Application of Thin-Layer Chromatography in Combination with Densitometry for the Determination of Diclofenac in Enteric Coated Tablets
Abstract
1. Introduction
2. Results and Discussion
2.1. Validation of TLC Method
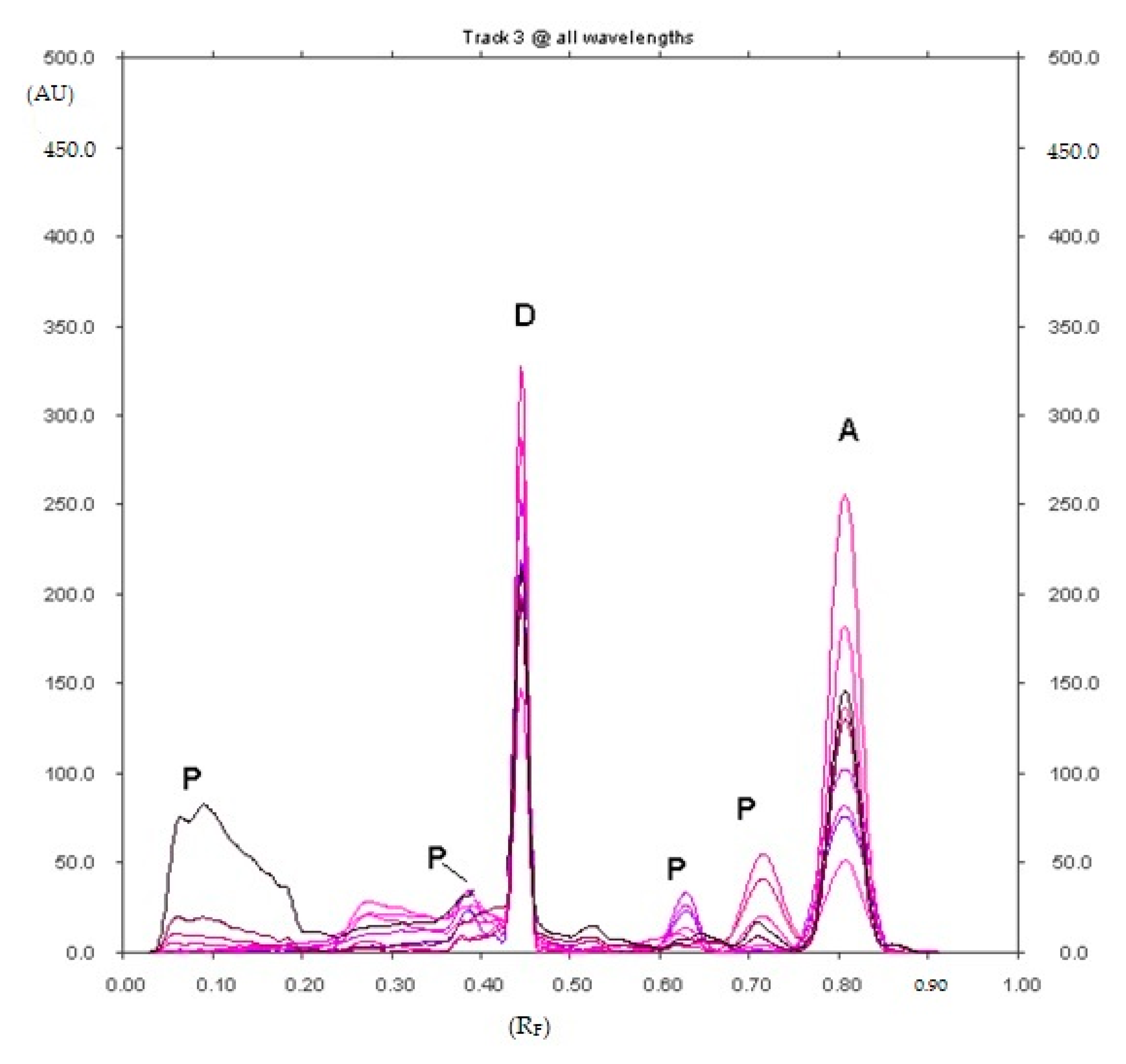
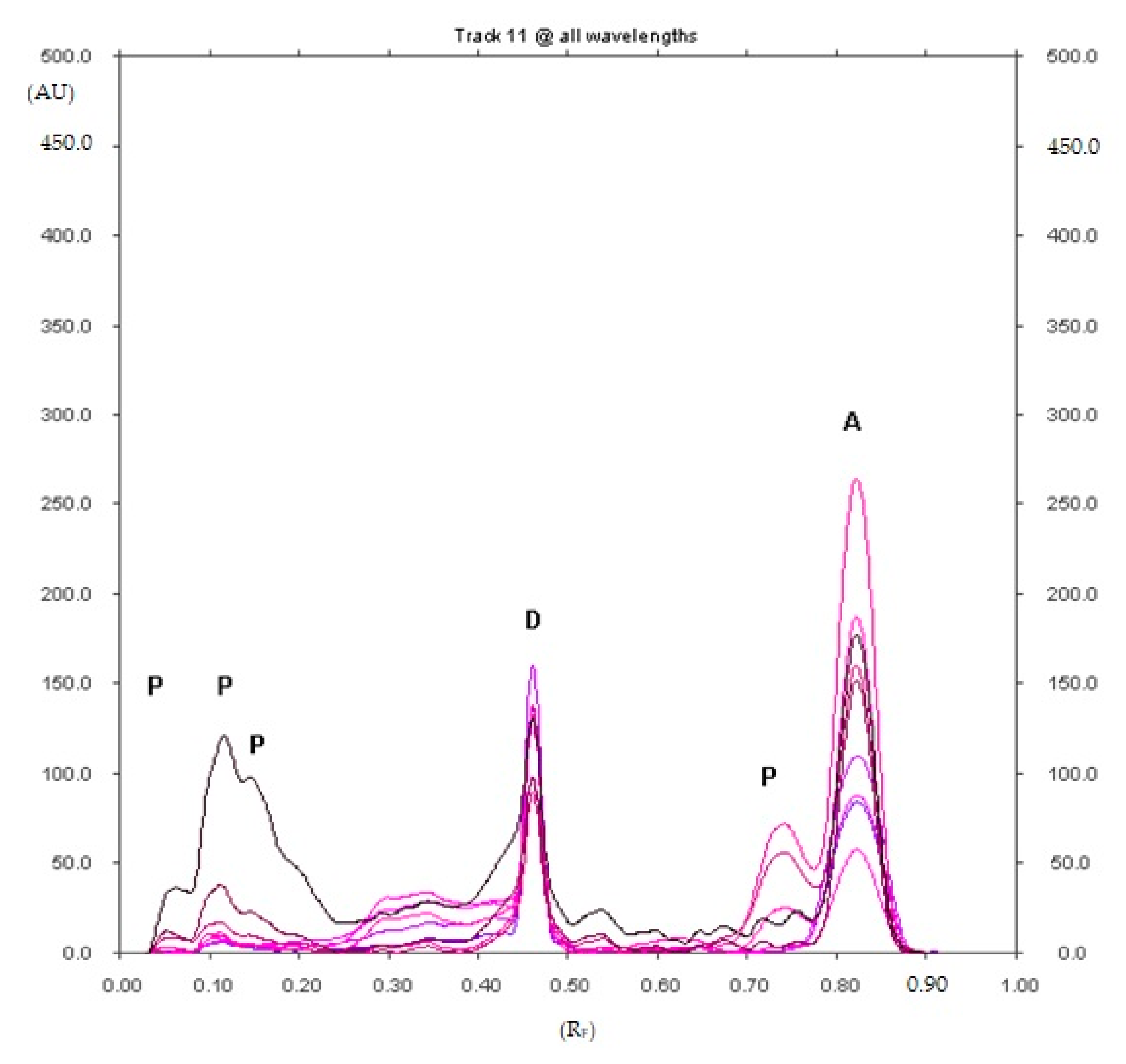
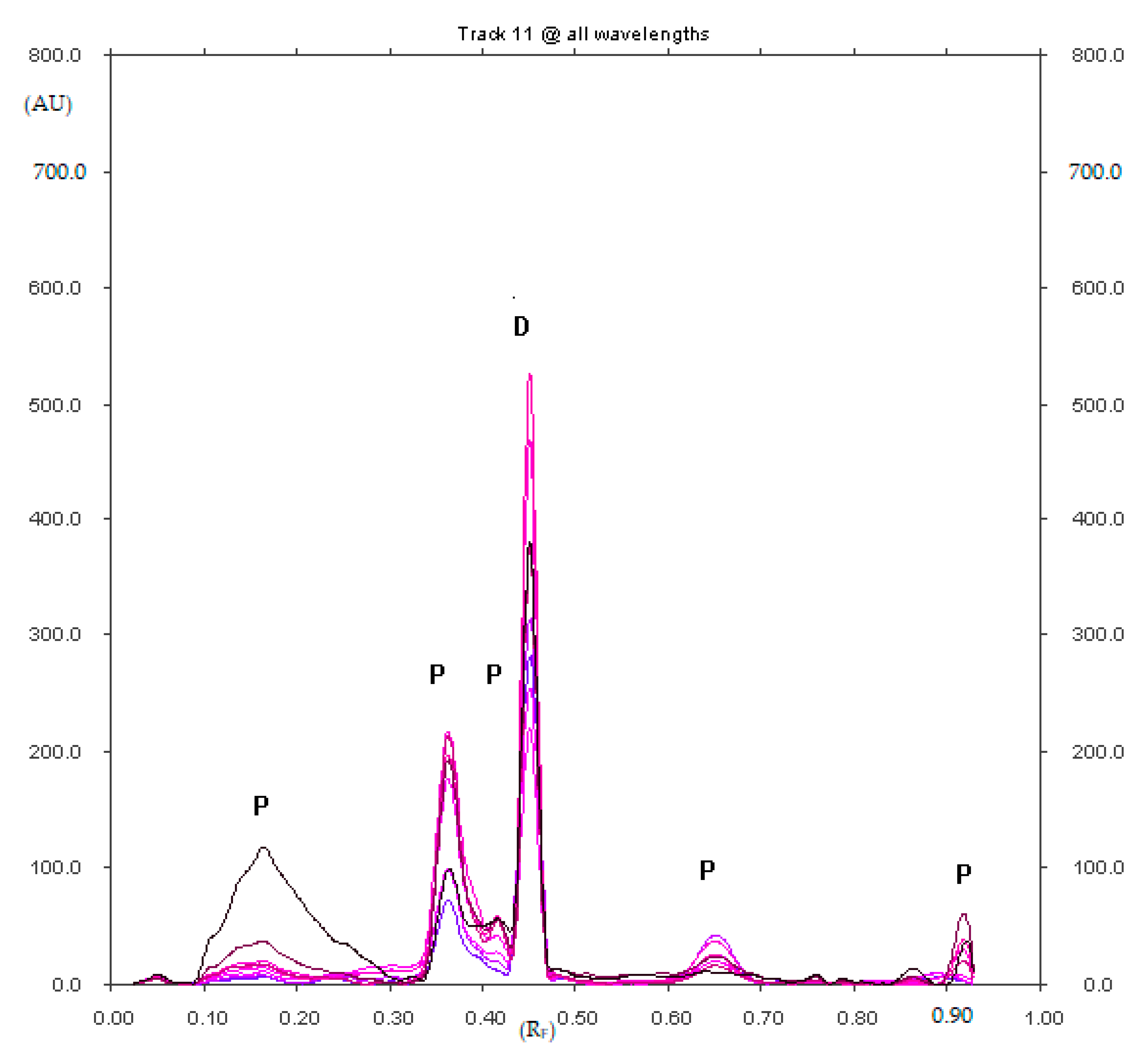
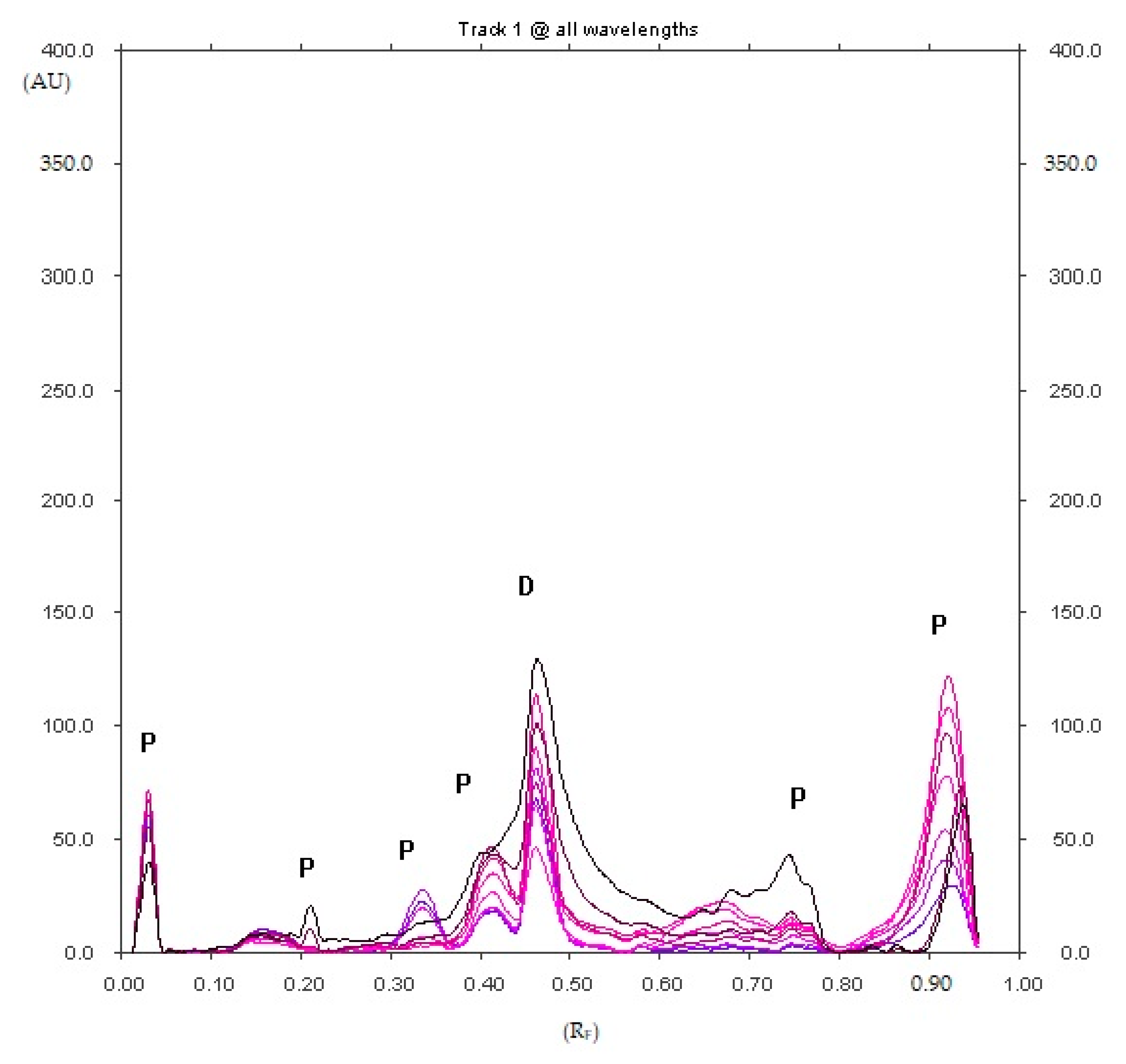
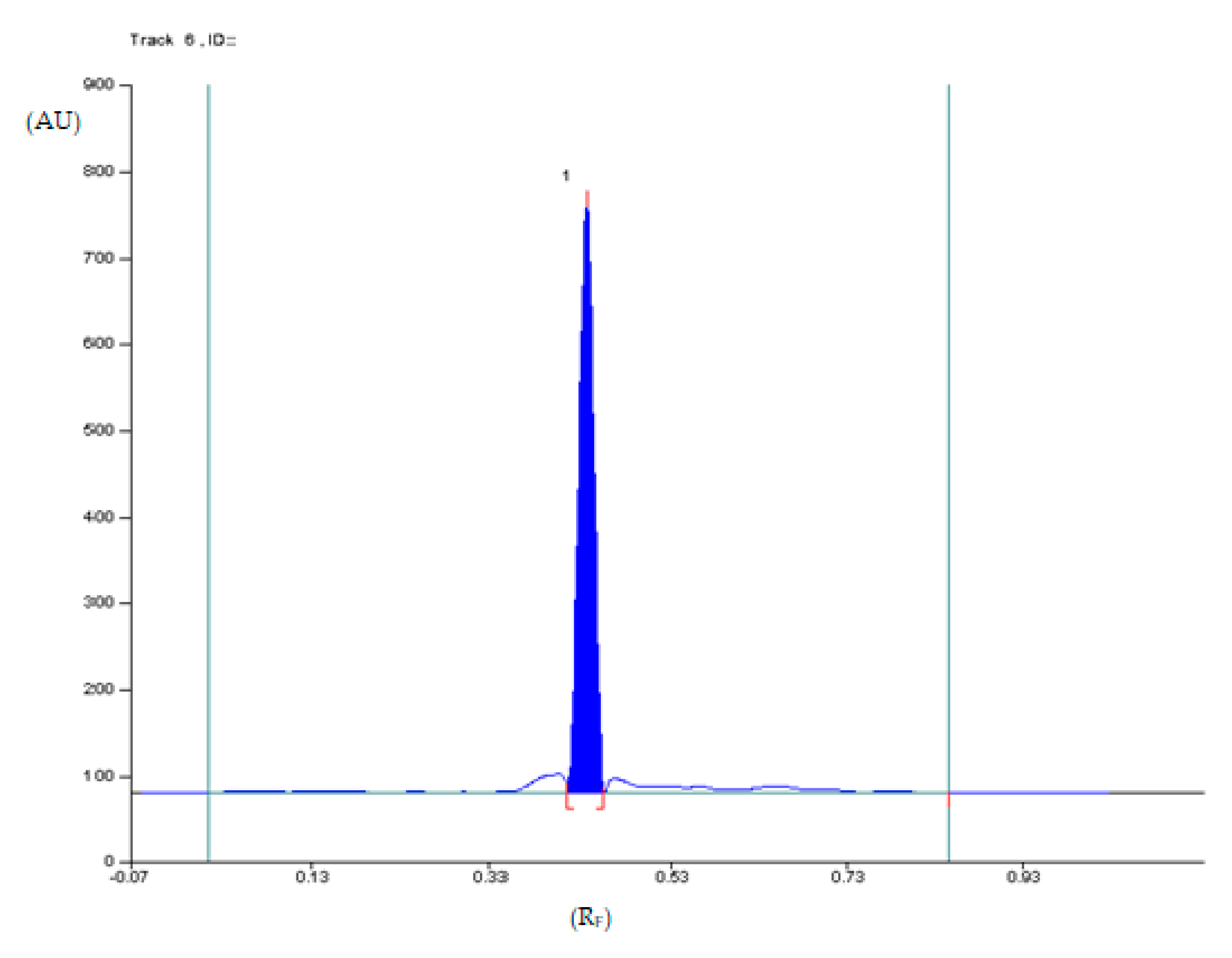
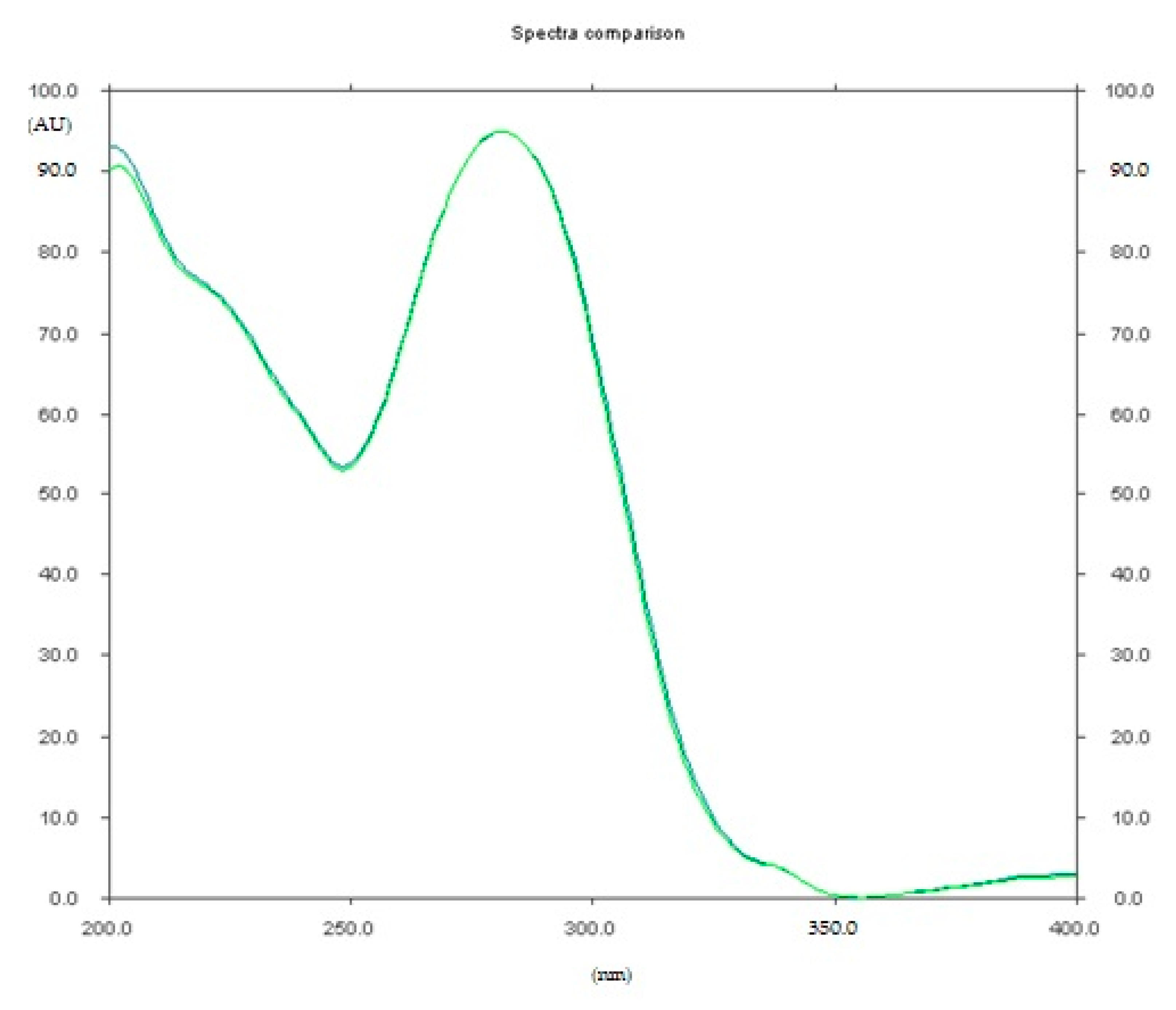
2.1.1. Optimization and Selectivity
2.1.2. Accuracy
2.1.3. Calibration and Range
2.1.4. Precision
2.1.5. Limit of Detection (LOD) and Limit of Quantification (LOQ) Based on the Calibration Curve
2.1.6. Robustness
2.1.7. Analysis of Diclofenac Sodium in Commercial Enteric Tablets
3. Materials and Methods
3.1. Apparatus
3.2. Pharmaceutical Reference Standards and Chemicals
3.3. Pharmaceutical Preparation
3.4. Preparation the Sample of Tablets
3.5. Preparation of Standard Solutions
3.6. Thin Layer Chromatography
3.7. Densitometric and Spectrodensitometric Study
3.8. Validation of the NP-TLC Method
3.8.1. Selectivity and Resolution
Preparation of the standard solution of diclofenac sodium:
Acidic hydrolysis:
Alkaline hydrolysis:
Oxidation:
Effects of UV radiation (254 nm):
These studies were carried out as follows:
- Diclofenac sodium solution with added hydrochloric acid or sodium hydroxide and hydrogen peroxide, respectively was placed in a volume flask on hot plate at 90 °C. Every half hour the solution was cooled and then 5 μL of it was applied onto chromatographic plate. This action was repeated for 5 h. Chromatographic plates were then developed by using the three best mobile phases. The examined solution was then applied to the plates after 3 days, 10 days and 2, 3, 4 and 10 weeks. Each chromatographic plate was developed also by means of the three best mobile phases.
- In studies on the influence of UV radiation on the stability of diclofenac sodium, the methanolic solution of this compound was placed in a beaker under UV lamp (λ = 254 nm). Every half hour the solution was cooled and then 5 μL of it was applied onto chromatographic plate. This procedure was repeated for 5 h. Chromatographic plates were then developed by using the three best mobile phases. The examined solution was then applied to the plates after 3 days, 10 days and 2, 3, 4 and 10 weeks. Each chromatographic plate was developed by means of the three best mobile phases
- The stability of diclofenac sodium on silica gel was performed by applying the standard solution of this compound on two plates. One was placed under UV lamp, the other in a laboratory dryer at temperature of 40 °C. Each hour, a new portion of standard solution was spotted onto chromatographic plate, in such a way that the first portion was exposed to light and heated for 5 h. Each chromatographic plate was developed by means of the three best mobile phases
3.8.2. Linearity and Range
3.8.3. Accuracy
3.8.4. Precision
3.8.5. Limit of Detection and Limit of Quantification Based on the Calibration Curve
3.8.6. Robustness Study
- The kind of chromatographic plates (1.05554 and 1.05570);
- Mobile phase volume (±5%);
- Temperature of the activation of the plates at 120 (±5) °C;
- Development distance (±5 mm);
- Time of saturation of chromatographic chamber (±5 min).
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, V.K.; Khan, Z.Y.; Ahmad, M. The concomitant consumption of cod liver oil causes a reduction in the daily diclofenac sodium usage in rheumatoid arthritis patients: A pilot study. Int. J. Clin. Diag. Res. 2013, 7, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Todd, P.; Sorkin, E. Diclofenac sodium: A reappraisal of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs 1988, 35, 244–285. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K. Comparative study of anti-inflammatory effect of diclofenac and aceclofenac on human. JMSCR 2019, 7, 804–805. [Google Scholar] [CrossRef]
- Budukova, L.A.; Kondratèva, T.S.; Uribe-Echevarria, V.D.; Arzamastsev, L.P.; Volchenok, V.I. Composition, preparation, analysis and stability studies of orthofen [diclofenac sodium] suppositories. Farmatsiya (Moscow) 1989, 38, 16–20. [Google Scholar]
- Kaale, E.; Nyamweru, B.C.; Manyanga, V.; Chambuso, M.; Layloff, T. The development and validation of a thin-layer chromatography densitometry method for the analysis of diclofenac sodium tablets. Int. J. Chem. Anal. Sci. 2013, 4, 73–79. [Google Scholar] [CrossRef]
- Ali, N.W.; Abdelwahab, N.S.; Abdelkawy, M.M.; Elgebaly, A.M. Different spectrophotometric and TLC-densitometric methods for determination of diclofenac Na and lidocaine HCl. Pharm. Innov. J. 2014, 3, 57–64. [Google Scholar]
- Starek, M.; Krzek, J. Densitometric determination of diclofenac, 1-(2,6-dichlorophenyl)indolin-2-one and indolin-2-one in pharmaceutical preparations and model solutions. J. Pharm. Biomed. Anal. 2002, 28, 227–243. [Google Scholar]
- Patel, D.S.; Captain, A.D.; Prajapati, P.P.; Shah, H.G. Development and validation of HPTLC method for simultaneous determination of tolperisone hydrochloride and diclofenac sodium in combined dosage form. Int. J. Pharm. Tech. Res. 2013, 5, 147–154. [Google Scholar]
- Seigel, A.; Schröck, A.; Hauser, R.; Spangenberg, B. Sensitive quantification of diclofenac and ibuprofen using thin-layer chromatography coupled with a Vibrio fischeri bioluminescence assay. J. Liq. Chrom. Relat. Technol. 2011, 34, 817–828. [Google Scholar] [CrossRef]
- Shah, H.J.; Rathod, I.S.; Shah, S.A.; Savale, S.S.; Shishoo, C.J. Sensitive HPTLC method for monitoring dissolution profiles of diclofenac from different tablets containing combined diclofenac and acetaminophen. J. Planar Chromatogr. Mod. TLC 2003, 16, 36–44. [Google Scholar] [CrossRef]
- Panchal, H.J.; Rathod, I.S.; Shah, S.A. Development of validated HPTLC method for quantitation of diclofenac in diclofenac gels. Ind. Drugs 2008, 45, 301–306. [Google Scholar]
- Dighe, V.V.; Sane, R.T.; Menon, S.N.; Tambe, H.N.; Pillai, S.; Gokarn, V.N. Simultaneous determination of diclofenac sodium and paracetamol in a pharmaceutical preparation and in bulk drug powder by high-performance thin-layer chromatography. J. Planar Chromatogr. Mod. TLC 2006, 19, 443–448. [Google Scholar] [CrossRef]
- Lala, L.G.; D’Mello, P.M.; Naik, S.R. HPTLC determination of diclofenac sodium from serum. J. Pharm. Biomed. Anal. 2002, 29, 539–544. [Google Scholar] [CrossRef]
- Thongchai, W.; Liawruangrath, B.; Thongpoon, C.; Machan, T. High performance thin layer chromatographic method for the determination of diclofenac sodium in pharmaceutical formulations. Chiang Mai J. Sci. 2006, 33, 123–128. [Google Scholar]
- Bhushan, R.; Gupta, D.; Mukherjee, A. Liquid chromatographic analysis of certain commercial formulations for non-opioid analgesics. Biomed. Chromatogr. 2007, 21, 1284–1290. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Dange, P.B.; Walode, S.G. Stability indicating thin-layer chromatographic determination of chlorzoxazone, diclofenac sodium and paracetamol as bulk drug: Application to forced degradation study. Der Pharmacia Sinica 2012, 3, 643–652. [Google Scholar]
- Chaholotiya, U.K.; Patel, D.B.; Shah, D.A.; Mehta, F.A.; Bhatt, K.K. TLC method for simultaneous quantification of chlorzoxazone, paracetamol, famotidine and diclofenac potassium in their combined dosage form. Austin Chromatogr. 2017, 4, 1–6. [Google Scholar]
- Mohammad, A.; Sharma, S. Separation of co-existing paracetamol and diclofenac sodium on silica gel ’H’ layers using surfactant mediated mobile phases: Identification of diclofenac sodium from human urine. Farmacia 2009, 57, 201–211. [Google Scholar]
- Kasperek, R. Determination of diclofenac sodium and papaverine hydrovchloride in tablets by HPLC method. Acta Poloniae Pharmaceutica 2008, 65, 403–408. [Google Scholar]
- Dhaneshwar, S.R.; Bhusari, V.K. Validated HPLC method for simultaneous quantitation of diclofenac sodium and misoprostol in bulk drug and formulation. Der Chemica Sinica 2010, 1, 110–118. [Google Scholar]
- Elzayat, E.M.; Ibrahim, M.F.; Abdel-Rachman, A.A.; Ahmed, S.M.; Alanazi, F.K.; Habib, W.A. A validated stability-indicating UPLC method for determination of diclofenac sodium in its pure form and matrix formulations. Arab. J. Chem. 2017, 10, 3245–3254. [Google Scholar] [CrossRef]
- Yilmaz, B.; Ciltas, U. Determination of diclofenac in pharmaceutical preparations by voltammetry and gas chromatography methods. J. Pharm. Anal. 2015, 5, 153–160. [Google Scholar] [CrossRef]
- Aurora-Prado, M.; Steppe, M.; Tavares, M.; Kedor-Hackmann, E.; Santoro, M. Comparison between capillary electrophoresis and liquid chromatography for the determination of diclofenac sodium in a pharmaceutical tablet. J. AOAC Int. 2002, 85, 333–340. [Google Scholar]
- Damiani, P.; Bearzotti, M.; Cabezón, M.; Olivieri, A. Spectrofluorometric determination of diclofenac in tablets and ointments. J. Pharm. Biomed. Anal. 1999, 20, 587–590. [Google Scholar] [CrossRef]
- Arancibia, J.; Boldrini, M.; Escandar, G. Spectrofluorimetric determination of diclofenac in the presence of α-cyclodextrin. Talanta 2000, 52, 261–268. [Google Scholar] [CrossRef]
- Barry, A.; Mahood, M.; Hamezh, M. Spectrophotometric determination of diclofenac sodium in pharmaceutical preparations. J. Kerbala Univ. 2009, 7, 310–316. [Google Scholar]
- Matin, A.; Farajzadeh, M.; Jouyban, A. A simple spectrophotometric method for determination of sodium diclofenac in pharmaceutical formulations. Il Farmaco 2005, 60, 855–858. [Google Scholar] [CrossRef]
- Pérez-Ruiz, T.; Martínez-Lozano, C.; Sanz, A.; San Miguel, M. Flow extraction spectrophotometric method for the determination of diclofenac sodium in pharmaceutical preparations. J. Pharm. Biomed. Anal. 1997, 16, 249–254. [Google Scholar] [CrossRef]
- Lyra, W.; Sanches, F.; Cunha, F.; Diniz, P.; Lemos, S.; Cirino, E.; Araujo, M. Indirect determination of sodium diclofenac, sodium dipyrone and calcium gluconate in injection drugs using digital image-based (webcam) flame emission spectrometric method. Anal. Methods 2011, 3, 1975–1980. [Google Scholar] [CrossRef]
- Santini, A.O.; Pezza, H.R.; Pezza, L. Determination of diclofenac in pharmaceutical preparations using a potentiometric sensor immobilized in a graphite matrix. Talanta 2006, 68, 636–642. [Google Scholar] [CrossRef]
- Lenik, J. A new potentiometric electrode incorporating functionalized β-cyclodextrins for diclofenac determination. Mater. Sci. Eng. C 2014, 45, 109–116. [Google Scholar] [CrossRef]
- Afkhami, A.; Bahiraei, A.; Madrakian, T. Gold nanoparticle/multi-walled carbon nanotube modified glassy carbon electrode as a sensitive voltammetric sensor for the determination of diclofenac sodium. Mater. Sci. Eng. C. 2016, 59, 168–176. [Google Scholar] [CrossRef]
- Da Cunha, C.E.P.; Rodrigues, E.S.B.; Fernandes Alecrim, M.; Thomaz, D.V.; Macêdo, I.Y.L.; Garcia, L.F.; de Oliveira Neto, J.R.; Moreno, E.K.G.; Ballaminut, N.; de Souza Gil, E. Voltammetric evaluation of diclofenac tablets samples through carbon black-based electrodes. Pharmaceuticals 2019, 12, 83. [Google Scholar] [CrossRef]
- Bucci, R.; Magrì, A.D.; Magrì, A.L. DSC in the chemical analysis of drugs. Determination of diclofenac in pharmaceutical formulations. J. Therm. Anal. Calorim. 2000, 61, 369–376. [Google Scholar] [CrossRef]
- Tubino, M.; Souza, R. Determination of diclofenac in pharmaceutical preparations by diffuse reflectance photometry. Talanta 2006, 68, 776–800. [Google Scholar] [CrossRef]
- USP. The United States Pharmacopeia, 34th ed.; United States Pharmacopeial Convention: Rockville, MD, USA, 2011. [Google Scholar]
- Polish Pharmaceutical Society. Polish Pharmacopoeia, 11th ed.; Polish Pharmaceutical Society: Warsaw, Poland, 2017. [Google Scholar]
- TSO. European Pharmacopoeia, 6th ed.; The Stationery Office: Strasbourg, France, 2008. [Google Scholar]
- Fornstedt, T.; Forssén, P.; Westerlund, D. Basic HPLC theory and definitions: Retention, thermodynamics, selectivity, zone spreading, kinetics, and resolution. In Analytical Separation Science, 1st ed.; Anderson, J.L., Berthod, A., Pino, V., Stalcup, A.M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 1–24. [Google Scholar]
- Enmark, M.; Rova, M.; Samuelsson, J.; Örnskov, E.; Schweikart, F.; Fornstedt, T. Investigation of factors influencing the separation of diastereomers of phosphorothioated oligonucleotides. Anal. Bioanal. Chem. 2019, 411, 3383–3394. [Google Scholar] [CrossRef]
- ICH. ICH Harmonised Tripartite Guideline: Validation of Analytical Procedures: Text and Methodology, Q2(R1); ICH: Geneva, Switzerland, 2005; Available online: http://www.ich.org/products/guidelines/efficacy/article/ efficacy-guidelines.html (accessed on 28 May 2019).
- Azougagha, M.; Elkarbanea, M.; Bakhousb, K.; Issmaili, S.; Skalli, A.; Iben Moussadd, S.; Benajic, B. A novel stability-indicating UPLC method development and validation for the determination of seven impurities in various diclofenac pharmaceutical dosage forms. Ann. Pharm. Fr. 2016, 74, 358–369. [Google Scholar] [CrossRef]
- Ioele, G.; De Luca, M.; Tavano, L.; Ragno, G. The difficulties for a photolabile drug in topical formulations: The case of diclofenac. Int. J. Pharmaceut. 2014, 465, 284–290. [Google Scholar] [CrossRef]
- Rubim, A.M.; Rubenick, J.B.; Laporta, L.V.; Bueno Rolim, C.M. A simple method for the quantification of diclofenac potassium in oral suspension by high-performance liquid chromatography with UV-detection. Braz. J. Pharm. Sci. 2013, 49, 589–597. [Google Scholar] [CrossRef]
- Alquadeib, B.T. Development and validation of a new HPLC analytical method for the determination of diclofenac in tablets. Saudi Pharm. J. 2019, 27, 66–70. [Google Scholar] [CrossRef]
- Ferenczi-Fodor, K.; Renger, B.; Végh, Z. The frustrated reviewer—Recurrent failures in manuscripts describing validation of quantitative TLC/HPTLC procedures for analysis of pharmaceuticals. J. Planar Chromatogr. Mod. TLC 2010, 23, 173–179. [Google Scholar] [CrossRef]
- EMA Committee for Medicinal Products for Human Use (CHMP). Guideline on Bioanalytical Method Validation; European Medicines Agency (EMA): London, UK, 2011; pp. 1–23. [Google Scholar]
- Lindholm, J.; Johansson, M.; Fornstedt, T. Guidelines for analytical method development and validation of biotechnological synthesis of drugs. Production of a hydroxyprogesterone as model. J. Chromatogr. B 2003, 791, 323–336. [Google Scholar] [CrossRef]
- USP. United States Pharmacopeia, 23th ed.; United States Pharmacopeial Convention: North Bethesda, MD, USA, 1995. [Google Scholar]
- Konieczka, P. Namieśnik, Assessment and Quality Control of Analytical Results (Polish); Scientific Publisher (PWN): Warsaw, Poland, 2017; ISBN 978-83-0119-444-4. Available online: https://www.poczytaj.pl/ksiazka/ocena-i-kontrola-jakosci-wynikow-pomiarow-analitycznych (accessed on 15 December 2019).
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; McNaught, A.D., Wilkinson, A., Eds.; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar]
- Christophe, A.B. Valey to peak ratio as a measure for the separation of two chromatographic peaks. Chromatographia 1971, 4, 455–458. [Google Scholar] [CrossRef]
| Method Characteristic | Results | |
|---|---|---|
| Selectivity | Selective | |
| Range (μg/spot) | 4.00–18.00 | |
| Linearity | n = 8; r = 0.994; s = 160.6; F = 491.1; p < 0.0001 | |
| Limit of detection (LOD; μg/spot) Limit of quantification (LOQ; μg/spot) | 0.28 0.84 | |
| For tablets Accuracy and precision | ||
| Accuracy (n = 6) | ||
| for 50% standard added (n = 6) for 100% standard added (n = 6) for 150% standard added (n = 6) | R = 100.4%; CV = 2.48% R = 100.0%; CV = 2.81% R = 99.4%; CV = 2.81% | |
| Quantity of Precision (CV, (%)) n = 3 | ||
| Interday | 6.00 μg/spot | 0.72 |
| 10.00 μg/spot | 1.54 | |
| 16.00 μg/spot | 2.15 | |
| Intraday | 6.00 μg/spot | 1.26 |
| 10.00 μg/spot | 1.57 | |
| 16.00 μg/spot | 2.69 | |
| Robustness | Robust | |
| Parameter | % RSD of Peak Area |
|---|---|
| The kind of chromatographic plates | 0.88 |
| Mobile phase volume (±5%) | 0.78 |
| Temperature of the activation of the plates at 120 (±5) °C | 0.82 |
| Development distance (±5 mm) | 1.35 |
| Time of saturation of chromatographic chamber (±5 min) | 0.89 |
| Number of Analysis | Diclofenac Sodium Determined by NP-TLC with Densitometry |
|---|---|
| 1 | 50.1 |
| 2 | 48.6 |
| 3 | 49.7 |
| 4 | 47.3 |
| 5 | 47.8 |
| 6 | 51.9 |
| 7 | 50.5 |
| 8 | 50.3 |
| 9 | 48.9 |
| 10 | 49.2 |
| Average amount (mg/tablet) | 49.4 |
| The label claim (mg/tablet) | 50 |
| Standard deviation (SD) | 1.36 |
| Coefficient of variation (CV%) | 2.75 |
| Confidence interval of arithmetic mean with confidence level equal 95% | μ = 49.4 ± 1.0 |
| Amount of diclofenac sodium (%) in relations to the label claim | 98.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parys, W.; Pyka-Pająk, A.; Dołowy, M. Application of Thin-Layer Chromatography in Combination with Densitometry for the Determination of Diclofenac in Enteric Coated Tablets. Pharmaceuticals 2019, 12, 183. https://doi.org/10.3390/ph12040183
Parys W, Pyka-Pająk A, Dołowy M. Application of Thin-Layer Chromatography in Combination with Densitometry for the Determination of Diclofenac in Enteric Coated Tablets. Pharmaceuticals. 2019; 12(4):183. https://doi.org/10.3390/ph12040183
Chicago/Turabian StyleParys, Wioletta, Alina Pyka-Pająk, and Małgorzata Dołowy. 2019. "Application of Thin-Layer Chromatography in Combination with Densitometry for the Determination of Diclofenac in Enteric Coated Tablets" Pharmaceuticals 12, no. 4: 183. https://doi.org/10.3390/ph12040183
APA StyleParys, W., Pyka-Pająk, A., & Dołowy, M. (2019). Application of Thin-Layer Chromatography in Combination with Densitometry for the Determination of Diclofenac in Enteric Coated Tablets. Pharmaceuticals, 12(4), 183. https://doi.org/10.3390/ph12040183