Parthenolide as Cooperating Agent for Anti-Cancer Treatment of Various Malignancies
Abstract
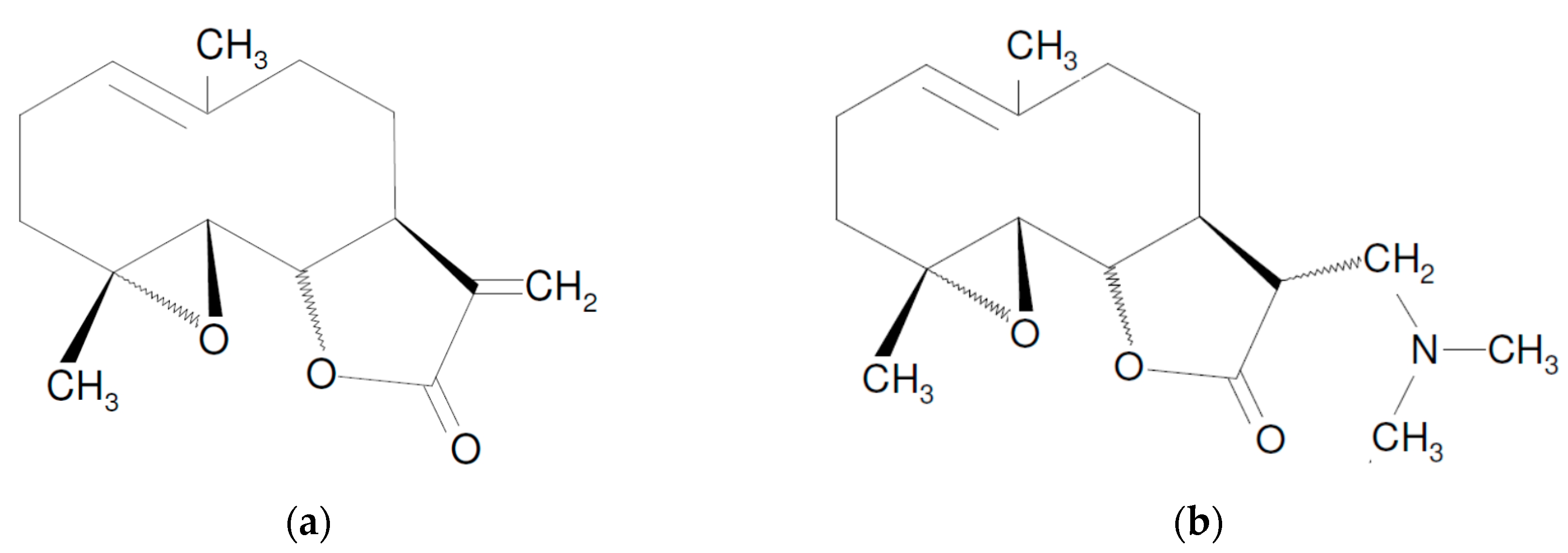
1. Introduction
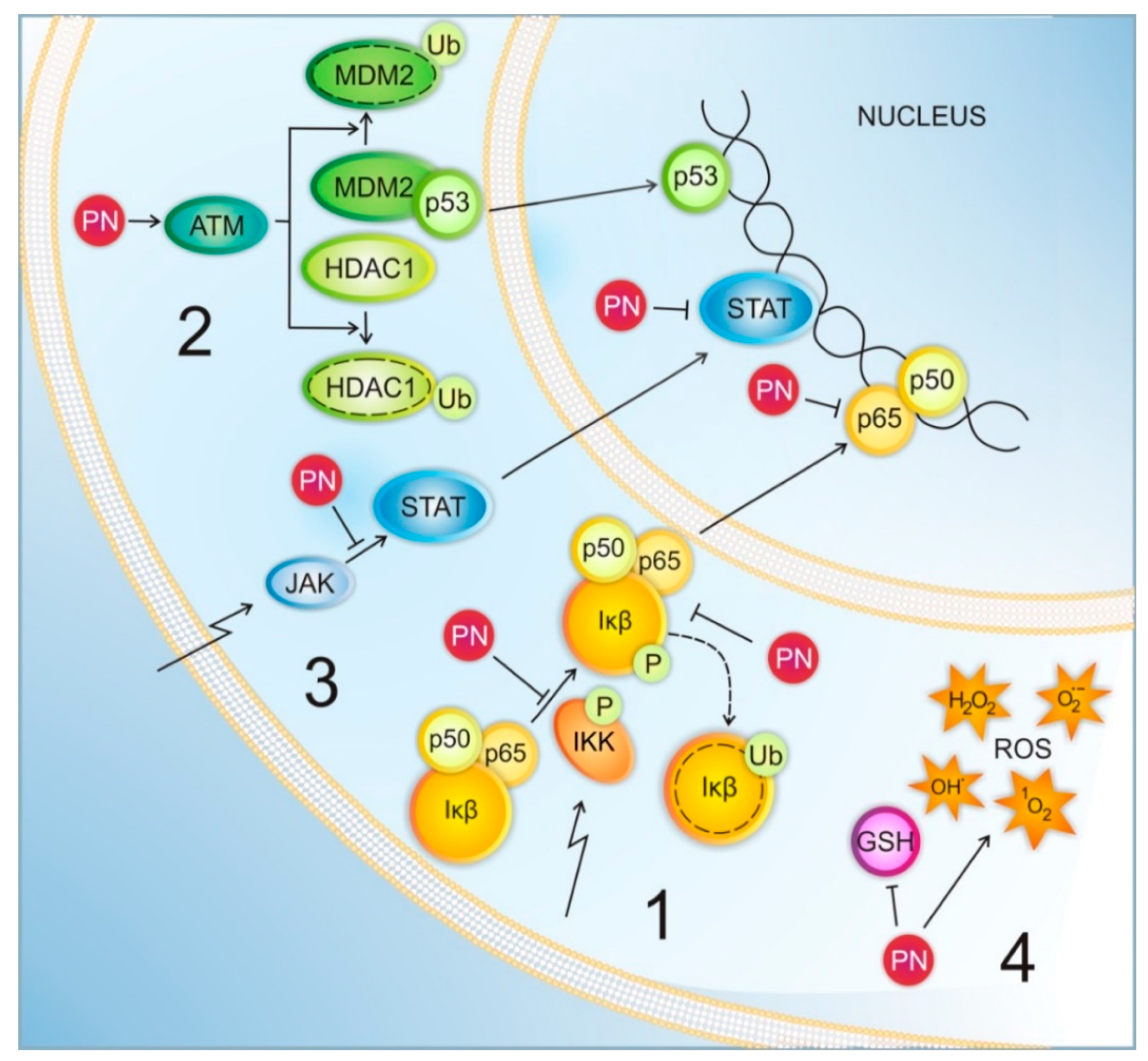
2. Mechanisms of PN Action
3. Parthenolide in Combination with Anticancer Agents
3.1. Parthenolide Combined with Tubulin-Directed Agents
3.2. Parthenolide Combined with TRAIL
3.3. Parthenolide Combined with Anti-Inflammatory Drugs
3.4. Parthenolide Combined with Hormonal Agents
3.5. Parthenolide Combined with Anthracyclines
3.6. Parthenolide Combined with Alkylating Agents
3.7. Parthenolide Combined with Antimetabolites
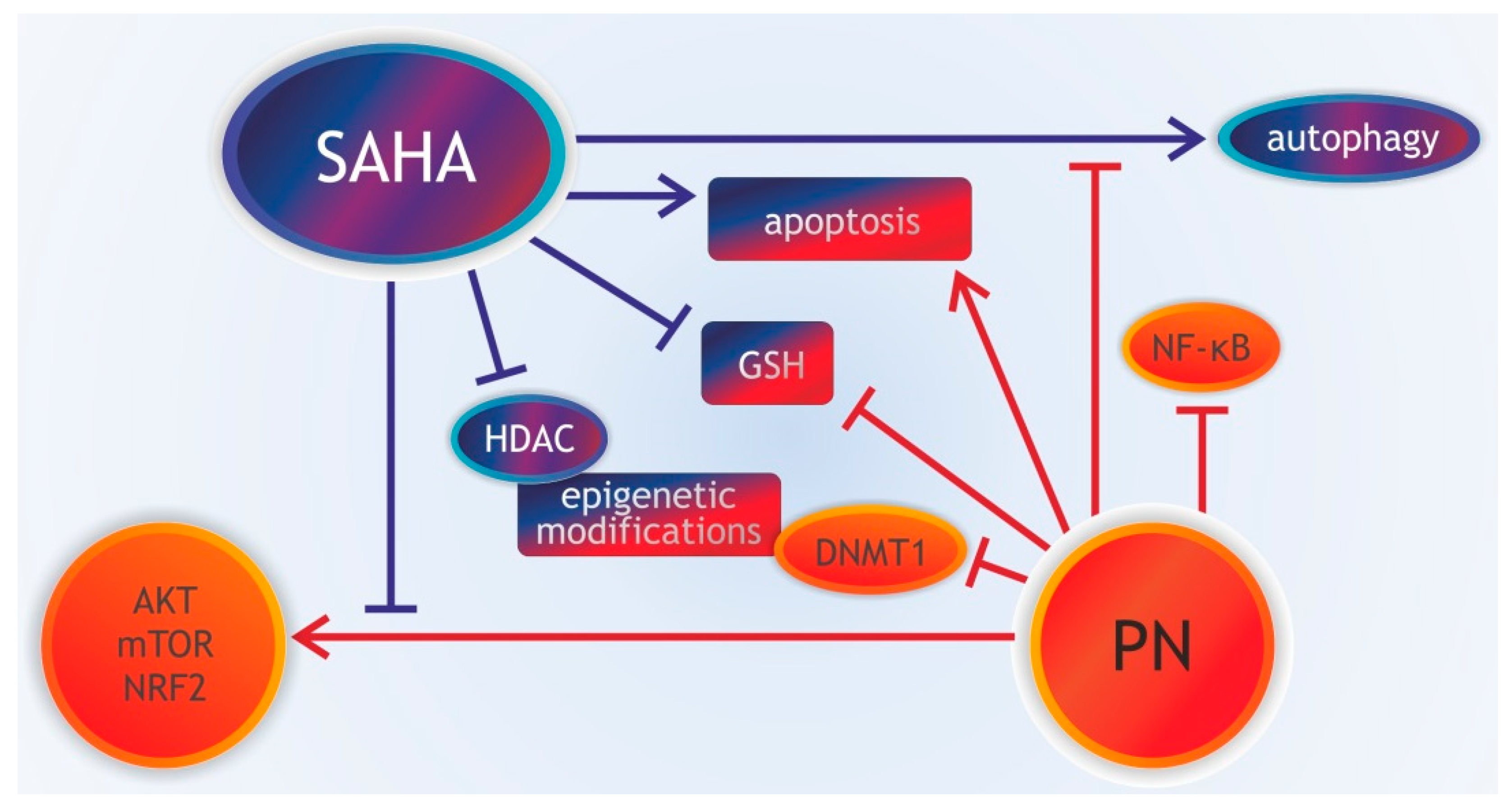
3.8. Parthenolide Combined with Histone Deacetylase Inhibitors
3.9. Parthenolide Combined with mTOR Inhibitors
3.10. Parthenolide Combined with Retinoids
3.11. Parthenolide Combined with Inducers of Reactive Oxygen Species (ROS)
3.12. Parthenolide Combined with Other Drugs
4. Parthenolide in Combination with Radio- and Thermotherapy
4.1. Parthenolide Combined with Radiotherapy
4.2. Parthenolide Combined with Thermotherapy
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| ABCB5 | ATP-binding cassette sub-family B member-5 |
| AML | acute myeloid leukemia |
| ATM | ataxia telangiectasia mutated serine/threonine kinase |
| ATRA | all-trans retinoic acid |
| BL | Burkitt lymphoma |
| CAC | colitis-associated colon cancer |
| CC | cholangiocarcinoma |
| COX | cyclooxygenase |
| CRC | colorectal cancer |
| DHEA | dehydroepiandrosterone |
| DMAPT | dimethylamino-parthenolide |
| DNMT1 | DNA methyltransferase 1 |
| Dox | doxorubicin |
| DR | death receptor |
| DTIC | dacarbazine |
| EBV | Epstein-Barr virus |
| ER | estrogen receptor |
| FLT3 | FMS-like tyrosine kinase 3 |
| GBM | glioblastoma multiforme |
| GSH | glutathione in reduced state |
| HCC | hepatocellular carcinoma |
| HDAC1 | histone deacetylase 1 |
| HDACIs | histone deacetylase inhibitors |
| HSP | heat-shock proteins |
| IĸB | inhibitor-of-ĸB |
| IKK | IĸB complex kinase |
| JNK | c-Jun N-terminal kinase |
| MDM2 | mouse double minute 2 homolog |
| MGMT | O6-methylguanine-DNA methyltransferase |
| MITF | microphthalmia-associated transcription factor |
| MMP9 | matrix metalloproteinase 9 |
| Mn-SOD | manganese superoxide dismutase |
| NF-ĸB | nuclear transcription factor-kappa B |
| NSAID | nonsteroidal anti-inflammatory drugs |
| NSCLC | non-small-cell lung cancer |
| PARP | poly (ADP-ribose) polymerase |
| PN | parthenolide |
| ROS | reactive oxygen species |
| SAHA | suberoylanilide hydroxamic acid, vorinostat |
| SP | side-population of cells |
| TNF-α | tumor necrosis factor α |
| TRAIL | tumor necrosis factor-related apoptosis-inducing ligand |
| TRAMP | transgenic adenocarcinoma of the mouse prostate |
| VEGF | vascular endothelial growth factor |
References
- Bork, P.M.; Schmitz, M.L.; Kuhnt, M.; Escher, C.; Heinrich, M. Sesquiterpene lactone containing Mexican Indian medical plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-κB. FEBS Lett. 1997, 402, 85–90. [Google Scholar] [CrossRef]
- Juliana, C.; Fernandes-Alnemri, T.; Wu, J.; Datta, P.; Solorzano, L.; Yu, J.W.; Meng, R.; Quong, A.A.; Latz, E.; Scott, C.P.; et al. Anti-inflammatory compounds parthenolide and Bay 11- 7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 2010, 285, 9792–9802. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.O.; Ferguson, J.E.; Hunsaker, L.A.; Deck, L.M.; Vander Jagt, D.L. Natural products inhibit LPS-induced activation of pro-inflammatory cytokines in peripheral blood mononuclear cells. Nat. Prod. Res. 2010, 24, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Tenci, B.; Zanardelli, M.; Maidecchi, A.; Lugli, A.; Mattoli, L.; Ghelardini, C. Widespread pain reliever profile of a flower extract of Tanacetum parthenium. Phytomedicine 2015, 22, 752–758. [Google Scholar] [CrossRef]
- Ghantous, A.; Gali-Muhtasib, H.; Vuorela, H.; Saliba, N.A.; Darwiche, N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today 2007, 15, 668–678. [Google Scholar] [CrossRef]
- Carlisi, D.; D’Anneo, A.; Angileri, L.; Lauricella, M.; Emanuele, S.; Santulli, A.; Vento, R.; Tesoriere, G. Parthenolide sensitizes hepatocellular carcinoma cells to TRAIL by inducing the expression of death receptors through inhibition of STAT3 activation. J. Cell. Physiol. 2011, 226, 1632–1641. [Google Scholar] [CrossRef]
- Wen, J.; You, K.R.; Lee, S.Y.; Song, C.H.; Kim, D.G. Oxidative stress-mediated apoptosis. The anticancer effect of the sesquiterpene lactone parthenolide. J. Biol. Chem. 2002, 277, 38954–38964. [Google Scholar] [CrossRef]
- Zunino, S.J.; Ducore, J.M.; Storms, D.H. Parthenolide induces significant apoptosis and production of reactive oxygen species in high-risk pre-B leukemia cells. Cancer Lett. 2007, 254, 119–127. [Google Scholar] [CrossRef]
- Duechler, M.; Stanczyk, M.; Czyz, M.; Stepnik, M. Potentiation of arsenic trioxide cytotoxicity by Parthenolide and buthionine sulfoximine in murine and human leukemic cells. Cancer Chemother. Pharmacol. 2008, 61, 727–737. [Google Scholar] [CrossRef]
- Suvannasankha, A.; Crean, C.D.; Shanmugam, R.; Farag, S.S.; Abonour, R.; Boswell, H.S.; Nakshatri, H. Antimyeloma effects of a sesquiterpene lactone parthenolide. Clin. Cancer Res. 2008, 14, 1814–1822. [Google Scholar] [CrossRef]
- Dai, Y.; Guzman, M.L.; Chen, S.; Wang, L.; Yeung, S.K.; Pei, X.Y.; Dent, P.; Jordan, C.T.; Grant, S. The NF (Nuclear factor)-κB inhibitor parthenolide interacts with histone deacetylase inhibitors to induce MKK7/JNK1-dependent apoptosis in human acute myeloid leukaemia cells. Br. J. Haematol. 2010, 151, 70–83. [Google Scholar] [CrossRef]
- Czyz, M.; Lesiak-Mieczkowska, K.; Koprowska, K.; Szulawska-Mroczek, A.; Wozniak, M. Cell context-dependent activities of parthenolide in primary and metastatic melanoma cells. Br. J. Pharmacol. 2010, 160, 1144–1157. [Google Scholar] [CrossRef] [PubMed]
- Lesiak, K.; Koprowska, K.; Zalesna, I.; Nejc, D.; Düchler, M.; Czyz, M. Parthenolide, a sesquiterpene lactone from the medical herb feverfew, shows anticancer activity against human melanoma cells in vitro. Melanoma Res. 2010, 20, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Czyz, M.; Koprowska, K.; Sztiller-Sikorska, M. Parthenolide reduces the frequency of ABCB5-positive cells and clonogenic capacity of melanoma cells from anchorage independent melanospheres. Cancer Biol. Ther. 2013, 14, 135–145. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, J.; Kinghorn, A.D. Development of Anticancer Agents from Plant-Derived Sesquiterpene Lactones. Curr. Med. Chem. 2016, 23, 2397–2420. [Google Scholar] [CrossRef]
- Baranello, M.P.; Bauer, L.; Jordan, C.T.; Benoit, D.S.W. Micelle Delivery of Parthenolide to Acute Myeloid Leukemia Cells. Cell. Mol. Bioeng. 2015, 8, 455–470. [Google Scholar] [CrossRef]
- Carlisi, D.; Buttitta, G.; Di Fiore, R.; Scerri, C.; Drago-Ferrante, R.; Vento, R.; Tesoriere, G. Parthenolide and DMAPT exert cytotoxic effects on breast cancer stem-like cells by inducing oxidative stress, mitochondrial dysfunction and necrosis. Cell Death Dis. 2016, 7, e2194. [Google Scholar] [CrossRef]
- Curry, E.A., III; Murry, D.J.; Yoder, C.; Fife, K.; Armstrong, V.; Nakshatri, H.; O’Connell, M.; Sweeney, C.J. Phase I dose escalation trial of feverfew with standardized doses of parthenolide in patients with cancer. Invest. New Drugs 2004, 22, 299–305. [Google Scholar] [CrossRef] [PubMed]
- D’Anneo, A.; Carlisi, D.; Lauricella, M.; Puleio, R.; Martinez, R.; Di Bella, S.; Di Marco, P.; Emanuele, S.; Di Fiore, R.; Guercio, A.; et al. Parthenolide generates reactive oxygen species and autophagy in MDA-MB231 cells. A soluble parthenolide analogue inhibits tumour growth and metastasis in a xenograft model of breast cancer. Cell Death Dis. 2013, 4, e891. [Google Scholar] [CrossRef] [PubMed]
- Gopal, Y.N.; Arora, T.S.; Van Dyke, M.W. Parthenolide specifically depletes histone deacetylase 1 protein and induces cell death through ataxia telangiectasia mutated. Chem. Biol. 2007, 14, 813–823. [Google Scholar] [CrossRef]
- Gopal, Y.N.; Chanchorn, E.; Van Dyke, M.W. Parthenolide promotes the ubiquitination of MDM2 and activates p53 cellular functions. Mol. Cancer Ther. 2009, 8, 552–562. [Google Scholar] [CrossRef]
- Guzman, M.L.; Rossi, R.M.; Karnischky, L.; Li, X.; Peterson, D.R.; Howard, D.S.; Jordan, C.T. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood 2005, 105, 4163–4169. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.L.; Talar, B.; Sztiller-Sikorska, M.; Nejc, D.; Czyz, M. Parthenolide induces MITF-M downregulation and senescence in patient-derived MITF-M (high) melanoma cell populations. Oncotarget 2016, 7, 9026–9040. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Liu, L.; Lee, S.O.; Kim, Y.T.; You, K.R.; Kim, D.G. Susceptibility of cholangiocarcinoma cells to parthenolide-induced apoptosis. Cancer Res. 2005, 65, 6312–6320. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.L.; Liu, Y.C.; Park, Y.R.; Seo, S.Y.; Kim, S.H.; Kim, I.H.; Lee, S.O.; Lee, S.T.; Kim, D.G.; Kim, S.W. Parthenolide enhances sensitivity of colorectal cancer cells to TRAIL by inducing death receptor 5 and promotes TRAIL-induced apoptosis. Int. J. Oncol. 2015, 46, 1121–1130. [Google Scholar] [CrossRef]
- Koprowska, K.; Hartman, M.L.; Sztiller-Sikorska, M.; Czyz, M.E. Parthenolide enhances dacarbazine activity against melanoma cells. Anticancer Drugs 2013, 24, 835–845. [Google Scholar] [CrossRef]
- Li, H.; Lu, H.; Lv, M.; Wang, Q.; Sun, Y. Parthenolide facilitates apoptosis and reverses drug-resistance of human gastric carcinoma cells by inhibiting the STAT3 signaling pathway. Oncol. Lett. 2018, 15, 3572–3579. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Fu, M.; Yao, Q.; Zhuo, H.; Lu, Q.; Niu, X.; Zhang, P.; Pei, Y.; Zhang, K. Parthenolide induces apoptosis and lytic cytotoxicity in Epstein-Barr virus-positive Burkitt lymphoma. Mol. Med. Rep. 2012, 6, 477–482. [Google Scholar] [CrossRef]
- Liu, M.; Xiao, C.; Sun, M.; Tan, M.; Hu, L.; Yu, Q. Parthenolide Inhibits STAT3 Signaling by Covalently Targeting Janus Kinases. Molecules 2018, 23, E1478. [Google Scholar] [CrossRef]
- Mendonca, M.S.; Chin-Sinex, H.; Gomez-Millan, J.; Datzman, N.; Hardacre, M.; Comerford, K.; Nakshatri, H.; Nye, M.; Benjamin, L.; Mehta, S.; et al. PN sensitizes cells to X-ray-induced cell killing through inhibition of NF-kappaB and split-dose repair. Radiat. Res. 2007, 168, 689–697. [Google Scholar] [CrossRef]
- Morel, K.L.; Ormsby, R.J.; Bezak, E.; Sweeney, C.J.; Sykes, P.J. Parthenolide Selectively Sensitizes Prostate Tumor Tissue to Radiotherapy while Protecting Healthy Tissues In Vivo. Radiat. Res. 2017, 187, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, R.; Jayaprakasan, V.; Gokmen-Polar, Y.; Kelich, S.; Miller, K.D.; Yip-Schneider, M.; Cheng, L.; Bhat-Nakshatri, P.; Sledge, G.W., Jr.; Nakshatri, H.; et al. Restoring chemotherapy and hormone therapy sensitivity by parthenolide in a xenograft hormone refractory prostate cancer model. Prostate 2006, 66, 1498–1511. [Google Scholar] [CrossRef] [PubMed]
- Sobota, R.; Szwed, M.; Kasza, A.; Bugno, M.; Kordula, T. Parthenolide inhibits activation of signal transducers and activators of transcription (STATs) induced by cytokines of the IL-6 family. Biochem. Biophys. Res. Commun. 2000, 267, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Sohma, I.; Fujiwara, Y.; Sugita, Y.; Yoshioka, A.; Shirakawa, M.; Moon, J.H.; Takiguchi, S.; Miyata, H.; Yamasaki, M.; Mori, M.; et al. Parthenolide, an NF-κB inhibitor, suppresses tumor growth and enhances response to chemotherapy in gastric cancer. Cancer Genomics Proteomics 2011, 8, 39–47. [Google Scholar]
- Sun, Y.; St Clair, D.K.; Xu, Y.; Crooks, P.A.; St Clair, W.H. A NADPH oxidase-dependent redox signaling pathway mediates the selective radiosensitization effect of parthenolide in prostate cancer cells. Cancer Res. 2010, 70, 2880–2890. [Google Scholar] [CrossRef]
- Wozniak, M.; Szulawska-Mroczek, A.; Hartman, M.L.; Nejc, D.; Czyz, M. Parthenolide complements the cell death-inducing activity of doxorubicin in melanoma cells. Anticancer Res. 2013, 33, 3205–3212. [Google Scholar]
- Yang, C.; Yang, Q.O.; Kong, Q.J.; Yuan, W.; Ou Yang, Y.P. Parthenolide Induces Reactive Oxygen Species-Mediated Autophagic Cell Death in Human Osteosarcoma Cells. Cell. Physiol. Biochem. 2016, 40, 146–154. [Google Scholar] [CrossRef]
- Zhang, S.; Ong, C.N.; Shen, H.M. Critical roles of intracellular thiols and calcium in parthenolide-induced apoptosis in human colorectal cancer cells. Cancer Lett. 2004, 208, 143–153. [Google Scholar] [CrossRef]
- Hassane, D.C.; Sen, S.; Minhajuddin, M.; Rossi, R.M.; Corbett, C.A.; Balys, M.; Wei, L.; Crooks, P.A.; Guzman, M.L.; Jordan, C.T. Chemical genomic screening reveals synergism between parthenolide and inhibitors of the PI-3 kinase and mTOR pathways. Blood 2010, 116, 5983–5990. [Google Scholar] [CrossRef]
- Diamanti, P.; Cox, C.V.; Moppett, J.P.; Blair, A. Parthenolide eliminates leukemia-initiating cell populations and improves survival in xenografts of childhood acute lymphoblastic leukemia. Blood 2013, 121, 1384–1393. [Google Scholar] [CrossRef]
- Spagnuolo, P.A.; Hurren, R.; Gronda, M.; MacLean, N.; Datti, A.; Basheer, A.; Lin, F.H.; Wang, X.; Wrana, J.; Schimmer, A.D. Inhibition of intracellular dipeptidyl peptidases 8 and 9 enhances parthenolide’s anti-leukemic activity. Leukemia 2013, 27, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Uddin, S.; Mohammad, R.M. Targeting acute myeloid leukemia stem cell signaling by natural products. Mol. Cancer 2017, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.L.; Rossi, R.M.; Neelakantan, S.; Li, X.; Corbett, C.A.; Hassane, D.C.; Becker, M.W.; Bennett, J.M.; Sullivan, E.; Lachowicz, J.L.; et al. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood 2007, 110, 4427–4435. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, B.T.; Hurt, E.M.; Kalathur, M.; Duhagon, M.A.; Milner, J.A.; Kim, Y.S.; Farrar, W.L. Effects of the sesquiterpene lactone parthenolide on prostate tumor initiating cells: An integrated molecular profiling approach. Prostate 2009, 69, 827–837. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, H.; Gu, P.; Bai, J.; Margolick, J.B.; Zhang, Y. NF-kappaB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res. Treat. 2008, 111, 419–427. [Google Scholar] [CrossRef]
- Flores-Lopez, G.; Moreno-Lorenzana, D.; Ayala-Sanchez, M.; Aviles-Vazquez, S.; Torres-Martinez, H.; Crooks, P.A.; Guzman, M.L.; Mayani, H.; Chávez-González, A. Parthenolide and DMAPT induce cell death in primitive CML cells through reactive oxygen species. J. Cell Mol. Med. 2018, 22, 4899–4912. [Google Scholar] [CrossRef]
- Neelakantan, S.; Nasim, S.; Guzman, M.L.; Jordan, C.T.; Crooks, P.A. Aminoparthenolides as novel anti-leukemic agents: Discovery of the NF-kappaB inhibitor, DMAPT (LC-1). Bioorg. Med. Chem. Lett. 2009, 19, 4346–4349. [Google Scholar] [CrossRef]
- Song, J.M.; Qian, X.; Upadhyayya, P.; Hong, K.H.; Kassie, F. Dimethylaminoparthenolide, a water soluble parthenolide, suppresses lung tumorigenesis through down-regulating the STAT3 signaling pathway. Curr. Cancer Drug Targets 2014, 14, 59–69. [Google Scholar] [CrossRef]
- Nakshatri, H.; Appaiah, H.N.; Anjanappa, M.; Gilley, D.; Tanaka, H.; Badve, S.; Crooks, P.A.; Mathews, W.; Sweeney, C.; Bhat-Nakshatri, P. NF-kappa B-dependent and -independent epigenetic modulation using the novel anti-cancer agent DMAPT. Cell Death Dis. 2015, 6, e1608. [Google Scholar] [CrossRef]
- Penthala, N.R.; Janganati, V.; Alpe, T.L.; Apana, S.M.; Berridge, M.S.; Crooks, P.A.; Borrelli, M.J. N-[11CH3] Dimethylaminoparthenolide (DMAPT) uptake into orthotopic 9LSF glioblastoma tumors in the rat. Bioorg. Med. Chem. Lett. 2016, 26, 5883–5886. [Google Scholar] [CrossRef]
- Kwok, B.H.; Koh, B.; Ndubuisi, M.I.; Elofsson, M.; Crews, C.M. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem. Biol. 2001, 8, 759–766. [Google Scholar] [CrossRef]
- Nagel, D.; Vincendeau, M.; Eitelhuber, A.C.; Krappmann, D. Mechanisms and consequences of constitutive NF-κB activation in B-cell lymphoid malignancies. Oncogene 2014, 33, 5655–5665. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, K.A.; Kaergel, E.; Heinig, M.; Fontaine, J.F.; Patone, G.; Muro, E.M.; Mathas, S.; Hummel, M.; Andrade-Navarro, M.A.; Hübner, N.; et al. A roadmap of constitutive NF-κB activity in Hodgkin lymphoma: Dominant roles of p50 and p52 revealed by genome-wide analyses. Genome Med. 2016, 8, 28. [Google Scholar] [CrossRef]
- Thu, Y.M.; Su, Y.; Yang, J.; Splittgerber, R.; Na, S.; Boyd, A.; Mosse, C.; Simons, C.; Richmond, A. NF-κB inducing kinase (NIK) modulates melanoma tumorigenesis by regulating expression of pro-survival factors through the β-catenin pathway. Oncogene 2012, 31, 2580–2592. [Google Scholar] [CrossRef] [PubMed]
- Tilborghs, S.; Corthouts, J.; Verhoeven, Y.; Arias, D.; Rolfo, C.; Trinh, X.B.; van Dam, P.A. The role of Nuclear Factor-kappa B signaling in human cervical cancer. Crit. Rev. Oncol. Hematol. 2017, 120, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.S. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-nB. J. Clin. Investig. 2001, 107, 241–246. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Wang, W.; Liu, Y.; Li, Y.; Gao, J.; Wang, C.; Zhou, M.; Liu, R.; Xu, G.; et al. ZBTB7 evokes 5-fluorouracil resistance in colorectal cancer through the NF-κB signaling pathway. Int. J. Oncol. 2018, 53, 2102–2110. [Google Scholar] [CrossRef]
- Tian, M.; Tian, D.; Qiao, X.; Li, J.; Zhang, L. Modulation of Myb-induced NF-kB -STAT3 signaling and resulting cisplatin resistance in ovarian cancer by dietary factors. J. Cell. Physiol. 2019, 234, 21126–21134. [Google Scholar] [CrossRef]
- García-Piñeres, A.J.; Lindenmeyer, M.T.; Merfort, I. Role of cysteine residues of p65/NF-kappaB on the inhibition by the sesquiterpene lactone parthenolide and N-ethyl maleimide, and on its transactivating potential. Life Sci. 2004, 75, 841–856. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, Z.N.; Yang, C.F.; Shi, X.; Ong, C.N.; Shen, H.M. Suppressed NF-kappaB and sustained JNK activation contribute to the sensitization effect of parthenolide to TNF-alpha-induced apoptosis in human cancer cells. Carcinogenesis 2004, 25, 2191–2199. [Google Scholar] [CrossRef]
- Nakshatri, H.; Rice, S.E.; Bhat-Nakshatri, P. Antitumor agent parthenolide reverses resistance of breast cancer cells to tumor necrosis factor-related apoptosis-inducing ligand through sustained activation of c-Jun N-terminal kinase. Oncogene 2004, 23, 7330–7344. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, B.; Schnyder-Candrian, S.; Panski, A.; Bömmel, H.; Heim, M.; Duschl, A.; Moser, R. Phytochemical inhibition of interleukin-4-activated Stat6 and expression of VCAM-1. Biochem. Biophys. Res. Commun. 2002, 292, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kong, L.; Yang, Q.; Duan, A.; Ju, X.; Cai, B.; Chen, L.; An, T.; Li, Y. Parthenolide inhibits ubiquitin-specific peptidase 7 (USP7), Wnt signaling, and colorectal cancer cell growth. J. Biol. Chem. 2020, 295, 3576–3589. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Adachi, M.; Kawamura, R.; Sakamoto, H.; Hayashi, T.; Ishida, T.; Imai, K.; Shinomura, Y. Parthenolide-induced apoptosis in multiple myeloma cells involves reactive oxygen species generation and cell sensitivity depends on catalase activity. Apoptosis 2006, 11, 2225–2235. [Google Scholar] [CrossRef]
- Fonrose, X.; Ausseil, F.; Soleilhac, E.; Masson, V.; David, B.; Pouny, I.; Cintrat, J.C.; Rousseau, B.; Barette, C.; Massiot, G.; et al. Parthenolide inhibits tubulin carboxypeptidase activity. Cancer Res. 2007, 67, 3371–3378. [Google Scholar] [CrossRef]
- Freund, R.R.A.; Gobrecht, P.; Rao, Z.; Gerstmeier, J.; Schlosser, R.; Görls, H.; Werz, O.; Fischer, D.; Arndt, H.D. Stereoselective total synthesis of parthenolides indicates target selectivity for tubulin carboxypeptidase activity. Chem. Sci. 2019, 10, 7358–7364. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, S.; Xie, Z.; Pavlovicz, R.E.; Wu, J.; Chen, P.; Aimiuwu, J.; Pang, J.; Bhasin, D.; Neviani, P.; et al. Modulation of DNA methylation by a sesquiterpene lactone parthenolide. J. Pharmacol. Exp. Ther. 2009, 329, 505–514. [Google Scholar] [CrossRef]
- Umemura, K.; Itoh, T.; Hamada, N.; Fujita, Y.; Akao, Y.; Nozawa, Y.; Matsuura, N.; Iinuma, M.; Ito, M. Preconditioning by sesquiterpene lactone enhances H2O2-induced Nrf2/ARE activation. Biochem. Biophys. Res. Commun. 2008, 368, 948–954. [Google Scholar] [CrossRef]
- Hartman, M.L.; Rozanski, M.; Osrodek, M.; Zalesna, I.; Czyz, M. Vemurafenib and trametinib reduce expression of CTGF and IL-8 in V600EBRAF melanoma cells. Lab. Invest. 2017, 97, 217–227. [Google Scholar] [CrossRef]
- Braig, S.; Wallner, S.; Junglas, B.; Fuchshofer, R.; Bosserhoff, A.K. CTGF is overexpressed in malignant melanoma and promotes cell invasion and migration. Br. J. Cancer. 2011, 105, 231–238. [Google Scholar] [CrossRef]
- Rowinsky, E.K. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu. Rev. Med. 1997, 48, 353–374. [Google Scholar] [CrossRef] [PubMed]
- Joerger, M. Treatment regimens of classical and newer taxanes. Cancer Chemother. Pharmacol. 2016, 77, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Ojima, I.; Lichtenthal, B.; Lee, S.; Wang, C.; Wang, X. Taxane anticancer agents: A patent perspective. Expert Opin. Ther. Pat. 2016, 26, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mekhail, T.M.; Markman, M. Paclitaxel in cancer therapy. Expert Opin. Pharmacother. 2002, 3, 755–766. [Google Scholar] [PubMed]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef]
- Patel, N.M.; Nozaki, S.; Shortle, N.H.; Bhat-Nakshatri, P.; Newton, T.R.; Rice, S.; Gelfanov, V.; Boswell, S.H.; Goulet, R.J., Jr.; Sledge, G.W., Jr.; et al. Paclitaxel sensitivity of breast cancer cells with constitutively active NF-kappaB is enhanced by IkappaBalpha super-repressor and parthenolide. Oncogene 2000, 19, 4159–4169. [Google Scholar] [CrossRef]
- Riedel, R.F.; Porrello, A.; Pontzer, E.; Chenette, E.J.; Hsu, D.S.; Balakumaran, B.; Potti, A.; Nevins, J.; Febbo, P.G. A genomic approach to identify molecular pathways associated with chemotherapy resistance. Mol. Cancer Ther. 2008, 7, 3141–3149. [Google Scholar] [CrossRef]
- O’Neill, A.J.; Prencipe, M.; Dowling, C.; Fan, Y.; Mulrane, L.; Gallagher, W.M.; O’Connor, D.; O’Connor, R.; Devery, A.; Corcoran, C.; et al. Characterisation and manipulation of docetaxel resistant prostate cancer cell lines. Mol. Cancer 2011, 10, 126. [Google Scholar] [CrossRef]
- Gao, Z.W.; Zhang, D.L.; Guo, C.B. Paclitaxel efficacy is increased by parthenolide via nuclear factor-kappaB pathways in in vitro and in vivo human non-small cell lung cancer models. Curr. Cancer Drug Targets 2010, 10, 705–715. [Google Scholar] [CrossRef]
- Jin, X.; Qiu, L.; Zhang, D.; Zhang, M.; Wang, Z.; Guo, Z.; Deng, C.; Guo, C. Chemosensitization in non-small cell lung cancer cells by IKK inhibitor occurs via NF-kappaB and mitochondrial cytochrome c cascade. J. Cell. Mol. Med. 2009, 13, 4596–4607. [Google Scholar] [CrossRef]
- Zhang, D.; Qiu, L.; Jin, X.; Guo, Z.; Guo, C. Nuclear factor-kappaB inhibition by parthenolide potentiates the efficacy of Taxol in non-small cell lung cancer in vitro and in vivo. Mol. Cancer Res. 2009, 7, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Gill, K.K.; Kaddoumi, A.; Nazzal, S. Mixed micelles of PEG(2000)-DSPE and vitamin-E TPGS for concurrent delivery of paclitaxel and parthenolide: Enhanced chemosenstization and antitumor efficacy against non-small cell lung cancer (NSCLC) cell lines. Eur. J. Pharm. Sci. 2012, 46, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.J.; Mehrotra, S.; Sadaria, M.R.; Kumar, S.; Shortle, N.H.; Roman, Y.; Sheridan, C.; Campbell, R.A.; Murry, D.J.; Badve, S.; et al. The sesquiterpene lactone parthenolide in combination with docetaxel reduces metastasis and improves survival in a xenograft model of breast cancer. Mol. Cancer Ther. 2005, 4, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Parrondo, R.; de las Pozas, A.; Reiner, T.; Rai, P.; Perez-Stable, C. NF-kappaB activation enhances cell death by antimitotic drugs in human prostate cancer cells. Mol. Cancer 2010, 9, 182. [Google Scholar] [CrossRef]
- Xu, Y.C.; Wang, H.X.; Tang, L.; Ma, Y.; Zhang, F.C. A systematic review of vinorelbine for the treatment of breast cancer. Breast, J. 2013, 19, 180–188. [Google Scholar] [CrossRef]
- Faller, B.A.; Pandit, T.N. Safety and efficacy of vinorelbine in the treatment of non-small cell lung cancer. Clin. Med. Insights Oncol. 2011, 5, 131–144. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, W.L.; Guo, J.; Du, J.; Li, T.; Wu, J.W.; Wang, G.L.; Wang, J.C.; Zhang, X.; Zhang, Q. A potential target associated with both cancer and cancer stem cells: A combination therapy for eradication of breast cancer using vinorelbine stealthy liposomes plus parthenolide stealthy liposomes. J. Control. Release 2008, 129, 18–25. [Google Scholar] [CrossRef]
- Gonzalvez, F.; Ashkenazi, A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene 2010, 29, 4752–4765. [Google Scholar] [CrossRef]
- Mert, U.; Sanlioglu, A.D. Intracellular localization of DR5 and related regulatory pathways as a mechanism of resistance to TRAIL in cancer. Cell. Mol. Life Sci. 2017, 74, 245–255. [Google Scholar] [CrossRef]
- Trivedi, R.; Mishra, D.P. Trailing TRAIL Resistance: Novel Targets for TRAIL Sensitization in Cancer Cells. Front. Oncol. 2015, 5, 69. [Google Scholar] [CrossRef]
- Trang, K.T.; Kim, S.L.; Park, S.B.; Seo, S.Y.; Choi, C.H.; Park, J.K.; Moon, J.C.; Lee, S.T.; Kim, S.W. Parthenolide Sensitizes Human Colorectal Cancer Cells to Tumor Necrosis Factor-related Apoptosis-inducing Ligand through Mitochondrial and Caspase Dependent Pathway. Intest. Res. 2014, 12, 34–41. [Google Scholar] [CrossRef]
- Tang, S.C.; Chen, Y.C. Novel therapeutic targets for pancreatic cancer. World J. Gastroenterol. 2014, 20, 10825–10844. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.Y.; Hurst, E.A.; Argyle, D.J. Cyclooxygenase-2: A Role in Cancer Stem Cell Survival and Repopulation of Cancer Cells during Therapy. Stem Cells Int. 2016, 2016, 2048731. [Google Scholar] [CrossRef] [PubMed]
- Yip-Schneider, M.T.; Nakshatri, H.; Sweeney, C.J.; Marshall, M.S.; Wiebke, E.A.; Schmidt, C.M. Parthenolide and sulindac cooperate to mediate growth suppression and inhibit the nuclear factor-kappa B pathway in pancreatic carcinoma cells. Mol. Cancer Ther. 2005, 4, 587–594. [Google Scholar] [CrossRef]
- Yip-Schneider, M.T.; Wu, H.; Ralstin, M.; Yiannoutsos, C.; Crooks, P.A.; Neelakantan, S.; Noble, S.; Nakshatri, H.; Sweeney, C.J.; Schmidt, C.M. Suppression of pancreatic tumor growth by combination chemotherapy with sulindac and LC-1 is associated with cyclin D1 inhibition in vivo. Mol. Cancer Ther. 2007, 6, 1736–1744. [Google Scholar] [CrossRef] [PubMed]
- Yip-Schneider, M.T.; Wu, H.; Hruban, R.H.; Lowy, A.M.; Crooks, P.A.; Schmidt, C.M. Efficacy of dimethylaminoparthenolide and sulindac in combination with gemcitabine in a genetically engineered mouse model of pancreatic cancer. Pancreas 2013, 42, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Yip-Schneider, M.T.; Wu, H.; Njoku, V.; Ralstin, M.; Holcomb, B.; Crooks, P.A.; Neelakantan, S.; Sweeney, C.J.; Schmidt, C.M. Effect of celecoxib and the novel anti-cancer agent, dimethylamino-parthenolide, in a developmental model of pancreatic cancer. Pancreas 2008, 37, e45–e53. [Google Scholar] [CrossRef]
- Ralstin, M.C.; Gage, E.A.; Yip-Schneider, M.T.; Klein, P.J.; Wiebke, E.A.; Schmidt, C.M. Parthenolide cooperates with NS398 to inhibit growth of human hepatocellular carcinoma cells through effects on apoptosis and G0-G1 cell cycle arrest. Mol. Cancer Res. 2006, 4, 387–400. [Google Scholar] [CrossRef][Green Version]
- Baker, D.E. Safety of balsalazide therapy in the treatment of inflammatory bowel disease. Rev. Gastroenterol. Disord. 2005, 5, 135–141. [Google Scholar]
- Kim, H.Y.; Kim, S.L.; Park, Y.R.; Liu, Y.C.; Seo, S.Y.; Kim, S.H.; Kim, I.H.; Lee, S.O.; Lee, S.T.; Kim, S.W. Balsalazide Potentiates Parthenolide-Mediated Inhibition of Nuclear Factor-κB Signaling in HCT116 Human Colorectal Cancer Cells. Intest. Res. 2015, 13, 233–241. [Google Scholar] [CrossRef]
- Kim, S.L.; Kim, S.H.; Park, Y.R.; Liu, Y.C.; Kim, E.M.; Jeong, H.J.; Kim, Y.N.; Seo, S.Y.; Kim, I.H.; Lee, S.O.; et al. Combined Parthenolide and Balsalazide Have Enhanced Antitumor Efficacy Through Blockade of NF-κB Activation. Mol. Cancer Res. 2017, 15, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Liu, M.C.; Bouker, K.B.; Gu, Z.; Lee, R.Y.; Zhu, Y.; Skaar, T.C.; Gomez, B.; O’Brien, K.; Wang, Y.; et al. Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene 2003, 22, 7316–7339. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Eppenberger-Castori, S.; Marx, C.; Yau, C.; Scott, G.K.; Eppenberger, U.; Benz, C.C. Activation of nuclear factor-kappaB (NFkappaB) identifies a high-risk subset of hormone-dependent breast cancers. Int. J. Biochem. Cell Biol. 2005, 37, 1130–1144. [Google Scholar] [CrossRef] [PubMed]
- de Graffenried, L.A.; Chandrasekar, B.; Friedrichs, W.E.; Donzis, E.; Silva, J.; Hidalgo, M.; Freeman, J.W.; Weiss, G.R. NF-kappa B inhibition markedly enhances sensitivity of resistant breast cancer tumor cells to tamoxifen. Ann. Oncol. 2004, 15, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yau, C.; Gray, J.W.; Chew, K.; Dairkee, S.H.; Moore, D.H.; Eppenberger, U.; Eppenberger-Castori, S.; Benz, C.C. Enhanced NF kappa B and AP-1 transcriptional activity associated with antiestrogen resistant breast cancer. BMC Cancer 2007, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Nehra, R.; Riggins, R.B.; Shajahan, A.N.; Zwart, A.; Crawford, A.C.; Clarke, R. BCL2 and CASP8 regulation by NF-kappaB differentially affect mitochondrial function and cell fate in antiestrogen-sensitive and resistant breast cancer cells. FASEB J. 2010, 24, 2040–2055. [Google Scholar] [CrossRef]
- Nobert, G.S.; Kraak, M.M.; Crawford, S. Estrogen dependent growth inhibitory effects of tamoxifen but not genistein in solid tumors derived from estrogen receptor positive (ER+) MCF7: Single agent and novel combined treatment approaches. Bull. Cancer 2006, 93, E59–E66. [Google Scholar]
- Nathan, M.R.; Schmid, P. A Review of Fulvestrant in Breast Cancer. Oncol. Ther. 2017, 5, 17–29. [Google Scholar] [CrossRef]
- Gu, Z.; Lee, R.Y.; Skaar, T.C.; Bouker, K.B.; Welch, J.N.; Lu, J.; Liu, A.; Zhu, Y.; Davis, N.; Leonessa, F.; et al. Association of interferon regulatory factor-1, nucleophosmin, nuclear factor-kappaB, and cyclic AMP response element binding with acquired resistance to Faslodex (ICI 182,780). Cancer Res. 2002, 62, 3428–3437. [Google Scholar]
- Riggins, R.B.; Zwart, A.; Nehra, R.; Clarke, R. The nuclear factor kappa B inhibitor parthenolide restores ICI 182,780 (Faslodex; fulvestrant)-induced apoptosis in antiestrogen-resistant breast cancer cells. Mol. Cancer Ther. 2005, 4, 33–41. [Google Scholar]
- Aragno, M.; Mastrocola, R.; Brignardello, E.; Catalano, M.; Robino, G.; Manti, R.; Parola, M.; Danni, O.; Boccuzzi, G. Dehydroepiandrosterone modulates nuclear factor-kappaB activation in hippocampus of diabetic rats. Endocrinology 2002, 143, 3250–3258. [Google Scholar] [CrossRef]
- Taguchi, T.; Takao, T.; Iwasaki, Y.; Nishiyama, M.; Asaba, K.; Hashimoto, K. Suppressive effects of dehydroepiandrosterone and the nuclear factor-kappaB inhibitor parthenolide on corticotroph tumor cell growth and function in vitro and in vivo. J. Endocrinol. 2006, 188, 321–331. [Google Scholar] [CrossRef]
- Frank, N.Y.; Margaryan, A.; Huang, Y.; Schatton, T.; Waaga-Gasser, A.M.; Gasser, M.; Sayegh, M.H.; Sadee, W.; Frank, M.H. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005, 65, 4320–4333. [Google Scholar] [CrossRef] [PubMed]
- Smylie, M.G.; Wong, R.; Mihalcioiu, C.; Lee, C.; Pouliot, J.F. A phase II, open label, monotherapy study of liposomal doxorubicin in patients with metastatic malignant melanoma. Invest. New Drugs 2007, 25, 155–159. [Google Scholar] [CrossRef]
- Zanotto-Filho, A.; Braganhol, E.; Schröder, R.; de Souza, L.H.; Dalmolin, R.J.; Pasquali, M.A.; Gelain, D.P.; Battastini, A.M.; Moreira, J.C. NFκB inhibitors induce cell death in glioblastomas. Biochem. Pharmacol. 2011, 81, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Bellarosa, D.; Binaschi, M.; Maggi, C.A.; Goso, C. Sabarubicin- (MEN 10755) and paclitaxel show different kinetics in nuclear factor-kappaB (NF-kB) activation: Effect of parthenolide on their cytotoxicity. Anticancer Res. 2005, 25, 2119–2128. [Google Scholar]
- Carlisi, D.; De Blasio, A.; Drago-Ferrante, R.; Di Fiore, R.; Buttitta, G.; Morreale, M.; Scerri, C.; Vento, R.; Tesoriere, G. Parthenolide prevents resistance of MDA-MB231 cells to doxorubicin and mitoxantrone: The role of Nrf2. Cell Death Discov. 2017, 3, 17078. [Google Scholar] [CrossRef] [PubMed]
- Mishra, H.; Mishra, P.K.; Ekielski, A.; Jaggi, M.; Iqbal, Z.; Talegaonkar, S. Melanoma treatment: From conventional to nanotechnology. J. Cancer Res. Clin. Oncol. 2018, 144, 2283–2302. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Mason, W.P. Emerging drugs for malignant glioma. Expert Opin. Emerg. Drugs 2008, 13, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Hegi, M.E.; Diserens, A.C.; Godard, S.; Dietrich, P.Y.; Regli, L.; Ostermann, S.; Otten, P.; Van Melle, G.; de Tribolet, N.; Stupp, R. Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin. Cancer Res. 2004, 10, 1871–1874. [Google Scholar] [CrossRef] [PubMed]
- Bredel, M.; Bredel, C.; Juric, D.; Duran, G.E.; Yu, R.X.; Harsh, G.R.; Vogel, H.; Recht, L.D.; Scheck, A.C.; Sikic, B.I. Tumor necrosis factor-alpha-induced protein 3 as a putative regulator of nuclear factor-kappaB-mediated resistance to O6-alkylating agents in human glioblastomas. J. Clin. Oncol. 2006, 24, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Lavon, I.; Fuchs, D.; Zrihan, D.; Efroni, G.; Zelikovitch, B.; Fellig, Y.; Siegal, T. Novel mechanism whereby nuclear factor kappaB mediates DNA damage repair through regulation of O(6)-methylguanine-DNA-methyltransferase. Cancer Res. 2007, 67, 8952–8959. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Chen, Y.; Wang, S.; Li, P.; Zhou, G.; Yuan, Y. Inhibition of NF-κB results in anti-glioma activity and reduces temozolomide-induced chemoresistance by down-regulating MGMT gene expression. Cancer Lett. 2018, 428, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Fang, L.J.; Shao, X.T.; Wang, S.; Lu, G.H.; Xu, T.; Zhou, J.Y. Sesquiterpene lactone parthenolide markedly enhances sensitivity of human A549 cells to low-dose oxaliplatin via inhibition of NF-kappaB activation and induction of apoptosis. Planta Med. 2010, 76, 258–264. [Google Scholar] [CrossRef]
- Xi, X.; Liu, N.; Wang, Q.; Chu, Y.; Yin, Z.; Ding, Y.; Lu, Y. ACT001, a novel PAI-1 inhibitor, exerts synergistic effects in combination with cisplatin by inhibiting PI3K/AKT pathway in glioma. Cell Death Dis. 2019, 10, 757. [Google Scholar] [CrossRef]
- Yuan, J.; Llamas Luceño, N.; Sander, B.; Golas, M.M. Synergistic anti-cancer effects of epigenetic drugs on medulloblastoma cells. Cell. Oncol. Dordr 2017, 40, 263–279. [Google Scholar] [CrossRef]
- Izumi, K.M.; Kieff, E.D. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-kappaB. Proc. Natl. Acad. Sci. USA 1997, 94, 12592–12597. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.L.; Kim, S.H.; Trang, K.T.; Kim, I.H.; Lee, S.O.; Lee, S.T.; Kim, D.G.; Kang, S.B.; Kim, S.W. Synergistic antitumor effect of 5-fluorouracil in combination with parthenolide in human colorectal cancer. Cancer Lett. 2013, 335, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, Y.; Liu, M.; Ran, L.; Li, Y. Reversing resistance of multidrug-resistant hepatic carcinoma cells with parthenolide. Future Oncol. 2013, 9, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, S.; Ge, W.; Liu, Z.; Zhang, X.; Wang, M.; Chen, T.; Chen, Y.; Zhang, Q. Design and synthesis of parthenolide and 5-fluorouracil conjugates as potential anticancer agents against drug resistant hepatocellular carcinoma. Eur. J. Med. Chem. 2019, 183, 111706. [Google Scholar] [CrossRef]
- Gesto, D.S.; Cerqueira, N.M.; Fernandes, P.A.; Ramos, M.J. Gemcitabine: A critical nucleoside for cancer therapy. Curr. Med. Chem. 2012, 19, 1076–1087. [Google Scholar] [CrossRef]
- Holcomb, B.K.; Yip-Schneider, M.T.; Waters, J.A.; Beane, J.D.; Crooks, P.A.; Schmidt, C.M. Dimethylamino parthenolide enhances the inhibitory effects of gemcitabine in human pancreatic cancer cells. J. Gastrointest. Surg. 2012, 16, 1333–1340. [Google Scholar] [CrossRef]
- Marks, P.A.; Jiang, X. Histone deacetylase inhibitors in programmed cell death and cancer therapy. Cell Cycle 2005, 4, 549–551. [Google Scholar] [CrossRef]
- Ruefli, A.A.; Ausserlechner, M.J.; Bernhard, D.; Sutton, V.R.; Tainton, K.M.; Kofler, R.; Smyth, M.J.; Johnstone, R.W. The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc. Natl. Acad. Sci. USA 2001, 98, 10833–10838. [Google Scholar] [CrossRef]
- Rosato, R.R.; Grant, S. Histone deacetylase inhibitors in clinical development. Expert Opin. Investig. Drugs 2004, 13, 21–38. [Google Scholar] [CrossRef]
- Mayo, M.W.; Denlinger, C.E.; Broad, R.M.; Yeung, F.; Reilly, E.T.; Shi, Y.; Jones, D.R. Ineffectiveness of histone deacetylase inhibitors to induce apoptosis involves the transcriptional activation of NF-kappa B through the Akt pathway. J. Biol. Chem. 2003, 278, 18980–18989. [Google Scholar] [CrossRef]
- Rundall, B.K.; Denlinger, C.E.; Jones, D.R. Combined histone deacetylase and NF-kappaB inhibition sensitizes non-small cell lung cancer to cell death. Surgery 2004, 136, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Yeow, W.S.; Ziauddin, M.F.; Maxhimer, J.B.; Shamimi-Noori, S.; Baras, A.; Chua, A.; Schrump, D.S.; Nguyen, D.M. Potentiation of the anticancer effect of valproic acid, an antiepileptic agent with histone deacetylase inhibitory activity, by the kinase inhibitor Staurosporine or its clinically relevant analogue UCN-01. Br. J. Cancer 2006, 94, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Blaheta, R.A.; Cinatl, J., Jr. Anti-tumor mechanisms of valproate: A novel role for an old drug. Med. Res. Rev. 2002, 22, 492–511. [Google Scholar] [CrossRef] [PubMed]
- Carlisi, D.; Lauricella, M.; D’Anneo, A.; Buttitta, G.; Emanuele, S.; di Fiore, R.; Martinez, R.; Rolfo, C.; Vento, R.; Tesoriere, G. The synergistic effect of SAHA and parthenolide in MDA-MB231 breast cancer cells. J. Cell. Physiol. 2015, 230, 1276–1289. [Google Scholar] [CrossRef]
- Ge, W.; Liu, Z.; Sun, Y.; Wang, T.; Guo, H.; Chen, X.; Li, S.; Wang, M.; Chen, Y.; Ding, Y.; et al. Design and synthesis of parthenolide-SAHA hybrids for intervention of drug-resistant acute myeloid leukemia. Bioorg. Chem. 2019, 87, 699–713. [Google Scholar] [CrossRef]
- Chen, L.F.; Fischle, W.; Verdin, E.; Greene, W.C. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science 2001, 293, 1653–1657. [Google Scholar] [CrossRef]
- Law, A.Y.; Lai, K.P.; Lui, W.C.; Wan, H.T.; Wong, C.K. Histone deacetylase inhibitor-induced cellular apoptosis involves stanniocalcin-1 activation. Exp. Cell Res. 2008, 314, 2975–2984. [Google Scholar] [CrossRef]
- Pei, S.; Minhajuddin, M.; D’Alessandro, A.; Nemkov, T.; Stevens, B.M.; Adane, B.; Khan, N.; Hagen, F.K.; Yadav, V.K.; De, S.; et al. Rational Design of a Parthenolide-based Drug Regimen That Selectively Eradicates Acute Myelogenous Leukemia Stem Cells. J. Biol. Chem. 2016, 291, 21984–22000. [Google Scholar] [CrossRef]
- Sen, S.; Hassane, D.C.; Corbett, C.; Becker, M.W.; Jordan, C.T.; Guzman, M.L. Novel mTOR inhibitory activity of ciclopirox enhances parthenolide antileukemia activity. Exp. Hematol. 2013, 41, 799–807.e4. [Google Scholar] [CrossRef]
- Cicconi, L.; Fenaux, P.; Kantarjian, H.; Tallman, M.; Sanz, M.A.; Lo-Coco, F. Molecular remission as a therapeutic objective in acute promyelocytic leukemia. Leukemia 2018, 32, 1671–1678. [Google Scholar] [CrossRef]
- Duprez, E.; Wagner, K.; Koch, H.; Tenen, D.G. C/EBPbeta: A major PML-RARA-responsive gene in retinoic acid-induced differentiation of APL cells. EMBO J. 2003, 22, 5806–5816. [Google Scholar] [CrossRef]
- Kim, S.H.; Danilenko, M.; Kim, T.S. Differential enhancement of leukaemia cell differentiation without elevation of intracellular calcium by plant-derived sesquiterpene lactone compounds. Br. J. Pharmacol. 2008, 155, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Kim, S.H.; Cho, K.M.; Hwang, S.Y.; Kim, H.J.; Kim, T.S. Analysis of gene profiles involved in the enhancement of all-trans retinoic acid-induced HL-60 cell differentiation by sesquiterpene lactones identifies asparagine synthetase as a novel target for differentiation-inducing therapy. Int. J. Oncol. 2014, 44, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Liu, L.; Kim, I.H.; Kim, J.H.; You, K.R.; Kim, D.G. Identification of the genes involved in enhanced fenretinide-induced apoptosis by parthenolide in human hepatoma cells. Cancer Res. 2005, 65, 2804–2814. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Degrande, E. Vildagliptin: A new oral treatment for type 2 diabetes mellitus. Vasc. Health Risk Manag. 2008, 4, 1349–1360. [Google Scholar] [CrossRef]
- Hoonjan, M.; Jadhav, V.; Bhatt, P. Arsenic trioxide: Insights into its evolution to an anticancer agent. J. Biol. Inorg. Chem. 2018, 23, 313–329. [Google Scholar] [CrossRef]
- Kouhpaikar, H.; Sadeghian, M.H.; Rafatpanah, H.; Kazemi, M.; Iranshahi, M.; Delbari, Z.; Khodadadi, F.; Ayatollahi, H.; Rassouli, F.B. Synergy between parthenolide and arsenic trioxide in adult T-cell leukemia/lymphoma cells in vitro. Iran. J. Basic Med. Sci. 2020, 23, 616–622. [Google Scholar]
- Wang, W.; Adachi, M.; Zhang, R.; Zhou, J.; Zhu, D. A novel combination therapy with arsenic trioxide and parthenolide against pancreatic cancer cells. Pancreas 2009, 38, e114–e123. [Google Scholar] [CrossRef]
- Fu, L.L.; Zhao, X.Y.; Ji, L.D.; Xu, J. Okadaic acid (OA): Toxicity, detection and detoxification. Toxicon 2019, 160, 1–7. [Google Scholar] [CrossRef]
- Di Fiore, R.; Drago-Ferrante, R.; D’Anneo, A.; Augello, G.; Carlisi, D.; De Blasio, A.; Giuliano, M.; Tesoriere, G.; Vento, R. In human retinoblastoma Y79 cells okadaic acid-parthenolide co-treatment induces synergistic apoptotic effects, with PTEN as a key player. Cancer Biol. Ther. 2013, 14, 922–931. [Google Scholar] [CrossRef]
- Antar, A.I.; Otrock, Z.K.; Jabbour, E.; Mohty, M.; Bazarbachi, A. FLT3 inhibitors in acute myeloid leukemia: Ten frequently asked questions. Leukemia 2020, 34, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, J.; Wang, Y.; Bai, S.; Wang, Y.; Wang, L.; Sheng, G. Combined effects of FLT3 and NF-κB selective inhibitors on acute myeloid leukemia in vivo. J. Biochem. Mol. Toxicol. 2012, 26, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, L.; Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 2005, 5, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Kim, Y.J.; Lee, S.A.; Myung, S.C.; Kim, W. Combined effect of Hsp90 inhibitor geldanamycin and parthenolide via reactive oxygen species-mediated apoptotic process on epithelial ovarian cancer cells. Basic Clin. Pharmacol. Toxicol. 2012, 111, 173–181. [Google Scholar] [CrossRef]
- Yun, B.R.; Lee, M.J.; Kim, J.H.; Kim, I.H.; Yu, G.R.; Kim, D.G. Enhancement of parthenolide-induced apoptosis by a PKC-alpha inhibition through heme oxygenase-1 blockage in cholangiocarcinoma cells. Exp. Mol. Med. 2010, 42, 787–797. [Google Scholar] [CrossRef]
- Sobell, H.M. Actinomycin and DNA transcription. Proc. Natl. Acad. Sci. USA 1985, 82, 5328–5331. [Google Scholar] [CrossRef]
- Hill, C.R.; Cole, M.; Errington, J.; Malik, G.; Boddy, A.V.; Veal, G.J. Characterisation of the clinical pharmacokinetics of actinomycin D and the influence of ABCB1 pharmacogenetic variation on actinomycin D disposition in children with cancer. Clin. Pharmacokinet. 2014, 53, 741–751. [Google Scholar] [CrossRef]
- Lamture, G.; Crooks, P.A.; Borrelli, M.J. Actinomycin-D and dimethylamino-parthenolide synergism in treating human pancreatic cancer cells. Drug Dev. Res. 2018, 79, 287–294. [Google Scholar] [CrossRef]
- de Thé, H. Differentiation therapy revisited. Nat. Rev. Cancer 2018, 18, 117–127. [Google Scholar] [CrossRef]
- Kang, S.N.; Kim, S.H.; Chung, S.W.; Lee, M.H.; Kim, H.J.; Kim, T.S. Enhancement of 1 alpha,25-dihydroxyvitamin D(3)-induced differentiation of human leukaemia HL-60 cells into monocytes by parthenolide via inhibition of NF-kappa B activity. Br. J. Pharmacol. 2002, 135, 1235–1244. [Google Scholar] [CrossRef]
- Cory, A.H.; Cory, J.G. Lactacystin, a proteasome inhibitor, potentiates the apoptotic effect of parthenolide, an inhibitor of NFkappaB activation, on drug-resistant mouse leukemia L1210 cells. Anticancer Res. 2002, 22, 3805–3809. [Google Scholar] [PubMed]
- Wu, C.; Chen, F.; Rushing, J.W.; Wang, X.; Kim, H.J.; Huang, G.; Haley-Zitlin, V.; He, G. Antiproliferative activities of parthenolide and golden feverfew extract against three human cancer cell lines. J. Med. Food 2006, 9, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.E.; Paget, J.T.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Veuger, S.J.; Hunter, J.E.; Durkacz, B.W. Ionizing radiation-induced NF-kappaB activation requires PARP-1 function to confer radioresistance. Oncogene 2009, 28, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.; Yu, T.; Chen, G.; Brown, S.A.; Wang, Y.; Thompson, J.S.; Zhou, D. Cellularorigin of ionizing radiation-induced NF-kappaB activation in vivo and role of NFkappaB in ionizing radiation-induced lymphocyte apoptosis. Int. J. Radiat. Biol. 2003, 79, 849–861. [Google Scholar] [CrossRef]
- Watson, C.; Miller, D.A.; Chin-Sinex, H.; Losch, A.; Hughes, W.; Sweeney, C.; Mendonca, M.S. Suppression of NF-kappaB activity by parthenolide induces X-ray sensitivity through inhibition of split-dose repair in TP53 null prostate cancer cells. Radiat. Res. 2009, 171, 389–396. [Google Scholar] [CrossRef]
- Sun, Y.; St Clair, D.K.; Fang, F.; Warren, G.W.; Rangnekar, V.M.; Crooks, P.A.; St Clair, W.H. The radiosensitization effect of parthenolide in prostate cancer cells is mediated by nuclear factor-kappaB inhibition and enhanced by the presence of PTEN. Mol. Cancer Ther. 2007, 6, 2477–2486. [Google Scholar] [CrossRef]
- Deraska, P.V.; O’Leary, C.; Reavis, H.D.; Labe, S.; Dinh, T.K.; Lazaro, J.B.; Sweeney, C.; D’Andrea, A.D.; Kozono, D. NF-κB inhibition by dimethylaminoparthenolide radiosensitizes non-small-cell lung carcinoma by blocking DNA double-strand break repair. Cell Death Discov. 2018, 4, 10. [Google Scholar] [CrossRef]
- Estabrook, N.C.; Chin-Sinex, H.; Borgmann, A.J.; Dhaemers, R.M.; Shapiro, R.H.; Gilley, D.; Huda, N.; Crooks, P.; Sweeney, C.; Mendonca, M.S. Inhibition of NF-κB and DNA double-strand break repair by DMAPT sensitizes non-small-cell lung cancers to X-rays. Free Radic. Biol. Med. 2011, 51, 2249–2258. [Google Scholar] [CrossRef]
- Mendonca, M.S.; Turchan, W.T.; Alpuche, M.E.; Watson, C.N.; Estabrook, N.C.; Chin-Sinex, H.; Shapiro, J.B.; Imasuen-Williams, I.E.; Rangel, G.; Gilley, D.P.; et al. DMAPT inhibits NF-κB activity and increases sensitivity of prostate cancer cells to X-rays in vitro and in tumor xenografts in vivo. Free Radic. Biol. Med. 2017, 112, 318–326. [Google Scholar] [CrossRef]
- Meazza, C.; Scanagatta, P. Metastatic osteosarcoma: A challenging multidisciplinary treatment. Expert Rev. Anticancer Ther. 2016, 16, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Zuch, D.; Giang, A.H.; Shapovalov, Y.; Schwarz, E.; Rosier, R.; O’Keefe, R.; Eliseev, R.A. Targeting radioresistant osteosarcoma cells with PN. J. Cell Biochem. 2012, 113, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Sugiyasu, K.; Nanno, K.; Tamai, N.; Hashimoto, N.; Kishida, Y.; Yoshikawa, H.; Myoui, A. Radio-sensitization of the murine osteosarcoma cell line LM8 with parthenolide, a natural inhibitor of NF-κB. Oncol. Lett. 2011, 2, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, J.; Shukla, H.D.; Soman, S.; Samanta, S.; Singh, P.; Kamlapurkar, S.; Saeed, A.; Amin, N.P.; Vujaskovic, Z. Immunotherapy, Radiotherapy, and Hyperthermia: A Combined Therapeutic Approach in Pancreatic Cancer Treatment. Cancers (Basel) 2018, 10, E469. [Google Scholar] [CrossRef]
- Poland, J.; Schadendorf, D.; Lage, H.; Schnölzer, M.; Celis, J.E.; Sinha, P. Study of therapy resistance in cancer cells with functional proteome analysis. Clin. Chem. Lab. Med. 2002, 40, 221–234. [Google Scholar] [CrossRef]
- Habash, R.W.Y. Therapeutic hyperthermia. Handb. Clin. Neurol. 2018, 157, 853–868. [Google Scholar]
- Hayashi, S.; Hatashita, M.; Hayashi, A.; Matsumoto, H.; Shioura, H.; Kitai, R. Thermosensitization by PN in human lung adenocarcinoma A549 cells and p53- and hsp72-independent apoptosis induction via the nuclear factor-kappaB signal pathway. Int. J. Mol. Med. 2008, 21, 585–592. [Google Scholar]
- Hayashi, S.; Sakurai, H.; Hayashi, A.; Tanaka, Y.; Hatashita, M.; Shioura, H. Inhibition of NF-kappaB by combination therapy with PN and hyperthermia and kinetics of apoptosis induction and cell cycle arrest in human lung adenocarcinoma cells. Int. J. Mol. Med. 2010, 25, 81–87. [Google Scholar]
- Hayashi, S.; Koshiba, K.; Hatashita, M.; Sato, T.; Jujo, Y.; Suzuki, R.; Tanaka, Y.; Shioura, H. Thermosensitization and induction of apoptosis or cell-cycle arrest via the MAPK cascade by PN, an NF-κB inhibitor, in human prostate cancer androgen-independent cell lines. Int. J. Mol. Med. 2011, 28, 1033–1042. [Google Scholar]
- Kruk, P.J. Beneficial effect of additional treatment with widely available anticancer agents in advanced small lung cell carcinoma: A case report. Mol. Clin. Oncol. 2018, 9, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Madieh, S.; Augsburger, L.L. The solution and solid state stability and excipient compatibility of parthenolide in feverfew. AAPS PharmSciTech. 2007, 8, E105. [Google Scholar] [CrossRef] [PubMed]
- Nasim, S.; Crooks, P.A. Antileukemic activity of aminoparthenolide analogs. Bioorg. Med. Chem. Lett. 2008, 18, 3870–3873. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Ding, Y.H.; Wang, P.P.; Zhang, Q.; Chen, Y. Protection-group-free semisyntheses of parthenolide and its cyclopropyl analogue. J. Org. Chem. 2013, 78, 10512–10518. [Google Scholar] [CrossRef]
- Long, J.; Zhang, S.F.; Wang, P.P.; Zhang, X.M.; Yang, Z.J.; Zhang, Q.; Chen, Y. Total syntheses of parthenolide and its analogues with macrocyclic stereocontrol. J. Med. Chem. 2014, 57, 7098–7112. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Kuang, B.; Kang, N.; Ding, Y.; Ge, W.; Lian, L.; Gao, Y.; Wei, Y.; Chen, Y.; Zhang, Q. Synthesis and anti-acute myeloid leukemia activity of C-14 modified parthenolide derivatives. Eur. J. Med. Chem. 2017, 127, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Ge, W.Z.; Li, Q.Y.; Lu, Y.; Gong, J.M.; Kuang, B.J.; Xi, X.; Wu, H.; Zhang, Q.; Chen, Y. Syntheses and Biological Evaluation of Costunolide, Parthenolide, and Their Fluorinated Analogues. J. Med. Chem. 2015, 58, 7007–7020. [Google Scholar] [PubMed]
- Zhang, Q.; Lu, Y.; Ding, Y.; Zhai, J.; Ji, Q.; Ma, W.; Yang, M.; Fan, H.; Long, J.; Tong, Z.; et al. Guaianolide sesquiterpene lactones, a source to discover agents that selectively inhibit acute myelogenous leukemia stem and progenitor cells. J. Med. Chem. 2012, 55, 8757–8769. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, Z.; Ge, W.; Kuang, B.; Xu, J.; Yang, J.; Chen, Y.; Zhang, Q. Synthesis and biological evaluation of dithiocarbamate esters of parthenolide as potential anti-acute myelogenous leukaemia agents. J. Enzyme Inhib. Med. Chem. 2018, 33, 1376–1391. [Google Scholar] [CrossRef]
- Jia, X.; Liu, Q.; Wang, S.; Zeng, B.; Du, G.; Zhang, C.; Li, Y. Synthesis, cytotoxicity, and in vivo antitumor activity study of parthenolide semicarbazones and thiosemicarbazones. Bioorg. Med. Chem. 2020, 28, 115557. [Google Scholar] [CrossRef]
- Taleghani, A.; Nasseri, M.A.; Iranshahi, M. Synthesis of dual-action parthenolide prodrugs as potent anticancer agents. Bioorg Chem. 2017, 71, 128–134. [Google Scholar] [CrossRef]
- Lickliter, J. A Phase 1 Dose-Escalation Study to Evaluate the Safety, Tolerability and Pharmacokinetics of ACT001 in Patients with Advanced Solid Tumors. Available online: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616000228482p.
- Tong, L.; Li, J.; Li, Q.; Wang, X.; Medikonda, R.; Zhao, T.; Li, T.; Ma, H.; Yi, L.; Liu, P.; et al. ACT001 reduces the expression of PD-L1 by inhibiting the phosphorylation of STAT3 in glioblastoma. Theranostics 2020, 10, 5943–5956. [Google Scholar] [CrossRef] [PubMed]
- Ridolfo, R.; Ede, B.C.; Diamanti, P.; White, P.B.; Perriman, A.W.; van Hest, J.C.M.; Blair, A.; Williams, D.S. Biodegradable, Drug-Loaded Nanovectors via Direct Hydration as a New Platform for Cancer Therapeutics. Small 2018, 14, e1703774. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Xu, Y.; Mustafa, T.; Kannarpady, G.; Bratton, S.M.; Radominska-Pandya, A.; Crooks, P.A.; Biris, A.S. Nanodelivery of Parthenolide Using Functionalized Nanographene Enhances its Anticancer Activity. RSC Adv. 2015, 5, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Darwish, N.H.E.; Sudha, T.; Godugu, K.; Bharali, D.J.; Elbaz, O.; El-Ghaffar, H.A.A.; Azmy, E.; Anber, N.; Mousa, S.A. Novel Targeted Nano-Parthenolide Molecule against NF-kB in Acute Myeloid Leukemia. Molecules 2019, 24, 2103. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Li, L.; Zhang, X.; Luo, L.; He, Y.; Cong, C.; Gao, D. Nanomagnetic liposome-encapsulated parthenolide and indocyanine green for targeting and chemo-photothermal antitumor therapy. Nanomedicine (Lond.) 2020, 15, 871–890. [Google Scholar] [CrossRef]
- Mathema, V.B.; Koh, Y.S.; Thakuri, B.C.; Sillanpa, M. Parthenolide, a sesquiterpene lactone, expresses multipleanti-cancer and anti-inflammatory activities. Inflammation 2012, 35, 560–565. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, R.T.; Zhang, P.; Zhang, N.; Yang, C.L.; Yue, L.T.; Li, X.L.; Liu, Y.; Li, H.; Du, J.; et al. Parthenolide inhibits the initiation of experimental autoimmune neuritis. J. Neuroimmunol. 2017, 15, 154–161. [Google Scholar] [CrossRef]
- Bahrami, M.; Kamalinejad, M.; Latifi, S.A.; Seif, F.; Dadmehr, M. Cytokine storm in COVID-19 and parthenolide: Preclinical evidence. Phytother. Res. 2020, 10, 1002. [Google Scholar] [CrossRef]
| Co-Treatment with PN | Main Mechanism of Action | In Vitro Cell Line (PN Concentration) | In Vivo Model (PN Dose) | References |
|---|---|---|---|---|
| Leukemias | ||||
| 1α,25-dihdroxyvitamin D3 | differentiation inducer | human HL-60 (0.625–10 μM) | − | [170] |
| ATO (arsenic trioxide) | reactive oxygen species inducer | human Jurkat, human HL-60, human K-562 (10 μM) | − | [9] |
| ATRA (all-trans retinoic acid) | differentiation inducer | human HL-60 (0.25–10 μM) | − | [152,153] |
| ciclopirox | mTOR inhibitor translation initiation factor eIF5A inhibitor | human Kasumi-1, human primary AML specimens (2.5–10 μM) | − | [149] |
| SC-203048 | FLT3 selective inhibitor | − | human THP-1 in athymic BALB/c nude mice (10 μg/kg 7x each 2nd day) | [162] |
| lactacystin | proteasome inhibitor | murine L1210 (Y8) (5 μM) | − | [171] |
| rapamycin/ temsirolimus | mTOR inhibitors | human primary AML specimens (5 μM) | human primary AML cells in NOD/SCID mice (100 mg DMAPT/kg 3x daily) | [39] |
| SAHA (suberoylanilide hydroxamic acid) LBH589 | pan-histone deacetylase inhibitor | human U-937, human HL-60, human NB4, human MV-4-11, human MOLM-13(3–8 μM) | − | [11] |
| vildagliptin | dipeptidyl peptidase IV inhibitor | human TEX, human OCI-AML2 (2.5–5 μM) | − | [41] |
| wortmannin | PI3Ks inhibitor | human primary AML specimens (2.5–10 μM) | − | [39] |
| Breast Cancers | ||||
| 4HT (4-hydroxytamoxifen) | selective estrogen receptor modulator | human MCF7/RR, human MCF7/LCC1, human MCF7/LCC9 (0.5 μM) | − | [106] |
| docetaxel | microtubules stabilizer | human HBL-100, human MDA-MB-231 (0.5–5 μM) | human MDA-MB-231 in female nude mice (40 mg/kg daily) | [83] |
| doxorubicin mitoxantrone | topoisomerase II inhibitor | human MDA-MB-231 (1–10 μM) | − | [117] |
| faslodex (fulvestrant) | estrogen receptor down-regulator | human MCF7/LCC9 (0.6 μM) | − | [110] |
| paclitaxel (Taxol) | microtubules stabilizer | human MDA-MB-231 (0.1–5 μM) | − | [76] |
| SAHA | pan-histone deacetylase inhibitor | human MDA-MB-231 (2.5–25 μM) | − | [144] |
| tamoxifen | selective estrogen receptor modulator | human MCF7, human MCF7/HER2, human BT-474 (1–50 μM) | − | [103,104,105,107] |
| TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) | apoptosis inducer | human MDA-MB-231 (1–5 μM) | − | [61] |
| vinorelbine | microtubule assembly blocker mitosis inhibitor | human MCF7, human MDA-MB-231 (0.5–10 μM) | human MCF7 in female BALB/c nude mice (10 mg/kg 5th and 8th day after inoculation) | [87] |
| Colorectal Cancers | ||||
| 5-fluorouracil | thymidylate synthase inhibitor | human SW620 (10 μM) | human SW620 in nude mice (2.5 mg/kg 3x weekly) | [132] |
| balsalazide | anti-inflammatory agent | human HCT116, human SW480, human HT-29 (5–10 μM) | azoxymethane-induced CAC in BALB/c female mice (2 mg/kg 3x weekly) | [100,101] |
| sabarubicin | topoisomerase II inhibitor | human HCT116 (10–20 μM) | − | [116] |
| TRAIL | apoptosis inducer | human HT-29, human HCT116 (10 μM) | − | [91] |
| trichostatin A | histone deacetylase inhibitor | human HT-29 (10 μM) | − | [147] |
| Thoracic Cancers | ||||
| oxaliplatin | inter- and intra-strand DNA cross-linker | human A549 (10–500 μM) | − | [127] |
| paclitaxel (Taxol) | microtubules stabilizer | human A549, H446, human A549-T24, human H460 (5 μM) | human A549 and H460 in athymic nude mice (5 mg/kg 3x weekly) | [79,81,82] |
| valproic acid | histone deacetylase inhibitor nitric oxide synthase inhibitor | human TE2, human TE12, human H322, human H460, human H513, human H211 (20–30 μM) | − | [142] |
| Pancreatic Cancers | ||||
| actinomycin-D | RNA polymerase inhibitor | human PANC-1 (3–24 μM DMAPT) | − | [168] |
| ATO | reactive oxygen species inducer | human PANC-1, human BxPC-3 (2.5–5 μM) | human PANC-1 in athymic nude mice (0.8 mM/25 μL 2x weekly) | [158] |
| celecoxib | COX-2 inhibitor | − | cancer induced in hamster by N-nitroso bis(2-oxopropyl) amine (20–40 mg DMAPT/kg daily) | [97] |
| gemcitabine | ribonucleotide reductase inhibitor | human BxPC-3, human PANC-1, human MIA PaCa-2 (1–10 μM DMAPT) | − | [136] |
| sulindac | prostaglandin synthesis inhibitor | human BxPC-3, human PANC-1, human MIA PaCa-2 (2.5–10 μM) | − | [94] |
| Melanomas | ||||
| DTIC (dacarbazine) | DNA alkylator | human A-375, patient derived melanoma (3–24 μM) | − | [26] |
| doxorubicin | topoisomerase II inhibitor | human A-375, human 1205Lu, patient derived melanoma (10–24 μM) | − | [36] |
| Hepatomas | ||||
| 5-fluorouracil | thymidylate synthase inhibitor | human BEL-7402 (5–100 μM) | − | [133] |
| fenretinide | reactive oxygen species inducer | human Hep 3B, human SK-HEP-1 (4 μM) | − | [154] |
| NS398 | COX-2 inhibitor | human Hep 3B, human Hep G2, human PLC (5 μM) | − | [98] |
| Ro317549 | protein kinase C-alpha inhibitor | human Choi-CK, human Cho-CK, human JCK1, human SCK (10–40 μM) | human Choi-CK and human SCK in BALB/cByJ-Hfh11null nude mice (2.5 mg/kg daily) | [165] |
| llTRAIL | apoptosis inducer | human Hep 3B, human Hep G2, human SK-HEP-1 (15 μM) | − | [6] |
| Brain Malignancies | ||||
| 5-aza-2′-deoxycytidine | DNA methyltransferase 1 inhibitor | human Daoy, human D283 Med (2–5 μM) | − | [129] |
| cisplatin | inter- and intra-strand DNA cross-linker | rat C6, human U-138 MG (10–25 μM), human U-118 MG (1.875–60 μM ACT001) | human U-118 MG in female nude BALB/c mice (200 mg ACT001/kg 6x each 7th day) | [115,128] |
| DHEA (dehydroepiandrosterone) | NF-κB signaling pathway inhibitor | murine AtT-20 (1–10 μM) | male BALB/c nude mice (200 μg/mouse daily) | [112] |
| doxorubicin | topoisomerase II inhibitor | rat C6, human U-138 MG (10–25 μM) | − | [115] |
| temozolomide | DNA alkylator | human LN-18, human T98G (10 μM) | human T98G in SCID mice (2.5–10 mg/kg daily) | [124] |
| Gastric Cancers | ||||
| cisplatin | inter- and intra-strand DNA cross-linker | human MKN-28, human MKN-45, human MKN-74/5-15 μM, human SGC-7901 (5–10 μM) | − | [27,34] |
| paclitaxel | microtubules stabilizer | human MKN-28, human MKN-45, human MKN-74 (3–9 μM) | human MKN-45 in female BALBc nu/nu mice (0.25–4 mg/kg daily) | [34] |
| Other Cancers | ||||
| ganciclovir Burkitt’s lymphoma | DNA polymerases inhibitor | human Raji (4–6 μM) | − | [28] |
| bicalutamide prostate cancer | androgen receptor blocker | − | human CWR22Rv1 in nude athymic mice (40 mg/kg daily) | [32] |
| Docetaxel prostate cancer | microtubules stabilizer | human CWR22Rv1 (0.5–10 μM) | human CWR22Rv1 in nude athymic mice (40 mg/kg daily) | [32] |
| geldanamycin ovarian carcinoma | Hsp90 inhibitor | human OVCAR-3, human SK-OV-3 (1–8 μM) | − | [164] |
| OKA (okadaic acid) retinoblastoma | phosphoserine/threonine protein phosphatase 1 and 2a inhibitor | human Y79 (0.25–0.5 μM) | − | [160] |
| Phase | Purpose | Clinical Trial Registry | |
|---|---|---|---|
| PN | I | pharmacokinetics and toxicity | none [18] |
| DMAPT | I | AML, ALL, and other blood-lymph tumors | none [unpublished] (United Kingdom) |
| ACT001 | I/II | safety, tolerability, pharmacokinetics, recurrent glioblastoma | ACTRN12616000228482 (Australia & New Zealand) ChiCTR-OIC-17013604 (China) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sztiller-Sikorska, M.; Czyz, M. Parthenolide as Cooperating Agent for Anti-Cancer Treatment of Various Malignancies. Pharmaceuticals 2020, 13, 194. https://doi.org/10.3390/ph13080194
Sztiller-Sikorska M, Czyz M. Parthenolide as Cooperating Agent for Anti-Cancer Treatment of Various Malignancies. Pharmaceuticals. 2020; 13(8):194. https://doi.org/10.3390/ph13080194
Chicago/Turabian StyleSztiller-Sikorska, Malgorzata, and Malgorzata Czyz. 2020. "Parthenolide as Cooperating Agent for Anti-Cancer Treatment of Various Malignancies" Pharmaceuticals 13, no. 8: 194. https://doi.org/10.3390/ph13080194
APA StyleSztiller-Sikorska, M., & Czyz, M. (2020). Parthenolide as Cooperating Agent for Anti-Cancer Treatment of Various Malignancies. Pharmaceuticals, 13(8), 194. https://doi.org/10.3390/ph13080194





