Bactericidal and In Vitro Cytotoxicity of Moringa oleifera Seed Extract and Its Elemental Analysis Using Laser-Induced Breakdown Spectroscopy
Abstract
1. Introduction
2. Results
2.1. Qualitative Analysis of MOS Using LIBS
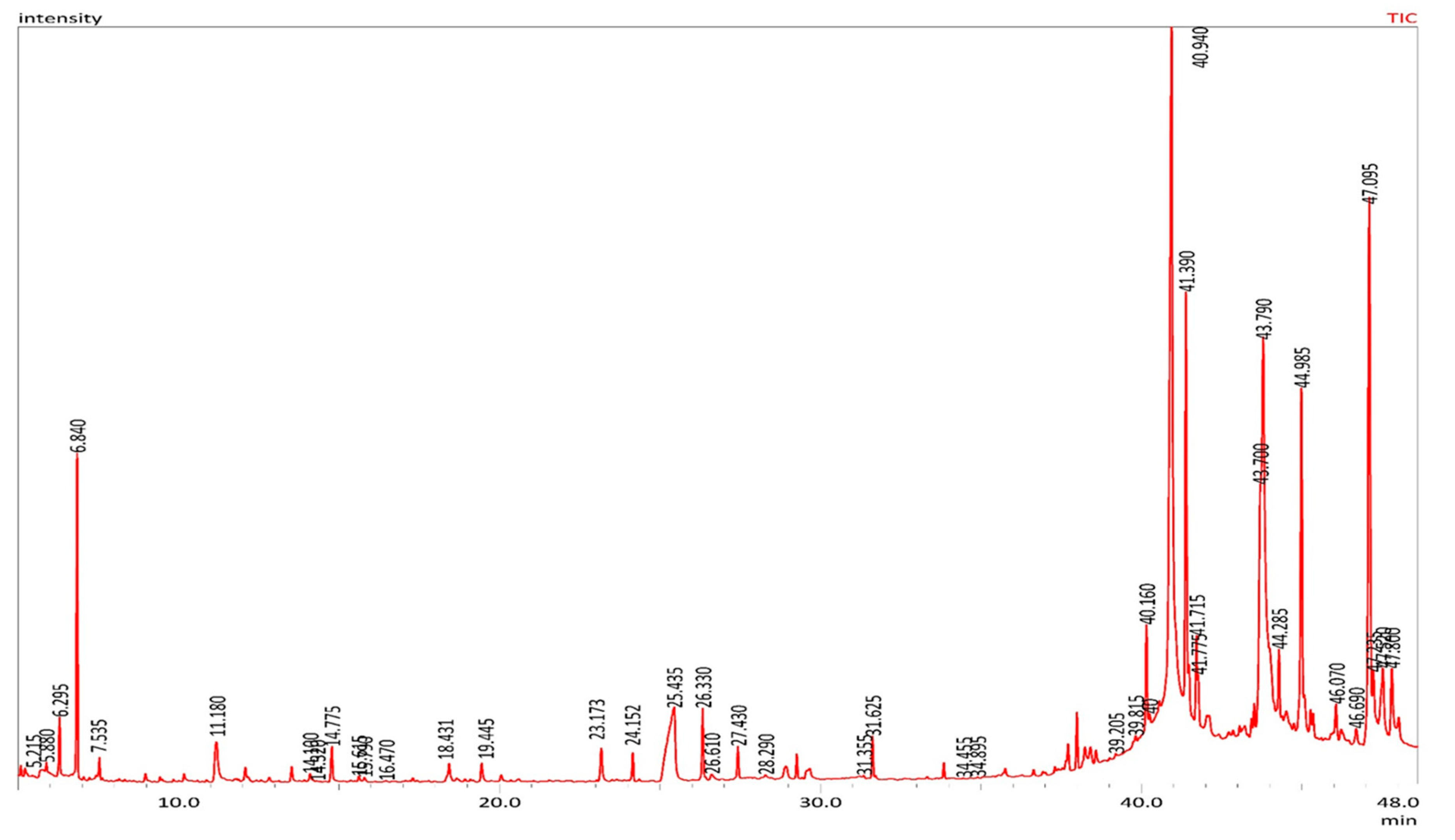
2.2. Volatile Content Analyses of MOS using GC-MS
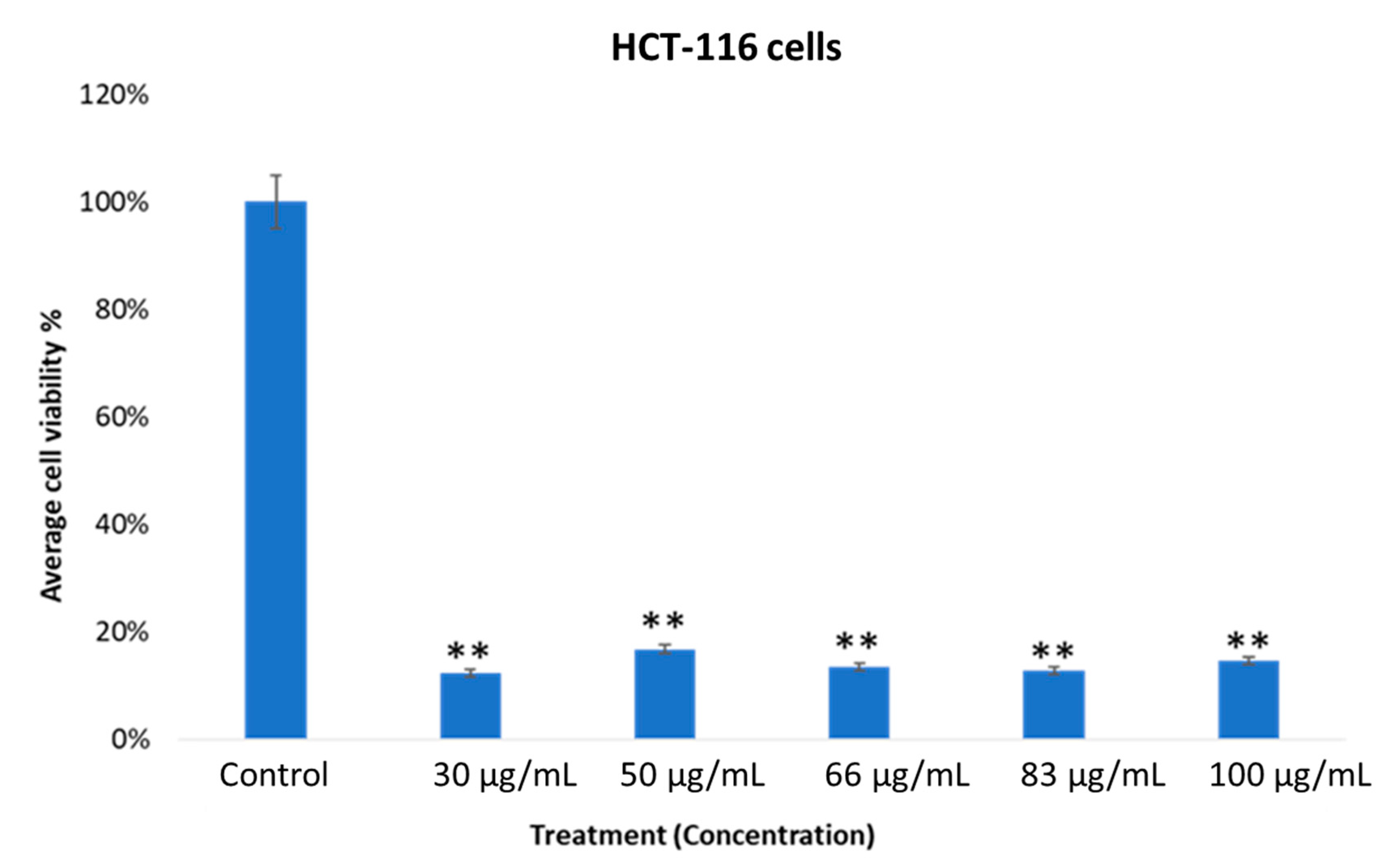
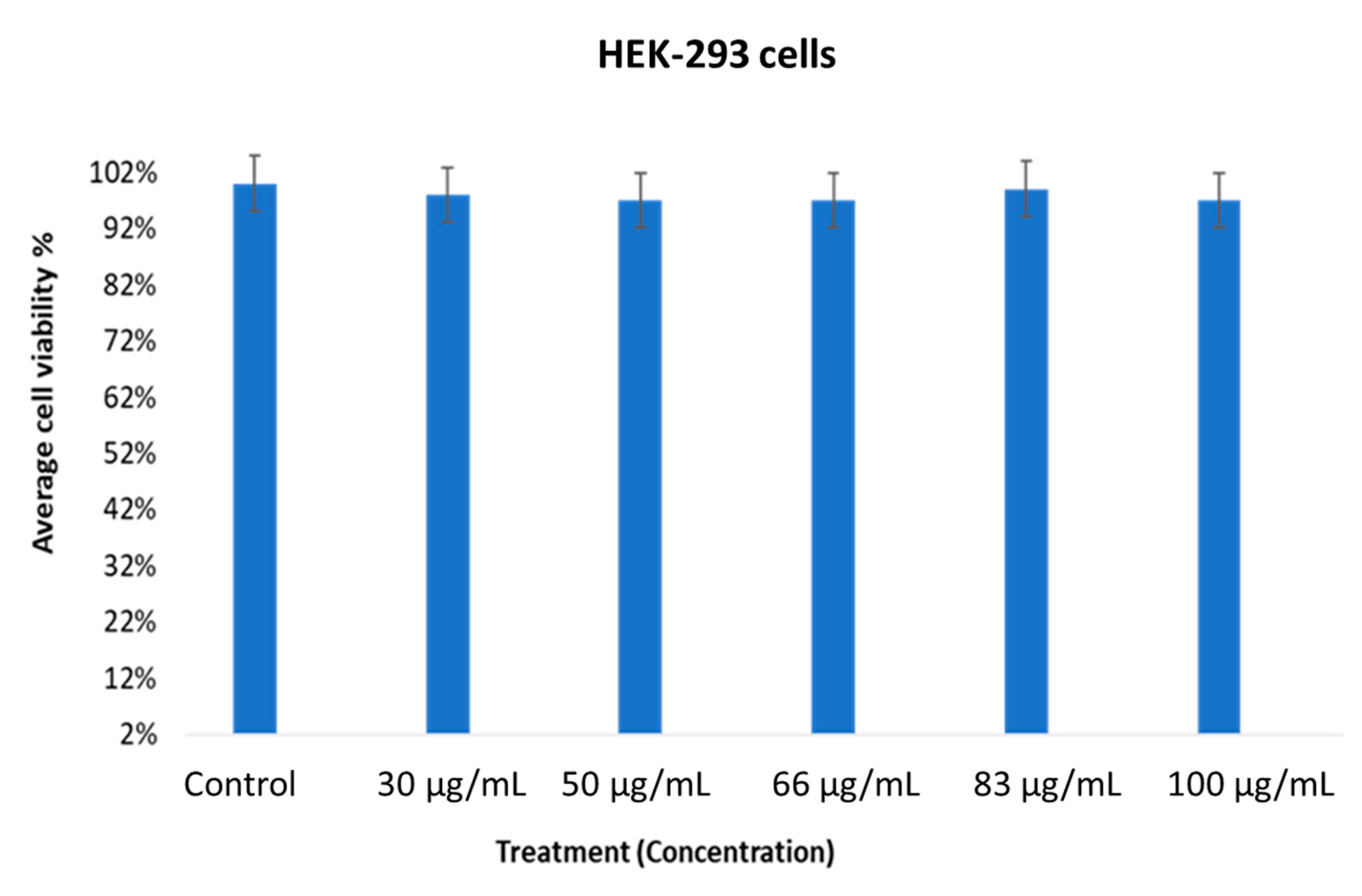
2.3. In vitro Cytotoxic Activity of MOS
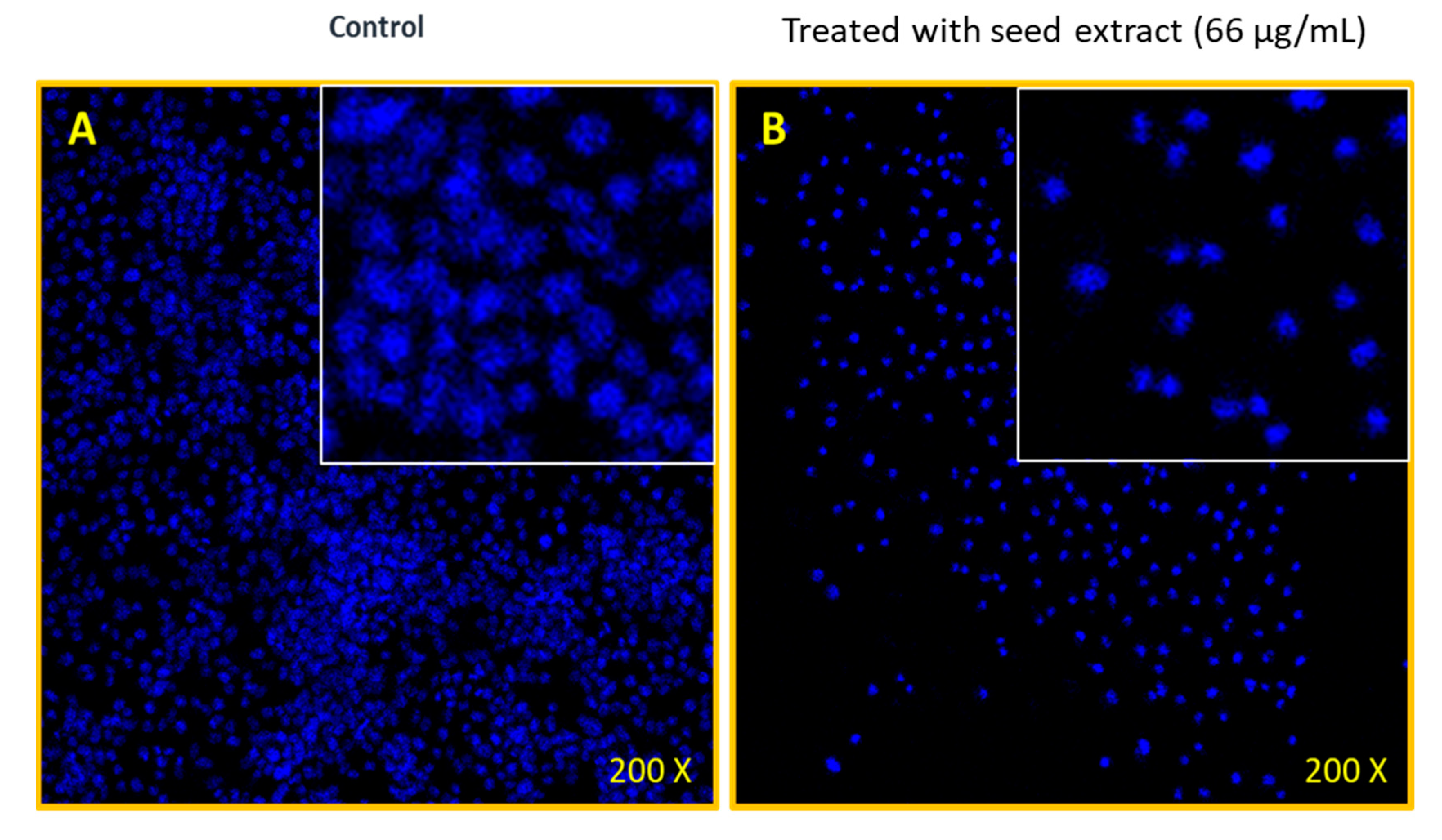
2.4. Nuclear Breakdown of MOS Extract-Treated Cancerous Cells
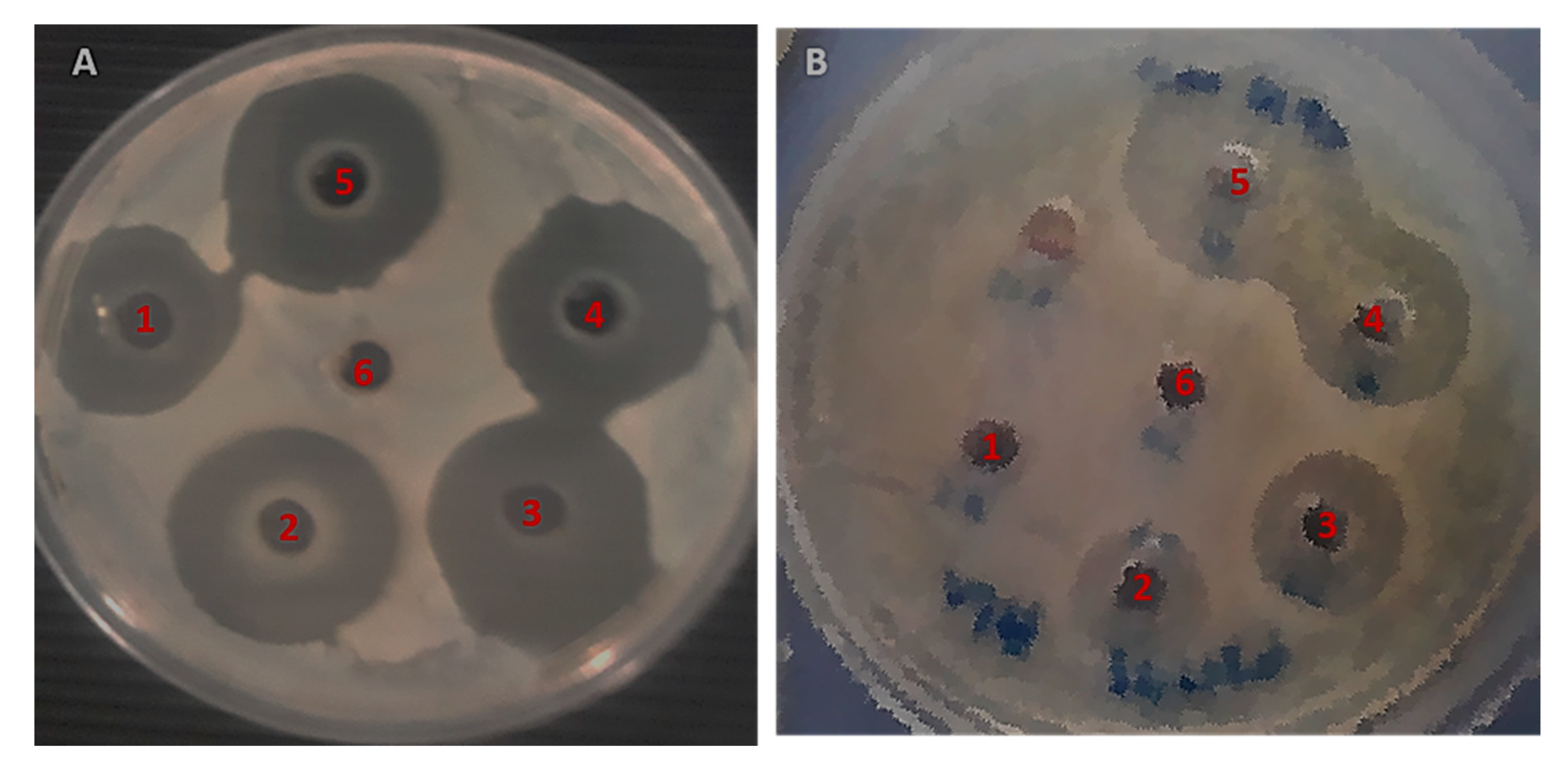
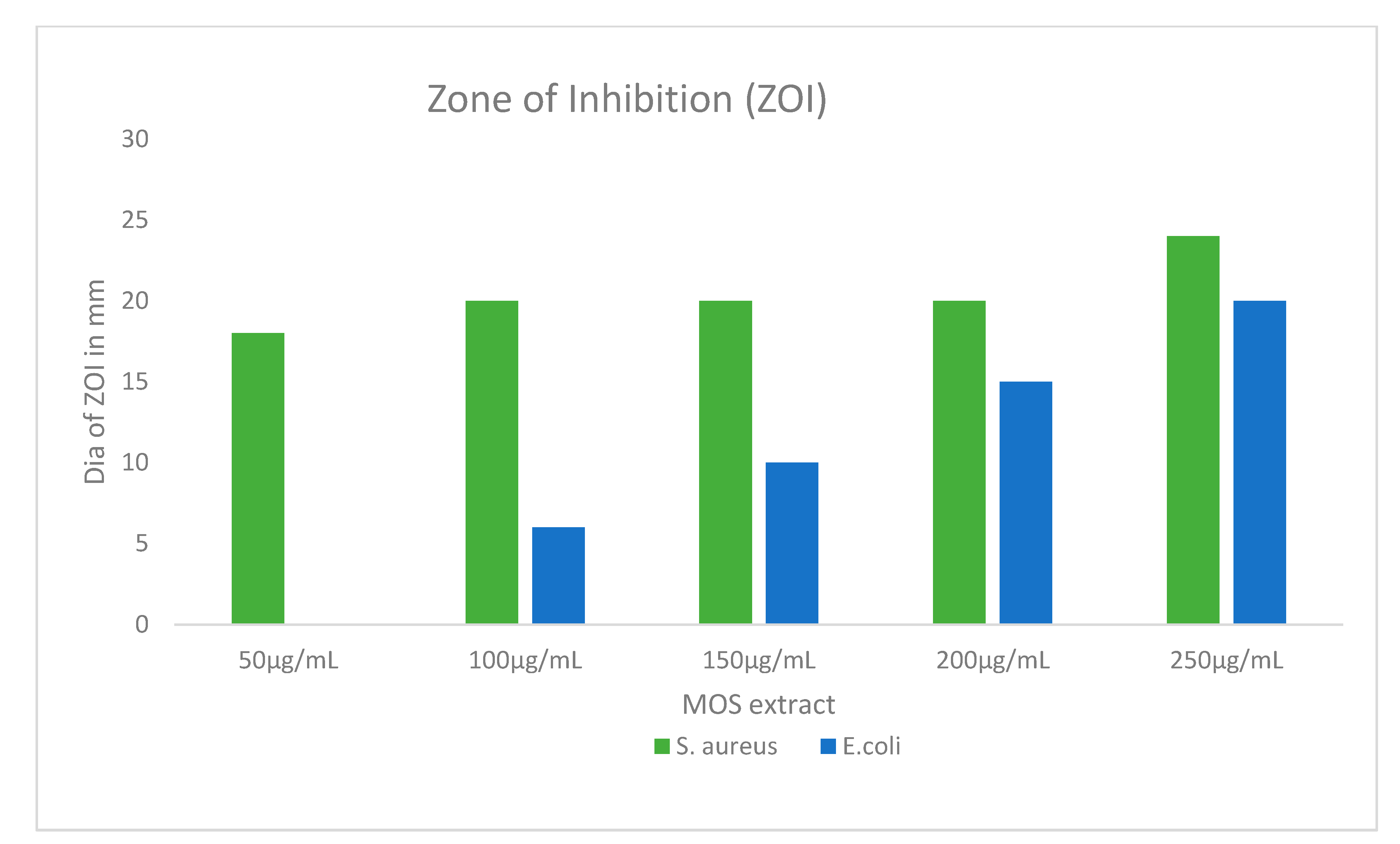
2.5. Antibacterial Efficacy of MOS Extract
3. Discussion
4. Materials and Procedures
4.1. Seed Assembly and Extract Preparation
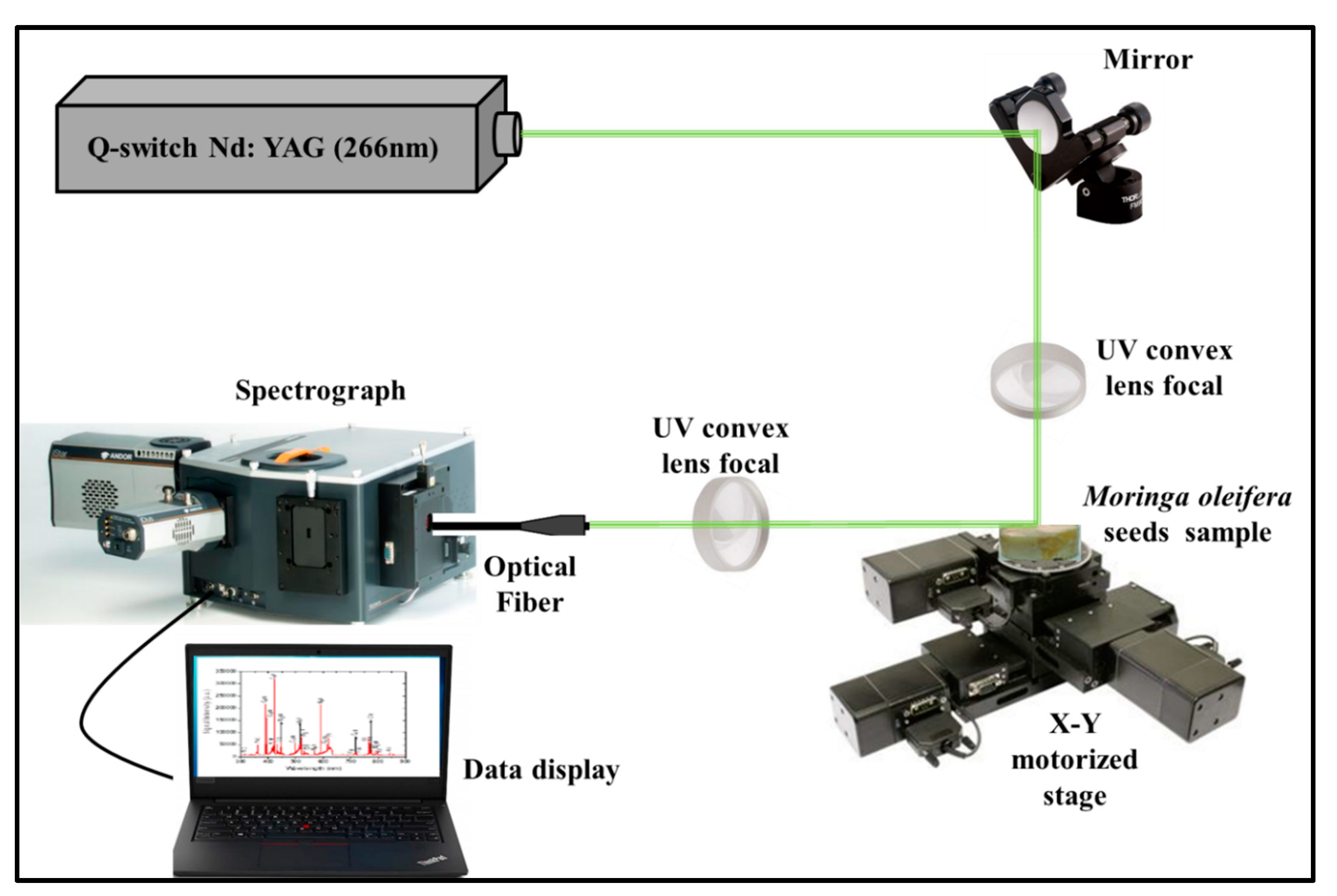
4.2. LIBS Setup
4.3. GC-MS Measurements
4.4. Anticancer Activity of MOS Extract
4.4.1. In Vitro Cell Viability and Cell Culture Assay
4.4.2. Nuclear Staining via DAPI
4.5. Antimicrobial Activity Assessment of MOS Extract
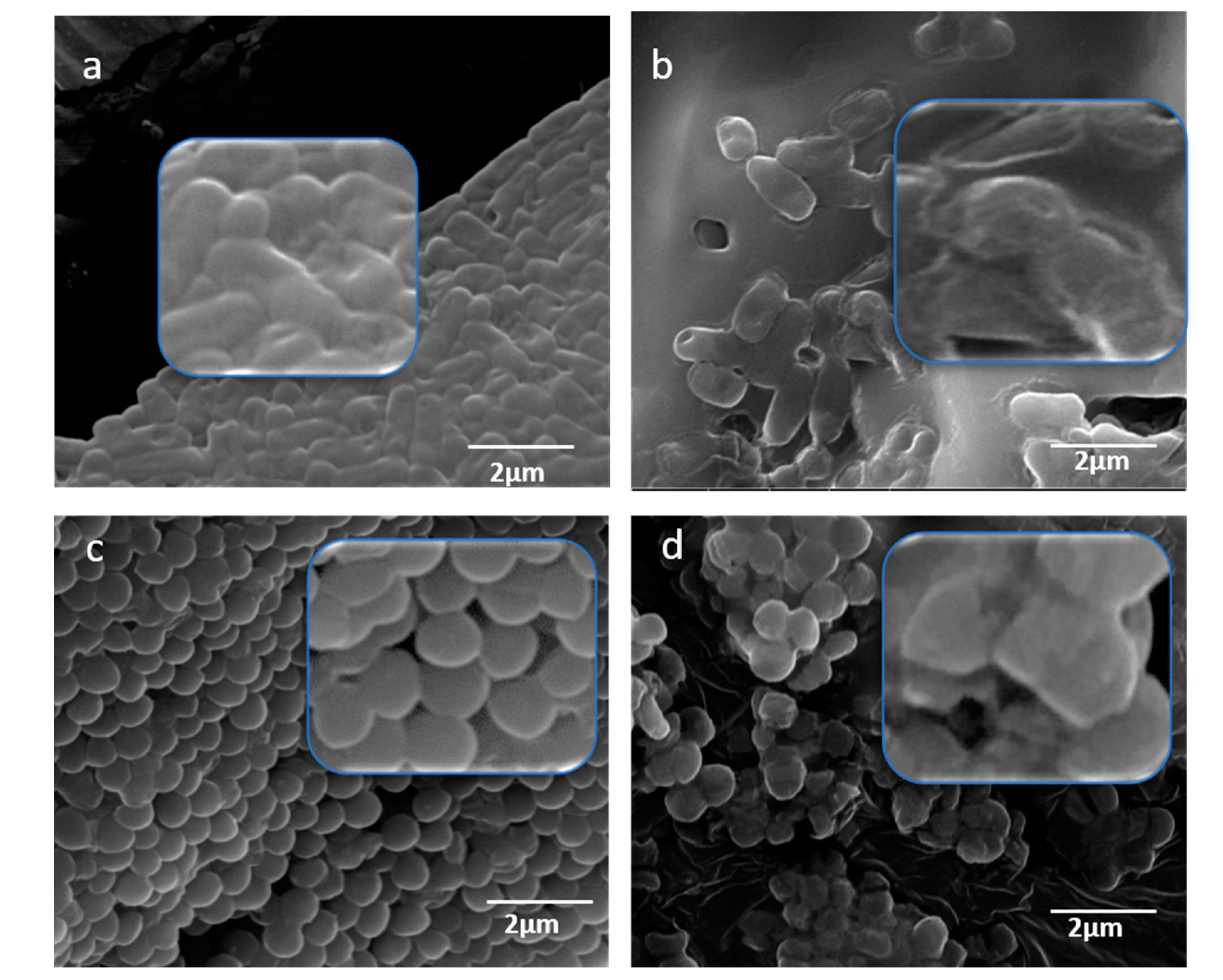
4.6. Antimicrobial Activity Assessment of MOS Extract Using SEM
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shale, T.L.; Stirk, W.A.; van Staden, J. Screening of medicinal plants used in Lesotho for anti-bacterial and anti-inflammatory activity. J. Ethnopharmacol. 1999. [Google Scholar] [CrossRef]
- Macéé, S.R.H.; Truelstrup, H.L. Anti-bacterial activity of phenolic compounds against Streptococcus pyogenes. Medicines 2017, 4, 25. [Google Scholar] [CrossRef]
- Nakhjavan, M.; Palethorpe, H.M.; Tomita, Y.; Smith, E.; Price, T.G.; Yool, A.J.; Pei, V.J.; Townsend, A.R.; Hardingham, G.H. Stereoselective anti-cancer activities of ginsenoside rg3 on triple negative breast cancer cell models. Pharmaceuticals 2019, 12, 117. [Google Scholar] [CrossRef]
- Khor, K.Z.; Lim, V.; Moses, E.J.; Samad, N.A. The in Vitro and in Vivo Anticancer Properties of Moringa oleifera. Evid.-based Complement. Altern. Med. 2018, 2018, 14. [Google Scholar] [CrossRef]
- Oduro, I.; Ellis, W.O.; Owusu, D. Nutritional potential of two leafy vegetables: Moringa oleifera and Ipomoea batatas leaves. Sci. Res. Essays 2008, 3, 57–60. [Google Scholar] [CrossRef]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef]
- Natarajan, S.; Joshi, J.A. Characterisation of moringa (Moringa oleifera Lam.) genotypes for growth, pod and seed characters and seed oil using morphological and molecular markers. Vegetos 2015, 28, 64–71. [Google Scholar] [CrossRef]
- Stohs, S.J.; Hartman, M.J. Review of the safety and efficacy of Moringa oleifera. Phytother. Res. 2015, 29, 796–804. [Google Scholar] [CrossRef]
- Retta, N.; Awoke, T.; Mekonnen, Y. Comparison of total phenolic content, free radical scavenging potential and anti-hyperglycemic condition from leaves extract of Moringa stenopetala and Moringa oliefera. Ethiop. J. Public Heal. Nutr. 2019, 1, 20–27. [Google Scholar]
- El-Hack, M.E.A.; Alagawany, M.; Elrys, A.S.; Desoky, E.-S.M.; Tolba, H.M.N.; Elnahal, A.S.M.; Elnesr, S.S.; Swelum, A.A. Effect of forage moringa oleifera l. (moringa) on animal health and nutrition and its beneficial applications in soil, plants and water purification. Agriculture (Switzerland) 2018, 8, 145. [Google Scholar] [CrossRef]
- Ferreira, P.M.P.; Farias, D.F.; Oliveira, J.T.D.A.; Carvalho, A.D.F.U. Moringa oleifera: Bioactive compounds and nutritional potential. Revista de Nutricao 2008, 21. [Google Scholar] [CrossRef]
- Anwar, F.; Zafar, S.N.; Rashid, U. Characterization of Moringa oleifera seed oil from drought and irrigated regions of Punjab, Pakistan. Grasasy Aceites 2006. [Google Scholar] [CrossRef]
- Silva, M.O.; Camacho, F.P.; Ferreira-Pinto, L.; Giufrida, W.M.; Vieira, A.M.S.; Visentaine, J.V.; Vedoy, D.R.L. Cardozo-Filho, L. Extraction and phase behaviour of Moringa oleifera seed oil using compressed propane. Can. J. Chem. Eng. 2016, 94, 2195–2201. [Google Scholar] [CrossRef]
- Gondal, M.A.; Hussain, T.; Yamani, Z.H.; Baig, M.A. Detection of heavy metals in Arabian crude oil residue using laser induced breakdown spectroscopy. Talanta 2006, 69, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Sabsabi, M.; Cielo, P. Quantitative analysis of aluminum alloys by Laser-induced breakdown spectroscopy and plasma characterization. Appl. Spectrosc. 1995, 49. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D. An experimental investigation into the solubility of Moringa oleifera oil in supercritical carbon dioxide. J. Food Eng. 2014. [Google Scholar] [CrossRef]
- Yamaguchi, F.; Takata, M.; Kamitori, K.; Ninaka, M.; Dong, Y.; Tokuda, M. Rare sugar D-allose induces specific up-regulation of TXNIP and subsequent G1 cell cycle arrest in hepatocellular carcinoma cells by stabilization of p27kip1. Int. J. Oncol. 2008, 32, 377–385. [Google Scholar] [CrossRef]
- Sharma, S.; Shukla, N.; Bharti, A.S.; Uttam, K.N. Simultaneous Multielemental Analysis of the Leaf of Moringa oleifera by Direct Current Arc Optical Emission Spectroscopy. Natl. Acad. Sci. Lett. 2018, 41, 65–68. [Google Scholar] [CrossRef]
- Osuntokun, O.T.; Yusuf-Babatunde, M.A.; Fasila, O.O. Components and Bioactivity of Ipomoea batatas (L.) (Sweet Potato) Ethanolic Leaf Extract. Asian J. Adv. Res. Rep. 2020, 10, 10–26. [Google Scholar] [CrossRef]
- Welch, R.; Tietje, A. Investigation of Moringa oleifera leaf extract and its cancer-selective antiproliferative properties. J. South. Carolina Acad. Sci. 2017, 15, 4. [Google Scholar]
- Menendez, J.; Lupu, R. Mediterranean Dietary Traditions for the Molecular Treatment of Human Cancer: Anti-Oncogenic Actions of the Main Olive Oils Monounsaturated Fatty Acid Oleic Acid (18:1n-9). Curr. Pharm. Biotechnol. 2006. [Google Scholar] [CrossRef]
- Fahey, J. Moringa oleifera: A Review of the Medical Evidence for Its Nutritional, Therapeutic, and Prophylactic Properties. Part 1. Trees Life J. 2005, 10, 602–608. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, Y.; Yang, R.; Liu, X.; Yang, Q.; Qin, X. The application of ultrasound and microwave to increase oil extraction from Moringa oleifera seeds. Ind. Crops Prod. 2018, 120, 1–10. [Google Scholar] [CrossRef]
- Rai, A.; Mohanty, B.; Bhargava, R. Experimental modeling and simulation of supercritical fluid extraction of Moringa oleifera seed oil by carbon dioxide. Chem. Eng. Commun. 2017, 204, 957–964. [Google Scholar] [CrossRef]
- Belo, Y.N.; Al-Hamimi, S.; Chimuka, L.; Turner, C. Ultrahigh-pressure supercritical fluid extraction and chromatography of Moringa oleifera and Moringa peregrina seed lipids. Anal Bioanal Chem. 2019, 411, 3685–3693. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Altuwiriqi, R.; Gondal, M.A.; AlDakheel, R.K.; Alotaibi, H.F. Qualitative and quantitative analysis of human nails to find correlation between nutrients and vitamin D deficiency using LIBS and ICP-AES. Talanta 2018, 185, 61–70. [Google Scholar] [CrossRef]
- Mehta, S.; Rai, P.K.; Rai, N.K.; Rai, A.K.; Bicanic, D.; Watal, G. Role of Spectral Studies in Detection of Antibacterial Phytoelements and Phytochemicals of Moringa oleifera. Food Biophys. 2011. [Google Scholar] [CrossRef]
- Pasquini, C.; Cortez, J.; Silva, L.M.C.; Gonzaga, F.B. Laser induced breakdown spectroscopy. J. Braz. Chem. Soc. 2007, 18, 463–512. [Google Scholar] [CrossRef]
- Rehman, S.; Asiri, S.M.; Khan, F.A.; Jermy, B.R.; Ravinayagam, V.; Alsalem, Z.; Jindan, R.A.; Qurashi, A. Biocompatible Tin Oxide Nanoparticles: Synthesis, Antibacterial, Anticandidal and Cytotoxic Activities. ChemistrySelect 2019, 4, 4013–4017. [Google Scholar] [CrossRef]
- Rehman, S.; Asiri, S.M.; Khan, F.A.; Jermy, B.R.; Ravinayagam, V.; Alsalem, Z.; Jindan, R.A.; Qurashi, A. Anticandidal and In Vitro Anti-Proliferative Activity of Sonochemically synthesized Indium Tin Oxide Nanoparticles. Sci. Rep. 2020. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; Rodríguez-Herrera, R.; Aguilar, C.N. Essential Oils: A Natural Alternative to Combat Antibiotics Resistance. A Natural Alternative to Combat Antibiotics Resistance. Antibiot. Resist. Mech. New Antimicrob. Approaches 2016, 11, 227–235. [Google Scholar]
- Paduch, R.; Kandefer-Szerszeń, M.; Trytek, M.; Fiedurek, J. Terpenes: Substances useful in human healthcare. Arch. Immunol. et Ther. Exp. 2007, 55, 27–315. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Baek, K.H.; Kang, S.C. Control of Salmonella in foods by using essential oils: A review. Food Res. Int. 2012, 45, 722–734. [Google Scholar] [CrossRef]
- Friedly, E.C.; Crandall, P.G.; Ricke, S.C.; Roman, M.; O’Bryan, C.; Chalova, V.I. In vitro antilisterial effects of citrus oil fractions in combination with organic acids. J. Food Sci. 2009, 74, M67–M72. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005. [Google Scholar] [CrossRef]
- Nithyanand, P.; Shafreen, R.M.B.; Muthamil, S.; Murugan, R.; Pandian, S.K. Essential oils from commercial and wild Patchouli modulate Group A Streptococcal biofilms. Ind. Crops Prod. 2015, 69, 1–492. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
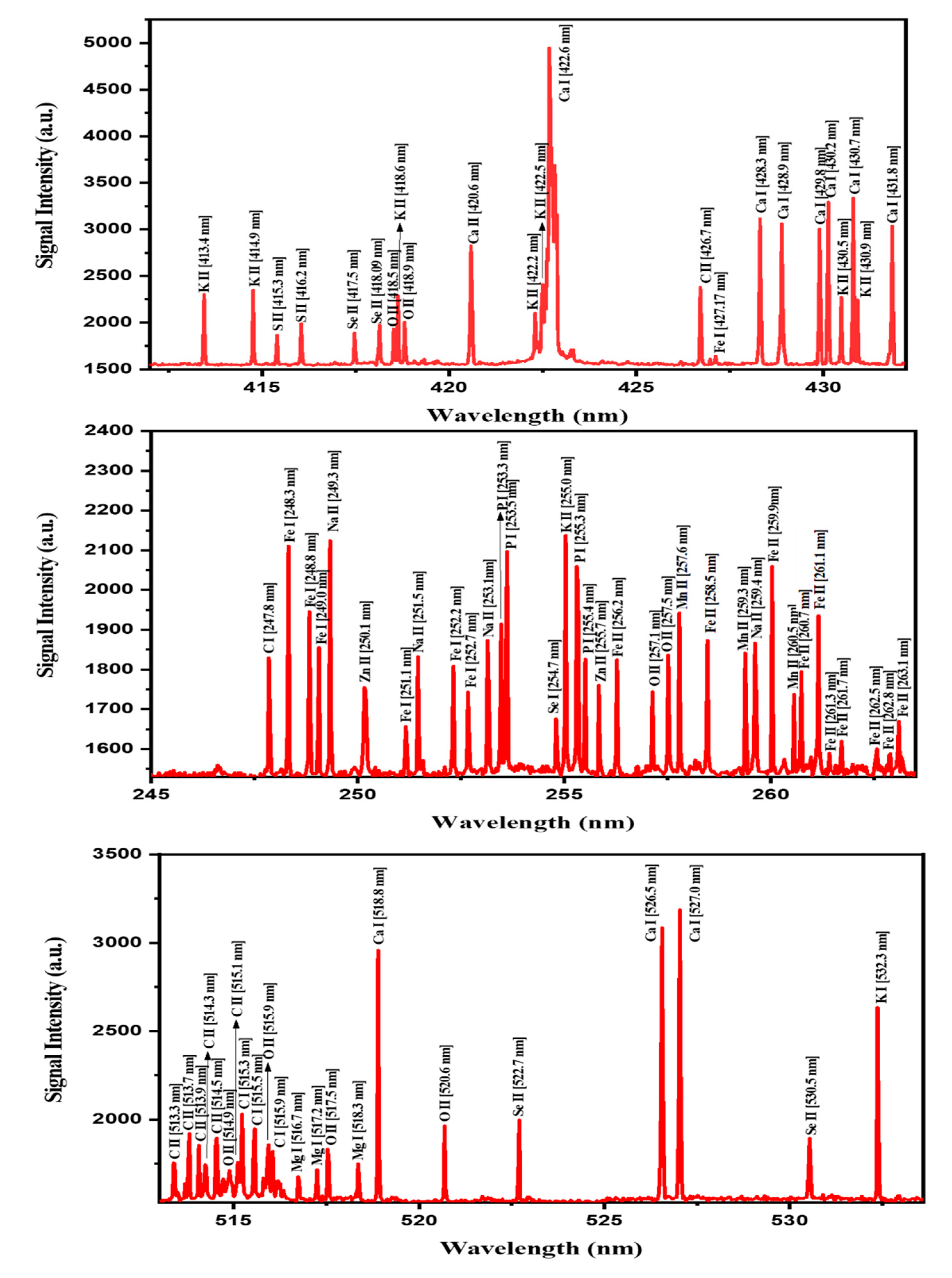

| Name of Element | Wavelength (nm) | Transition Configuration | LIBS Signal Intensity (arbitrary unit(a.u.)) |
|---|---|---|---|
| Ca | 422.6 | 3p6 4s2 1S0→3p6 4s 4p 1P°1 | 4960.9 |
| 518.8 | 3p6 4s 4p 1P°1→3p6 4s 5d 1D2 | 2967.1 | |
| 527.0 | 3p6 3d 4s 3D3→>3p6 3d 4p 3P°2 | 3196.3 | |
| Fe | 248.3 | 3d6 4s2 5D4→3d6 (5D) 4s 4p (1P°) 5F°5 | 2114.1 |
| 252.2 | 3d6 4s2 5D4→3d6 (5D) 4s 4p (1P°) 5D°4 | 1810.1 | |
| 259. 9 | 3d6 (5D) 4s 6D9/2→3d6 (5D) 4p 6D°9/2 | 2061.9 | |
| K | 414.9 | 3p5 3d3P°0→3p5 4p 3D1 | 2347.6 |
| 430.5 | 3p5 3d3P°2→3p5 4p 1D2 | 2270.9 | |
| 532.3 | 3p6 4p2P°1/2→3p6 8s 2S1/2 | 2639.7 | |
| Mg | 518.3 | 3s 3p 3P°2→3s 4s 3S1 | 1753.5 |
| Mn | 259.3 | 3d5 (6S) 4s 7S3→3d5 (6S) 4p (7P°3) | 1843.9 |
| Na | 249.3 | 2s2 2p5 3s 1P°1→2s2 2p5 3p 1S0 | 2128.4 |
| 251.5 | 2s2 2p5 3p 3S1→2s2 2p5 (2P°1/2) 3d 2[→]°2 | 1834.7 | |
| P | 253.5 | 3s2 3p3 2P°3/2→3s2 3p2 (3P) 4s 2P3/2 | 2100.0 |
| 255.3 | 3s2 3p3 2P°1/2→3s2 3p2 (3P) 4s 2P1/2 | 2032.1 | |
| S | 416.2 | 3s2 3p2 (3P) 4p 4D°7/2→3s2 3p2 (3P) 4d 4F9/2 | 1987.8 |
| Se | 418.09 | 4s2 4p2 (3P) 5p 4D°7/2→4s2 4p2 (3P) 5d 4F9/2 | 1969.7 |
| 522.7 | 4s2 4p2 (3P) 5s 4P5/2→4s2 4p2 (3P) 5p 4D°7/2 | 2002.0 | |
| Zn | 250.1 | 3d10 4p 2P°1/2→3d10 5s 2S1/2 | 1751.4 |
| 255.7 | 3d10 4p 2P°3/2→3d10 5s 2S1/2 | 1762.3 | |
| C | 426.7 | 2s2 3d 2D5/2→2s2 4f 2F°7/2 | 2379.0 |
| 247.8 | 2s2 2p2 1S0→2s2 2p 3s 1P°1 | 1831.6 | |
| O | 418.9 | 2s2 2p2 (1D) 3p 2F°7/2→2s2 2p2 (1D) 3d 2G9/2 | 2003.4 |
| No. | Compounds | RT | Peak area (%) |
|---|---|---|---|
| Esters | |||
| 1 | Propanoic acid, 2-oxo-, methyl ester | 6.293 | 0.76 |
| 2 | Acetic acid, ethoxyhydroxy-, ethyl ester | 7.043 | 0.04 |
| 3 | Diethoxymethyl acetate | 12.827 | 0.09 |
| 4 | 2-Propenoic acid, 2-methyl-, 2-hydroxypropyl ester | 13.134 | 0.02 |
| 5 | 6,9,12-Octadecatrienoic acid, phenylmethyl ester, (Z,Z,Z)- | 17.797 | 0.01 |
| 6 | Cyclopentanecarboxylic acid, 4-tridecyl ester | 19.179 | 0.03 |
| 7 | 1-Cyclohexene-1-carboxylic acid, 2,6,6-trimethyl-, methyl ester | 20.058 | 0.13 |
| 8 | Hexanoic acid, 4-hexadecyl ester | 28.291 | 0.07 |
| 9 | Phthalic acid, diethyl ester | 29.568 | 0.14 |
| 10 | 2-Propenoic acid, pentadecyl ester | 31.625 | 0.59 |
| 11 | Acetic acid, 3,7,11,15-tetramethyl-hexadecyl ester | 33.627 | 0.02 |
| 12 | Phthalic acid, dibutyl ester | 35.692 | 0.07 |
| 13 | Phthalic acid, diisobutyl ester | 35.760 | 0.15 |
| 14 | Palmitoleic acid, methyl ester | 36.285 | 0.03 |
| 15 | Pentadecanoic acid, 13-methyl-, methyl ester | 36.642 | 0.09 |
| 16 | 9-Hexadecenoic acid, ethyl ester | 37.652 | 0.15 |
| 17 | Hexadecanoic acid, ethyl ester (Ethyl palmitate) | 37.993 | 0.78 |
| 18 | Heptadecanoic acid, ethyl ester | 38.416 | 0.46 |
| 19 | Propanoic acid, 3-mercapto-, dodecyl ester | 38.588 | 0.25 |
| 20 | 9-Octadecenoic acid, methyl ester, (E)- (Methyl elaidate) | 40.159 | 1.52 |
| 21 | Oleic acid, methyl ester (Methyl oleate) | 40.255 | 0.15 |
| 22 | l-(+)-Ascorbic acid 2,6-dihexadecanoate | 40.561 | 0.10 |
| 23 | Ethyl oleate | 41.389 | 6.03 |
| 24 | Octadecanoic acid, ethyl ester (Ethyl stearate) | 41.774 | 0.53 |
| 25 | 9-octadecenyl ester (Oleyl oleate) | 43.516 | 0.34 |
| 26 | 2,3-dihydroxypropyl elaidate | 43.794 | 13.48 |
| 27 | 9-Octadecenoic acid, 1,2,3-propanetriyl ester | 44.287 | 0.90 |
| 28 | Docosanoic acid, ethyl ester | 45.273 | 0.37 |
| 29 | Oleoyl chloride | 46.066 | 0.65 |
| 30 | 9-Octadecenoic acid (Z)-, 2,3-dihydroxypropyl ester | 47.099 | 11.35 |
| 31 | Glycidol stearate | 47.522 | 1.76 |
| 32 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 47.803 | 1.57 |
| Alcohols | |||
| 33 | 1,4-Cyclohexanediol, trans- | 7.224 | 0.02 |
| 34 | 1,2-Propanediol, 3-methoxy- | 7.430 | 0.05 |
| 35 | Ethanol, 2,2-diethoxy- | 7.535 | 0.31 |
| 36 | 1,2,4-Butanetriol | 9.496 | 0.04 |
| 37 | Glycerin | 11.180 | 1.35 |
| 38 | 1-Butanol, 4-(ethylthio)- | 11.742 | 0.02 |
| 39 | Methoxyacetaldehyde diethyl acetal | 11.883 | 0.02 |
| 40 | 1-Dodecanol | 18.431 | 0.48 |
| 41 | 1-Tetradecanol | 24.152 | 0.42 |
| 42 | 1,3-Propanediol, 2-ethyl-2-(hydroxymethyl)- | 25.444 | 5.52 |
| 43 | 1-Tridecanol | 26.331 | 1.06 |
| 44 | 3-Hexadecanol | 31.348 | 0.05 |
| 45 | 3-Heptadecanol | 35.561 | 0.06 |
| Aldehydes | |||
| 46 | Butanal, 3-hydroxy- | 8.375 | 0.00 |
| 47 | Heptanal | 10.006 | 0.02 |
| 48 | Octanal | 12.567 | 0.04 |
| 49 | Nonanal | 15.790 | 0.09 |
| 50 | 2-Methyl-oct-2-enedial | 20.611 | 0.04 |
| 51 | Undecanal | 23.521 | 0.02 |
| 52 | Tridecanal | 34.455 | 0.01 |
| 53 | 10-Octadecenal | 37.418 | 0.09 |
| 54 | 9-Octadecenamide | 37.551 | 0.09 |
| 55 | cis-9-Hexadecenal | 38.241 | 0.55 |
| 56 | cis-13-Octadecenal | 47.228 | 1.92 |
| Ketones | |||
| 57 | 2-Propanone, 1,1-dimethoxy- | 6.841 | 3.86 |
| 58 | 2-Butanone | 8.151 | 0.03 |
| 59 | Dihydroxyacetone | 8.969 | 0.14 |
| 60 | 1,2-Cyclopentanedione | 10.172 | 0.14 |
| 61 | 2-Propanone, 1-(1,3-dioxolan-2-yl)- | 10.867 | 0.04 |
| 62 | 1,3-Dioxol-2-one,4,5-dimethyl- | 13.528 | 0.28 |
| 63 | 2-Heptanol, 5-ethyl- | 14.306 | 0.01 |
| 64 | 2-Methyl-4-octanone | 16.466 | 0.03 |
| 65 | 2-Pentanone, 3,4-epoxy- | 18.925 | 0.03 |
| 66 | 1-Oxa-spiro[4.5]deca-6,9-diene-2,8-dione | 37.006 | 0.05 |
| 67 | Z-11-Pentadecenol | 39.209 | 0.05 |
| 68 | Cyclopentadecanone, 2-hydroxy- | 39.822 | 0.13 |
| 69 | Cyclopentadecanone | 43.430 | 0.21 |
| 70 | 2-Tetradecanone | 46.691 | 0.34 |
| Acids | |||
| 71 | Acetic acid, (acetyloxy)- | 5.884 | 0.18 |
| 72 | Butanoic acid, 3-hydroxy- | 10.594 | 0.03 |
| 73 | Octanoic acid | 17.421 | 0.03 |
| 74 | Nonanoic acid | 20.363 | 0.02 |
| 75 | n-Hexadecanoic acid (Palmitic acid) | 37.305 | 0.05 |
| 76 | Oleic acid | 40.943 | 22.53 |
| Furans and lactones | |||
| 77 | Furfural | 7.624 | 0.02 |
| 78 | 2(5H)-Furanone | 9.840 | 0.04 |
| 79 | 2-Hydroxy-gamma-butyrolactone | 12.081 | 0.23 |
| 80 | 2,5-Dimethyl-4-hydroxy-3(2H)-furanone | 14.099 | 0.19 |
| 81 | 1,2-Ethanediol, 1-(2-furanyl) | 19.096 | 0.03 |
| 82 | 5-Hydroxymethylfurfural | 19.445 | 0.36 |
| 83 | 3-Deoxy-d-mannoic lactone | 28.924 | 0.52 |
| Nitrogen-containing compounds | |||
| 84 | N,N-Dimethylaminoethanol | 5.216 | 0.12 |
| 85 | 1,3,5-Triazine-2,4,6-triamine | 14.778 | 0.68 |
| 86 | Acetic acid, 2-(N-methyl-N-phosphonatomethyl)amino- | 15.617 | 0.11 |
| 87 | 1-Heptadecanamine | 18.667 | 0.07 |
| 88 | Nonanamide | 23.034 | 0.02 |
| 89 | Dodecanamide (Lauryl amide) | 33.331 | 0.04 |
| 90 | Tetradecanamide (Myristic amide) | 37.717 | 0.44 |
| 91 | Hexadecanamide (Palmitic amide) | 41.718 | 1.26 |
| 92 | Docosenamide | 44.987 | 6.04 |
| 93 | Nonadecanamide | 45.352 | 0.32 |
| Sulfur-containing compounds | |||
| 94 | Sulfurous acid, cyclohexylmethyl hexadecyl ester | 23.173 | 0.67 |
| Hydrocarbons | |||
| 95 | 1-Butene, 4,4-diethoxy-2-methyl- | 9.425 | 0.07 |
| 96 | 1-Methyl-2-octylcyclopropane | 12.171 | 0.07 |
| 97 | 2-Trifluoroacetoxytridecane | 13.923 | 0.03 |
| 98 | trans-2,3-Epoxynonane | 23.642 | 0.03 |
| 99 | 2-Heptafluorobutyroxypentadecane | 23.887 | 0.04 |
| 100 | 1,2-Epoxyundecane | 24.016 | 0.02 |
| 101 | Heptacosane | 24.357 | 0.03 |
| 102 | 1-Heptadecene | 29.262 | 0.33 |
| 103 | Octadecane, 1,1’-[(1-methyl-1,2-ethanediyl)bis(oxy)]bis- | 29.426 | 0.01 |
| 104 | 1-Nonadecene | 33.847 | 0.22 |
| Pyrans | |||
| 105 | Tetrahydro-4H-pyran-4-ol | 17.074 | 0.03 |
| 106 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 17.198 | 0.02 |
| Others | |||
| 107 | 5,6-Dihydroxypiperazine-2,3-dione dioxime | 8.320 | 0.02 |
| 108 | Tetraethyl silicate | 11.807 | 0.04 |
| 109 | Silanediol, dimethyl-, diacetate | 17.300 | 0.07 |
| 110 | D-Allose | 26.609 | 0.19 |
| 111 | Phenol, 2,4-bis(1,1-dimethylethyl)- | 27.430 | 0.51 |
| 112 | Oxirane, hexadecyl- | 34.898 | 0.02 |
| 113 | Ethyl iso-allocholate | 36.936 | 0.06 |
| 114 | Oleic anhydride | 43.700 | 3.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldakheel, R.K.; Rehman, S.; Almessiere, M.A.; Khan, F.A.; Gondal, M.A.; Mostafa, A.; Baykal, A. Bactericidal and In Vitro Cytotoxicity of Moringa oleifera Seed Extract and Its Elemental Analysis Using Laser-Induced Breakdown Spectroscopy. Pharmaceuticals 2020, 13, 193. https://doi.org/10.3390/ph13080193
Aldakheel RK, Rehman S, Almessiere MA, Khan FA, Gondal MA, Mostafa A, Baykal A. Bactericidal and In Vitro Cytotoxicity of Moringa oleifera Seed Extract and Its Elemental Analysis Using Laser-Induced Breakdown Spectroscopy. Pharmaceuticals. 2020; 13(8):193. https://doi.org/10.3390/ph13080193
Chicago/Turabian StyleAldakheel, Reem K., Suriya Rehman, Munirah A. Almessiere, Firdos A. Khan, Mohammed A. Gondal, Ahmed Mostafa, and Abdulhadi Baykal. 2020. "Bactericidal and In Vitro Cytotoxicity of Moringa oleifera Seed Extract and Its Elemental Analysis Using Laser-Induced Breakdown Spectroscopy" Pharmaceuticals 13, no. 8: 193. https://doi.org/10.3390/ph13080193
APA StyleAldakheel, R. K., Rehman, S., Almessiere, M. A., Khan, F. A., Gondal, M. A., Mostafa, A., & Baykal, A. (2020). Bactericidal and In Vitro Cytotoxicity of Moringa oleifera Seed Extract and Its Elemental Analysis Using Laser-Induced Breakdown Spectroscopy. Pharmaceuticals, 13(8), 193. https://doi.org/10.3390/ph13080193








