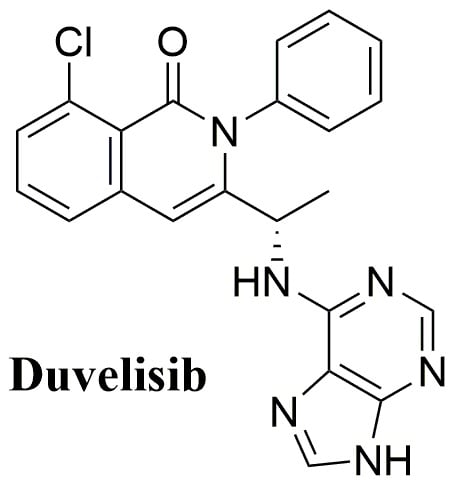
Duvelisib: A 2018 Novel FDA-Approved Small Molecule Inhibiting Phosphoinositide 3-Kinases
Abstract
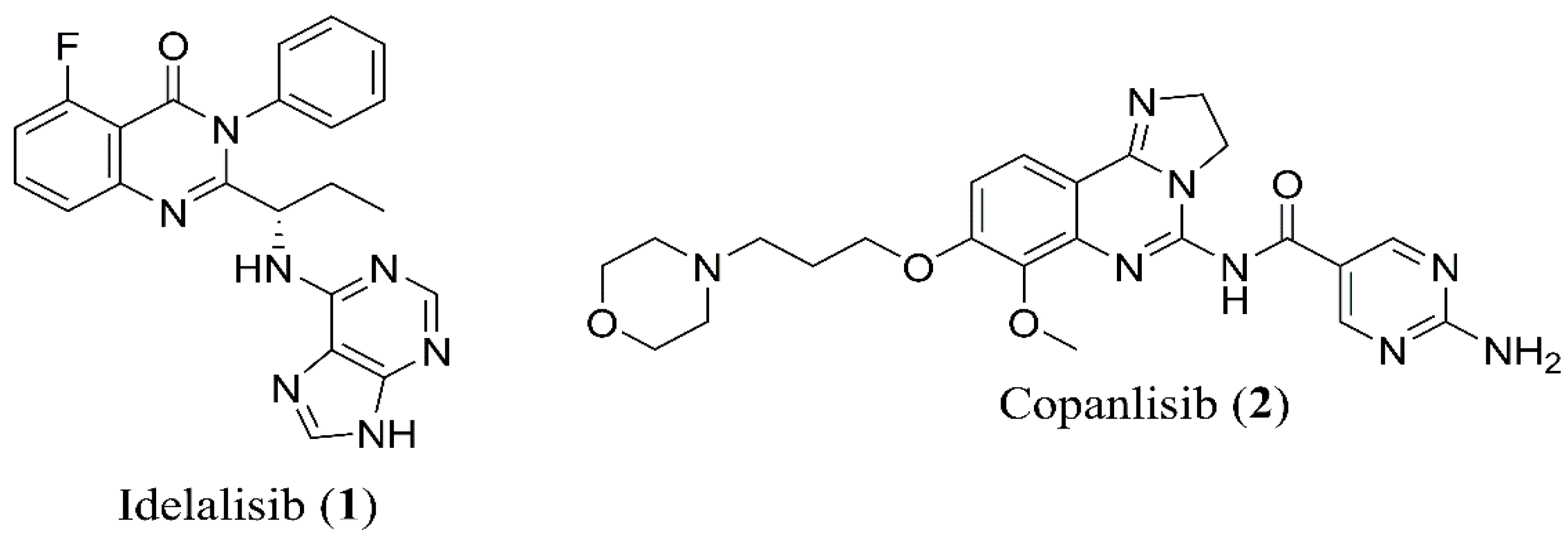
1. Introduction
2. Duvelisib
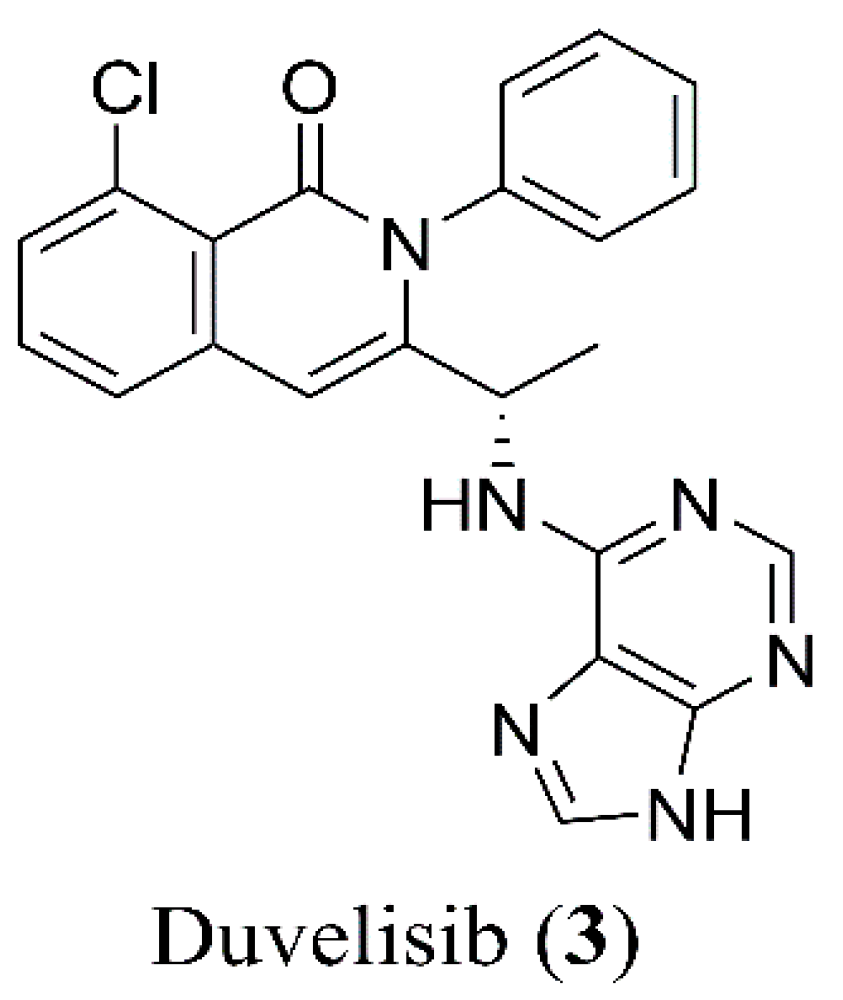
2.1. Names and Structure
2.2. Uses
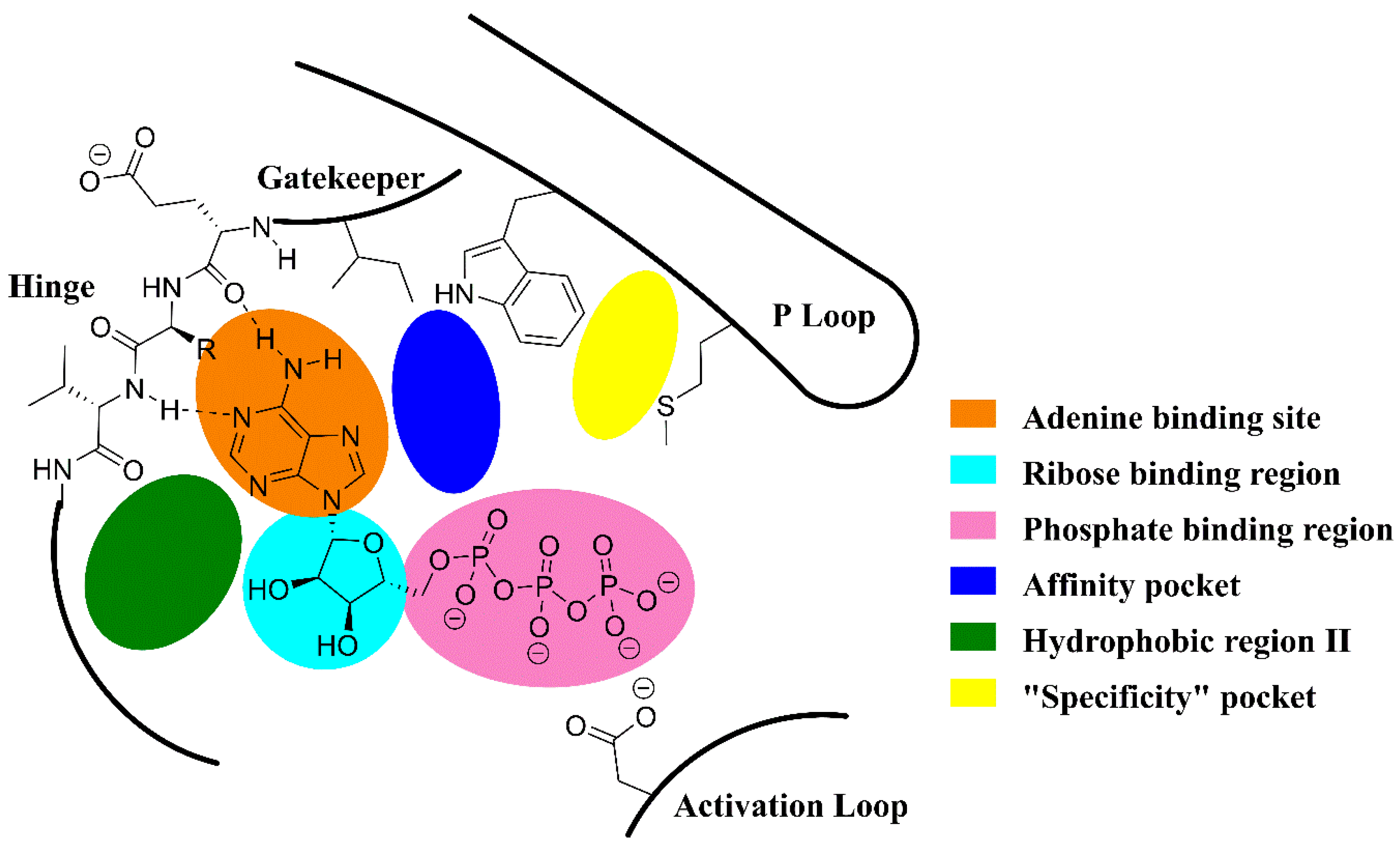
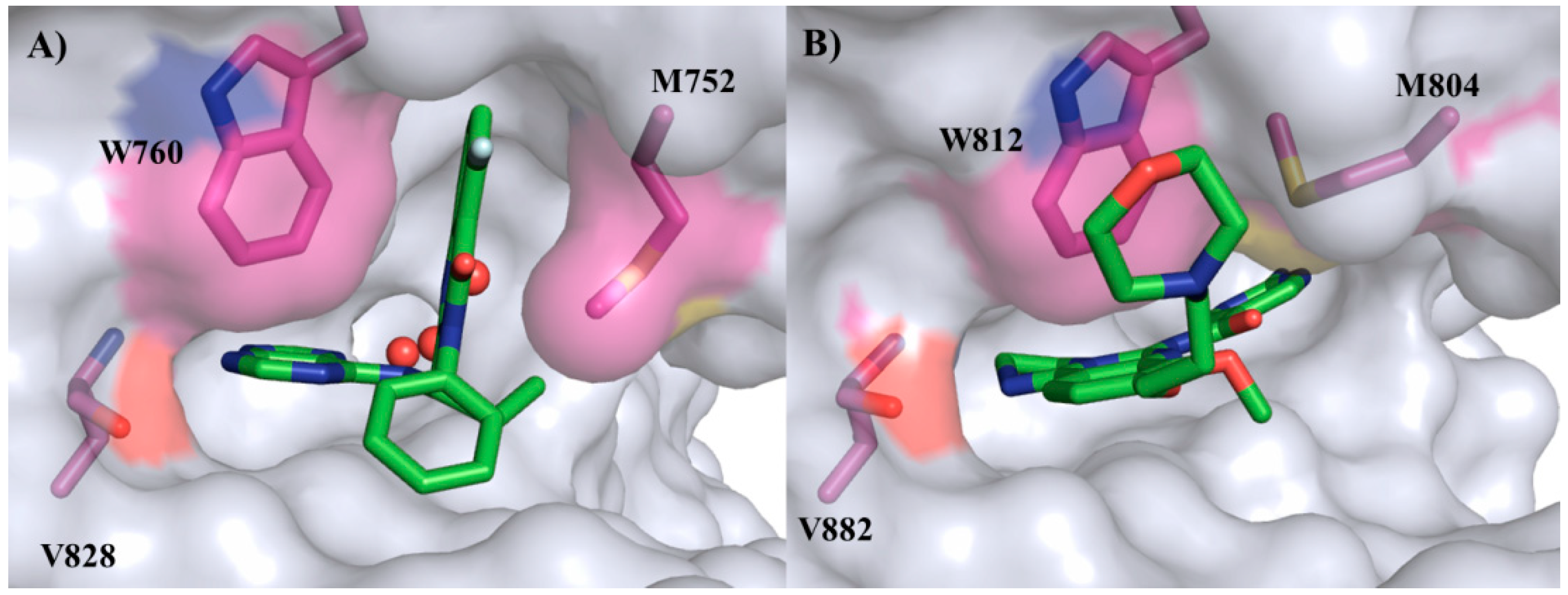
2.3. Targets and Selectivity
2.4. Clinical Trials
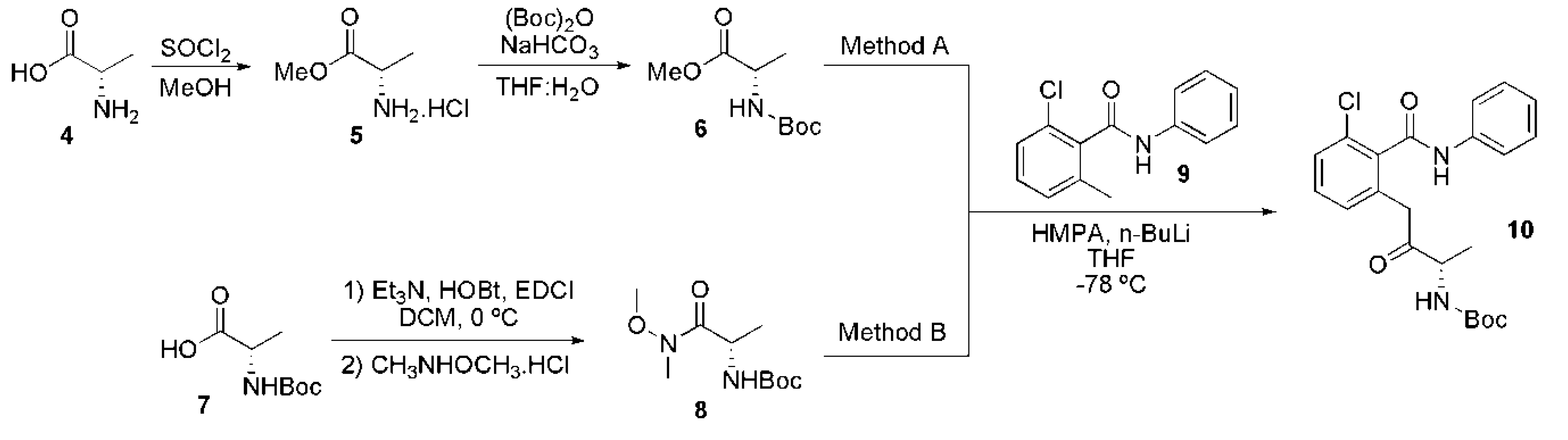
2.5. Syntheses
3. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Markham, A. Idelalisib: First global approval. Drugs 2014, 74, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Copanlisib: First Global Approval. Drugs 2017, 77, 2057–2062. [Google Scholar] [CrossRef] [PubMed]
- Scott, W.J.; Hentemann, M.F.; Rowley, R.B.; Bull, C.O.; Jenkins, S.; Bullion, A.M.; Johnson, J.; Redman, A.; Robbins, A.H.; Esler, W.; et al. Discovery and SAR of novel 2,3-dihydroimidazo[1,2-c]quinazoline PI3K inhibitors: Identification of copanlisib (BAY 80-6946). ChemMedChem 2016, 11, 1517–1530. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Duvelisib: First global approval. Drugs 2018, 78, 1847–1853. [Google Scholar] [CrossRef]
- Flinn, I.W.; Hillmen, P.; Montillo, M.; Nagy, Z.; Illes, A.; Etienne, G.; Delgado, J.; Kuss, B.J.; Tam, C.S.; Gasztonyi, Z.; et al. The phase 3 DUO trial: Duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood 2018, 132, 2446–2455. [Google Scholar] [CrossRef]
- A Phase 3 Study of Duvelisib Versus Ofatumumab in Patients with Relapsed or Refractory CLL/SLL (DUO). Available online: https://clinicaltrials.gov/ct2/show/NCT02004522 (accessed on 4 April 2019).
- Study of Duvelisib in Combination with Rituximab vs Rituximab in Subjects with Previously Treated Follicular Lymphoma (DYNAMO + R). Available online: https://clinicaltrials.gov/ct2/show/NCT02204982 (accessed on 4 April 2019).
- Walker, E.H.; Perisic, O.; Ried, C.; Stephens, L.; Williams, R.L. Structural insights into phosphoinositide 3-kinase catalysis and signalling. Nature 1999, 402, 313–320. [Google Scholar] [CrossRef]
- Hennessy, B.T.; Smith, D.L.; Ram, P.T.; Lu, Y.; Mills, G.B. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005, 4, 988–1004. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Thorpe, L.M.; Yuzugullu, H.; Zhao, J.J. PI3K in cancer: Divergent roles of isoforms, modes of activation and therapeutic targeting. Nat. Rev. Cancer 2015, 15, 7–24. [Google Scholar] [CrossRef]
- Garces, A.E.; Stocks, M.J. Class 1 PI3K clinical candidates and recent inhibitor design strategies: A medicinal chemistry perspective. J. Med. Chem. 2018. [Google Scholar] [CrossRef]
- Somoza, J.R.; Koditek, D.; Villasenor, A.G.; Novikov, N.; Wong, M.H.; Liclican, A.; Xing, W.; Lagpacan, L.; Wang, R.; Schultz, B.E.; et al. Structural, biochemical, and biophysical characterization of idelalisib binding to phosphoinositide 3-kinase delta. J. Biol. Chem. 2015, 290, 8439–8446. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.S.; Thompson, P.E.; Gabelli, S.B. Structural determinants of isoform selectivity in PI3K inhibitors. Biomolecules 2019, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Winkler, D.G.; Faia, K.L.; DiNitto, J.P.; Ali, J.A.; White, K.F.; Brophy, E.E.; Pink, M.M.; Proctor, J.L.; Lussier, J.; Martin, C.M.; et al. PI3K-delta and PI3K-gamma inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem. Biol. 2013, 20, 1364–1374. [Google Scholar] [CrossRef]
- Walker, E.H.; Pacold, M.E.; Perisic, O.; Stephens, L.; Hawkins, P.T.; Wymann, M.P.; Williams, R.L. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol. Cell. 2000, 6, 909–919. [Google Scholar] [CrossRef]
- Huang, C.H.; Mandelker, D.; Schmidt-Kittler, O.; Samuels, Y.; Velculescu, V.E.; Kinzler, K.W.; Vogelstein, B.; Gabelli, S.B.; Amzel, L.M. The structure of a human p110alpha/p85alpha complex elucidates the effects of oncogenic PI3Kalpha mutations. Science 2007, 318, 1744–1748. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Berndt, A.; Miller, S.; Hon, W.C.; Zhang, X. Form and flexibility in phosphoinositide 3-kinases. Biochem. Soc. Trans. 2009, 37 Pt 4, 615–626. [Google Scholar] [CrossRef]
- Traxler, P.; Furet, P. Strategies toward the design of novel and selective protein tyrosine kinase inhibitors. Pharmacol. Ther. 1999, 82, 195–206. [Google Scholar] [CrossRef]
- Berndt, A.; Miller, S.; Williams, O.; Le, D.D.; Houseman, B.T.; Pacold, J.I.; Gorrec, F.; Hon, W.C.; Liu, Y.; Rommel, C.; et al. The p110 delta structure: Mechanisms for selectivity and potency of new PI(3)K inhibitors. Nat. Chem. Biol. 2010, 6, 117–124. [Google Scholar] [CrossRef]
- Katso, R.; Okkenhaug, K.; Ahmadi, K.; White, S.; Timms, J.; Waterfield, M.D. Cellular function of phosphoinositide 3-kinases: Implications for development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 2001, 17, 615–675. [Google Scholar] [CrossRef]
- Cushing, T.D.; Metz, D.P.; Whittington, D.A.; McGee, L.R. PI3Kdelta and PI3Kgamma as targets for autoimmune and inflammatory diseases. J. Med. Chem. 2012, 55, 8559–8581. [Google Scholar] [CrossRef]
- Perry, M.W.D.; Abdulai, R.; Mogemark, M.; Petersen, J.; Thomas, M.J.; Valastro, B.; Eriksson, A.W. Evolution of PI3Kgamma and delta inhibitors for inflammatory and autoimmune diseases. J. Med. Chem. 2018. [Google Scholar] [CrossRef]
- Ali, A.Y.; Wu, X.; Eissa, N.; Hou, S.; Ghia, J.E.; Murooka, T.T.; Banerji, V.; Johnston, J.B.; Lin, F.; Gibson, S.B.; et al. Distinct roles for phosphoinositide 3-kinases gamma and delta in malignant B cell migration. Leukemia 2018, 32, 1958–1969. [Google Scholar] [CrossRef] [PubMed]
- Flinn, I.W.; O’Brien, S.; Kahl, B.; Patel, M.; Oki, Y.; Foss, F.F.; Porcu, P.; Jones, J.; Burger, J.A.; Jain, N.; et al. Duvelisib, a novel oral dual inhibitor of PI3K-delta,gamma, is clinically active in advanced hematologic malignancies. Blood 2018, 131, 877–887. [Google Scholar] [CrossRef]
- O’Brien, S.; Patel, M.; Kahl, B.S.; Horwitz, S.M.; Foss, F.M.; Porcu, P.; Jones, J.; Burger, J.; Jain, N.; Allen, K.; et al. Duvelisib, an oral dual PI3K-delta,gamma inhibitor, shows clinical and pharmacodynamic activity in chronic lymphocytic leukemia and small lymphocytic lymphoma in a phase 1 study. Am. J. Hematol. 2018, 93, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.M.; Koch, R.; Porcu, P.; Oki, Y.; Moskowitz, A.; Perez, M.; Myskowski, P.; Officer, A.; Jaffe, J.D.; Morrow, S.N.; et al. Activity of the PI3K-delta,gamma inhibitor duvelisib in a phase 1 trial and preclinical models of T-cell lymphoma. Blood 2018, 131, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Verastem Inc. Copiktra (Duvelisib), Capsules for Oral Use: US Prescribing Information. 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211155s000lbl.pdf (accessed on 29 April 2019).
- Flinn, I.W.; Patel, M.; Oki, Y.; Horwitz, S.; Foss, F.F.; Allen, K.; Douglas, M.; Stern, H.; Sweeney, J.; Kharidia, J.; et al. Duvelisib, an oral dual PI3K-delta, gamma inhibitor, shows clinical activity in indolent non-Hodgkin lymphoma in a phase 1 study. Am. J. Hematol. 2018, 93, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Trial of Duvelisib in Combination with Either Romidepsin or Bortezomib in Relapsed/Refractory T-Cell Lymphomas. Available online: https://clinicaltrials.gov/ct2/show/NCT02783625 (accessed on 4 April 2019).
- Duvelisib and Venetoclax in Relapsed or Refractory CLL or SLL. Available online: https://clinicaltrials.gov/ct2/show/NCT03534323 (accessed on 4 April 2019).
- A Phase 2a, Efficacy and Safety Study of Duvelisib in Mild Asthmatic Subjects. Available online: https://clinicaltrials.gov/ct2/show/NCT01653756 (accessed on 4 April 2019).
- A Double-Blind Study Evaluating Duvelisib in Subjects with Moderate to Severe Rheumatoid Arthritis and an Inadequate Response to Methotrexate Alone (ASPIRA). Available online: https://clinicaltrials.gov/ct2/show/NCT01851707 (accessed on 4 April 2019).
- Ren, P.; Liu, Y.; Wilson, T.E.; Li, L.; Chan, K.; Rommel, C. Certain Chemical Entities, Compositions and Methods. US2009312319A1, 14 December 2009. [Google Scholar]
- Ren, P.; Liu, Y.; Wilson, T.E.; Li, L.; Chan, K.; Rommel, C. Certain Chemical Entities, Compositions and Methods. WO2011008302A1, 20 January 2011. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, D.A.; Sagrillo, F.S.; Fraga, C.A.M. Duvelisib: A 2018 Novel FDA-Approved Small Molecule Inhibiting Phosphoinositide 3-Kinases. Pharmaceuticals 2019, 12, 69. https://doi.org/10.3390/ph12020069
Rodrigues DA, Sagrillo FS, Fraga CAM. Duvelisib: A 2018 Novel FDA-Approved Small Molecule Inhibiting Phosphoinositide 3-Kinases. Pharmaceuticals. 2019; 12(2):69. https://doi.org/10.3390/ph12020069
Chicago/Turabian StyleRodrigues, Daniel A., Fernanda S. Sagrillo, and Carlos A. M. Fraga. 2019. "Duvelisib: A 2018 Novel FDA-Approved Small Molecule Inhibiting Phosphoinositide 3-Kinases" Pharmaceuticals 12, no. 2: 69. https://doi.org/10.3390/ph12020069
APA StyleRodrigues, D. A., Sagrillo, F. S., & Fraga, C. A. M. (2019). Duvelisib: A 2018 Novel FDA-Approved Small Molecule Inhibiting Phosphoinositide 3-Kinases. Pharmaceuticals, 12(2), 69. https://doi.org/10.3390/ph12020069