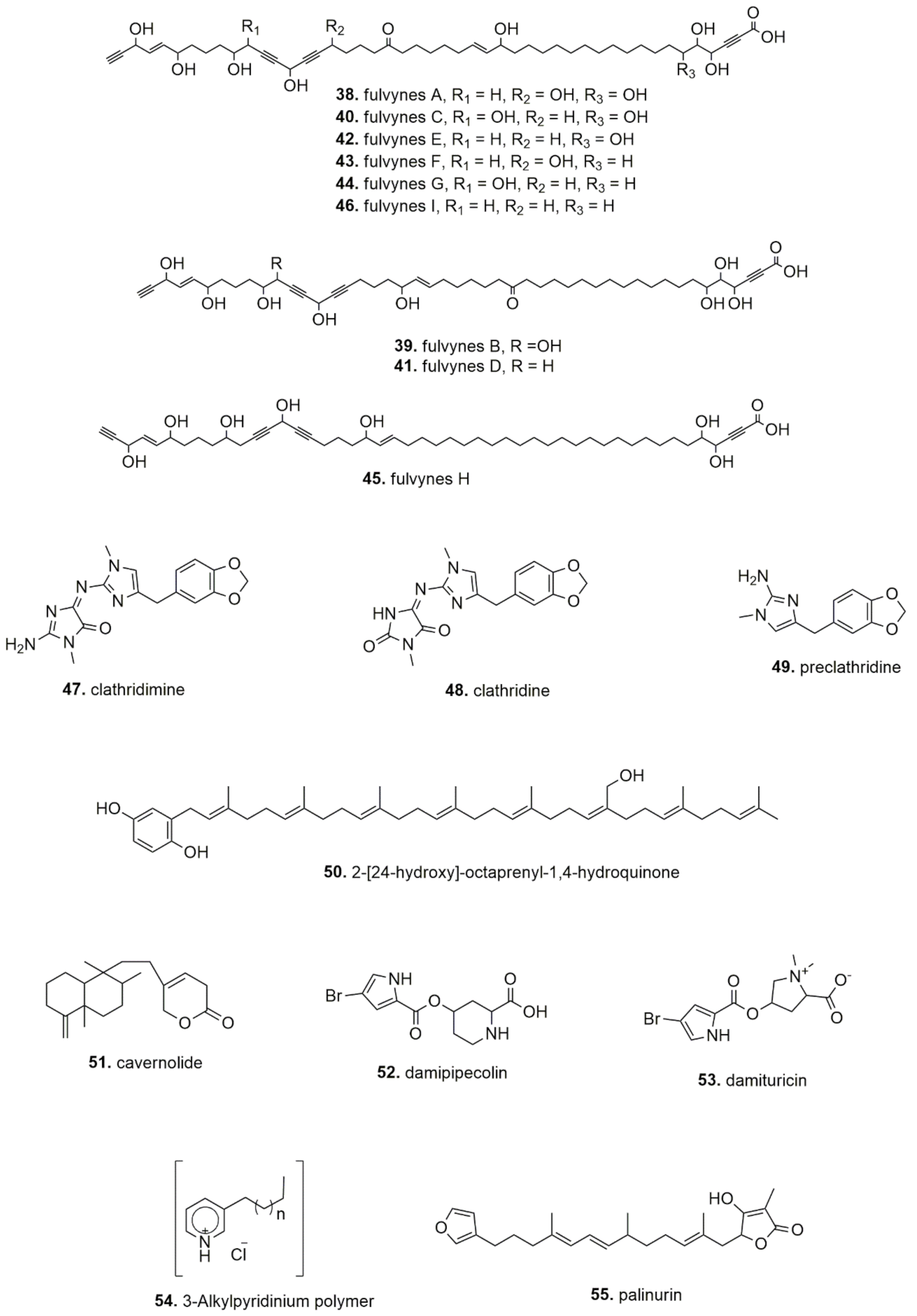
| Haliclona fulva | Butanolic extract, Fulvynes A-I (linear polyoxygenated acetylene) (Bay of Naples, Italy) | In vitro, chloramphenicol-resistant Bacillus subtilis | Antimicrobial | IC50: 60–12 µM | [50] |
| Haliclona (Soestella) mucosa | Panicein A hydroquinone (Villefranche-sur-Mer, France) | In vitro: MeWo cells | Anticancer | Panicein A: IC50 = >30 µM | [51] |
| Ircinia dendroides | Palinurin (Mediterranean Sea, unspecified area) | In vitro, neuroblastoma SH-SY5Y cells | rGSK-3b inhibitory activity | IC50 = 2.6 µM | [52] |
| Ircinia fasciculata | Polyprenyl-1,4-hydroquinone derivates (hexa-, hepta- and nona- prenyl-1,4-hydroquinone) (Fethiye, Turkey) | In vitro: H4IIE cells | Anticancer | Heptaprenyl hydroquinone: IC50 = 2.5 μM | [53] |
| Ircinia muscarum | Polyprenyl-hydroquinones; furanoterpenoids (Baleares, Spain) | In vitro: Inhibition of CDC25 phosphatase | Anticancer | 4-OH-3 Tetraprenylphenylacetic acid: IC50 = 0.4–4 µM; 2-Octaprenylhydroquinone: IC50 = 400 µM | [54] |
| Ircinia spinulosa | Polyprenyl-hydroquinones; furanoterpenoids(Bodrum, Turkey; Naples, Italy; Sutomiscica, Croatia) | In vitro: Inhibition of CDC25 phosphatase | Anticancer | 4-OH-3 Tetraprenylphenylacetic acid: IC50 = 0.4–4 µM;2-Octaprenylhydroquinone: IC50 = 400 µM | [54] |
| lrcinia spinosula | IS2, IS3 (Bay of Naples, Italy) | In vitro: human recombinant enzyme | Inhibition of synovial PLA2 | IC50: 48.7 and 48 µM | [55] |
| In vitro: human neutrophils | Inhibition of LTB4 production and TXB2 synthesis and release | IC50: 23.1 and 7.4 µM (LTB4); IC50 3.9 and 3.4 µM (TXB2) | |||
| In vivo, mouse | Reduction of ear inflammation | 250 µg/ear and 125 µg/ear (topically) | |||
| Petrosia ficiformis sp. | Methanol extract (Portofino’s promontory, Italy) | In vitro: LAN5 and SK-N-BE(2)-C cells | Anticancer | 10–20 ppm significantly increased cell death | [24] |
| Rhaphisia iacazei | Topsentin B1 and B2 (Ustica, Italy) | In vitro: NSCLC-N6 cells | Anticancer | IC50 = 6.3 (B1) and 12 (B2) µg/mL | [56] |
| Reniera fulva | Paniceins A, Panicein B3, Panicein C (Favignana island, Italy) | In vitro: CCRF-CEM, NCI-H522 | Anticancer | −log10 I50: 5.11–5.48 | [57] |
| Reniera sarai | 3-Alkylpyridinium polymers (Northern Adriatic Sea) | In vitro, isolated enzyme | AChE inhibition | 50% inhibition induced by 0.06 (human erythrocyte AChE), 0.08 (electric eel AChE), 0.7 (insect recombinant AChE) and 0.14 µg/mL (horse serum butyrylcholinesterase) | [58] |
| 3-Alkylpyridinium oligomers and polymers (Northern Adriatic Sea) | Ex vivo, mouse skeletal muscle | AChE inhibition; blockade of the neuromuscular transmission | IC50 = 18.5 μM (mouse muscle twitch), 18.5 μM (tetanic contraction) | [59] | |
| Sarcotragus fasciculatus | Furanosesterterpene tetronic acids and Polyprenyl-hydroquinones (Monastir, Tunisia) | In vitro: A549, Hs683, MCF-7, SKMEL-28, U373, B16F10) | Anticancer | Furanosesterpene tetronic acids IC50=> 80 µM; Polyprenyl-hydroquinones IC50 = 3–23 µM | [60] |
| Sarcotragus foetidus | Furanosesterterpene tetronic acids and Polyprenyl-hydroquinones (Cap Zebib, Tunisia) | In vitro: A549, Hs683, MCF-7, SKMEL-28, U373, B16F10) | Anticancer | Furanosesterpene tetronic acids IC50 => 80 µM; Polyprenyl-hydroquinones IC50 = 3–23 µM | [60] |
| Sarcotragus muscarum | Polyprenyl-1,4-hydroquinone derivates (hexa-, hepta- and nona- prenyl-1,4-hydroquinone)(Mersin, Turkey) | In vitro: H4IIE cells | Anticancer | Heptaprenylhydroquinone: IC50 = 2.5 μM | [53] |
| Sarcotragus spinosulus | Hydroxylated nonaprenylhydroquinone; hepta- and octa-prenylhydroquinone (Callejones, Spain) | In vitro: K562 cells | Anticancer (apoptosis) | Hepta- and octa-prenylhydroquinone: 8 and 10 µM; Hydroxylated nonaprenylhydroquinone 193 µM | [61] |
| Furanosesterterpene tetronic acids and Polyprenyl-hydroquinones (Tabarka and Monastir, Tunisia) | In vitro: A549, Hs683, MCF-7, SKMEL-28, U373, B16F10) | Anticancer (apoptosis) | Furanosesterpene tetronic acids IC50 => 80 µM; Polyprenyl-hydroquinones IC50 = 3–23 µM | [60] | |
| Spongia officinalis | Methanol and fractions (0%, 50% and 80% MeOH in water)(Monastir, Tunisia) | In vitro: A549, HCT15, MCF7 cells | Anticancer | F3 fraction greater potency: IC50 = 212–572 µg/mL | [62] |
| Furospongin-5; Cyclofurospongin-2; demethylfurospongin-4 (Cadiz, Spain) | In vitro: P-388 cells | Anticancer | Furospongin: IC50 = 5 µg/mL | [63] | |
| Polyprenyl-hydroquinones; furanoterpenoids (Bodrum, Turkey) | In vitro: Inhibition of CDC25 phosphatase | Anticancer | 4-OH-3 Tetraprenylphenylacetic acid: IC50 = 0.4–4 µM; 2-Octaprenylhydroquinone: IC50 = 400 µM | [54] | |
| Methanol/water, crude extract and fractions (Tunisia) | In vivo, mice | Anticovulsant and analgesic | 600 mg/kg crude extract; 100 and 200 mg/kg 50% methanol fractions | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Cesare Mannelli, L.; Palma Esposito, F.; Sangiovanni, E.; Pagano, E.; Mannucci, C.; Polini, B.; Ghelardini, C.; Dell’Agli, M.; Izzo, A.A.; Calapai, G.; et al. Pharmacological Activities of Extracts and Compounds Isolated from Mediterranean Sponge Sources. Pharmaceuticals 2021, 14, 1329. https://doi.org/10.3390/ph14121329
Di Cesare Mannelli L, Palma Esposito F, Sangiovanni E, Pagano E, Mannucci C, Polini B, Ghelardini C, Dell’Agli M, Izzo AA, Calapai G, et al. Pharmacological Activities of Extracts and Compounds Isolated from Mediterranean Sponge Sources. Pharmaceuticals. 2021; 14(12):1329. https://doi.org/10.3390/ph14121329
Chicago/Turabian StyleDi Cesare Mannelli, Lorenzo, Fortunato Palma Esposito, Enrico Sangiovanni, Ester Pagano, Carmen Mannucci, Beatrice Polini, Carla Ghelardini, Mario Dell’Agli, Angelo Antonio Izzo, Gioacchino Calapai, and et al. 2021. "Pharmacological Activities of Extracts and Compounds Isolated from Mediterranean Sponge Sources" Pharmaceuticals 14, no. 12: 1329. https://doi.org/10.3390/ph14121329
APA StyleDi Cesare Mannelli, L., Palma Esposito, F., Sangiovanni, E., Pagano, E., Mannucci, C., Polini, B., Ghelardini, C., Dell’Agli, M., Izzo, A. A., Calapai, G., de Pascale, D., & Nieri, P. (2021). Pharmacological Activities of Extracts and Compounds Isolated from Mediterranean Sponge Sources. Pharmaceuticals, 14(12), 1329. https://doi.org/10.3390/ph14121329