[68Ga]Ga-DOTA-TOC: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging
Abstract
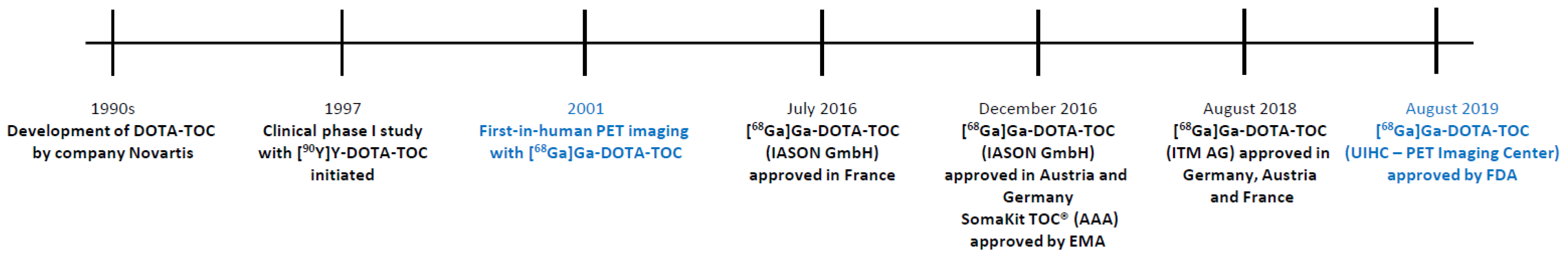
1. Introduction
2. Chemical Overview
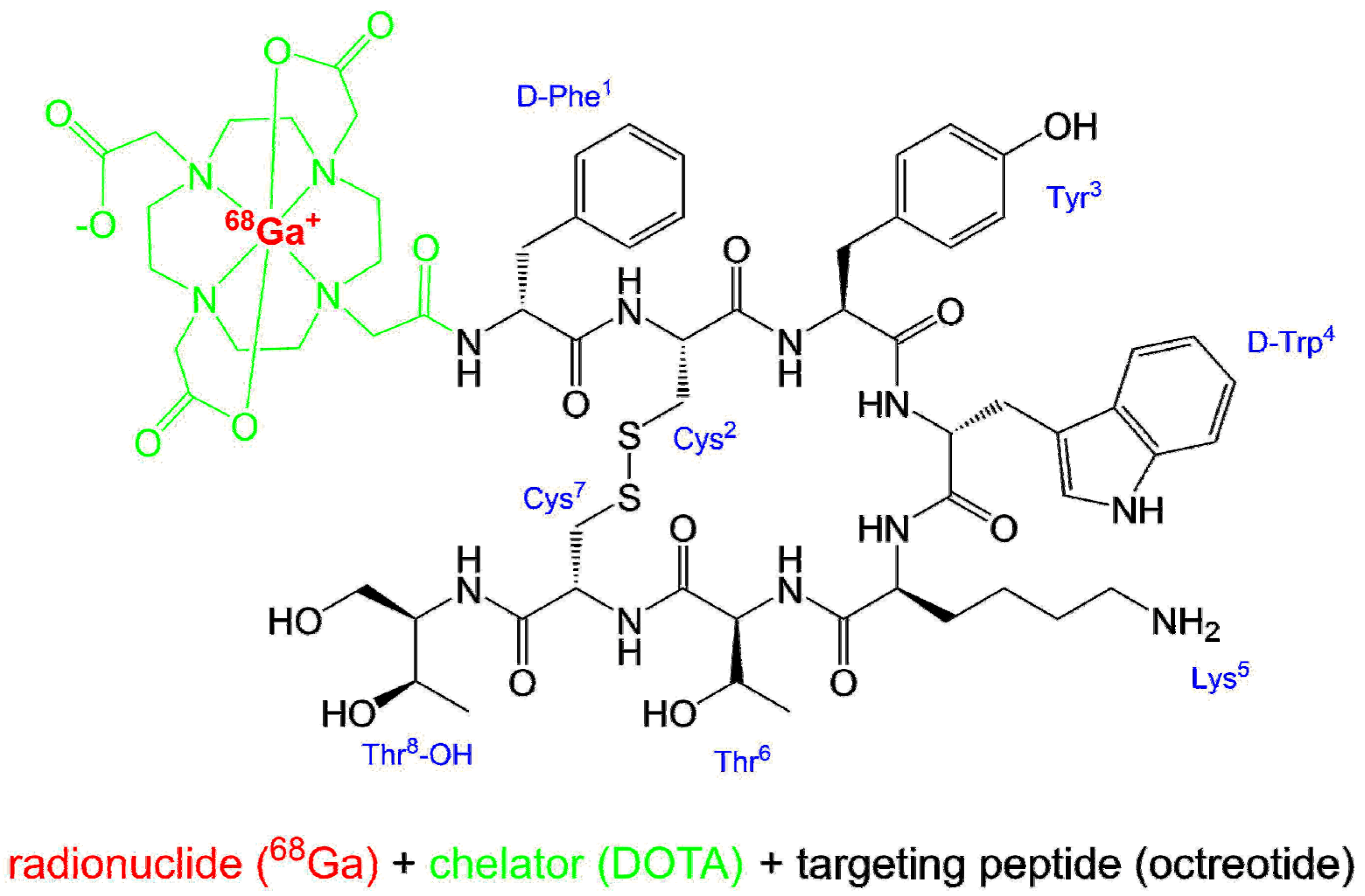
2.1. Names and Structure
[68Ga]gallium-N-[(4,7,10-tricarboxymethyl-1,4,7,10-tetraazacyclododec-1-yl)acetyl]-D-phenylalanyl-L-cysteinyl-L-tyrosyl-D-tryptophyl-L-lysyl-L-threonyl-N-[(1R,2R)-2-hydroxy-1-(hydroxymethyl)propyl]-L-cysteinamide cyclic(2-7)disulfide.
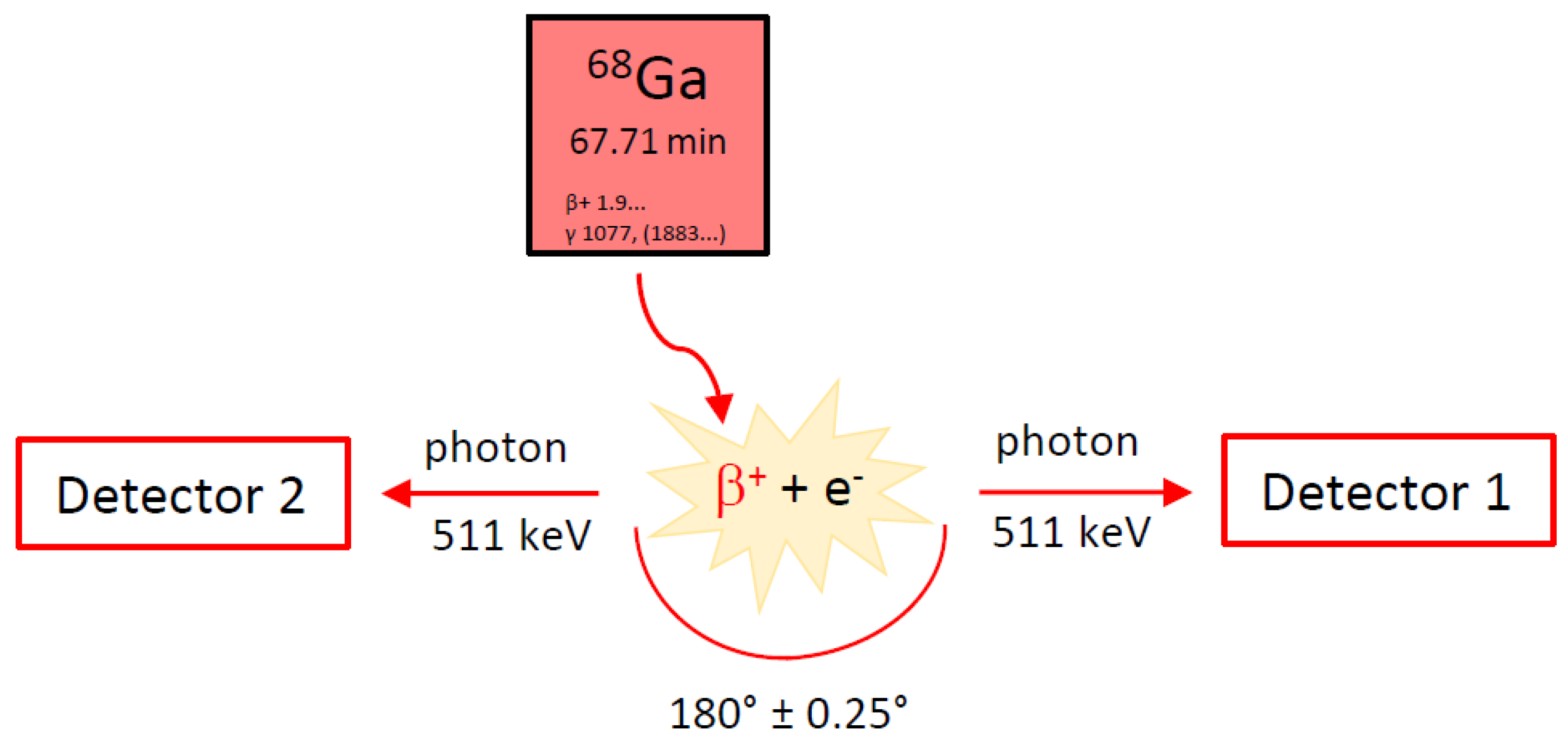
2.2. Gallium-68
2.3. Manufacturing and Quality Criteria
3. Medicinal and Pharmaceutical Overview
3.1. Clinical Indication
3.2. Application
3.3. Pharmacology and Pharmacokinetics
4. Perspective
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FDA Letter of Approval for [68Ga]Ga-DOTA-TOC. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2019/210828Orig1s000ltr.pdf (accessed on 19 September 2019).
- Authorization Details of IasoTOC®. Available online: http://www.iason.eu/produkte/iasotoc.html#page-top (accessed on 5 November 2019).
- Authorization of TOCscan®. Available online: https://isotope-technologies-munich.com/news/press-releases/press-releases-detail?tx_news_pi1%5Baction%5D=detail&tx_news_pi1%5Bcontroller%5D=News &tx_news_pi1%5Bnews%5D=62&cHash=a33a4ba0e17471259b3884fbadf88416 (accessed on 16 December 2019).
- Authorization Details for SomaKit TOC® in Europe. Available online: https://www.ema.europa.eu/ en/medicines/human/EPAR/somakit-toc#authorisation-details-section (accessed on 11 October 2019).
- FDA Letter of Approval for NETSPOTTM. Available online: https://www.accessdata.fda.gov/ drugsatfda_docs/appletter/2016/208547Orig1s000ltr.pdf (accessed on 28 October 2019).
- Gambhir, S.S. Molecular Imaging of Cancer with Positron Emission Tomography. Nat. Rev. Cancer 2002, 2, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Notni, J.; Wester, H.-J. Re-thinking the role of radiometal isotopes: Towards a future concept for theranostic radiopharmaceuticals. J. Label. Compd. Radiopharm. 2018, 61, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.H. An introduction to the clinical practice of theranostics in oncology. Br. J. Radiol. 2018, 91, 20180440. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, U.; Kopka, K. Lutathera®: The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals 2019, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Authorization Details for Lutathera® in Europe. Available online: https://www.ema.europa.eu/en/ medicines/human/EPAR/lutathera#authorisation-details-section (accessed on 31 October 2019).
- FDA Letter of Approval for LUTATHERA®. Available online: https://www.accessdata.fda.gov/ drugsatfda_docs/appletter/2018/208700Orig1s000ltr.pdf (accessed on 31 October 2019).
- Reubi, J.C.; Schär, J.-C.; Waser, B.; Wenger, S.; Heppeler, A.; Schmitt, J.S.; Mäcke, H.R. Affinity profiles for human somatostatin receptor subtypes SST1–SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use, Eur. J. Nucl. Med. 2000, 27, 273–282. [Google Scholar] [CrossRef]
- Poeppel, T.D.; Binse, I.; Petersenn, S.; Lahner, H.; Schott, M.; Antoch, G.; Brandau, W.; Bockisch, A.; Boy, C. 68Ga-DOTATOC Versus 68Ga-DOTATATE PET/CT in Functional Imaging of Neuroendocrine Tumors. J. Nucl. Med. 2011, 52, 1864–1870. [Google Scholar] [CrossRef]
- Product Monograph LUTATHERA®. Available online: https://www.samnordic.se/wp-content/uploads /2018/05/LUTATHERA-MONOGRAPH-120218.pdf (accessed on 18 November 2019).
- Xu, C.; Zhang, H. Somatostatin Receptor Based Imaging and Radionuclide Therapy. BioMed. Res. Int. 2015, 1–14. [Google Scholar] [CrossRef]
- Jacobs, S.; Schulz, S. Intracellular trafficking of somatostatin receptors. Mol. Cell Endocrinol. 2008, 286, 58–62. [Google Scholar] [CrossRef]
- Dash, A.; Chakraborty, S.; Pillai, M.R.A.; Knapp, F.F., Jr. Peptide Receptor Radionuclide Therapy: An Overview. Cancer Biother. Radiopharm. 2015, 30, 47–71. [Google Scholar] [CrossRef]
- Brazeau, P.; Vale, W.; Burgus, R.; Ling, N.; Butcher, M.; Rivier, J.; Guillemin, R. Hypothalamic Polypeptide That Inhibits the Secretion of Immunoreactive Pituitary Growth Hormone. Science 1973, 179, 77–79. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; Kam, B.L.; van Essen, M.; Teunissen, J.J.M.; van Eijck, C.H.J.; Valkema, R.; de Jong, M.; de Herder, W.W.; Krenning, E.P. Somatostatin receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr.-Relat. Cancer 2010, 17, R53–R73. [Google Scholar] [CrossRef] [PubMed]
- Krenning, E.P.; Bakker, W.H.; Breeman, W.A.; Koper, J.W.; Kooij, P.P.; Ausema, L.; Lameris, J.S.; Reubi, J.C.; Lamberts, S.W. Localization of endocrine related tumors with radioiodinated analogue of somatostatin. Lancet 1989, 1, 242–245. [Google Scholar] [CrossRef]
- Pepe, G.; Moncayo, R.; Bombardieri, E.; Chiti, A. Somatostatin receptor SPECT. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, S41–S51. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Maecke, H. Peptide-Based Probes for Cancer Imaging. J. Nucl. Med. 2008, 49, 1735–1738. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C. Peptide Receptors as Molecular Targets for Cancer Diagnosis and Therapy. Endocr. Rev. 2003, 24, 389–427. [Google Scholar] [CrossRef]
- Johnbeck, C.B.; Knigge, U.; Kjaer, A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: Current status and review of the literature. Future Oncol. 2014, 10, 2259–2277. [Google Scholar] [CrossRef]
- Otte, A.; Jermann, E.; Béhé, M.; Goetze, M.; Bucher, H.C.; Roser, H.W.; Heppeler, A.; Mueller-Brand, J.; Mäcke, H.R. DOTATOC: A powerful new tool for receptor-mediated radionuclide therapy. Eur. J. Nucl. Med. 1997, 24, 792–795. [Google Scholar] [CrossRef][Green Version]
- Otte, A.; Mueller-Brand, J.; Dellas, S.; Nitzsche, E.U.; Herrmann, R.; Mäcke, H.R. Yttrium-90-labelled somatostatinanalogue for cancer treatment. Lancet 1998, 351, 417–418. [Google Scholar] [CrossRef]
- Heppeler, A.; Froidevaux, S.; Mäcke, H.R.; Jermann, E.; Béhé, M.; Powell, P.; Hennig, M. Radiometal-Labelled Macrocyclic Chelator-Derivatised Somatostatin Analogue with Superb Tumour-Targeting Properties and Potential for Receptor-Mediated Internal Radiotherapy. Chem. Eur. J. 1999, 5, 1974–1981. [Google Scholar] [CrossRef]
- Hofmann, M.; Maecke, H.; Börner, A.R.; Weckesser, E.; Schöffski, P.; Oei, M.L.; Schumacher, J.; Henze, M.; Heppeler, A.; Meyer, G.J.; et al. Biokinetics and imaging with the somatostatin receptor PET radioligand 68Ga-DOTATOC: Prelimary data. Eur. J. Nucl. Med. 2001, 28, 1751–1757. [Google Scholar] [CrossRef]
- Graham, M.M.; Gu, X.; Ginader, T.; Breheny, P.; Sunderland, J.J. 68Ga-DOTATOC Imaging of Neuroendocrine Tumors: A Systematic Review and Metaanalysis. J. Nucl. Med. 2017, 58, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; van der Meulen, N.P.; Müller, C.; Klette, I.; Kulkarni, H.R.; Türler, A.; Schibli, R.; Baum, R.P. First-in-Human PET/CT Imaging of Metastatic Neuroendocrine Neoplasms with Cyclotron-Produced 44Sc-DOTATOC: A Proof-of-Concept Study. Cancer Biother Radiopharm 2017, 32, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Singh, A.; Benešová, M.; Vermeulen, C.; Gnesin, S.; Koster, U.; Johnston, K.; Müller, D.; Senftleben, S.; Kulkarni, H.R.; et al. Clinical evaluation of the radiolanthanide terbium-152: First-in-human PET/CT with 152Tb-DOTATOC. Dalton Trans. 2017, 46, 14638–14646. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: A first-in-human experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. Ac-225-DOTATOC-An empiric dose finding for alpha particle emitter based radionuclide therapy of neuroendocrine tumors. J. Nucl. Med. 2015, 56, 1232. [Google Scholar]
- Navalkissoor, S.; Grossman, A. Targeted Alpha Particle Therapy for Neuroendocrine Tumours: The Next Generation of Peptide Receptor Radionuclide Therapy. Neuroendocrinology 2019, 108, 256–264. [Google Scholar] [CrossRef]
- Wadas, T.J.; Pandya, D.N.; Solingapuram Sai, K.K.; Mintz, A. Molecular Targeted α-Particle Therapy for Oncologic Applications. AJR Am. J. Roentgenol. 2014, 203, 253–260. [Google Scholar] [CrossRef]
- Stolz, B.; Weckbecker, G.; Smith-Jones, P.M.; Albert, R.; Raulf, F.; Bruns, C. The somatostatin receptor-targeted radiotherapeutic [90Y-DOTA-DPhe1,Tyr3]octreotide (90Y-SMT 487) eradicates experimental rat pancreatic CA 20948 tumours. Eur. J. Nucl. Med. 1998, 25, 668–674. [Google Scholar] [CrossRef]
- Kubicek, V.; Havlickova, J.; Kotek, J.; Tircso, G.; Hermann, P.; Toth, E.; Lukes, I. Gallium(III) Complexes of DOTA and DOTA−Monoamide: Kinetic and Thermodynamic Studies. Inorg. Chem. 2010, 49, 10960–10969. [Google Scholar] [CrossRef]
- Nomenclature of Inorganic Chemistry, IUPAC Recommendations 2005, IR-9 Coordination Compounds. Available online: https://iupac.org/wp-content/uploads/2016/07/Red_Book_2005.pdf (accessed on 19 September 2019).
- Decay Characteristics of 68Ga. Available online: https://www.nndc.bnl.gov/nudat2/dec_searchi.jsp (accessed on 19 September 2019).
- Sanchez-Crespo, A. Comparison of Gallium-68 and Fluorine-18 imaging characteristics in positron emission tomography. Appl. Rad. Isot. 2013, 76, 55–62. [Google Scholar] [CrossRef]
- Brandt, M.; Cardinale, J.; Aulsebrook, M.L.; Gasser, G.; Mindt, T.L. An Overview of PET Radiochemistry, Part 2: Radiometals. J. Nucl. Med. 2018, 59, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Gallium-68 Cyclotron Production. IAEA-TECDOC-1863. Available online: https://www.researchgate.net/publication/331035585_Gallium-68_Cyclotron_Production (accessed on 25 February 2020).
- Protocol: A Phase II Theranostics Trial: Dosimetry-Guided Peptide Receptor Radiotherapy (PRRT) with 90Y-DOTA-tyr3-Octreotide (90Y-DOTATOC) in Children and Adults with Neuroendocrine and Other Somatostatin Receptor Expressing Tumors as Determined by 68Ga-DOTA-tyr3-Octreotide (68Ga-DOTATOC) PET/CT. Available online: https://clinicaltrials.gov/ProvidedDocs/88/NCT02441088/ Prot_SAP_000.pdf (accessed on 1 October 2019).
- Monograph “Gallium (68Ga) Edotreotide Injection” (Monograph Number 2485), European Pharmacopeia 9th Edition 2019. Available online: online6.edqm.eu/ep908 (accessed on 2 March 2020).
- Product Quality Review of [68Ga]Ga-DOTA-TOC. Available online: https://www.accessdata.fda.gov/ drugsatfda_docs/nda/2019/210828Orig1s000ChemR.pdf (accessed on 1 October 2019).
- Label for “Ga 68 DOTATOC Injection”. Available online: https://www.accessdata.fda.gov/ drugsatfda_docs/label/2019/210828s000lbl.pdf (accessed on 1 October 2019).
- Product Monograph for SomaKit TOC®. Available online: https://www.ema.europa.eu/en/documents/ product-information/somakit-toc-epar-product-information_en.pdf (accessed on 11 November 2019).
- Fani, M.; Peitl, P.K.; Velikyan, I. Current Status of Radiopharmaceuticals for the Theranostics of Neuroendocrine Neoplasms. Pharmaceuticals 2017, 10, 30. [Google Scholar] [CrossRef]
- Sandström, M.; Velikyan, I.; Garske-Román, U.; Sörensen, J.; Eriksson, B.; Granberg, D.; Lundqvist, H.; Sundin, A.; Lubberink, M. Comparative Biodistribution and Radiation Dosimetry of 68Ga-DOTATOC and 68Ga-DOTATATE in Patients with Neuroendocrine Tumors, J. Nucl. Med. 2013, 54, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Schäfer, M.; Bauder-Wüst, U.; Hull, W.-E.; Wängler, C.; Mier, W.; Haberkorn, U.; Eisenhut, M. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug. Chem. 2012, 23, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Haberkorn, U.; Eder, M.; Eisenhut, M.; Zechmann, C.M. [68Ga]gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: Comparison with 18F-FECH. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Neels, O.; Müller, M.; Bauder-Wüst, U.; Remde, Y.; Schäfer, M.; Hennrich, U.; Eisenhut, M.; Afshar-Oromieh, A.; Haberkorn, U.; et al. Novel Preclinical and Radiopharmaceutical Aspects of [68Ga]Ga-PSMA-HBED-CC: A New PET Tracer for Imaging of Prostate Cancer. Pharmaceuticals 2014, 7, 779–796. [Google Scholar] [CrossRef]
- Waldmann, C.M.; Stuparu, A.D.; van Dam, R.M.; Slavik, R. The Search for an Alternative to [68Ga]Ga-DOTA-TATE in Neuroendocrine Tumor Theranostics: Current State of 18F-labeled Somatostatin Analog Development. Theranostics 2019, 9, 1336–1347. [Google Scholar] [CrossRef]
- Dubash, S.R.; Barwick, T.; Mauri, F.A.; Kozlowski, K.; Frilling, A.; Valle, J.W.; Lamarca, A.; Sharma, R.; Aboagye, E. [18F]FET-βAG-TOCA versus [68Ga]DOTATATE PET/CT in functional imaging of neuroendocrine tumours. J. Clin. Oncol. 2018, 36, e24193. [Google Scholar] [CrossRef]
- Laverman, P.; D’Souza, C.A.; Eek, A.; McBride, W.J.; Sharkey, R.M.; Oyen, W.J.G.; Goldenberg, D.M.; Boerman, O.C. Optimized labeling of NOTA-conjugated octreotide with F-18. Tumor Biol. 2012, 33, 427–434. [Google Scholar] [CrossRef]
- Long, T.; Yang, N.; Zhou, M.; Chen, D.; Li, Y.; Li, J.; Tang, Y.; Liu, Z.; Li, Z.; Hu, S. Clinical Application of 18F-AlF-NOTA-Octreotide PET/CT in Combination With 18F-FDG PET/CT for Imaging Neuroendocrine Neoplasms. Clin. Nucl. Med. 2019, 44, 452–458. [Google Scholar] [CrossRef]
- Pauwels, E.; Cleeren, F.; Tshibangu, T.; Koole, M.; Serdons, K.; Dekerve, J.; Van Cutsem, E.; Verslype, C.; Van Laere, K.; Bormans, G.; et al. Al18F-NOTA-octreotide: First comparison with 68Ga-DOTATATE in a neuroendocrine tumour patient. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2398–2399. [Google Scholar] [CrossRef] [PubMed]
- Tshibangu, T.; Cawthorne, C.; Serdons, K.; Pauwels, E.; Gsell, W.; Bormans, G.; Deroose, C.M.; Cleeren, F. Automated GMP compliant production of [18F]AlF-NOTA-octreotide. EJNMMI Radiopharm. Chem. 2020, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, J.; Schäfer, M.; Benešová, M.; Bauder-Wüst, U.; Leotta, K.; Eder, M.; Neels, O.C.; Haberkorn, U.; Giesel, F.L.; Kopka, K. Preclinical evaluation of 18FPSMA-1007: A new PSMA ligand for prostate cancer imaging. J. Nucl. Med. 2017, 58, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Hadaschik, B.; Cardinale, J.; Radtke, J.; Vinsensia, M.; Lehnert, W.; Kesch, C.; Tolstov, Y.; Singer, S.; Grabe, N.; et al. F-18 labelled PSMA-1007: Biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 678–688. [Google Scholar] [CrossRef]
- Cardinale, J.; Martin, R.; Remde, Y.; Schäfer, M.; Hienzsch, A.; Hübner, S.; Zerges, A.-M.; Marx, H.; Hesse, R.; Weber, K.; et al. Procedures for the GMP-Compliant Production and Quality Control of [18F]PSMA-1007: A Next Generation Radiofluorinated Tracer for the Detection of Prostate Cancer. Pharmaceuticals 2017, 10, 77. [Google Scholar] [CrossRef]
| Organ | Absorbed Dose/mGy/MBq (n = 9) | |
|---|---|---|
| Mean | SD | |
| Urinary bladder wall | 0.119 | 0.058 |
| Spleen | 0.108 | 0.065 |
| Kidney | 0.082 | 0.020 |
| Adrenal gland | 0.077 | 0.028 |
| Liver | 0.041 | 0.014 |
| Red Marrow | 0.016 | 0.003 |
| Gallbladder wall | 0.015 | 0.001 |
| Lungs | 0.007 | 0.001 |
| Total Body | 0.014 | 0.002 |
| Effective Dose/mSv/MBq | 0.021 | 0.003 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hennrich, U.; Benešová, M. [68Ga]Ga-DOTA-TOC: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging. Pharmaceuticals 2020, 13, 38. https://doi.org/10.3390/ph13030038
Hennrich U, Benešová M. [68Ga]Ga-DOTA-TOC: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging. Pharmaceuticals. 2020; 13(3):38. https://doi.org/10.3390/ph13030038
Chicago/Turabian StyleHennrich, Ute, and Martina Benešová. 2020. "[68Ga]Ga-DOTA-TOC: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging" Pharmaceuticals 13, no. 3: 38. https://doi.org/10.3390/ph13030038
APA StyleHennrich, U., & Benešová, M. (2020). [68Ga]Ga-DOTA-TOC: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging. Pharmaceuticals, 13(3), 38. https://doi.org/10.3390/ph13030038






