2-Nitroimidazole-Furanoside Derivatives for Hypoxia Imaging—Investigation of Nucleoside Transporter Interaction, 18F-Labeling and Preclinical PET Imaging
Abstract
1. Introduction
2. Results
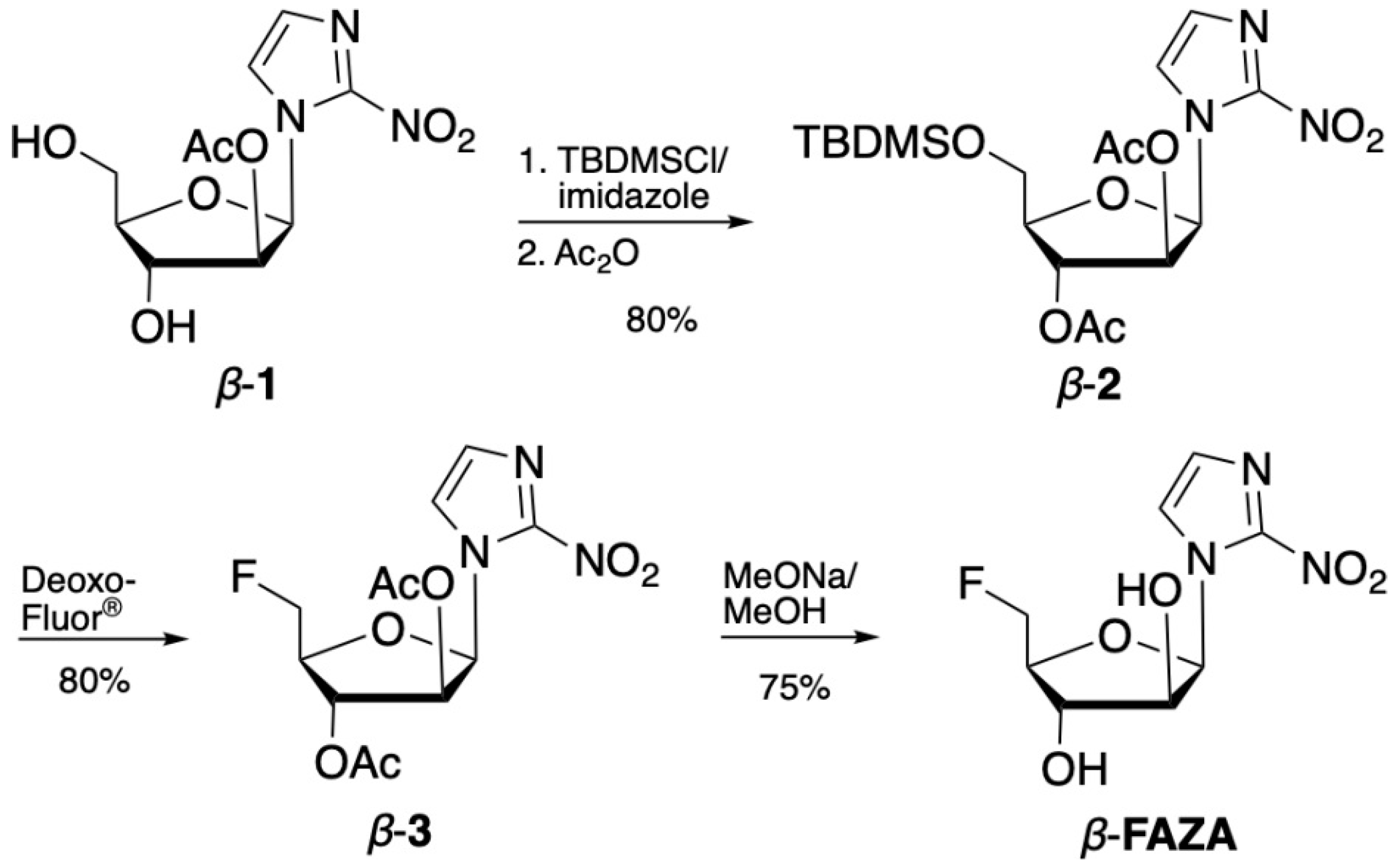
2.1. Organic Chemistry
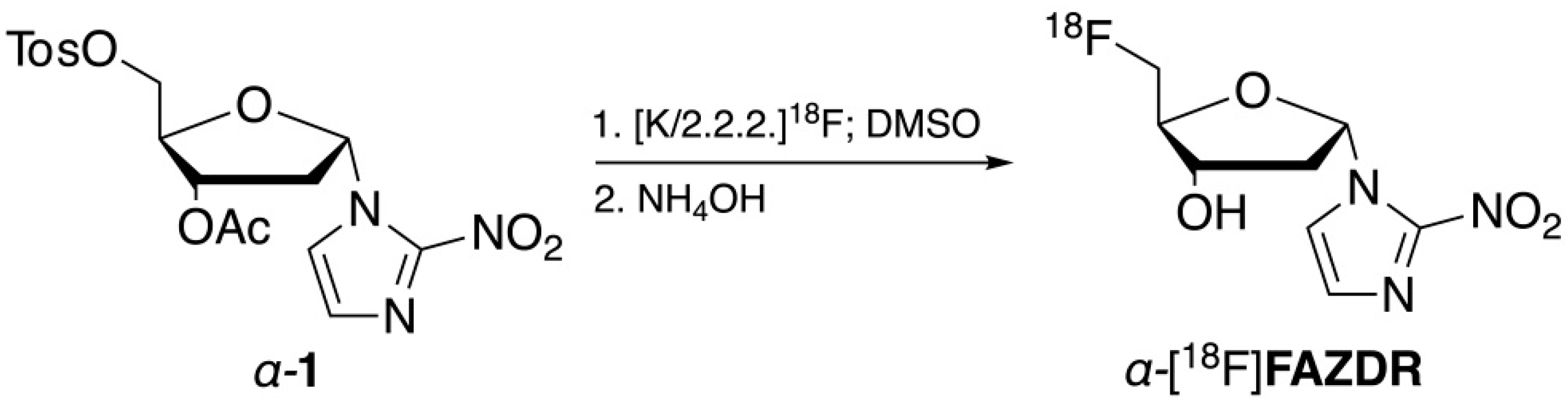
2.2. Radiochemistry
2.3. Transporter Studies in Saccharomyces cerevisiae
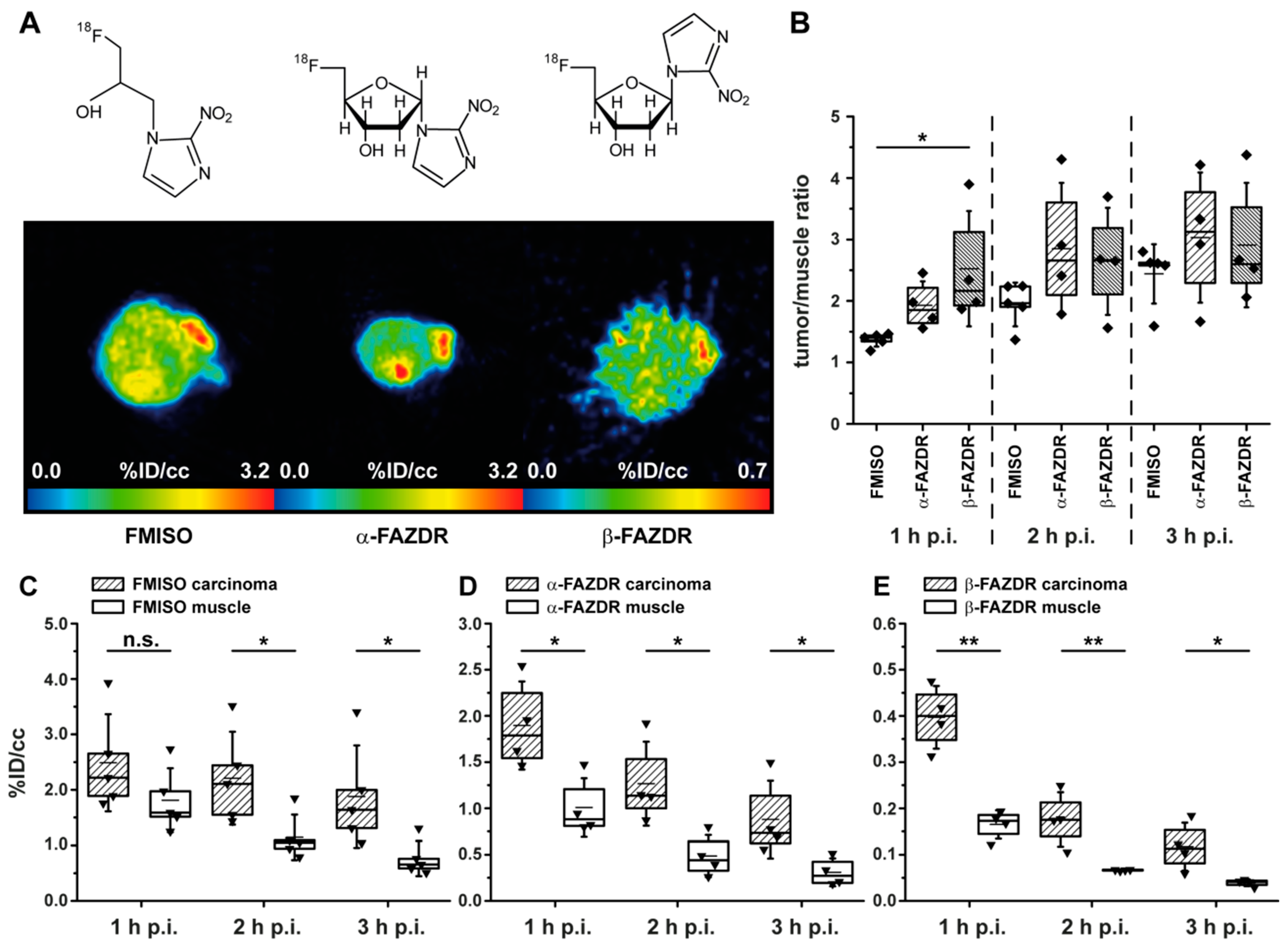
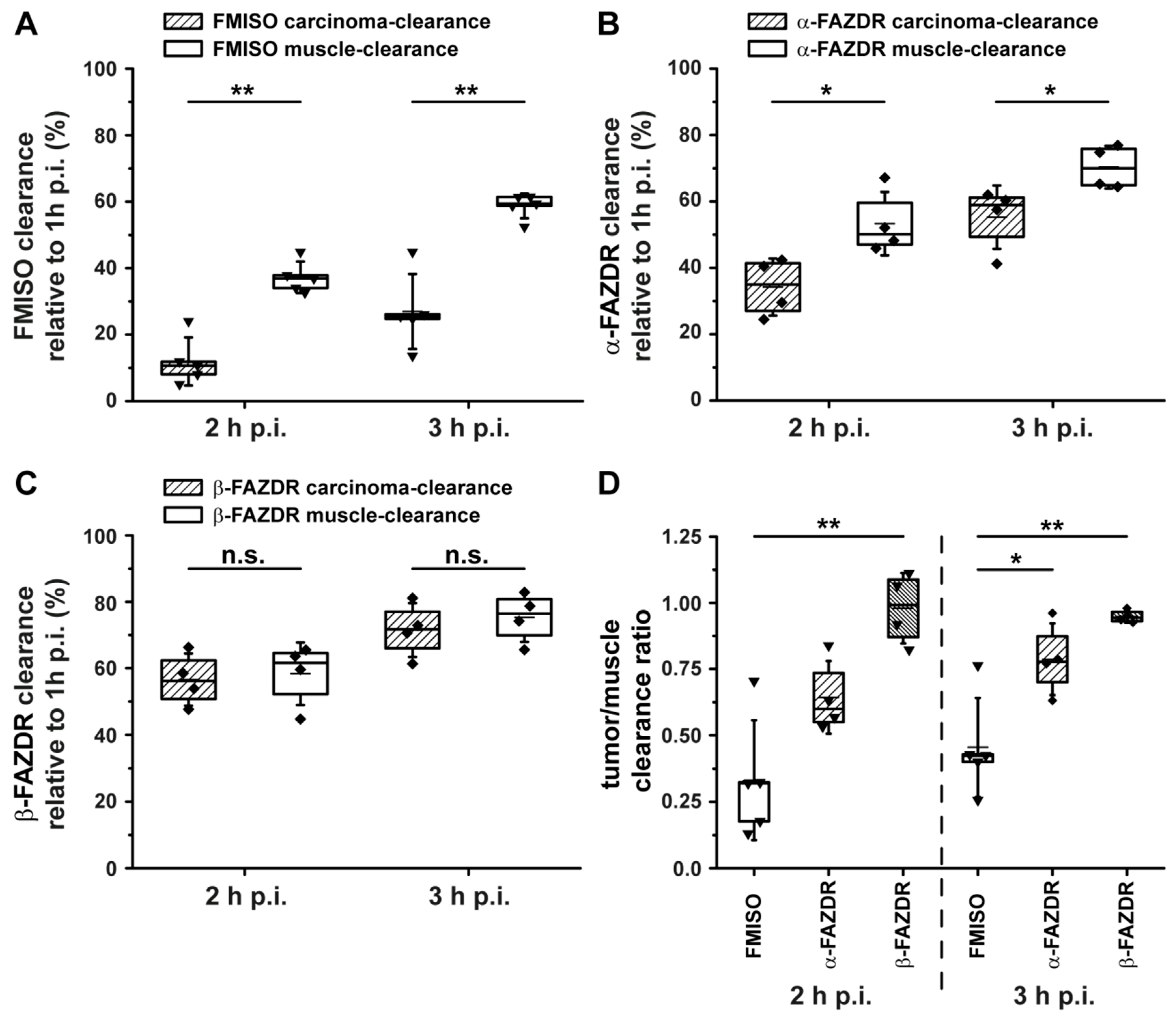
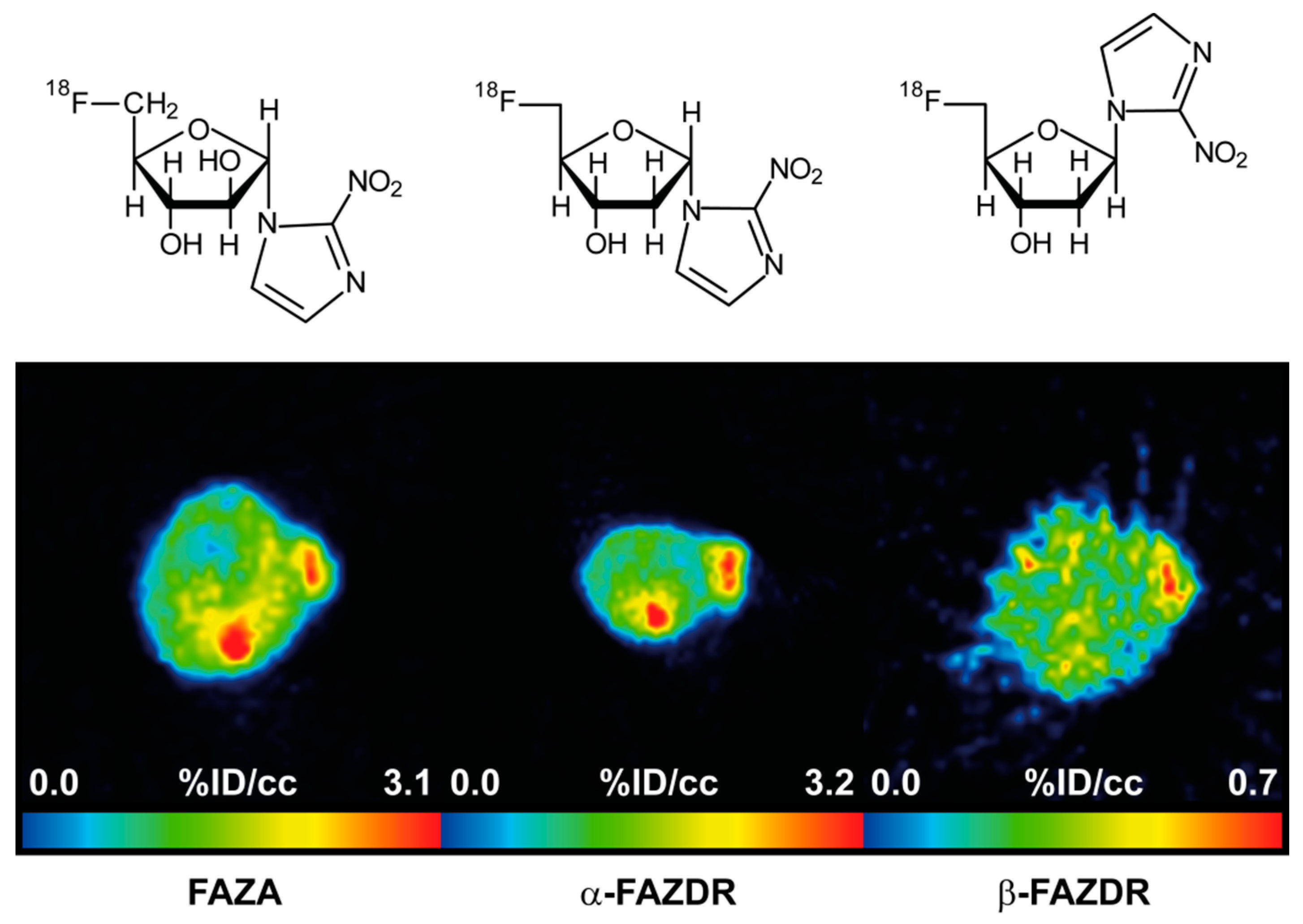
2.4. PET Hypoxia Imaging of CT26 Colon Carcinoma Bearing Mice
3. Discussion
4. Materials and Methods
4.1. General
4.2. Synthesis of 1-(5′-Deoxy-5′-fluoro-β-D-arabinofuranosyl)-2-nitroimidazole (β-FAZA)
4.3. Radiosynthetic Procedures
4.4. Transporter Studies in Saccharomyces cerevisiae
4.5. Cell Culture
4.6. Animals
4.7. PET Imaging
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stieb, S.; Eleftheriou, A.; Warnock, G.; Guckenberger, M.; Riesterer, O. Longitudinal PET imaging of tumor hypoxia during the course of radiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2201–2217. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, X.F.; Zou, H.; Sun, X.; Shen, B. (18)F-Fluoromisonidazole in tumor hypoxia imaging. Oncotarget 2017, 8, 94969–94979. [Google Scholar] [CrossRef] [PubMed]
- Colliez, F.; Gallez, B.; Jordan, B.F. Assessing Tumor Oxygenation for Predicting Outcome in Radiation Oncology: A Review of Studies Correlating Tumor Hypoxic Status and Outcome in the Preclinical and Clinical Settings. Front. Oncol. 2017, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Parolia, A.; Dunphy, M.P.; Venneti, S. Non-invasive metabolic imaging of brain tumours in the era of precision medicine. Nat. Rev. Clin. Oncol. 2016, 13, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Vordermark, D.; Horsman, M.R. Hypoxia as a Biomarker and for Personalized Radiation Oncology. Recent Results Cancer Res. 2016, 198, 123–142. [Google Scholar] [PubMed]
- O’Connor, J.P.; Rose, C.J.; Waterton, J.C.; Carano, R.A.; Parker, G.J.; Jackson, A. Imaging intratumor heterogeneity: Role in therapy response, resistance, and clinical outcome. Clin. Cancer Res. 2015, 21, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Widmer, D.S.; Hoek, K.S.; Cheng, P.F.; Eichhoff, O.M.; Biedermann, T.; Raaijmakers, M.I.G.; Hemmi, S.; Dummer, R.; Levesque, M.P. Hypoxia Contributes to Melanoma Heterogeneity by Triggering HIF1α-Dependent Phenotype Switching. J. Investig. Dermatol. 2013, 133, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Z.; Chen, S.F.; Nieh, S.; Benner, C.; Ger, L.P.; Jan, C.I.; Ma, L.; Chen, C.H.; Hishida, T.; Chang, H.T.; et al. Hypoxia Drives Breast Tumor Malignancy through a TET-TNFalpha-p38-MAPK Signaling Axis. Cancer Res. 2015, 75, 3912–3924. [Google Scholar] [CrossRef] [PubMed]
- Maier, F.C.; Kneilling, M.; Reischl, G.; Cay, F.; Bukala, D.; Schmid, A.; Judenhofer, M.S.; Röcken, M.; Machulla, H.J.; Pichler, B.J. Significant impact of different oxygen breathing conditions on noninvasive in vivo tumor-hypoxia imaging using [18F]-fluoro-azomycinarabino-furanoside ([18F]FAZA). Radiat. Oncol. 2011, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- Souvatzoglou, M.; Grosu, A.L.; Röper, B.; Krause, B.J.; Beck, R.; Reischl, G.; Picchio, M.; Machulla, H.J.; Wester, H.J.; Piert, M. Tumour hypoxia imaging with [18F]FAZA PET in head and neck cancer patients: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Mahling, M.; Fuchs, K.; Thaiss, W.M.; Maier, F.C.; Feger, M.; Bukala, D.; Harant, M.; Eichner, M.; Reutershan, J.; Lang, F.; et al. A Comparative pO2 Probe and [18F]Fluoro-Azomycinarabino-Furanoside ([18F]FAZA) PET Study Reveals Anesthesia-Induced Impairment of Oxygenation and Perfusion in Tumor and Muscle. PLoS ONE 2015, 10, e0124665. [Google Scholar] [CrossRef] [PubMed]
- Piert, M.; Machulla, H.J.; Picchio, M.; Reischl, G.; Ziegler, S.; Kumar, P.; Wester, H.J.; Beck, R.; McEwan, A.J.; Wiebe, L.I.; et al. Hypoxia-specific tumor imaging with 18F-fluoroazomycin arabinoside. J. Nucl. Med. 2005, 46, 106–113. [Google Scholar] [PubMed]
- Schweifer, A.; Maier, F.C.; Ehrlichmann, W.; Lamparter, D.; Kneilling, M.; Pichler, B.J.; Hammerschmidt, F.; Reischl, G. [18F]Fluoro-azomycin-2-deoxy-beta-d-ribofuranoside—A new imaging agent for tumor hypoxia in comparison with [18F]FAZA. Nucl. Med. Biol. 2016, 43, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Roselt, P.; Reischl, G.; Cullinane, C.; Beiki, D.; Ehrlichmann, W.; Binns, D.; Naimi, E.; Yang, J.; Hicks, R.; et al. beta-[18F]Fluoro Azomycin Arabinoside (beta-[18F]FAZA): Synthesis, Radiofluorination and Preliminary PET Imaging of Murine A431 Tumors. Curr. Radiopharm. 2017, 10, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Wanek, T.; Kreis, K.; Krizkova, P.; Schweifer, A.; Denk, C.; Stanek, J.; Mairinger, S.; Filip, T.; Sauberer, M.; Edelhofer, P.; et al. Synthesis and preclinical characterization of 1-(6’-deoxy-6’-[18F]fluoro-beta-d-allofuranosyl)-2-nitroimidazole (beta-6’-[18F]FAZAL) as a positron emission tomography radiotracer to assess tumor hypoxia. Bioorg. Med. Chem. 2016, 24, 5326–5339. [Google Scholar] [CrossRef] [PubMed]
- Schweifer, A.; Malová Križková, P.; Mereiter, K.; Hammerschmidt, F. Preparation of Nonradioactive Standards and a Precursor for a Hypoxia 18F PET Tracer Derived from 1-(β-d-Galactopyranosyl)-2-nitroimidazole. Synthesis 2017, 49, 2933–2938. [Google Scholar]
- Young, J.D.; Yao, S.Y.; Baldwin, J.M.; Cass, C.E.; Baldwin, S.A. The human concentrative and equilibrative nucleoside transporter families, SLC28 and SLC29. Mol. Asp. Med. 2013, 34, 529–547. [Google Scholar] [CrossRef] [PubMed]
- Damaraju, V.L.; Sawyer, M.B.; Mackey, J.R.; Young, J.D.; Cass, C.E. Human nucleoside transporters: Biomarkers for response to nucleoside drugs. Nucleosides Nucleotides Nucleic Acids 2009, 28, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Visser, F.; King, K.M.; Baldwin, S.A.; Young, J.D.; Cass, C.E. The role of nucleoside transporters in cancer chemotherapy with nucleoside drugs. Cancer Metastasis Rev. 2007, 26, 85–110. [Google Scholar] [CrossRef]
- Hendrickson, K.; Phillips, M.; Smith, W.; Peterson, L.; Krohn, K.; Rajendran, J. Hypoxia imaging with [F-18]FMISO-PET in head and neck cancer: Potential for guiding intensity modulated radiation therapy in overcoming hypoxia-induced treatment resistance. Radiother. Oncol. 2011, 101, 369–375. [Google Scholar] [CrossRef]
- Boeke, S.; Thorwarth, D.; Monnich, D.; Pfannenberg, C.; Reischl, G.; La Fougère, C.; Nikolaou, K.; Mauz, P.S.; Paulsen, F.; Zips, D.; et al. Geometric analysis of loco-regional recurrences in relation to pre-treatment hypoxia in patients with head and neck cancer. Acta Oncol. 2017, 56, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Damaraju, V.L.; Damaraju, S.; Young, J.D.; Baldwin, S.A.; Mackey, J.; Sawyer, M.B.; Cass, C.E. Nucleoside anticancer drugs: The role of nucleoside transporters in resistance to cancer chemotherapy. Oncogene 2003, 22, 7524–7536. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Stypinski, D.; Xia, H.; McEwan, A.J.B.; Machulla, H.-J.; Wiebe, L.I. Fluoroazomycin arabinoside (FAZA): Synthesis, 2H and 3H-labelling and preliminary biological evaluation of a novel 2-nitroimidazole marker of tissue hypoxia. J. Label. Compd. Radiopharm. 1999, 42, 3–16. [Google Scholar] [CrossRef]
- Zanato, C.; Testa, A.; Zanda, M. Improved synthesis of the hypoxia probe 5-deutero-5-fluoro-5-deoxy-azomycin arabinoside (FAZA) as a model process for tritium radiolabeling. J. Fluor. Chem. 2013, 155, 110–117. [Google Scholar] [CrossRef]
- Kumar, P.; Ohkura, K.; Beiki, D.; Wiebe, L.I.; Seki, K. Synthesis of 1-beta-d-(5-deoxy-5-iodoarabinofuranosyl)-2-nitroimidazole (beta-IAZA): A novel marker of tissue hypoxia. Chem. Pharm. Bull. 2003, 51, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Al-Maharik, N.; O-Hagan, D. Organofluorine chemistry: Deoxyfluorination reagents for C-F bond synthesis. Aldrichim. Acta 2011, 44, 65–79. [Google Scholar]
- Fujibayashi, Y.; Taniuchi, H.; Yonekura, Y.; Ohtani, H.; Konishi, J.; Yokoyama, A. Copper-62-ATSM: A new hypoxia imaging agent with high membrane permeability and low redox potential. J. Nucl. Med. 1997, 38, 1155–1160. [Google Scholar] [PubMed]
- Busk, M.; Munk, O.L.; Jakobsen, S.; Wang, T.; Skals, M.; Steiniche, T.; Horsman, M.R.; Overgaard, J. Assessing hypoxia in animal tumor models based on pharmocokinetic analysis of dynamic FAZA PET. Acta Oncol. 2010, 49, 922–933. [Google Scholar] [CrossRef]
- Busk, M.; Horsman, M.R.; Jakobsen, S.; Bussink, J.; van der Kogel, A.; Overgaard, J. Cellular uptake of PET tracers of glucose metabolism and hypoxia and their linkage. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 2294–2303. [Google Scholar] [CrossRef]
- Li, F.; Joergensen, J.T.; Hansen, A.E.; Kjaer, A. Kinetic modeling in PET imaging of hypoxia. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 490–506. [Google Scholar]
- Shi, K.; Souvatzoglou, M.; Astner, S.T.; Vaupel, P.; Nüsslin, F.; Wilkens, J.J.; Ziegler, S.I. Quantitative assessment of hypoxia kinetic models by a cross-study of dynamic 18F-FAZA and 15O-H2O in patients with head and neck tumors. J. Nucl. Med. 2010, 51, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Verwer, E.E.; Bahce, I.; van Velden, F.H.P.; Yaqub, M.; Schuit, R.C.; Windhorst, A.D.; Raijmakers, P.; Hoekstra, O.S.; Lammertsma, A.A.; Smit, E.F.; et al. Parametric Methods for Quantification of 18F-FAZA Kinetics in Non–Small Cell Lung Cancer Patients. J. Nucl. Med. 2014, 55, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Verwer, E.E.; van Velden, F.H.P.; Bahce, I.; Yaqub, M.; Schuit, R.C.; Windhorst, A.D.; Raijmakers, P.; Lammertsma, A.A.; Smit, E.F.; Boellaard, R. Pharmacokinetic analysis of [18F]FAZA in non-small cell lung cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Mikenda, W. Solid films and layers deposited on silicon—A versatile infrared sampling technique. Vib. Spectrosc. 1992, 3, 327–330. [Google Scholar] [CrossRef]
- Patt, M.; Kuntzsch, M.; Machulla, H.J. Preparation of [18F]fluoromisonidazole by nucleophilic substitution on THP-protected precursor: Yield dependence on reaction parameters. J. Radioanal. Nucl. Chem. 1999, 240, 925–927. [Google Scholar] [CrossRef]
- Vickers, M.F.; Kumar, R.; Visser, F.; Zhang, J.; Charania, J.; Raborn, R.T.; Baldwin, S.A.; Young, J.D.; Cass, C.E. Comparison of the interaction of uridine, cytidine, and other pyrimidine nucleoside analogues with recombinant human equilibrative nucleoside transporter 2 (hENT2) produced in Saccharomyces cerevisiae. Biochem. Cell Biol. 2002, 80, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Visser, F.; Vickers, M.F.; Lang, T.; Robins, M.J.; Nielsen, L.P.; Nowak, I.; Baldwin, S.A.; Young, J.D.; Cass, C.E. Uridine binding motifs of human concentrative nucleoside transporters 1 and 3 produced in Saccharomyces cerevisiae. Mol. Pharmacol. 2003, 64, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Vickers, M.F.; Zhang, J.; Visser, F.; Tackaberry, T.; Robins, M.J.; Nielsen, L.P.; Nowak, I.; Baldwin, S.A.; Young, J.D.; Cass, C.E. Uridine recognition motifs of human equilibrative nucleoside transporters 1 and 2 produced in Saccharomyces cerevisiae. Nucleosides Nucleotides Nucleic Acids 2004, 23, 361–373. [Google Scholar] [CrossRef] [PubMed]
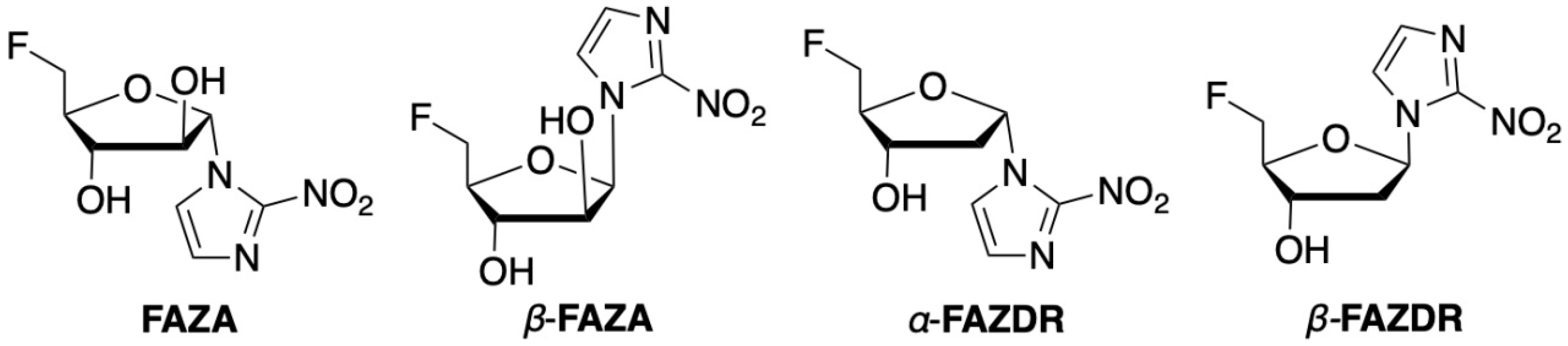
| Compound | Transporter Interaction | 18F-Radiolabeling | PET In Vivo Data (18F-Labeled; Small Animal Studies) |
|---|---|---|---|
| FAZA | * | [12] | [9,10,11,12,13] |
| β-FAZA | * | [14] | [14] |
| α-FAZDR | * | * | * |
| β-FAZDR | * | [13] | [13] and * |
| Compound | hENT1 | hENT2 | hCNT1 | hCNT2 | hCNT3 |
|---|---|---|---|---|---|
| (SLC29A1) | (SLC29A2) | (SLC28A1) | (SLC28A2) | (SLC28A3) | |
| IC50 (µM) | |||||
| FAZA | NI | NI | 290 ± 44 | 640 ± 10 | 1385 ± 100 |
| β-FAZA | 2070 ± 50 | >3000 | >3000 | >3000 | 2309 ± 352 |
| β-FAZDR | NI | >3000 | 269 ± 4 | NI | 124 ± 33 |
| α-FAZDR | NI | NI | NI | NI | NI |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maier, F.C.; Schweifer, A.; Damaraju, V.L.; Cass, C.E.; Bowden, G.D.; Ehrlichmann, W.; Kneilling, M.; Pichler, B.J.; Hammerschmidt, F.; Reischl, G. 2-Nitroimidazole-Furanoside Derivatives for Hypoxia Imaging—Investigation of Nucleoside Transporter Interaction, 18F-Labeling and Preclinical PET Imaging. Pharmaceuticals 2019, 12, 31. https://doi.org/10.3390/ph12010031
Maier FC, Schweifer A, Damaraju VL, Cass CE, Bowden GD, Ehrlichmann W, Kneilling M, Pichler BJ, Hammerschmidt F, Reischl G. 2-Nitroimidazole-Furanoside Derivatives for Hypoxia Imaging—Investigation of Nucleoside Transporter Interaction, 18F-Labeling and Preclinical PET Imaging. Pharmaceuticals. 2019; 12(1):31. https://doi.org/10.3390/ph12010031
Chicago/Turabian StyleMaier, Florian C., Anna Schweifer, Vijaya L. Damaraju, Carol E. Cass, Gregory D. Bowden, Walter Ehrlichmann, Manfred Kneilling, Bernd J. Pichler, Friedrich Hammerschmidt, and Gerald Reischl. 2019. "2-Nitroimidazole-Furanoside Derivatives for Hypoxia Imaging—Investigation of Nucleoside Transporter Interaction, 18F-Labeling and Preclinical PET Imaging" Pharmaceuticals 12, no. 1: 31. https://doi.org/10.3390/ph12010031
APA StyleMaier, F. C., Schweifer, A., Damaraju, V. L., Cass, C. E., Bowden, G. D., Ehrlichmann, W., Kneilling, M., Pichler, B. J., Hammerschmidt, F., & Reischl, G. (2019). 2-Nitroimidazole-Furanoside Derivatives for Hypoxia Imaging—Investigation of Nucleoside Transporter Interaction, 18F-Labeling and Preclinical PET Imaging. Pharmaceuticals, 12(1), 31. https://doi.org/10.3390/ph12010031






