Flaxseed Lignans as Important Dietary Polyphenols for Cancer Prevention and Treatment: Chemistry, Pharmacokinetics, and Molecular Targets
Abstract
1. Introduction
2. Growing Use of Naturally Derived Products
3. Cancer, the Unmet Medical Need
4. Cancer Prevention
5. Alternate Approaches to Malignant Disease
6. Potential of Dietary Phytochemicals for Malignant Disease
6.1. Dietary Polyphenols as Principal Phytochemicals for Malignant Disease
6.2. General Properties of Polyphenols and Evidence on Health
6.2.1. General Pharmacodynamic (or Nutridynamic) Effects of Polyphenols
6.2.2. General Pharmacokinetic (Or Nutrikinetic) Characteristics of Polyphenols
7. Challenges Associated with Cancer Prevention and Dietary Polyphenols
8. Polyphenols of Flaxseed as Important Phytochemicals in Malignant Disease
8.1. Lignans of Flaxseed
8.2. Chemistry and Pharmacokinetics (or Nutrikinetics) of Lignans
8.3. Lignans as Therapeutic Agents for Cancer
8.4. Linking Benefits of Flaxseed with Cancer Associated Chronic Diseases
8.5. Purposing Lignans into Established Models of Cancer Characteristics
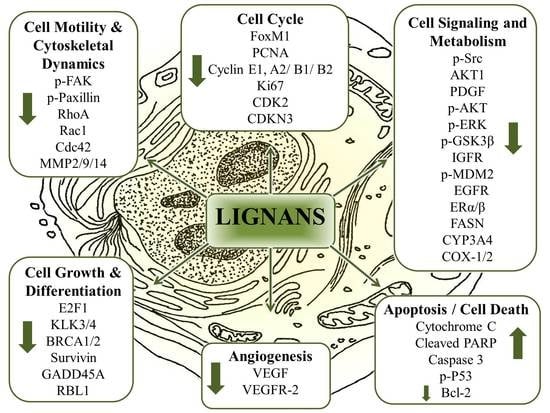
8.6. The Multitarget Effects of Lignans in Cancer (Nutridynamics)
8.6.1. Antioxidant and Anti-Inflammatory Properties
8.6.2. Anticarcinogenic and Antimutagenic Properties
8.6.3. Anti-proliferative properties
8.6.4. Dysregulated cellular metabolism
8.6.5. Antiangiogenic Properties
8.6.6. Anti-invasive and Antimigratory Properties
8.6.7. Induction of Apoptosis and Cell Death
9. Final Remarks
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Apc-Min | Mouse tumor model (intestinal and mammary) |
| AKT1 | AKT (RAC-alpha) serine/threonine kinase 1 |
| ASECO | Anhydro-secoisolariciresinol |
| Bax | Bcl-2-associated X protein |
| BC | Breast cancer |
| Biphasic | ↑lower/↓higher concentration |
| CC | Cervical Cancer |
| TR | Transfected |
| COC | Colon cancer |
| ALP | alkaline phosphatase |
| miR | micro RNA |
| ERK | Extracellular signal–regulated kinases |
| subG1 | DNA profile representing cells in the G1 stage of the cell cycle |
| F-actin | Filamentous actin |
| BIRC5 | Survivin |
| BRCA1 | breast cancer type 1 (BCT1) susceptibility protein |
| BRCA2 | BCT2 susceptibility protein |
| CCNB1 | Cyclin B1 |
| CCNB2 | Cyclin B2 |
| CCND2 | Cyclin D2 |
| CCNF | Cyclin F |
| CCNG1 | Cyclin G1 |
| CCNH | Cyclin H |
| CDC2 | Cell division cycle 2 |
| CDC20 | Cell division cycle 20 |
| CDK2 | Cyclin dependent kinase 2 |
| CDK4 | Cyclin dependent kinase 4 |
| CDK5R1 | CDK5 regulatory subunit 1 |
| CDKN1B | Cyclin dependent kinase inhibitor 1B/p27/Kip1 |
| CDKN3 | Cyclin dependent inhibitor 3/Cip2 |
| CDT1 | DNA replication factor Cdt1 |
| CHEK1 | CHK1 checkpoint homolog |
| CKS1B | CDC28 protein kinase regulatory subunit 1B |
| CKS2 | CDC28 protein kinase regulatory subunit 2 |
| COX1/2 | Cyclooxygenase 1 & 2 |
| CRP | C-reactive protein |
| CYP | Cytochrome P450 |
| DDX11 | DEAD/H box polypeptide 11 |
| DNMT | DNA methyl transferases E2F1, Retinoblastoma protein transcription factor |
| F4/80 (EMR1) | EGF-like module-containing mucin-like hormone receptor-like 1 |
| GADD45A | Growth arrest and DNA-damage-inducible, alpha |
| GMNN | Geminin |
| GPER | G protein-coupled estrogen receptor 1 |
| HDACs | Histone deacetylases |
| HPV | Human papillomavirus |
| ITGA2 | Integrin subunit alpha 2 |
| KLK3 | Prostate-specific antigen (PSA)/kallikrein-3 |
| KLK4 | Kallikrein 4 |
| KNTC1 | Kinetochore associated 1 |
| KPNA2 | Karyopherin alpha 2 |
| LIV-1 | Zinc transporter SLC39A6 |
| MAD2L1 | MAD2 mitotic arrest deficient-like 1 |
| MBD2 | Methyl-CpG-Binding domain protein |
| MCM2 | Mini-chromosome maintenance 2/mitotin |
| MCM2/7 | Mini-chromosome maintenance complex component 2/7 |
| MCM3 | Mini-chromosome maintenance 3 |
| MCM4 | Mini-chromosome maintenance 4 |
| MCM5 | Mini-chromosome maintenance 5 |
| MCM2/7 | Mini-chromosome maintenance complex component 2/7 |
| MKI67 | Antigen identified by mAb Ki-67 |
| MBD2 | Methyl-CpG-Binding domain protein |
| MO | Mouse orthotopic |
| MRE11A | Meiotic recombination 11 homolog A |
| 16/2-OHE1 | 16/2-hydroxyestrone |
| OVX | Ovariectomized rat |
| p21 (p21WAF1/Cip1) | Cyclin-dependent kinase inhibitor 1 (CDK-interacting protein 1) |
| P53 | Tumor protein p53 (aka “guardian of the genome”) |
| p65 | Transcription factor p65 (nuclear factor NF-kappa-B p65 subunit) |
| p70S6K1 | Ribosomal protein S6 kinase beta-1 (S6K1)/p70S6 kinase 1 |
| PARP | poly-ADP ribose polymerase |
| PC | Prostate cancer |
| PCNA | Proliferating cell nuclear antigen |
| PDGF | Platelet-derived growth factor |
| PH | Prostatic hyperplasia |
| PIAS1 | E3 SUMO-protein ligase PIAS1 |
| PRKCD | Protein kinase C delta type |
| PRKCH | Protein kinase C eta type |
| Prostaglandin E2 | Dinoprostone |
| PS | Prostate Stromal |
| pS2 (TFF1) | Trefoil factor family 1 |
| RASSF1 | Ras association domain-containing protein 1 |
| RBL1 | Retinoblastoma-like 1/p107 |
| RPA3 | Replication protein A3 |
| SKP2 | S-phase kinase-associated protein |
| SLC43A1 | Large neutral amino acid transporter small subunit 3 |
| TPM1 | Tropomyosin alpha-1 |
| VEGFR | Vascular endothelial growth factor receptor |
| XM | Xenograft model |
| YAMC | Young adult mouse colon |
| ZIP2 | Zinc transporter SLC39A2 |
| ZnT-1 | Zinc transporter protein 1 |
References
- Saarinen, N.M.; Tuominen, J.; Pylkkänen, L.; Santti, R. Assessment of Information to Substantiate a Health Claim on the Prevention of Prostate Cancer by Lignans. Nutrients 2010, 2, 99–115. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Lee, J.; Khor, T.; Shu, L.; Su, Z.; Fuentes, F.; Kong, A. Dietary phytochemicals and cancer prevention: Nrf2 signaling, epigenetics, and cell death mechanisms in blocking cancer initiation and progression. Pharmacol. Ther. 2013, 137, 53–171. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef]
- DeLuca, J.A.A.; Garcia-Villatoro, E.L.; Allred, C.D. Flaxseed Bioactive Compounds and Colorectal Cancer Prevention. Curr. Oncol. Rep. 2018, 20, 59. [Google Scholar] [CrossRef] [PubMed]
- Kezimana, P.; Dmitriev, A.A.; Kudryavtseva, A.V.; Romanova, E.V.; Melnikova, N.V. Secoisolariciresinol Diglucoside of Flaxseed and Its Metabolites: Biosynthesis and Potential for Nutraceuticals. Front. Genet. 2018, 9, 641. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K. Flaxseed and cardiovascular health. J. Cardiovasc. Pharmacol. 2009, 54, 369–377. [Google Scholar] [CrossRef]
- Calado, A.; Neves, P.M.; Santos, T.; Ravasco, P. The Effect of Flaxseed in Breast Cancer: A Literature Review. Front. Nutr. 2018, 5, 4. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Liu, Y.; Tian, H.; Flickinger, B.; Empie, M.W.; Sun, S.Z. Dietary flaxseed lignan extract lowers plasma cholesterol and glucose concentrations in hypercholesterolaemic subjects. Br. J. Nutr. 2008, 99, 1301–1309. [Google Scholar] [CrossRef]
- Zanwar, A.A.; Hegde, M.V.; Rojatkar, S.R.; Sonawane, K.B.; Rajamohanan, P.R.; Bodhankar, S.L. Isolation, characterization and antihyperlipidemic activity of secoisolariciresinol diglucoside in poloxamer-407-induced experimental hyperlipidemia. Pharm. Biol. 2014, 52, 1094–1103. [Google Scholar] [CrossRef]
- Thompson, L.U.; Rickard, S.E.; Orcheson, L.J.; Seidl, M.M. Flaxseed and its lignan and oil components reduce mammary tumor growth at a late stage of carcinogenesis. Carcinogenesis 1996, 17, 1373–1376. [Google Scholar] [CrossRef]
- Pilar, B.; Gullich, A.; Oliveira, P.; Stroher, D.; Piccoli, J.; Manfredini, V. Protective Role of Flaxseed Oil and Flaxseed Lignan Secoisolariciresinol Diglucoside Against Oxidative Stress in Rats with Metabolic Syndrome. J. Food Sci. 2017, 82, 3029–3036. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Thompson, L.U. The inhibitory effect of flaxseed on the growth and metastasis of estrogen receptor negative human breast cancer xenograftsis attributed to both its lignan and oil components. Int. J. Cancer 2005, 116, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Power, K.A.; Thompson, L.U. Can the combination of flaxseed and its lignans with soy and its isoflavones reduce the growth stimulatory effect of soy and its isoflavones on established breast cancer? Mol. Nutr. Food Res. 2007, 51, 845–856. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [CrossRef]
- Kajla, P.; Sharma, A.; Sood, D.R. Flaxseed-a potential functional food source. J. Food Sci. Technol. 2015, 52, 1857–1871. [Google Scholar] [CrossRef]
- Rodriguez-Leyva, D.; Dupasquier, C.M.C.; McCullough, R.; Pierce, G.N. The cardiovascular effects of flaxseed and its omega-3 fatty acid, alpha-linolenic acid. Can. J. Cardiol. 2010, 26, 489–496. [Google Scholar] [CrossRef]
- Shirvani, H.; Rahmati-Ahmadabad, S. Irisin interaction with adipose tissue secretions by exercise training and flaxseed oil supplement. Lipids Health Disease 2019, 18, 15. [Google Scholar] [CrossRef]
- Bassett, C.M.; Rodriguez-Leyva, D.; Pierce, G.N. Experimental and clinical research findings on the cardiovascular benefits of consuming flaxseed. Appl. Physiol. Nutr. Metab. 2009, 34, 965–974. [Google Scholar] [CrossRef]
- Gillingham, L.G.; Gustafson, J.A.; Han, S.-Y.; Jassal, D.S.; Jones, P.J.H. High-oleic rapeseed (canola) and flaxseed oils modulate serum lipids and inflammatory biomarkers in hypercholesterolaemic subjects. Br. J. Nutr. 2010, 105, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.K.; Thompson, L.U. Flaxseed and its lignan and oil components: Can they play a role in reducing the risk of and improving the treatment of breast cancer? Appl. Physiol. Nutr. Metab. 2014, 39, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Danbara, N.; Yuri, T.; Tsujita-Kyutoku, M.; Tsukamoto, R.; Uehara, N.; Tsubura, A. Enterolactone Induces Apoptosis and Inhibits Growth of Colo 201 Human Colon Cancer Cells both In Vitro and In Vivo. Anticancer Res. 2005, 25, 2269–2276. [Google Scholar]
- Kuijsten, A.; Arts, I.C.W.; Hollman, P.C.H.; van’t Veer, P.; Kampman, E. Plasma Enterolignans Are Associated with Lower Colorectal Adenoma Risk. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Zanwar, A.A.; Hegde, M.V.; Bodhankar, S.L. Chapter 71-Flax Lignan in the Prevention of Atherosclerotic Cardiovascular Diseases. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 915–921. [Google Scholar] [CrossRef]
- Adolphe, J.L.; Whiting, S.J.; Juurlink, B.H.J.; Thorpe, L.U.; Alcorn, J. Health effects with consumption of the flax lignan secoisolariciresinol diglucoside. Br. J. Nutr. 2009, 103, 929–938. [Google Scholar] [CrossRef]
- Puukila, S.; Fernandes, R.O.; Turck, P.; Carraro, C.C.; Bonetto, J.H.P.; de Lima-Seolin, B.G.; da Rosa Araujo, A.S.; Bello-Klein, A.; Boreham, D.; Khaper, N. Secoisolariciresinol diglucoside attenuates cardiac hypertrophy and oxidative stress in monocrotaline-induced right heart dysfunction. Mol. Cell Biochem. 2017, 432, 33–39. [Google Scholar] [CrossRef]
- Prasad, K.; Jadhav, A. Prevention and treatment of atherosclerosis with flaxseed-derived compound secoisolariciresinol diglucoside. Curr. Pharm. Des. 2016, 22, 214–220. [Google Scholar] [CrossRef]
- Imran, M.; Ahmad, N.; Anjum, F.M.; Khan, M.K.; Mushtaq, Z.; Nadeem, M.; Hussain, S. Potential protective properties of flax lignan secoisolariciresinol diglucoside. Nutr. J. 2015, 14, 71. [Google Scholar] [CrossRef]
- Jenab, M.; Thompson, L.U. The influence of flaxseed and lignans on colon carcinogenesis and beta-glucuronidase activity. Carcinogenesis 1996, 17, 1343–1348. [Google Scholar] [CrossRef]
- Jenab, M.; Rickard, S.E.; Orcheson, L.J.; Thompson, L.U. Flaxseed and lignans increase cecal beta-glucuronidase activity in rats. Nutr. Cancer 1999, 33, 154–158. [Google Scholar] [CrossRef]
- Serraino, M.; Thompson, L.U. Flaxseed supplementation and early markers of colon carcinogenesis. Cancer Lett. 1992, 63, 159–165. [Google Scholar] [CrossRef]
- Li, D.; Yee, J.A.; Thompson, L.U.; Yan, L. Dietary supplementation with secoisolariciresinol diglycoside (SDG) reduces experimental metastasis of melanoma cells in mice. Cancer Lett. 1999, 142, 91–96. [Google Scholar] [CrossRef]
- Truan, J.S.; Chen, J.M.; Thompson, L.U. Comparative effects of sesame seed lignan and flaxseed lignan in reducing the growth of human breast tumors (MCF-7) at high levels of circulating estrogen in athymic mice. Nutr. Cancer 2012, 64, 65–71. [Google Scholar] [CrossRef]
- Wiggins, A.K.; Mason, J.K.; Thompson, L.U. Beneficial Influence of Diets Enriched with Flaxseed and Flaxseed Oil on Cancer. In Cancer Chemoprevention and Treatment by Diet Therapy; Cho, W.C.S., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 55–89. [Google Scholar] [CrossRef]
- Rhee, Y.; Brunt, A. Flaxseed supplementation improved insulin resistance in obese glucose intolerant people: A randomized crossover design. Nutr. J. 2011, 10, 44. [Google Scholar] [CrossRef]
- Zhu, Y.; Kawaguchi, K.; Kiyama, R. Differential and directional estrogenic signaling pathways induced by enterolignans and their precursors. PLoS ONE 2017, 12, e0171390. [Google Scholar] [CrossRef]
- Krajčová, A.; Schulzová, V.; Hajšlová, J.; Bjelková, M. Lignans in Flaxseed Lignans in Flaxseed. Czech J. Food Sci. 2009, 27, S252–S255. [Google Scholar] [CrossRef]
- Touré, A.; Xueming, X. Flaxseed Lignans: Source, Biosynthesis, Metabolism, Antioxidant Activity, Bio-Active Components, and Health Benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J.; Adlercreutz, H.; Scalbert, A.; Jacques, P.; McCullough, M.L. Dietary lignans: Physiology and potential for cardiovascular disease risk reduction. Nutr. Rev. 2010, 68, 571–603. [Google Scholar] [CrossRef]
- Bagniewska-Zadworna, A.; Barakat, A.; Lakomy, P.; Smolinski, D.J.; Zadworny, M. Lignin and lignans in plant defence: Insight from expression profiling of cinnamyl alcohol dehydrogenase genes during development and following fungal infection in Populus. Plant Sci. 2014, 229, 111–121. [Google Scholar] [CrossRef]
- Zhu, H.-Y.; Li, M.-X.; Yang, D.-H.; Tao, Y.-L.; Zhang, Y.; Liu, S.-L. Biotransformation of the SDG in defatted flaxseed into END co-cultured by three single bacterial colonies. Process Biochem. 2014, 49, 19–24. [Google Scholar] [CrossRef]
- Wang, L.Q.; Meselhy, M.R.; Li, Y.; Qin, G.W.; Hattori, M. Human intestinal bacteria capable of transforming secoisolariciresinol diglucoside to mammalian lignans, enterodiol and enterolactone. Chem. Pharm. Bull. 2000, 48, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Bannwart, C.; Adlercreutz, H.; Wahala, K.; Brunow, G.; Hase, T. Detection and identification of the plant lignans lariciresinol, isolariciresinol and secoisolariciresinol in human urine. Clin. Chim. Acta 1989, 180, 293–301. [Google Scholar] [CrossRef]
- Saarinen, N.M.; Warri, A.; Makela, S.I.; Eckerman, C.; Reunanen, M.; Ahotupa, M.; Salmi, S.M.; Franke, A.A.; Kangas, L.; Santti, R. Hydroxymatairesinol, a novel enterolactone precursor with antitumor properties from coniferous tree (Picea abies). Nutr. Cancer 2000, 36, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Borriello, S.P.; Setchell, K.D.; Axelson, M.; Lawson, A.M. Production and metabolism of lignans by the human faecal flora. J. Appl. Bacteriol. 1985, 58, 37–43. [Google Scholar] [CrossRef]
- Heinonen, S.; Nurmi, T.; Liukkonen, K.; Poutanen, K.; Wahala, K.; Deyama, T.; Nishibe, S.; Adlercreutz, H. In vitro metabolism of plant lignans: New precursors of mammalian lignans enterolactone and enterodiol. J. Agric. Food Chem. 2001, 49, 3178–3186. [Google Scholar] [CrossRef]
- Jin, J.-S.; Hattori, M. A new mammalian lignan precursor, asarinin. Food Chem. 2011, 124, 895–899. [Google Scholar] [CrossRef]
- US-Department-of-Health-and-Human-Services. Complementary, Alternative, or Integrative Health: What’s in a Name? Available online: https://nccih.nih.gov/health/integrative-health (accessed on 30 March 2019).
- Zhang, J.; Onakpoya, I.J.; Posadzki, P.; Eddouks, M. The safety of herbal medicine: From prejudice to evidence. Evid. Based Complement. Alternat. Med. 2015, 2015, 316706. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Raynor, D.K.; Dickinson, R.; Knapp, P.; Long, A.F.; Nicolson, D.J. Buyer beware? Does the information provided with herbal products available over the counter enable safe use? BMC Med. 2011, 9, 94. [Google Scholar] [CrossRef]
- Pawar, R.S.; Grundel, E. Overview of regulation of dietary supplements in the USA and issues of adulteration with phenethylamines (PEAs). Drug Test. Anal. 2017, 9, 500–517. [Google Scholar] [CrossRef]
- Laeeque, H.; Boon, H.; Kachan, N.; Cohen, J.C.; D’Cruz, J. The Canadian Natural Health Products (NHP) regulations: Industry perceptions and compliance factors. BMC Health Serv. Res. 2006, 6, 63. [Google Scholar] [CrossRef]
- Tamayo, C.; Ann, H. Canada’s Natural Health Products: A Regulatory Overview. Pharm. Regul. Aff. Open Access 2016, 5, 1. [Google Scholar]
- Di, Y. Flaxseed Lignan Supplementation as Possible Adjuvant Therapy for Prostate and Breast Cancer. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2017. [Google Scholar]
- Weeks, C. Health Canada Rules Ask for Science behind Natural Health Products’ Claims. Available online: https://www.theglobeandmail.com/life/health-and-fitness/health/health-canada-rules-ask-for-science-behind-natural-products-claims-health/article33287337/ (accessed on 12 December 2017).
- Wilson, V.L. Carcinogenesis as the Sum of Its Parts. Disrupt. Sci. Technol. 2012, 1, 110–115. [Google Scholar] [CrossRef]
- Block, K.I.; Gyllenhaal, C.; Lowe, L.; Amedei, A.; Amin, A.R.; Amin, A.; Aquilano, K.; Arbiser, J.; Arreola, A.; Arzumanyan, A.; et al. Designing a broad-spectrum integrative approach for cancer prevention and treatment. Semin. Cancer Biol. 2015, 35, S276–S304. [Google Scholar] [CrossRef]
- Block, K.I.; Gyllenhaal, C.; Lowe, L.; Amedei, A.; Amin, A.R.; Amin, A.; Aquilano, K.; Arbiser, J.; Arreola, A.; Arzumanyan, A.; et al. A Broad-Spectrum Integrative Design for Cancer Prevention and Therapy. Semin. Cancer Biol. 2015, 35, S276–S304. [Google Scholar] [CrossRef]
- Huerta, E.; Grey, N. Cancer control opportunities in low- and middle-income countries. CA Cancer J. Clin. 2007, 57, 72–74. [Google Scholar] [CrossRef][Green Version]
- Ginsburg, O.M. Breast and cervical cancer control in low and middle-income countries: Human rights meet sound health policy. J. Cancer Policy 2013, 1, e35–e41. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Steward, W.P.; Brown, K. Cancer chemoprevention: A rapidly evolving field. Br. J. Cancer 2013, 109, 1–7. [Google Scholar] [CrossRef]
- Mocanu, M.M.; Nagy, P.; Szollosi, J. Chemoprevention of Breast Cancer by Dietary Polyphenols. Molecules 2015, 20, 22578–22620. [Google Scholar] [CrossRef]
- Othman, N.H. Honey and cancer: Sustainable inverse relationship particularly for developing nations-a review. Evid. Based Complement. Alternat. Med. 2012, 2012, 410406. [Google Scholar] [CrossRef]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef] [PubMed]
- Pitot, H.C. The molecular biology of carcinogenesis. Cancer 1993, 72, 962–970. [Google Scholar] [CrossRef]
- Fajardo, A.M.; Piazza, G.A. Chemoprevention in gastrointestinal physiology and disease. Anti-inflammatory approaches for colorectal cancer chemoprevention. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G59–G70. [Google Scholar] [CrossRef] [PubMed]
- Al Rabadi, L.; Bergan, R. A Way Forward for Cancer Chemoprevention: Think Local. Cancer Prev. Res. 2017, 10, 14–35. [Google Scholar] [CrossRef] [PubMed]
- Todoric, J.; Antonucci, L.; Karin, M. Targeting Inflammation in Cancer Prevention and Therapy. Cancer Prev. Res. 2016, 9, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.S.; Mann, M.; DuBois, R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999, 18, 7908–7916. [Google Scholar] [CrossRef]
- Gurpinar, E.; Grizzle, W.E.; Piazza, G.A. NSAIDs inhibit tumorigenesis, but how? Clin. Cancer Res. 2014, 20, 1104–1113. [Google Scholar] [CrossRef]
- Harris, R.E.; Beebe-Donk, J.; Doss, H.; Burr Doss, D. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: A critical review of non-selective COX-2 blockade (review). Oncol. Rep. 2005, 13, 559–583. [Google Scholar] [CrossRef]
- Umamaheswaran, S.; Dasari, S.K.; Yang, P.; Lutgendorf, S.K.; Sood, A.K. Stress, inflammation, and eicosanoids: An emerging perspective. Cancer Metastasis. Rev. 2018, 37, 203–211. [Google Scholar] [CrossRef]
- Aravindaram, K.; Yang, N.S. Anti-inflammatory plant natural products for cancer therapy. Planta Med. 2010, 76, 1103–1117. [Google Scholar] [CrossRef]
- Tan, A.C.; Konczak, I.; Sze, D.M.; Ramzan, I. Molecular pathways for cancer chemoprevention by dietary phytochemicals. Nutr. Cancer 2011, 63, 495–505. [Google Scholar] [CrossRef]
- Ramos, S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J. Nutr. Biochem. 2007, 18, 427–442. [Google Scholar] [CrossRef]
- Institute-for-Work-and-Health-Toronto. Primary, Secondary and Tertiary Prevention. Available online: https://www.iwh.on.ca/what-researchers-mean-by/primary-secondary-and-tertiary-prevention (accessed on 30 March 2019).
- Gapstur, S.M.; Drope, J.M.; Jacobs, E.J.; Teras, L.R.; McCullough, M.L.; Douglas, C.E.; Patel, A.V.; Wender, R.C.; Brawley, O.W. A blueprint for the primary prevention of cancer: Targeting established, modifiable risk factors. CA Cancer J. Clin. 2018, 68, 446–470. [Google Scholar] [CrossRef]
- Blackburn, E.H. Highlighting the Science of Cancer Prevention. Cancer Prev. Res. 2010, 3, 393. [Google Scholar] [CrossRef]
- Schoenberg, M.H. Physical Activity and Nutrition in Primary and Tertiary Prevention of Colorectal Cancer. Visc. Med. 2016, 32, 199–204. [Google Scholar] [CrossRef]
- Rock, C.L.; Yang, C.S.; Alberts, D.S.; Meyskens, F.L., Jr.; Mukhtar, H.; Cuzick, J.; Ramsey, S.D.; Lippman, S.M.; Kensler, T.W. Cancer Prevention: Obstacles, Challenges, and the Road Ahead. JNCI J. Natl. Cancer Inst. 2015, 108. [Google Scholar] [CrossRef]
- Berrino, F. Life style prevention of cancer recurrence: The yin and the yang. Cancer Treat. Res. 2014, 159, 341–351. [Google Scholar] [CrossRef]
- De Flora, S.; Izzotti, A.; D’Agostini, F.; Balansky, R.M.; Noonan, D.; Albini, A. Multiple points of intervention in the prevention of cancer and other mutation-related diseases. Mutat. Res. 2001, 480–481, 9–22. [Google Scholar] [CrossRef]
- De Flora, S.; Ferguson, L.R. Overview of mechanisms of cancer chemopreventive agents. Mutat. Res. 2005, 591, 8–15. [Google Scholar] [CrossRef]
- Sporn, M.B.; Dunlop, N.; Newton, D.; Smith, J. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids). Feder. Proc. 1976, 35, 1332–1338. [Google Scholar]
- Wattenberg, L.W. Inhibition of carcinogenesis by minor anutrient constituents of the diet. Proc. Nutr. Soc. 1990, 49, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.G.; Murillo, G.; Naithani, R.; Peng, X. Cancer Chemoprevention by Natural Products: How Far Have We Come? Pharm. Res. 2010, 27, 950–961. [Google Scholar] [CrossRef]
- Amawi, H.; Ashby, C.R., Jr.; Tiwari, A.K. Cancer chemoprevention through dietary flavonoids: what’s limiting? Chin. J. Cancer 2017, 36, 50. [Google Scholar] [CrossRef] [PubMed]
- Benetou, V.; Lagiou, A.; Lagiou, P. Chemoprevention of cancer: Current evidence and future prospects. F1000Research 2015, 4, 916. [Google Scholar] [CrossRef]
- Naomi, G.; Gad, R. Beyond aspirin—Cancer prevention with statins, metformin and bisphosphonates. Nat. Rev. Clin. Oncol. 2013, 10, 625. [Google Scholar] [CrossRef]
- Bosland, M.C. Is There a Future for Chemoprevention of Prostate Cancer? Cancer Prev. Res. 2016, 9, 642–647. [Google Scholar] [CrossRef]
- Kreuger, M.R.; Grootjans, S.; Biavatti, M.W.; Vandenabeele, P.; D’Herde, K. Sesquiterpene lactones as drugs with multiple targets in cancer treatment: Focus on parthenolide. Anticancer Drugs 2012, 23, 883–896. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Kingston, D.G. Modern natural products drug discovery and its relevance to biodiversity conservation. J. Nat. Prod. 2011, 74, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef]
- Vue, B.; Zhang, S.; Chen, Q.H. Flavonoids with Therapeutic Potential in Prostate Cancer. Anticancer Agents Med. Chem. 2016, 16, 1205–1229. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Tachibana, H.; Yamada, K. Inhibition of environmental estrogen-induced proliferation of human breast carcinoma MCF-7 cells by flavonoids. In Vitro Cell. Dev. Biol. Anim. 2001, 37, 275–282. [Google Scholar] [PubMed]
- Yin, F.; Giuliano, A.E.; Van Herle, A.J. Signal pathways involved in apigenin inhibition of growth and induction of apoptosis of human anaplastic thyroid cancer cells (ARO). Anticancer Res. 1999, 19, 4297–4303. [Google Scholar]
- Cho, H.J.; Suh, D.S.; Moon, S.H.; Song, Y.J.; Yoon, M.S.; Park, D.Y.; Choi, K.U.; Kim, Y.K.; Kim, K.H. Silibinin inhibits tumor growth through downregulation of extracellular signal-regulated kinase and Akt in vitro and in vivo in human ovarian cancer cells. J. Agric. Food Chem. 2013, 61, 4089–4096. [Google Scholar] [CrossRef]
- Petrick, J.L.; Steck, S.E.; Bradshaw, P.T.; Trivers, K.F.; Abrahamson, P.E.; Engel, L.S.; He, K.; Chow, W.H.; Mayne, S.T.; Risch, H.A.; et al. Dietary intake of flavonoids and oesophageal and gastric cancer: Incidence and survival in the United States of America (USA). Br. J. Cancer 2015, 112, 1291–1300. [Google Scholar] [CrossRef]
- Rossi, M.; Rosato, V.; Bosetti, C.; Lagiou, P.; Parpinel, M.; Bertuccio, P.; Negri, E.; La Vecchia, C. Flavonoids, proanthocyanidins, and the risk of stomach cancer. Cancer Causes Control. 2010, 21, 1597–1604. [Google Scholar] [CrossRef]
- Zhou, Y. Vitexins, nature-derived lignan compounds, induce apoptosis and suppress tumor growth. Clin. Cancer Res. 2009, 15. [Google Scholar] [CrossRef]
- Yatkin, E.; Polari, L.; Laajala, T.D.; Smeds, A.; Eckerman, C.; Holmbom, B.; Saarinen, N.M.; Aittokallio, T.; Mäkelä, S.I. Novel lignan and stilbenoid mixture shows anticarcinogenic efficacy in preclinical PC-3M-luc2 prostate cancer model. PLoS ONE 2014, 9, e93764. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Yee, J.A.; Li, D.; McGuire, M.H.; Thompson, L.U. Dietary flaxseed supplementation and experimental metastasis of melanoma cells in mice. Cancer Lett. 1998, 124, 181–186. [Google Scholar] [CrossRef]
- Westcott, N.D.; Muir, A.D. Flax seed lignan in disease prevention and health promotion. Phytochem. Rev. 2003, 2, 401–417. [Google Scholar] [CrossRef]
- Boyland, E. Critical review of problems of chemotherapy. Proc. R. Soc. Med. 1963, 56, 640–642. [Google Scholar] [PubMed]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Block, K.I.; Block, P.B.; Gyllenhaal, C. Integrative Therapies in Cancer:Modulating a Broad Spectrum of Targets for Cancer Management. Integr. Cancer Ther. 2015, 14, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Marchand, L. Integrative and complementary therapies for patients with advanced cancer. Ann. Palliat. Med. 2014, 3, 160–171. [Google Scholar]
- Academic Consortium for Integrative Medicine and, H. Definition of Integrative Medicine and Health. Available online: https://imconsortium.org/ (accessed on 30 March 2019).
- Eran, B.-A.; Noah, S.; Ofer, L. Integrative Medicine for Female Patients with Gynecologic Cancer. J. Alternat. Complement. Med. 2018, 24, 881–889. [Google Scholar] [CrossRef]
- E Alessandra Strada, R.K.P. Psychological, Rehabilitative, and Integrative Therapies for Cancer Pain—UpToDate. Available online: https://www.uptodate.com/contents/psychological-rehabilitative-and-integrative-therapies-for-cancer-pain (accessed on 30 March 2019).
- Redondo-Blanco, S.; Fernández, J.; Gutiérrez-del-Río, I.; Villar, C.J.; Lombó, F. New Insights toward Colorectal Cancer Chemotherapy Using Natural Bioactive Compounds. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Saxena, A.; Becker, D.; Preeshagul, I.; Lee, K.; Katz, E.; Levy, B. Therapeutic Effects of Repurposed Therapies in Non-Small Cell Lung Cancer: What Is Old Is New Again. Oncologist 2015, 20, 934–945. [Google Scholar] [CrossRef]
- Farnsworth, N.R.; Akerele, O.; Bingel, A.S.; Soejarto, D.D.; Guo, Z. Medicinal plants in therapy. Bull. World Health Organ. 1985, 63, 965–981. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Campbell, I.W. Metformin—Life begins at 50 A symposium held on the occasion of the 43rd Annual Meeting of the European Association for the Study of Diabetes, Amsterdam, The Netherlands, September 2007. Br. J. Diabetes Vasc. Disease 2007, 7, 247–252. [Google Scholar] [CrossRef]
- Duthie, G.G.; Wood, A.D. Natural salicylates: Foods, functions and disease prevention. Food Funct. 2011, 2, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Gui, Y.; Chen, L.; Yuan, G.; Lu, H.-Z.; Xu, X. Use of natural products as chemical library for drug discovery and network pharmacology. PLoS ONE 2013, 8, e62839. [Google Scholar] [CrossRef]
- Fox, S.; Farr-Jones, S.; Sopchak, L.; Boggs, A.; Nicely, H.W.; Khoury, R.; Biros, M. High-throughput screening: Update on practices and success. J. Biomol. Screen. 2006, 11, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, C.; Gupta, A.; Kanjilal, S.; Katiyar, S. Drug discovery from plant sources: An integrated approach. Ayu 2012, 33, 10–19. [Google Scholar] [CrossRef]
- Jacobson, K.A. New paradigms in GPCR drug discovery. Biochem Pharmacol 2015, 98, 541–555. [Google Scholar] [CrossRef]
- Pathania, S.; Ramakrishnan, S.M.; Bagler, G. Phytochemica: A platform to explore phytochemicals of medicinal plants. Database 2015, 2015. [Google Scholar] [CrossRef]
- Tarkang, P.A.; Appiah-Opong, R.; Ofori, M.F.; Ayong, L.S.; Nyarko, A.K. Application of multi-target phytotherapeutic concept in malaria drug discovery: A systems biology approach in biomarker identification. Biomark. Res. 2016, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Bhanot, A.; Sharma, R.; Noolvi, M.N. Natural sources as potential anti-cancer agents: A review. Int. J. Phytomed. 2011, 3, 18. [Google Scholar]
- Willett, W.C. Diet and Cancer. Oncol. 2000, 5, 393–404. [Google Scholar] [CrossRef]
- Li, M.; Cima, M.J.; Milner, D.A., Jr. If It’s Not One Thing, It’s Another: An Inverse Relationship of Malignancy and Atherosclerotic Disease. PLoS ONE 2015, 10, e0126855. [Google Scholar] [CrossRef]
- Doll, R.; Peto, R. The causes of cancer: Quantitative estimates of avoidable risks of cancer in the United States today. JNCI J. Natl. Cancer Inst. 1981, 66, 1192–1308. [Google Scholar] [CrossRef]
- Cojocneanu Petric, R.; Braicu, C.; Raduly, L.; Zanoaga, O.; Dragos, N.; Monroig, P.; Dumitrascu, D.; Berindan-Neagoe, I. Phytochemicals modulate carcinogenic signaling pathways in breast and hormone-related cancers. Oncol. Targets Ther. 2015, 8, 2053–2066. [Google Scholar] [CrossRef]
- Simonsen, H.T.; Drew, D.P.; Lunde, C. Perspectives on Using Physcomitrella Patens as an Alternative Production Platform for Thapsigargin and Other Terpenoid Drug Candidates. Perspect. Med. Chem. 2009, 3, 1–6. [Google Scholar] [CrossRef]
- Lampe, J.W.; Chang, J.L. Interindividual differences in phytochemical metabolism and disposition. Semin. Cancer Biol. 2007, 17, 347–353. [Google Scholar] [CrossRef]
- Adlercreutz, H. Lignans and human health. Crit. Rev. Clin. Lab. Sci. 2007, 44, 483–525. [Google Scholar] [CrossRef] [PubMed]
- Fantini, M.; Benvenuto, M.; Masuelli, L.; Frajese, G.V.; Tresoldi, I.; Modesti, A.; Bei, R. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: Perspectives on cancer treatment. Int. J. Mol. Sci. 2015, 16, 9236–9282. [Google Scholar] [CrossRef]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Lall, R.K.; Syed, D.N.; Adhami, V.M.; Khan, M.I.; Mukhtar, H. Dietary polyphenols in prevention and treatment of prostate cancer. Int. J. Mol. Sci. 2015, 16, 3350–3376. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Estrada, B.A.; Gutierrez-Uribe, J.A.; Serna-Saldivar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Macia, A.; Motilva, M.J. Metabolic and Microbial Modulation of the Large Intestine Ecosystem by Non-Absorbed Diet Phenolic Compounds: A Review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479s–3485s. [Google Scholar] [CrossRef] [PubMed]
- Kausar, H.; Jeyabalan, J.; Aqil, F.; Chabba, D.; Sidana, J.; Singh, I.P.; Gupta, R.C. Berry anthocyanidins synergistically suppress growth and invasive potential of human non-small-cell lung cancer cells. Cancer Lett. 2012, 325, 54–62. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Tang, L.; Chen, H.; Wu, C.; Zhao, M.; Yang, Y.; Chen, X.; Liu, G. Resveratrol inhibits TGF-beta1-induced epithelial-to-mesenchymal transition and suppresses lung cancer invasion and metastasis. Toxicology 2013, 303, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Rigalli, J.P.; Tocchetti, G.N.; Arana, M.R.; Villanueva, S.S.; Catania, V.A.; Theile, D.; Ruiz, M.L.; Weiss, J. The phytoestrogen genistein enhances multidrug resistance in breast cancer cell lines by translational regulation of ABC transporters. Cancer Lett. 2016, 376, 165–172. [Google Scholar] [CrossRef]
- Shi, J.; Liu, F.; Zhang, W.; Liu, X.; Lin, B.; Tang, X. Epigallocatechin-3-gallate inhibits nicotine-induced migration and invasion by the suppression of angiogenesis and epithelial-mesenchymal transition in non-small cell lung cancer cells. Oncol. Rep. 2015, 33, 2972–2980. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, S.; Li, H.-B.; Deng, G.-F.; Ling, W.-H.; Wu, S.; Xu, X.-R.; Chen, F. Antiproliferative activity of peels, pulps and seeds of 61 fruits. J. Funct. Foods 2013, 5, 1298–1309. [Google Scholar] [CrossRef]
- Li, F.; Li, S.; Li, H.B.; Deng, G.F.; Ling, W.H.; Xu, X.R. Antiproliferative activities of tea and herbal infusions. Food Funct. 2013, 4, 530–538. [Google Scholar] [CrossRef]
- Agoston, V.; Csermely, P.; Pongor, S. Multiple weak hits confuse complex systems: A transcriptional regulatory network as an example. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2005, 71, 051909. [Google Scholar] [CrossRef]
- Csermely, P. Strong links are important, but weak links stabilize them. Trends Biochem. Sci. 2004, 29, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Van Duynhoven, J.P.M.; van Velzen, E.J.J.; Westerhuis, J.A.; Foltz, M.; Jacobs, D.M.; Smilde, A.K. Nutrikinetics: Concept, technologies, applications, perspectives. Trends Food Sci. Technol. 2012, 26, 4–13. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, M.J.; Ahn, J.; Lee, S.H.; Lee, H.; Kim, J.H.; Park, S.H.; Jang, Y.J.; Ha, T.Y.; Jung, C.H. Nutrikinetics of Isoflavone Metabolites After Fermented Soybean Product (Cheonggukjang) Ingestion in Ovariectomized Mice. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- De Vos, W.M.; Castenmiller, J.J.M.; Hamer, R.J.; Brummer, R.J.M. Nutridynamics—Studying the dynamics of food components in products and in the consumer. Curr. Opin. Biotechnol. 2006, 17, 217–225. [Google Scholar] [CrossRef]
- Serrano, J.C.; Jove, M.; Gonzalo, H.; Pamplona, R.; Portero-Otin, M. Nutridynamics: Mechanism(s) of action of bioactive compounds and their effects. Int. J. Food Sci. Nutr. 2015, 66, S22–S30. [Google Scholar] [CrossRef]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A dietary antioxidant for health promotion. Antioxid. Redox Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5. [Google Scholar] [CrossRef]
- Singh, A.; Holvoet, S.; Mercenier, A. Dietary polyphenols in the prevention and treatment of allergic diseases. Clin. Exp. Allergy 2011, 41, 1346–1359. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64, S112. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Holst, B. Dietary reference intake (DRI) value for dietary polyphenols: Are we heading in the right direction? Br. J. Nutr. 2008, 99, S55–S58. [Google Scholar] [CrossRef]
- Crowe, K.M.; Francis, C. Position of the academy of nutrition and dietetics: Functional foods. J. Acad. Nutr. Diet. 2013, 113, 1096–1103. [Google Scholar] [CrossRef]
- Auclair, S.; Chironi, G.; Milenkovic, D.; Hollman, P.; Renard, C.; Megnien, J.; Gariepy, J.; Paul, J.; Simon, A.; Scalbert, A. The regular consumption of a polyphenol-rich apple does not influence endothelial function: A randomised double-blind trial in hypercholesterolemic adults. Eur. J. Clin. Nutr. 2010, 64, 1158. [Google Scholar] [CrossRef]
- Habas, K.; Brinkworth, M.H.; Anderson, D. Diethylstilbestrol induces oxidative DNA damage, resulting in apoptosis of spermatogonial stem cells in vitro. Toxicology 2017, 382, 117–121. [Google Scholar] [CrossRef]
- Anderson, A.L.; Harris, T.B.; Tylavsky, F.A.; Perry, S.E.; Houston, D.K.; Lee, J.S.; Kanaya, A.M.; Sahyoun, N.R. Dietary patterns, insulin sensitivity and inflammation in older adults. Eur. J. Clin. Nutr. 2012, 66, 18. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Jirillo, E. Influence of polyphenols on allergic immune reactions: Mechanisms of action. Proc. Nutr. Soc. 2012, 71, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Saeidnia, S.; Abdollahi, M. Antioxidants: Friends or foe in prevention or treatment of cancer: The debate of the century. Toxicol. Appl. Pharmacol. 2013, 271, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Dixit, M. Role of Polyphenols and Other Phytochemicals on Molecular Signaling. Oxid. Med. Cell. Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef]
- Wang, S.; Lin, H.; Cong, W. Chinese Medicines Improve Perimenopausal Symptoms Induced by Surgery, Chemoradiotherapy, or Endocrine Treatment for Breast Cancer. Front. Pharmacol. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Sang, S.; Lu, A.Y.; Yang, C.S. Metabolism of dietary polyphenols and possible interactions with drugs. Curr. Drug Metab. 2007, 8, 499–507. [Google Scholar] [CrossRef]
- Tham, D.M.; Gardner, C.D.; Haskell, W.L. Clinical review 97: Potential health benefits of dietary phytoestrogens: A review of the clinical, epidemiological, and mechanistic evidence. J. Clin Endocrinol. Metab. 1998, 83, 2223–2235. [Google Scholar] [CrossRef][Green Version]
- Limonta, P.; Moretti, R.M.; Marzagalli, M.; Fontana, F.; Raimondi, M.; Montagnani Marelli, M. Role of Endoplasmic Reticulum Stress in the Anticancer Activity of Natural Compounds. Int. J. Mol. Sci. 2019, 20, 961. [Google Scholar] [CrossRef] [PubMed]
- Hasima, N.; Ozpolat, B. Regulation of autophagy by polyphenolic compounds as a potential therapeutic strategy for cancer. Cell Death Disease 2014, 5, e1509. [Google Scholar] [CrossRef]
- Rajamanickam, S.; Agarwal, R. Natural products and colon cancer: Current status and future prospects. Drug Dev. Res. 2008, 69, 460–471. [Google Scholar] [CrossRef]
- Taxvig, C.; Elleby, A.; Sonne-Hansen, K.; Bonefeld-Jørgensen, E.C.; Vinggaard, A.M.; Lykkesfeldt, A.E.; Nellemann, C. Effects of Nutrition Relevant Mixtures of Phytoestrogens on Steroidogenesis, Aromatase, Estrogen, and Androgen Activity. Nutr. Cancer 2009, 62, 122–131. [Google Scholar] [CrossRef]
- Wang, L.Q. Mammalian phytoestrogens: Enterodiol and enterolactone. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 777, 289–309. [Google Scholar] [CrossRef]
- Richter, D.; Abarzua, S.; Chrobak, M.; Vrekoussis, T.; Weissenbacher, T.; Kuhn, C.; Schulze, S.; Kupka, M.S.; Friese, K.; Briese, V.; et al. Effects of phytoestrogen extracts isolated from pumpkin seeds on estradiol production and ER/PR expression in breast cancer and trophoblast tumor cells. Nutr. Cancer 2013, 65, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Teiten, M.-H.; Gaascht, F.; Cronauer, M.; Henry, E.; Dicato, M.; Diederich, M. Anti-proliferative potential of curcumin in androgen-dependent prostate cancer cells occurs through modulation of the Wingless signaling pathway. Int. J. Oncol. 2011, 38, 603–611. [Google Scholar] [PubMed]
- Masuda, M.; Suzui, M.; Lim, J.T.; Deguchi, A.; Soh, J.W.; Weinstein, I.B. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J. Exp. Ther. Oncol. 2002, 2, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.J.C.; Paliyath, G. Functional Foods And Nutraceuticals; John Wiley & Sons: New York, NY, USA, 2011; pp. 11–43. [Google Scholar] [CrossRef]
- Morbidelli, L. Polyphenol-based nutraceuticals for the control of angiogenesis: Analysis of the critical issues for human use. Pharmacol. Res. 2016, 111, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, J.; Li, L.; Shi, W.; Yuan, X.; Wu, L. The Natural Occurring Compounds Targeting Endoplasmic Reticulum Stress. Evid.-Based Complement. Alternat. Med. eCAM 2016, 2016, 7831282. [Google Scholar] [CrossRef]
- Fu, Y.; Chang, H.; Peng, X.; Bai, Q.; Yi, L.; Zhou, Y.; Zhu, J.; Mi, M. Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/β-catenin signaling pathway. PLoS ONE 2014, 9, e102535. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Sureda, A.; Dehpour, A.R.; Shirooie, S.; Silva, A.S.; Devi, K.P.; Ahmed, T.; Ishaq, N.; Hashim, R.; Sobarzo-Sánchez, E.; et al. Regulation of autophagy by polyphenols: Paving the road for treatment of neurodegeneration. Biotechnol. Adv. 2018, 36, 1768–1778. [Google Scholar] [CrossRef]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Aryaeian, N.; Sedehi, S.K.; Arablou, T. Polyphenols and their effects on diabetes management: A review. Med. J. Islam. Republ. Iran 2017, 31, 134. [Google Scholar] [CrossRef]
- Sharma, S.; Rana, S.; Patial, V.; Gupta, M.; Bhushan, S.; Padwad, Y.S. Antioxidant and hepatoprotective effect of polyphenols from apple pomace extract via apoptosis inhibition and Nrf2 activation in mice. Hum. Exp. Toxicol. 2016, 35, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Quan, M.; Li, Q.; Zhao, P.; Tian, C. Chemical composition and hepatoprotective effect of free phenolic extract from barley during malting process. Sci. Rep. 2018, 8, 4460. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.H.; Labib, R.M.; Hamid, N.S.A.; Hamed, M.A.; Ross, S.A. Hepatoprotective and antioxidant polyphenols from a standardized methanolic extract of the leaves of Liquidambar styraciflua L. Bull. Faculty Pharm. Cairo Univ. 2015, 53, 117–127. [Google Scholar] [CrossRef]
- Shivashankara, A.R.; Kumar, A.; Ravi, R.; Simon, P.; Rai, P.; Francis, A.; Baliga, M.S. Chapter 55-Hepatoprotective Effects of Green Tea and its Polyphenols: Preclinical Observations. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 715–721. [Google Scholar] [CrossRef]
- Sun, L.; Meng, Y.; Sun, J.; Guo, Y. Characterization, antioxidant activities and hepatoprotective effects of polysaccharides from pre-pressing separation Fuji apple peel. CyTA-J. Food 2017, 15, 307–319. [Google Scholar] [CrossRef]
- Holst, B.; Williamson, G. A critical review of the bioavailability of glucosinolates and related compounds. Nat. Prod. Rep. 2004, 21, 425–447. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230s–242s. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I.; Meririnne, E.; Alfthan, G.; Aro, A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J. Nutr. 2001, 131, 235–241. [Google Scholar] [CrossRef]
- Shephard, S.E.; Zogg, M.; Burg, G.; Panizzon, R.G. Measurement of 5-methoxypsoralen and 8-methoxypsoralen in saliva of PUVA patients as a noninvasive, clinically relevant alternative to monitoring in blood. Arch. Dermatol. Res. 1999, 291, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Kuijsten, A.; Arts, I.C.; Vree, T.B.; Hollman, P.C. Pharmacokinetics of enterolignans in healthy men and women consuming a single dose of secoisolariciresinol diglucoside. J. Nutr. 2005, 135, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Undevia, S.D.; Gomez-Abuin, G.; Ratain, M.J. Pharmacokinetic variability of anticancer agents. Nat. Rev. Cancer 2005, 5, 447–458. [Google Scholar] [CrossRef]
- Goldin, B.R. In situ bacterial metabolism and colon mutagens. Annu. Revi. Microbiol. 1986, 40, 367–393. [Google Scholar] [CrossRef] [PubMed]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Keppler, K.; Humpf, H.U. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorg. Med. Chem. 2005, 13, 5195–5205. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Yanez-Gascon, M.J.; Selma, M.V.; Gonzalez-Sarrias, A.; Toti, S.; Ceron, J.J.; Tomas-Barberan, F.; Dolara, P.; Espin, J.C. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J. Agric. Food Chem. 2009, 57, 2211–2220. [Google Scholar] [CrossRef]
- Anhe, F.F.; Roy, D.; Pilon, G.; Dudonne, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Calvo, M.; Lisnock, J.; Bull, H.G.; Hawes, B.E.; Burnett, D.A.; Braun, M.P.; Crona, J.H.; Davis, H.R., Jr.; Dean, D.C.; Detmers, P.A.; et al. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc. Natl. Acad. Sci. USA 2005, 102, 8132–8137. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, A.; Hapangama, N.; Yuan, Y.; Achanfuo-Yeboah, J.; Iannucci, R.; Chowdhury, S.; Alton, K.; Patrick, J.E.; Zbaida, S. Identification of human UDP-glucuronosyltransferase enzyme(s) responsible for the glucuronidation of ezetimibe (Zetia). Drug Metab. Dispos. 2004, 32, 314–320. [Google Scholar] [CrossRef]
- Patel, J.; Sheehan, V.; Gurk-Turner, C. Ezetimibe (Zetia): A new type of lipid-lowering agent. Proc. Bayl. Univ. Med. Cent. 2003, 16, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Temsamani, J.; Bonnafous, C.; Rousselle, C.; Fraisse, Y.; Clair, P.; Granier, L.A.; Rees, A.R.; Kaczorek, M.; Scherrmann, J.M. Improved brain uptake and pharmacological activity profile of morphine-6-glucuronide using a peptide vector-mediated strategy. J. Pharmacol. Exp. Ther. 2005, 313, 712–719. [Google Scholar] [CrossRef]
- Klimas, R.; Mikus, G. Morphine-6-glucuronide is responsible for the analgesic effect after morphine administration: A quantitative review of morphine, morphine-6-glucuronide, and morphine-3-glucuronide. Br. J. Anaesth. 2014, 113, 935–944. [Google Scholar] [CrossRef]
- MacDougall, J.M.; Zhang, X.D.; Polgar, W.E.; Khroyan, T.V.; Toll, L.; Cashman, J.R. Design, chemical synthesis, and biological evaluation of thiosaccharide analogues of morphine- and codeine-6-glucuronide. J. Med. Chem. 2004, 47, 5809–5815. [Google Scholar] [CrossRef]
- Gordon, H.L. Morphine intoxication in renal failure: The role of morphine-6-glucuronide. Br. Med. J. Clin. Res. Ed. 1986, 293, 818–819. [Google Scholar] [CrossRef] [PubMed]
- Mukker, J. Pharmacokinetic and Pharmacodynamic Studies on Flaxseed Lignans. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2013. [Google Scholar]
- Brand, W.; Schutte, M.E.; Williamson, G.; van Zanden, J.J.; Cnubben, N.H.; Groten, J.P.; van Bladeren, P.J.; Rietjens, I.M. Flavonoid-mediated inhibition of intestinal ABC transporters may affect the oral bioavailability of drugs, food-borne toxic compounds and bioactive ingredients. Biomed. Pharmacother. 2006, 60, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Batra, P.; Sharma, A.K. Anti-cancer potential of flavonoids: Recent trends and future perspectives. 3 Biotech 2013, 3, 439–459. [Google Scholar] [CrossRef] [PubMed]
- Sesink, A.L.; Arts, I.C.; de Boer, V.C.; Breedveld, P.; Schellens, J.H.; Hollman, P.C.; Russel, F.G. Breast cancer resistance protein (Bcrp1/Abcg2) limits net intestinal uptake of quercetin in rats by facilitating apical efflux of glucuronides. Mol. Pharmacol. 2005, 67, 1999–2006. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, J.; Zeng, S. Involvement of P-glycoprotein in regulating cellular levels of Ginkgo flavonols: Quercetin, kaempferol, and isorhamnetin. J. Pharm. Pharmacol. 2005, 57, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Miguel, V.; Otero, J.A.; Garcia-Villalba, R.; Tomas-Barberan, F.; Espin, J.C.; Merino, G.; Alvarez, A.I. Role of ABCG2 in transport of the mammalian lignan enterolactone and its secretion into milk in Abcg2 knockout mice. Drug Metab. Dispos. 2014, 42, 943–946. [Google Scholar] [CrossRef]
- Cascorbi, I. Role of pharmacogenetics of ATP-binding cassette transporters in the pharmacokinetics of drugs. Pharmacol. Ther. 2006, 112, 457–473. [Google Scholar] [CrossRef]
- Kerb, R. Implications of genetic polymorphisms in drug transporters for pharmacotherapy. Cancer Lett. 2006, 234, 4–33. [Google Scholar] [CrossRef]
- Ieiri, I.; Takane, H.; Hirota, T.; Otsubo, K.; Higuchi, S. Genetic polymorphisms of drug transporters: Pharmacokinetic and pharmacodynamic consequences in pharmacotherapy. Expert Opin. Drug Metab. Toxicol. 2006, 2, 651–674. [Google Scholar] [CrossRef]
- Figueira, I.; Menezes, R.; Macedo, D.; Costa, I.; Dos Santos, C.N. Polyphenols Beyond Barriers: A Glimpse into the Brain. Curr. Neuropharmacol. 2017, 15, 562–594. [Google Scholar] [CrossRef]
- Gee, J.M.; DuPont, M.S.; Day, A.J.; Plumb, G.W.; Williamson, G.; Johnson, I.T. Intestinal transport of quercetin glycosides in rats involves both deglycosylation and interaction with the hexose transport pathway. J. Nutr. 2000, 130, 2765–2771. [Google Scholar] [CrossRef]
- Hussain, S.A.; Sulaiman, A.A.; Alhaddad, H.; Alhadidi, Q. Natural polyphenols: Influence on membrane transporters. J. Intercult. Ethnopharmacol. 2016, 5, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; de Vries, J.H.; van Leeuwen, S.D.; Mengelers, M.J.; Katan, M.B. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995, 62, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef]
- Janle, E.M.; Lila, M.A.; Grannan, M.; Wood, L.; Higgins, A.; Yousef, G.G.; Rogers, R.B.; Kim, H.; Jackson, G.S.; Ho, L.; et al. Pharmacokinetics and tissue distribution of 14C-labeled grape polyphenols in the periphery and the central nervous system following oral administration. J. Med. Food 2010, 13, 926–933. [Google Scholar] [CrossRef]
- Chen, L.; Lee, M.J.; Li, H.; Yang, C.S. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab. Dispos. 1997, 25, 1045–1050. [Google Scholar] [PubMed]
- Gester, S.; Wuest, F.; Pawelke, B.; Bergmann, R.; Pietzsch, J. Synthesis and biodistribution of an 18F-labelled resveratrol derivative for small animal positron emission tomography. Amino Acids 2005, 29, 415–428. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Vari, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Chen, L. Polyphenols and Bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2018. [Google Scholar] [CrossRef]
- Murray, T.; Kang, J.; Astheimer, L.; Price, W.E. Tissue distribution of lignans in rats in response to diet, dose-response, and competition with isoflavones. J. Agric. Food Chem. 2007, 55, 4907–4912. [Google Scholar] [CrossRef]
- Saarinen, N.M.; Thompson, L.U. Prolonged administration of secoisolariciresinol diglycoside increases lignan excretion and alters lignan tissue distribution in adult male and female rats. Br. J. Nutr. 2010, 104, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Mukker, J.K.; Singh, R.S.P.; Muir, A.D.; Krol, E.S.; Alcorn, J. Comparative pharmacokinetics of purified flaxseed and associated mammalian lignans in male Wistar rats. Br. J. Nutr. 2015, 113, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Kurlbaum, M.; Hogger, P. Plasma protein binding of polyphenols from maritime pine bark extract (USP). J. Pharm. Biomed. Anal. 2011, 54, 127–132. [Google Scholar] [CrossRef]
- Xiao, J.; Zhao, Y.; Wang, H.; Yuan, Y.; Yang, F.; Zhang, C.; Yamamoto, K. Noncovalent interaction of dietary polyphenols with common human plasma proteins. J. Agric. Food Chem. 2011, 59, 10747–10754. [Google Scholar] [CrossRef]
- Xiao, J.; Kai, G. A review of dietary polyphenol-plasma protein interactions: Characterization, influence on the bioactivity, and structure-affinity relationship. Crit. Rev. Food Sci. Nutr. 2012, 52, 85–101. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Treatment with inhibitors of caspases, that are substrates of drug transporters, selectively permits chemotherapy-induced apoptosis in multidrug-resistant cells but protects normal cells. Leukemia 2001, 15, 936–941. [Google Scholar] [CrossRef]
- Wu, C.P.; Calcagno, A.M.; Hladky, S.B.; Ambudkar, S.V.; Barrand, M.A. Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5). FEBS J. 2005, 272, 4725–4740. [Google Scholar] [CrossRef] [PubMed]
- Jodoin, J.; Demeule, M.; Beliveau, R. Inhibition of the multidrug resistance P-glycoprotein activity by green tea polyphenols. Biochim. biophys. Acta 2002, 1542, 149–159. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, M. Natural polyphenol disposition via coupled metabolic pathways. Expert Opin. Drug Metab. Toxicol. 2007, 3, 389–406. [Google Scholar] [CrossRef]
- Scheepens, A.; Tan, K.; Paxton, J.W. Improving the oral bioavailability of beneficial polyphenols through designed synergies. Genes Nutr. 2010, 5, 75–87. [Google Scholar] [CrossRef]
- Hu, M.; Wu, B.; Liu, Z. Bioavailability of Polyphenols and Flavonoids in the Era of Precision Medicine. Mol. Pharm. 2017, 14, 2861–2863. [Google Scholar] [CrossRef]
- Amararathna, M.; Johnston, M.R.; Rupasinghe, H.P. Plant Polyphenols as Chemopreventive Agents for Lung Cancer. Int. J. Mol. Sci. 2016, 17, 1352. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Maier, C.S. The Chemistry of Gut Microbial Metabolism of Polyphenols. Phytochem. Rev. 2016, 15, 425–444. [Google Scholar] [CrossRef]
- Marin, L.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. Biomed. Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef]
- Most types of cancer not due to ‘bad luck’ IARC responds to scientific article claiming that environmental and lifestyle factors account for less than one third of cancers. Centr. Eur. J. Public Health 2015, 23, 87.
- Weinberg, C.R.; Zaykin, D. Is bad luck the main cause of cancer? J. Natl. Cancer Inst 2015, 107. [Google Scholar] [CrossRef]
- Rozhok, A.I.; Wahl, G.M.; DeGregori, J. A Critical Examination of the “Bad Luck” Explanation of Cancer Risk. Cancer Prev. Res. 2015, 8, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, C.; Vogelstein, B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 2015, 347, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zhang, C.; Xu, Y. Somatic mutations may not be the primary drivers of cancer formation. Int. J. Cancer 2015, 137, 2762–2765. [Google Scholar] [CrossRef]
- Lichtenstein, A.V. Cancer: Bad Luck or Punishment? Biochem. Biokhim. 2017, 82, 75–80. [Google Scholar] [CrossRef]
- Gleichenhagen, M.; Schieber, A. Current challenges in polyphenol analytical chemistry. Curr. Opin. Food Sci. 2016, 7, 43–49. [Google Scholar] [CrossRef]
- Plaza, M.; Domínguez-Rodríguez, G.; Castro-Puyana, M.; Marina, M.L. 6-Polyphenols analysis and related challenges. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 177–232. [Google Scholar] [CrossRef]
- Opara, E.I.; Chohan, M. Culinary Herbs and Spices: Their Bioactive Properties, the Contribution of Polyphenols and the Challenges in Deducing Their True Health Benefits. Int. J. Mol. Sci. 2014, 15, 19183–19202. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Arseneault, M.; Sanderson, T.; Murthy, V.; Ramassamy, C. Challenges for research on polyphenols from foods in Alzheimer’s disease: Bioavailability, metabolism, and cellular and molecular mechanisms. J. Agric. Food Chem. 2008, 56, 4855–4873. [Google Scholar] [CrossRef] [PubMed]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Smoliga, J.M.; Vang, O.; Baur, J.A. Challenges of translating basic research into therapeutics: Resveratrol as an example. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 158–167. [Google Scholar] [CrossRef]
- Vittorio, O.; Curcio, M.; Cojoc, M.; Goya, G.F.; Hampel, S.; Iemma, F.; Dubrovska, A.; Cirillo, G. Polyphenols delivery by polymeric materials: Challenges in cancer treatment. Drug Deliv. 2017, 24, 162–180. [Google Scholar] [CrossRef]
- Gao, S.; Hu, M. Bioavailability challenges associated with development of anti-cancer phenolics. Mini Rev. Med. Chem. 2010, 10, 550–567. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cao, Y.L.; Jiang, J.G.; Lin, Q.S.; Chen, J.; Zhu, L. Response surface optimization of ultrasound-assisted flavonoids extraction from the flower of Citrus aurantium L. var. amara Engl. J. Sep. Sci. 2010, 33, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Y.; Chen, G.; Yue, W.; Liang, Q.; Wu, Q. Optimisation of ultrasound assisted extraction of phenolic compounds from Sparganii rhizoma with response surface methodology. Ultrason. Sonochem. 2013, 20, 846–854. [Google Scholar] [CrossRef]
- Kurepa, J.; Nakabayashi, R.; Paunesku, T.; Suzuki, M.; Saito, K.; Woloschak, G.E.; Smalle, J.A. Direct isolation of flavonoids from plants using ultra-small anatase TiO(2) nanoparticles. Plant J. Cell Mol. Biol. 2014, 77, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.L.; Wang, D.; Li, Y.Y.; Peng, H.Y.; Yuan, M.Y.; Gao, F. Ultrasound-assisted extraction of total flavonoids from Aconitum gymnandrum. Pharmacogn. Mag. 2014, 10, S141–S146. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Du, G.; Chen, J. Novel fermentation processes for manufacturing plant natural products. Curr. Opin. Biotechnol. 2014, 25, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Du, G.; Zhou, J.; Chen, J. Systems metabolic engineering of microorganisms to achieve large-scale production of flavonoid scaffolds. J. Biotechnol. 2014, 188, 72–80. [Google Scholar] [CrossRef]
- Santos, C.N.; Koffas, M.; Stephanopoulos, G. Optimization of a heterologous pathway for the production of flavonoids from glucose. Metab. Eng. 2011, 13, 392–400. [Google Scholar] [CrossRef]
- Wu, J.; Du, G.; Zhou, J.; Chen, J. Metabolic engineering of Escherichia coli for (2S)-pinocembrin production from glucose by a modular metabolic strategy. Metab. Eng. 2013, 16, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.; Yu, O. Metabolic engineering of flavonoids in plants and microorganisms. Appl. Microbiol. Biotechnol. 2011, 91, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Vannelli, T.; Wei Qi, W.; Sweigard, J.; Gatenby, A.A.; Sariaslani, F.S. Production of p-hydroxycinnamic acid from glucose in Saccharomyces cerevisiae and Escherichia coli by expression of heterologous genes from plants and fungi. Metab. Eng. 2007, 9, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Chen, Y.C.; Su, C.Y.; Hong, C.S.; Ho, H.O.; Sheu, M.T. Development and characterization of self-assembling lecithin-based mixed polymeric micelles containing quercetin in cancer treatment and an in vivo pharmacokinetic study. Int. J. Nanomed. 2016, 11, 1557–1566. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, G.; Kumar, R.; Singh, B.; Malik, R.; Katare, O.P.; Raza, K. Promises of a biocompatible nanocarrier in improved brain delivery of quercetin: Biochemical, pharmacokinetic and biodistribution evidences. Int. J. Pharm. 2016, 515, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.S.; Quelhas, S.; Silva, A.M.; Souto, E.B. Nanoemulsions for delivery of flavonoids: Formulation and in vitro release of rutin as model drug. Pharm. Dev. Technol. 2014, 19, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Liu, C.; Zhang, J.; Wang, F.; Wang, J.; Zhang, J. A new drug nanocrystal self-stabilized Pickering emulsion for oral delivery of silybin. Eur. J. Pharm. Sci. 2017, 96, 420–427. [Google Scholar] [CrossRef]
- Di, Y.; Ji, S.; Wolf, P.; Krol, E.S.; Alcorn, J. Enterolactone glucuronide and β-glucuronidase in antibody directed enzyme prodrug therapy for targeted prostate cancer cell treatment. AAPS PharmSciTech 2017, 18, 2336–2345. [Google Scholar] [CrossRef]
- Docampo, M.; Olubu, A.; Wang, X.; Pasinetti, G.; Dixon, R.A. Glucuronidated Flavonoids in Neurological Protection: Structural Analysis and Approaches for Chemical and Biological Synthesis. J. Agric. Food Chem. 2017, 65, 7607–7623. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wan, S.B.; Yang, H.; Yuan, J.; Chan, T.H.; Dou, Q.P. EGCG, green tea polyphenols and their synthetic analogs and prodrugs for human cancer prevention and treatment. Adv. Clin. Chem. 2011, 53, 155–177. [Google Scholar]
- Terao, J.; Murota, K.; Kawai, Y. Conjugated quercetin glucuronides as bioactive metabolites and precursors of aglycone in vivo. Food Funct. 2011, 2, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Hollman, P.C.; Vree, T.B.; Katan, M.B. Bioavailabilities of quercetin-3-glucoside and quercetin-4’-glucoside do not differ in humans. J. Nutr. 2000, 130, 1200–1203. [Google Scholar] [CrossRef]
- Tatiraju, D.V.; Bagade, V.B.; Karambelkar, P.J.; Jadhav, V.M.; Kadam, V. Natural bioenhancers: An overview. Available online: http://www.phytojournal.com/vol2Issue3/15.1.html (accessed on 30 March 2019).
- Rinwa, P.; Kumar, A. Quercetin along with piperine prevents cognitive dysfunction, oxidative stress and neuro-inflammation associated with mouse model of chronic unpredictable stress. Arch. Pharm. Res. 2017, 40, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Hong, J.; Kim, D.H.; Mishin, V.M.; Yang, C.S. Piperine enhances the bioavailability of the tea polyphenol (-)-epigallocatechin-3-gallate in mice. J. Nutr. 2004, 134, 1948–1952. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, J.B.; Walle, T. Cellular uptake and efflux of the tea flavonoid (-)epicatechin-3-gallate in the human intestinal cell line Caco-2. J. Pharmacol. Exp. Ther. 2003, 307, 745–752. [Google Scholar] [CrossRef]
- Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Althagafy, H.S.; Graf, T.N.; Sy-Cordero, A.A.; Gufford, B.T.; Paine, M.F.; Wagoner, J.; Polyak, S.J.; Croatt, M.P.; Oberlies, N.H. Semisynthesis, cytotoxicity, antiviral activity, and drug interaction liability of 7-O-methylated analogues of flavonolignans from milk thistle. Bioorg. Med. Chem. 2013, 21, 3919–3926. [Google Scholar] [CrossRef]
- Vue, B.; Zhang, S.; Zhang, X.; Parisis, K.; Zhang, Q.; Zheng, S.; Wang, G.; Chen, Q.H. Silibinin derivatives as anti-prostate cancer agents: Synthesis and cell-based evaluations. Eur. J. Med. Chem. 2016, 109, 36–46. [Google Scholar] [CrossRef]
- Grande, F.; Parisi, O.I.; Mordocco, R.A.; Rocca, C.; Puoci, F.; Scrivano, L.; Quintieri, A.M.; Cantafio, P.; Ferla, S.; Brancale, A.; et al. Quercetin derivatives as novel antihypertensive agents: Synthesis and physiological characterization. Eur. J. Pharm. Sci. 2016, 82, 161–170. [Google Scholar] [CrossRef]
- Kim, M.K.; Park, K.S.; Lee, C.; Park, H.R.; Choo, H.; Chong, Y. Enhanced stability and intracellular accumulation of quercetin by protection of the chemically or metabolically susceptible hydroxyl groups with a pivaloxymethyl (POM) promoiety. J. Med. Chem. 2010, 53, 8597–8607. [Google Scholar] [CrossRef] [PubMed]
- Patra, N.; De, U.; Kang, J.A.; Kim, J.M.; Ahn, M.Y.; Lee, J.; Jung, J.H.; Chung, H.Y.; Moon, H.R.; Kim, H.S. A novel epoxypropoxy flavonoid derivative and topoisomerase II inhibitor, MHY336, induces apoptosis in prostate cancer cells. Eur. J. Pharmacol. 2011, 658, 98–107. [Google Scholar] [CrossRef]
- He, Z.; Xu, M.; Zeng, M.; Qin, F.; Chen, J. Interactions of milk alpha- and beta-casein with malvidin-3-O-glucoside and their effects on the stability of grape skin anthocyanin extracts. Food Chem. 2016, 199, 314–322. [Google Scholar] [CrossRef]
- Arroyo-Maya, I.J.; Campos-Teran, J.; Hernandez-Arana, A.; McClements, D.J. Characterization of flavonoid-protein interactions using fluorescence spectroscopy: Binding of pelargonidin to dairy proteins. Food Chem. 2016, 213, 431–439. [Google Scholar] [CrossRef]
- Tang, L.; Li, S.; Bi, H.; Gao, X. Interaction of cyanidin-3-O-glucoside with three proteins. Food Chem. 2016, 196, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Rawat, M.S.; Semalty, A.; Semalty, M. Quercetin-phospholipid complex: An amorphous pharmaceutical system in herbal drug delivery. Curr. Drug Discov. Technol. 2012, 9, 17–24. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, M.; Liu, Z.; Zhang, Y.; Gu, L.; Hu, G.; Chen, X.; Jia, J. Development of quercetin-phospholipid complex to improve the bioavailability and protection effects against carbon tetrachloride-induced hepatotoxicity in SD rats. Fitoterapia 2016, 113, 102–109. [Google Scholar] [CrossRef]
- Cimino, S.; Sortino, G.; Favilla, V.; Castelli, T.; Madonia, M.; Sansalone, S.; Russo, G.I.; Morgia, G. Polyphenols: Key Issues Involved in Chemoprevention of Prostate Cancer. Oxid. Med. Cell. Longev. 2012, 2012, 8. [Google Scholar] [CrossRef]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomas-Barberan, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Kobuchi, H.; Roy, S.; Sen, C.K.; Nguyen, H.G.; Packer, L. Quercetin inhibits inducible ICAM-1 expression in human endothelial cells through the JNK pathway. Am. J. Physiol.-Cell Physiol. 1999, 277, C403–C411. [Google Scholar] [CrossRef]
- Kong, A.N.; Yu, R.; Chen, C.; Mandlekar, S.; Primiano, T. Signal transduction events elicited by natural products: Role of MAPK and caspase pathways in homeostatic response and induction of apoptosis. Arch. Pharm. Res. 2000, 23, 1–16. [Google Scholar] [CrossRef]
- Spencer, J.P.; Schroeter, H.; Crossthwaithe, A.J.; Kuhnle, G.; Williams, R.J.; Rice-Evans, C. Contrasting influences of glucuronidation and O-methylation of epicatechin on hydrogen peroxide-induced cell death in neurons and fibroblasts. Free Radic. Biol. Med. 2001, 31, 1139–1146. [Google Scholar] [CrossRef]
- Schroeter, H.; Spencer, J.P.; Rice-Evans, C.; Williams, R.J. Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem. J. 2001, 358, 547–557. [Google Scholar] [CrossRef]
- Muir, A.E.; Westcott, N.; Hardman, R. Flax: The Genus Linum; CRC Press: London, UK, 2003; p. 320. [Google Scholar]
- Bernacchia, R.; Preti, R.; Vinci, G. Chemical Composition and Health Benefits of Flaxseed. Austin J. Nutr. Food Sci. 2014, 2. [Google Scholar]
- Shim, Y.Y.; Gui, B.; Arnison, P.G.; Wang, Y.; Reaney, M.J.T. Flaxseed (Linum usitatissimum L.) bioactive compounds and peptide nomenclature: A review. Trends Food Sci. Technol. 2014, 38, 5–20. [Google Scholar] [CrossRef]
- Tarpila, S.; Tarpila, A.; Grohn, P.; Silvennoinen, T.; Lindberg, L. Efficacy of ground flaxseed on constipation in patients with irritable bowel syndrome. Curr. Top. Nutraceut. Res. 2004, 2, 119–125. [Google Scholar]
- Health-Canada. Monograph: Flaxseed. Available online: http://webprod.hc-sc.gc.ca/nhpid-bdipsn/monoReq.do?id=219 (accessed on 2 January 2018).
- Rajaram, S. Health benefits of plant-derived alpha-linolenic acid. Am. J. Clin. Nutr 2014, 100, 443S–448S. [Google Scholar] [CrossRef]
- Health-Canada. Summary of Health Canada’s Assessment of a Health Claim about Ground Whole Flaxseed (Bureau of Nutritional Sciences-Food Directorate, Health Products and Food Branch). Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-labelling/health-claims/assessments/ground-whole-flaxseed-blood-cholesterol-lowering-nutrition-health-claims-food-labelling.html (accessed on 2 January 2018).
- Wong, H.; Chahal, N.; Manlhiot, C.; Niedra, E.; McCrindle, B.W. Flaxseed in pediatric hyperlipidemia: A placebo-controlled, blinded, randomized clinical trial of dietary flaxseed supplementation for children and adolescents with hypercholesterolemia. JAMA Pediatr. 2013, 167, 708–713. [Google Scholar] [CrossRef]
- Patade, A.; Devareddy, L.; Lucas, E.A.; Korlagunta, K.; Daggy, B.P.; Arjmandi, B.H. Flaxseed reduces total and LDL cholesterol concentrations in Native American postmenopausal women. J. Women’s Health 2008, 17, 355–366. [Google Scholar] [CrossRef]
- Lucas, E.A.; Wild, R.D.; Hammond, L.J.; Khalil, D.A.; Juma, S.; Daggy, B.P.; Stoecker, B.J.; Arjmandi, B.H. Flaxseed improves lipid profile without altering biomarkers of bone metabolism in postmenopausal women. J. Clin. Endocrinol. Metab. 2002, 87, 1527–1532. [Google Scholar] [CrossRef]
- Arjmandi, B.H.; Khan, D.A.; Juma, S.; Drum, M.L.; Venkatesh, S.; Sohn, E.; Wei, L.; Derman, R. Whole flaxseed consumption lowers serum LDL-cholesterol and lipoprotein(a) concentrations in postmenopausal women. Nutr. Rese. 1998, 18, 1203–1214. [Google Scholar] [CrossRef]
- Cunnane, S.C.; Hamadeh, M.J.; Liede, A.C.; Thompson, L.U.; Wolever, T.M.; Jenkins, D.J. Nutritional attributes of traditional flaxseed in healthy young adults. Am. J. Clin. Nutr. 1995, 61, 62–68. [Google Scholar] [CrossRef]
- U.S.-National-Institutes-of-Health. Flaxseed and Flaxseed Oil. Available online: https://www.ncbi.nlm.nih.gov/pubmed/ (accessed on 30 March 2019).
- Flax-Council-of-Canada. Flax: A Healthy Food. Available online: https://flaxcouncil.ca/resources/nutrition/general-nutrition-information/flax-a-healthy-food/ (accessed on 30 March 2019).
- Healthyflax.org. HomeHealthy Flax. Available online: https://healthyflax.org/ (accessed on 29 March 2019).
- AmeriFlax. Flax FAQs—AmeriFlax. Available online: https://www.ameriflax.com/flax-faqs (accessed on 29 March 2019).
- Kaur, P.; Waghmare, R.; Kumar, V.; Rasane, P.; Kaur, S.; Gat, Y. Recent advances in utilization of flaxseed as potential source for value addition. OCL 2018, 25, A304. [Google Scholar] [CrossRef]
- Benedetti, E.; Pedone, C. Cyclolinopeptide A: Inhibitor, immunosuppressor or other? J. Pept. Sci. 2005, 11, 268–272. [Google Scholar] [CrossRef]
- Gallo, P.; Rossi, F.; Saviano, M.; Pedone, C.; Colonna, G.; Ragone, R. Specific Interaction between Bovine Cyclophilin A and Synthetic Analogues of Cyclolinopeptide A. J. Biochem. 1998, 124, 880–885. [Google Scholar] [CrossRef]
- Okinyo-Owiti, D.P.; Dong, Q.; Ling, B.; Jadhav, P.D.; Bauer, R.; Maley, J.M.; Reaney, M.J.T.; Yang, J.; Sammynaiken, R. Evaluating the cytotoxicity of flaxseed orbitides for potential cancer treatment. Toxicol. Rep. 2015, 2, 1014–1018. [Google Scholar] [CrossRef]
- Korhonen, H. Technology options for new nutritional concepts. Int. J. Dairy Technol. 2002, 55, 79–88. [Google Scholar] [CrossRef]
- Di, Y.; Jones, J.; Mansell, K.; Whiting, S.; Fowler, S.; Thorpe, L.; Billinsky, J.; Viveky, N.; Cheng, P.C.; Almousa, A.; et al. Influence of Flaxseed Lignan Supplementation to Older Adults on Biochemical and Functional Outcome Measures of Inflammation. J. Am. Coll. Nutr. 2017, 36, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Billinsky, J.; Glew, R.A.; Cornish, S.M.; Whiting, S.J.; Thorpe, L.U.; Alcorn, J.; Paus-Jenssen, L.; Hadjistavropoulos, T.; Chilibeck, P.D. No evidence of hypoglycEemia or hypotension in older adults during 6 months of flax lignan supplementation in a randomized controlled trial: A safety evaluation. Pharm. Biol. 2013, 51, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Sonestedt, E.; Wirfält, E. Enterolactone and breast cancer: Methodological issues may contribute to conflicting results in observational studies. Nutr. Res. 2010, 30, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Lamuela-Raventos, R.; Ray, S.; Micek, A.; Pajak, A.; Sciacca, S.; D’Orazio, N.; Del Rio, D.; Galvano, F. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol. Nutr. Food Res. 2017, 61, 1600930. [Google Scholar] [CrossRef] [PubMed]
- Wallstrom, P.; Drake, I.; Sonestedt, E.; Gullberg, B.; Bjartell, A.; Olsson, H.; Adlercreutz, H.; Tikkanen, M.J.; Wirfalt, E. Plasma enterolactone and risk of prostate cancer in middle-aged Swedish men. Eur. J. Nutr. 2018, 57, 2595–2606. [Google Scholar] [CrossRef]
- Johnsen, N.F.; Hausner, H.; Olsen, A.; Tetens, I.; Christensen, J.; Knudsen, K.E.; Overvad, K.; Tjonneland, A. Intake of whole grains and vegetables determines the plasma enterolactone concentration of Danish women. J. Nutr. 2004, 134, 2691–2697. [Google Scholar] [CrossRef][Green Version]
- Stattin, P.; Adlercreutz, H.; Tenkanen, L.; Jellum, E.; Lumme, S.; Hallmans, G.; Harvei, S.; Teppo, L.; Stumpf, K.; Luostarinen, T.; et al. Circulating enterolactone and prostate cancer risk: A Nordic nested case-control study. Int. J. Cancer 2002, 99, 124–129. [Google Scholar] [CrossRef]
- Chen, J.; Thompson, L.U. Lignans and tamoxifen, alone or in combination, reduce human breast cancer cell adhesion, invasion and migration in vitro. Breast Cancer Res. Treat. 2003, 80, 163–170. [Google Scholar] [CrossRef]
- Saarinen, N.M.; Abrahamsson, A.; Dabrosin, C. Estrogen-induced angiogenic factors derived from stromal and cancer cells are differently regulated by enterolactone and genistein in human breast cancer in vivo. Int. J. Cancer 2010, 127, 737–745. [Google Scholar] [CrossRef]
- Hedelin, M.; Balter, K.A.; Chang, E.T.; Bellocco, R.; Klint, A.; Johansson, J.E.; Wiklund, F.; Thellenberg-Karlsson, C.; Adami, H.O.; Gronberg, H. Dietary intake of phytoestrogens, estrogen receptor-beta polymorphisms and the risk of prostate cancer. Prostate 2006, 66, 1512–1520. [Google Scholar] [CrossRef]
- Hedelin, M.; Klint, A.; Chang, E.T.; Bellocco, R.; Johansson, J.E.; Andersson, S.O.; Heinonen, S.M.; Adlercreutz, H.; Adami, H.O.; Gronberg, H.; et al. Dietary phytoestrogen, serum enterolactone and risk of prostate cancer: The cancer prostate Sweden study (Sweden). Cancer Causes Control 2006, 17, 169–180. [Google Scholar] [CrossRef]
- Rhee, Y. Flaxseed Lignan Metabolite, Enterolactone, Down-regulated DNA Methyltransferase, Histone Deacetylase, and Methyl-CpG-binding Domain Protein Expression in Murine Adipocytes. FASEB J. 2016, 30. [Google Scholar] [CrossRef]
- McCann, M.J.; Gill, C.I.; Linton, T.; Berrar, D.; McGlynn, H.; Rowland, I.R. Enterolactone restricts the proliferation of the LNCaP human prostate cancer cell line in vitro. Mol. Nutr. Food Res. 2008, 52, 567–580. [Google Scholar] [CrossRef]
- McCann, S.E.; Hootman, K.C.; Weaver, A.M.; Thompson, L.U.; Morrison, C.; Hwang, H.; Edge, S.B.; Ambrosone, C.B.; Horvath, P.J.; Kulkarni, S.A. Dietary intakes of total and specific lignans are associated with clinical breast tumor characteristics. J. Nutr. 2012, 142, 91–98. [Google Scholar] [CrossRef]
- Chen, J.; Saggar, J.K.; Corey, P.; Thompson, L.U. Flaxseed cotyledon fraction reduces tumour growth and sensitises tamoxifen treatment of human breast cancer xenograft (MCF-7) in athymic mice. Br. J. Nutr. 2011, 105, 339–347. [Google Scholar] [CrossRef]
- Sacco, S.M.; Chen, J.; Ganss, B.; Thompson, L.U.; Ward, W.E. Flaxseed enhances the beneficial effect of low-dose estrogen therapy at reducing bone turnover and preserving bone microarchitecture in ovariectomized rats. Appl. Physiol. Nutr. Metab. 2014, 39, 801–810. [Google Scholar] [CrossRef]
- Chen, J.; Saggar, J.K.; Ward, W.E.; Thompson, L.U. Effects of flaxseed lignan and oil on bone health of breast-tumor-bearing mice treated with or without tamoxifen. J. Toxicol. Environ. Health A 2011, 74, 757–768. [Google Scholar] [CrossRef]
- McCann, S.E.; Thompson, L.U.; Nie, J.; Dorn, J.; Trevisan, M.; Shields, P.G.; Ambrosone, C.B.; Edge, S.B.; Li, H.F.; Kasprzak, C.; et al. Dietary lignan intakes in relation to survival among women with breast cancer: The Western New York Exposures and Breast Cancer (WEB) Study. Breast Cancer Res. Treat. 2010, 122, 229–235. [Google Scholar] [CrossRef]
- Saggar, J.K.; Chen, J.; Corey, P.; Thompson, L.U. Dietary flaxseed lignan or oil combined with tamoxifen treatment affects MCF-7 tumor growth through estrogen receptor- and growth factor-signaling pathways. Mol. Nutr. Food Res. 2010, 54, 415–425. [Google Scholar] [CrossRef]
- Saggar, J.K.; Chen, J.; Corey, P.; Thompson, L.U. The effect of secoisolariciresinol diglucoside and flaxseed oil, alone and in combination, on MCF-7 tumor growth and signaling pathways. Nutr. Cancer 2010, 62, 533–542. [Google Scholar] [CrossRef]
- Truan, J.S.; Chen, J.M.; Thompson, L.U. Flaxseed oil reduces the growth of human breast tumors (MCF-7) at high levels of circulating estrogen. Mol. Nutr. Food Res. 2010, 54, 1414–1421. [Google Scholar] [CrossRef]
- Chen, J.; Saggar, J.K.; Corey, P.; Thompson, L.U. Flaxseed and pure secoisolariciresinol diglucoside, but not flaxseed hull, reduce human breast tumor growth (MCF-7) in athymic mice. J. Nutr. 2009, 139, 2061–2066. [Google Scholar] [CrossRef] [PubMed]
- Serraino, M.; Thompson, L.U. The effect of flaxseed supplementation on early risk markers for mammary carcinogenesis. Cancer Lett. 1991, 60, 135–142. [Google Scholar] [CrossRef]
- Power, K.A.; Ward, W.E.; Chen, J.M.; Saarinen, N.M.; Thompson, L.U. Flaxseed and soy protein isolate, alone and in combination, differ in their effect on bone mass, biomechanical strength, and uterus in ovariectomized nude mice with MCF-7 human breast tumor xenografts. J. Toxicol. Environ. Health A 2007, 70, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Sacco, S.M.; Power, K.A.; Chen, J.; Ward, W.E.; Thompson, L.U. Interaction of sesame seed and tamoxifen on tumor growth and bone health in athymic mice. Exp. Biol. Med. 2007, 232, 754–761. [Google Scholar]
- Herchi, W.; Arraez-Roman, D.; Trabelsi, H.; Bouali, I.; Boukhchina, S.; Kallel, H.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Phenolic compounds in flaxseed: A review of their properties and analytical methods. An overview of the last decade. J. Oleo Sci. 2014, 63, 7–14. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Mazur, W. Phyto-oestrogens and Western diseases. Ann. Med. 1997, 29, 95–120. [Google Scholar] [CrossRef]
- Brash, A.R.; Song, W.C. Structure-function features of flaxseed allene oxide synthase. J. Lipid Mediat. Cell Signal. 1995, 12, 275–282. [Google Scholar] [CrossRef]
- Meagher, L.P.; Beecher, G.R.; Flanagan, V.P.; Li, B.W. Isolation and Characterization of the Lignans, Isolariciresinol and Pinoresinol, in Flaxseed Meal. J. Agric. Food Chem. 1999, 47, 3173–3180. [Google Scholar] [CrossRef]
- Thompson, L.U.; Boucher, B.A.; Liu, Z.; Cotterchio, M.; Kreiger, N. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr. Cancer 2006, 54, 184–201. [Google Scholar] [CrossRef]
- Bloedon, L.T.; Szapary, P.O. Flaxseed and cardiovascular risk. Nutr. Rev. 2004, 62, 18–27. [Google Scholar] [CrossRef]
- Struijs, K. The Lignan Macromolecule from Flaxseed: Structure and Bioconversion of Lignans. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2008. [Google Scholar]
- Ghose, K.; Selvaraj, K.; McCallum, J.; Kirby, C.W.; Sweeney-Nixon, M.; Cloutier, S.J.; Deyholos, M.; Datla, R.; Fofana, B. Identification and functional characterization of a flax UDP-glycosyltransferase glucosylating secoisolariciresinol (SECO) into secoisolariciresinol monoglucoside (SMG) and diglucoside (SDG). BMC Plant Biol. 2014, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, T. Diversity in lignan biosynthesis. Phytochem. Rev. 2003, 2, 371–390. [Google Scholar] [CrossRef]
- Cortes, C.; Gagnon, N.; Benchaar, C.; da Silva, D.; Santos, G.T.; Petit, H.V. In vitro metabolism of flax lignans by ruminal and faecal microbiota of dairy cows. J. Appl. Microbiol. 2008, 105, 1585–1594. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Shouman, S.A.; Elkhoely, A.; Attia, Y.M.; Elsesy, M.S.; El Senousy, A.S.; Choucry, M.A.; El Gayed, S.H.; El Sayed, A.A.; Sattar, E.A.; et al. Anticancer potentiality of lignan rich fraction of six Flaxseed cultivars. Sci. Rep. 2018, 8, 544. [Google Scholar] [CrossRef]
- Garros, L.; Drouet, S.; Corbin, C.; Decourtil, C.; Fidel, T.; Lebas de Lacour, J.; Leclerc, E.A.; Renouard, S.; Tungmunnithum, D.; Doussot, J.; et al. Insight into the Influence of Cultivar Type, Cultivation Year, and Site on the Lignans and Related Phenolic Profiles, and the Health-Promoting Antioxidant Potential of Flax (Linum usitatissimum L.) Seeds. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Thompson, L.U.; Rickard, S.E.; Cheung, F.; Kenaschuk, E.O.; Obermeyer, W.R. Variability in anticancer lignan levels in flaxseed. Nutr. Cancer 1997, 27, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, P.; Kamal-Eldin, A.; Lundgren, L.N.; Aman, P. HPLC method for analysis of secoisolariciresinol diglucoside in flaxseeds. J. Agric. Food Chem. 2000, 48, 5216–5219. [Google Scholar] [CrossRef]
- Setchell, K.D.; Lawson, A.M.; Mitchell, F.L.; Adlercreutz, H.; Kirk, D.N.; Axelson, M. Lignans in man and in animal species. Nature 1980, 287, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Lawson, A.M.; Conway, E.; Taylor, N.F.; Kirk, D.N.; Cooley, G.; Farrant, R.D.; Wynn, S.; Axelson, M. The definitive identification of the lignans trans-2,3-bis(3-hydroxybenzyl)-gamma-butyrolactone and 2,3-bis(3-hydroxybenzyl)butane-1,4-diol in human and animal urine. Biochem. J. 1981, 197, 447–458. [Google Scholar] [CrossRef]
- Axelson, M.; Sjovall, J.; Gustafsson, B.E.; Setchell, K.D. Origin of lignans in mammals and identification of a precursor from plants. Nature 1982, 298, 659–660. [Google Scholar] [CrossRef]
- Saleem, M.; Kim, H.J.; Ali, M.S.; Lee, Y.S. An update on bioactive plant lignans. Nat. Prod. Rep. 2005, 22, 696–716. [Google Scholar] [CrossRef]
- Lignans: chemical, biological and clinical properties. Comp. Biochem. Physiol. Part A Physiol. 1991, 100, 231. [CrossRef]
- Hatfield, R.; Vermerris, W. Lignin formation in plants. The dilemma of linkage specificity. Plant Physiol. 2001, 126, 1351–1357. [Google Scholar] [CrossRef]
- DeVries, J.W. On defining dietary fibre. Proc. Nutr. Soc. 2003, 62, 37–43. [Google Scholar] [CrossRef]
- Lewis, L.B.D.; Michaël, J.; Ann, M.P.; Kye-Won, K.; Daniel, G.V.; Norman, G. Dissection of lignin macromolecular configuration and assembly: Comparison to related biochemical processes in allyl/propenyl phenol and lignan biosynthesis. Nat. Prod. Rep. 2008, 25, 1015–1090. [Google Scholar] [CrossRef]
- Smeds, A.I.; Eklund, P.C.; Sjoholm, R.E.; Willfor, S.M.; Nishibe, S.; Deyama, T.; Holmbom, B.R. Quantification of a broad spectrum of lignans in cereals, oilseeds, and nuts. J. Agric. Food Chem. 2007, 55, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Kamal-Eldin, A.; Peerlkamp, N.; Johnsson, P.; Andersson, R.; Andersson, R.E.; Lundgren, L.N.; Åman, P. An oligomer from flaxseed composed of secoisolariciresinoldiglucoside and 3-hydroxy-3-methyl glutaric acid residues. Phytochemistry 2001, 58, 587–590. [Google Scholar] [CrossRef]
- Struijs, K.; Vincken, J.P.; Doeswijk, T.G.; Voragen, A.G.; Gruppen, H. The chain length of lignan macromolecule from flaxseed hulls is determined by the incorporation of coumaric acid glucosides and ferulic acid glucosides. Phytochemistry 2009, 70, 262–269. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Klaes, M.; Sendker, J. Lignans in seeds of Linum species. Phytochemistry 2012, 82, 89–99. [Google Scholar] [CrossRef]
- Struijs, K.; Vincken, J.P.; Verhoef, R.; Voragen, A.G.; Gruppen, H. Hydroxycinnamic acids are ester-linked directly to glucosyl moieties within the lignan macromolecule from flaxseed hulls. Phytochemistry 2008, 69, 1250–1260. [Google Scholar] [CrossRef]
- Ford, J.D.; Huang, K.-S.; Wang, H.-B.; Davin, L.B.; Lewis, N.G. Biosynthetic Pathway to the Cancer Chemopreventive Secoisolariciresinol Diglucoside-Hydroxymethyl Glutaryl Ester-Linked Lignan Oligomers in Flax (Linum u sitatissimum) Seed. J. Nat. Prod. 2001, 64, 1388–1397. [Google Scholar] [CrossRef]
- Yuan, J.P.; Li, X.; Xu, S.P.; Wang, J.H.; Liu, X. Hydrolysis kinetics of secoisolariciresinol diglucoside oligomers from flaxseed. J. Agric. Food Chem. 2008, 56, 10041–10047. [Google Scholar] [CrossRef] [PubMed]
- Struijs, K.; Vincken, J.P.; Verhoef, R.; van Oostveen-van Casteren, W.H.; Voragen, A.G.; Gruppen, H. The flavonoid herbacetin diglucoside as a constituent of the lignan macromolecule from flaxseed hulls. Phytochemistry 2007, 68, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Kosinska, A.; Penkacik, K.; Wiczkowski, W.; Amarowicz, R. Presence of caffeic acid in flaxseed lignan macromolecule. Plant. Foods Hum. Nutr. 2011, 66, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Faughnan, M.; Hoey, L.; Wahala, K.; Williamson, G.; Cassidy, A. Bioavailability of phyto-oestrogens. Br. J. Nutr. 2003, 89, S45–S58. [Google Scholar] [CrossRef]
- Clavel, T.; Henderson, G.; Engst, W.; Dore, J.; Blaut, M. Phylogeny of human intestinal bacteria that activate the dietary lignan secoisolariciresinol diglucoside. FEMS Microbiol. Ecol. 2006, 55, 471–478. [Google Scholar] [CrossRef]
- Schogor, A.L.; Huws, S.A.; Santos, G.T.; Scollan, N.D.; Hauck, B.D.; Winters, A.L.; Kim, E.J.; Petit, H.V. Ruminal Prevotella spp. may play an important role in the conversion of plant lignans into human health beneficial antioxidants. PLoS ONE 2014, 9, e87949. [Google Scholar] [CrossRef]
- Wang, C.Z.; Ma, X.Q.; Yang, D.H.; Guo, Z.R.; Liu, G.R.; Zhao, G.X.; Tang, J.; Zhang, Y.N.; Ma, M.; Cai, S.Q.; et al. Production of enterodiol from defatted flaxseeds through biotransformation by human intestinal bacteria. BMC Microbiol. 2010, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Clavel, T.; Henderson, G.; Alpert, C.A.; Philippe, C.; Rigottier-Gois, L.; Dore, J.; Blaut, M. Intestinal bacterial communities that produce active estrogen-like compounds enterodiol and enterolactone in humans. Appl. Environ. Microbiol. 2005, 71, 6077–6085. [Google Scholar] [CrossRef] [PubMed]
- Clavel, T.; Borrmann, D.; Braune, A.; Dore, J.; Blaut, M. Occurrence and activity of human intestinal bacteria involved in the conversion of dietary lignans. Anaerobe 2006, 12, 140–147. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, G.; Han, Z.; Yao, W.; Zhu, W. Metabolism of flaxseed lignans in the rumen and its impact on ruminal metabolism and flora. Anim. Feed Sci. Technol. 2009, 150, 18–26. [Google Scholar] [CrossRef]
- Jung, E.; Choi, K.-Y.; Jung, D.-h.; Yun, H.; Kim, B.-G. Ortho-hydroxylation of mammalian lignan enterodiol by cytochrome P450s from Actinomycetes sp. Korean J. Chem. Eng. 2014, 32, 471–477. [Google Scholar] [CrossRef]
- Qiu, S.-X.; Lu, Z.-Z.; Luyengi, L.; Lee, S.K.; Pezzuto, J.M.; Farnsworth, N.R.; Thompson, L.U.; Fong, H.H.S. Isolation and Characterization of Flaxseed (Linum usitatissimum) Constituents. Pharm. Biol. 2008, 37, 1–7. [Google Scholar] [CrossRef]
- Penalvo, J.L.; Nurmi, T. Application of coulometric electrode array detection to the analysis of isoflavonoids and lignans. J. Pharm. Biomed. Anal. 2006, 41, 1497–1507. [Google Scholar] [CrossRef]
- Eeckhaut, E.; Struijs, K.; Possemiers, S.; Vincken, J.-P.; Keukeleire, D.D.; Verstraete, W. Metabolism of the lignan macromolecule into enterolignans in the gastrointestinal lumen as determined in the simulator of the human intestinal microbial ecosystem. J. Agric. Food Chem. 2008, 56, 4806–4812. [Google Scholar] [CrossRef] [PubMed]
- Kilkkinen, A.; Pietinen, P.; Klaukka, T.; Virtamo, J.; Korhonen, P.; Adlercreutz, H. Use of oral antimicrobials decreases serum enterolactone concentration. Am. J. Epidemiol. 2002, 155, 472–477. [Google Scholar] [CrossRef]
- Muir, A.D. Flax LignansAnalytical Methods and How They Influence Our Understanding of Biological Activity. J. AOAC Int. 2006, 89, 1147–1157. [Google Scholar]
- Mukker, J.K.; Michel, D.; Muir, A.D.; Krol, E.S.; Alcorn, J. Permeability and Conjugative Metabolism of Flaxseed Lignans by Caco-2 Human Intestinal Cells. J. Nat. Prod. 2014, 77, 29–34. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Brown, N.M.; Zimmer-Nechemias, L.; Wolfe, B.; Jha, P.; Heubi, J.E. Metabolism of secoisolariciresinol-diglycoside the dietary precursor to the intestinally derived lignan enterolactone in humans. Food Funct. 2014, 5, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, A.M.; Lampe, J.W.; Martini, M.C.; Campbell, D.R.; Slavin, J.L. Vegetables, fruits, and legumes: Effect on urinary isoflavonoid phytoestrogen and lignan excretion. J. Am. Diet. Assoc. 1995, 95, 769–774. [Google Scholar] [CrossRef]
- Jansen, G.H.E.; Arts, I.C.W.; Nielen, M.W.F.; Müller, M.; Hollman, P.C.H.; Keijer, J. Uptake and metabolism of enterolactone and enterodiol by human colon epithelial cells. Arch. Biochem. Biophys. 2005, 435, 74–82. [Google Scholar] [CrossRef]
- Niemeyer, H.B.; Honig, D.; Lange-Böhmer, A.; Jacobs, E.; Kulling, S.E.; Metzler, M. Oxidative Metabolites of the Mammalian Lignans Enterodiol and Enterolactone in Rat Bile and Urine. J. Agric. Food Chem. 2000, 48, 2910–2919. [Google Scholar] [CrossRef]
- Jacobs, E.; Metzler, M. Oxidative metabolism of the mammalian lignans enterolactone and enterodiol by rat, pig, and human liver microsomes. J. Agric. Food Chem. 1999, 47, 1071. [Google Scholar] [CrossRef]
- Jacobs, E.; Kulling, S.E.; Metzler, M. Novel metabolites of the mammalian lignans enterolactone and enterodiol in human urine. J. Steroid Biochem. Mol. Biol. 1999, 68, 211–218. [Google Scholar] [CrossRef]
- Knust, U.; Hull, W.E.; Spiegelhalder, B.; Bartsch, H.; Strowitzki, T.; Owen, R.W. Analysis of enterolignan glucuronides in serum and urine by HPLC-ESI-MS. Food Chem. Toxicol. 2006, 44, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Dean, B.; Chang, S.; Doss, G.A.; King, C.; Thomas, P.E. Glucuronidation, oxidative metabolism, and bioactivation of enterolactone in rhesus monkeys. Arch. Biochem. Biophys. 2004, 429, 244–251. [Google Scholar] [CrossRef]
- Billinsky, J. Oxidative Metabolism and Cytochrome P450 Enzyme Inhibition Potential of Creosote Bush and Flaxseed Lignans; Krol, E., Alcorn, J., Blakley, B., Bandy, B., Janz, D., Siraki, A., Eds.; ProQuest Dissertations Publishing: Saskatoon, SK, Canada, 2009. [Google Scholar]
- Jacobs, M.N.; Nolan, G.T.; Hood, S.R. Lignans, bacteriocides and organochlorine compounds activate the human pregnane X receptor (PXR). Toxicol. Appl. Pharmacol. 2005, 209, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Kemperman, R.A.; Bolca, S.; Roger, L.C.; Vaughan, E.E. Novel approaches for analysing gut microbes and dietary polyphenols: Challenges and opportunities. Microbiology 2010, 156, 3224–3231. [Google Scholar] [CrossRef]
- Laerke, H.N.; Mortensen, M.A.; Hedemann, M.S.; Bach Knudsen, K.E.; Penalvo, J.L.; Adlercreutz, H. Quantitative aspects of the metabolism of lignans in pigs fed fibre-enriched rye and wheat bread. Br. J. Nutr. 2009, 102, 985–994. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Hockerstedt, K.; Bannwart, C.; Bloigu, S.; Hamalainen, E.; Fotsis, T.; Ollus, A. Effect of dietary components, including lignans and phytoestrogens, on enterohepatic circulation and liver metabolism of estrogens and on sex hormone binding globulin (SHBG). J. Steroid Biochem. 1987, 27, 1135–1144. [Google Scholar] [CrossRef]
- Beaud, D.; Tailliez, P.; Anba-Mondoloni, J. Genetic characterization of the beta-glucuronidase enzyme from a human intestinal bacterium, Ruminococcus gnavus. Microbiology 2005, 151, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D.; Yuan, Y.V.; Wijewickreme, A.N.; Thompson, L.U. Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol. Cell Biochem. 1999, 202, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Corsini, E.; Dell’Agli, M.; Facchi, A.; De Fabiani, E.; Lucchi, L.; Boraso, M.S.; Marinovich, M.; Galli, C.L. Enterodiol and enterolactone modulate the immune response by acting on nuclear factor-kappaB (NF-kappaB) signaling. J. Agric. Food Chem. 2010, 58, 6678–6684. [Google Scholar] [CrossRef] [PubMed]
- Axelson, M.; Setchell, K.D.R. The excretion of lignans in rats - evidence for an intestinal bacterial source for this new group of compounds. FEBS Lett. 1981, 123, 337–342. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Fotsis, T.; Kurzer, M.S.; Wahala, K.; Makela, T.; Hase, T. Isotope dilution gas chromatographic-mass spectrometric method for the determination of unconjugated lignans and isoflavonoids in human feces, with preliminary results in omnivorous and vegetarian women. Anal. Biochem. 1995, 225, 101–108. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Fotsis, T.; Lampe, J.; Wähälä, K.; Mäkelä, T.; Brunow, G.; Hase, T. Quantitative Determination of Lignans and Isoflavonoids in Plasma of Omnivorous and Vegetarian Women by Isotope Dilution Gas Chromatographγ-Mass Spectrometry. Scand. J. Clin. Lab. Investig. 1993, 53, 5–18. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Serena, A.; Kjaer, A.K.; Tetens, I.; Heinonen, S.M.; Nurmi, T.; Adlercreutz, H. Rye bread in the diet of pigs enhances the formation of enterolactone and increases its levels in plasma, urine and feces. J. Nutr. 2003, 133, 1368–1375. [Google Scholar] [CrossRef]
- Raffaelli, B.; Hoikkala, A.; Leppala, E.; Wahala, K. Enterolignans. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 777, 29–43. [Google Scholar] [CrossRef]
- Axelson, M.; Setchell, K.D. Conjugation of lignans in human urine. FEBS Lett. 1980, 122, 49–53. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Honjo, H.; Higashi, A.; Fotsis, T.; Hamalainen, E.; Hasegawa, T.; Okada, H. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am. J. Clin. Nutr. 1991, 54, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Nurmi, T.; Mursu, J.; Penalvo, J.L.; Poulsen, H.E.; Voutilainen, S. Dietary intake and urinary excretion of lignans in Finnish men. Br. J. Nutr. 2010, 103, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H.; Fotsis, T.; Heikkinen, R.; Dwyer, J.T.; Woods, M.; Goldin, B.R.; Gorbach, S.L. Excretion of the lignans enterolactone and enterodiol and of equol in omnivorous and vegetarian postmenopausal women and in women with breast cancer. Lancet 1982, 2, 1295–1299. [Google Scholar] [CrossRef]
- Smeds, A.I.; Willfor, S.M.; Pietarinen, S.P.; Peltonen-Sainio, P.; Reunanen, M.H. Occurrence of “mammalian” lignans in plant and water sources. Planta 2007, 226, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Kuhnle, G.G.; Dell’Aquila, C.; Aspinall, S.M.; Runswick, S.A.; Mulligan, A.A.; Bingham, S.A. Phytoestrogen content of foods of animal origin: Dairy products, eggs, meat, fish, and seafood. J. Agric. Food Chem. 2008, 56, 10099–10104. [Google Scholar] [CrossRef]
- Gagnon, N.; Cortes, C.; da Silva, D.; Kazama, R.; Benchaar, C.; dos Santos, G.; Zeoula, L.; Petit, H.V. Ruminal metabolism of flaxseed (Linum usitatissimum) lignans to the mammalian lignan enterolactone and its concentration in ruminal fluid, plasma, urine and milk of dairy cows. Br. J. Nutr. 2009, 102, 1015–1023. [Google Scholar] [CrossRef]
- Kilkkinen, A.; Stumpf, K.; Pietinen, P.; Valsta, L.M.; Tapanainen, H.; Adlercreutz, H. Determinants of serum enterolactone concentration. Am. J. Clin. Nutr. 2001, 73, 1094–1100. [Google Scholar] [CrossRef]
- Vanharanta, M.; Voutilainen, S.; Nurmi, T.; Kaikkonen, J.; Roberts, L.J.; Morrow, J.D.; Adlercreutz, H.; Salonen, J.T. Association between low serum enterolactone and increased plasma F2-isoprostanes, a measure of lipid peroxidation. Atherosclerosis 2002, 160, 465–469. [Google Scholar] [CrossRef]
- Bhakta, D.; Higgins, C.D.; Sevak, L.; Mangtani, P.; Adlercreutz, H.; McMichael, A.J.; dos Santos Silva, I. Phyto-oestrogen intake and plasma concentrations in South Asian and native British women resident in England. Br. J. Nutr. 2006, 95, 1150–1158. [Google Scholar] [CrossRef]
- Morton, M.S.; Chan, P.S.; Cheng, C.; Blacklock, N.; Matos-Ferreira, A.; Abranches-Monteiro, L.; Correia, R.; Lloyd, S.; Griffiths, K. Lignans and isoflavonoids in plasma and prostatic fluid in men: Samples from Portugal, Hong Kong, and the United Kingdom. Prostate 1997, 32, 122–128. [Google Scholar] [CrossRef]
- Damdimopoulou, P.; Nurmi, T.; Salminen, A.; Damdimopoulos, A.E.; Kotka, M.; van der Saag, P.; Strauss, L.; Poutanen, M.; Pongratz, I.; Makela, S. A single dose of enterolactone activates estrogen signaling and regulates expression of circadian clock genes in mice. J. Nutr. 2011, 141, 1583–1589. [Google Scholar] [CrossRef] [PubMed]
- Jan, K.C.; Chang, Y.W.; Hwang, L.S.; Ho, C.T. Tissue distribution and cytochrome P450 inhibition of sesaminol and its tetrahydrofuranoid metabolites. J. Agric. Food Chem. 2012, 60, 8616–8623. [Google Scholar] [CrossRef] [PubMed]
- Boccardo, F.; Lunardi, G.L.; Petti, A.R.; Rubagotti, A. Enterolactone in breast cyst fluid: Correlation with EGF and breast cancer risk. Breast Cancer Res. Treat. 2003, 79, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, N.; Cortes, C.; Petit, H.V. Weekly excretion of the mammalian lignan enterolactone in milk of dairy cows fed flaxseed meal. J. Dairy Res. 2009, 76, 455–458. [Google Scholar] [CrossRef]
- Rickard, S.E.; Thompson, L.U. Chronic exposure to secoisolariciresinol diglycoside alters lignan disposition in rats. J. Nutr. 1998, 128, 615–623. [Google Scholar] [CrossRef]
- DeSilva, S.F. Albumin Permeability in Experimental Brain Metastases of Breast Cancer and the Differential Effects on Astrocytes, Neurons, and Metastatic Tumor Cells. Master’s Thesis, West Texas A & M University, Canyon, TX, USA, 2012. [Google Scholar]
- Lockman, P.R.; Mittapalli, R.K.; Taskar, K.S.; Rudraraju, V.; Gril, B.; Bohn, K.A.; Adkins, C.E.; Roberts, A.; Thorsheim, H.R.; Gaasch, J.A.; et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin. Cancer Res. 2010, 16, 5664–5678. [Google Scholar] [CrossRef] [PubMed]
- Adkins, C.E.; Mohammad, A.S.; Terrell-Hall, T.B.; Dolan, E.L.; Shah, N.; Sechrest, E.; Griffith, J.; Lockman, P.R. Characterization of passive permeability at the blood-tumor barrier in five preclinical models of brain metastases of breast cancer. Clin. Exp. Metast. 2016, 33, 373–383. [Google Scholar] [CrossRef]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Unraveling the mysteries of serum albumin-more than just a serum protein. Front. Physiol. 2014, 5, 299. [Google Scholar] [CrossRef]
- Miele, E.; Spinelli, G.P.; Miele, E.; Tomao, F.; Tomao, S. Albumin-bound formulation of paclitaxel (Abraxane(®) ABI-007) in the treatment of breast cancer. Int. J. Nanomed. 2009, 4, 99–105. [Google Scholar]
- Almousa, M.A.; Krol, E.; Alcorn, J. Enterolactone-glucuronide upregulates Insulin Induced Gene-1 (INSIG-1) to modulate cholesterol metabolism in Caco-2 cells. In Proceedings of the Canadian Society for Pharmaceutical Sciences (CSPS) 20th Annual Symposium, Montreal, QC, Canada, 22–27 May 2019. [Google Scholar]
- Perez-Vizcaino, F.; Duarte, J.; Santos-Buelga, C. The flavonoid paradox: Conjugation and deconjugation as key steps for the biological activity of flavonoids. J. Sci. Food Agric. 2012, 92, 1822–1825. [Google Scholar] [CrossRef]
- Sperker, B.; Werner, U.; Murdter, T.E.; Tekkaya, C.; Fritz, P.; Wacke, R.; Adam, U.; Gerken, M.; Drewelow, B.; Kroemer, H.K. Expression and function of beta-glucuronidase in pancreatic cancer: Potential role in drug targeting. Naunyn Schmiedebergs Arch. Pharmacol. 2000, 362, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Sperker, B.; Backman, J.T.; Kroemer, H.K. The role of β-glucuronidase in drug disposition and drug targeting in humans. Clin. Pharmacokinet. 1997, 33, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jin, Y.H. Intestinal bacterial beta-glucuronidase activity of patients with colon cancer. Arch. Pharm. Res. 2001, 24, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Fishman, W.H.; Anlyan, A.J. The presence of high beta-glucuronidase activity in cancer tissue. J. Biol. Chem. 1947, 169, 449. [Google Scholar] [PubMed]
- Menendez, C.; Duenas, M.; Galindo, P.; Gonzalez-Manzano, S.; Jimenez, R.; Moreno, L.; Zarzuelo, M.J.; Rodriguez-Gomez, I.; Duarte, J.; Santos-Buelga, C.; et al. Vascular deconjugation of quercetin glucuronide: The flavonoid paradox revealed? Mol. Nutr. Food Res. 2011, 55, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Vore, M.; Hoffman, T. Carrier-mediated electrogenic transport of estradiol-17 beta-glucuronide in rat liver BMV. Am. J. Physiol. 1994, 267, G546–G551. [Google Scholar] [CrossRef]
- Teiten, M.H.; Eifes, S.; Dicato, M.; Diederich, M. Curcumin-the paradigm of a multi-target natural compound with applications in cancer prevention and treatment. Toxins 2010, 2, 128–162. [Google Scholar] [CrossRef]
- Farrand, L.; Oh, S.W.; Song, Y.S.; Tsang, B.K. Phytochemicals: A multitargeted approach to gynecologic cancer therapy. Biomed. Res. Int. 2014, 2014, 890141. [Google Scholar] [CrossRef]
- Thomasset, S.C.; Berry, D.P.; Garcea, G.; Marczylo, T.; Steward, W.P.; Gescher, A.J. Dietary polyphenolic phytochemicals—Promising cancer chemopreventive agents in humans? A review of their clinical properties. Int. J. Cancer 2007, 120, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.L.; Baum, P.D.; Gu, M.; Jorgensen, E.M.; Clark, S.G.; Garriga, G.C. elegans AP-2 and retromer control Wnt signaling by regulating mig-14/Wntless. Dev. Cell 2008, 14, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Martinchik, A.N.; Baturin, A.K.; Zubtsov, V.V.; Molofeev, V. Nutritional value and functional properties of flaxseed. Vopr. Pitan. 2012, 81, 4–10. [Google Scholar] [PubMed]
- Bylund, A.; Zhang, J.X.; Bergh, A.; Damber, J.E.; Widmark, A.; Johansson, A.; Adlercreutz, H.; Aman, P.; Shepherd, M.J.; Hallmans, G. Rye bran and soy protein delay growth and increase apoptosis of human LNCaP prostate adenocarcinoma in nude mice. Prostate 2000, 42, 304–314. [Google Scholar] [CrossRef]
- Landstrom, M.; Zhang, J.X.; Hallmans, G.; Aman, P.; Bergh, A.; Damber, J.E.; Mazur, W.; Wahala, K.; Adlercreutz, H. Inhibitory effects of soy and rye diets on the development of Dunning R3327 prostate adenocarcinoma in rats. Prostate 1998, 36, 151–161. [Google Scholar] [CrossRef]
- Lin, X.; Gingrich, J.R.; Bao, W.; Li, J.; Haroon, Z.A.; Demark-Wahnefried, W. Effect of flaxseed supplementation on prostatic carcinoma in transgenic mice. Urology 2002, 60, 919–924. [Google Scholar] [CrossRef]
- Bergman Jungestrom, M.; Thompson, L.U.; Dabrosin, C. Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin. Cancer Res. 2007, 13, 1061–1067. [Google Scholar] [CrossRef]
- Buck, K.; Vrieling, A.; Zaineddin, A.K.; Becker, S.; Hüsing, A.; Kaaks, R.; Linseisen, J.; Flesch-Janys, D.; Chang-Claude, J. Serum Enterolactone and Prognosis of Postmenopausal Breast Cancer. J. Clin. Oncol. 2011, 29, 3730–3738. [Google Scholar] [CrossRef]
- Schroder, L.; Richter, D.U.; Piechulla, B.; Chrobak, M.; Kuhn, C.; Schulze, S.; Abarzua, S.; Jeschke, U.; Weissenbacher, T. Effects of Phytoestrogen Extracts Isolated from Elder Flower on Hormone Production and Receptor Expression of Trophoblast Tumor Cells JEG-3 and BeWo, as well as MCF7 Breast Cancer Cells. Nutrients 2016, 8, 616. [Google Scholar] [CrossRef]
- Guglielmini, P.; Rubagotti, A.; Boccardo, F. Serum enterolactone levels and mortality outcome in women with early breast cancer: A retrospective cohort study. Breast Cancer Res. Treat. 2012, 132, 661–668. [Google Scholar] [CrossRef]
- Olsen, A.; Christensen, J.; Knudsen, K.E.; Johnsen, N.F.; Overvad, K.; Tjonneland, A. Prediagnostic plasma enterolactone levels and mortality among women with breast cancer. Breast Cancer Res. Treat. 2011, 128, 883–889. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sonestedt, E.; Ivarsson, M.I.; Harlid, S.; Ericson, U.; Gullberg, B.; Carlson, J.; Olsson, H.; Adlercreutz, H.; Wirfalt, E. The protective association of high plasma enterolactone with breast cancer is reasonably robust in women with polymorphisms in the estrogen receptor alpha and beta genes. J. Nutr. 2009, 139, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Martinchik, A.N.; Zubtsov, V.V. Phytoestrogenis properties of flaxseed lignans. Vopr. Pitan. 2012, 81, 61–66. [Google Scholar]
- Viveky, N.; Thorpe, L.; Alcorn, J.; Hadjistavropoulos, T.; Whiting, S. Safety evaluation of flaxseed lignan supplementation in older adults residing in long-term care homes. JNHR-J. Nurs. Home Res. 2015, 1, 84–88. [Google Scholar]
- Hemmings, S.J.; Westcott, N.; Muir, A.; Czechowicz, D. The effects of dietary flaxseed on the Fischer 344 rat: II. Liver gamma-glutamyltranspeptidase activity. Cell. Biochem. Funct. 2004, 22, 225–231. [Google Scholar] [CrossRef]
- Prasad, K. Effect of chronic administration of lignan complex isolated from flaxseed on the hemopoietic system. Mol. Cell. Biochem. 2005, 270, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Woo, G. Secoisolariciresinol Diglucoside Effects in Diet-Induced Hyperlipidemic Rats. Ph.D. Thesis, University of Saskatchewan, Saskatchewan, SK, Canada, 2006. [Google Scholar]
- Ward, W.E.; Jiang, F.O.; Thompson, L.U. Exposure to flaxseed or purified lignan during lactation influences rat mammary gland structures. Nutr. Cancer 2000, 37, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Tou, J.C.; Chen, J.; Thompson, L.U. Flaxseed and its lignan precursor, secoisolariciresinol diglycoside, affect pregnancy outcome and reproductive development in rats. J. Nutr. 1998, 128, 1861–1868. [Google Scholar] [CrossRef]
- Ward, W.E.; Chen, J.; Thompson, L.U. Exposure to flaxseed or its purified lignan during suckling only or continuously does not alter reproductive indices in male and female offspring. J. Toxicol. Environ. health part A 2001, 64, 567–577. [Google Scholar] [CrossRef]
- Collins, T.F.; Sprando, R.L.; Black, T.N.; Olejnik, N.; Wiesenfeld, P.W.; Babu, U.S.; Bryant, M.; Flynn, T.J.; Ruggles, D.I. Effects of flaxseed and defatted flaxseed meal on reproduction and development in rats. Food Chem. Toxicol. 2003, 41, 819–834. [Google Scholar] [CrossRef]
- Tang, R.; Chen, M.; Zhou, K.; Chen, D.; Yu, J.; Hu, W.; Song, L.; Hang, B.; Wang, X.; Xia, Y. Prenatal lignan exposures, pregnancy urine estrogen profiles and birth outcomes. Environ. Pollut. 2015, 205, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, J.; Wang, S.; Zeng, Z.; Li, T.; Liu, Y.; Mastriani, E.; Li, Q.H.; Bao, H.X.; Zhou, Y.J.; et al. Enterolactone has stronger effects than enterodiol on ovarian cancer. J. Ovarian Res. 2017, 10, 49. [Google Scholar] [CrossRef]
- Hutchins, A.M.; Martini, M.C.; Olson, B.A.; Thomas, W.; Slavin, J.L. Flaxseed influences urinary lignan excretion in a dose-dependent manner in postmenopausal women. Cancer Epidemiol. Biomark. Prev. 2000, 9, 1113–1118. [Google Scholar]
- Pietinen, P.; Stumpf, K.; Mannisto, S.; Kataja, V.; Uusitupa, M.; Adlercreutz, H. Serum enterolactone and risk of breast cancer: A case-control study in eastern Finland. Cancer Epidemiol. Biomark. Prev. 2001, 10, 339–344. [Google Scholar]
- Shavers, V.L.; Underwood, W.; Moser, R.P. Race/ethnicity and the perception of the risk of developing prostate cancer. Am. J. Prev. Med. 2009, 37, 64–67. [Google Scholar] [CrossRef][Green Version]
- Azrad, M.; Vollmer, R.T.; Madden, J.; Dewhirst, M.; Polascik, T.J.; Snyder, D.C.; Ruffin, M.T.; Moul, J.W.; Brenner, D.E.; Demark-Wahnefried, W. Flaxseed-derived enterolactone is inversely associated with tumor cell proliferation in men with localized prostate cancer. J. Med. Food 2013, 16, 357–360. [Google Scholar] [CrossRef]
- Heald, C.L.; Ritchie, M.R.; Bolton-Smith, C.; Morton, M.S.; Alexander, F.E. Phyto-oestrogens and risk of prostate cancer in Scottish men. Br. J. Nutr. 2007, 98, 388–396. [Google Scholar] [CrossRef] [PubMed]
- McCann, S.E.; Ambrosone, C.B.; Moysich, K.B.; Brasure, J.; Marshall, J.R.; Freudenheim, J.L.; Wilkinson, G.S.; Graham, S. Intakes of selected nutrients, foods, and phytochemicals and prostate cancer risk in western New York. Nutr. Cancer 2005, 53, 33–41. [Google Scholar] [CrossRef]
- Bylund, A.; Lundin, E.; Zhang, J.X.; Nordin, A.; Kaaks, R.; Stenman, U.H.; Aman, P.; Adlercreutz, H.; Nilsson, T.K.; Hallmans, G.; et al. Randomised controlled short-term intervention pilot study on rye bran bread in prostate cancer. Eur. J. Cancer Prev. 2003, 12, 407–415. [Google Scholar] [CrossRef]
- Buck, K.; Zaineddin, A.K.; Vrieling, A.; Linseisen, J.; Chang-Claude, J. Meta-analyses of lignans and enterolignans in relation to breast cancer risk. Am. J. Clin. Nutr. 2010, 92, 141–153. [Google Scholar] [CrossRef]
- Velentzis, L.S.; Cantwell, M.M.; Cardwell, C.; Keshtgar, M.R.; Leathem, A.J.; Woodside, J.V. Lignans and breast cancer risk in pre- and post-menopausal women: Meta-analyses of observational studies. Br. J. Cancer 2009, 100, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary Flavonoid and Lignan Intake and Mortality in Prospective Cohort Studies: Systematic Review and Dose-Response Meta-Analysis. Am. J. Epidemiol. 2017, 185, 1304–1316. [Google Scholar] [CrossRef]
- Horn-Ross, P.L. Phytoestrogen Consumption and Breast Cancer Risk in a Multiethnic Population: The Bay Area Breast Cancer Study. Am. J. Epidemiol. 2001, 154, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Verheus, M.; van Gils, C.H.; Keinan-Boker, L.; Grace, P.B.; Bingham, S.A.; Peeters, P.H. Plasma phytoestrogens and subsequent breast cancer risk. J. Clin. Oncol. 2007, 25, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Ward, H.; Chapelais, G.; Kuhnle, G.G.; Luben, R.; Khaw, K.T.; Bingham, S.; European Prospective into Cancer-Norfolk, c. Breast cancer risk in relation to urinary and serum biomarkers of phytoestrogen exposure in the European Prospective into Cancer-Norfolk cohort study. Breast Cancer Res. 2008, 10, R32. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Yao, S.; Tritchler, D.; Hullar, M.A.; Lampe, J.W.; Thompson, L.U.; McCann, S.E. Genetic Variation in Steroid and Xenobiotic Metabolizing Pathways and Enterolactone Excretion Before and After Flaxseed Intervention in African American and European American Women. Cancer Epidemiol. Biomark. Prev. 2019, 28, 265–274. [Google Scholar] [CrossRef]
- Tu, H.; Wen, C.P.; Tsai, S.P.; Chow, W.-H.; Wen, C.; Ye, Y.; Zhao, H.; Tsai, M.K.; Huang, M.; Dinney, C.P.; et al. Cancer risk associated with chronic diseases and disease markers: Prospective cohort study. BMJ 2018, 360, k134. [Google Scholar] [CrossRef] [PubMed]
- Rao Kondapally Seshasai, S.; Kaptoge, S.; Thompson, A.; Di Angelantonio, E.; Gao, P.; Sarwar, N.; Whincup, P.H.; Mukamal, K.J.; Gillum, R.F.; Holme, I.; et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 2011, 364, 829–841. [Google Scholar] [CrossRef]
- Jee, S.H.; Ohrr, H.; Sull, J.W.; Yun, J.E.; Ji, M.; Samet, J.M. Fasting serum glucose level and cancer risk in Korean men and women. JAMA 2005, 293, 194–202. [Google Scholar] [CrossRef]
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and cancer: A consensus report. CA Cancer J. Clin. 2010, 60, 207–221. [Google Scholar] [CrossRef]
- Song, Y.M.; Sung, J.; Kim, J.S. Which cholesterol level is related to the lowest mortality in a population with low mean cholesterol level: A 6.4-year follow-up study of 482,472 Korean men. Am. J. Epidemiol. 2000, 151, 739–747. [Google Scholar] [CrossRef][Green Version]
- Strohmaier, S.; Edlinger, M.; Manjer, J.; Stocks, T.; Bjorge, T.; Borena, W.; Haggstrom, C.; Engeland, A.; Nagel, G.; Almquist, M.; et al. Total serum cholesterol and cancer incidence in the Metabolic syndrome and Cancer Project (Me-Can). PLoS ONE 2013, 8, e54242. [Google Scholar] [CrossRef] [PubMed]
- Schatzkin, A.; Hoover, R.N.; Taylor, P.R.; Ziegler, R.G.; Carter, C.L.; Larson, D.B.; Licitra, L.M. Serum cholesterol and cancer in the NHANES I epidemiologic followup study. National Health and Nutrition Examination Survey. Lancet 1987, 2, 298–301. [Google Scholar] [CrossRef]
- Sherwin, R.W.; Wentworth, D.N.; Cutler, J.A.; Hulley, S.B.; Kuller, L.H.; Stamler, J. Serum cholesterol levels and cancer mortality in 361,662 men screened for the Multiple Risk Factor Intervention Trial. JAMA 1987, 257, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Iso, H.; Ikeda, A.; Inoue, M.; Sato, S.; Tsugane, S. Serum cholesterol levels in relation to the incidence of cancer: The JPHC study cohorts. Int. J. Cancer 2009, 125, 2679–2686. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Berrington de Gonzalez, A.; Freedman, N.D.; Huxley, R.; Mok, Y.; Jee, S.H.; Samet, J.M. Total cholesterol and cancer risk in a large prospective study in Korea. J. Clin. Oncol. 2011, 29, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Jouven, X.; Escolano, S.; Celermajer, D.; Empana, J.P.; Bingham, A.; Hermine, O.; Desnos, M.; Perier, M.C.; Marijon, E.; Ducimetiere, P. Heart rate and risk of cancer death in healthy men. PLoS ONE 2011, 6, e21310. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, G.; Shaper, A.G.; Macfarlane, P.W. Heart rate, physical activity, and mortality from cancer and other noncardiovascular diseases. Am. J. Epidemiol. 1993, 137, 735–748. [Google Scholar] [CrossRef]
- Stocks, T.; Bjorge, T.; Ulmer, H.; Manjer, J.; Haggstrom, C.; Nagel, G.; Engeland, A.; Johansen, D.; Hallmans, G.; Selmer, R.; et al. Metabolic risk score and cancer risk: Pooled analysis of seven cohorts. Int. J. Epidemiol. 2015, 44, 1353–1363. [Google Scholar] [CrossRef]
- Batty, G.D.; Shipley, M.J.; Marmot, M.G.; Davey Smith, G. Blood pressure and site-specific cancer mortality: Evidence from the original Whitehall study. Br. J. Cancer 2003, 89, 1243–1247. [Google Scholar] [CrossRef]
- Goon, P.K.; Stonelake, P.S.; Lip, G.Y. Hypertension, anti-hypertensive therapy and neoplasia. Curr. Pharm. Des. 2007, 13, 2539–2544. [Google Scholar] [CrossRef] [PubMed]
- Grossman, E.; Messerli, F.H.; Boyko, V.; Goldbourt, U. Is there an association between hypertension and cancer mortality? Am. J. Med. 2002, 112, 479–486. [Google Scholar] [CrossRef]
- Stocks, T.; Van Hemelrijck, M.; Manjer, J.; Bjorge, T.; Ulmer, H.; Hallmans, G.; Lindkvist, B.; Selmer, R.; Nagel, G.; Tretli, S.; et al. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension 2012, 59, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Strasak, A.M.; Lang, S.; Kneib, T.; Brant, L.J.; Klenk, J.; Hilbe, W.; Oberaigner, W.; Ruttmann, E.; Kaltenbach, L.; Concin, H.; et al. Use of penalized splines in extended Cox-type additive hazard regression to flexibly estimate the effect of time-varying serum uric acid on risk of cancer incidence: A prospective, population-based study in 78,850 men. Ann. Epidemiol. 2009, 19, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.A.; Elias, A.; Johnson, R.J.; Wright, R.M. Contribution of uric acid to cancer risk, recurrence, and mortality. Clin. Transl. Med. 2012, 1, 16. [Google Scholar] [CrossRef]
- Taghizadeh, N.; Vonk, J.M.; Boezen, H.M. Serum uric acid levels and cancer mortality risk among males in a large general population-based cohort study. Cancer Causes Control 2014, 25, 1075–1080. [Google Scholar] [CrossRef]
- Strasak, A.M.; Rapp, K.; Hilbe, W.; Oberaigner, W.; Ruttmann, E.; Concin, H.; Diem, G.; Pfeiffer, K.P.; Ulmer, H. Serum uric acid and risk of cancer mortality in a large prospective male cohort. Cancer Causes Control 2007, 18, 1021–1029. [Google Scholar] [CrossRef]
- Strasak, A.M.; Rapp, K.; Hilbe, W.; Oberaigner, W.; Ruttmann, E.; Concin, H.; Diem, G.; Pfeiffer, K.P.; Ulmer, H. The role of serum uric acid as an antioxidant protecting against cancer: Prospective study in more than 28 000 older Austrian women. Ann. Oncol. 2007, 18, 1893–1897. [Google Scholar] [CrossRef]
- Stengel, B. Chronic kidney disease and cancer: A troubling connection. J. Nephrol. 2010, 23, 253–262. [Google Scholar]
- Lowrance, W.T.; Ordoñez, J.; Udaltsova, N.; Russo, P.; Go, A.S. CKD and the Risk of Incident Cancer. J. Am. Soc. Nephrol. 2014, 25, 2327–2334. [Google Scholar] [CrossRef]
- Wong, G.; Hayen, A.; Chapman, J.R.; Webster, A.C.; Wang, J.J.; Mitchell, P.; Craig, J.C. Association of CKD and Cancer Risk in Older People. J. Am. Soc. Nephrol. 2009, 20, 1341–1350. [Google Scholar] [CrossRef]
- Weng, P.-H.; Hung, K.-Y.; Huang, H.-L.; Chen, J.-H.; Sung, P.-K.; Huang, K.-C. Cancer-Specific Mortality in Chronic Kidney Disease: Longitudinal Follow-Up of a Large Cohort. Clin. J. Am. Soc. Nephrol. 2011, 6, 1121–1128. [Google Scholar] [CrossRef]
- Iff, S.; Craig, J.C.; Turner, R.; Chapman, J.R.; Wang, J.J.; Mitchell, P.; Wong, G. Reduced estimated GFR and cancer mortality. Am. J. Kidney Diseases 2014, 63, 23–30. [Google Scholar] [CrossRef]
- Fried, L.F.; Katz, R.; Sarnak, M.J.; Shlipak, M.G.; Chaves, P.H.M.; Jenny, N.S.; Stehman-Breen, C.; Gillen, D.; Bleyer, A.J.; Hirsch, C.; et al. Kidney Function as a Predictor of Noncardiovascular Mortality. J. Am. Soc. Nephrol. 2005, 16, 3728–3735. [Google Scholar] [CrossRef]
- Brenner, D.R.; McLaughlin, J.R.; Hung, R.J. Previous lung diseases and lung cancer risk: A systematic review and meta-analysis. PLoS ONE 2011, 6, e17479. [Google Scholar] [CrossRef]
- Tomaz Pacheco, J.; Beltrame Daleprame, J.; Teles Boaventura, G. Impact of dietary flaxseed (linum usitatissimum) supplementation on biochemical profile in healthy rats. Nutr. Hosp. 2011, 26, 798–802. [Google Scholar] [CrossRef]
- Dupasquier, C.M.C.; Weber, A.-M.; Ander, B.P.; Rampersad, P.P.; Steigerwald, S.; Wigle, J.T.; Mitchell, R.W.; Kroeger, E.A.; Gilchrist, J.S.C.; Moghadasian, M.M.; et al. Effects of dietary flaxseed on vascular contractile function and atherosclerosis during prolonged hypercholesterolemia in rabbits. Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, H2987–H2996. [Google Scholar] [CrossRef]
- Parikh, M.; Pierce, G.N. Dietary flaxseed: What we know and don’t know about its effects on cardiovascular disease. Can. J. Physiol. Pharmacol. 2018, 97, 75–81. [Google Scholar] [CrossRef]
- Parikh, M.; Netticadan, T.; Pierce, G.N. Flaxseed: Its bioactive components and their cardiovascular benefits. Am. J. Physiol.-Heart Circ. Physiol. 2018, 314, H146–H159. [Google Scholar] [CrossRef]
- Ander, B.P.; Weber, A.R.; Rampersad, P.P.; Gilchrist, J.S.; Pierce, G.N.; Lukas, A. Dietary flaxseed protects against ventricular fibrillation induced by ischemia-reperfusion in normal and hypercholesterolemic Rabbits. J. Nutr. 2004, 134, 3250–3256. [Google Scholar] [CrossRef]
- Caligiuri, S.P.; Edel, A.L.; Aliani, M.; Pierce, G.N. Flaxseed for hypertension: Implications for blood pressure regulation. Curr. Hypertens. Rep. 2014, 16, 499. [Google Scholar] [CrossRef]
- Khalesi, S.; Irwin, C.; Schubert, M. Flaxseed Consumption May Reduce Blood Pressure: A Systematic Review and Meta-Analysis of Controlled Trials. J. Nutr. 2015, 145, 758–765. [Google Scholar] [CrossRef]
- Rodriguez-Leyva, D.; Weighell, W.; Edel, A.L.; LaVallee, R.; Dibrov, E.; Pinneker, R.; Maddaford, T.G.; Ramjiawan, B.; Aliani, M.; Guzman, R.; et al. Potent Antihypertensive Action of Dietary Flaxseed in Hypertensive Patients. Hypertension 2013, 62, 1081–1089. [Google Scholar] [CrossRef]
- Pan, A.; Sun, J.; Chen, Y.; Ye, X.; Li, H.; Yu, Z.; Wang, Y.; Gu, W.; Zhang, X.; Chen, X.; et al. Effects of a flaxseed-derived lignan supplement in type 2 diabetic patients: A randomized, double-blind, cross-over trial. PLoS ONE 2007, 2, e1148. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K. Secoisolariciresinol diglucoside from flaxseed delays the development of type 2 diabetes in Zucker rat. J. Lab. Clin. Med. 2001, 138, 32–39. [Google Scholar] [CrossRef]
- Prasad, K. Suppression of phosphoenolpyruvate carboxykinase gene expression by secoisolariciresinol diglucoside (SDG), a new antidiabetic agent. Int. J. Angiol. 2002, 11, 107–109. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Bhathena, S.J. Beneficial role of dietary phytoestrogens in obesity and diabetes. The Am. J. Clin. Nutr. 2002, 76, 1191–1201. [Google Scholar] [CrossRef]
- Javidi, A.; Mozaffari-Khosravi, H.; Nadjarzadeh, A.; Dehghani, A.; Eftekhari, M.H. The effect of flaxseed powder on insulin resistance indices and blood pressure in prediabetic individuals: A randomized controlled clinical trial. J. Res. Med. Sci. 2016, 21, 70. [Google Scholar] [CrossRef]
- Barre, D.E.; Mizier-Barre, K.A.; Stelmach, E.; Hobson, J.; Griscti, O.; Rudiuk, A.; Muthuthevar, D. Flaxseed Lignan Complex Administration in Older Human Type 2 Diabetics Manages Central Obesity and Prothrombosis—An Invitation to Further Investigation into Polypharmacy Reduction. J. Nutr. Metab. 2012, 2012, 7. [Google Scholar] [CrossRef]
- Soltanian, N.; Janghorbani, M. A randomized trial of the effects of flaxseed to manage constipation, weight, glycemia, and lipids in constipated patients with type 2 diabetes. Nutr. Metab. 2018, 15, 36. [Google Scholar] [CrossRef]
- Raeisi-Dehkordi, H.; Mohammadi-Sartang, M.; Mazloom, Z.; Barati-Boldaji, R.; Sohrabi, Z. Flaxseed supplementation on glucose control and insulin sensitivity: A systematic review and meta-analysis of 25 randomized, placebo-controlled trials. Nutr. Rev. 2017, 76, 125–139. [Google Scholar] [CrossRef]
- Caligiuri, S.P.; Aukema, H.M.; Ravandi, A.; Pierce, G.N. Elevated levels of pro-inflammatory oxylipins in older subjects are normalized by flaxseed consumption. Exp. Gerontol. 2014, 59, 51–57. [Google Scholar] [CrossRef]
- Dupasquier, C.M.C.; Dibrov, E.; Kneesh, A.L.; Cheung, P.K.M.; Lee, K.G.Y.; Alexander, H.K.; Yeganeh, B.K.; Moghadasian, M.H.; Pierce, G.N. Dietary flaxseed inhibits atherosclerosis in the LDL receptor-deficient mouse in part through antiproliferative and anti-inflammatory actions. Am. J. Physiolo.-Heart Circ. Physiol. 2007, 293, H2394–H2402. [Google Scholar] [CrossRef]
- Prasad, K. Dietary flax seed in prevention of hypercholesterolemic atherosclerosis. Atherosclerosis 1997, 132, 69–76. [Google Scholar] [CrossRef]
- Prasad, K. Antioxidant Activity of Secoisolariciresinol Diglucoside-derived Metabolites, Secoisolariciresinol, Enterodiol, and Enterolactone. Int. J. Angiol. 2000, 9, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Carreau, C.; Flouriot, G.; Bennetau-Pelissero, C.; Potier, M. Enterodiol and enterolactone, two major diet-derived polyphenol metabolites have different impact on ERα transcriptional activation in human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2008, 110, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yuan, Y.V.; Kitts, D.D. Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem. Toxicol. 2007, 45, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Tülüce, Y.; Özkol, H.; Koyuncu, İ. Photoprotective effect of flax seed oil (Linum usitatissimum L.) against ultraviolet C-induced apoptosis and oxidative stress in rats. Toxicol. Ind. Health 2012, 28, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Faseehuddin Shakir, K.A.; Madhusudhan, B. Effects of flaxseed (Linum usitatissimum) chutney on gamma-glutamyl transpeptidase and micronuclei profile in azoxymethane treated rats. Indian J. Clin. Biochem. IJCB 2007, 22, 129–131. [Google Scholar] [CrossRef]
- Shakir, K.A.; Madhusudhan, B. Hypocholesterolemic and hepatoprotective effects of flaxseed chutney: Evidence from animal studies. Indian J. Clin. Biochem. IJCB 2007, 22, 117–121. [Google Scholar] [CrossRef]
- Sacco, S.M.; Thompson, L.U.; Ganss, B.; Ward, W.E. Accessibility of (3)H-secoisolariciresinol diglycoside lignan metabolites in skeletal tissue of ovariectomized rats. J. Med. Food 2011, 14, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K. Flax lignan complex slows down the progression of atherosclerosis in hyperlipidemic rabbits. J. Cardiovasc. Pharmacol. Ther. 2009, 14, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Dzuvor, C.K.O.; Taylor, J.T.; Acquah, C.; Pan, S.; Agyei, D. Bioprocessing of Functional Ingredients from Flaxseed. Molecules 2018, 23, 2444. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Harjai, K.; Mohan, H.; Punia, R.P.; Chhibber, S. Long-term flaxseed oil supplementation diet protects BALB/c mice against Streptococcus pneumoniae infection. Med. Microbiol. Immunol. 2010, 199, 27–34. [Google Scholar] [CrossRef]
- Spence, J.D.; Thornton, T.; Muir, A.D.; Westcott, N.D. The effect of flax seed cultivars with differing content of alpha-linolenic acid and lignans on responses to mental stress. J. Am. Coll. Nutr. 2003, 22, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Sumalla Cano, S.; Elio, I.; Masias Vergara, M.; Giampieri, F.; Battino, M. Associations between Sleep, Cortisol Regulation, and Diet: Possible Implications for the Risk of Alzheimer Disease. Adv. Nutr. 2016, 7, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, R.; Zhao, X.; Zhang, C.; Sun, J.; Li, J.; Zhang, L.; Shao, T.; Ruan, L.; Chen, L.; et al. Antidepressant-like effect of flaxseed secoisolariciresinol diglycoside in ovariectomized mice subjected to unpredictable chronic stress. Metab. Brain Disease 2013, 28, 77–84. [Google Scholar] [CrossRef]
- Hu, P.; Mei, Q.Y.; Ma, L.; Cui, W.G.; Zhou, W.H.; Zhou, D.S.; Zhao, Q.; Xu, D.Y.; Zhao, X.; Lu, Q.; et al. Secoisolariciresinol diglycoside, a flaxseed lignan, exerts analgesic effects in a mouse model of type 1 diabetes: Engagement of antioxidant mechanism. Eur. J. Pharmacol. 2015, 767, 183–192. [Google Scholar] [CrossRef]
- McCann, S.E.; Edge, S.B.; Hicks, D.G.; Thompson, L.U.; Morrison, C.D.; Fetterly, G.; Andrews, C.; Clark, K.; Wilton, J.; Kulkarni, S. A pilot study comparing the effect of flaxseed, aromatase inhibitor, and the combination on breast tumor biomarkers. Nutr. Cancer 2014, 66, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.U.; Seidl, M.M.; Rickard, S.E.; Orcheson, L.J.; Fong, H.H. Antitumorigenic effect of a mammalian lignan precursor from flaxseed. Nutr. Cancer 1996, 26, 159–165. [Google Scholar] [CrossRef]
- Chen, J.; Tan, K.P.; Ward, W.E.; Thompson, L.U. Exposure to flaxseed or its purified lignan during suckling inhibits chemically induced rat mammary tumorigenesis. Exp. Biol. Med. 2003, 228, 951–958. [Google Scholar] [CrossRef]
- Devaraj, S.; Hemarajata, P.; Versalovic, J. The human gut microbiome and body metabolism: Implications for obesity and diabetes. Clin. Chem. 2013, 59, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Nieuwdorp, M.; Gilijamse, P.W.; Pai, N.; Kaplan, L.M. Role of the microbiome in energy regulation and metabolism. Gastroenterology 2014, 146, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Arora, T.; Rudenko, O.; Egerod, K.L.; Husted, A.S.; Kovatcheva-Datchary, P.; Akrami, R.; Kristensen, M.; Schwartz, T.W.; Bäckhed, F. Microbial fermentation of flaxseed fibers modulates the transcriptome of GPR41-expressing enteroendocrine cells and protects mice against diet-induced obesity. Am. J. Physiol.-Endocrinol. Metab. 2019, 316, E453–E463. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A.; Kinzler, K.W. Cancer Genome Landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
- Bergers, G.; Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Block, K. The Block Center Program for Integrative Cancer Treatment. In Life Over Cancer; Random House Publishing Group: New York, NY, USA, 2009; p. 594. [Google Scholar]
- Devi, N.S.; Ramanan, M.; Paragi-Vedanthi, P.; Doble, M. Phytochemicals as multi-target inhibitors of the inflammatory pathway- A modeling and experimental study. Biochem. Biophys. Res. Commun. 2017, 484, 467–473. [Google Scholar] [CrossRef]
- Thomas, E.; Egon, K. Complex Interactions between Phytochemicals. The Multi-Target Therapeutic Concept of Phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef]
- Wang, H.; Khor, T.O.; Shu, L.; Su, Z.; Fuentes, F.; Lee, J.-H.; Kong, A.-N.T. Plants Against Cancer: A Review on Natural Phytochemicals in Preventing and Treating Cancers and Their Druggability. Anti-Cancer Agents Med. Chem. 2012, 12, 1281–1305. [Google Scholar] [CrossRef]
- Lee, K.W.; Bode, A.M.; Dong, Z. Molecular targets of phytochemicals for cancer prevention. Nat. Rev. Cancer 2011, 11, 211–218. [Google Scholar] [CrossRef]
- Broad-spectrum Therapy. Available online: http://www.gettingtoknowcancer.org/broad-spectrum_chemotherapy.php (accessed on 12 December 2017).
- Bishayee, A. The Inflammation and Liver Cancer. In Inflammation and Cancer; Aggarwal, B.B., Sung, B., Gupta, S.C., Eds.; Springer: Basel, Switzerland, 2014; pp. 401–435. [Google Scholar] [CrossRef]
- Muqbil, I.; Bao, G.W.; El-Kharraj, R.; Shah, M.; Mohammad, R.M.; Sarkar, F.H.; Azmi, A.S. Systems and Network Pharmacology Approaches to Cancer Stem Cells Research and Therapy. J. Stem Cell Res. Ther. 2012, 7, 10413. [Google Scholar] [CrossRef] [PubMed]
- Teponno, R.B.; Kusari, S.; Spiteller, M. Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef]
- Stocker, R.; Perrella, M.A. Heme oxygenase-1: A novel drug target for atherosclerotic diseases? Circulation 2006, 114, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, A.M.; Kansanen, E.; Jyrkkänen, H.-K.; Nurmi, T.; Ylä-Herttuala, S.; Levonen, A.-L. Enterolactone Induces Heme Oxygenase-1 Expression through Nuclear Factor-E2-Related Factor 2 Activation in Endothelial Cells. J. Nutr. 2008, 138, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Rajesha, J.; Murthy, K.N.; Kumar, M.K.; Madhusudhan, B.; Ravishankar, G.A. Antioxidant potentials of flaxseed by in vivo model. J. Agric. Food Chem. 2006, 54, 3794–3799. [Google Scholar] [CrossRef] [PubMed]
- Cortes, C.; Palin, M.F.; Gagnon, N.; Benchaar, C.; Lacasse, P.; Petit, H.V. Mammary gene expression and activity of antioxidant enzymes and concentration of the mammalian lignan enterolactone in milk and plasma of dairy cows fed flax lignans and infused with flax oil in the abomasum. Br. J. Nutr. 2012, 108, 1390–1398. [Google Scholar] [CrossRef]
- Lee, J.C.; Krochak, R.; Blouin, A.; Kanterakis, S.; Chatterjee, S.; Arguiri, E.; Vachani, A.; Solomides, C.C.; Cengel, K.A.; Christofidou-Solomidou, M. Dietary flaxseed prevents radiation-induced oxidative lung damage, inflammation and fibrosis in a mouse model of thoracic radiation injury. Cancer Biol. Ther. 2009, 8, 47–53. [Google Scholar] [CrossRef]
- Slavova-Kazakova, A.; Karamać, M.; Kancheva, V.; Amarowicz, R. Antioxidant Activity of Flaxseed Extracts in Lipid Systems. Molecules 2016, 21, 17. [Google Scholar] [CrossRef]
- Hosseinian, F.S.; Muir, A.D.; Westcott, N.D.; Krol, E.S. Antioxidant capacity of flaxseed lignans in two model systems. J. Am. Oil Chem. Soc. 2006, 83, 835. [Google Scholar] [CrossRef]
- Kasote, D. Flaxseed phenolics as natural antioxidants. Int. Food Res. J. 2013, 20, 27. [Google Scholar]
- Barthet, V.J.; Klensporf-Pawlik, D.; Przybylski, R. Antioxidant activity of flaxseed meal components. Can. J. Plant Sci. 2014, 94, 593–602. [Google Scholar] [CrossRef]
- Cort, A.; Ozben, T.; Saso, L.; De Luca, C.; Korkina, L. Redox Control of Multidrug Resistance and Its Possible Modulation by Antioxidants. Oxid. Med. Cell. Longev. 2016, 2016, 4251912. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.; Pischke, S.E.; Zhou, Z.; Davis, R.J.; Flavell, R.A.; Loop, T.; Otterbein, S.L.; Otterbein, L.E.; Choi, A.M. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1. J. Biol. Chem. 2003, 278, 36993–36998. [Google Scholar] [CrossRef] [PubMed]
- Ryu, E.Y.; Park, S.Y.; Kim, S.G.; Park, D.J.; Kang, J.S.; Kim, Y.H.; Seetharaman, R.; Choi, Y.W.; Lee, S.J. Anti-inflammatory effect of heme oxygenase-1 toward Porphyromonas gingivalis lipopolysaccharide in macrophages exposed to gomisins A, G, and J. J. Med. Food 2011, 14, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Sawant, S.H.; Bodhankar, S.L. Flax lignan concentrate attenuate hypertension and abnormal left ventricular contractility via modulation of endogenous biomarkers in two-kidney-one-clip (2K1C) hypertensive rats. Revista Brasileira de Farmacognosia 2016, 26, 601–610. [Google Scholar] [CrossRef]
- Velalopoulou, A.; Tyagi, S.; Pietrofesa, R.A.; Arguiri, E.; Christofidou-Solomidou, M. The Flaxseed-Derived Lignan Phenolic Secoisolariciresinol Diglucoside (SDG) Protects Non-Malignant Lung Cells from Radiation Damage. Int. J. Mol. Sci. 2015, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Christofidou-Solomidou, M.; Tyagi, S.; Pietrofesa, R.; Dukes, F.; Arguiri, E.; Turowski, J.; Grieshaber, P.A.; Solomides, C.C.; Cengel, K.A. Radioprotective role in lung of the flaxseed lignan complex enriched in the phenolic secoisolariciresinol diglucoside (SDG). Radiat. Res. 2012, 178, 568–580. [Google Scholar] [CrossRef]
- Mishra, O.P.; Popov, A.V.; Pietrofesa, R.A.; Christofidou-Solomidou, M. Gamma-irradiation produces active chlorine species (ACS) in physiological solutions: Secoisolariciresinol diglucoside (SDG) scavenges ACS - A novel mechanism of DNA radioprotection. Biochimica et biophysica acta 2016, 1860, 1884–1897. [Google Scholar] [CrossRef] [PubMed]
- Mishra, O.P.; Pietrofesa, R.; Christofidou-Solomidou, M. Novel synthetic (S,S) and (R,R)-secoisolariciresinol diglucosides (SDGs) protect naked plasmid and genomic DNA From gamma radiation damage. Radiat. Res. 2014, 182, 102–110. [Google Scholar] [CrossRef]
- Braun, S.; Bitton-Worms, K.; LeRoith, D. The Link between the Metabolic Syndrome and Cancer. Int. J. Biol. Sci. 2011, 7, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Philip, M.; Rowley, D.A.; Schreiber, H. Inflammation as a tumor promoter in cancer induction. Semin. Cancer Biol. 2004, 14, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Pietrofesa, R.; Turowski, J.; Tyagi, S.; Dukes, F.; Arguiri, E.; Busch, T.M.; Gallagher-Colombo, S.M.; Solomides, C.C.; Cengel, K.A.; Christofidou-Solomidou, M. Radiation mitigating properties of the lignan component in flaxseed. BMC Cancer 2013, 13, 179. [Google Scholar] [CrossRef] [PubMed]
- Pietrofesa, R.A.; Velalopoulou, A.; Arguiri, E.; Menges, C.W.; Testa, J.R.; Hwang, W.T.; Albelda, S.M.; Christofidou-Solomidou, M. Flaxseed lignans enriched in secoisolariciresinol diglucoside prevent acute asbestos-induced peritoneal inflammation in mice. Carcinogenesis 2016, 37, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, A.; Gomes Filho, M.A.; Eilati, E.; McGee, S.; Small, C.; Gao, C.; Klug, T.; Hales, D.B. Flaxseed reduces the pro-carcinogenic micro-environment in the ovaries of normal hens by altering the PG and oestrogen pathways in a dose-dependent manner. Br. J. Nutr. 2015, 113, 1384–1395. [Google Scholar] [CrossRef]
- Christofidou-Solomidou, M.; Pietrofesa, R.; Arguiri, E.; McAlexander, M.A.; Witwer, K.W. Dietary flaxseed modulates the miRNA profile in irradiated and non-irradiated murine lungs: A novel mechanism of tissue radioprotection by flaxseed. Cancer Biol. Ther. 2014, 15, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Verghese, M.; Walker, L.T.; Boateng, J.; Shackelford, L.; Chawan, C.B. Flax seed oil and flax seed meal reduce the formation of aberrant crypt foci (ACF) in azoxymethane-induced colon cancer in Fisher 344 male rats. Food Chem. Toxicol. 2007, 45, 153–159. [Google Scholar] [CrossRef]
- Curry, C.; Garcia-Villatoro, E.; Hales, D.B.; Allred, C.D. The Chemoprotective Effects of Mammalian Lignans Enterodiol and Enterolactone in Non-Transformed Colonocytes. FASEB J. 2016, 30, 691.38. [Google Scholar] [CrossRef]
- Curry, C.A. Chemoprotective Effects of Flaxseed Lignans Enterodiol and Enterolactone in Non-Transformed Colonocytes. Ph.D. Thesis, Texas A & M University, College Station, TX, USA, 2015. [Google Scholar]
- Awad, K.S. Inhibition of Human Papilloma Virus E6 Oncogene Function by Mammalian Lignans Activates p53 Tumor Supressor Protein and Induces Apoptosis in Cervical Cancer Cells. Ph.D. Thesis, Kent State University, Kent, OH, USA, 2007. [Google Scholar]
- Spurgers, K.B.; Coombes, K.R.; Meyn, R.E.; Gold, D.L.; Logothetis, C.J.; Johnson, T.J.; McDonnell, T.J. A comprehensive assessment of p53-responsive genes following adenoviral-p53 gene transfer in Bcl-2-expressing prostate cancer cells. Oncogene 2004, 23, 1712–1723. [Google Scholar] [CrossRef]
- Alimirah, F.; Panchanathan, R.; Davis, F.J.; Chen, J.; Choubey, D. Restoration of p53 expression in human cancer cell lines upregulates the expression of Notch1: Implications for cancer cell fate determination after genotoxic stress. Neoplasia 2007, 9, 427–434. [Google Scholar] [CrossRef]
- Feig, D.I.; Reid, T.M.; Loeb, L.A. Reactive oxygen species in tumorigenesis. Cancer Res. 1994, 54, 1890s–1894s. [Google Scholar] [PubMed]
- Xue, J.Y.; Liu, G.T.; Wei, H.L.; Pan, Y. Antioxidant activity of two dibenzocyclooctene lignans on the aged and ischemic brain in rats. Free Radic. Biol. Med. 1992, 12, 127–135. [Google Scholar] [CrossRef]
- McCann, M.J.; Rowland, I.R.; Roy, N.C. The anti-proliferative effects of enterolactone in prostate cancer cells: Evidence for the role of DNA licencing genes, mi-R106b cluster expression, and PTEN dosage. Nutrients 2014, 6, 4839–4855. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Dhar, A. Flaxseed and Diabetes. Curr. Pharm. Des. 2016, 22, 141–144. [Google Scholar] [CrossRef]
- Torkan, M.; Entezari, M.H.; Siavash, M. Effect of flaxseed on blood lipid level in hyperlipidemic patients. Rev. Recent Clin. Trials 2015, 10, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Almario, R.U.; Karakas, S.E. Lignan content of the flaxseed influences its biological effects in healthy men and women. J. Am. Coll. Nutr. 2013, 32, 194–199. [Google Scholar] [CrossRef]
- Ding, E.L.; Hu, F.B. Cancer and cholesterol: Understanding the V-shaped association in patients with diabetes. CMAJ 2008, 179, 403–404. [Google Scholar] [CrossRef][Green Version]
- Radisauskas, R.; Kuzmickiene, I.; Milinaviciene, E.; Everatt, R. Hypertension, serum lipids and cancer risk: A review of epidemiological evidence. Medicina 2016, 52, 89–98. [Google Scholar] [CrossRef]
- Vulcan, A.; Manjer, J.; Ohlsson, B. High blood glucose levels are associated with higher risk of colon cancer in men: A cohort study. BMC Cancer 2017, 17, 842. [Google Scholar] [CrossRef]
- Ryu, T.Y.; Park, J.; Scherer, P.E. Hyperglycemia as a risk factor for cancer progression. Diabetes Metab. J. 2014, 38, 330–336. [Google Scholar] [CrossRef]
- Cui, G.; Zhang, T.; Ren, F.; Feng, W.M.; Yao, Y.; Cui, J.; Zhu, G.L.; Shi, Q.L. High Blood Glucose Levels Correlate with Tumor Malignancy in Colorectal Cancer Patients. Med Sci Monit 2015, 21, 3825–3833. [Google Scholar] [CrossRef]
- May-Wilson, S.; Sud, A.; Law, P.J.; Palin, K.; Tuupanen, S.; Gylfe, A.; Hanninen, U.A.; Cajuso, T.; Tanskanen, T.; Kondelin, J.; et al. Pro-inflammatory fatty acid profile and colorectal cancer risk: A Mendelian randomisation analysis. Eur. J. Cancer 2017, 84, 228–238. [Google Scholar] [CrossRef]
- Ribas, V.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondria, cholesterol and cancer cell metabolism. Clin. Transl. Med. 2016, 5, 22. [Google Scholar] [CrossRef]
- Kuzu, O.F.; Noory, M.A.; Robertson, G.P. The Role of Cholesterol in Cancer. Cancer Res. 2016, 76, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P.E.; Filigheddu, N.; Pedro, J.M.B.; Kroemer, G.; Galluzzi, L. Mitochondrial metabolism and cancer. Cell Res. 2018, 28, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef] [PubMed]
- Drygalski, K.; Berk, K.; Charytoniuk, T.; Ilowska, N.; Lukaszuk, B.; Chabowski, A.; Konstantynowicz-Nowicka, K. Does the enterolactone (ENL) affect fatty acid transporters and lipid metabolism in liver? Nutr. Metab. 2017, 14, 69. [Google Scholar] [CrossRef]
- Di, Y.; De Silva, F.; Krol, E.S.; Alcorn, J. Flaxseed Lignans Enhance the Cytotoxicity of Chemotherapeutic Agents against Breast Cancer Cell Lines MDA-MB-231 and SKBR3. Nutr. Cancer 2018, 70, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, Y.; Furukawa, T.; Oyama, N.; Hasegawa, Y.; Kiyono, Y.; Nishii, R.; Waki, A.; Tsuji, A.B.; Sogawa, C.; Wakizaka, H.; et al. Fatty acid synthase is a key target in multiple essential tumor functions of prostate cancer: Uptake of radiolabeled acetate as a predictor of the targeted therapy outcome. PLoS ONE 2013, 8, e64570. [Google Scholar] [CrossRef]
- Fukumitsu, S.; Aida, K.; Ueno, N.; Ozawa, S.; Takahashi, Y.; Kobori, M. Flaxseed lignan attenuates high-fat diet-induced fat accumulation and induces adiponectin expression in mice. Br. J. Nutr. 2008, 100, 669–676. [Google Scholar] [CrossRef]
- Rietjens, I.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef]
- Key, T.J. Hormones and cancer in humans. Mutat. Res. 1995, 333, 59–67. [Google Scholar] [CrossRef]
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Martinez-Chantar, M.L.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015, 35, S25–S54. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.O.; Simon, S.; Chae, K.; Metzler, M.; Korach, K.S. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol. Sci. 2004, 80, 14–25. [Google Scholar] [CrossRef]
- Saarinen, N.M.; Power, K.; Chen, J.; Thompson, L.U. Flaxseed attenuates the tumor growth stimulating effect of soy protein in ovariectomized athymic mice with MCF-7 human breast cancer xenografts. Int. J. Cancer 2006, 119, 925–931. [Google Scholar] [CrossRef]
- Chen, J.; Hui, E.; Ip, T.; Thompson, L.U. Dietary flaxseed enhances the inhibitory effect of tamoxifen on the growth of estrogen-dependent human breast cancer (mcf-7) in nude mice. Clin. Cancer Res. 2004, 10, 7703–7711. [Google Scholar] [CrossRef]
- Chen, J.; Power, K.A.; Mann, J.; Cheng, A.; Thompson, L.U. Flaxseed alone or in combination with tamoxifen inhibits MCF-7 breast tumor growth in ovariectomized athymic mice with high circulating levels of estrogen. Exp. Biol. Med. 2007, 232, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Fang, J.; Sun, Z.; Li, H.; Wu, Y.; Demark-Wahnefried, W.; Lin, X. Enterolactone inhibits insulin-like growth factor-1 receptor signaling in human prostatic carcinoma PC-3 cells. J. Nutr. 2009, 139, 653–659. [Google Scholar] [CrossRef]
- Rosner, W.; Hryb, D.J.; Khan, M.S.; Nakhla, A.M.; Romas, N.A. Sex hormone-binding globulin mediates steroid hormone signal transduction at the plasma membrane. J. Steroid Biochem. Mol. Biol. 1999, 69, 481–485. [Google Scholar] [CrossRef]
- Schottner, M.; Spiteller, G.; Gansser, D. Lignans interfering with 5 alpha-dihydrotestosterone binding to human sex hormone-binding globulin. J. Nat. Prod. 1998, 61, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H.; Mousavi, Y.; Clark, J.; Hockerstedt, K.; Hamalainen, E.; Wahala, K.; Makela, T.; Hase, T. Dietary phytoestrogens and cancer: In vitro and in vivo studies. J. Steroid Biochem. Mol. Biol. 1992, 41, 331–337. [Google Scholar] [CrossRef]
- Shoulars, K.; Rodrigues, M.A.; Crowley, J.R.; Turk, J.; Thompson, T.; Markaverich, B.M. Nuclear type II [3H]estradiol binding sites: A histone H3-H4 complex. J. Steroid Biochem. Mol. Biol. 2005, 96, 19–30. [Google Scholar] [CrossRef]
- Shoulars, K.; Brown, T.; Alejandro, M.A.; Crowley, J.; Markaverich, B.M. Identification of nuclear type II [3H]estradiol binding sites as histone H4. Biochem. Biophys. Res. Commun. 2002, 296, 1083–1090. [Google Scholar] [CrossRef]
- Brodie, A.M.; Lu, Q.; Long, B.J.; Fulton, A.; Chen, T.; Macpherson, N.; DeJong, P.C.; Blankenstein, M.A.; Nortier, J.W.; Slee, P.H.; et al. Aromatase and COX-2 expression in human breast cancers. J. Steroid Biochem. Mol. Biol. 2001, 79, 41–47. [Google Scholar] [CrossRef]
- Poutanen, M.; Isomaa, V.; Lehto, V.P.; Vihko, R. Immunological analysis of 17β-hydroxysteroid dehydrogenase in benign and malignant human breast tissue. Int. J. Cancer 1992, 50, 386–390. [Google Scholar] [CrossRef]
- Evans, B.A.; Griffiths, K.; Morton, M.S. Inhibition of 5 alpha-reductase in genital skin fibroblasts and prostate tissue by dietary lignans and isoflavonoids. J. Endocrinol. 1995, 147, 295–302. [Google Scholar] [CrossRef]
- Xiong, X.Y.; Hu, X.J.; Li, Y.; Liu, C.M. Inhibitory Effects of Enterolactone on Growth and Metastasis in Human Breast Cancer. Nutr. Cancer 2015, 67, 1324–1332. [Google Scholar] [CrossRef]
- Chikara, S.; Lindsey, K.; Borowicz, P.; Christofidou-Solomidou, M.; Reindl, K.M. Enterolactone alters FAK-Src signaling and suppresses migration and invasion of lung cancer cell lines. BMC Complement. Alternat. Med. 2017, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Peuhu, E.; Rivero-Muller, A.; Stykki, H.; Torvaldson, E.; Holmbom, T.; Eklund, P.; Unkila, M.; Sjoholm, R.; Eriksson, J.E. Inhibition of Akt signaling by the lignan matairesinol sensitizes prostate cancer cells to TRAIL-induced apoptosis. Oncogene 2010, 29, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, D.J.; Kim, Y.H.; Kim, Y.; Kim, S.G.; Shon, K.J.; Choi, Y.W.; Lee, S.J. Upregulation of heme oxygenase-1 via PI3K/Akt and Nrf-2 signaling pathways mediates the anti-inflammatory activity of Schisandrin in Porphyromonas gingivalis LPS-stimulated macrophages. Immunol. Lett. 2011, 139, 93–101. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, D.J.; Kim, Y.H.; Kim, Y.; Choi, Y.W.; Lee, S.J. Schisandra chinensis alpha-iso-cubebenol induces heme oxygenase-1 expression through PI3K/Akt and Nrf2 signaling and has anti-inflammatory activity in Porphyromonas gingivalis lipopolysaccharide-stimulated macrophages. Int. Immunopharmacol. 2011, 11, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Fang, J.; Li, H.; Demark-Wahnefried, W.; Lin, X. Enterolactone induces apoptosis in human prostate carcinoma LNCaP cells via a mitochondrial-mediated, caspase-dependent pathway. Mol. Cancer Ther. 2007, 6, 2581–2590. [Google Scholar] [CrossRef] [PubMed]
- Gacche, R.N.; Meshram, R.J. Targeting tumor micro-environment for design and development of novel anti-angiogenic agents arresting tumor growth. Prog. Biophys. Mol. Biol. 2013, 113, 333–354. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Adjei, A.A. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef]
- Dimova, I.; Popivanov, G.; Djonov, V. Angiogenesis in cancer—General pathways and their therapeutic implications. J. BUON 2014, 19, 15–21. [Google Scholar]
- Azzi, S.; Hebda, J.K.; Gavard, J. Vascular permeability and drug delivery in cancers. Front. Oncol. 2013, 3, 211. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Thompson, L.U. Flaxseed and its components reduce metastasis after surgical excision of solid human breast tumor in nude mice. Cancer Lett. 2006, 234, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Magee, P.J.; McGlynn, H.; Rowland, I.R. Differential effects of isoflavones and lignans on invasiveness of MDA-MB-231 breast cancer cells in vitro. Cancer Lett. 2004, 208, 35–41. [Google Scholar] [CrossRef]
- Bartsch, J.E.; Staren, E.D.; Appert, H.E. Matrix metalloproteinase expression in breast cancer. J. Surg. Res. 2003, 110, 383–392. [Google Scholar] [CrossRef]
- Mali, A.V.; Wagh, U.V.; Hegde, M.V.; Chandorkar, S.S.; Surve, S.V.; Patole, M.V. In vitro anti-metastatic activity of enterolactone, a mammalian lignan derived from flax lignan, and down-regulation of matrix metalloproteinases in MCF-7 and MDA MB 231 cell lines. Indian J. Cancer 2012, 49, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Mali, A.V.; Joshi, A.A.; Hegde, M.V.; Kadam, S.S. Enterolactone Suppresses Proliferation, Migration and Metastasis of MDA-MB-231 Breast Cancer Cells Through Inhibition of uPA Induced Plasmin Activation and MMPs-Mediated ECM Remodeling. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Kraljevic Pavelic, S.; Sedic, M.; Bosnjak, H.; Spaventi, S.; Pavelic, K. Metastasis: New perspectives on an old problem. Mol. Cancer 2011, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Y.; Feng, C.; Wu, G.; Ye, Y.; Tian, J. Calycosin Inhibits the Migration and Invasion of Human Breast Cancer Cells by Down-Regulation of Foxp3 Expression. Cell. Physiol. Biochem. 2017, 44, 1775–1784. [Google Scholar] [CrossRef]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed]
- Mali, A.V.; Joshi, A.A.; Hegde, M.V.; Kadam, S.S. Enterolactone modulates the ERK/NF-κB/Snail signaling pathway in triple-negative breast cancer cell line MDA-MB-231 to revert the TGF-β-induced epithelial-mesenchymal transition. Cancer Biol. Med. 2018, 15, 137–156. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Wang, X.L.; Sun, D.X.; Liu, X.X.; Hu, X.Y.; Kong, F. Regulation of zinc transporters by dietary flaxseed lignan in human breast cancer xenografts. Mol. Biol. Rep. 2008, 35, 595–600. [Google Scholar] [CrossRef]
- Lineberger, C.G.; Bowers, L.W.; Ford, N.A.; Rossi, E.L.; Kimler, B.K.; Fabian, C.J.; Hursting, S.D. Abstract 231: The polyphenolic plant lignan secoisolariciresinol diglycoside reduces mammary tumor growth, possibly via inhibition of local inflammatory signaling. Cancer Res. 2017, 77, 231. [Google Scholar] [CrossRef]
- Feng, J.; Shi, Z.; Ye, Z. Effects of Metabolites of the Lignans Enterolactone and Enterodiol on Osteoblastic Differentiation of MG-63 Cells. Biol. Pharm. Bull. 2008, 31, 1067–1070. [Google Scholar] [CrossRef]
- Ren, G.Y.; Chen, C.Y.; Chen, W.G.; Huang, Y.; Qin, L.Q.; Chen, L.H. The treatment effects of flaxseed-derived secoisolariciresinol diglycoside and its metabolite enterolactone on benign prostatic hyperplasia involve the G protein-coupled estrogen receptor 1. Appl. Physiol. Nutr. Metab. 2016, 41, 1303–1310. [Google Scholar] [CrossRef]
- Bommareddy, A.; Zhang, X.; Schrader, D.; Kaushik, R.S.; Zeman, D.; Matthees, D.P.; Dwivedi, C. Effects of dietary flaxseed on intestinal tumorigenesis in Apc(Min) mouse. Nutr. Cancer 2009, 61, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Lehraiki, A.; Attoumbre, J.; Bienaime, C.; Matifat, F.; Bensaddek, L.; Nava-Saucedo, E.; Fliniaux, M.A.; Ouadid-Ahidouch, H.; Baltora-Rosset, S. Extraction of lignans from flaxseed and evaluation of their biological effects on breast cancer MCF-7 and MDA-MB-231 cell lines. J. Med. Food 2010, 13, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Chan, D.S.M.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2011, 343, d6617. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.; Doll, R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int. J. Cancer 1975, 15, 617–631. [Google Scholar] [CrossRef]
- Kolonel, L.N. Cancer patterns of four ethnic groups in Hawaii. J. Natl. Cancer Inst. 1980, 65, 1127–1139. [Google Scholar]
- Kono, S. Secular trend of colon cancer incidence and mortality in relation to fat and meat intake in Japan. Eur. J. Cancer Prev. 2004, 13, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Cagan, R.; Meyer, P. Rethinking cancer: Current challenges and opportunities in cancer research. Disease Models Mech. 2017, 10, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Molina-Montes, E.; Sánchez, M.-J.; Zamora-Ros, R.; Bueno-de-Mesquita, H.B.A.; Wark, P.A.; Obon-Santacana, M.; Kühn, T.; Katzke, V.; Travis, R.C.; Ye, W.; et al. Flavonoid and lignan intake and pancreatic cancer risk in the European prospective investigation into cancer and nutrition cohort. Int. J. Cancer 2016, 139, 1480–1492. [Google Scholar] [CrossRef]
- Mattiello, A.; Hjartåker, A.; Mulligan, A.A.; Tjønneland, A.; Trichopoulou, A.; Agudo, A.; Scalbert, A.; Teucher, B.; Saieva, C.; González, C.A.; et al. Dietary flavonoid and lignan intake and gastric adenocarcinoma risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am. J. Clin. Nutr. 2012, 96, 1398–1408. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Sacerdote, C.; Ricceri, F.; Weiderpass, E.; Roswall, N.; Buckland, G.; St-Jules, D.E.; Overvad, K.; Kyro, C.; Fagherazzi, G.; et al. Flavonoid and lignan intake in relation to bladder cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Cancer 2014, 111, 1870–1880. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Fedirko, V.; Trichopoulou, A.; Gonzalez, C.A.; Bamia, C.; Trepo, E.; Nothlings, U.; Duarte-Salles, T.; Serafini, M.; Bredsdorff, L.; et al. Dietary flavonoid, lignan and antioxidant capacity and risk of hepatocellular carcinoma in the European prospective investigation into cancer and nutrition study. Int. J. Cancer 2013, 133, 2429–2443. [Google Scholar] [CrossRef] [PubMed]
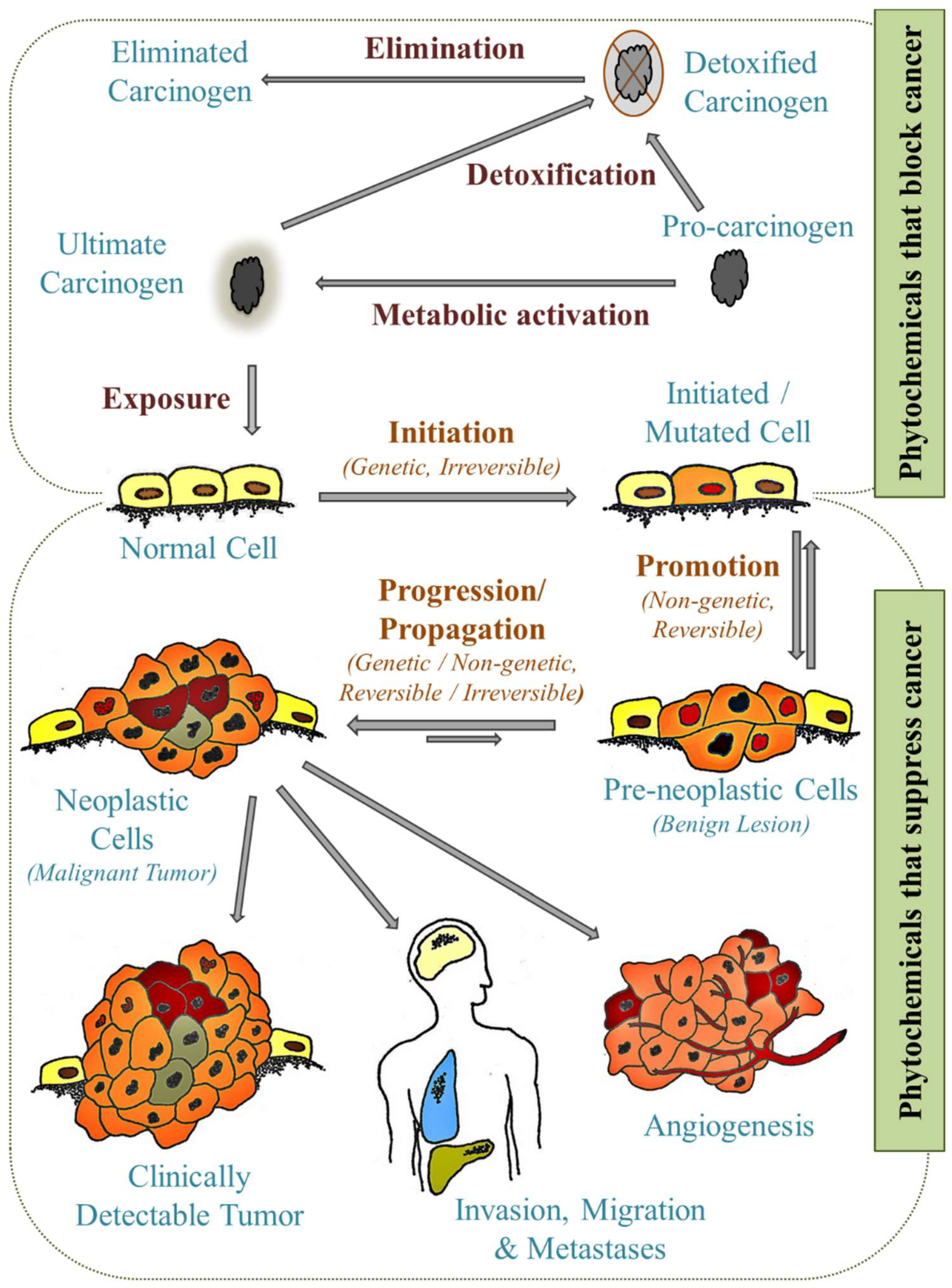
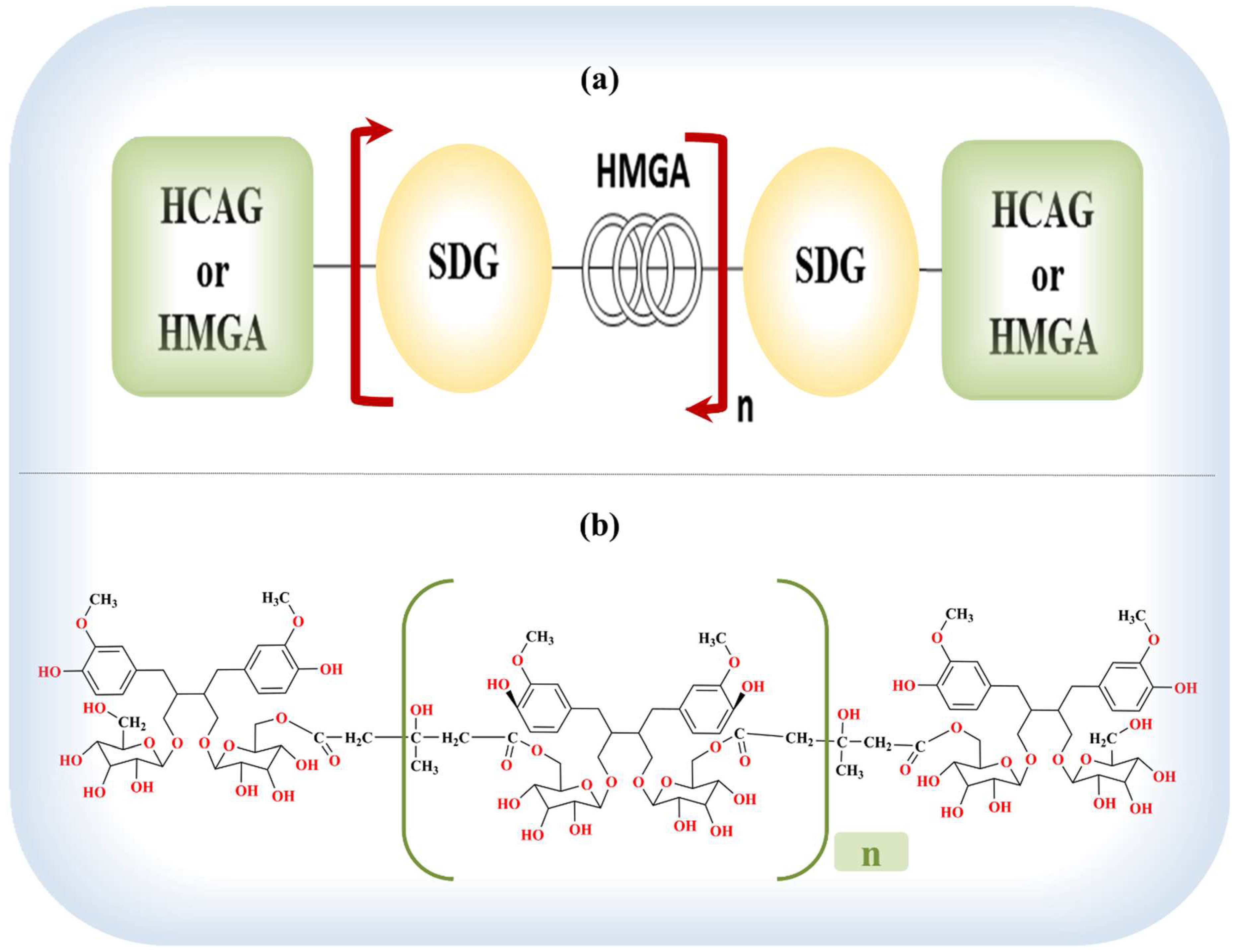
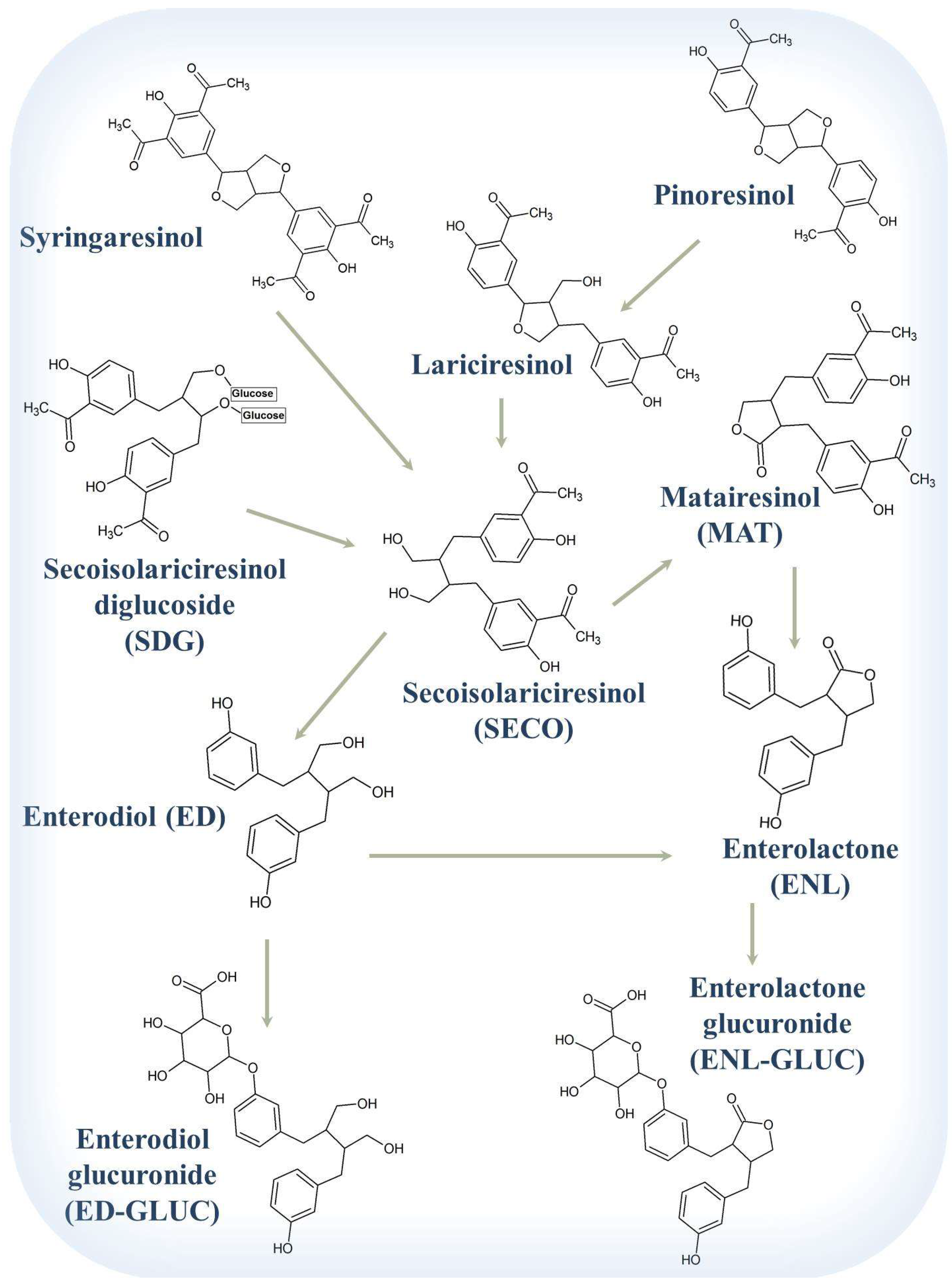
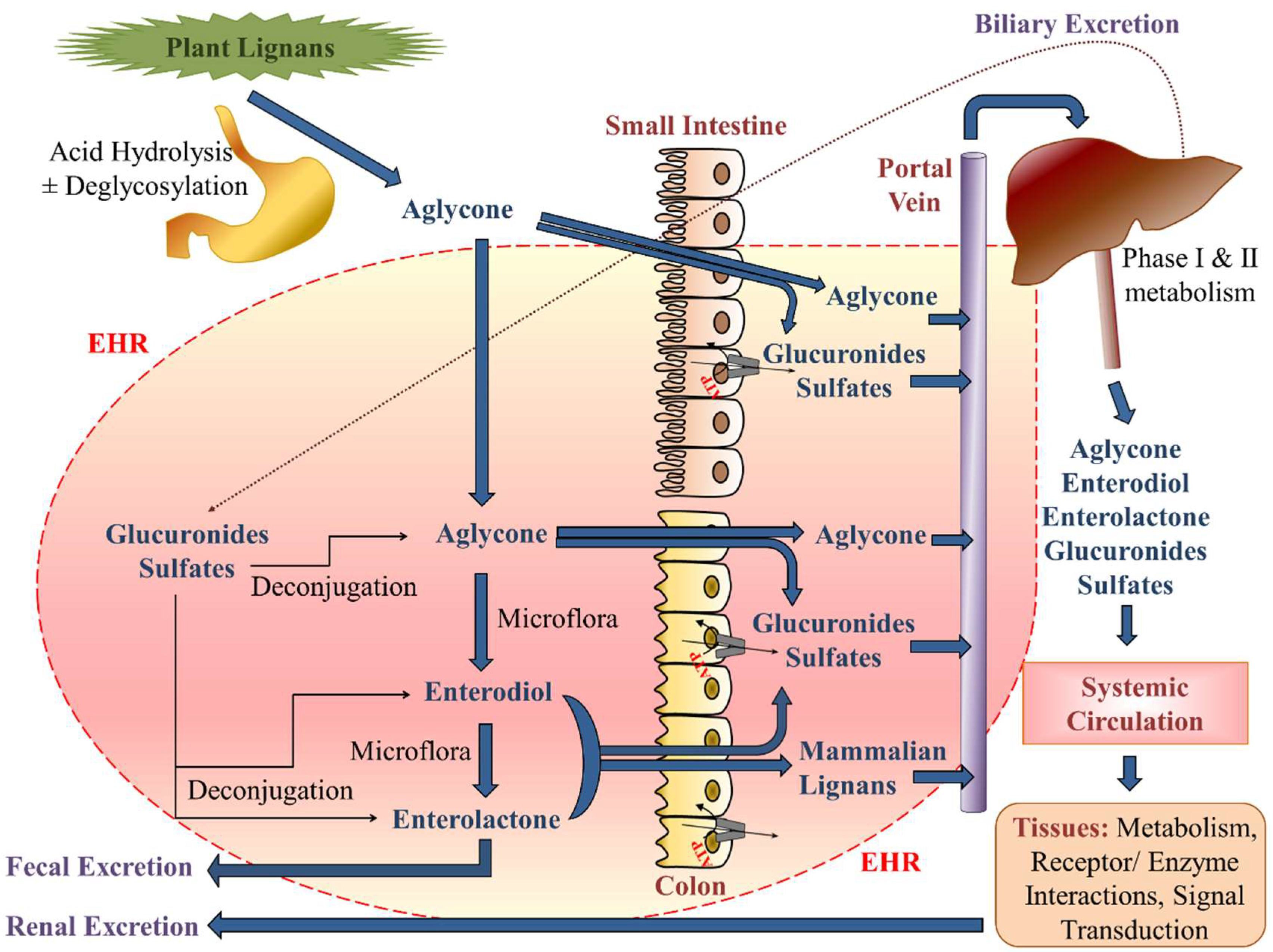
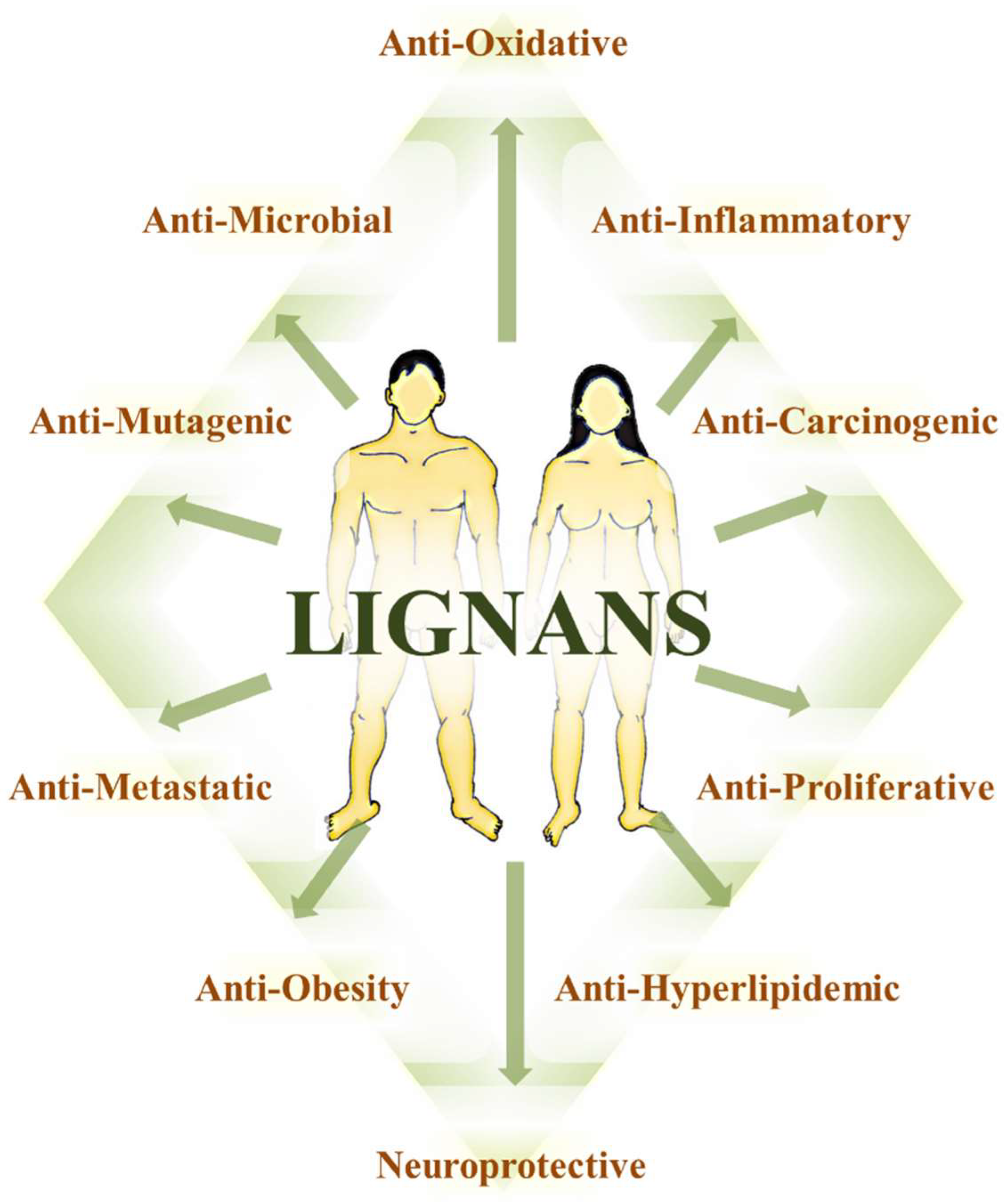
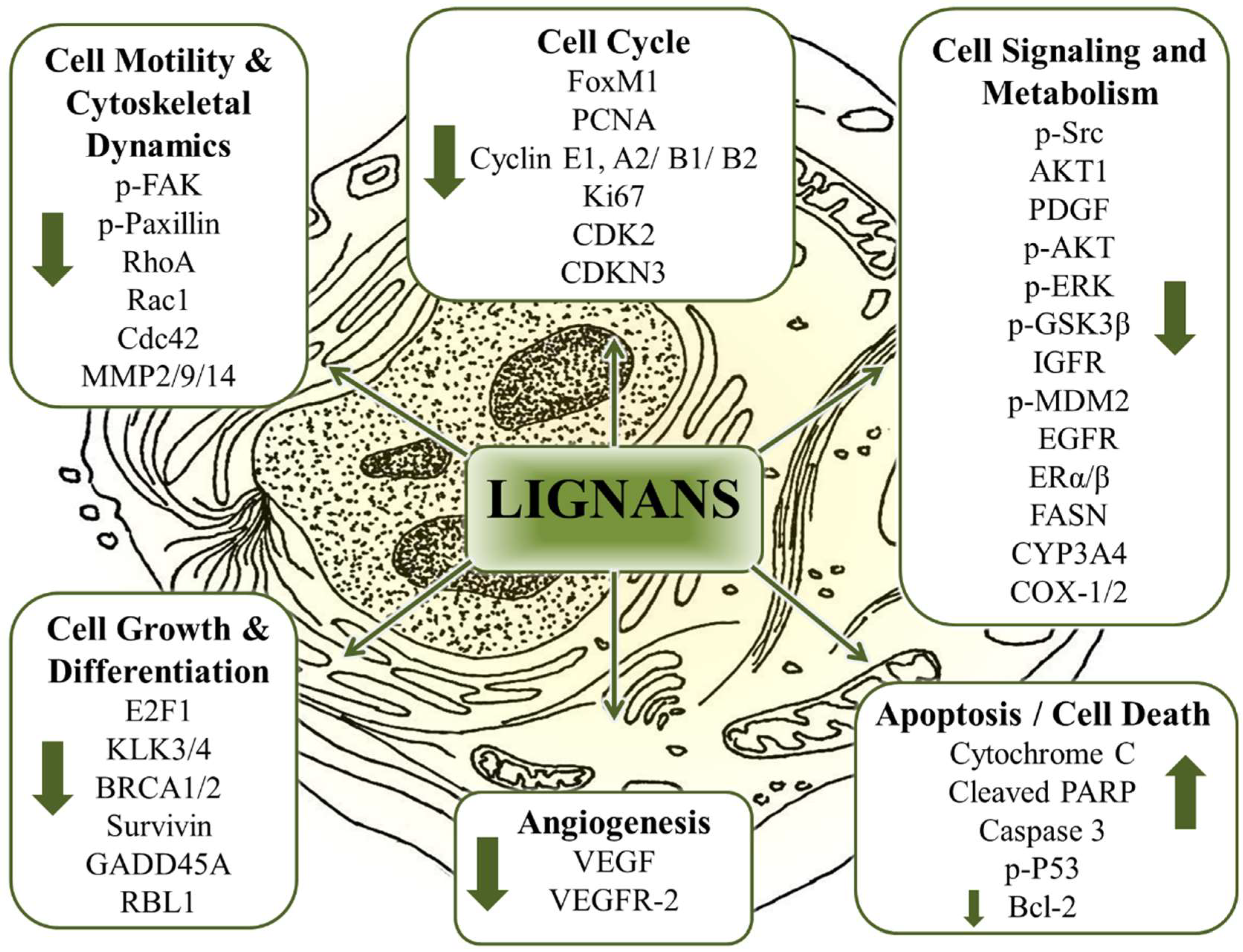
| Classification | Representative Members | Examples of Dietary Sources | ||
|---|---|---|---|---|
| Poly-Phenolics | Phenolic Acids | Hydroxycinnamic acids | p-Coumaric, caffeic, ferulic, sinapic | Barley, eucalyptus, coffee, Arabidopsis, Hibiscus, cereal grains |
| Hydroxybenzoic acids | Gallic, vanillic, syringic, ellagic | Chestnuts (boiled or roasted), witch hazel, tea leaves, oak bark, rhubarb, pomegranate, grapes, chocolate, wine | ||
| Lignans | Plant Lignans | sesamin, secoisolariciresinol diglucoside, lariciresinol, isolariciresinol, 7-hydroxymatairesinol, matairesinol, pinoresinol, arctigenin, syringaresinol, asarinin | Flaxseed, pumpkin, sunflower, poppy, rye, oats, barley, wheat, oat, rye, berries | |
| Mammalian Lignans (enterolignans) | Enterodiol, enterolactone | |||
| Stilbenes | Grapes | |||
| Other Phenolics | Coumarins | Tonka bean, vanilla grass | ||
| Tannins | Eucalyptus, geranium | |||
| Flavonoids | Flavonols | Quercetin, kaempferol, myricetin | Aloe Vera, European elderberry, soy, St John’s wort, tomatoes, red onions | |
| Flavones | Apigenin, luteolin | Celery, parsley, chamomile tea, green peppers, thyme, oregano | ||
| Flavanols (catechins) | Catechin, epicatechin, epigallocatechin gallate | White tea, green tea, persimmon, pomegranate, cocoa beans | ||
| Flavanones | Eriodictyol, hesperetin | Citrus fruits, rose hip, mountain balm | ||
| Anthocyanidins | Cyanidin, pelargonidin, malvidin | Grapes, berries, red cabbage, red onions, plums, kidney beans, geranium | ||
| Isoflavonoids | Genistein, glycitein | Lupin, fava beans, soy, coffee | ||
| Alkaloids | Poppy, tomatoes, potatoes | |||
| Carotenoids | α-carotene, β-carotene, lutein, zeaxanthin, lycopene | Carrots, broccoli, spinach, zucchini | ||
| Organosulfur compounds | Isothiocyanates, indoles, allyl sulfur compounds | Cabbage, broccoli, spinach, garlic, onions | ||
| Experimental System and Lignan * | Targets: Molecules (Protein/Gene) | Block’s Model | Hanahan and Weinberg’s Model | Vogelstein et al., Model |
|---|---|---|---|---|
| MDA-MB231 (BC) ENL * | ↓Ki67, ↓PCNA, ↓FoxM1, ↓Cyclin E1, ↓Cyclin A2, ↓Cyclin B1 ↓Cyclin B2 [627] | Proliferation, Immortality, Treatment resistance | Sustaining proliferative signaling, Evading growth suppressors | Cell survival |
| ↓pFAK, ↓pPaxillin [627] ↓ERK-1/2, ↓NF-κB, ↓MAPK-p38, ↓CD44 [647] | Proliferation, Metastasis, Cell-to-cell communication and Immortality | Activating invasion & metastasis, Sustaining proliferative signaling | Cell survival, Cell fate | |
| ↓uPA, ↓MMP-2, ↓MMP-9, ↑PAI-1, ↑TIMP-1, ↑TIMP-2 [643] ↓N-cadherin, ↓vimentin, ↑E-cadherin, ↑occludin, ↓Snail [647] | Differentiation, Metastasis | Activating invasion & metastasis | Cell fate | |
| XM (MDA-MB231) SDG * | ↑LIV-1, ↑↓ ZIP2, ZnT-1 [648] | Proliferation | Sustaining proliferative signaling | Cell survival |
| MO (basal-like BC) SDG * | ↓Proinflammatory markers (F4/80, CRP), ↓p-p65 [649] | Inflammation | Tumor promoting inflammation | Cell survival |
| MO (MCF7) (BC) ENL * | ↓VEGF, ↑PIGF [331] | Proliferation, Treatment resistance, Angiogenesis | Inducing angiogenesis | Cell survival |
| OVX MO (MCF-7) SDG * | ↓ERα, ↓ERβ, ↓EGFR, ↓pS2, ↓IGF-1R, ↓BCL2 [341] | Apoptosis, Proliferation, Glycemia | Sustaining proliferative signaling, Resisting cell death | Cell survival |
| ↓pMAPK [341] | Proliferation | Sustaining proliferative signaling | Cell survival | |
| MCF7, MDA-MB231 ENL * | ↓MMP2, ↓MMP9 ↓MMP14, ± MMP11 [642] | Differentiation, Metastasis | Activating invasion & metastasis | Cell fate |
| A549, H60 (Lung cancer) ENL * | ↓pFAK, ↓pSrc, ↓pPaxillin [628] | Proliferation, Metastasis, Cell-to-cell communication | Activating invasion & metastasis, Sustaining proliferative signaling | Cell survival, Cell fate |
| ↓RhoA, ↓Rac1, ↓Cdc42 [628] | Metastasis, Cell-to-cell communication | Activating invasion & metastasis | Cell fate | |
| ↑↓FAK, PDGF signaling (AKT1, CCND3). ↓RhoA, Rac1, Cdc42, ↑ITGA2 [628] | Metastasis, Differentiation, Proliferation, Cell-to-cell communication | Activating invasion & metastasis, Sustaining proliferative signaling | Cell survival, Cell fate | |
| MG-63 (Osteosarcoma) ENL and ED * | Biphasic (↑↓) – osteonectin, collagen I [650] | Proliferation, Differentiation, Cell-to-cell communication | Activating invasion & metastasis | Cell fate |
| ↑ALP, ↑osteopontin, ↑osteocalcin [650] | Proliferation, Differentiation, Metastasis, Cell-to-cell communication | Activating invasion & metastasis | Cell fate | |
| WPMY-1 (PS) ENL * | ↑GPER, ↑p-ERK, ↑P53, ↑P21, ↓Cyclin D1 [651] | Proliferation, Immortality | Sustaining proliferative signaling | Cell survival |
| Rat prostate SDG * | ↑GPER [651] | Proliferation, Immortality | Sustaining proliferative signaling | Cell survival |
| WPE1-NA22, WPE1-NB14, WPE1-NB11, WPE1-NB26 and LNCaP (PC) ENL * | ↑↓DNA licensing genes (GMNN, CDT1, MCM2, MCM7) [593] | Proliferation, Immortality, Treatment resistance | Sustaining proliferative signaling | Cell survival, Genome maintenance |
| ↓miR-106b cluster (miR-106b, miR-93, miR-25),↑PTEN [593] | Proliferation, Angiogenesis | Sustaining proliferative signaling | Cell survival | |
| LNCaP ENL * | ↓BRCA1, ↓CDK2, ↓CDKN3, ↓E2F1, ↓KLK3, ↓KLK4, ↓PCNA, ↓PIAS1, ↓PRKCD, ↓PRKCH, ↓RASSF1, ↓TPM1, ↓SLC43A1 [335] | Proliferation, Immortality, Differentiation, Treatment resistance | Sustaining proliferative signaling, Replicative immortality, Evading growth suppressors | Cell survival, Genome maintenance Cell fate |
| ↓BIRC5, ↓BRCA1, ↓BRCA2, ↓CCNB1, ↓CCNB2, ↓CCNF, ↓CCNG1, ↓CCNH, ↓CDC2, ↓CDC20, ↓CDK2, ↓CDK4, ↓CDK5R1, ↓CDKN1B, ↓CDKN3, ↓CHEK1, CKS1B, ↓CKS2, ↓DDX11, ↓GADD45A, ↓KNTC1, ↓KPNA2, ↓MAD2L1, ↓MCM2, ↓MCM3, ↓MCM4, ↓MCM5, ↓MKI67, ↓MRE11A, ↓PCNA, ↓RBL1, ↓RPA3, ↓SKP2, ↑CCND2 [335] | Proliferation, Immortality, Treatment resistance, Stress chemistry | Sustaining proliferative signaling, Evading growth suppressors | Genome maintenance, Cell survival | |
| LNCaP MAT * | ↓pAKT [629] | Treatment resistance, Apoptosis, Proliferation, Glycemia | Sustaining proliferative signaling | Cell survival |
| ↓DR4 [629] | Apoptosis, Proliferation, Immortality | Resisting cell death | Cell survival, Cell fate | |
| ↓TRAIL-DISC proteins (c-FLIPL/S, caspase-8) [629] | Apoptosis, Proliferation | Sustaining proliferative signaling, Resisting cell death | Cell survival, Cell fate | |
| ↑TRAIL-induced BID cleavage [629] | Apoptosis, Proliferation | Resisting cell death | Cell survival | |
| LNCaP ENL * | ↑Cytochrome c release, ↑cleaved caspase-3, ↑PARP [634] | Apoptosis, Proliferation, Glycemia, Immortality, Oxidation | Deregulated cellular energetics, and Genome instability and mutation | Cell survival |
| ↓pAKT, ↓pGSK-3β, ↓pMDM2, ↑P53 [634] | Apoptosis, Immortality, Proliferation | Sustaining proliferative signaling, Evading growth suppressors, Enabling replicative immortality | Cell survival | |
| ↑Caspase cell death [634] | Apoptosis | Resisting cell death | Cell survival | |
| PC3 (PC) ENL * | ↓pIGF-R(IGF-1), ↓pAKT, ↓p-p70S6K1, ↓pGSK3β, ↓pCyclinD1, ↓pERK ½ [618] | Proliferation, Glycemia, Immortality | Sustaining proliferative signaling, Activating invasion & metastasis | Cell survival, Cell fate |
| ↓IGF-1 signaling [618] | Proliferation, Glycemia | Sustaining proliferative signaling | Cell survival, Cell fate | |
| ↓FASN [213] | Proliferation, Treatment resistance | Sustaining proliferative signaling, Deregulated cellular energetics | Cell survival | |
| HUVEC (endothelial) ENL * | ↓VEGFR-2 [331] | Proliferation, Angiogenesis | Inducing angiogenesis | Cell survival |
| Adipocytes ENL * | ↓ROS - oxidative damage, ↓DNMTs, ↓HDACs, ↓MBD2 [334] | Proliferation, Oxidation, Inflammation, Stress chemistry, Immortality | Cell fate, Genome maintenance | |
| Colonocytes-YAMC ENL and ED * | ↓Cyclin D1, ↓Bcl-2 [586] | Proliferation, Immortality, Apoptosis | Sustaining proliferative signaling, Resisting cell death | Cell survival |
| Colo201 (COC) ENL * | ↓Bcl-2, ↓PCNA, ↑cleaved caspase-3 [22] | Apoptosis, Proliferation | Resisting cell death | Cell survival |
| Apc-Min (intestinal) Diet (flaxseed) * | ↓COX-1, COX-2 [652] | Proliferation, Immortality, Inflammation | Sustaining proliferative signaling, Tumor promoting inflammation | Cell survival |
| Hens Flaxseed supplement * | ↓COX-2 [583] | Proliferation, Immortality, Inflammation | Tumor promoting inflammation | Cell survival |
| ↓Prostaglandin E2, ↓ERα, ↓CYP3A4, ↓CYP1B1, ↓16-OHE1, ↑CYP1A1, ↑2-OHE1 [583] | Proliferation, Inflammation, Treatment resistance, Stress chemistry | Tumor promoting inflammation | Cell survival | |
| Hela (CC) ENL * | ↓Viral oncogene E6 [588] | Proliferation | Evading growth suppressors | Cell survival |
| ↓Survivin [588] | Apoptosis, Proliferation | Resisting cell death, Sustaining proliferative signaling | Cell survival | |
| ↑pHistone H2AX [588] | Apoptosis, Immortality, Proliferation | Resisting cell death | Cell survival, Cell fate | |
| Hela ED * | ↑Caspase 3 [588] | Apoptosis | Resisting cell death | Cell survival |
| CaSki (CC) ENL * | ↓Viral oncogene E7 [588] | Proliferation | Evading growth suppressors | Cell survival |
| ↓Bcl-2 [588] | Apoptosis, Treatment resistance | Resisting cell death | Cell survival | |
| Hela and CaSki ENL * | ↑P53 [588] | Proliferation, Apoptosis | Evading growth suppressors | Cell survival, Genome maintenance |
| ↑Bax [588] | Apoptosis, Treatment resistance | Resisting cell death | Cell survival | |
| Targets: Cellular Processors | ||||
| SKBR3 and MDA-MB231 (BC) ENL * | ↓Cell viability with anticancer agents [55,608] | Proliferation, Treatment resistance, Stress chemistry, Apoptosis | Resisting cell death, Sustaining proliferative signaling, Evading growth suppressors | Cell survival |
| MDA-MB231 ENL * | ↑Cell cycle S phase, ↓cell viability [627] | Apoptosis, Immortality, Proliferation | Sustaining proliferative signaling, Evading growth suppressors | Cell survival, Genome maintenance, Cell fate |
| ↓Actin cytoskeleton organization [627,647] ↓Epithelial–mesenchymal transition [647] | Proliferation, Metastasis | Sustaining proliferative signaling, Activating invasion & metastasis | Cell survival, Cell fate | |
| ↓Migration, invasion [627,642] | Metastasis | Activating invasion & metastasis | Cell fate | |
| ↓Actin, filopodia, lamellipodia [642] | Proliferation, Metastasis | Sustaining proliferative signaling, Activating invasion & metastasis | Cell survival, Cell fate | |
| Anticancer/metastatic/ proliferative/migratory/clonogenic [643] | Metastasis | Activating invasion & metastasis | Cell fate | |
| MCF7 and MDA-MB231 SDG and ASECO * | ↓Growth [653] | Proliferation | Sustaining proliferative signaling | Cell survival |
| ER+ BC (XM) ENL and ED * | ↓Angiogenesis [451] | Angiogenesis | Inducing angiogenesis | Cell survival |
| WPMY-1 ENL * | ↓proliferation, arrested cell cycle (G0/G1) [651] | Proliferation | Sustaining proliferative signaling | Cell survival |
| Rat model (PH) SDG * | ↓Prostate enlargement, # papillary projections, thickness of cell layers [651] | Proliferation | Sustaining proliferative signaling | Cell survival |
| WPE1-NA22, WPE1-NB14, WPE1-NB11, WPE1-NB26 and LNCaP ENL * | ↓Metabolic activity,↑doubling time [593] | Proliferation, Stress chemistry, Oxidation | Sustaining proliferative signaling, Deregulated cellular energetics, Evading growth suppressors | Cell survival, Cell fate |
| Modulated cell cycle [593] | Proliferation, Immortality | Evading growth suppressors, Sustaining proliferative signaling | Cell survival, Genome maintenance | |
| ↑Apoptosis [593] | Immortality, Apoptosis | Sustaining proliferative signaling, Resisting cell death | Cell survival | |
| LNCaP ENL * | ↑Sub-G0 and S, ↓G0/G1, ↓G2/M cell cycle [335] | Proliferation, Immortality | Sustaining proliferative signaling, Evading growth suppressors | Cell survival, Cell fate |
| ↓Cell density, ↓metabolic activity, ↓PSA, ↑apoptosis [335] | Proliferation, Apoptosis | Sustaining proliferative signaling, Resisting cell death, Deregulated cellular energetics | Cell survival, Cell fate | |
| ↑Apoptosis with anticancer agents [335] | Apoptosis | Resisting cell death | Cell survival | |
| ↓Mitochondrial membrane potential [634] | Treatment resistance, Stress chemistry, Glycemia, Oxidation, Proliferation, Apoptosis | Deregulated cellular energetics | Cell survival | |
| LNCaP MAT * | Death receptor sensitizer (sensitizes TRAIL-induced apoptosis) [629] | Proliferation, Apoptosis | Sustaining proliferative signaling, Evading growth suppressors, Resisting cell death | Cell survival, Cell fate |
| ↑TRAIL-induced mitochondrial depolarization [629] | Proliferation, Apoptosis | Resisting cell death, Deregulated cellular energetics | Cell survival | |
| PC3 ENL * | ↓IGF-1 induced proliferation, ↓cell cycle arrest (G0/G1) [618] | Proliferation | Sustaining proliferative signaling, Evading growth suppressors | Cell survival |
| ↓IGF-1 induced migration [618] | Metastasis | Activating invasion & metastasis | Cell survival, Cell fate | |
| A549 and H60 ENL * | ↓Migration, invasion [628] | Metastasis | Activating invasion & metastasis | Cell fate |
| ↓Density F-actin fibers [628] | Metastasis, Proliferation | Activating invasion & metastasis, Sustaining proliferative signaling | Cell survival, Cell fate | |
| YAMC ENL and ED * | ↓Cell growth, ↑ apoptosis [586] | Proliferation, Apoptosis | Resisting cell death, Sustaining proliferative signaling, Evading growth suppressors | Cell survival |
| MG-63 ENL and ED * | Biphasic (↓↑)- cell viability, ALP activity [650] | Proliferation | Sustaining proliferative signaling | Cell survival, Cell fate |
| Mouse model ENL * | ↓Estradiol-induced endothelial cell infiltration [331] | Metastasis | Activating invasion & metastasis | Cell survival, Cell fate |
| Colo201 ENL * | ↑Apoptosis (sub-G1 cells),↑cell viability [22] | Proliferation, Apoptosis | Sustaining proliferative signaling, Evading growth suppressors, Resisting cell death | Cell survival |
| CC cells ENL * | ↑Cell death, ↓ metabolic activity in p53+ [588] | Immortality, Proliferation, Treatment resistance, Glycemia, Apoptosis | Evading growth suppressors, Resisting cell death, Deregulated cellular energetics | Cell survival, Genome maintenance |
| ↑Apoptosis (Hela) [588] | Apoptosis | Resisting cell death | Cell survival | |
| CC cells ENL and ED * | ↓Cell survival [588] | Immortality, Proliferation, Apoptosis | Sustaining proliferative signaling, Evading growth suppressors | Cell survival, Genome maintenance |
| TR C33-A (CC) ENL and ED * | ↓Promoter activity (Episomal, HPV oncoproteins) [588] | Proliferation | Sustaining proliferative signaling, Evading growth suppressors | Cell survival |
| Hela ENL * | ↑p53 activity [588] | Immortality, Proliferation | Evading growth suppressors | Cell survival, Genome maintenance |
| No DNA-breaks (genotoxicity) [588] | Proliferation, Apoptosis | Resisting cell death, Evading growth suppressors | Cell survival | |
| Hela/CaSki ENL * | ↑Apoptosis (Caspase 9, Caspase 3) [588] | Proliferation, Apoptosis | Resisting cell death | Cell survival |
| CaSki ED * | ↑Caspase 3 activity [588] | Proliferation, Apoptosis | Resisting cell death | Cell survival |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Silva, S.F.; Alcorn, J. Flaxseed Lignans as Important Dietary Polyphenols for Cancer Prevention and Treatment: Chemistry, Pharmacokinetics, and Molecular Targets. Pharmaceuticals 2019, 12, 68. https://doi.org/10.3390/ph12020068
De Silva SF, Alcorn J. Flaxseed Lignans as Important Dietary Polyphenols for Cancer Prevention and Treatment: Chemistry, Pharmacokinetics, and Molecular Targets. Pharmaceuticals. 2019; 12(2):68. https://doi.org/10.3390/ph12020068
Chicago/Turabian StyleDe Silva, S. Franklyn, and Jane Alcorn. 2019. "Flaxseed Lignans as Important Dietary Polyphenols for Cancer Prevention and Treatment: Chemistry, Pharmacokinetics, and Molecular Targets" Pharmaceuticals 12, no. 2: 68. https://doi.org/10.3390/ph12020068
APA StyleDe Silva, S. F., & Alcorn, J. (2019). Flaxseed Lignans as Important Dietary Polyphenols for Cancer Prevention and Treatment: Chemistry, Pharmacokinetics, and Molecular Targets. Pharmaceuticals, 12(2), 68. https://doi.org/10.3390/ph12020068