Role of TRPV1 and TRPA1 Ion Channels in Inflammatory Bowel Diseases: Potential Therapeutic Targets?
Abstract
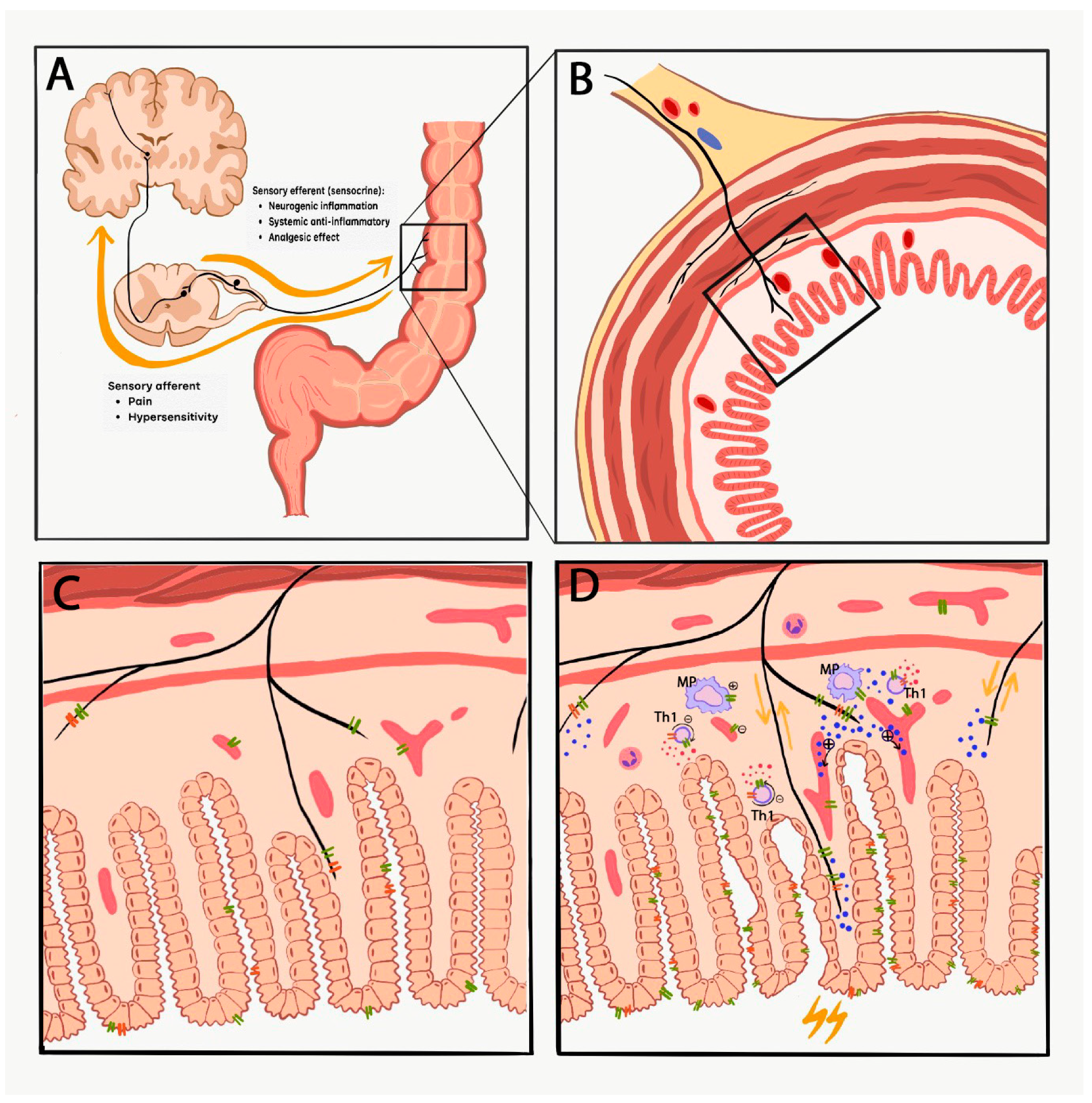
1. Introduction
2. Inflammatory Bowel Diseases (IBD) and Related Pain
3. Transient Receptor Potential Vanilloid 1 and Ankyrin 1 Pain Sensing Ion Channels
TRPV1 and TRPA1 in IBD Patients
4. Animal Models of IBD
4.1. Expression of TRPV1 and TRPA1 in Animal Colon
4.2. Role of TRP Channels in Animal Models of Colitis
5. Conclusions, Drug Developmental Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Torres, J.; Mehandru, S.; Colombel, J.-F.; Peyrin-Biroulet, L. Crohn’s Disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative Colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; von Martels, J.Z.H.; de Vos, P.; Faber, K.N.; Dijkstra, G. Increased Fecal Calprotectin Levels in Crohn’s Disease Correlate with Elevated Serum Th1- and Th17-Associated Cytokines. PLoS ONE 2018, 13, e0193202. [Google Scholar] [CrossRef]
- Kidd, B.L.; Urban, L.A. Mechanisms of Inflammatory Pain. Br. J. Anaesth. 2001, 87, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Bielefeldt, K.; Davis, B.; Binion, D.G. Pain and Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2011, 15, 778–788. [Google Scholar] [CrossRef]
- Beckers, A.B.; Weerts, Z.Z.R.M.; Helyes, Z.; Masclee, A.A.M.; Keszthelyi, D. Review Article: Transient Receptor Potential Channels as Possible Therapeutic Targets in Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2017, 46, 938–952. [Google Scholar] [CrossRef]
- Simren, M.; Axelsson, J.; Gillberg, R.; Abrahamsson, H.; Svedlund, J.; Björnsson, E.S. Quality of Life in Inflammatory Bowel Disease in Remission: The Impact of IBS-Like Symptoms and Associated Psychological Factors. Am. J. Gastroenterol. 2002, 97, 389–396. [Google Scholar] [CrossRef]
- Farthing, M.J.G.; Lennard-Jones, J.E. Sensibility of the Rectum to Distension and the Anorectal Distension Reflex in Ulcerative Colitis. Gut 1978, 19, 64–69. [Google Scholar] [CrossRef]
- Rao, S.S.C.; Read, N.W.; Davison, P.A.; Bannister, J.J.; Holdsworth, C.D. Anorectal Sensitivity and Responses to Rectal Distention in Patients with Ulcerative Colitis. Gastroenterology 1987, 93, 1270–1275. [Google Scholar] [CrossRef]
- Loening-Baucke, V.; Metcalf, A.M.; Shirazi, S. Anorectal Manometry in Active and Quiescent Ulcerative Colitis. Am. J. Gastroenterol. 1989, 84, 892–897. [Google Scholar]
- van Hoboken, E.A.; Thijssen, A.Y.; Verhaaren, R.; van der Veek, P.P.J.; Prins, F.A.; Verspaget, H.W.; Masclee, A.A.M. Symptoms in Patients with Ulcerative Colitis in Remission Are Associated with Visceral Hypersensitivity and Mast Cell Activity. Scand. J. Gastroenterol. 2011, 46, 981–987. [Google Scholar] [CrossRef]
- Keszthelyi, D.; Jonkers, D.M.; Hamer, H.M.; Masclee, A.A.M. Letter: The Role of Sub-Clinical Inflammation and TRPV1 in the in Ulcerative Colitis in Remission. Aliment. Pharmacol. Ther. 2013, 38, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Niazi, N.; Robert, M.; Mertz, H.; Kodner, A.; Munakata, J.; Naliboff, B.; Mayer, E.A. Rectal Afferent Function in Patients with Inflammatory and Functional Intestinal Disorders. Pain 1996, 66, 151–161. [Google Scholar] [CrossRef]
- Chang, L.; Munakata, J.; Mayer, E.A.; Schmulson, M.J.; Johnson, T.D.; Bernstein, C.N.; Saba, L.; Naliboff, B.; Anton, P.A.; Matin, K. Perceptual Responses in Patients with Inflammatory and Functional Bowel Disease. Gut 2000, 47, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, W.E.; Delvaux, M. Standardization of Barostat Procedures for Testing Smooth Muscle Tone and Sensory Thresholds in the Gastrointestinal Tract. Dig. Dis. Sci. 1997, 42, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, F.S.; Owsianik, G.; Nilius, B. TRP Channels: An Overview. Cell Calcium 2005, 38, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Abramowitz, J.; Yildirim, E.; Birnbaumer, L. The TRPC Family of Ion Channels: Relation to the TRP Superfamily and Role in Receptor- and Store-Operated Calcium Entry. In TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; Liedtke, W.B., Heller, S., Eds.; CRC Press: Boca Raton, FL, USA, 2007; Chapter 1. [Google Scholar]
- Latorre, R.; Zaelzer, C.; Brauchi, S. Structure-Functional Intimacies of Transient Receptor Potential Channels. Q. Rev. Biophys. 2009, 42, 201–246. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Fernandes, M.A.; Keeble, J.E. The Functions of TRPA1 and TRPV1: Moving Away from Sensory Nerves. Br. J. Pharmacol. 2012, 166, 510–521. [Google Scholar] [CrossRef]
- Ruparel, N.B.; Patwardhan, A.M.; Akopian, A.N.; Hargreaves, K.M. Homologous and Heterologous Desensitization of Capsaicin and Mustard Oil Responses Utilize Different Cellular Pathways in Nociceptors. Pain 2008, 135, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like Channel Expressed in Nociceptive Neurons, Is Activated by Cold Temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- Amadesi, S.; Nie, J.; Vergnolle, N.; Cottrell, G.S.; Grady, E.F.; Trevisani, M.; Manni, C.; Geppetti, P.; McRoberts, J.A.; Ennes, H.; et al. Protease-Activated Receptor 2 Sensitizes the Capsaicin Receptor Transient Receptor Potential Vanilloid Receptor 1 to Induce Hyperalgesia. J. Neurosci. 2004, 24, 4300–4312. [Google Scholar] [CrossRef]
- Cattaruzza, F.; Lyo, V.; Jones, E.; Pham, D.; Hawkins, J.; Kirkwood, K.; Valdez-Morales, E.; Ibeakanma, C.; Vanner, S.J.; Bogyo, M.; et al. Cathepsin S Is Activated During Colitis and Causes Visceral Hyperalgesia by a PAR2-Dependent Mechanism in Mice. Gastroenterology 2011, 141, 1864–1874.el–3. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Cho, H.; Hwang, S.W.; Jung, J.; Shin, C.Y.; Lee, S.; Kim, S.H.; Lee, M.G.; Choi, Y.H.; Kim, J.; et al. Bradykinin-12-Lipoxygenase-VR1 Signaling Pathway for Inflammatory Hyperalgesia. Proc. Natl. Acad. Sci. USA 2002, 99, 10150–10155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, J.; Mcnaughton, P.A. NGF Rapidly Increases Membrane Expression of TRPV1 Heat-Gated Ion Channels. EMBO J. 2005, 24, 4211–4223. [Google Scholar] [CrossRef]
- Zhang, N.; Inan, S.; Cowan, A.; Sun, R.; Wang, J.M.; Rogers, T.J.; Caterina, M.; Oppenheim, J.J. A Proinflammatory Chemokine, CCL3, Sensitizes the Heat- and Capsaicin-Gated Ion Channel TRPV1. Proc. Natl. Acad. Sci. USA 2005, 102, 4536–4541. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Higashi, T.; Togashi, K.; Iida, T.; Segi, E.; Sugimoto, Y.; Tominaga, T.; Narumiya, S.; Tominaga, M. Sensitization of TRPV1 by EP 1 and IP Reveals Peripheral Nociceptive Mechanism of Prostaglandins. Mol. Pain 2005, 13, 1–13. [Google Scholar] [CrossRef]
- Okajima, F. Regulation of Inflammation by Extracellular Acidification and Proton-Sensing GPCRs. Cell. Signal. 2013, 25, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Rukwied, R.; Chizh, B.A.; Lorenz, U.; Obreja, O.; Margarit, S.; Schley, M.; Schmelz, M. Potentiation of Nociceptive Responses to Low PH Injections in Humans by Prostaglandin E2. J. Pain 2019, 8, 443–451. [Google Scholar] [CrossRef]
- Jones, N.G.; Slater, R.; Cadiou, H.; Mcnaughton, P.; Mcmahon, S.B. Acid-Induced Pain and Its Modulation in Humans. J. Neurosci. 2004, 24, 10974–10979. [Google Scholar] [CrossRef]
- Yiangou, Y.; Facer, P.; Dyer, N.H.C.; Chan, C.L.H.; Knowles, C.; Williams, N.S.; Anand, P. Vanilloid Receptor 1 Immunoreactivity in Inflamed Human Bowel. Lancet 2001, 357, 1338–1339. [Google Scholar] [CrossRef]
- Luo, C.; Wang, Z.; Mu, J.; Zhu, M.; Zhen, Y.; Zhang, H. Upregulation of the Transient Receptor Potential Vanilloid 1 in Colonic Epithelium of Patients with Active Inflammatory Bowel Disease. Int. J. Clin. Exp. Pathol. 2017, 10, 11335–11344. [Google Scholar]
- Kun, J.; Szitter, I.; Kemény, Á.; Perkecz, A.; Kereskai, L.; Pohóczky, K.; Vincze, Á.; Szabó, I.; Szolcsányi, J.; Pintér, E.; Helyes, Z. Upregulation of the Transient Receptor Potential Ankyrin 1 Ion Channel in the Inflamed Human and Mouse Colon and Its Protective Roles. PLoS ONE 2014, 9, e108164. [Google Scholar] [CrossRef] [PubMed]
- Rizopoulos, T.; Papadaki-Petrou, H.; Assimakopoulou, M. Expression Profiling of the Transient Receptor Potential Vanilloid (TRPV) Channels 1, 2, 3 and 4 in Mucosal Epithelium of Human Ulcerative Colitis. Cells 2018, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Yiangou, Y.; Facer, P.; Brydon, W.G.; Walters, J.R.F.; Anand, P.; Ghosh, S. Expression of the TRPV1 Receptor Differs in Quiescent Inflammatory Bowel Disease with or without Abdominal Pain. Gut 2010, 59, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Keszthelyi, D.; Troost, F.J.; Jonkers, D.M.; Helyes, Z.; Hamer, H.M.; Ludidi, S.; Vanhoutvin, S.; Venema, K.; Dekker, J.; Szolcsányi, J.; et al. Alterations in Mucosal Neuropeptides in Patients with Irritable Bowel Syndrome and Ulcerative Colitis in Remission: A Role in Pain Symptom Generation? Eur. J. Pain 2013, 17, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Bertin, S.; Aoki-Nonaka, Y.; Lee, J.; de Jong, P.R.; Kim, P.; Han, T.; Yu, T.; To, K.; Takahashi, N.; Boland, B.S.; et al. The TRPA1 Ion Channel Is Expressed in CD4+ T Cells and Restrains T Cell-Mediated Colitis through Inhibition of TRPV1. Gut 2017, 66, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, K.; Kurahara, L.-H.; Sumiyoshi, M.; Hu, Y.; Koga, K.; Onitsuka, M.; Kojima, D.; Yue, L.; Takedatsu, H.; Jian, Y.-W.; et al. Daikenchuto (Da-Jian-Zhong-Tang) Ameliorates Intestinal Fibrosis by Activating Myofibroblast Transient Receptor Potential Ankyrin 1 Channel. World J. Gastroenterol. 2018, 24, 4036–4053. [Google Scholar] [CrossRef]
- Kurahara, L.H.; Hiraishi, K.; Hu, Y.; Koga, K.; Onitsuka, M.; Doi, M.; Aoyagi, K.; Takedatsu, H.; Kojima, D.; Fujihara, Y.; et al. Activation of Myofibroblast TRPA1 by Steroids and Pirfenidone Ameliorates Fibrosis in Experimental Crohn’s Disease. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 299–318. [Google Scholar] [CrossRef]
- Valatas, V.; Bamias, G.; Kolios, G. Experimental Colitis Models: Insights into the Pathogenesis of in Fl Ammatory Bowel Disease and Translational Issues. Eur. J. Pharmacol. 2015, 759, 253–264. [Google Scholar] [CrossRef]
- Low, D.; Nguyen, D.D.; Mizoguchi, E. Animal Models of Ulcerative Colitis and Their Application in Drug Research. Drug Des. Dev. Ther. 2013, 7, 1341–1357. [Google Scholar]
- Perse, M.; Cerar, A. Dextran Sodium Sulphate Colitis Mouse Model: Traps and Tricks. J. Biomed. Biotechnol. 2012, 718617. [Google Scholar]
- Kawada, M.; Arihiro, A.; Mizoguchi, E. Insights from Advances in Research of Chemically Induced Experimental Models of Human Inflammatory Bowel Disease. World J. Gastroenterol. 2007, 13, 5581–5593. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Ueno, Y.; Yajima, T.; Okamoto, S.; Hayashi, T.; Yamazaki, M.; Iwao, Y.; Ishii, H.; Habu, S.; Uehira, M.; et al. Interleukin 7 Transgenic Mice Develop Chronic Colitis with Decreased Interleukin 7 Protein Accumulation in the Colonic Mucosa. J. Exp. Med. 1998, 187, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Mombaerts, P.; Mizoguchi, E.; Grusby, M.J.; Glimcher, L.H.; Bhan, A.K.; Tonegawa, S. Spontaneous Development of Inflammatory Bowel Disease in T Cell Receptor Mutant Mice. Cell 1993, 75, 275–282. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Maillard, M.H.; Cotta-de-Almeida, V.; Mizoguchi, E.; Klein, C.; Fuss, I.; Nagler, C.; Mizoguchi, A.; Bhan, A.K.; Snapper, S.B. Lymphocyte-Dependent and Th2 Cytokine-Associated Colitis in Mice Deficient in Wiskott-Aldrich Syndrome Protein. Gastroenterology 2007, 133, 1188–1197. [Google Scholar] [CrossRef]
- Panwala, C.M.; Jones, J.C.; Viney, J.L. A Novel Model of Inflammatory Bowel Disease: Mice Deficient for the Multiple Drug Resistance Gene, Mdr1a, Spontaneously Develop Colitis. J. Immunol. 1998, 161, 5733–5744. [Google Scholar]
- Sadlack, B.; Merz, H.; Schorle, H.; Schimpl, A.; Feller, A.C.; Horak, I. Ulcerative Colitis-like Disease in Mice with a Disrupted Interleukin-2 Gene. Cell 1993, 75, 253–261. [Google Scholar] [CrossRef]
- Rudolph, U.; Finegold, M.J.; Rich, S.S.; Harriman, G.R.; Srinivasan, Y.; Brabet, P.; Boulay, G.; Bradley, A.; Birnbaumer, L. Ulcerative Colitis and Adenocarcinoma of the Colon in Gαi2-Deficient Mice. Nat. Genet. 1995, 10, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Kurosawa, E.; Terui, H.; Hosoya, T.; Tashima, K.; Murayama, T.; Priestley, J.V.; Horie, S. Localization of TRPV1 and Contractile Effect of Capsaicin in Mouse Large Intestine: High Abundance and Sensitivity in Rectum and Distal Colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.A.; Khalil, M.; Mueller-Tribbensee, S.M.; Becker, C.; Neuhuber, W.L.; Neurath, M.F.; Reeh, P.W. The Proximodistal Aggravation of Colitis Depends on Substance P Released from TRPV1-Expressing Sensory Neurons. J. Gastroenterol. 2012, 47, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Anavi-Goffer, S.; Mckay, N.G.; Ashford, M.L.J.; Coutts, A.A. Vanilloid Receptor Type 1-Immunoreactivity Is Expressed by Intrinsic Afferent Neurones in the Guinea-Pig Myenteric Plexus. Neurosci. Lett. 2002, 319, 53–57. [Google Scholar] [CrossRef]
- Poole, D.P.; Pelayo, J.C.; Cattaruzza, F.; Kuo, Y.-M.; Gai, G.; Chiu, J.V.; Bron, R.; Furness, J.B.; Grady, E.F.; Bunnett, N.W. Transient Receptor Potential Ankyrin 1 Is Expressed by Inhibitory Motoneurons of the Mouse Intestine. Gastroenterology 2011, 141, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Kaji, I.; Yasuoka, Y.; Karaki, S.; Kuwahara, A. Activation of TRPA1 by Luminal Stimuli Induces EP 4-Mediated Anion Secretion in Human and Rat Colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 302, 690–701. [Google Scholar] [CrossRef]
- Bertin, S.; Aoki-Nonaka, Y.; de Jong, P.R.; Nohara, L.L.; Xu, H.; Stanwood, S.R.; Srikanth, S.; Lee, J.; To, K.; Abramson, L.; et al. The Ion Channel TRPV1 Regulates the Activation and Proinflammatory Properties of CD4+ T Cells. Nat. Immunol. 2014, 15, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Kihara, N.; de la Fuente, S.G.; Fujino, K.; Takahashi, T.; Pappas, T.N.; Mantyh, C.R. Vanilloid Receptor-1 Containing Primary Sensory Neurones Mediate Dextran Sulphate Sodium Induced Colitis in Rats. Gut 2003, 52, 713–719. [Google Scholar] [CrossRef]
- Kimball, E.S.; Wallace, N.H.; Schneider, C.R.; D’Andrea, M.R.; Hornby, P.J. Vanilloid Receptor 1 Antagonists Attenuate Disease Severity in Dextran Sulphate Sodium-Induced Colitis in Mice. Neurogastroenterol. Motil. 2004, 16, 811–818. [Google Scholar] [CrossRef]
- Fujino, K.; Takami, Y.; de la Fuente, S.G.; Ludwig, K.A.; Mantyh, C.R. Inhibition of the Vanilloid Receptor Subtype-1 Attenuates TNBS-Colitis. J. Gastrointest. Surg. 2004, 7, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, W.; De Man, J.; De Schepper, H.U.; Bult, H.; Moreels, T.G.; Pelckmans, P.A.; De Winter, B.Y. Role of TRPV1 and TRPA1 in Visceral Hypersensitivity to Colorectal Distension during Experimental Colitis in Rats. Eur. J. Pharmacol. 2013, 698, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Goso, C.; Evangelista, S.; Tramontana, M.; Manzini, S.; Blumberg, P.M.; Szallasi, A. Topical Capsaicin Administration Protects against Trinitrobenzene Sulfonic Acid-Induced Colitis in the Rat. Eur. J. Pharmacol. 1993, 249, 185–190. [Google Scholar] [CrossRef]
- Martelli, L.; Ragazzi, E.; Di Mario, F.; Martelli, M.; Castagliuolo, I.; Dal Maschio, M.; Palu, G.; Maschietto, M.; Scorzeto, M.; Vassanelli, S.; et al. A Potential Role for the Vanilloid Receptor TRPV1 in the Therapeutic Effect of Curcumin in Dinitrobenzene Sulphonic Acid-Induced Colitis in Mice. Neurogastroenterol. Motil. 2007, 19, 668–674. [Google Scholar] [CrossRef]
- Yang, M.; Wang, J.; Yang, C.; Han, H.; Rong, W.; Zhang, G. Oral Administration of Curcumin Attenuates Visceral Hyperalgesia through Inhibiting Phosphorylation of TRPV1 in Rat Model of Ulcerative Colitis. Mol. Pain 2017, 13, 1744806917726416. [Google Scholar] [CrossRef]
- Szitter, I.; Pozsgai, G.; Sandor, K.; Elekes, K.; Kemeny, A.; Perkecz, A.; Szolcsanyi, J.; Helyes, Z.; Pinter, E. The Role of Transient Receptor Potential Vanilloid 1 (Trpv1) Receptors in Dextran Sulfate-Induced Colitis in Mice. J. Mol. Neurosci. 2010, 42, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, D.; Matsumoto, K.; Tsukahara, T.; Amagase, K.; Tominaga, M.; Kato, S. Transient Receptor Potential Vanilloid 1 and Transient Receptor Potential Ankyrin 1 Contribute to the Progression of Colonic Inflammation in Dextran Sulfate Sodium-Induced Colitis in Mice: Links to Calcitonin Gene-Related Peptide and Substance P. J. Pharmacol. Sci. 2018, 136, 121–132. [Google Scholar] [CrossRef]
- Massa, F.; Sibaev, A.; Marsicano, G.; Blaudzun, H.; Storr, M.; Lutz, B. Vanilloid Receptor (TRPV1)-Deficient Mice Show Increased Susceptibility to Dinitrobenzene Sulfonic Acid Induced Colitis. J. Mol. Med. 2006, 84, 142–146. [Google Scholar] [CrossRef]
- Engel, M.A.; Leffler, A.; Niedermirtl, F.; Babes, A.; Mueller-Tribbensee, S.M.; Khalil, M.; Siklosi, N.; Nau, C.; Ivanovic-Burmazovic, I.; Neuhuber, W.L.; et al. TRPA1 and Substance P Mediate Colitis in Mice. Gastroenterology 2011, 141, 1346–1358. [Google Scholar] [CrossRef]
- Lapointe, T.K.; Basso, L.; Iftinca, M.C.; Flynn, R.; Chapman, K.; Dietrich, G.; Vergnolle, N.; Altier, C. TRPV1 Sensitization Mediates Postinflammatory Visceral Pain Following Acute Colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, 87–99. [Google Scholar] [CrossRef]
- Lee, J.; Yamamoto, T.; Kuramoto, H.; Kadowaki, M. TRPV1 Expressing Extrinsic Primary Sensory Neurons Play a Protective Role in Mouse Oxazolone-Induced Colitis. Auton. Neurosci. Basic Clin. 2012, 166, 72–76. [Google Scholar] [CrossRef]
- Szitter, I.; Pintér, E.; Perkecz, A.; Kemény, Á.; Kun, J.; Kereskai, L.; Pietra, C.; Quinn, J.P.; Zimmer, A.; Berger, A.; Paige, C.J.; et al. Role of Neurokinin 1 Receptors in Dextran Sulfate-Induced Colitis: Studies with Gene-Deleted Mice and the Selective Receptor Antagonist Netupitant. Inflamm. Res. 2014, 63, 399–409. [Google Scholar] [CrossRef]
- Nalli, M.; Ortar, G.; Moriello, A.S.; Marzo, V.D.; Petrocellis, L.D. Effects of Curcumin and Curcumin Analogues on TRP Channels. Fitoterapia 2017, 122, 126–131. [Google Scholar] [CrossRef]
- Leamy, A.W.; Shukla, P.; Mcalexander, M.A.; Carr, M.J.; Ghatta, S. Crucumin ((E,E)-1,7-Bis(4-Hydroxy-3-Methoxyphenyl)-1,6-Heptadiene-3,5-Dione) Activates and Desensitizes the Nociceptor Ion Channel TRPA1. Neurosci. Lett. 2011, 503, 157–162. [Google Scholar] [CrossRef]
- Larmonier, C.B.; Midura-Kiela, M.T.; Ramalingam, R.; Laubitz, D.; Janikashvili, N.; Larmonier, N.; Ghishan, F.K.; Kiela, P.R. Modulation of Neutrophil Motility by Curcumin: Implications for Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2011, 17, 503–515. [Google Scholar] [CrossRef]
- Zhi, L.; Dong, L.; Kong, D.; Sun, B.; Sun, Q.; Grundy, D.; Zhang, G.; Rong, W. Curcumin Acts via Transient Receptor Potential Vanilloid-1 Receptors to Inhibit Gut Nociception and Reverses Visceral Hyperalgesia. Neurogastroenterol. Motil. 2013, 25, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Hanai, H.; Iida, T.; Takeuchi, K.; Watanabe, F.; Maruyama, Y.; Andoh, A.; Tsujikawa, T.; Fujiyama, Y.; Mitsuyama, K.; Sata, M.; et al. Curcumin Maintenance Therapy for Ulcerative Colitis: Randomized, Multicenter, Double-Blind, Placebo-Controlled Trial. Clin. Gastroenterol. Hepatol. 2006, 4, 1502–1506. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, G.; Valenti, M.; Scaglione, G.; Cosenza, V.; Sorrentini, I.; Di Marzo, V. Up-Regulation of Anandamide Levels as an Endogenous Mechanism and a Pharmacological Strategy to Limit Colon Inflammation. FASEB J. 2006, 20, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Hasenoehrl, C.; Taschler, U.; Storr, M.; Schicho, R. The Gastrointestinal Tract—A Central Organ of Cannabinoid Signaling in Health and Disease. Neurogastroenterol. Motil. 2016, 28, 1765–1780. [Google Scholar] [CrossRef] [PubMed]
- Couch, D.G.; Tasker, C.; Theophilidou, E.; Lund, J.N.; O’Sullivan, S.E. Cannabidiol and Palmitoylethanolamide Are Anti-Inflammatory in the Acutely Inflamed Human Colon. Clin. Sci. 2017, 131, 2611–2626. [Google Scholar] [CrossRef]
- Petrosino, S.; Moriello, A.S.; Cerrato, S.; Fusco, M.; Puigdemont, A.; De Petrocellis, L.; Di Marzo, V. The Anti-Inflammatory Mediator Palmitoylethanolamide Enhances the Levels of 2-Arachidonoyl-Glycerol and Potentiates Its Actions at TRPV1 Cation Channels. Br. J. Pharmacol. 2016, 173, 1154–1162. [Google Scholar] [CrossRef]
- Petrosino, S.; Cristino, L.; Karsak, M.; Gaffal, E.; Ueda, N.; Tüting, T.; Bisogno, T.; De Filippis, D.; D’Amico, A.; Saturnino, C.; et al. Protective Role of Palmitoylethanolamide in Contact Allergic Dermatitis. Exp. Allergy Immunol. 2010, 65, 698–711. [Google Scholar] [CrossRef]
- Swanson, D.M.; Dubin, A.E.; Shah, C.; Nasser, N.; Chang, L.; Dax, S.L.; Jetter, M.; Breitenbucher, J.G.; Liu, C.; Mazur, C.; et al. Identification and Biological Evaluation of 4-(3-Trifluoromethylpyridin-2-Yl)Piperazine-1-Carboxylic Acid (5-Trifluoromethylpyridin-2-Yl) Amide, a High Affinity TRPV1 (VR1) Vanilloid Receptor Antagonist. J. Med. Chem. 2005, 48, 1857–1872. [Google Scholar] [CrossRef]
- Steiner, A.A.; Turek, V.F.; Almeida, M.C.; Burmeister, J.J.; Oliveira, D.L.; Roberts, J.L.; Bannon, A.W.; Norman, M.H.; Louis, J.; Treanor, J.J.S.; et al. Nonthermal Activation of Transient Receptor Potential Vanilloid-1 Channels in Abdominal Viscera Tonically Inhibits Autonomic Cold-Defense Effectors. J. Neurosci. 2007, 27, 7459–7468. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Harris, S.; Whiteside, G.T.; Hummel, M.; Knappenberger, T.; O’Keefe, S.; Kapil, R.; Kyle, D. A Randomized, Double-Blind, Positive-Controlled, 3-Way Cross-over Human Experimental Pain Study of a Trpv1 Antagonist (V116517) in Healthy Volunteers and Comparison with Preclinical Profile. Pain 2016, 157, 2057–2067. [Google Scholar] [CrossRef] [PubMed]
- Gavva, N.R.; Treanor, J.J.S.; Garami, A.; Fang, L.; Surapaneni, S.; Akrami, A.; Alvarez, F.; Bak, A.; Darling, M.; Gore, A.; et al. Pharmacological Blockade of the Vanilloid Receptor TRPV1 Elicits Marked Hyperthermia in Humans. Pain 2008, 136, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hackos, D.H. TRPA1 as a Drug Target—Promise and Challenges. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015, 388, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Chiche, D.; Brown, W.; Walker, P. NEO6860, a Novel Modality Selective TRPV1 Antagonist: Results from a Phase I, Double-Blind, Placebo-Controlled Study in Healthy Subjects. J. Pain 2016, 17, S79. [Google Scholar] [CrossRef]
- Kaneko, Y.; Szallasi, A. Transient Receptor Potential (TRP) Channels: A Clinical Perspective. Br. J. Pharmacol. 2013, 171, 2474–2507. [Google Scholar] [CrossRef]
| Disease Activity | Ion Channel | Sampling Method/Location | Methods | Results; Number of Patients | Relation to Abdominal Complaints and/or Disease severity | Ref |
|---|---|---|---|---|---|---|
| Active CD | TRPV1 | Resection (colectomy) | IHC (computerized image analysis) | upregulated in submucosa; n = 6 | Not reported | [31] |
| Colon biopsy–affected and non-affected regions | IHC (computerized image analysis) | upregulated in mucosa and infiltrating inflammatory cells; n = 30 | No significant correlation between disease severity and TRPV1 expression | [32] | ||
| Distal colon biopsy | IHC, qPCR | downregulated mRNA n = not reported | Not reported | [33] | ||
| Colon biopsy | IF | downregulated mRNA n = 6 | Not reported | [37] | ||
| TRPA1 | Distal colon biopsy | IHC, qPCR | upregulated mRNA n = not reported | Not reported | [33] | |
| Colon biopsy | IF | upregulated mRNA n = 7 | Not reported | [37] | ||
| CD in remission | TRPV1 | Distal colon biopsy | IHC, qPCR | downregulated mRNA n = not reported | Not reported | [33] |
| Rectosigmoid biopsy | IHC | upregulated in symptomatic quiescent patients; 3.9-fold increase in median number of TRPV1-immunoreactive fibers (CD and UC combined) n = 9 | Significant correlation between TRPV1 expression and abdominal pain score | [35] | ||
| CD – disease activity unknown | TRPA1 | Surgical samples of fibrotic regions (colon) | IHC | Denser immunoreactivity in mucosal and submucosal layers n = 3 | Not reported | [38] |
| Biopsy from fibrotic regions (colon) | IHC, RT-PCR | upregulated mRNA and protein levels n = 8 | Not reported | [39] |
| Disease Activity | Ion Channel | Sampling Method/Location | Methods | Results; Number of Patients | Relation to Abdominal Complaints and/or Disease Severity | Ref |
|---|---|---|---|---|---|---|
| Active UC | TRPV1 | Resection (colectomy) | IHC (computerized image analysis) | upregulated in submucosa n = 3 | Not reported | [31] |
| Colon biopsy–affected and non-affected regions | IHC (computerized image analysis) | upregulated in mucosa and infiltrating inflammatory cells; n = 30 | No significant correlation between disease severity and TRPV1 expression | [32] | ||
| Distal colon biopsy | IHC, qPCR | downregulated mRNA | Not reported | [33] | ||
| Colon biopsy | IHC (manual counting by two observers) | downregulated protein n = 26 | No significant correlation between clinical features and TRPV1 expression | [34] | ||
| UC in remission | TRPV1 | Distal colon biopsy | IHC, qPCR | downregulated mRNA | Not reported | [33] |
| Colon biopsy | IHC (manual counting by two observers) | downregulated protein n = 24 | No significant correlation between clinical features and TRPV1 expression | [34] | ||
| Rectosigmoid biopsy | IHC | upregulated in patients with IBS-like symptoms; 3.9-fold increase in median number of TRPV1-immunoreactive fibers (CD and UC combined) n = 11 | Significant correlation between TRPV1 expression and abdominal pain score | [35] | ||
| Rectosigmoid biopsy | qPCR | No significant difference in mRNA levels between asymptomatic patients and healthy controls n = 34 | Not reported | [36] |
| mRNA | Location | Method | Model, Animal Species/Strain | Ref |
|---|---|---|---|---|
| TRPV1 | isolated crypts, submucosal and muscle layers of distal, middle and proximal colon | qPCR | intact male Wistar rats | [54] |
| upregulated in colonic DRG to the distal colon in DSS-colitis | 2.5% DSS-treated C57BL/6 mice | [51] | ||
| unaltered in distal colon, cell type not specified | DSS colitis - male C57BL/6 mice | [33] | ||
| CD4+ T cells | primary cell culture from C57BL/6 spleen | [55] | ||
| TRPA1 | muscularis externa and mucosa of duodenum, ileum and colon; cell type not specified | intact C57BL/6 mice | [53] | |
| surface epithelium of middle colon | ISH | intact male Wistar rats | [54] | |
| isolated crypts, submucosal and muscle layers of distal, middle and proximal colon | qPCR | intact male Wistar rats | [54] | |
| upregulated in distal colon, cell type not specified | DSS colitis - male C57BL/6 mice | [33] |
| Protein | Location | Method | Model, Animal Species/Strain | Ref |
|---|---|---|---|---|
| TRPV1 | intrinsic sensory neurons of the myenteric plexus-longitudinal muscle of ileum and colon | IHC | intact Sprague-Dawley rats and Dunkin-Hartley guinea pigs of both sexes | [52] |
| mucosa, submucosal layers, myenteric plexus and mucosal layer of rectum, distal, transverse and proximal colon | male ddY mice | [50] | ||
| immunopositive neuron fiber density is higher in the distal than the proximal colon | intact and 2.5% DSS-treated C57BL/6 mice colon | [51] | ||
| enteric ganglia, epithelial cells of the distal colon, myenteric and submucosal plexuses, mucosal macrophages, leukocytes | male C57BL/6 mice | [33] | ||
| membrane of resting CD4+ T cells | immunoblotting, flow cytometry, confocal microscopy | primary cell culture from C57BL/6 spleen | [55] | |
| TRPA1 | distal colonic epithelial cells, myenteric and submucosal plexuses, interstitial macrophages | IHC | male C57BL/6 mice | [33] |
| myenteric and submucosal ganglia; surface epithelial cells of small and large intestines | intact C57BL/6 mice | [53] | ||
| surface epithelium of middle colon | intact male Wistar rats | [54] | ||
| membrane of resting CD4+ T cells | IHC, confocal microscopy | primary cell culture from C57BL/6 spleen | [37] |
| Approaches | Results | Animal Strain/Species | Model | Ref |
|---|---|---|---|---|
| TRPV1 antagonist | reduces colitis severity | Sprague-Dawley rats | 5% DSS + capsazepine | [56] |
| female BALB/c mice | 5% DSS + capsazepine/JNJ 10185734 | [57] | ||
| Sprague-Dawley rats | TNBS + capsazepine | [58] | ||
| female Wistar rats | TNBS + BCTC | [59] | ||
| IL10−/−Trpv1−/− mice | IL10−/− -induced spontaneous colitis + SB366791 | [55] | ||
| TRPV1 agonist | attenuates colitis/visceral hyperalgesia | male Sprague-Dawley rats | TNBS + capsaicin | [60] |
| male BALB/c mice | DNBS + curcumin | [61] | ||
| male Sprague-Dawley rats | 5% DSS + curcumin | [62] | ||
| TRPV1 gene deletion | decreases colitis | female Trpv1−/−mice | 2% DSS | [63] |
| IL10−/−Trpv1−/− mice | IL10−/−-induced spontaneous colitis | [55] | ||
| male Trpv1−/−mice | 2% DSS | [64] | ||
| aggravates colitis | female Trpv1−/−mice | DNBS | [65] | |
| does not affect colitis severity | female Trpv1−/−mice | 5% DSS | [63] | |
| Trpv1−/− mice | TNBS | [66] | ||
| Trpv1−/− mice | 2.5% DSS | [67] | ||
| protects against chronic pain during recovery | Trpv1−/− mice | 2.5% DSS | [67] | |
| decreases CD4+ T cell activation and cytokine production | IL10−/−Trpv1−/− mice | IL10−/−-induced spontaneous colitis | [55] | |
| TRPA1 antagonist | reduces colitis severity | C57BL/6 mice | TNBS + HC-030031; DSS + HC-030031 | [66] |
| reverses visceromotor response | female Wistar rats | TNBS/ethanol + TCS-5861528 | [59] | |
| TRPA1 gene deletion | decreases colitis | Trpa1−/− mice | TNBS, 2% DSS | [66] |
| male Trpa1−/− mice | 2% DSS | [64] | ||
| aggravates colitis | male Trpa1−/− mice | 2% DSS | [33] | |
| IL10−/−Trpa1−/− mice | IL10−/−-induced spontaneous colitis | [37] | ||
| increases TRPV1 channel activity in CD4+ T cells, increases CD4+ T cell activation and proinflammatory cytokine production | IL10−/−Trpa1−/− mice | IL10−/−-induced spontaneous colitis | [37] | |
| Capsaicin-induced sensory desensitization | aggravates colitis | female BALB/c mice | oxazolone | [68] |
| male Trpv1−/−, Trpa1−/− mice | 2% DSS | [64] | ||
| alleviates colitis | Sprague-Dawley rats | 5% DSS | [56] | |
| RTX-denervation | alleviates colitis | C57BL/6 mice | TNBS, 2% DSS | [66] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csekő, K.; Beckers, B.; Keszthelyi, D.; Helyes, Z. Role of TRPV1 and TRPA1 Ion Channels in Inflammatory Bowel Diseases: Potential Therapeutic Targets? Pharmaceuticals 2019, 12, 48. https://doi.org/10.3390/ph12020048
Csekő K, Beckers B, Keszthelyi D, Helyes Z. Role of TRPV1 and TRPA1 Ion Channels in Inflammatory Bowel Diseases: Potential Therapeutic Targets? Pharmaceuticals. 2019; 12(2):48. https://doi.org/10.3390/ph12020048
Chicago/Turabian StyleCsekő, Kata, Bram Beckers, Daniel Keszthelyi, and Zsuzsanna Helyes. 2019. "Role of TRPV1 and TRPA1 Ion Channels in Inflammatory Bowel Diseases: Potential Therapeutic Targets?" Pharmaceuticals 12, no. 2: 48. https://doi.org/10.3390/ph12020048
APA StyleCsekő, K., Beckers, B., Keszthelyi, D., & Helyes, Z. (2019). Role of TRPV1 and TRPA1 Ion Channels in Inflammatory Bowel Diseases: Potential Therapeutic Targets? Pharmaceuticals, 12(2), 48. https://doi.org/10.3390/ph12020048






