Omadacycline: A Newly Approved Antibacterial from the Class of Tetracyclines
Abstract
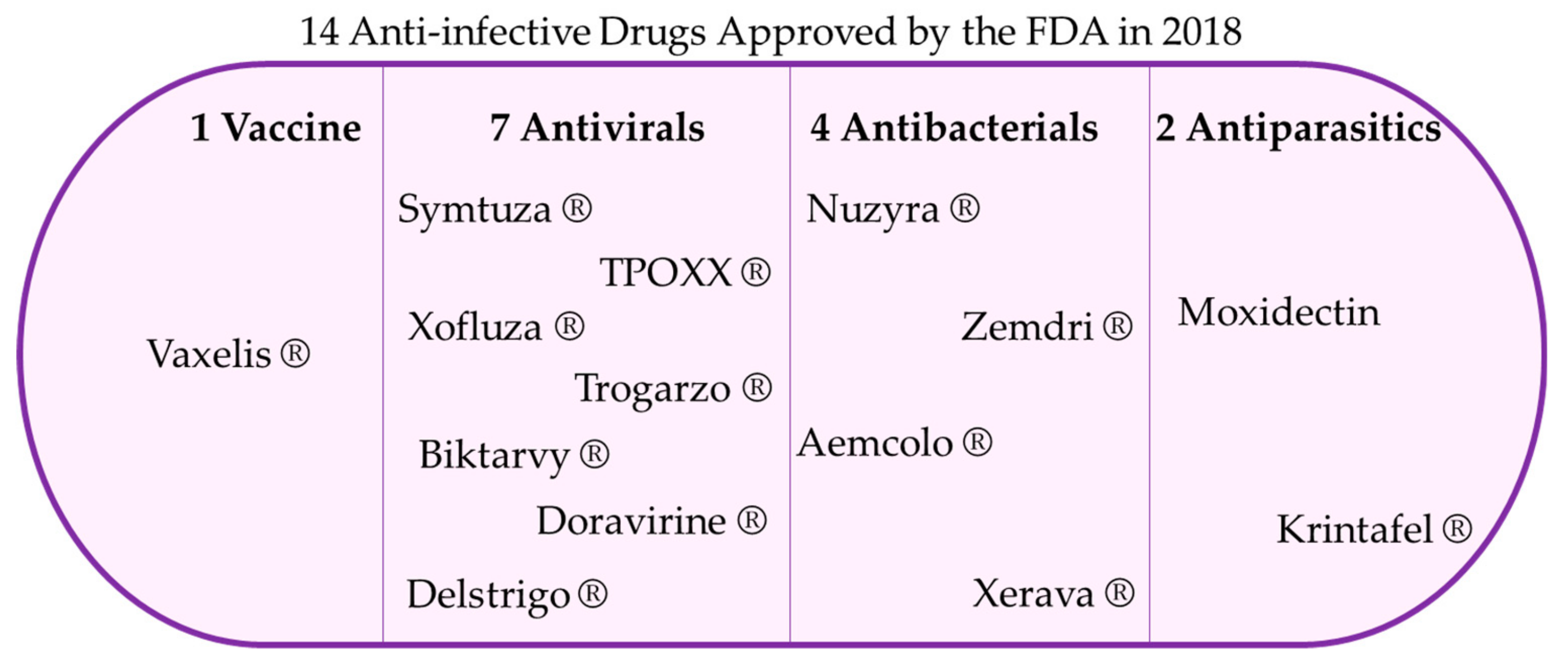
1. Introduction
2. Omadacycline
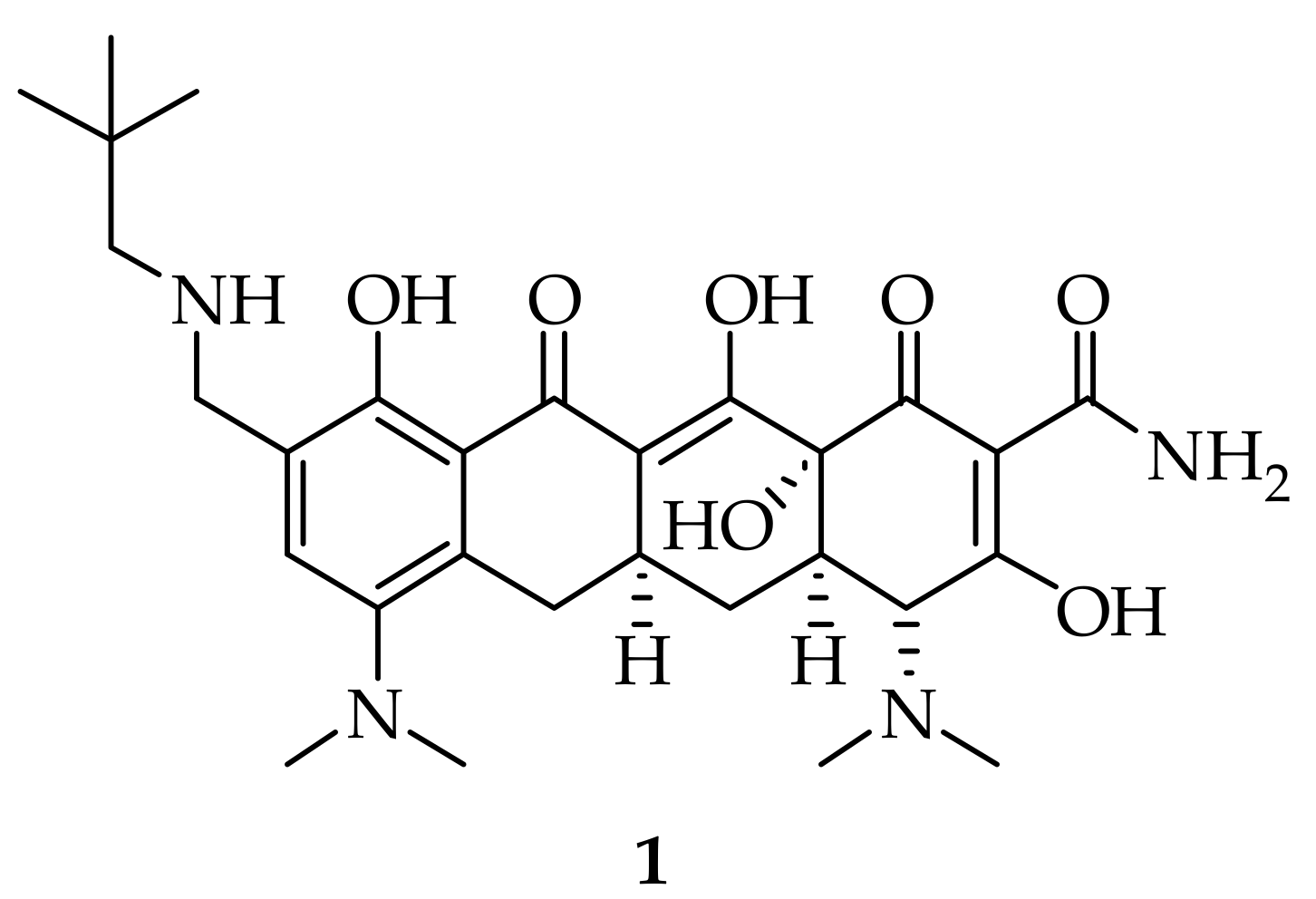
2.1. Names and Structure
2.2. Uses
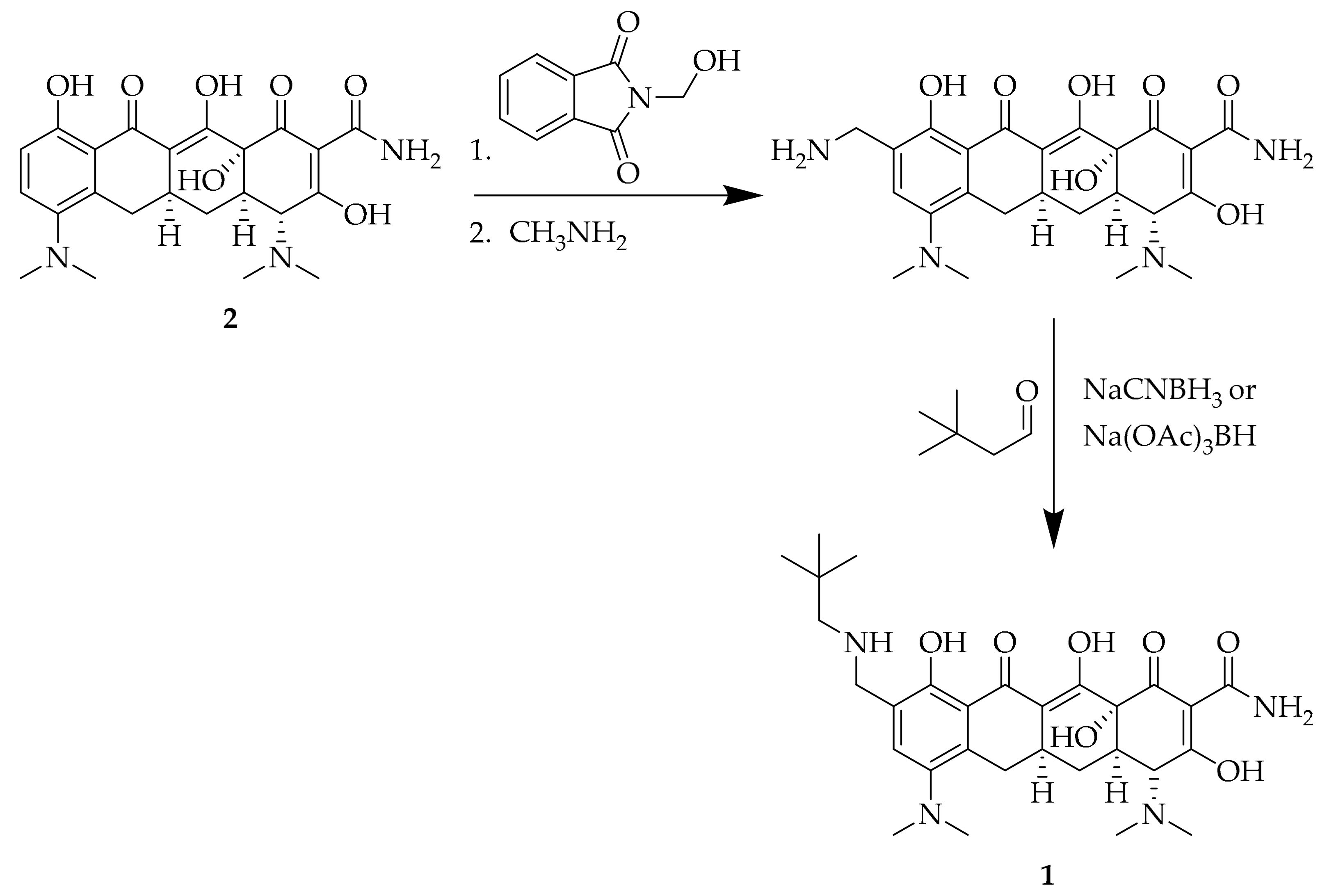
2.3. Synthesis
2.4. Mechanism Action and Resistance
2.5. Antibacterial Activity
2.6. Clinical Studies
3. Conclusions
Funding
Conflicts of Interest
References
- Aminov, R.I. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef]
- Duraes, F.; Pinto, M.; Sousa, E. Medicinal chemistry updates on bacterial efflux pump modulators. Curr. Med. Chem. 2018, 25, 6030–6069. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Novel Drug Approvals for 2018. Available online: https://www.fda.gov/drugs/developmentapprovalprocess/druginnovation/ucm592464.htm (accessed on 24 February 2019).
- Macone, A.B.; Caruso, B.K.; Leahy, R.G.; Donatelli, J.; Weir, S.; Draper, M.P.; Tanaka, S.K.; Levy, S.B. In vitro and in vivo antibacterial activities of omadacycline, a novel aminomethylcycline. Antimicrob. Agents Chemother. 2014, 58, 1127–1135. [Google Scholar] [PubMed]
- Stets, R.; Popescu, M.; Gonong, J.R.; Mitha, I.; Nseir, W.; Madej, A.; Kirsch, C.; Das, A.F.; Garrity-Ryan, L.; Steenbergen, J.N.; et al. Omadacycline for community-acquired bacterial pneumonia. N. Engl. J. Med. 2019, 380, 517–527. [Google Scholar] [CrossRef]
- Dougherty, J.A.; Sucher, A.J.; Chahine, E.B.; Shihadeh, K.C. Omadacycline: A new tetracycline antibiotic. Ann. Pharmacother. 2019, 53, 486–500. [Google Scholar] [CrossRef]
- Kollef, M.H.; Betthauser, K.D. New antibiotics for community-acquired pneumonia. Curr. Opin. Infect. Dis. 2019, 32, 169–175. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, W.; Green, S.; Overcash, J.S.; Puljiz, I.; Metallidis, S.; Gardovskis, J.; Garrity-Ryan, L.; Das, A.F.; Tzanis, E.; Eckburg, P.B.; et al. Omadacycline for acute bacterial skin and skin-structure infections. N. Engl. J. Med. 2019, 380, 528–538. [Google Scholar]
- Chambers, H.F. Omadacycline—The newest tetracycline. N. Engl. J. Med. 2019, 380, 588–589. [Google Scholar] [CrossRef] [PubMed]
- Montravers, P.; Tran-Dinh, A.; Tanaka, S. The role of omadacycline in skin and soft tissue infections. Curr. Opin. Infect. Dis. 2018, 31, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Markham, A.; Keam, S.J. Omadacycline: First global approval. Drugs 2018, 78, 1931–1937. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.R.; Deresinski, S. Omadacycline: A novel tetracycline derivative with oral and intravenous formulations. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019. [Google Scholar] [CrossRef] [PubMed]
- Honeyman, L.; Ismail, M.; Nelson, M.L.; Bhatia, B.; Bowser, T.E.; Chen, J.; Mechiche, R.; Ohemeng, K.; Verma, A.K.; Cannon, E.P.; et al. Structure-activity relationship of the aminomethylcyclines and the discovery of omadacycline. Antimicrob. Agents Chemother. 2015, 59, 7044–7053. [Google Scholar] [CrossRef]
- Tanaka, S.K.; Steenbergen, J.; Villano, S. Discovery, pharmacology, and clinical profile of omadacycline, a novel aminomethylcycline antibiotic. Bioorg. Med. Chem. 2016, 24, 6409–6419. [Google Scholar] [CrossRef] [PubMed]
- Draper, M.P.; Weir, S.; Macone, A.; Donatelli, J.; Trieber, C.A.; Tanaka, S.K.; Levy, S.B. Mechanism of action of the novel aminomethylcycline antibiotic omadacycline. Antimicrob. Agents Chemother. 2014, 58, 1279–1283. [Google Scholar] [CrossRef]
- Cho, J.C.; Childs-Kean, L.M.; Zmarlicka, M.T.; Crotty, M.P. Return of the tetracyclines: Omadacycline, a novel aminomethylcycline antimicrobial. Drugs Today 2018, 54, 209–217. [Google Scholar] [CrossRef]
- Heidrich, C.G.; Mitova, S.; Schedlbauer, A.; Connell, S.R.; Fucini, P.; Steenbergen, J.N.; Berens, C. The novel aminomethylcycline omadacycline has high specificity for the primary tetracycline-binding site on the bacterial ribosome. Antibiotics 2016, 5, 32. [Google Scholar] [CrossRef]
- Markley, J.L.; Wencewicz, T.A. Tetracycline-inactivating enzymes. Front. Microbiol. 2018, 9, 1058. [Google Scholar] [CrossRef]
- Markley, J.L.; Fang, L.; Gasparrini, A.J.; Symister, C.T.; Kumar, H.; Tolia, N.H.; Dantas, G.; Wencewicz, T.A. Semisynthetic analogues of anhydrotetracycline as inhibitors of tetracycline destructase enzymes. ACS Infect. Dis. 2019, 5, 618–633. [Google Scholar] [CrossRef]
- Villano, S.; Steenbergen, J.; Loh, E. Omadacycline: Development of a novel aminomethylcycline antibiotic for treating drug-resistant bacterial infections. Future Microbiol. 2016, 11, 1421–1434. [Google Scholar]
- Barber, K.E.; Bell, A.M.; Wingler, M.J.B.; Wagner, J.L.; Stover, K.R. Omadacycline enters the ring: A new antimicrobial contender. Pharmacotherapy 2018, 38, 1194–1204. [Google Scholar]
- Traczewski, M.M.; Brown, S.D. PTK 0796 (BAY 73-6944): In vitro potency and spectrum of activity compared to ten other antimicrobial compounds. In Proceedings of the 43rd Interscience Congress on Antimicrobial Agents and Chemotherapy, Chicago, IL, USA, 14–17 September 2003. [Google Scholar]
- Fluit, A.C.; van Gorkum, S.; Vlooswijk, J. Minimal inhibitory concentration of omadacycline and doxycycline against bacterial isolates with known tetracycline resistance determinants. Diagn. Microbiol. Infect. Dis. 2018, 94, 78–80. [Google Scholar] [CrossRef]
- Kohlhoff, S.A.; Huerta, N.; Hammerschlag, M.R. In vitro activity of omadacycline against Chlamydia pneumoniae. Antimicrob. Agents Chemother. 2019, 63, e01907–e01918. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Huband, M.D.; Shortridge, D.; Flamm, R.K. Surveillance of omadacycline activity tested against clinical isolates from the united states and europe as part of the 2016 SENTRY antimicrobial surveillance program. Antimicrob. Agents Chemother. 2018, 62, e02327-17. [Google Scholar]
- Stapert, L.; Wolfe, C.; Shinabarger, D.; Marra, A.; Pillar, C. In vitro activities of omadacycline and comparators against anaerobic bacteria. Antimicrob. Agents Chemother. 2018, 62, e00047-18. [Google Scholar]
- Chernova, O.A.; Medvedeva, E.S.; Mouzykantov, A.A.; Baranova, N.B.; Chernov, V.M. Mycoplasmas and their antibiotic resistance: The problems and prospects in controlling infections. Acta Nat. 2016, 8, 24–34. [Google Scholar]
- Xiaotian, Z.; Stella, L.; Rangaraj, S.; Xuan, Q.; Yi-Wei, T.; Jeffrey, S.; Tao, H.; Kathleen, T.; Amy, E.R.; Donna, M.C.; et al. Macrolide-resistant Mycoplasma pneumoniae, United States. Emerg. Infect. Dis. J. 2015, 21, 1470. [Google Scholar]
- Waites, K.B.; Crabb, D.M.; Liu, Y.; Duffy, L.B. In vitro activities of omadacycline (PTK 0796) and other antimicrobial agents against human mycoplasmas and ureaplasmas. Antimicrob. Agents Chemother. 2016, 60, 7502. [Google Scholar]
- Horn, K.; Gotfried, M.H.; Steenbergen, J.; Villano, S.; Tzanis, E.; Garrity-Ryan, L.; Chitra, S.; Manley, A.; Tanaka, K.; Rodvold, K. Comparison of omadacycline (OMC) and tigecycline (TGC) pharmacodynamics (PD) in the plasma, epithelial lining fluid (ELF), and alveolar macrophages (AM) in healthy subjects. In Proceedings of the 27th European Congress Clinical Microbiology and Infectious Diseases, Vienna, Austria, 22–25 April 2017. [Google Scholar]
- Gotfried, M.H.; Horn, K.; Garrity-Ryan, L.; Villano, S.; Tzanis, E.; Chitra, S.; Manley, A.; Tanaka, S.K.; Rodvold, K.A. Comparison of omadacycline and tigecycline pharmacokinetics in the plasma, epithelial lining fluid, and alveolar cells of healthy adult subjects. Antimicrob. Agents Chemother. 2017, 61, e01135-17. [Google Scholar] [CrossRef] [PubMed]
- Shoen, C.; Benaroch, D.; Sklaney, M.; Cynamon, M. In vitro activities of omadacycline against rapidly growing mycobacteria. Antimicrob. Agents Chemother. 2019. [Google Scholar] [CrossRef]
- Noel, G.J.; Draper, M.P.; Hait, H.; Tanaka, S.K.; Arbeit, R.D. A randomized, evaluator-blind, phase 2 study comparing the safety and efficacy of omadacycline to those of linezolid for treatment of complicated skin and skin structure infections. Antimicrob. Agents Chemother. 2012, 56, 5650–5654. [Google Scholar]
- Abrahamian, F.M.; Sakoulas, G.; Tzanis, E.; Manley, A.; Steenbergen, J.N.; Das, A.; Eckburg, P.; McGovern, P. 1347. Omadacycline for acute bacterial skin and skin structure infections: Integrated analysis of randomized clinical trials. Open Forum Infect. Dis. 2018, 5, S412. [Google Scholar] [CrossRef][Green Version]
- ClinicalTrials.gov. Oral Omadacycline Vs. Oral Nitrofurantoin for the Treatment of Cystitis. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03425396?term=omadacycline&rank=3 (accessed on 8 March 2019).
- ClinicalTrials.gov. Iv or iv/po omadacycline vs. Iv/po levofloxacin for the treatment of acute pyelonephritis. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03757234?term=omadacycline&rank=1 (accessed on 8 March 2019).
- Overcash, J.S.; Bhiwandi, P.; Garrity-Ryan, L.; Steenbergen, J.; Bai, S.; Chitra, S.; Manley, A.; Tzanis, E. Pharmacokinetics, safety, and clinical outcomes of omadacycline in women with cystitis: Results from a phase 1 study. Antimicrob. Agents Chemother. 2019. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durães, F.; Sousa, E. Omadacycline: A Newly Approved Antibacterial from the Class of Tetracyclines. Pharmaceuticals 2019, 12, 63. https://doi.org/10.3390/ph12020063
Durães F, Sousa E. Omadacycline: A Newly Approved Antibacterial from the Class of Tetracyclines. Pharmaceuticals. 2019; 12(2):63. https://doi.org/10.3390/ph12020063
Chicago/Turabian StyleDurães, Fernando, and Emília Sousa. 2019. "Omadacycline: A Newly Approved Antibacterial from the Class of Tetracyclines" Pharmaceuticals 12, no. 2: 63. https://doi.org/10.3390/ph12020063
APA StyleDurães, F., & Sousa, E. (2019). Omadacycline: A Newly Approved Antibacterial from the Class of Tetracyclines. Pharmaceuticals, 12(2), 63. https://doi.org/10.3390/ph12020063





